Abstract
Background
In US clinical practice, many patients who undergo placement of an implantable cardioverter‐defibrillator (ICD) for primary prevention of sudden cardiac death receive dual‐chamber devices. The superiority of dual‐chamber over single‐chamber devices in reducing the risk of inappropriate ICD shocks in clinical practice has not been established. The objective of this study was to compare risk of adverse outcomes, including inappropriate shocks, between single‐ and dual‐chamber ICDs for primary prevention.
Methods and Results
We identified patients receiving a single‐ or dual‐chamber ICD for primary prevention who did not have an indication for pacing from 15 hospitals within 7 integrated health delivery systems in the Longitudinal Study of Implantable Cardioverter‐Defibrillators from 2006 to 2009. The primary outcome was time to first inappropriate shock. ICD shocks were adjudicated for appropriateness. Other outcomes included all‐cause hospitalization, heart failure hospitalization, and death. Patient, clinician, and hospital‐level factors were accounted for using propensity score weighting methods. Among 1042 patients without pacing indications, 54.0% (n=563) received a single‐chamber device and 46.0% (n=479) received a dual‐chamber device. In a propensity‐weighted analysis, device type was not significantly associated with inappropriate shock (hazard ratio, 0.91; 95% confidence interval, 0.59–1.38 [P=0.65]), all‐cause hospitalization (hazard ratio, 1.03; 95% confidence interval, 0.87–1.21 [P=0.76]), heart failure hospitalization (hazard ratio, 0.93; 95% confidence interval, 0.72–1.21 [P=0.59]), or death (hazard ratio, 1.19; 95% confidence interval, 0.93–1.53 [P=0.17]).
Conclusions
Among patients who received an ICD for primary prevention without indications for pacing, dual‐chamber devices were not associated with lower risk of inappropriate shock or differences in hospitalization or death compared with single‐chamber devices. This study does not justify the use of dual‐chamber devices to minimize inappropriate shocks.
Keywords: defibrillator shocks, implantable cardioverter‐defibrillators, outcomes research
Subject Categories: Sudden Cardiac Death, Quality and Outcomes
Clinical Perspective
What Is New?
In this large observational study of a contemporary population of patients receiving an implantable cardioverter‐defibrillator for primary prevention who did not have an indication for pacing, there was no association between the use of a dual‐chamber defibrillator compared with a single‐chamber device with respect to the risk of inappropriate shocks, hospitalization, or death.
What Are the Clinical Implications?
Given the higher costs and known higher risks of complications associated with dual‐chamber devices, this study does not support their use in patients receiving a primary prevention ICD when there is no indication for pacing.
Introduction
Among patients receiving an implantable cardioverter‐defibrillator (ICD) for primary prevention of sudden cardiac death, a central decision is whether to implant a single‐ or dual‐chamber device. Dual‐chamber devices are appropriate in patients who require pacing. However, they are used in more than half of patients without a pacing indication, a practice that varies widely by institution and is largely unrelated to patient characteristics.1, 2 Compared with single‐chamber devices, dual‐chamber devices are more complex and costly and are associated with more complications and no difference in hospitalizations or mortality.3 Yet, dual‐chamber devices may theoretically offer improved arrhythmia discrimination and reduction in inappropriate therapies.
Dual‐chamber ICDs might be used in patients without an indication for pacing in the hopes of reducing the risks of inappropriate shocks, an important complication that can cause pain, anxiety, depression, and impaired quality of life.4, 5 Further, inappropriate shocks may be proarrhythmic and are associated with higher mortality.6, 7, 8 The most common cause for inappropriate shocks is supraventricular tachycardia incorrectly identified by the device as a lethal ventricular tachyarrhythmia.9 Dual‐chamber ICDs may have enhanced arrhythmia discrimination, which, in theory, might reduce inappropriate shocks caused by supraventricular tachycardia. However, existing data regarding the benefits of dual‐chamber devices with respect to inappropriate therapies are inconclusive.10, 11, 12, 13, 14, 15, 16, 17
Accordingly, the primary aim of this study was to compare the risks of inappropriate ICD shocks between single‐ and dual‐chamber devices among patients without an indication for pacing in the LS‐ICD (Longitudinal Study of Implantable Cardioverter‐Defibrillators),18 in a community‐based cohort of patients receiving primary prevention ICDs. We further evaluated outcomes of hospitalization and death.
Methods
Data Sources
The Cardiovascular Research Network (CVRN) LS‐ICD has been previously described in detail.18 The data for this study were derived from: (1) the National Cardiovascular Data Registry (NCDR) ICD Registry; (2) the CVRN virtual data warehouse, which includes information from each health plan's electronic medical record and administrative databases; and (3) a database of ICD‐delivered therapies. Pacing indications, device type, clinical characteristics, and provider characteristics were collected from the NCDR ICD Registry.19 The NCDR implements a data quality program that includes data and range checks, outlier analysis, and random audits.20 All hospitals participating in this study report data to the NCDR on all ICD implants regardless of payer or indication. Data on outcomes of hospitalization and death were obtained from the CVRN virtual data warehouse.
Data on inappropriate shocks were obtained from the ICD therapy database.18 In brief, data on arrhythmia episodes resulting in device therapies were collected from device interrogation reports and medical records at the study sites. Board‐certified electrophysiologists reviewed intracardiac electrograms and relevant clinical notes to adjudicate each therapy as appropriate, inappropriate, or uncertain. Two adjudicators reviewed each therapy independently. Discrepancies in appropriateness determinations were resolved by conference of reviewer pairs or review by an additional adjudicator. Institutional review boards at participating sites approved the study and a waiver of informed consent was obtained because of the study design.
Study Population
Consecutive adult patients (aged >18 years) undergoing first‐time ICD implantation for the primary prevention of sudden cardiac death in 15 hospitals within 7 integrated healthcare delivery systems in the CVRN between January 1, 2006, and December 31, 2009 (n=2787) were identified. We excluded patients not enrolled in one of the 7 CVRN health plans at the time of implantation (n=431), those with a left ventricular ejection fraction >0.35 (n=57), those with a prior tachycardic arrest (n=34), and those who received a biventricular device (n=722). We further excluded those with a documented indication for pacing (n=501). Pacing indications were ascertained from NCDR data and included: second‐ or third‐degree heart block, previous bradycardic arrest, abnormal sinus node function, or a documented paced rhythm. The final analytic cohort included 1042 patients (Figure 1).
Figure 1.
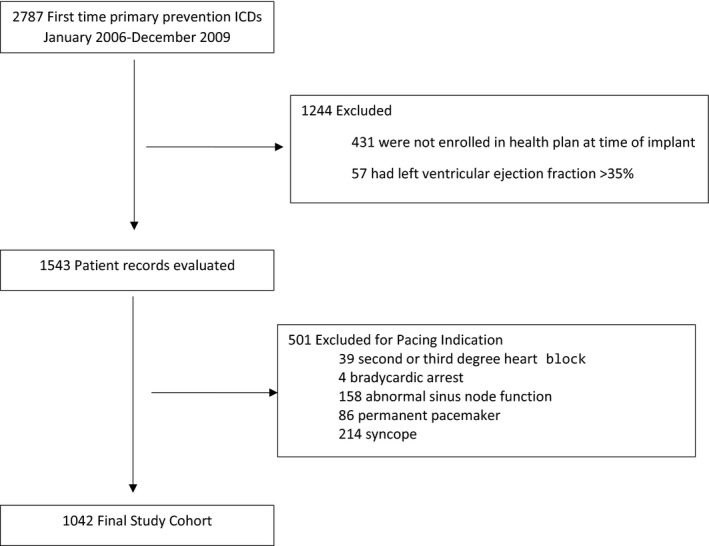
Assembly of study cohort of adults receiving single‐ vs dual‐chamber implantable cardioverter‐defibrillator (ICD) therapy for primary prevention of sudden cardiac death and no indication for pacing.
Outcomes
The primary outcome was time to first inappropriate shock. Therapies were deemed appropriate, inappropriate or uncertain using standardized definitions developed by the expert panel based on literature. Follow‐up time for device therapy was as long as 3 years.
Secondary end points included time to all‐cause hospitalization, heart failure hospitalization, and all‐cause death. Hospitalizations were ascertained from the virtual data warehouse, which includes diagnoses for all hospitalizations, including those outside of health plan facilities. Heart failure hospitalizations were identified using the principal discharge diagnosis International Classification of Diseases, Ninth Edition codes 428, 402.01, 402.11, 402.91, 404.91, 404.01, 404.03, 404.11, 404.13, 404.93, and 398.91. Death was ascertained through the virtual data warehouse, which employs health system databases, Social Security Administration vital status files, and state death certificate registries, depending on the site. Follow‐up time for death and hospitalization was up to 6 years.
Patient Characteristics
Patient characteristics included demographics, reason for admission at time of implant, cardiovascular history, family history of sudden death, comorbidities, laboratory values and systolic blood pressure at the time of implantation, and cardiovascular studies (electrophysiology study performed [yes/no], left ventricular ejection fraction, QRS duration, QRS morphology, first‐degree atrioventricular block). Heart failure medications prescribed at discharge following device implantation were also considered, as they are known to influence mortality and hospitalization outcomes and may also influence inappropriate shocks.
To avoid case‐wise deletion, missing values were imputed. Variables were generally missing infrequently (<0.5%); in these cases, continuous variables were imputed using the median value of known measurements in the population and categorical variables were imputed as the most common response in the population. Because atrioventricular conduction (6.0%) and intraventricular conduction (5.9%) were more frequently missing, a dummy variable for missing status was employed.
Device setting data were available in 971 (93.2%) patients. Device settings were abstracted from surgical implant notes and device interrogation reports and included arrhythmia detection enhancements (on/off) and the tachycardia rate threshold for delivery of ICD therapy. The lowest programmed rate threshold, regardless of arrhythmia detection zone, was categorized as <180, 180 to 199, and >200 beats per minute. When available, settings at the time of implant were considered. If device settings were missing at the time of implant, but available by interrogation report at the time of inappropriate shock, those settings were considered.
Statistical Analysis
Baseline characteristics of patients receiving single‐ and dual‐chamber devices were compared using t tests for continuous variables and chi‐square tests for categorical variables. Death was considered as a competing risk for inappropriate shock and hospitalization outcomes. Cox regression was used to estimate the cause‐specific hazard of device‐type effects. The crude hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were estimated accounting for clustering by hospital by including hospital as a random effect.
To create more comparable treatment groups, we fit a propensity score model using logistic regression for treatment with a dual‐chamber (versus single‐chamber) device, and then applied the stabilized inverse probability of treatment weight in the analysis of outcomes. In the propensity of treatment model, the linearity and function forms of continuous variables were verified by graphic evaluation. Colinearity among variables was also examined. The probability that a patient received a dual‐chamber ICD was modeled as a function of the patient, clinician, and hospital characteristics in Table 1, except for medications prescribed at discharge, which were subsequently accounted for in Cox models. Hospitals were included as a random effect in the propensity model to account for the clustering of patients within the same hospitals. The propensity score model was evaluated for its overlap of propensity score distribution. Because of a few outliers of propensity score in our cohort, the trimmed stabilized inverse probability of treatment weight was calculated for each patient. The trim was based on 5 and 95 percentiles of propensity scores. All patients were included in the weighted analysis. Standardized differences were examined in the weighted sample to assess the balance of measured baseline covariates between patients receiving single‐ and dual‐chamber ICDs. Small absolute differences in standardized mean differences (<0.10) support the assumption of balance of observed variables between treatment groups.21
Table 1.
Baseline Patient, Physician, and Hospital Characteristics by Single‐ and Dual‐Chamber ICD Device Type
| Total (N=1042) | Single‐Chamber (n=563) | Dual‐Chamber (n=479) | P Value | |
|---|---|---|---|---|
| Age, mean±SD, y | 65.1±11.6 | 62.4±12.1 | 68.2±10.2 | <0.001 |
| Women, No. (%) | 232 (22.3) | 134 (23.8) | 98 (20.5) | 0.196 |
| Race/ethnicity, No. (%) | 0.020 | |||
| White | 618 (59.3) | 355 (63.1) | 263 (54.9) | |
| Black | 171 (16.4) | 80 (14.2) | 91 (19.0) | |
| Hispanic | 169 (16.2) | 80 (14.2) | 89 (18.6) | |
| Other/unknown | 84 (8.1) | 48 (8.5) | 36 (7.5) | |
| Reason for admission | <0.001 | |||
| Admitted for ICD, No. (%) | 920 (88.3) | 521 (92.5) | 399 (83.3) | |
| Other | 122 (11.7) | 42 (7.5) | 80 (16.7) | |
| History and risk factors, No. (%) | ||||
| Cerebrovascular disease | 136 (13.1) | 67 (11.9) | 69 (14.4) | 0.232 |
| Chronic lung disease | 183 (17.6) | 78 (13.9) | 105 (21.9) | 0.001 |
| Diabetes mellitus | 444 (42.6) | 227 (40.3) | 217 (45.3) | 0.105 |
| Hypertension | 742 (71.2) | 352 (62.5) | 390 (81.4) | <0.001 |
| Renal failure––dialysis | 20 (1.9) | 14 (2.5) | 6 (1.3) | 0.148 |
| Family history of sudden death | 38 (3.6) | 27 (4.8) | 11 (2.3) | 0.032 |
| NYHA, No. (%) | 0.006 | |||
| Class I | 178 (17.1) | 80 (14.2) | 98 (20.5) | |
| Class II | 671 (64.4) | 386 (68.6) | 285 (59.5) | |
| Class III or IV | 193 (18.5) | 97 (17.2) | 96 (20.0) | |
| Atrial fibrillation/atrial flutter, No. (%) | 219 (21.0) | 118 (21.0) | 101 (21.1) | 0.960 |
| Ventricular tachycardia, any | 179 (17.2) | 80 (14.2) | 99 (20.7) | 0.006 |
| Nonischemic dilated cardiomyopathy | 361 (34.6) | 219 (38.9) | 142 (29.6) | 0.002 |
| Ischemic heart disease | 673 (64.6) | 343 (60.9) | 330 (68.9) | 0.007 |
| Myocardial infarction | 602 (57.8) | 310 (55.1) | 292 (61.0) | 0.055 |
| Coronary artery bypass surgery | 309 (29.7) | 169 (30.0) | 140 (29.2) | 0.781 |
| Percutaneous coronary intervention | 357 (34.3) | 189 (33.6) | 168 (35.1) | 0.610 |
| Valve repair/replacement | 48 (4.6) | 32 (5.7) | 16 (3.3) | 0.072 |
| Heart failure | 909 (87.2) | 481 (85.4) | 428 (89.4) | 0.059 |
| Electrophysiology study performed | 24 (2.3) | 15 (2.7) | 9 (1.9) | 0.400 |
| Ejection fraction, mean±SD | 25.0±6.0 | 24.8±5.8 | 25.2±6.2 | 0.164 |
| QRS duration, mean±SD, ms | 113.8±26.8 | 111.3±25.0 | 116.6±28.5 | 0.012 |
| Atrioventricular conduction, No. (%) | <0.001 | |||
| Normal | 818 (78.5) | 444 (78.9) | 374 (78.1) | |
| First‐degree atrioventricular block | 161 (15.5) | 65 (11.5) | 96 (20.0) | |
| Missing | 63 (6.0) | 54 (9.6) | 9 (1.9) | |
| Intraventricular conduction, No. (%) | ||||
| Normal | 610 (58.5) | 332 (59.0) | 278 (58.0) | <0.001 |
| Left bundle branch block | 145 (13.9) | 73 (13.0) | 72 (15.0) | |
| Right bundle branch block | 85 (8.2) | 33 (5.9) | 52 (10.9) | |
| Other | 141 (13.5) | 73 (13.0) | 68 (14.2) | |
| Missing | 61 (5.9) | 52 (9.2) | 9 (1.9) | |
| Creatinine mg/dL, mean±SD | 1.3±1.0 | 1.3±0.8 | 1.4±1.1 | 0.053 |
| Blood urea nitrogen, mg/dL, mean±SD | 23.3±12.6 | 23.2±13.2 | 23.5±11.9 | 0.387 |
| Sodium, mEq/L, mean±SD | 139.0±3.2 | 139.2±3.3 | 138.7±3.0 | 0.001 |
| Systolic blood pressure, mm Hg, mean±SD | 123.3±19.0 | 120.6±18.1 | 126.3±19.6 | <0.001 |
| Discharge medications | ||||
| ARB | 210 (20.2) | 126 (22.4) | 84 (17.5) | 0.052 |
| ACEI | 731 (70.2) | 392 (69.6) | 339 (70.8) | 0.687 |
| β‐Blocker | 947 (90.9) | 518 (92.0) | 429 (89.6) | 0.172 |
| Aldosterone | 355 (34.1) | 196 (34.8) | 159 (33.2) | 0.583 |
| Physician characteristics | ||||
| Physician annual ICD implants, No. (%) | <0.001 | |||
| <25 | 153 (14.7) | 42 (7.5) | 111 (23.2) | |
| 25 to ≤100 | 794 (76.2) | 499 (88.6) | 295 (61.6) | |
| >100 | 95 (9.1) | 22 (3.9) | 73 (15.2) | |
| Physician training adult electrophysiology | 778 (74.7) | 412 (73.2) | 366 (76.4) | 0.232 |
| Hospital characteristics, No. (%) | ||||
| Hospital annual ICD implants | <0.001 | |||
| ≤50 | 215 (20.6) | 158 (28.1) | 57 (11.9) | |
| >50 to ≤200 | 199 (19.1) | 118 (21.0) | 81 (16.9) | |
| >200 | 628 (60.3) | 287 (51.0) | 341 (71.2) | |
| Patients beds | <0.001 | |||
| ≤500 | 844 (81.0) | 431 (76.6) | 413 (86.2) | |
| >500 | 198 (19.0) | 132 (23.4) | 66 (13.8) | |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ICD, implantable cardioverter‐defibrillator; NYHA, New York Heart Association classification.
The associations of ICD type with the outcomes were evaluated in multivariable Cox proportional hazards models using trimmed stabilized weights. Models further adjusted for unbalanced covariates as well as medications (β‐blockers, angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers, and spironolactone) prescribed at discharge because these therapies could influence future risk of inappropriate shock. To account for clustering of patients by hospital, frailty models were used with hospital included as a random effect. For inappropriate shock, patients were not censored if they had an appropriate or uncertain shock. For the outcomes of time to first inappropriate shock and all‐cause hospitalization, patients were censored at health plan disenrollment or death. For the outcome of death, patients were censored at the time of last documented encounter regardless of the continuity of health plan enrollment. The proportional hazards assumption for ICD treatment was evaluated by testing the interaction of treatment and time. A secondary analysis of time to first inappropriate shock was performed including device settings in the model.
Because 13.5% of shocks (51/377) were classified as uncertain by the adjudication panel, a sensitivity analysis was performed. In the “best‐case” scenario (an assumption favoring dual‐chamber devices), all uncertain shocks among dual‐chamber devices were classified as appropriate and all uncertain shocks among single‐chamber devices were classified as inappropriate. In the “worst‐case” scenario (an assumption favoring single‐chamber devices), all uncertain shocks among dual‐chamber devices were classified as inappropriate and all uncertain shocks among single‐chamber devices were classified as appropriate. All statistical analyses were performed using SAS version 9.4 software (SAS Institute Inc).
Results
The study population consisted of 1042 patients receiving a first time primary prevention ICD with no pacing indication. There were 563 (54.0%) single‐chamber devices and 479 (46.0%) dual‐chamber devices, with use of dual‐chamber devices ranging from 8.4% to 85.8% by site. Patients who received dual‐chamber devices were older; more likely to be black or Hispanic; have a history of chronic lung disease, hypertension, ischemic heart disease, or ventricular tachycardia; and have first‐degree atrioventricular block and a wider QRS duration (Table 1). In the weighted sample, the absolute value of standardized difference in measured covariates between device types was <0.10 for all except 3 of the 33 total variables (Table 2).
Table 2.
Standardized Difference in Patient, Physician, and Hospital Characteristics Between Single‐ and Dual‐Chamber Device Type in a Propensity Score Weighted Sample
| Single | Dual | Standardized Difference | |
|---|---|---|---|
| Age, mean, y | 64.8 | 64.5 | 0.029 |
| Women | 24.0 | 22.1 | 0.046 |
| Race/ethnicity | |||
| White | 62.9 | 59.2 | 0.075 |
| Black | 12.9 | 16.8 | −0.107 |
| Hispanic | 15.4 | 14.3 | 0.031 |
| Other/unknown | 8.8 | 9.7 | −0.032 |
| Reason for admission | |||
| Admitted for ICD | 86.7 | 87.5 | −0.022 |
| Other | 13.3 | 12.5 | 0.022 |
| History and risk factors | |||
| Cerebrovascular disease | 11.6 | 12.4 | −0.026 |
| Chronic lung disease | 17.5 | 21.6 | −0.104 |
| Diabetes mellitus | 38.2 | 37.3 | 0.019 |
| Hypertension | 67.7 | 69.0 | −0.028 |
| Dialysis | 1.9 | 2.0 | −0.006 |
| Family history of sudden death | 3.6 | 3.6 | 0.005 |
| NYHA | |||
| Class I | 15.7 | 12.4 | 0.094 |
| Class II | 65.6 | 65.9 | −0.006 |
| Class III and IV | 18.7 | 21.6 | −0.074 |
| Atrial fibrillation/atrial flutter | 27.1 | 28.5 | −0.03 |
| Ventricular tachycardia, any | 18.3 | 17.3 | 0.026 |
| Ventricular tachycardia, none | 81.7 | 82.7 | −0.026 |
| Nonischemic dilated cardiomyopathy | 37.0 | 37.7 | −0.014 |
| Ischemic heart disease | 62.0 | 61.6 | 0.007 |
| Myocardial infarction | 53.1 | 52.2 | 0.019 |
| Coronary artery bypass surgery | 26.3 | 30.5 | −0.093 |
| Percutaneous coronary intervention | 31.8 | 27.5 | 0.094 |
| Valve replacement/repair | 6.1 | 6.6 | −0.017 |
| Heart failure | 86.5 | 87.8 | −0.04 |
| Electrophysiology study performed | 3.4 | 2.3 | 0.068 |
| Ejection fraction, mean, % | 25.1 | 25.1 | 0.001 |
| QRS duration, mean, ms | 113.359 | 113.168 | 0.007 |
| Atrioventricular conduction | |||
| Normal | 82.4 | 80.6 | 0.046 |
| First‐degree atrioventricular block | 11.0 | 13.6 | −0.082 |
| Missing | 6.6 | 5.7 | 0.037 |
| Intraventricular conduction | |||
| Normal | 59.0 | 59.2 | −0.005 |
| Left bundle branch block | 13.5 | 12.1 | 0.043 |
| Right bundle branch block | 6.9 | 7.3 | −0.015 |
| Other | 14.2 | 15.7 | −0.041 |
| Missing | 6.4 | 5.7 | 0.028 |
| Creatinine, mg/dL, mean | 1.31 | 1.35 | −0.057 |
| Blood urea nitrogen, mg/dL, mean | 24.6 | 24.2 | 0.03 |
| Sodium, mEq/L, mean | 138.9 | 139.2 | −0.086 |
| Systolic blood pressure, mm Hg, mean | 121.9 | 122.6 | −0.037 |
| Physician characteristics | |||
| No. of physician annual ICD implants | |||
| <25 | 15.2 | 15.8 | −0.017 |
| >25 to ≤100 | 77.0 | 75.0 | 0.046 |
| >100 | 7.8 | 9.1 | −0.048 |
| Adult electrophysiology training | 72.9 | 76.4 | −0.083 |
| Hospital characteristics | |||
| No. of hospital annual ICD implants | |||
| ≤50 | 23.1 | 15.9 | 0.182 |
| >50 to ≤200 | 19.7 | 19.2 | 0.014 |
| >200 | 57.2 | 64.9 | −0.159 |
| Patient beds | |||
| ≤500 | 79.9 | 81.7 | −0.046 |
| >500 | 20.1 | 18.3 | 0.046 |
ICD indicates implantable cardioverter‐defibrillator; NYHA, New York Heart Association Classification.
During a median follow‐up of 2.0 years for device therapies, 17.3% received any shock, 6.6% (n=69) received at least one inappropriate shock (6.9% for single versus 6.3% for dual, P=0.67), and 3.6% (n=37) received at least one shock of uncertain appropriateness (4.3% for single versus 2.7% for dual, P=0.18). The most common cause of inappropriate shock was atrial fibrillation/supraventricular tachycardia (81.0%), followed by sensing abnormality (17.5%). The cause of inappropriate shock did not differ by device type (P=0.72). The total number of inappropriate shocks did not differ by device type. Among those with a single‐chamber device, 52% (n=125) of shocks were appropriate, 33% (n=80) were inappropriate, and 14% (n=35) were uncertain. Among those with a dual‐chamber device, 47% (n=64) of shocks were appropriate, 42% (n=57) were inappropriate, and 12% (n=16) were uncertain (P=0.26 for comparison by device type).22 There was no difference between device type in unadjusted cumulative incidence analysis of time to first inappropriate shock (Gray's test for equality, P=0.75; Figure 2) or in an unadjusted Cox model accounting for clustering by hospital (HR, 0.91; 95% CI, 0.60–1.38 [P=0.65] for dual‐ versus single‐chamber ICD). In multivariable analysis, the risk of inappropriate shock was not significantly different for dual‐ versus single‐chamber devices (HR, 0.91; 95% CI, 0.59–1.38 [P=0.65]).
Figure 2.
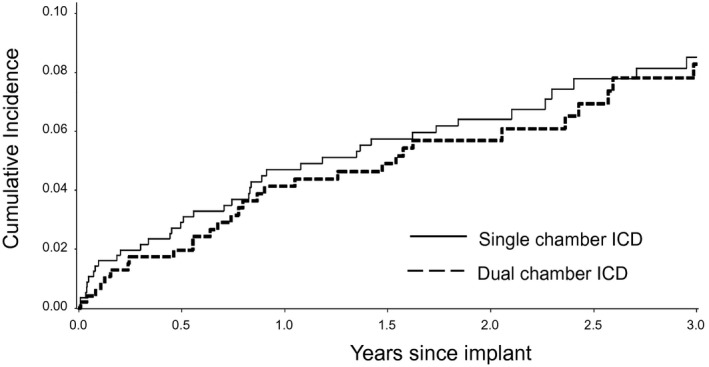
Cumulative incidence curves of time to each outcome by device type. ICD indicates implantable cardioverter‐defibrillator.
A secondary analysis was performed among the 981 (93%) patients with device setting data. After accounting for device settings, the hazard of inappropriate shock was not significantly different between single‐ and dual‐chamber devices (HR, 0.89; 95% CI, 0.58–1.38 [P=0.65]).
We also performed secondary analyses assigning all uncertain shocks as appropriate or inappropriate as specified above. In the best‐case scenario for dual‐chamber devices, there was a lower hazard of inappropriate shock with dual‐chamber devices, but the difference was of marginal statistical significance (HR, 0.67; 95% CI, 0.45–1.00 [P=0.05]). In the worst‐case scenario, dual‐chamber devices were not significantly associated with inappropriate shock (HR, 1.08; 95% CI, 0.72–1.62 [P=0.72]). The estimates in the best‐ and worst‐case scenarios restricted to patients with recorded device settings were similar.
During a median follow‐up of 2.4 years, all‐cause hospitalization and heart failure hospitalization were not significantly different by device type in unadjusted analysis (HR, 1.13; 95% CI, 0.92–1.39 [P=0.26] and HR, 1.00; 95% CI, 0.70–1.43 [P=1.0], respectively) or in multivariable analysis, (HR, 1.03; 95% CI, 0.87–1.21 [P=0.76] for all‐cause hospitalization and HR, 0.93; 95% CI, 0.72–1.21 [P=0.59] for heart failure hospitalization).
The overall mortality rate was 24.6% (n=256) over a median of 2.9 years. Mortality was not significantly different by device type in unadjusted analysis (HR, 1.25; 95% CI, 0.98–1.60 [P=0.07]) or in multivariable analysis (HR, 1.19; 95% CI, 0.93–1.53 [P=0.17]).
Discussion
In this cohort of patients without an indication for pacing receiving an ICD for primary prevention in clinical practice, the use of a dual‐chamber device was not associated with significantly lower risks of inappropriate shock, death, or hospitalization. Even after accounting for device programming, inappropriate shocks did not differ between single‐ and dual‐chamber devices. These results have important implications for the standard of care for patients receiving primary prevention ICDs.
Randomized trials of single‐ versus dual‐chamber devices to reduce inappropriate therapies have been inconclusive.10, 11, 12 Similarly, clinical trials comparing settings that simulate a single‐chamber device with settings that utilize both leads among patients who have all received dual‐chamber devices are also conflicting.14, 15, 16, 17 A limitation and one potential explanation for the variable results of these trials is that they included patients who received an ICD for secondary prevention. Arrhythmia characteristics and shock rates may differ among patients with secondary prevention, among whom ventricular tachycardia tends to occur at a slower rate with greater overlap with supraventricular tachycardia.9 This limits the value of these trials in directing device selection in patients who receive devices for primary prevention, who comprise the majority of patients undergoing device implantation.23 A recent trial limited to patients with primary prevention without a pacing indication and using standardized optimal programming strategies for single‐ and dual‐chamber ICDs found a 2% inappropriate shock rate over 1 year and no difference between single‐ and dual‐chamber devices.13
Another potential explanation for the mixed results of prior trials is differences in the programming of devices. Recent trials of programming strategies demonstrate that higher detection rates and longer detection intervals reduce inappropriate therapy and mortality among primary prevention patients.24, 25 These advanced programming strategies to reduce shock burden were not used in previous comparisons of dual‐ versus single‐chamber ICDs. However, these effective programming strategies do not require a dual‐chamber device. Although the programming strategies have evolved since the time the devices in this study were implanted, one would expect any benefit of dual‐chamber devices to be more apparent in a cohort with older programming strategies. Our study was not designed to evaluate the mechanistic performance of single‐ versus dual‐chamber rhythm discrimination, but rather to evaluate the real‐world effectiveness and outcomes of a treatment strategy of dual‐chamber selection. As such, our findings add materially to the current understanding of the role of dual‐chamber devices in reducing inappropriate shocks among primary prevention recipients in contemporary community practice.
Data comparing single‐ and dual‐chamber devices with regard to inappropriate shock in clinical practice are also limited. A report from the Danish ICD Registry of 1609 patients receiving primary prevention ICDs from 2007 to 2011 found a higher risk of inappropriate shock with dual‐chamber compared with single‐chamber devices.26 However, device settings were not collected, the population was not limited to those without a pacing indication, and patients who received cardiac resynchronization therapy were included. Another report from a French registry of 2538 primary prevention devices implanted from 2002 to 2012 found a higher rate of procedural complications and generator replacements with dual‐chamber devices and no differences in appropriate shocks, inappropriate shocks, or mortality. However, the registry did not exclude those with pacing indications; no adjustment was made for patient, provider, or hospital characteristics; and device programming was not collected in the registry. Thus, our study expands the current understanding of the outcomes of patients undergoing the strategy of dual‐chamber devices in contemporary practice in the United States.
Given the higher cost of dual‐chamber devices, the absence of benefit in outcomes suggests that the use of dual‐chamber devices without a pacing indication does not represent high‐value health care. Despite the absence of compelling evidence to support the use of dual‐chamber ICDs, they are used in a large proportion of patients in the United States who receive primary prevention ICD therapy who do not have an indication for pacing.1 The routine implantation of single‐chamber ICDs rather than dual‐chamber devices could save an estimated $200 million annually in the United States.13 The costs of generator replacements, complications, and lead replacements should also be considered, as battery life is typically shorter, complications are more common, and the potential for lead failure is greater with dual‐chamber devices.1, 27 Our study provides additional information that in real‐world settings, dual‐chamber devices do not reduce inappropriate shocks. Additional evidence is needed to identify the subset of patients who systematically benefit from dual‐chamber devices, which would greatly improve the cost‐benefit ratio of this therapy.
An important observation in our study was the relatively low rate of inappropriate shocks compared with the rates reported in the primary prevention trials that were published a decade or more ago.28, 29 The lower shock rate observed in more contemporary cohorts may be related to optimized programming such as higher rate detection thresholds.26, 30 The lower rates of inappropriate shock in contemporary practice, while beneficial to patients, increase the challenges of demonstrating the benefits of additional interventions such as dual‐chamber devices. However, given the greater cost and risk, the benefit of dual‐chamber devices should be proven to justify their use.
Based on data from randomized controlled trials, in which the inappropriate shock rate was ≈20%, this sample size provided 80% power to detect a minimum absolute difference in rates of 7.3%. However, the rate of inappropriate shock in this cohort was much lower. With our sample size and observed inappropriate shock rate of 6.6%, we had 80% power to detect a minimum absolute difference of ≈4.5%. The low rate of inappropriate shock in our study highlights the challenges in identifying the benefits of strategies designed to further reduce the risks of inappropriate shocks. Ultimately, treatment strategies associated with higher costs and complications should be proven effective before they are widely implemented. In the case of dual‐chamber ICDs among patients without pacing indications, the proof of effectiveness in improving outcomes, including inappropriate shocks, is not available.
Study Limitations and Strengths
The results of this study should be interpreted in the context of the following limitations. First, 38 patients received at least one shock for which the appropriateness of shock therapy was uncertain based on available interrogation and clinical record data. However, results were consistent in a sensitivity analysis assigning uncertain shocks based on best‐case and worst‐case scenarios. Further, the availability of adjudicated shock data is a significant strength of this study and is unique to this clinical practice population. Second, programming of devices was not standardized. However, we were able to account for programmed detection rate and discrimination algorithms among a large subset of the cohort studied. Similarly, longitudinal data on device settings were not available. However, implant settings remain relevant for the outcome of time to first inappropriate shock. Reprogramming of the device may occur at any time but is more likely following an inappropriate shock. Third, follow‐up was limited to up to 3 years, while the risk of inappropriate shocks persists over a lifetime. However, the median follow‐up of 2 years is aligned with other studies of outcomes in patients with left ventricular systolic dysfunction. Third, we did not capture lead or generator revisions. Within the follow‐up time, patients may have undergone lead or generator revisions, including upgrades from single‐ to dual‐chamber devices or to cardiac resynchronization therapy. However, we expect the rate of revisions for the purpose of upgrade to be low within this time frame. Finally, selection bias may be present in those who received single‐ versus dual‐chamber devices because treating physicians may have been more likely to implant a dual‐chamber device in patients who they felt might have a greater need for rhythm discrimination. Thus, the similar rate of inappropriate shock observed between single‐ and dual‐chamber devices in this study may not be the same if patients were randomized to single‐ or dual‐chamber devices. However, propensity score weighting should mitigate this concern. Similarly, the data are observational and residual confounding by unmeasured confounders cannot be fully excluded despite our ability to account for a wide range of measured potential confounders.
Conclusions
Among a cohort of patients without pacing indications who received an ICD for primary prevention in contemporary community practice, dual‐chamber ICDs were not associated with a lower risk of inappropriate shocks, hospitalization, or death. This study adds to the literature comparing single‐ versus dual‐chamber ICDs with regard to inappropriate shocks, informing the selection of devices in patients undergoing primary prevention ICD therapy and supporting the use of simpler, less expensive single‐chamber devices unless a compelling indication for pacing is identified.
Sources of Funding
Dr Peterson was supported by grant K08HS019814‐01 from the Agency for Healthcare Research and Quality. This project was funded under contract No. 290201000008I from the Agency for Healthcare Research and Quality, US Department of Health and Human Services as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program, and by the American College of Cardiology Foundation, with support from the National Heart, Lung, and Blood Institute (U19HL091179). While the sponsoring organizations have been involved in discussions of this research as it has progressed and have provided oversight and guidance, the authors of this report are solely responsible for its content. Sponsorship may not be construed as an endorsement of all statements in the report by the Agency for Healthcare Research and Quality; the National Heart, Lung, and Blood Institute; and the US Department of Health and Human Services or the American College of Cardiology Foundation. The views expressed in this article are those of the authors and do not necessarily reflect the view of the Department of Veterans Affairs.
Disclosures
Dr Masoudi has a contract with the American College of Cardiology Foundation. Dr Hayes serves as a consultant for Medtronic and Boston Scientific. All other authors have no disclosures.
Acknowledgments
Dr Zeng and Ms Glenn had access to all data and affirm its accuracy. The views expressed in this article are those of the authors and do not necessarily reflect the view of the Department of Veterans Affairs.
(J Am Heart Assoc. 2017;6:e006937 DOI: 10.1161/JAHA.117.006937.)29122811
This article was handled independently by N.A. Mark Estes III, MD, as a guest editor.
References
- 1. Dewland TA, Pellegrini CN, Wang Y, Marcus GM, Keung E, Varosy PD. Dual‐chamber implantable cardioverter‐defibrillator selection is associated with increased complication rates and mortality among patients enrolled in the NCDR implantable cardioverter‐defibrillator registry. J Am Coll Cardiol. 2011;58:1007–1013. [DOI] [PubMed] [Google Scholar]
- 2. Matlock DD, Peterson PN, Wang Y, Curtis JP, Reynolds MR, Varosy PD, Masoudi FA. Variation in use of dual‐chamber implantable cardioverter‐defibrillators: results from the National Cardiovascular Data Registry. Arch Intern Med. 2012;172:634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peterson PN, Varosy PD, Heidenreich PA, Wang Y, Dewland TA, Curtis JP, Go AS, Greenlee RT, Magid DJ, Normand SL, Masoudi FA. Association of single‐ vs dual‐chamber ICDs with mortality, readmissions, and complications among patients receiving an ICD for primary prevention. JAMA. 2013;309:2025–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schron EB, Exner DV, Yao Q, Jenkins LS, Steinberg JS, Cook JR, Kutalek SP, Friedman PL, Bubien RS, Page RL, Powell J. Quality of life in the antiarrhythmics versus implantable defibrillators trial: impact of therapy and influence of adverse symptoms and defibrillator shocks. Circulation. 2002;105:589–594. [DOI] [PubMed] [Google Scholar]
- 5. Bourke JP, Turkington D, Thomas G, McComb JM, Tynan M. Florid psychopathology in patients receiving shocks from implanted cardioverter‐defibrillators. Heart. 1997;78:581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Germano JJ, Reynolds M, Essebag V, Josephson ME. Frequency and causes of implantable cardioverter‐defibrillator therapies: is device therapy proarrhythmic? Am J Cardiol. 2006;97:1255–1261. [DOI] [PubMed] [Google Scholar]
- 7. Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, Reddy RK, Marchlinski FE, Yee R, Guarnieri T, Talajic M, Wilber DJ, Fishbein DP, Packer DL, Mark DB, Lee KL, Bardy GH. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Rees JB, Borleffs CJ, de Bie MK, Stijnen T, Van EL, Bax JJ, Schalij MJ. Inappropriate implantable cardioverter‐defibrillator shocks: incidence, predictors, and impact on mortality. J Am Coll Cardiol. 2011;57:556–562. [DOI] [PubMed] [Google Scholar]
- 9. Wilkoff BL, Hess M, Young J, Abraham WT. Differences in tachyarrhythmia detection and implantable cardioverter defibrillator therapy by primary or secondary prevention indication in cardiac resynchronization therapy patients. J Cardiovasc Electrophysiol. 2004;15:1002–1009. [DOI] [PubMed] [Google Scholar]
- 10. Almendral J, Arribas F, Wolpert C, Ricci R, Adragao P, Cobo E, Navarro X, Quesada A; DATAS Steering Committee, DATAS Writing Committee, DATAS I . Dual‐chamber defibrillators reduce clinically significant adverse events compared with single‐chamber devices: results from the DATAS (Dual chamber and Atrial Tachyarrhythmias Adverse events Study) trial. Europace. 2008;10:528–535. [DOI] [PubMed] [Google Scholar]
- 11. Deisenhofer I, Kolb C, Ndrepepa G, Schreieck J, Karch M, Schmieder S, Zrenner B, Schmitt C. Do current dual chamber cardioverter defibrillators have advantages over conventional single chamber cardioverter defibrillators in reducing inappropriate therapies? A randomized, prospective study. J Cardiovasc Electrophysiol. 2001;12:134–142. [DOI] [PubMed] [Google Scholar]
- 12. Ruwald AC, Sood N, Ruwald MH, Jons C, Clyne CA, McNitt S, Wang P, Zareba W, Moss AJ. Frequency of inappropriate therapy in patients implanted with dual‐ versus single‐chamber ICD devices in the ICD arm of MADIT‐CRT. J Cardiovasc Electrophysiol. 2013;24:672–679. [DOI] [PubMed] [Google Scholar]
- 13. Friedman PA, Bradley D, Koestler C, Slusser J, Hodge D, Bailey K, Kusumoto F, Munger TM, Militanu A, Glikson M. A prospective randomized trial of single‐ or dual‐chamber implantable cardioverter‐defibrillators to minimize inappropriate shock risk in primary sudden cardiac death prevention. Europace. 2014;16:1460–1468. [DOI] [PubMed] [Google Scholar]
- 14. Theuns DAMJ, Klootwijk AP, Goedhart DM, Jordaens LJ. Prevention of inappropriate therapy in implantable cardioverter‐defibrillators: results of a prospective, randomized study of tachyarrhythmia detection algorithms. J Am Coll Cardiol. 2004;44:2362–2367. [DOI] [PubMed] [Google Scholar]
- 15. Friedman PA, McClelland RL, Bamlet WR, Acosta H, Kessler D, Munger TM, Kavesh NG, Wood M, Daoud E, Massumi A, Schuger C, Shorofsky S, Wilkoff B, Glikson M. Dual‐chamber versus single‐chamber detection enhancements for implantable defibrillator rhythm diagnosis: the detect supraventricular tachycardia study. Circulation. 2006;113:2871–2879. [DOI] [PubMed] [Google Scholar]
- 16. Kolb C, Sturmer M, Sick P, Reif S, Davy JM, Molon G, Schwab JO, Mantovani G, Dan D, Lennerz C, Borri‐Brunetto A, Babuty D. Reduced risk for inappropriate implantable cardioverter‐defibrillator shocks with dual‐chamber therapy compared with single‐chamber therapy: results of the randomized OPTION study. JACC Heart Fail. 2014;2:611–619. [DOI] [PubMed] [Google Scholar]
- 17. Olshansky B, Day JD, Moore S, Gering L, Rosenbaum M, McGuire M, Brown S, Lerew DR. Is dual‐chamber programming inferior to single‐chamber programming in an implantable cardioverter‐defibrillator? Results of the INTRINSIC RV (Inhibition of Unnecessary RV Pacing With AVSH in ICDs) study. Circulation. 2007;115:9–16. [DOI] [PubMed] [Google Scholar]
- 18. Masoudi FA, Go AS, Magid DJ, Cassidy‐Bushrow AE, Doris JM, Fiocchi F, Garcia‐Montilla R, Glenn KA, Goldberg RJ, Gupta N, Gurwitz JH, Hammill SC, Hayes JJ, Jackson N, Kadish A, Lauer M, Miller AW, Multerer D, Peterson PN, Reifler LM, Reynolds K, Saczynski JS, Schuger C, Sharma PP, Smith DH, Suits M, Sung SH, Varosy PD, Vidaillet HJ, Greenlee RT. Longitudinal study of implantable cardioverter‐defibrillators: methods and clinical characteristics of patients receiving implantable cardioverter‐defibrillators for primary prevention in contemporary practice. Circ Cardiovasc Qual Outcomes. 2012;5:e78–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hammill SC, Stevenson LW, Kadish AH, Kremers MS, Heidenreich P, Lindsay BD, Mirro MJ, Radford MJ, Wang Y, Lang CM, Harder JC, Brindis RG. Review of the registry's first year, data collected, and future plans. Heart Rhythm. 2007;4:1260–1263. [DOI] [PubMed] [Google Scholar]
- 20. Messenger JC, Ho KK, Young CH, Slattery LE, Draoui JC, Curtis JP, Dehmer GJ, Grover FL, Mirro MJ, Reynolds MR, Rokos IC, Spertus JA, Wang TY, Winston SA, Rumsfeld JS, Masoudi FA; NCDR Science and Quality Oversight Committee Data Quality Workgroup . The National Cardiovascular Data Registry (NCDR) data quality brief: the NCDR Data Quality Program in 2012. J Am Coll Cardiol. 2012;60:1484–1488. [DOI] [PubMed] [Google Scholar]
- 21. Cohen J. The t test for means In: Cohen J, ed. Statistical Power Analysis for the Behavioral Sciences. Toronto, Canada: Academic Press Inc.; 1977:19–74. [Google Scholar]
- 22. Defaye P, Boveda S, Klug D, Beganton F, Piot O, Narayanan K, Perier MC, Gras D, Fauchier L, Bordachar P, Algalarrondo V, Babuty D, Deharo JC, Leclercq C, Marijon E, Sadoul N. Dual‐ vs. single‐chamber defibrillators for primary prevention of sudden cardiac death: long‐term follow‐up of the Defibrillateur Automatique Implantable‐Prevention Primaire registry. Europace. 2017;19:1478–1484. [DOI] [PubMed] [Google Scholar]
- 23. Kremers MS, Hammill SC, Berul CI, Koutras C, Curtis JS, Wang Y, Beachy J, Blum ML, Conyers del M, Reynolds MR, Heidenreich PA, Al‐Khatib SM, Pina IL, Blake K, Norine WM, Wilkoff BL, Shalaby A, Masoudi FA, Rumsfeld J. The National ICD Registry Report: version 2.1 including leads and pediatrics for years 2010 and 2011. Heart Rhythm. 2013;10:e59–e65. [DOI] [PubMed] [Google Scholar]
- 24. Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes NA, Greenberg H, Hall WJ, Huang DT, Kautzner J, Klein H, McNitt S, Olshansky B, Shoda M, Wilber D, Zareba W. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275–2283. [DOI] [PubMed] [Google Scholar]
- 25. Gasparini M, Proclemer A, Klersy C, Kloppe A, Lunati M, Ferrer JB, Hersi A, Gulaj M, Wijfels MC, Santi E, Manotta L, Arenal A. Effect of long‐detection interval vs standard‐detection interval for implantable cardioverter‐defibrillators on antitachycardia pacing and shock delivery: the ADVANCE III randomized clinical trial. JAMA. 2013;309:1903–1911. [DOI] [PubMed] [Google Scholar]
- 26. Weeke P, Johansen JB, Jorgensen OD, Nielsen JC, Moller M, Videbaek R, Hojgaard MV, Riahi S, Jacobsen PK. Mortality and appropriate and inappropriate therapy in patients with ischaemic heart disease and implanted cardioverter‐defibrillators for primary prevention: data from the Danish ICD Register. Europace. 2013;15:1150–1157. [DOI] [PubMed] [Google Scholar]
- 27. Thijssen J, Borleffs CJW, van Rees JB, Man S, de Bie MK, Venlet J, van der Velde ET, van Erven L, Schalij MJ. Implantable cardioverter‐defibrillator longevity under clinical circumstances: an analysis according to device type, generation, and manufacturer. Heart Rhythm. 2012;9:513–519. [DOI] [PubMed] [Google Scholar]
- 28. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML; Multicenter Automatic Defibrillator Implantation Trial II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 29. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp‐Channing N, Davidson‐Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH; the Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) Investigators . Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 30. Wilkoff BL, Fauchier L, Stiles MK, Morillo CA, Al‐Khatib SM, Almendral J, Aguinaga L, Berger RD, Cuesta A, Daubert JP, Dubner S, Ellenbogen KA, Estes NA III, Fenelon G, Garcia FC, Gasparini M, Haines DE, Healey JS, Hurtwitz JL, Keegan R, Kolb C, Kuck KH, Marinskis G, Martinelli M, Mcguire M, Molina LG, Okumura K, Proclemer A, Russo AM, Singh JP, Swerdlow CD, Teo WS, Uribe W, Viskin S, Wang CC, Zhang S; Document Review . 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter‐defibrillator programming and testing. Europace. 2016;18:159–183. [DOI] [PubMed] [Google Scholar]


