Stable coronary artery disease1 (CAD) or stable ischemic heart disease2 are terms preferred in Europe and the United States, respectively, that generally refer to the same patients––those with angina, its equivalent, or no symptoms who experience episodes of reversible myocardial supply:demand mismatch in the absence of acute myocardial infarction (MI) or unstable angina. Although the exact number is unknown, nearly 300 000 percutaneous coronary interventions (PCIs) for stable CAD are performed annually on inpatients in the United States.3, 4 A significant and increasing number of additional PCIs for stable CAD are performed on outpatients, but these numbers are not available. Despite randomized controlled trials and meta‐analyses of these trials demonstrating that an initial strategy of PCI for stable CAD does not improve survival or prevent MI beyond what is achieved by optimal medical therapy (OMT),5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 more than half of these procedures are performed on patients not treated with OMT.19 Numerous explanations related to physician behavior or the healthcare environment have been advanced to explain the deviation of practice from the evidence.20 However, a broader perspective indicates that the current stable CAD paradigm wherein the epicardial stenosis is the proximate cause of angina, ischemia, MI, and death and those outcomes can be prevented by revascularization is the product of 500 years of scientific thought.
History of the Current Paradigm
Although atherosclerosis has been described since antiquity, the intellectual birth of the epicardial stenosis paradigm can be traced to the early 1500s when Leonardo da Vinci recognized the degeneration of blood vessels with age during autopsies he personally performed. He described the continuous narrowing of the lumen associated with the “thickening of the coats” of arteries.21 While he determined that this process led to death in some individuals, he attributed it to the natural aging process rather than disease.21 Unfortunately, his observations were recorded in private journals that were not published until the 1800s.
In 1698, 70 years after Harvey conceptualized the circulatory system,22 Chirac demonstrated the importance of coronary blood flow on cardiac function when he observed that the canine heart stopped beating after the coronary arteries were ligated (Figure 1).23 William Heberden subsequently characterized the syndrome of angina pectoris in 176824 but was unable to connect it to Chirac's previous work or to determine the underlying cause.
Figure 1.
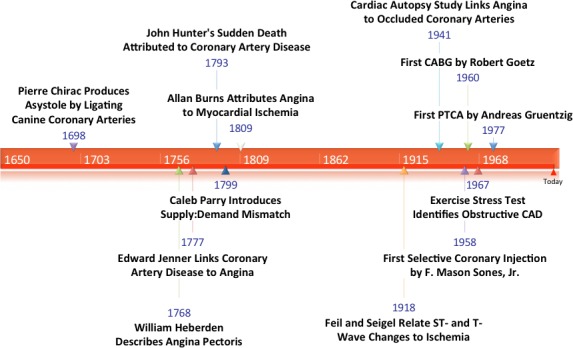
Historical development of the epicardial stenosis paradigm. CABG indicates coronary artery bypass graft; CAD, coronary artery disease; PTCA, percutaneous transluminal coronary angioplasty.
The link to atherosclerosis, that da Vinci had initially hinted at in the 1500s, would be discovered by Edward Jenner when he first ascribed angina to underlying atherosclerosis after correlating clinical symptoms with autopsy findings.24 In a 1777 letter to Heberden, Jenner described “a kind of firm, fleshy tube, formed within the vessel, with a considerable quantity of ossific matter dispersed irregularly through it.”24 Jenner's schoolmate, Caleb Parry, reasoned that a supply:demand mismatch during exertion caused angina, likely related to flow limitations through the obstructed coronary arteries that Jenner described.25 Ironically, Jenner's mentor, the renowned anatomist John Hunter, developed angina and died suddenly after an argument at St. George's Hospital in 1793.24 Jenner noted marked atheroma during Hunter's autopsy.25
In 1809, Allen Burns, a lecturer in anatomy in Glasgow, elaborated on Parry's ideas and asserted that myocardial ischemia was the likely cause of angina pectoris.25 This theory would be linked to electrocardiographic findings in 1928 when Feil and Siegel attributed ST‐ and T‐wave changes during exercise in patients with angina to a decrease in blood flow to the heart.26
The primacy of the epicardial obstruction was emphasized in a review of 355 cardiac autopsies in 1941 that noted that every patient who developed angina had an occlusion or marked narrowing of a coronary artery.27 The serendipitous discovery of coronary angiography by Sones in 1958 allowed visualization of epicardial CAD in living patients for the first time.28 Subsequent landmark angiographic studies documented the natural history of epicardial CAD and its adverse impact on survival.29 Around the same time, Robb and coworkers demonstrated reduced survival in patients with an abnormal stress test.30 Other studies correlated abnormal exercise stress test findings with the presence of obstructive CAD.31
With the first human coronary artery bypass by Goetz in 196032 and the first percutaneous transluminal coronary angioplasty (PTCA) by Grüntzig in 1977,33 physicians could, for the first time, bypass or dilate the epicardial stenosis and, consistent with the existing paradigm, alter the course of the disease. By the early 1980s, through a unique collaboration of physicians, hospitals, and industry that has been referred to as the medical‐industrial complex,34 an efficient system had been created to identify and treat the millions of patients with obstructive CAD. Despite early warnings that the enthusiasm for revascularization surpassed the evidence,35, 36 coronary bypass surgery and PCI soon became two of the most commonly performed procedures in the United States and throughout the world.
Challenges to the Paradigm
Prevention of Death and MI
The first trial comparing PTCA with medical therapy in patients with stable CAD, the 1992 ACME (Angioplasty Compared to Medicine) study randomized 212 patients with single‐vessel CAD to PTCA or medical therapy.5 PTCA resulted in greater freedom from angina (64% versus 46%) and exercise tolerance at 6 months but did not reduce mortality or MI, although the study was not powered for these outcomes. Subsequent trials were consistent in demonstrating greater but far from universal short‐term angina relief with PTCA than medical therapy but no reduction in mortality or MI.6, 7, 8, 9, 10
The results of the early studies led some to argue that the failure of PTCA to improve hard outcomes (death or MI) was attributable to the inclusion of patients at low risk. MASS (Medicine Angioplasty and Surgery Study), therefore, only included patients with proximal left anterior descending coronary artery (LAD) stenosis, yet still found no reduction in mortality or MI with PTCA.6 These early PTCA trials came before the advent of stenting; medical therapy in this era was limited to antianginal medications such as β‐blockers and nitrates.
Among these early trials, the RITA‐2 (second Randomised Intervention Treatment of Angina) was the first large (>1000 patients) investigation to compare PTCA with medical therapy. Despite medical therapy that was rudimentary by today's standards, death or definite MI were less frequent with medical therapy than PTCA (3.3% versus 6.3%) (relative risk, 1.92; 95% confidence interval [CI], 1.08–3.41 [P=0.02]). Angina improved in both groups but more so with PTCA. Compared with the PTCA group, there was a 16.5% absolute excess of grade 2 or worse angina in the medical group 3 months after randomization (P<0.001), which attenuated to 7.6% after 2 years. Bare‐metal stents were introduced at the end of the trial and were only used in 9% of procedures.
It was anticipated that the newly available bare‐metal stents would improve outcomes by providing a larger lumen and a more durable angiographic result than PTCA. The first trial to more uniformly include stents, MASS‐II, randomized 611 patients with stable CAD and proximal LAD stenosis to coronary artery bypass grafting (CABG), PCI (72% received stents), or medical therapy.11 Compared with PCI, medical therapy reduced the composite end point of cardiac mortality, MI, or refractory angina. MASS‐II, along with the trial by Hambrecht13 also published in 2004, were the first trials to include disease‐modifying statins and angiotensin‐converting enzyme inhibitors in the medical therapy regimen. In MASS‐II, there remained no difference in overall mortality between the 3 groups after 10 years of follow‐up.
The landmark COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial, published in 2007, was a multicenter randomized trial that compared an initial strategy of PCI with protocol‐mandated optimal medical therapy (OMT) with OMT alone in 2287 patients with angina or objective evidence of ischemia and significant CAD (>70% stenosis).12 Patients randomized to OMT were permitted to cross over to PCI for refractory angina. After 4.6 years, there was no difference in death or nonfatal MI between the two groups (hazard ratio [HR] for the PCI group, 1.05; 95% CI, 0.87–1.27; P=0.62), with a 30% cross‐over rate from the OMT group to PCI.
None of the trials comparing an initial strategy of PCI with stents and medical therapy to medical therapy alone in stable CAD11, 12, 13, 14, 15, 16 nor a 2012 meta‐analysis17 have shown that PCI improves survival (combined odds ratio [OR], 0.98; 95% CI, 0.83–1.15)17 or reduces MI (combined OR, 1.12; 95% CI, 0.93–1.34).17 We performed an updated meta‐analysis of all published trials randomizing patients to an initial strategy of PCI (with or without stents) in addition to medical therapy compared with medical therapy alone using the longest published follow‐up available.8, 9, 12, 13, 14, 15, 37, 38, 39, 40, 41, 42, 43 The analysis continues to show no improvement in survival (Figure 2A) and no reduction in nonfatal MI (Figure 2B). These results persisted even after limiting the analysis to the trials that only included stents (data not shown).
Figure 2.
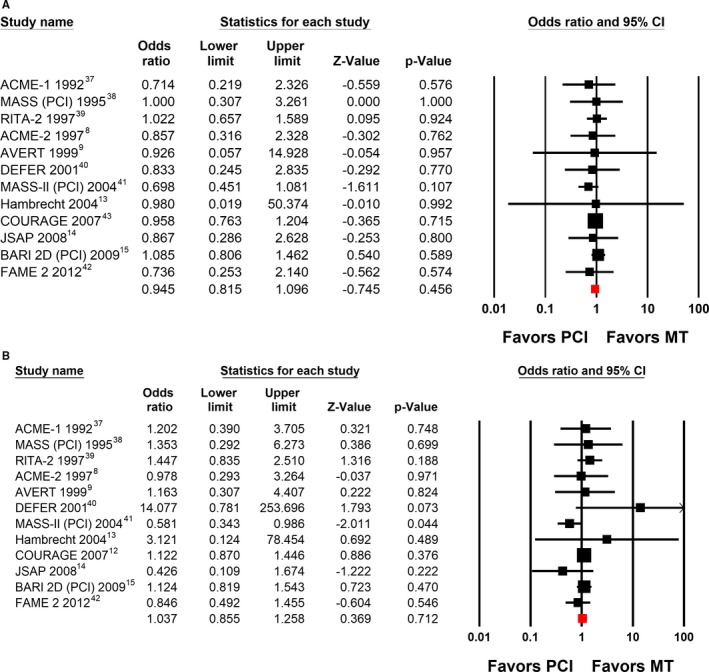
A, Difference in survival in randomized controlled trials comparing initial percutaneous coronary intervention (PCI) vs medical therapy (MT). A systematic search of published studies in any language in MEDLINE, Cochrane, and PubMed from 1970 to October 2017 was performed independently by both authors using the following search terms: stent, medical therapy, stable angina, coronary artery disease (CAD), and combinations of these terms. Patient outcomes (death from any cause and nonfatal myocardial infarction) were systematically reviewed and recorded independently by both authors. A meta‐analysis of summary statistics from individual trials was performed using Comprehensive Meta‐Analysis software, version 2 (Biostat Inc). Summary odds ratios (ORs) were calculated using a random‐effects model. Results from the longest reported follow‐up are shown.8, 9, 13, 14, 15, 37, 38, 39, 40, 41, 42, 43 All included studies are listed by name along with point estimates of the ORs and respective 95% confidence intervals (CIs). The red squares represent the overall findings in each plot. B, Difference in nonfatal myocardial infarction in randomized controlled trials comparing initial PCI vs MT. Results from the longest reported follow‐up are shown.8, 9, 12, 13, 14, 15, 37, 38, 39, 40, 41, 42 All included studies are listed by name along with point estimates of the ORs and respective 95% CIs. The red squares represent the overall findings in each plot.
Angina
PCI has been shown to provide incremental, short‐term relief of angina compared with medical therapy that is far from universal as would be expected according to the prevailing paradigm. In MASS‐II,11 the BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) trial44 and COURAGE45 only 7% to 17% more patients in the PCI arm were free from angina at 12 months compared with patients randomized to medical therapy alone. In MASS‐II, the incremental benefit of PCI persisted for 120 months.41 By 24 months in BARI 2D44 and by 36 months in COURAGE,45 freedom from angina was not significantly different between PCI and medical therapy groups. The percentage of patients treated with PCI who continued to have angina at 1 year ranged from 45% in MASS‐II (class II or III angina) to 60% in BARI 2D to 68% in COURAGE.
One month after relieving the flow‐limiting stenosis by stent placement in COURAGE, 79% of patients with baseline angina still had angina45 (Figure 3), suggesting that, in most COURAGE patients, the epicardial stenosis was not the cause of angina. There was only an 11% advantage of PCI over the medical therapy arm in angina relief at 1 month with a diminishing benefit over time. The recently reported ORBITA (Objective Randomised Blinded Investigation With Optimal Medical Therapy of Angioplasty) trial, a randomized double‐blind, sham‐controlled trial of PCI and medical therapy in patients with angina and single‐vessel CAD refractory to OMT found no benefit of PCI in change in exercise time, angina relief, angina frequency, angina stability, time to 1 mm of ST depression, quality of life or treatment satisfaction.46 These results suggest that prior studies that reported greater angina relief from PCI compared to medical therapy were confounded by the placebo effect associated with unblinded PCI and raise the question of whether PCI is associated with any incremental improvement in angina relief compared to OMT.46
Figure 3.
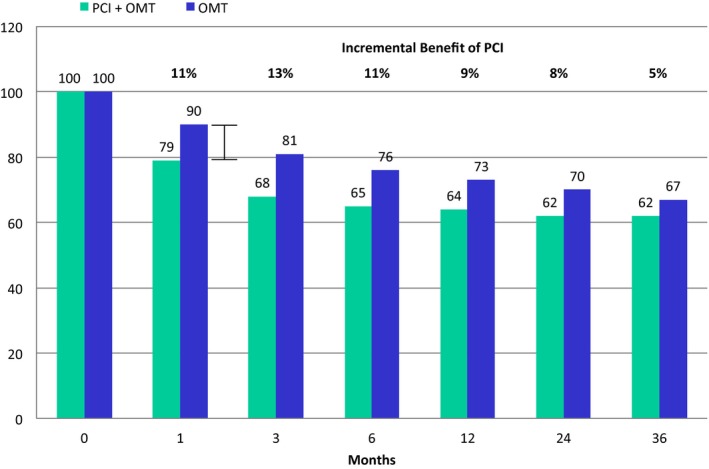
The incremental benefit of percutaneous coronary intervention (PCI) and optimal medical therapy (OMT) compared with OMT alone in patients with baseline angina in the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial. The percentage of patients with angina over time from baseline to 36 months is displayed. One month after PCI, 79% of patients with baseline angina still had angina in the PCI arm, an 11% incremental benefit relative to the OMT arm. At 36 months, there was no significant difference between groups.
High‐Risk Subgroups
As was the case with the early PTCA trials, the results of randomized trials of PCI and medical therapy were downplayed because it was believed that patients at high risk had not been enrolled. However, patients at high risk including those with diabetes mellitus, proximal LAD lesions, reduced ejection fraction, multivessel CAD, increased age, chronic kidney disease, and significant myocardial ischemia have been included in recent trials.
Diabetes mellitus
BARI 2D randomized 2368 patients with type 2 diabetes mellitus and stable CAD to prompt revascularization with intensive medical therapy or intensive medical therapy alone.15 After 5 years, there was no difference in mortality or in major adverse cardiovascular events between initial PCI and medical therapy groups. Subsequent analyses found no difference between initial PCI and medical therapy regardless of the number of diseased vessels, the amount of jeopardized myocardium, the number of stenotic lesions, the presence of a total occlusion, proximal LAD disease, prior revascularization, or an abnormal left ventricular ejection fraction.47 While the patients at higher risk with diabetes mellitus had more adverse cardiovascular events, those events were not prevented or reduced by PCI.
Proximal LAD
In COURAGE, although survival free of death, MI, or acute coronary syndrome was reduced in all patients with a proximal LAD stenosis >90%, PCI did not improve these outcomes (P=0.79).48 These results align with the earlier findings of MASS,6 which included patients with >80% proximal LAD stenosis, and MASS‐II,11 which required >70% proximal LAD stenosis and documented ischemia. Neither study found that PCI resulted in any reduction in cardiovascular events.
Three‐vessel CAD and low ejection fraction
In a subgroup analysis of COURAGE, an increasing number of diseased vessels and/or the presence of reduced ejection fraction were associated with significant reductions in event‐free survival.49 In the patients at highest risk with 3‐vessel CAD and reduced ejection fraction (n=124), there was no benefit from initial PCI and OMT compared with OMT alone (P=0.59).
Other high‐risk groups
Additional analyses of COURAGE have found no benefit for an initial strategy of PCI in patients with a recent acute coronary syndrome or class III angina,50 older patients51 or patients with chronic kidney disease.52 As expected, each high‐risk subgroup experienced worse outcomes, but PCI did not improve these outcomes for any subgroup. Although these hypothesis‐generating post hoc analyses are limited by their small sample size, the sample sizes are larger than the anecdotal experience of most individual physicians, and unlike anecdotal experience, they are not subject to selection or recall bias. Across the numerous high‐risk subsets evaluated, there has never been any signal suggestive of an improvement in outcomes with PCI that would justify an appropriately powered randomized trial.
CAD before vascular surgery
Given the hemodynamic stress of vascular and other major noncardiac surgeries, prophylactic coronary revascularization is often pursued in the hope of reducing postoperative ischemic events. No benefit of prophylactic PCI (or CABG) was shown in the CARP (Coronary Artery Revascularization Prophylaxis) trial in which patients with >70% stenosis of at least one coronary vessel (n=510) were randomized to revascularization before vascular surgery for an expanding abdominal aortic aneurysm (33%) or arterial occlusive disease of the legs (67%).53 In the revascularization group, 59% underwent PCI and 41% underwent CABG. There was no difference in postoperative mortality between the revascularization and medical therapy groups. No high‐risk subset that benefited from revascularization could be identified, including patients with a moderate or large degree of ischemia, a greater revised cardiac risk index, or 3‐vessel CAD and left ventricular dysfunction.
The Ischemia‐PCI Reflex: Pathophysiology or Mythology?
Myocardial ischemia on stress testing has long been associated with increased mortality.54 In a recent review it was asserted that “the presence and extent of ischemia is the most important factor related to outcome and that all functionally significant stenoses should be revascularized to relieve ischemia.”55 However, closer examination of the evidence base used to support this opinion finds it is of insufficient quality to justify such a reflexive approach to treatment of patients with stable CAD and ischemia. Furthermore, closer review of the data suggests that ischemia is a marker for adverse outcomes rather than the cause of the adverse outcomes (analogous to ventricular ectopy after MI56).
The belief that ischemia necessitates revascularization to improve outcomes can be traced, in part, to a retrospective analysis of the CASS (Coronary Artery Surgery Study) registry that reported the benefit of bypass surgery was greatest in patients who were unable to exercise beyond stage 1 due to angina and displayed 1 mm of ST depression.57
Hachamovitch et al58 subsequently performed a single‐center, retrospective review of 10 627 patients who underwent an exercise or adenosine stress myocardial perfusion scan between 1991 and 1997. After a mean follow‐up of 1.9 years, Cox proportional hazards modeling indicated that the 671 (6.3%) patients who underwent revascularization (PCI in 346, CABG in 325) within 60 days experienced improved cardiac survival if their ischemic burden was ≈10% to 12.5%, but worse cardiac survival if their ischemic burden was <10%.
As a retrospective analysis, this study cannot assess causality. Furthermore, it was subject to methodological flaws including short follow‐up and significant selection bias that severely limited the interpretation of its findings. Their model included degrees of myocardial ischemia up to 50% that are not seen clinically. In addition, there was no uniformly prescribed medical therapy that would be considered optimal by modern standards. Although this article is widely quoted to justify revascularization in the presence of ischemia, to our knowledge it has never been used to justify avoidance of revascularization in patients with an ischemic burden <10%.
An analysis of 314 patients from COURAGE who underwent nonprotocol‐mandated rest/stress myocardial perfusion scans before and after randomization found that patients with >5% reduction in ischemia on follow‐up imaging (regardless of treatment strategy) had a lower risk for death or MI in an unadjusted analysis (P=0.037).59 However, this finding did not persist in an adjusted analysis (P=0.26). The unadjusted outcome data from this small cohort of patients continue to be used to justify the use of PCI for patients with ischemia.
The COURAGE investigators subsequently reviewed the 1381 randomized patients who underwent myocardial perfusion scans at baseline.60 Of these, 486 had moderate to severe ischemia, evenly divided between the randomized treatment groups. There was no difference in outcomes in patients with moderate to severe ischemia randomized to treatment with PCI and OMT compared with OMT alone. Similarly, the nuclear substudy of 1505 patients from BARI 2D found no relationship between ischemic myocardium and subsequent death or MI.60
A 2014 meta‐analysis reported outcomes of patients with baseline ischemia or an abnormal fractional flow reserve (FFR) in the randomized trials comparing PCI and medical therapy with medical therapy alone.18 There was no difference in death, MI, unplanned revascularization or angina between groups (Figure 4). The strong association of ischemia with impaired event‐free survival coupled with the lack of benefit of PCI in patients with ischemia suggests that ischemia is a marker of adverse outcomes rather than the cause of the adverse outcomes. The extent of ischemia is correlated with atherosclerotic plaque burden61 that, as the substrate for acute coronary syndromes, may be the underlying determinant of prognosis and the explanation for why PCI offers no incremental benefit beyond optimal disease‐modifying therapy. In further support of this theory, in COURAGE, atherosclerotic burden was independently predictive of adverse outcomes, whereas ischemic burden was not.62
Figure 4.
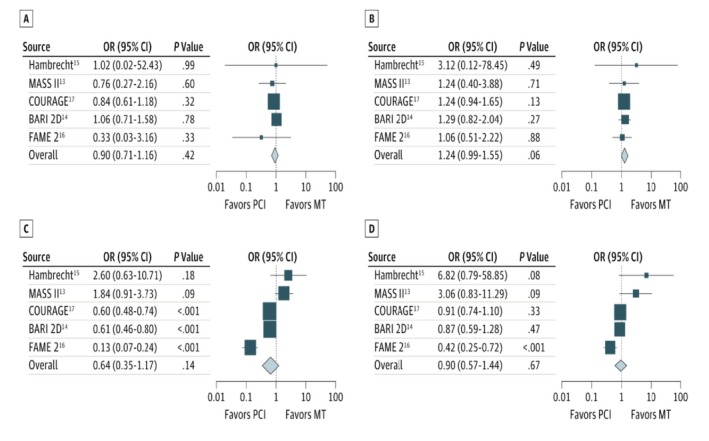
Comparison of percutaneous coronary intervention (PCI) and medical therapy (MT) vs MT alone in patients with documented myocardial ischemia. Each graph illustrates an outcome. A, Death; B, nonfatal myocardial infarction; C, unplanned revascularization; and D, angina during follow‐up. All included studies are listed by name along with point estimates of the odds ratios (ORs) and respective 95% confidence intervals (CIs). The sizes of the squares denoting the point estimate in each study are proportional to the weight of the study. The diamonds represent the overall findings in each plot. See text for full trial names. Reproduced from Stergiopoulos et al18 with permission. Copyright©2014 American Medical Association. All rights reserved.
Invasive Physiologic Tools to Assess Hemodynamic Significance of Epicardial CAD
FFR was developed as an invasive tool to quantify the hemodynamic significance of an angiographic stenosis under the assumption that revascularization of hemodynamically significant lesions will improve outcomes––in other words, to replace the “oculostenotic” reflex with an “ischemia‐PCI” reflex. FFR estimates flow across a lesion by measuring the change in pressures between the aorta and the coronary artery distal to a lesion during pharmacologically induced maximal coronary flow.63 The impetus for its development was recognition of the limited accuracy of angiographic assessment of lesion severity. FFR was calibrated against stress testing to derive a cutoff value that correlates best with ischemia. Originally an FFR <0.75 was found to accurately predict at least one positive stress test in patients undergoing an exercise test, thallium scan, and dobutamine stress echocardiogram.64 However, contemporary stress tests were validated against coronary angiography whose limitations stimulated the development of FFR in the first place. Thus, the logic justifying FFR appears circular, at best.
In the FAME (Fractional Flow Reserve versus Angiography for Multivessel Evaluation) trial, 1005 patients were randomized to angiographically guided PCI or PCI guided by an FFR <0.80 (it is not clear why 0.75 was not used). FFR‐guided PCI was associated with fewer PCIs and a reduction in the combined end point of death, MI, or revascularization at 2 years.65 Often overlooked, the FAME trial was the first study to demonstrate harm from unnecessary PCI in patients with stable CAD. It left unanswered where the line is drawn between necessary and unnecessary PCI.
The FAME 2 trial provided insight into where that line should be drawn. This unblinded trial randomized 888 patients with FFR <0.80 to initial PCI with second‐generation drug‐eluting stents and medical therapy versus medical therapy alone.16 There was no difference in death or MI between groups at a follow‐up of 2 years.42 FAME 2 enrolled a very low‐risk population: there were only 6 deaths (1.3%) in the PCI group and 8 deaths (1.8%) in the medical therapy group, including only 3 cardiac deaths in each group at 2 years (0.7%).40 In an effort to demonstrate some benefit of PCI on important end points, FAME 2 included a landmark analysis that excluded events that occurred in the first 7 days following randomization including periprocedural MI that can only occur in the PCI‐treated patients. By eliminating periprocedural MI from end point analysis, the authors showed a reduction of MI in the PCI group.40 However, FAME 2 defined MI, including periprocedural MI, as CKMB >10 times the upper limits of normal or >5 times upper limits of normal with an associated occluded artery or new Q waves, a threshold clearly associated with increased 30‐day mortality.63, 64
FAME 2 was initially designed to randomize 1600 patients with a planned follow‐up of 5 years. The study was terminated prematurely at the recommendation of the data safety monitoring board after 213 days of follow‐up with only 888 patients randomized because the composite end point favored the FFR‐guided PCI group, but the end point difference was driven solely by urgent revascularization. Notably, 52% of the “urgent” revascularizations in the initial analysis14 and 49% of urgent revascularizations at 2‐year follow‐up40 were not associated with either ischemic ST‐T–wave changes or positive cardiac biomarkers. The increase in urgent revascularization in the medical therapy group appears to have been largely driven by the subset of patients with FFR <0.65 (P=0.01 for interaction)14 indicative of such a severe stenosis that passage of the flow wire across the lesion without stent placement may have resulted in plaque disruption.62 Patients in the medical therapy arm were not treated with dual anti‐platelet therapy which may have protected against events caused by iatrogenic plaque injury. In addition, the unblinded nature of the trial may have prompted more aggressive evaluation and treatment of patients with very low FFR values who were randomized to medical therapy.
There were 42 more urgent revascularizations in the medical therapy arm than the FFR‐PCI arm (7 versus 49). By suggesting that the results of FAME 2 support PCI of every lesion with an FFR <0.8 is to suggest that to prevent urgent revascularizations (but not MI and death) in the additional 9.5% of patients who experienced them in the medical arm, 100% of patients with an abnormal FFR should undergo PCI. This is not a wise use of resources and recalls the lesson of FAME: unnecessary stenting leads to worse outcomes. Finally, it must be acknowledged that FFR is not a benign procedure. Coronary artery dissection, abrupt vessel closure, and death have been caused by measuring FFR. A French trial of FFR versus angiographic guidance for PCI in multivessel disease was terminated prematurely in 2016 because of excess deaths (17 versus 7) in the FFR arm at 12 months.66
Concern for Harm
Even with the lack of documented benefit for an initial PCI strategy in patients with stable CAD, some argue that it is reasonable to still pursue initial PCI since randomized trials have shown that it can improve or relieve angina in some patients with no increase in MI or death. It is important to note that PCI does carry with it a low, but real, risk of complications including death (0.65%), MI (15%), renal injury (13%), stroke (0.2%), contrast allergy (≤1%), and vascular complications (2–6%).4, 67, 68 While there was no difference in overall death or MI in the randomized trials of PCI versus medical therapy, the studies were not powered to detect an increase in uncommon but unavoidable procedural complications. These seemingly low risks are put in perspective when they are multiplied by the estimated 300 000 PCI procedures performed annually in the United States for stable CAD. Given the added cost69 and procedure‐related risks associated with PCI, as well as the limited improvement in angina compared with OMT, it is difficult to argue that equipoise exists in the selection of an initial treatment strategy for patients with stable CAD.
Paradigm Change
The centuries‐old paradigm that the epicardial stenosis leads to ischemia and angina has led to the labeling of patients with ischemia on stress testing but no epicardial stenosis as having a false‐positive stress test result. However, up to 50% of patients with angina and an abnormal stress test do not have obstructive CAD70 Despite the absence of obstructive CAD, these patients experience increased event rates including mortality.71, 72 These findings, in combination with the persistent inability of PCI to prevent death or MI or eliminate angina in patients with stable CAD, firmly argue against the current paradigm that the epicardial stenosis leads to ischemia and angina in a 1:1 relationship and, eventually, MI and death.
In his seminal book, The Structure of Scientific Revolutions, Thomas Kuhn argued that scientific progress was not simply an incremental process marked by the steady accumulation of more data.73 Instead, revolutionary periods in thought occur when the weight of new evidence forces the scientific community to abandon their formerly held beliefs. Tossing aside previous assumptions, scientists then form a new paradigm that encapsulates the entirety of the knowledge and understanding of the time. We and others believe the weight of data indicates it is time for construction of a new paradigm.74, 75
A New Paradigm: Chronic Ischemic Coronary Syndromes
Ischemia, by definition, is a reduction in blood supply to cells resulting in a lack of sufficient oxygen for oxidative metabolism. Ischemia can, therefore, result from dysfunction anywhere along the vascular delivery conduit from the aorta to the microvasculature. The recognition of the multiple mechanistic causes of ischemia, angina, and cardiovascular events in stable CAD suggest it would be more appropriately and accurately referred to as a “syndrome” than a “disease.” Thus, a chronic ischemic coronary syndrome (CICS) can originate from flow obstruction, endothelial dysfunction or spasm of the epicardial vessels, and/or the microvasculature that is responsible for 80% of the resistance to coronary flow (Table). Further elaboration on microvascular dysfunction is beyond the scope of this review but the topic has recently been thoroughly reviewed.76
Table 1.
Potential Causes of Chronic Ischemic Coronary Syndromes
| Location of Defect | Potential Mechanisms | Selected Etiologies |
|---|---|---|
| Coronary macrovessels | Flow‐limiting stenosis | Atherosclerosis |
| Endothelial dysfunction | Atherosclerosis, viruses | |
| Spasm | Atherosclerosis, cocaine | |
| Muscle bridging | ||
| Aberrant origin | ||
| Dissection | Pregnancy, trauma, Marfan syndrome | |
| Inflammation | Cardiac transplant, collagen diseases | |
| Coronary microvessels | Microvascular dysfunction | Atherosclerosis |
| Endothelial dysfunction | Atherosclerosis | |
| Spasm | Atherosclerosis | |
| Inflammation | Cardiac transplant, collagen diseases | |
| Microemboli | Atherosclerosis, atrial fibrillation | |
| Capillary insufficiency | Left ventricular hypertrophy | |
| Noncoronary arteries | Increased stiffness (increased afterload) | Calcification, aging, hypertension, chronic kidney disease |
Adapted from Pepine and Douglas74 with permission. Copyright©2012 Elsevier.
Fully adopting the new paradigm will require a seismic change in our approach to patients with CICS. The optimal initial approach to patients with a CICS that can safely rule out significant pathology, such as left main disease, but reduce unnecessary catheterizations and interventions needs to be determined. New diagnostic tools need to be created and refined to more accurately diagnose endothelial dysfunction, microvascular dysfunction, and coronary spasm.76 New therapies need to be developed that can better treat the multitude of pathologies that contribute to the CICS. Most importantly, we need to do a better job of using the tools we already have. OMT is a powerful intervention, yet only 29% of patients in the SYNTAX (Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery) trial77 at baseline were on OMT, with only 35% to 40% on OMT 5 years after revascularization. Patients receiving OMT experienced a 36% relative reduction in mortality over 5 years (HR, 0.64; 95% CI, 0.48–0.85 [P=0.0002]).
Indication for Revascularization
While we have focused on the lack of evidence for PCI improving clinical outcomes in patients with stable CAD and the need for a new paradigm, it is important to note that this discussion does not mean to imply that revascularization does not improve outcomes in many patients with CAD including those with acute coronary syndromes and severe stenosis of the left main coronary artery and selected patients with a CICS. In certain patients with CICS, CABG in combination with OMT has been shown to improve outcomes compared with OMT alone. For example, a patient‐level pooled analysis of 5034 patients with diabetes mellitus with stable CAD from BARI 2D, COURAGE, and the FREEDOM (Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease) trial78 demonstrated that CABG and OMT were superior to OMT alone for reduction in the primary outcome of death, MI, and stroke (HR, 0.79; 95% CI, 0.64–0.97 [P=0.022]) including a reduction of MI alone (HR, 0.55; 95% CI, 0.41–0.74 [P=0.0001]) at a median follow‐up of 4.5 years. The primary outcome was largely driven by the decrease in MI, with no difference in mortality between groups and a trend toward increased stroke in the CABG group. CABG plus OMT was superior to PCI plus OMT for the primary end point (HR, 0.71; 95% CI, 0.59–0.85 [P=0.0002]), death (HR, 0.76; 95% CI, 0.60–0.96 [P=0.024]), and MI (HR, 0.50; 95% CI, 0.38–0.67 [P=0.0001]), but not stroke (HR, 1.54; 95% CI, 0.96–2.48 [P=0.074]).
These results are consistent with the hypothesis that atherosclerotic burden is the primary determinant of outcome. PCI treats a discrete lesion in a coronary artery while leaving large portions of diseased coronary arteries untreated. CABG, on the other hand, provides a vascular conduit around much of the diseased vessel with its numerous atherosclerotic plaques that form the substrate for plaque disruption, thrombosis, and MI.
Conclusions
Despite multiple trials, there is no evidence that a strategy of PCI and OMT improves cardiovascular outcomes in patients with CICS compared with OMT alone. Furthermore, multiple analyses have failed to identify a single high‐risk subset that benefits from a strategy of initial PCI. The ORBITA study requires confirmation in other populations but calls into question the value of PCI in treating refractory angina. A more intense effort should be made toward improving preventive care and maximizing use of proven medical therapy, which remains woefully underutilized in the modern era in patients with CICS. Given the failings of the epicardial stenosis paradigm, it is time to embrace a new, more enlightened paradigm that considers the many other known causes of myocardial ischemia including vasospasm, microvascular angina, and endothelial dysfunction in the evaluation of every patient with angina or ischemia (Table). Curtailing unnecessary PCI has the potential to allow a shift of resources toward gaining a greater understanding of the causes, diagnosis, prevention, and treatment of the CICS (Table).
Disclosures
None.
(J Am Heart Assoc. 2017;6:e007006 DOI: 10.1161/JAHA.117.007006.)29133520
References
- 1. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner‐Banzhoff N, Erol C, Frank H, Funck‐Brentano C, Gaemperli O, Gonzalez‐Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Ryden L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 2. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB III, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr, Smith SC Jr, Spertus JA, Williams SV. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. [DOI] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dehmer GJ, Weaver D, Roe MT, Milford‐Beland S, Fitzgerald S, Hermann A, Messenger J, Moussa I, Garratt K, Rumsfeld J, Brindis RG. A contemporary view of diagnostic cardiac catheterization and percutaneous coronary intervention in the United States: a report from the CathPCI Registry of the National Cardiovascular Data Registry, 2010 through June 2011. J Am Coll Cardiol. 2012;60:2017–2031. [DOI] [PubMed] [Google Scholar]
- 5. Parisi AF, Folland ED, Hartigan P. A comparison of angioplasty with medical therapy in the treatment of single‐vessel coronary artery disease. Veterans Affairs ACME Investigators. N Engl J Med. 1992;326:10–16. [DOI] [PubMed] [Google Scholar]
- 6. Hueb WA, Bellotti G, de Oliveira SA, Arie S, de Albuquerque CP, Jatene AD, Pileggi F. The Medicine, Angioplasty or Surgery Study (MASS): a prospective, randomized trial of medical therapy, balloon angioplasty or bypass surgery for single proximal left anterior descending artery stenoses. J Am Coll Cardiol. 1995;26:1600–1605. [DOI] [PubMed] [Google Scholar]
- 7. Coronary angioplasty versus medical therapy for angina: the second Randomised Intervention Treatment of Angina (RITA‐2) trial. RITA‐2 trial participants. Lancet. 1997;350:461–468. [PubMed] [Google Scholar]
- 8. Folland ED, Hartigan PM, Parisi AF. Percutaneous transluminal coronary angioplasty versus medical therapy for stable angina pectoris: outcomes for patients with double‐vessel versus single‐vessel coronary artery disease in a Veterans Affairs Cooperative randomized trial. Veterans Affairs ACME InvestigatorS. J Am Coll Cardiol. 1997;29:1505–1511. [DOI] [PubMed] [Google Scholar]
- 9. Pitt B, Waters D, Brown WV, van Boven AJ, Schwartz L, Title LM, Eisenberg D, Shurzinske L, McCormick LS. Aggressive lipid‐lowering therapy compared with angioplasty in stable coronary artery disease. Atorvastatin versus Revascularization Treatment Investigators. N Engl J Med. 1999;341:70–76. [DOI] [PubMed] [Google Scholar]
- 10. Bech GJ, De Bruyne B, Pijls NH, de Muinck ED, Hoorntje JC, Escaned J, Stella PR, Boersma E, Bartunek J, Koolen JJ, Wijns W. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation. 2001;103:2928–2934. [DOI] [PubMed] [Google Scholar]
- 11. Hueb W, Soares PR, Gersh BJ, Cesar LA, Luz PL, Puig LB, Martinez EM, Oliveira SA, Ramires JA. The Medicine, Angioplasty, or Surgery Study (MASS‐II): a randomized, controlled clinical trial of three therapeutic strategies for multivessel coronary artery disease: one‐year results. J Am Coll Cardiol. 2004;43:1743–1751. [DOI] [PubMed] [Google Scholar]
- 12. Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 13. Hambrecht R, Walther C, Mobius‐Winkler S, Gielen S, Linke A, Conradi K, Erbs S, Kluge R, Kendziorra K, Sabri O, Sick P, Schuler G. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation. 2004;109:1371–1378. [DOI] [PubMed] [Google Scholar]
- 14. Nishigaki K, Yamazaki T, Kitabatake A, Yamaguchi T, Kanmatsuse K, Kodama I, Takekoshi N, Tomoike H, Hori M, Matsuzaki M, Takeshita A, Shimbo T, Fujiwara H. Percutaneous coronary intervention plus medical therapy reduces the incidence of acute coronary syndrome more effectively than initial medical therapy only among patients with low‐risk coronary artery disease a randomized, comparative, multicenter study. JACC Cardiovasc Interv. 2008;1:469–479. [DOI] [PubMed] [Google Scholar]
- 15. Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Mobius‐Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engstrom T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Juni P, Fearon WF. Fractional flow reserve‐guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. [DOI] [PubMed] [Google Scholar]
- 17. Stergiopoulos K, Brown DL. Initial coronary stent implantation with medical therapy vs medical therapy alone for stable coronary artery disease: meta‐analysis of randomized controlled trials. Arch Intern Med. 2012;172:312–319. [DOI] [PubMed] [Google Scholar]
- 18. Stergiopoulos K, Boden WE, Hartigan P, Mobius‐Winkler S, Hambrecht R, Hueb W, Hardison RM, Abbott JD, Brown DL. Percutaneous coronary intervention outcomes in patients with stable obstructive coronary artery disease and myocardial ischemia: a collaborative meta‐analysis of contemporary randomized clinical trials. JAMA Intern Med. 2014;174:232–240. [DOI] [PubMed] [Google Scholar]
- 19. Borden WB, Redberg RF, Mushlin AI, Dai D, Kaltenbach LA, Spertus JA. Patterns and intensity of medical therapy in patients undergoing percutaneous coronary intervention. JAMA. 2011;305:1882–1889. [DOI] [PubMed] [Google Scholar]
- 20. Lin GA, Dudley RA, Redberg RF. Cardiologists' use of percutaneous coronary interventions for stable coronary artery disease. Arch Intern Med. 2007;167:1604–1609. [DOI] [PubMed] [Google Scholar]
- 21. Keele KD. Leonardo da Vinci's views on arteriosclerosis. Med Hist. 1973;17:304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harvey W. Exercitatio Anatomica de Motu Cordis et Sanguinis in Animalibus. Springfield, IL: Thomas; 1928. [PubMed] [Google Scholar]
- 23. Chirac P. Petri Chirac Consiliarii Medici et Professoris. De Motu Cordis: Adversaria Analytica. Montpellier: Apud Joannem Martel; 1698. [Google Scholar]
- 24. Osler W. Lectures on Angina Pectoris and Allied States. New York, NY: D. Appleton and Company; 1897. [Google Scholar]
- 25. Kligfield P. The early pathophysiolic understanding of angina pectoris (Edward Jenner, Caleb Hillier Parry, Alan Burns). Am J Cardiol. 1982;50:1433–1435. [DOI] [PubMed] [Google Scholar]
- 26. Feil H, Siegel M. Electrocardiographic changes during attacks of angina pectoris. Am J Med Sci. 1928;175:255–269. [Google Scholar]
- 27. Blumgart HL, Schlesinger MJ, Zoll PM. Angina pectoris, coronary failure and acute myocardial infarction: the role of coronary occlusions and collateral circulation. JAMA. 1941;116:91–97. [Google Scholar]
- 28. Ryan TJ. The coronary angiogram and its seminal contributions to cardiovascular medicine over five decades. Circulation. 2002;106:752–756. [DOI] [PubMed] [Google Scholar]
- 29. Jones WB, Riley CP, Reeves TJ, Sheffield LT. Natural history of coronary artery disease. Bull N Y Acad Med. 1972;48:1109–1125. [PMC free article] [PubMed] [Google Scholar]
- 30. Robb GP, Marks HH, Mattingly TW. The value of the double standard two‐step exercise test in the detection of coronary disease; a clinical and statistical follow‐up study of military personnel and insurance applicants. Trans Assoc Life Insur Med Dir Am. 1956;40:52–80; discussion, 80–85. [PubMed] [Google Scholar]
- 31. Hultgren H, Calciano A, Platt F, Abrams H. A clinical evaluation of coronary arteriography. Am J Med. 1967;42:228–247. [DOI] [PubMed] [Google Scholar]
- 32. Konstantinov IE. Robert H. Goetz: the surgeon who performed the first successful clinical coronary artery bypass operation. Ann Thorac Surg. 2000;69:1966–1972. [DOI] [PubMed] [Google Scholar]
- 33. Gruntzig A. Transluminal dilatation of coronary‐artery stenosis. Lancet. 1978;1:263. [DOI] [PubMed] [Google Scholar]
- 34. Relman AS. The new medical‐industrial complex. N Engl J Med. 1980;303:963–970. [DOI] [PubMed] [Google Scholar]
- 35. Braunwald E. Coronary‐artery surgery at the crossroads. N Engl J Med. 1977;297:661–663. [DOI] [PubMed] [Google Scholar]
- 36. McIntosh HD. Benefits from aortocoronary bypass graft. JAMA. 1978;239:1197–1199. [PubMed] [Google Scholar]
- 37. Hartigan PM, Giacomini JC, Folland ED, Parisi AF. Two‐ to three‐year follow‐up of patients with single‐vessel coronary artery disease randomized to PTCA or medical therapy (results of a VA cooperative study). Veterans Affairs Cooperative Studies Program ACME Investigators. Angioplasty Compared to Medicine. Am J Cardiol. 1998;82:1445–1450. [DOI] [PubMed] [Google Scholar]
- 38. Hueb WA, Soares PR, Almeida De Oliveira S, Arie S, Cardoso RH, Wajsbrot DB, Cesar LA, Jatene AD, Ramires JA. Five‐year follow‐op of the Medicine, Angioplasty, or Surgery Study (MASS): a prospective, randomized trial of medical therapy, balloon angioplasty, or bypass surgery for single proximal left anterior descending coronary artery stenosis. Circulation. 1999;100:Ii107–Ii113. [DOI] [PubMed] [Google Scholar]
- 39. Henderson RA, Pocock SJ, Clayton TC, Knight R, Fox KA, Julian DG, Chamberlain DA. Seven‐year outcome in the RITA‐2 trial: coronary angioplasty versus medical therapy. J Am Coll Cardiol. 2003;42:1161–1170. [DOI] [PubMed] [Google Scholar]
- 40. Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van't Veer M, Bar F, Hoorntje J, Koolen J, Wijns W, de Bruyne B. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5‐year follow‐up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105–2111. [DOI] [PubMed] [Google Scholar]
- 41. Hueb W, Lopes N, Gersh BJ, Soares PR, Ribeiro EE, Pereira AC, Favarato D, Rocha AS, Hueb AC, Ramires JA. Ten‐year follow‐up survival of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation. 2010;122:949–957. [DOI] [PubMed] [Google Scholar]
- 42. De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, Jagic N, Mobius‐Winckler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engstrom T, Oldroyd K, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Limacher A, Nuesch E, Juni P. Fractional flow reserve‐guided PCI for stable coronary artery disease. N Engl J Med. 2014;371:1208–1217. [DOI] [PubMed] [Google Scholar]
- 43. Sedlis SP, Hartigan PM, Teo KK, Maron DJ, Spertus JA, Mancini GB, Kostuk W, Chaitman BR, Berman D, Lorin JD, Dada M, Weintraub WS, Boden WE. Effect of PCI on long‐term survival in patients with stable ischemic heart disease. N Engl J Med. 2015;373:1937–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dagenais GR, Lu J, Faxon DP, Kent K, Lago RM, Lezama C, Hueb W, Weiss M, Slater J, Frye RL. Effects of optimal medical treatment with or without coronary revascularization on angina and subsequent revascularizations in patients with type 2 diabetes mellitus and stable ischemic heart disease. Circulation. 2011;123:1492–1500. [DOI] [PubMed] [Google Scholar]
- 45. Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, Zhang W, Hartigan PM, Lewis C, Veledar E, Bowen J, Dunbar SB, Deaton C, Kaufman S, O'Rourke RA, Goeree R, Barnett PG, Teo KK, Boden WE, Mancini GB. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med. 2008;359:677–687. [DOI] [PubMed] [Google Scholar]
- 46. Al‐Lamee R, Thompson D, Dehbi H‐M, Sen S, Tang K, Davies J, Keeble T, Mielewczik M, Kaprielian R, Malik IS, Nijjer SS, Petraco R, Cook C, Ahmad Y, Howard J, Baker C, Sharp A, Gerber R, Talwar S, Assomull R, Mayet J, Wensel R, Collier D, Shun‐Shin M, Thom S, Davies JE, Francis DP on behalf of the ORBITA Investigators . Percutaneous coronary intervention in stable angina (ORBITA): a double‐blind, randomised controlled trial Lancet 2017. published online Nov 2. http://dx.doi.org/10.1016/S0140-6736(17)32714-9 [DOI] [PubMed] [Google Scholar]
- 47. Brooks MM, Chaitman BR, Nesto RW, Hardison RM, Feit F, Gersh BJ, Krone RJ, Sako EY, Rogers WJ, Garber AJ, King SB III, Davidson CJ, Ikeno F, Frye RL. Clinical and angiographic risk stratification and differential impact on treatment outcomes in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation. 2012;126:2115–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mancini GB, Bates ER, Maron DJ, Hartigan P, Dada M, Gosselin G, Kostuk W, Sedlis SP, Shaw LJ, Berman DS, Berger PB, Spertus J, Mavromatis K, Knudtson M, Chaitman BR, O'Rourke RA, Weintraub WS, Teo K, Boden WE. Quantitative results of baseline angiography and percutaneous coronary intervention in the COURAGE trial. Circ Cardiovasc Qual Outcomes. 2009;2:320–327. [DOI] [PubMed] [Google Scholar]
- 49. Mancini GB, Hartigan PM, Bates ER, Chaitman BR, Sedlis SP, Maron DJ, Kostuk WJ, Spertus JA, Teo KK, Dada M, Knudtson M, Berman DS, Booth DC, Boden WE, Weintraub WS. Prognostic importance of coronary anatomy and left ventricular ejection fraction despite optimal therapy: assessment of residual risk in the Clinical Outcomes Utilizing Revascularization and Aggressive DruG Evaluation Trial. Am Heart J. 2013;166:481–487. [DOI] [PubMed] [Google Scholar]
- 50. Maron DJ, Spertus JA, Mancini GB, Hartigan PM, Sedlis SP, Bates ER, Kostuk WJ, Dada M, Berman DS, Shaw LJ, Chaitman BR, Teo KK, O'Rourke RA, Weintraub WS, Boden WE. Impact of an initial strategy of medical therapy without percutaneous coronary intervention in high‐risk patients from the Clinical Outcomes Utilizing Revascularization and Aggressive DruG Evaluation (COURAGE) trial. Am J Cardiol. 2009;104:1055–1062. [DOI] [PubMed] [Google Scholar]
- 51. Teo KK, Sedlis SP, Boden WE, O'Rourke RA, Maron DJ, Hartigan PM, Dada M, Gupta V, Spertus JA, Kostuk WJ, Berman DS, Shaw LJ, Chaitman BR, Mancini GB, Weintraub WS. Optimal medical therapy with or without percutaneous coronary intervention in older patients with stable coronary disease: a pre‐specified subset analysis of the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive druG Evaluation) trial. J Am Coll Cardiol. 2009;54:1303–1308. [DOI] [PubMed] [Google Scholar]
- 52. Sedlis SP, Jurkovitz CT, Hartigan PM, Goldfarb DS, Lorin JD, Dada M, Maron DJ, Spertus JA, Mancini GB, Teo KK, O'Rourke RA, Boden WE, Weintraub WS. Optimal medical therapy with or without percutaneous coronary intervention for patients with stable coronary artery disease and chronic kidney disease. Am J Cardiol. 2009;104:1647–1653. [DOI] [PubMed] [Google Scholar]
- 53. McFalls EO, Ward HB, Moritz TE, Goldman S, Krupski WC, Littooy F, Pierpont G, Santilli S, Rapp J, Hattler B, Shunk K, Jaenicke C, Thottapurathu L, Ellis N, Reda DJ, Henderson WG. Coronary‐artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795–2804. [DOI] [PubMed] [Google Scholar]
- 54. McNeer JF, Margolis JR, Lee KL, Kisslo JA, Peter RH, Kong Y, Behar VS, Wallace AG, McCants CB, Rosati RA. The role of the exercise test in the evaluation of patients for ischemic heart disease. Circulation. 1978;57:64–70. [DOI] [PubMed] [Google Scholar]
- 55. Pijls NH, Sels JW. Functional measurement of coronary stenosis. J Am Coll Cardiol. 2012;59:1045–1057. [DOI] [PubMed] [Google Scholar]
- 56. Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias‐Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL, Huther ML, Richardson DW; Investigators C . Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. [DOI] [PubMed] [Google Scholar]
- 57. Weiner DA, Ryan TJ, McCabe CH, Chaitman BR, Sheffield LT, Fisher LD, Tristani F. The role of exercise testing in identifying patients with improved survival after coronary artery bypass surgery. J Am Coll Cardiol. 1986;8:741–748. [DOI] [PubMed] [Google Scholar]
- 58. Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short‐term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900–2907. [DOI] [PubMed] [Google Scholar]
- 59. Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, Weintraub WS, O'Rourke RA, Dada M, Spertus JA, Chaitman BR, Friedman J, Slomka P, Heller GV, Germano G, Gosselin G, Berger P, Kostuk WJ, Schwartz RG, Knudtson M, Veledar E, Bates ER, McCallister B, Teo KK, Boden WE. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–1291. [DOI] [PubMed] [Google Scholar]
- 60. Shaw LJ, Weintraub WS, Maron DJ, Hartigan PM, Hachamovitch R, Min JK, Dada M, Mancini GB, Hayes SW, O'Rourke RA, Spertus JA, Kostuk W, Gosselin G, Chaitman BR, Knudtson M, Friedman J, Slomka P, Germano G, Bates ER, Teo KK, Boden WE, Berman DS. Baseline stress myocardial perfusion imaging results and outcomes in patients with stable ischemic heart disease randomized to optimal medical therapy with or without percutaneous coronary intervention. Am Heart J. 2012;164:243–250. [DOI] [PubMed] [Google Scholar]
- 61. Diaz‐Zamudio M, Dey D, Schuhbaeck A, Nakazato R, Gransar H, Slomka PJ, Narula J, Berman DS, Achenbach S, Min JK, Doh JH, Koo BK. Automated quantitative plaque burden from coronary CT angiography noninvasively predicts hemodynamic significance by using fractional flow reserve in intermediate coronary lesions. Radiology. 2015;276:408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mancini GB, Hartigan PM, Shaw LJ, Berman DS, Hayes SW, Bates ER, Maron DJ, Teo K, Sedlis SP, Chaitman BR, Weintraub WS, Spertus JA, Kostuk WJ, Dada M, Booth DC, Boden WE. Predicting outcome in the COURAGE trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation): coronary anatomy versus ischemia. JACC Cardiovasc Interv. 2014;7:195–201. [DOI] [PubMed] [Google Scholar]
- 63. Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R, Camici PG, Cerqueira MD, Chow BJ, Di Carli MF, Dorbala S, Gewirtz H, Gropler RJ, Kaufmann PA, Knaapen P, Knuuti J, Merhige ME, Rentrop KP, Ruddy TD, Schelbert HR, Schindler TH, Schwaiger M, Sdringola S, Vitarello J, Williams KA Sr, Gordon D, Dilsizian V, Narula J. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision‐making. J Am Coll Cardiol. 2013;62:1639–1653. [DOI] [PubMed] [Google Scholar]
- 64. Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek JKJJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary‐artery stenoses. N Engl J Med. 1996;334:1703–1708. [DOI] [PubMed] [Google Scholar]
- 65. Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van' t Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. [DOI] [PubMed] [Google Scholar]
- 66. Rioufol G, Mewton N, Rabilloud M, et al. The FUnctional Testing Underlying Coronary REvascularization (FUTURE) Study: A “Real World” Comparison of Fractional Flow Reserve‐guided Management versus Conventional Management in Multi Vessel Coronary Artery Disease Patients. Paper presented at: American Heart Association Scientific Sessions 2016; November 12–16, 2016; New Orleans, LA. [Google Scholar]
- 67. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast‐induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. [DOI] [PubMed] [Google Scholar]
- 68. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44–e122. [DOI] [PubMed] [Google Scholar]
- 69. Weintraub WS, Boden WE, Zhang Z, Kolm P, Zhang Z, Spertus JA, Hartigan P, Veledar E, Jurkovitz C, Bowen J, Maron DJ, O'Rourke R, Dada M, Teo KK, Goeree R, Barnett PG. Cost‐effectiveness of percutaneous coronary intervention in optimally treated stable coronary patients. Circ Cardiovasc Qual Outcomes. 2008;1:12–20. [DOI] [PubMed] [Google Scholar]
- 70. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bigi R, Cortigiani L, Bax JJ, Colombo P, Desideri A, Sponzilli C, Fiorentini C. Stress echocardiography for risk stratification of patients with chest pain and normal or slightly narrowed coronary arteries. J Am Soc Echocardiogr. 2002;15:1285–1289. [DOI] [PubMed] [Google Scholar]
- 72. Sicari R, Palinkas A, Pasanisi EG, Venneri L, Picano E. Long‐term survival of patients with chest pain syndrome and angiographically normal or near‐normal coronary arteries: the additional prognostic value of dipyridamole echocardiography test (DET). Eur Heart J. 2005;26:2136–2141. [DOI] [PubMed] [Google Scholar]
- 73. Kuhn TS. The Structure of Scientific Revolutions (50th Anniversary Edition). Chicago, IL: The University of Chicago Press; 2012. [Google Scholar]
- 74. Pepine CJ, Douglas PS. Rethinking stable ischemic heart disease: is this the beginning of a new era? J Am Coll Cardiol. 2012;60:957–959. [DOI] [PubMed] [Google Scholar]
- 75. Marzilli M, Merz CN, Boden WE, Bonow RO, Capozza PG, Chilian WM, DeMaria AN, Guarini G, Huqi A, Morrone D, Patel MR, Weintraub WS. Obstructive coronary atherosclerosis and ischemic heart disease: an elusive link! J Am Coll Cardiol. 2012;60:951–956. [DOI] [PubMed] [Google Scholar]
- 76. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence‐based therapies and research agenda for the next decade. Circulation. 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Iqbal J, Zhang YJ, Holmes DR, Morice MC, Mack MJ, Kappetein AP, Feldman T, Stahle E, Escaned J, Banning AP, Gunn JP, Colombo A, Steyerberg EW, Mohr FW, Serruys PW. Optimal medical therapy improves clinical outcomes in patients undergoing revascularization with percutaneous coronary intervention or coronary artery bypass grafting: insights from the Synergy Between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) trial at the 5‐year follow‐up. Circulation. 2015;131:1269–1277. [DOI] [PubMed] [Google Scholar]
- 78. Mancini GB, Farkouh ME, Brooks MM, Chaitman BR, Boden WE, Vlachos H, Hartigan PM, Siami FS, Sidhu MS, Bittner V, Frye R, Fuster V. Medical treatment and revascularization options in patients with type 2 diabetes and coronary disease. J Am Coll Cardiol. 2016;68:985–995. [DOI] [PubMed] [Google Scholar]


