Abstract
Background
Whether the association of blood pressure (BP) during sleep (nocturnal BP) with cognition differs by race is unknown.
Methods and Results
Participants in the GENOA (Genetic Epidemiology Network of Arteriopathy) Study underwent ambulatory BP measurements, brain magnetic resonance imaging, and cognitive function testing (the Rey Auditory Verbal Learning Test, the Digit Symbol Substitution Task, and the Trail Making Test Part B) between 2000 and 2007. We examined multivariable linear regression models of the nocturnal BP‐cognition association. Among 755 participants (mean age, 63 years; 64% women; 42% self‐identified black race; 76% taking antihypertensive medication), mean nocturnal systolic BP (SBP)/diastolic BP was 126/69 mm Hg, daytime SBP/diastolic BP level was 139/82 mm Hg, and mean reduction in SBP from day to night (dipping) was 9%. Among the entire sample, a race interaction was observed in Digit Symbol Substitution Task and Trail Making Test Part B (both P<0.15). Race‐stratified analyses showed that a 1‐SD increase in nocturnal SBP levels was associated with poorer Digit Symbol Substitution Task and log‐transformed Trail Making Test Part B scores (unstandardized regression coefficient [95% confidence interval]: −1.98 [−3.28 to −0.69] and 0.06 [0.004–0.12]; both P<0.05) in black but not white individuals. Additional adjustments for white matter hyperintensity volumes or brain atrophy, measured via brain magnetic resonance imaging, did not change the results. Results were similar when nocturnal SBP dipping was assessed as the exposure, yet daytime SBP levels yielded no association with cognition.
Conclusions
Nocturnal SBP measurements may be useful in assessing the potential risk for lower cognitive function in middle‐aged and older adults, particularly in black individuals.
Keywords: blood pressure, cognition, nocturnal blood pressure, race and ethnicity
Subject Categories: High Blood Pressure, Risk Factors, Cognitive Impairment
Clinical Perspective
What Is New?
The association between higher nocturnal blood pressure and lower cognitive function may be stronger in blacks than in whites.
What Are the Clinical Implications?
Nocturnal systolic blood pressure measurements may be useful in assessing the potential risk for lower cognitive function in middle‐aged and older adults, particularly in black individuals.
During sleep, blood pressure (BP) decreases from wakeful levels. Higher nocturnal BP has been associated with lower cognitive function in middle‐aged and older adults, independently of clinic BP or 24‐hour BP levels.1, 2, 3, 4, 5 Although the underlying mechanisms remain unknown, higher nocturnal BP has correlated with brain structural alterations (eg, white matter hyperintensities [WMHs] and brain atrophy).5, 6, 7, 8 Therefore, we hypothesized that the nocturnal BP‐cognition association may be partly attributable to brain structural alterations.
Black individuals have higher nocturnal BP levels and less nocturnal BP dipping than white individuals,9, 10 potentially because of differences in socioeconomic status, psychological conditions, salt sensitivity, and autonomic function between these race groups.11, 12, 13, 14, 15 The racial difference in nocturnal BP phenotypes has been proposed as a potential contributor to racial disparities in cardiovascular outcomes.10 However, whether higher nocturnal BP in black individuals (versus white individuals) has a stronger association with lower cognition is unknown. We hypothesized that the association of higher nocturnal BP with lower cognition is stronger in black versus white individuals.
Using data from the GENOA (Genetic Epidemiology Network of Arteriopathy) Study, which recruited self‐identified black and white middle‐aged and older adults, we assessed whether nocturnal BP‐cognition association was independent of WMHs or brain atrophy and whether the association varied between black and white individuals.
Methods
GENOA is a multicenter study that started in 1995 and followed up a well‐characterized cohort of sibships from families with histories of hypertension.16 Participants were recruited from families in which at least 2 siblings developed hypertension before the age of 60 years. Self‐identified black and white individuals were recruited; no ancestry or genetic determinations of race were made. Details about the GENOA cohort are provided in Data S1. An ancillary study to GENOA, the GMBI (Genetics of Microangiopathic Brain Injury) Study, included brain magnetic resonance imaging (MRI) between August 2001 and February 2006 and cognitive function testing between December 2000 and May 2004. Among GMBI study participants, 755 underwent noninvasive 24‐hour ambulatory BP monitoring (ABPM) between October 2003 and September 2007. ABPM was conducted within a median of 12 months after brain MRI. This study was approved by the institutional review boards at the Mayo Clinic (Rochester, MN) and University of Mississippi Medical Center (Jackson, MS). All subjects provided written informed consent before participating.
BP and Other Measurements
Participants underwent 24‐hour ABPM using the SpaceLabs model 90202 device.6 The device was attached between 8:00 and 9:00 am, and BP readings were obtained over a 24‐hour period every 15 minutes between 6:00 am and 10:00 pm and every 30 minutes between 10:00 pm and 6:00 am. Participants recorded when they got into bed at night and when they got out of bed the next morning. Daytime and nocturnal BP levels were defined on the basis of these times. Nocturnal systolic BP (SBP) dipping was calculated as follows: (daytime SBP−nocturnal SBP)×100/daytime SBP.17 Nocturnal BP dipping, calculated by SBP, is strongly correlated with that calculated by diastolic BP (r=0.8, P<0.0001). Most of the prior literature has used SBP to calculate nocturnal BP dipping,1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 17 so we did as well. Clinic BP was measured 3 times with appropriately sized cuffs placed on the right arm and with the participant in a seated, resting state. Clinic BP was defined as the average of the second and third measurements. Hypertension was defined as a self‐reported physician diagnosis of hypertension and prescription antihypertensive medication use or an average clinic SBP ≥140 mm Hg or clinic diastolic BP ≥90 mm Hg.
Data on education, smoking and drinking status, physical activity, medication use, clinical history of coronary heart disease and stroke, and fasting laboratory values were collected using standardized protocols (Data S1).
Brain MRI Assessment
All MRI scans were performed on identically equipped Signa 1.5‐T MRI scanners, and images were centrally processed at the Mayo Clinic. Details of the assessment are included in Data S1. Briefly, total intracranial volume was measured from T1‐weighted spin‐echo sagittal images. Total brain and white matter lesion volumes (cm3) were also determined from axial fluid‐attenuated inversion recovery images. Brain atrophy was defined as brain volume subtracted from total intracranial volume. This measure was strongly correlated with brain atrophy, defined by brain normal tissue volume divided by intracranial volume (Pearson r=−0.89; P<0.0001). WMHs in the corona radiata and periventricular zone, as well as infarcts in the central gray matter, were included in the global white matter lesion volume measurements.
Cognitive Assessment
Participants underwent a neuropsychological assessment using a standardized protocol to assess global cognition and domains of memory, executive function, and processing speed. Details of each cognitive assessment are included in Data S1. Briefly, global cognition was assessed by the Mini‐Mental State Examination (MMSE; range, 0–30).18 Tests of memory included the Rey Auditory Verbal Learning Test (RAVLT) delayed recall (range, 0–15).19 Processing speed was measured using the Digit Symbol Substitution Task (DSST).20 In these tests, higher scores indicate better cognition. Executive function was assessed by the Trail Making Test Part B (TMT‐B).18 A greater time to completion (seconds) indicates poorer performance. We also included the Stroop test, a measure of both processing speed and cognitive flexibility.21 Higher scores indicate better cognition.
Statistical Analyses
Descriptive statistics are presented as means and SDs, proportions, and medians with interquartile ranges, where appropriate. Correlations between nocturnal BP and clinical characteristics were calculated via the Pearson correlation method. Linear and logistic models with generalized estimating equations were used to assess the association between nocturnal BP and each cognitive function, accounting for clustering according to sibship.22 Results were reported as unstandardized regression coefficients associated with a 1‐SD increase of exposures for continuous outcomes and odds ratios for categorical outcomes. The primary outcomes were the measures of cognition function. The primary exposure was nocturnal BP levels, with nocturnal SBP dipping considered as a secondary exposure. To evaluate the effect size of nocturnal BP on outcomes, for comparison, we provided the effect size associated with age, a robust contributor to lower cognition.23 Some investigations of nocturnal SBP dipping and outcomes have demonstrated nonlinear associations17, 24; thus, quartile analyses of nocturnal SBP dipping were also conducted. Possible violations of the assumptions of multiple linear regression were examined by visual inspection of the distribution of residuals through both histograms and normal probability plots. We further checked for deviations of linearity and homoscedasticity by visually inspecting scatterplots of standardized residuals by standardized predicted values. In addition, we assessed variance inflation factors to examine the possibility of multicollinearity; values >2.5 indicated collinearity. The TMT‐B scores were log transformed because of skewed distributions. The MMSE scores, as a continuous variable, did not meet our model diagnostic criteria, even after log transformation. Therefore, we assessed the MMSE as a categorical variable. The lowest quartile group of the distribution of the MMSE score was defined as the presence of low cognition.18, 20
Covariates included demographic variables (age, sex, race, and educational attainment) and clinical characteristics (body mass index, estimated glomerular filtration rate,25 prevalent diabetes mellitus, duration of hypertension, use of antihypertensive medications, prevalent stroke, and clinic SBP levels) (model 1). These covariates were selected a priori because they have known correlations with nocturnal SBP dipping17 and cognitive function and could potentially confound the association between these 2 variables.23 We further adjusted for WMH volumes (model 2) or the extent of brain atrophy (model 3). For the secondary exposure (ie, nocturnal SBP dipping), 24‐hour mean SBP was used as an adjustment factor.
Analyses for heterogeneity of effect between nocturnal BP and cognition by sex, race, or antihypertensive medication use were performed with inclusion of additive interaction terms. Stratified analyses were considered when an interaction was observed (P<0.15). We imputed missing data on cognitive function (Table S1), using multiple imputation chained equations with 20 iterations, as described by Raghunathan et al.26 We conducted sensitivity analyses by doing the following: (1) performing analyses without imputing missing cognitive function test scores; and (2) identifying whether the nocturnal BP‐cognition association was modified by adjustments for physical activity, smoking or drinking status, use of diuretics, renin‐angiotensin system inhibitors, or sympatholytic drugs, or sleep duration during ABPM. These variables were assessed as sensitivity analyses to avoid overfitting in regression models. All statistical analyses were performed with STATA version 12.1. Statistical significance was defined by P<0.05 using 2‐sided tests.
Results
Of the 775 participants, 64.1% were women, 41.6% were black, and 75.8% reported antihypertensive medication use; their mean±SD age at baseline was 63.3±6.7 years (Table 1). Lower educational attainment and smaller magnitude of nocturnal SBP dipping were observed in black versus white individuals, whereas daytime SBP was higher in whites (Table 1). The MMSE, RAVLT, Stroop test, and TMT‐B scores were lower in black versus white individuals, and the difference remained significant after adjusting for covariates, including educational attainment and nocturnal SBP level or dipping (all P<0.001). The distribution of nocturnal or daytime SBP level and nocturnal SBP dipping according to race is shown in Figure S1.
Table 1.
Clinical Characteristics of GENOA Study Cohort Participants
| Characteristic | Total (N=755) | Black Individuals (n=314) | White Individuals (n=441) | P Values |
|---|---|---|---|---|
| Age, mean±SD, y | 63.3±6.7 | 63.2±6.6 | 63.4±6.8 | 0.67 |
| Men, % | 35.9 | 29.0 | 40.8 | 0.001 |
| Black (self‐identified), % | 41.6 | 100 | 0 | ··· |
| Education: less than high school, % | 16.4 | 28.3 | 7.9 | <0.001 |
| Body mass index, mean±SD, kg/m2 | 30.0±5.1 | 30.0±4.7 | 30.0±5.3 | 0.87 |
| Ever smoker, % | 44.1 | 49.3 | 40.4 | 0.01 |
| Current drinker, % | 58.0 | 75.8 | 45.4 | <0.001 |
| Physical activity, mean±SD, score | −10.7±6.1 | −12.8±4.3 | −9.3±6.7 | <0.001 |
| Diabetes mellitus, % | 18.3 | 23.9 | 14.3 | 0.001 |
| Total cholesterol, mean±SD, mg/dL | 201.8±39.0 | 203.5±44.0 | 200.5±35.1 | 0.31 |
| eGFR, mean±SD, mL/min per 1.73 m2 | 85.4±21.4 | 94.5±22.7 | 79.0±17.9 | <0.001 |
| Hypertension, % | 82.6 | 84.8 | 79.6 | 0.06 |
| Duration of hypertension, mean±SD, y | 12.4±12.3 | 11.9±12.6 | 12.8±12.1 | 0.30 |
| Antihypertensive medication, % | 75.8 | 70.4 | 79.6 | 0.004 |
| Diuretics | 43.4 | 43.3 | 43.5 | 0.95 |
| Renin‐angiotensin system inhibitor | 37.1 | 38.2 | 36.3 | 0.59 |
| Sympatholytic drug | 2.0 | 3.5 | 0.9 | 0.01 |
| BP measures, mean±SD | ||||
| Clinic SBP, mm Hg | 135.5±17.8 | 137.3±19.9 | 134.1±16.0 | 0.02 |
| Clinic DBP, mm Hg | 76.5±10.2 | 79.8±10.8 | 74.1±8.9 | <0.001 |
| 24‐h SBP, mm Hg | 135.9±16.2 | 132.8±14.2 | 138.1±17.1 | <0.001 |
| 24‐h DBP, mm Hg | 78.9±9.2 | 78.7±8.9 | 79.1±9.4 | 0.49 |
| Daytime SBP, mm Hg | 138.9±16.2 | 135.2±14.1 | 141.5±17.1 | <0.001 |
| Daytime DBP, mm Hg | 81.9±9.5 | 81.7±9.1 | 82.1±9.8 | 0.62 |
| Nocturnal SBP, mm Hg | 126.2±18.3 | 125.2±16.1 | 126.9±19.7 | 0.22 |
| Nocturnal DBP, mm Hg | 69.4±10.0 | 69.3±10.2 | 69.5±9.9 | 0.71 |
| Nocturnal SBP dipping, % | 9.1±7.5 | 7.4±6.8 | 10.4±7.7 | <0.001 |
| Sleep duration, h | 8.3±1.3 | 8.3±1.1 | 8.3±1.4 | 0.78 |
| Prevalent stroke, % | 3.3 | 3.5 | 3.2 | 0.80 |
| Prevalent coronary heart disease, % | 6.5 | 4.1 | 8.2 | 0.03 |
| Brain MRI, mean±SD, cm3 | ||||
| White matter hyperintensity | 9.3±9.5 | 10.3±11.9 | 8.5±7.1 | 0.01 |
| Brain atrophy | 311.0±72.5 | 301.3±69.9 | 318.0±73.6 | 0.002 |
| Cognitive function | ||||
| MMSE, mean±SD, score | 28.2±1.9 | 28.7±1.5 | 27.5±2.2 | |
| MMSE, median (IQR), score | 29 (27–30) | 28.0 (26.1–29.0) | 29.0 (28.0–30.0) | <0.001 |
| Low MMSE (n=214), % | 28.3 | 42.4 | 19.7 | <0.001 |
| DSST, mean±SD, symbols | 42.1±14.4 | 33.2±12.7 | 48.5±12.0 | 0.86 |
| RAVLT, mean±SD, words | 7.9±3.5 | 6.8±3.5 | 8.7±3.2 | <0.001 |
| Stroop test, mean±SD, score | 177.6±36.0 | 158.2±34.5 | 191.4±30.1 | <0.001 |
| TMT‐B, mean±SD, s | 103.2±55.7 | 139.0±63.2 | 77.7±30.1 | <0.001 |
| TMT‐B, median (IQR), s | 81.0 (66.0–114.0) | 121.0 (82.0–190.0) | 71.0 (61.0–84.0) | |
| Log TMT‐B, mean±SD, s | 4.49±0.46 | 4.81±0.46 | 4.26±0.28 | <0.001 |
P values were calculated by unpaired t test or χ2 test. Low MMSE was defined as the lowest quartile group of the distribution of the MMSE score. The TMT‐B scores were log transformed because of skewed distributions. BP indicates blood pressure; DBP, diastolic BP; DSST, Digit Symbol Substitution Task; eGFR, estimated glomerular filtration rate; GENOA, Genetic Epidemiology Network of Arteriopathy; IQR, interquartile range; MMSE, Mini‐Mental State Examination; MRI, magnetic resonance imaging; RAVLT, Rey Auditory Verbal Learning Test; SBP, systolic BP; and TMT‐B, Trail Making Test Part B.
Tables S2 through S7 show the associations between nocturnal BP and clinical characteristics. In black and white individuals, age and prevalent diabetes mellitus were associated with higher nocturnal SBP and less nocturnal SBP dipping.
Nocturnal or daytime SBP level was not associated with the MMSE, RAVLT, DSST, Stroop test, or TMT‐B score (Table 2 and Table S8). However, interactions were found between race and nocturnal SBP level in association with the DSST, Stroop test, and TMT‐B scores (Table 2). In race‐specific regression models, higher nocturnal SBP level was associated with poorer DSST and TMT‐B scores in black but not white individuals (model 1 in Table 3). A nonsignificant trend toward higher nocturnal SBP level associated with poorer Stroop test scores was observed in black but not white individuals. Results were largely similar when we adjusted for WMH volumes (model 2) or the extent of brain atrophy (model 3). In model 1 of Table 3, the unstandardized regression coefficient (95% confidence interval) for DSST score associated with a 1‐year increase of age was −0.70 (−0.90 to −0.50; P<0.001) in black individuals.
Table 2.
Associations Between Nocturnal SBP Levels and Each Cognitive Function
| Variables | Low MMSE Score, OR (95% CI) | β (95% CI) | |||
|---|---|---|---|---|---|
| DSST | RAVLT | Stroop Test | Log TMT‐B | ||
| Model 1a | |||||
| Nocturnal SBP level |
1.17 (0.96 to 1.42) P=0.12 |
−0.91 (−1.64 to −0.18) P=0.01 |
−0.19 (−0.43 to 0.06) P=0.13 |
−1.00 (−3.13 to 1.12) P=0.35 |
0.24 (−0.003 to 0.05) P=0.08 |
| Model 2b | |||||
| Nocturnal SBP level |
1.16 (0.95 to 1.42) P=0.15 |
−0.88 (−1.62 to −0.14) P=0.02 |
−0.13 (−0.38 to 0.11) P=0.28 |
−0.98 (−3.15 to 1.17) P=0.37 |
0.02 (−0.005 to 0.05) P=0.10 |
| Model 3c | |||||
| Nocturnal SBP level |
1.16 (0.95 to 1.42) P=0.14 |
−0.91 (−1.64 to −0.19) P=0.01 |
−0.19 (−0.43 to 0.05) P=0.13 |
−1.01 (−3.13 to 1.11) P=0.35 |
0.02 (−0.003 to 0.05) P=0.08 |
| Model 4d | |||||
| Nocturnal SBP level×race |
1.00 (0.69 to 1.46) P=0.99 |
−1.63 (−3.15 to −0.12) P=0.03 |
−0.14 (−0.64 to 0.35) P=0.57 |
−3.31 (−7.48 to 0.85) P=0.12 |
0.05 (−0.01 to 0.11) P=0.13 |
N=755. Adjusted OR or β (95% CI) values associated with 1‐SD increase of nocturnal SBP levels (+18.3 mm Hg) are shown. Statistical significance was defined as P<0.05. β indicates unstandardized regression coefficient; CI, confidence interval; DSST, Digit Symbol Substitution Task; MMSE, Mini‐Mental State Examination; OR, odds ratio; RAVLT, Rey Auditory Verbal Learning Test; SBP, systolic blood pressure; and TMT‐B, Trail Making Test Part B.
Adjustment factors for model 1 included demographic variables (age, sex, race, and education) plus clinical characteristics (body mass index, estimated glomerular filtration rate, prevalent diabetes mellitus, duration of hypertension, use of antihypertensive medications, prevalent stroke, and clinic SBP levels).
Adjustment factors for model 2 included demographic variables, clinical characteristics, and white matter hyperintensity volumes.
Adjustment factors for model 3 included demographic variables, clinical characteristics, and brain atrophy.
Adjustment factors for model 4 included demographic variables, clinical characteristics, and nocturnal SBP levels×race.
Table 3.
Race‐Specific Associations Between Nocturnal SBP Levels and Each Cognitive Function
| Variables | DSST | Stroop Test | Log TMT‐B | |||
|---|---|---|---|---|---|---|
| Black Individuals (n=314) | White Individuals (n=441) | Black Individuals (n=314) | White Individuals (n=441) | Black Individuals (n=314) | White Individuals (n=441) | |
| Model 1a | ||||||
| Nocturnal SBP level |
−1.98 (−3.28 to −0.69) P=0.003 |
−0.41 (−1.27 to 0.44) P=0.35 |
−3.45 (−7.16 to 2.54) P=0.07 |
−0.45 (−3.06 to 2.16) P=0.74 |
0.06 (0.004–0.12) P=0.048 |
0.02 (−0.13 to 0.04) P=0.28 |
| Model 2b | ||||||
| Nocturnal SBP level |
−2.00 (−3.33 to −0.67) P=0.003 |
−0.37 (−1.24 to 0.49) P=0.40 |
−3.67 (−7.44 to 0.09) P=0.06 |
−0.38 (−3.01 to 2.24) P=0.78 |
0.06 (−0.003 to 0.12) P=0.06 |
0.01 (−0.01 to 0.04) P=0.31 |
| Model 3c | ||||||
| Nocturnal SBP level |
−1.96 (−3.26 to −0.65) P=0.003 |
−0.41 (−1.27 to 0.44) P=0.34 |
−3.38 (−7.04 to 0.27) P=0.07 |
−0.46 (−3.07 to 2.15) P=0.73 |
0.06 (−0.001 to 0.12) P=0.05 |
0.02 (−0.01 to 0.04) P=0.28 |
Data are given as adjusted unstandardized regression coefficient (95% CI) values associated with 1‐SD increase of nocturnal SBP levels (+18.3 mm Hg). Statistical significance was defined as P<0.05. DSST indicates Digit Symbol Substitution Task; SBP, systolic blood pressure; and TMT‐B, Trail Making Test Part B.
Adjustment factors for model 1 included demographic variables (age, sex, and education) plus clinical characteristics (body mass index, estimated glomerular filtration rate, prevalent diabetes mellitus, duration of hypertension, use of antihypertensive medications, prevalent stroke, and clinic SBP levels).
Adjustment factors for model 2 included demographic variables, clinical characteristics, and white matter hyperintensity volumes.
Adjustment factors for model 3 included demographic variables, clinical characteristics, and brain atrophy.
With adjustments for covariates, smaller nocturnal SBP dipping was associated with poorer RAVLT scores, but not with MMSE, DSST, and Stroop test scores (Table 4). Results were similar when we adjusted for 24‐hour SBP levels (model 2), WMH volumes (model 3), or the extent of brain atrophy (model 4). A nonsignificant trend toward smaller nocturnal SBP dipping associated with poorer TMT‐B scores was observed. In model 2 of Table 2, the unstandardized regression coefficient (95% confidence interval) for RAVLT score associated with a 1‐year increase of age was −0.10 (−0.14 to −0.06; all P<0.001). Quartile analyses of nocturnal SBP dipping did not show a J‐ or U‐shaped association between dipping and cognition (Figure). Interactions were found between race and nocturnal SBP dipping in association with the DSST, Stroop test, and TMT‐B scores (model 5 in Table 4). In race‐specific regression models, a smaller magnitude of nocturnal SBP dipping was associated with worse DSST or TMT‐B score in black but not white individuals (model 1 in Table 5). Results were largely similar when we adjusted for 24‐hour SBP levels (model 2), WMH volumes (model 3), or the extent of brain atrophy (model 4). Additional adjustments for physical activity; smoking or drinking status; use of diuretics, renin‐angiotensin system inhibitors, or sympatholytic drugs; or sleep duration during ABPM did not change the results (data not shown).
Table 4.
Associations Between Nocturnal SBP Dipping and Each Cognitive Function
| Variables | Low MMSE Score, OR (95% CI) | β (95% CI) | |||
|---|---|---|---|---|---|
| DSST | RAVLT | Stroop Test | Log TMT‐B | ||
| Model 1a | |||||
| Nocturnal SBP dipping |
0.92 (0.76 to 1.11) P=0.38 |
0.64 (−0.05 to 1.33) P=0.07 |
0.34 (0.12–0.56) P=0.003 |
0.84 (−1.10 to 2.78) P=0.39 |
−0.03 (−0.05 to −0.001) P=0.04 |
| Model 2b | |||||
| Nocturnal SBP dipping |
0.93 (0.77 to 1.13) P=0.48 |
0.57 (−0.12 to 1.26) P=0.11 |
0.34 (0.12–0.57) P=0.003 |
0.77 (−1.19 to 2.72) P=0.44 |
−0.03 (−0.05 to 0.003) P=0.05 |
| Model 3c | |||||
| Nocturnal SBP dipping |
0.95 (0.78 to 1.15) P=0.60 |
0.55 (−0.14 to 1.25) P=0.12 |
0.32 (0.09–0.54) P=0.006 |
0.76 (−1.21 to 2.73) P=0.45 |
−0.03 (−0.05 to 0.002) P=0.05 |
| Model 4d | |||||
| Nocturnal SBP dipping |
0.95 (0.78 to 1.15) P=0.59 |
0.54 (−0.16 to 1.25) P=0.13 |
0.33 (0.11–0.55) P=0.004 |
0.73 (−1.24 to 2.69) P=0.47 |
−0.03 (−0.05 to 0.001) P=0.05 |
| Model 5e | |||||
| Nocturnal SBP dipping×race |
0.76 (0.53 to 1.10) P=0.15 |
1.99 (0.52–3.46) P=0.008 |
0.06 (−0.43 to 0.54) P=0.82 |
3.64 (−0.51 to 7.80) P=0.09 |
−0.05 (−0.11 to −0.005) P=0.07 |
N=755. Adjusted OR or β (95% CI) values associated with 1‐SD increase of nocturnal SBP dipping (7.5% reduction of nocturnal SBP from daytime SBP) are shown. Statistical significance was defined as P<0.05. β indicates unstandardized regression coefficient; CI, confidence interval; DSST, Digit Symbol Substitution Task; MMSE, Mini‐Mental State Examination; OR, odds ratio; RAVLT, Rey Auditory Verbal Learning Test; SBP; systolic blood pressure; and TMT‐B, Trail Making Test Part B.
Adjustment factors for model 1 included demographic variables (age, sex, race, and education) plus clinical characteristics (body mass index, estimated glomerular filtration rate, prevalent diabetes mellitus, duration of hypertension, use of antihypertensive medications, prevalent stroke, and clinic SBP levels).
Adjustment factors for model 2 included demographic variables, clinical characteristics, and 24‐hour mean SBP levels.
Adjustment factors for model 3 included demographic variables, clinical characteristics, 24‐hour mean SBP levels, and white matter hyperintensity volumes.
Adjustment factors for model 4 included demographic variables, clinical characteristics, 24‐hour mean SBP levels, and brain atrophy.
Adjustment factors for model 5 included demographic variables, clinical characteristics, 24‐hour mean SBP levels, and nocturnal SBP dipping×race.
Figure 1.
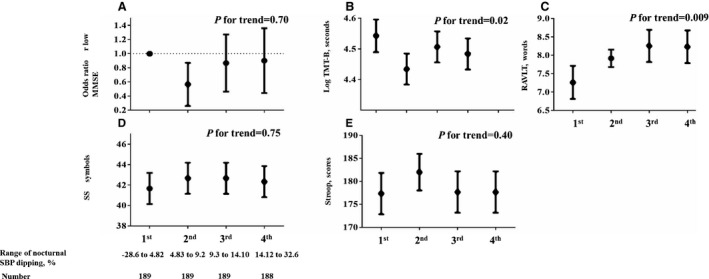
Cognitive function by nocturnal systolic blood pressure (SBP) dipping quartiles. A, Adjusted odds ratio (OR; 95% confidence interval [CI]) of low Mini‐Mental State Examination (MMSE; defined as the lowest quartile of the distribution of the MMSE scores) by nocturnal SBP dipping quartiles. The Trail Making Test Part B (TMT‐B) scores were log transformed because of skewed distributions. The first quartile group was defined as a reference. B through E, Adjusted means (95% CIs) of each cognitive test score by nocturnal SBP dipping quartile. The first quartile group was defined as a reference. Adjustment factors included age, sex, race, education, clinical characteristics (body mass index, estimated glomerular filtration rate, prevalent diabetes mellitus, duration of hypertension, use of antihypertensive medications, prevalent stroke, and clinic SBP levels), and 24‐hour mean SBP levels. All P values shown were for trend tests. Statistical significance was defined as P<0.05. DSST indicates Digit Symbol Substitution Task; and RAVLT, Rey Auditory Verbal Learning Test.
Table 5.
Race‐Specific Associations Between Nocturnal SBP Dipping and Each Cognitive Function
| Variables | DSST | Stroop Test | Log TMT‐B | |||
|---|---|---|---|---|---|---|
| Black Individuals (n=314) | White Individuals (n=441) | Black Individuals (n=314) | White Individuals (n=441) | Black Individuals (n=314) | White Individuals (n=441) | |
| Model 1a | ||||||
| Nocturnal SBP dipping |
1.49 (0.34 to 2.64) P=0.01 |
0.12 (−0.74 to 1.00) P=0.78 |
2.71 (−0.75 to 6.18) P=0.12 |
0.23 (−2.29 to 2.75) P=0.86 |
−0.05 (−0.10 to −0.002) P=0.04 |
−0.01 (−0.04 to 0.01) P=0.32 |
| Model 2b | ||||||
| Nocturnal SBP dipping |
1.41 (0.24 to 2.57) P=0.02 |
0.07 (−0.81 to 0.93) P=0.88 |
2.56 (−1.04 to 6.16) P=0.16 |
0.17 (−2.35 to 2.68) P=0.90 |
−0.05 (−0.10 to −0.00003) P=0.05 |
−0.01 (−0.04 to 0.02) P=0.36 |
| Model 3c | ||||||
| Nocturnal SBP dipping |
1.41 (0.24 to 2.58) P=0.02 |
−0.001 (−0.89 to 0.88) P=0.997 |
2.60 (−0.99 to 6.20) P=0.16 |
0.04 (−2.52 to 2.59) P=0.98 |
−0.05 (−0.09 to −0.001) P=0.05 |
−0.01 (−0.04 to 0.02) P=0.43 |
| Model 4d | ||||||
| Nocturnal SBP dipping |
1.37 (0.22 to 2.52) P=0.02 |
0.06 (−0.82 to 0.94) P=0.90 |
2.49 (−1.01 to 5.99) P=0.16 |
0.15 (−2.38 to 2.68) P=0.91 |
−0.05 (−0.10 to −0.002) P=0.06 |
−0.01 (−0.04 to 0.02) P=0.38 |
Data are given as adjusted unstandardized regression coefficient (95% CI) values associated with 1‐SD increase of nocturnal SBP dipping (7.5% reduction of nocturnal SBP from daytime SBP). Statistical significance was defined as P<0.05. DSST indicates Digit Symbol Substitution Task; SBP, systolic blood pressure; and TMT‐B, Trail Making Test Part B.
Adjustment factors for model 1 included demographic variables (age, sex, and education) plus clinical characteristics (body mass index, estimated glomerular filtration rate, prevalent diabetes mellitus, duration of hypertension, use of antihypertensive medications, prevalent stroke, and clinic SBP levels).
Adjustment factors for model 2 included demographic variables, clinical characteristics, and 24‐hour mean SBP levels.
Adjustment factors for model 3 included demographic variables, clinical characteristics, 24‐hour mean SBP levels, and white matter hyperintensity volumes.
Adjustment factors for model 4 included demographic variables, clinical characteristics, 24‐hour mean SBP levels, and brain atrophy.
There was no evidence of interaction of nocturnal SBP level or dipping with sex or antihypertensive medication use in association with any cognitive function score (all P>0.20). Results with and without imputing missing cognitive test scores were similar in terms of the point estimate for nocturnal SBP level and dipping (Tables S9 through S12).
Discussion
In this community‐based, biracial cohort of middle‐aged and older adults, higher nocturnal SBP levels and smaller nocturnal SBP dipping were associated with lower executive function (ie, higher TMT‐B scores) and with slower processing speed (ie, lower DSST scores) in self‐identified black but not white individuals. Smaller nocturnal SBP dipping was associated with lower memory (ie, lower RAVLT scores) in both black and white individuals. These associations were independent of brain structural alterations (ie, WMH volumes or brain atrophy). Daytime SBP levels were not associated with cognition in either racial group.
Nocturnal SBP levels and nocturnal SBP dipping were associated with TMT‐B and DSST scores only in black individuals. A nonsignificant trend was observed only in black individuals between higher nocturnal SBP and poorer Stroop test scores, which reflect white matter integrity in the frontal lobe and, thereby, executive function.21, 27 Nocturnal SBP levels and dipping were not associated with MMSE scores, potentially because of their lack of sensitivity to mild cognitive impairment.28 Whether the effects of nocturnal SBP on the brain are, in fact, regionally specific will require further investigation.29, 30 Structural changes related to the effects of high BP on the cerebral vasculature, including WMH and brain atrophy, have been proposed as a potential mechanistic link between high BP and cognitive dysfunction.29, 30 In our findings, the association between nocturnal SBP and cognition was slightly attenuated, but remained statistically significant even after adjustment for the extent of WMH and brain atrophy. More advanced imaging, taking into account microinfarcts, microbleeds, and cerebrovascular reactivity, and possible nonvascular pathological features (eg, β‐amyloid)31, 32 may shed additional light on the mechanistic links between higher nocturnal SBP and lower cognition.
Nocturnal BP compared with clinic or daytime BP could reflect existing pathophysiological features, including sympathovagal imbalance, volume retention, impaired salt excretion, and/or disturbed breathing during sleep.11, 12, 13, 14, 17 Spruill et al demonstrated that unmarried status and lower educational attainment were independently associated with nondipping and together accounted for 36% of the racial difference in nocturnal SBP dipping between black and white individuals.11 Psychological factors, including anger, hostility, depression, and stress, have been associated with nondipping in black individuals.12, 13 Higher nocturnal SBP levels or smaller nocturnal SBP dipping could be merely epiphenomenon of other contributing conditions. A stronger association of nocturnal BP with cognition in black compared with white individuals might be attributable to differences in the pathophysiological features of higher nocturnal BP across the racial groups. For example, adverse stressors (eg, psychosocial stress and sleep deprivation), sleep‐disordered breathing, and/or lower socioeconomic status, which are more common in black than white individuals, could lead to both higher nocturnal BP and lower cognitive function in black individuals.10, 11, 12, 13, 33, 34, 35 Determining the race‐specific mechanisms underlying higher nocturnal BP may lead to individualized interventions to prevent or slow cognitive decline.
The superiority of nocturnal BP dipping compared with nocturnal BP levels as a correlate of lower cognitive function has been demonstrated.36, 37 We found that nocturnal SBP dipping, but not nocturnal SBP levels, was associated with RAVLT scores, which reflect hippocampal (memory) function, in both black and white individuals. Despite strong correlation between nocturnal SBP dipping and nocturnal SBP levels (Pearson r=−0.6), the independent associations of these variables with brain function may differ.17 Nocturnal SBP dipping is determined by nocturnal BP levels and also by BP levels during daily activities, exercise, and postural change from sitting to standing.17, 38, 39 Although adjustment for physical activity did not materially change our findings, we cannot exclude possible residual confounding affecting the association of nocturnal BP dipping with memory. For example, orthostatic hypotension and psychological distress (eg, depression) may lead to both smaller nocturnal BP dipping and hippocampal damage.38, 40, 41, 42 Given that hippocampal neurons are highly vulnerable to disturbances of the cerebral circulation and adverse stressors,43, 44 nocturnal SBP dipping (versus nocturnal SBP levels) might have a stronger association with hippocampal function.
Strengths of this study include the well‐characterized, community‐based biracial cohort; the standardized data collection protocols and rigorous quality control of the GENOA Study; and application of a comprehensive standardized cognitive test battery. There are also several limitations. First, because the findings of this study are based on a cross‐sectional analysis, we are unable to determine the direction of the relationships observed. Further longitudinal studies will be needed to corroborate and elucidate our findings. Second, participants were from limited sites in the United States and, thus, might not be representative of the general US population. Third, self‐identified race might not be as accurate as direct assessment of individual genomic information. Fourth, although statistically significant, the effect sizes of nocturnal SBP levels or dipping on cognition were relatively small. Nevertheless, these effect sizes corresponded to age differences of ≈3 years. Fifth, participants taking antihypertensive medication were included. Of participants, 76% received antihypertensive medications; thus, stratified analyses by the presence or absence of medications were not conducted. Furthermore, the number of medications that required more than a single daily dose was unknown in this study. Medications with short durations of action might be related to higher nocturnal BP and, subsequently, lower cognitive function. Sixth, we have only a single measurement of ABPM, and the reproducibility of nocturnal BP dipping may be limited. Some participants might have had sleep deprivation during the overnight BP monitoring, although there was evidence that sleep quality did not affect the dipping‐cognition association in a prior community‐based cohort.1 In addition, we used self‐reported bedtimes from participants who underwent ABPM, which may be less accurate than an objective measurement. These limitations potentially dilute any true association between nocturnal BP and cognition.
We highlight the clinical relevance of nocturnal SBP levels and dipping on the brain in middle‐aged and older black and white individuals (ie, both nocturnal SBP levels and circadian rhythm in SBP appear important in identifying risk for lower cognitive function). This may be important for black individuals in particular. Further studies are warranted to assess whether reductions in nocturnal SBP or restoration of normal circadian BP variation can help to limit declines in cognition in later life. This hypothesis will need to be confirmed in interventional trials, with consideration of all the complex issues at play, including cost‐effectiveness, availability, and patient perspectives.
Sources of Funding
The GENOA (Genetic Epidemiology Network of Arteriopathy) and GMBI (Genetics of Microangiopathic Brain Injury) studies were supported by US Public Health Service grants (U01HL054463, U01HL054464, and R01NS41558). Yano is partially supported by the National Institute of General Medical Sciences of the National Institutes of Health (award P20GM104357). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
None.
Supporting information
Data S1. Supplemental Methods.
Table S1. Number and Type of Imputed Variables
Table S2. Race‐Specific Associations Between Nocturnal SBP Levels and Demographic Variables and Clinical Characteristics (n=755)
Table S3. Race‐Specific Associations Between Nocturnal SBP Levels and Demographic Variables and Clinical Characteristics (n=755)
Table S4. Race‐Specific Associations Between Nocturnal DBP Levels and Demographic Variables and Clinical Characteristics (n=755)
Table S5. Associations Between Nocturnal DBP Levels and Demographic Variables and Clinical Characteristics (n=755)
Table S6. Race‐Specific Associations Between Nocturnal SBP Dipping and Demographic Variables and Clinical Characteristics (n=755)
Table S7. Associations Between Nocturnal SBP Dipping and Demographic Variables and Clinical Characteristics (n=755)
Table S8. Associations Between Daytime SBP Levels and Each Cognitive Function (n=755)
Table S9. Associations Between Nocturnal SBP Levels and Each Cognitive Function (Without Multiple Imputation)
Table S10. Race‐Specific Associations Between Nocturnal SBP Levels and Each Cognitive Function (Without Multiple Imputation)
Table S11. Associations Between Nocturnal SBP Dipping and Each Cognitive Function (Without Multiple Imputation)
Table S12. Race‐Specific Associations Between Nocturnal SBP Dipping and Each Cognitive Function (Without Multiple Imputation)
Figure S1. Histogram of nocturnal SBP levels and dipping.
(J Am Heart Assoc.2017;6:e007022 DOI: 10.1161/JAHA.117.007022.)29079569
Contributor Information
Yuichiro Yano, Email: yyano@jichi.jp, Email: yyano@umc.edu.
Thomas H. Mosley, Email: tmosley@umc.edu.
References
- 1. Yano Y, Ning H, Muntner P, Reis JP, Calhoun DA, Viera AJ, Levine DA, Jacobs DR Jr, Shimbo D, Liu K, Greenland P, Lloyd‐Jones D. Nocturnal blood pressure in young adults and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Hypertens. 2015;28:1240–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yano Y, Inokuchi T, Hoshide S, Kanemaru Y, Shimada K, Kario K. Association of poor physical function and cognitive dysfunction with high nocturnal blood pressure level in treated elderly hypertensive patients. Am J Hypertens. 2011;24:285–291. [DOI] [PubMed] [Google Scholar]
- 3. Riba‐Llena I, Nafría C, Filomena J, Tovar JL, Vinyoles E, Mundet X, Jarca CI, Vilar‐Bergua A, Montaner J, Delgado P. High daytime and nighttime ambulatory pulse pressure predict poor cognitive function and mild cognitive impairment in hypertensive individuals. J Cereb Blood Flow Metab. 2016;36:253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kanemaru A, Kanemaru K, Kuwajima I. The effects of short‐term blood pressure variability and nighttime blood pressure levels on cognitive function. Hypertens Res. 2001;24:19–24. [DOI] [PubMed] [Google Scholar]
- 5. Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. J Hypertens. 2008;26:1636–1641. [DOI] [PubMed] [Google Scholar]
- 6. Schwartz GL, Bailey KR, Mosley T, Knopman DS, Jack CR Jr, Canzanello VJ, Turner ST. Association of ambulatory blood pressure with ischemic brain injury. Hypertension. 2007;49:1228–1234. [DOI] [PubMed] [Google Scholar]
- 7. Yamamoto Y, Akiguchi I, Oiwa K, Hayashi M, Kimura J. Adverse effect of nighttime blood pressure on the outcome of lacunar infarct patients. Stroke. 1998;29:570–576. [DOI] [PubMed] [Google Scholar]
- 8. White WB, Wolfson L, Wakefield DB, Hall CB, Campbell P, Moscufo N, Schmidt J, Kaplan RF, Pearlson G, Guttmann CR. Average daily blood pressure, not office blood pressure, is associated with progression of cerebrovascular disease and cognitive decline in older people. Circulation. 2011;124:2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muntner P, Lewis CE, Diaz KM, Carson AP, Kim Y, Calhoun D, Yano Y, Viera AJ, Shimbo D. Racial differences in abnormal ambulatory blood pressure monitoring measures: results from the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Hypertens. 2015;28:640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Profant J, Dimsdale JE. Race and diurnal blood pressure patterns: a review and meta‐analysis. Hypertension. 1999;33:1099–1104. [DOI] [PubMed] [Google Scholar]
- 11. Spruill TM, Gerin W, Ogedegbe G, Burg M, Schwartz JE, Pickering TG. Socioeconomic and psychosocial factors mediate race differences in nocturnal blood pressure dipping. Am J Hypertens. 2009;22:637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tomfohr L, Cooper DC, Mills PJ, Nelesen RA, Dimsdale JE. Everyday discrimination and nocturnal blood pressure dipping in black and white Americans. Psychosom Med. 2010;72:266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilson DK, Kliewer W, Teasley N, Plybon L, Sica DA. Violence exposure, catecholamine excretion, and blood pressure nondipping status in African American male versus female adolescents. Psychosom Med. 2002;64:906–915. [DOI] [PubMed] [Google Scholar]
- 14. Sachdeva A, Weder AB. Nocturnal sodium excretion, blood pressure dipping, and sodium sensitivity. Hypertension. 2006;48:527–533. [DOI] [PubMed] [Google Scholar]
- 15. Sherwood A, Steffen PR, Blumenthal JA, Kuhn C, Hinderliter AL. Nighttime blood pressure dipping: the role of the sympathetic nervous system. Am J Hypertens. 2002;15:111–118. [DOI] [PubMed] [Google Scholar]
- 16. FBPP Investigators . Multi‐center genetic study of hypertension: the Family Blood Pressure Program (FBPP). Hypertension. 2002;39:3–9. [DOI] [PubMed] [Google Scholar]
- 17. Yano Y, Kario K. Nocturnal blood pressure and cardiovascular disease: a review of recent advances. Hypertens Res. 2012;35:695–701. [DOI] [PubMed] [Google Scholar]
- 18. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 19. Spreen O, Strauss E. A Compendium of Neuropsychological Tests. 2nd ed New York, NY: Oxford University Press; 1998. [Google Scholar]
- 20. Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 21. Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 22. Epstein MP, Duncan R, Ware EB, Jhun MA, Bielak LF, Zhao W, Smith JA, Peyser PA, Kardia SL, Satten GA. A statistical approach for rare‐variant association testing in affected sibships. Am J Hum Genet. 2015;96:543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia . Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kario K, Matsuo T, Kobayashi H, Imiya M, Matsuo M, Shimada K. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients: advanced silent cerebrovascular damage in extreme dippers. Hypertension. 1996;27:130–135. [DOI] [PubMed] [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raghunathan TE, Lepkowski JM, van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27:85–95. [Google Scholar]
- 27. Wolf D, Zschutschke L, Scheurich A, Schmitz F, Lieb K, Tüscher O, Fellgiebel A. Age‐related increases in stroop interference: delineation of general slowing based on behavioral and white matter analyses. Hum Brain Mapp. 2014;35:2448–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tombaugh TN, McIntyre NJ. The Mini‐Mental State Examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. [DOI] [PubMed] [Google Scholar]
- 29. Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension. 2013;62:810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. [DOI] [PubMed] [Google Scholar]
- 31. Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, Gulati M, Kamel H, Knopman DS, Launer LJ, Saczynski JS, Seshadri S, Zeki Al Hazzouri A; American Heart Association Council on Hypertension; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council . Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension. 2016;68:e67–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tarumi T, Harris TS, Hill C, German Z, Riley J, Turner M, Womack KB, Kerwin DR, Monson NL, Stowe AM, Mathews D, Cullum CM, Zhang R. Amyloid burden and sleep blood pressure in amnestic mild cognitive impairment. Neurology. 2015;85:1922–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petrov ME, Lichstein KL. Differences in sleep between black and white adults: an update and future directions. Sleep Med. 2016;18:74–81. [DOI] [PubMed] [Google Scholar]
- 34. Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–252. [DOI] [PubMed] [Google Scholar]
- 35. Ivy JR, Oosthuyzen W, Peltz TS, Howarth AR, Hunter RW, Dhaun N, Al‐Dujaili EA, Webb DJ, Dear JW, Flatman PW, Bailey MA. Glucocorticoids induce nondipping blood pressure by activating the thiazide‐sensitive cotransporter. Hypertension. 2016;67:1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Boxtel MP, Gaillard C, Houx PJ, Buntinx F, de Leeuw PW, Jolles J. Is nondipping in 24 h ambulatory blood pressure related to cognitive dysfunction? J Hypertens. 1998;16:1425–1432. [DOI] [PubMed] [Google Scholar]
- 37. Birns J, Morris R, Jarosz J, Markus H, Kalra L. The structural and functional consequences of diurnal variations in blood pressure in treated patients with hypertensive cerebrovascular disease. J Hypertens. 2009;27:1042–1048. [DOI] [PubMed] [Google Scholar]
- 38. Mann S, Altman DG, Raftery EB, Bannister R. Circadian variation of blood pressure in autonomic failure. Circulation. 1983;68:477–483. [DOI] [PubMed] [Google Scholar]
- 39. Kario K, Schwartz JE, Pickering TG. Ambulatory physical activity as a determinant of diurnal blood pressure variation. Hypertension. 1999;34:685–691. [DOI] [PubMed] [Google Scholar]
- 40. Sunbul M, Sunbul EA, Kosker SD, Durmus E, Kivrak T, Ileri C, Oguz M, Sari I. Depression and anxiety are associated with abnormal nocturnal blood pressure fall in hypertensive patients. Clin Exp Hypertens. 2014;36:354–358. [DOI] [PubMed] [Google Scholar]
- 41. Wolters FJ, Mattace‐Raso FUS, Koudstaal PJ, Hofman A, Ikram MA; Heart Brain Connection Collaborative Research Group . Orthostatic hypotension and the long‐term risk of dementia: a population‐based study. PLoS Med. 2016;13:e1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sapolsky RM. Depression, antidepressants, and the shrinking hippocampus. Proc Natl Acad Sci U S A. 2001;98:12320–12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kalaria RN. Cerebrovascular disease and mechanisms of cognitive impairment: evidence from clinicopathological studies in humans. Stroke. 2012;43:2526–2534. [DOI] [PubMed] [Google Scholar]
- 44. Sabayan B, Wijsman LW, Foster‐Dingley JC, Stott DJ, Ford I, Buckley BM, Sattar N, Jukema JW, van Osch MJ, van der Grond J, van Buchem MA, Westendorp RG, de Craen AJ, Mooijaart SP. Association of visit‐to‐visit variability in blood pressure with cognitive function in old age: prospective cohort study. BMJ. 2013;347:f4600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Methods.
Table S1. Number and Type of Imputed Variables
Table S2. Race‐Specific Associations Between Nocturnal SBP Levels and Demographic Variables and Clinical Characteristics (n=755)
Table S3. Race‐Specific Associations Between Nocturnal SBP Levels and Demographic Variables and Clinical Characteristics (n=755)
Table S4. Race‐Specific Associations Between Nocturnal DBP Levels and Demographic Variables and Clinical Characteristics (n=755)
Table S5. Associations Between Nocturnal DBP Levels and Demographic Variables and Clinical Characteristics (n=755)
Table S6. Race‐Specific Associations Between Nocturnal SBP Dipping and Demographic Variables and Clinical Characteristics (n=755)
Table S7. Associations Between Nocturnal SBP Dipping and Demographic Variables and Clinical Characteristics (n=755)
Table S8. Associations Between Daytime SBP Levels and Each Cognitive Function (n=755)
Table S9. Associations Between Nocturnal SBP Levels and Each Cognitive Function (Without Multiple Imputation)
Table S10. Race‐Specific Associations Between Nocturnal SBP Levels and Each Cognitive Function (Without Multiple Imputation)
Table S11. Associations Between Nocturnal SBP Dipping and Each Cognitive Function (Without Multiple Imputation)
Table S12. Race‐Specific Associations Between Nocturnal SBP Dipping and Each Cognitive Function (Without Multiple Imputation)
Figure S1. Histogram of nocturnal SBP levels and dipping.


