Abstract
Background
Direct oral anticoagulants (DOACs) are noninferior to warfarin for stroke prevention in atrial fibrillation (AF). We aimed to determine the population risk of stroke and death in incident AF, stratified by anticoagulation status and type, and the temporal trends of oral anticoagulation practice in the post‐DOAC approval period.
Methods and Results
We conducted a population‐based cohort study of incident nonvalvular AF cases using administrative health data in Alberta, Canada. We used Cox proportional hazards modeling with anticoagulation status as a time‐varying exposure and adjusted for age (continuous), sex, congestive heart failure, hypertension, diabetes mellitus, prior transient ischemic attack or ischemic stroke, myocardial infarction, peripheral artery disease, and chronic kidney disease. Primary outcome was the composite of stroke and death. Among 34 965 patients with incident AF (56.0% male, median age 73 years), relative to warfarin, DOAC use was associated with decreased risk of all stroke and death (hazard ratio: 0.90; 95% confidence interval, 0.83–0.97) and decreased hemorrhagic stroke (hazard ratio: 0.60; 95% confidence interval, 0.40–0.91]) but a similar risk of ischemic stroke (hazard ratio: 1.12; 95% confidence interval, 0.94–1.34]). During this time period, DOAC use increased rapidly, surpassing warfarin, but the total oral anticoagulation use in the population remained stable, even in the subgroup with the highest thromboembolic risk.
Conclusions
In a real‐world population‐based study of patients with incident AF, anticoagulation with DOACs was associated with decreased risk of stroke and death compared with warfarin. Despite a rapid uptake of DOACs in clinical practice, the total proportion of AF patients on anticoagulation has remained stable, even in high‐risk patients.
Keywords: anticoagulant, atrial fibrillation, mortality, stroke
Subject Categories: Atrial Fibrillation, Anticoagulants, Ischemic Stroke, Intracranial Hemorrhage, Mortality/Survival
Clinical Perspective
What Is New?
In a population with access to universal health care, real‐world prescription patterns for oral anticoagulants in the post–direct oral anticoagulant approval period show that this treatment remains underused in patients with nonvalvular atrial fibrillation, even in the patients at high risk for thromboembolism.
What Are the Clinical Implications?
We confirm that direct oral anticoagulants are safer than warfarin but show that overall rates of stroke and death are unchanged despite having more choices of oral anticoagulation drugs, which highlights the need for prospective studies to understand the barriers of oral anticoagulation in atrial fibrillation.
The increased risk of stroke and death associated with atrial fibrillation (AF) can be effectively mitigated with anticoagulation.1, 2 Oral anticoagulation for nonvalvular AF has been revolutionized by the emergence of direct oral anticoagulants (DOACs) as alternatives to dose‐adjusted warfarin.3, 4, 5, 6 Meta‐analyses of randomized controlled trials7, 8 as well as observational data9, 10 confirm the efficacy and real‐life effectiveness of these agents. DOACs have the added benefit of favorable pharmacology resulting in convenience for patients, with rapid onset of action, fixed dosing, no laboratory monitoring, and fewer food and drug interactions.11 However, DOACs have higher drug costs and need adjustment based on renal function.
Population‐based analyses have reflected the effectiveness and safety of DOACs in routine clinical practice.12, 13, 14, 15, 16 A common feature of published population studies is that the exposure to the type of anticoagulant is determined at entry into the study and is assumed to be constant throughout follow‐up. In reality, anticoagulation status and type change with time. Furthermore, because dabigatran was the first DOAC to be approved, apixaban and rivaroxaban have been relatively less well studied.14, 15, 16 Finally, although some studies report declining temporal trends of AF‐related stroke and mortality in the population (1958–200717 and 1980–200018), more contemporary studies (2000–2010) show no further decline in the AF‐related stroke trends.19 Temporal trends of oral anticoagulation prescription pattern and AF‐related ischemic stroke, hemorrhagic stroke, and mortality in the post‐DOAC approval period are less well understood.
Using the complete population of Alberta, Canada, from 2009 to 2015, we aimed to determine the risk of stroke and death in incident AF, stratified by anticoagulation status and type, defined as a time‐varying exposure variable. An important secondary objective was to study the temporal trends in oral anticoagulation practice and outcomes during this post‐DOAC approval time period. We hypothesized that DOACs are associated with decreased risk of stroke and death compared with warfarin and that the temporal trends in the occurrence of these outcomes may decrease in response to an increase in DOAC use.
Methods
Using Alberta linked administrative data, we performed a population‐based cohort study of incident nonvalvular AF diagnosed between January 1, 2009 and June 30, 2015, and followed through December 31, 2015, allowing a minimum follow‐up of 6 months for each patient. All residents of Alberta (population of 4.2 million people) have access to publicly funded and universal health care. The Alberta Health Care Insurance Plan (AHCIP) provides medical coverage to most Alberta residents (>99%) with the rare exceptions of the members of the military, federal inmates, individuals who opt out of the AHCIP, and the Royal Canadian Mounted Police. Each resident covered by the plan is assigned a personal health number that acts as a unique lifetime identifier. There is no universal drug coverage in Alberta, and residents pay for drugs out of pocket, through private insurance (usually through employment), or through publicly funded drug programs for seniors (people aged ≥65 years) and a few selected groups administered through Alberta Blue Cross. Under the Alberta Blue Cross public program, DOACs are covered under specific circumstances: recurrent thromboembolism on warfarin, labile international normalized ratio, or difficult access to international normalized ratio test centers.
AF Cohort Identification
AF was identified using administrative data with the International Classification of Diseases (ICD) codes 427.3x (ICD‐9‐CM) or I48.x (ICD‐10‐CA) in any diagnosis field in any of the hospital inpatient, ambulatory, or emergency department encounters or physician claims databases.20, 21 Two diagnoses of AF were required at separate healthcare encounters >30 days apart within the first year of diagnosis to minimize misclassification of transient single episodes of AF or flutter. AF was defined as incident if no prior diagnosis of AF was made in Alberta from the date that the patient obtained an AHCIP number or April 1, 1994. We excluded valvular heart disease, defined as any of the following codes in any of the databases preceding the incidence date: mitral or aortic disease (ICD‐9 394‐396, 424.0, 424.1 or ICD‐10 I05, I06, I34, I35, I08.0, I08.1, I08.2, I08.3) or valve surgery (ICD‐9 35.0x, 35.2x, 35.96, 35.97, 35.99 and ICD‐10 Canadian Classification of Health Interventions (CCI) code 1.HT.89, 1.HV.80, 1.HU.80, 1.HT.80, 1.HS.80, 1.HV.90, 1.HU.90, 1.HT.90, 1.HS.90). Patients entered the study when the above case definition was met and no person‐time was contributed before AF was diagnosed.
Anticoagulation Status
Because a patient may change anticoagulation regimens during the follow‐up period, anticoagulation was considered a time‐varying exposure. If, for example, a patient was followed for 1 year and was on warfarin for 6 months and a DOAC for 6 months, the patient contributed 0.5 person‐year to each of the warfarin and DOAC‐exposed groups. Interruption in treatment was defined as a gap in prescription refills of ≥30 days between the date of the last refill plus the number of days of drugs dispensed and the date of the next refill. Treatment type was determined by the Pharmaceutical Information Network, which contains all drugs dispensed by 98% of the community pharmacies in Alberta regardless of insurance status. We considered warfarin, apixaban, rivaroxaban, and dabigatran (Anatomical Therapeutic Chemical codes B01AA, B01AF02, B01AF01, and B01AE07).
Statistical Analyses
The primary outcome was the composite of all stroke (ischemic and hemorrhagic) and all‐cause mortality. Secondary outcomes were the individual components of the composite outcomes, myocardial infarction, and hemorrhagic complications (gastrointestinal and subdural). We calculated the age–sex adjusted event rates per 1000 person‐years with 95% confidence intervals (CIs). We used Cox proportional hazards modeling and the mean of covariates and corrected group prognosis method to calculate risk‐adjusted event rates for patients on no anticoagulation, warfarin, and a DOAC.22 We adjusted for the elements of the CHADS2‐VASc score: age (continuous), sex, congestive heart failure, hypertension, diabetes mellitus, prior transient ischemic attack or ischemic stroke, prior myocardial infarction, peripheral artery disease, and chronic kidney disease, which could be a relative contraindication to treatment with DOAC. The proportionality assumption cannot be interpreted because we examined our exposure (anticoagulation status) as a time‐varying exposure covariate. We graphically examined the age–sex standardized rate ratios at different survival times (0–200 days, 201–400 days, etc.) to confirm that the actual hazard did not vary significantly over time or show any converging or diverging trends.
We performed a sensitivity analysis to additionally adjust for coverage by Alberta Blue Cross public drug insurance because it is possible that DOAC users with and without public insurance have different sociodemographic characteristics, such as age, employment, or socioeconomic status. The sensitivity analysis compares only warfarin versus DOAC and excludes the category of “never anticoagulated” because the type of reimbursement for a prescription can be determined only when a prescription is filled.
Outcomes and comorbidities were determined using administrative data codes (Table 1).21, 23, 24, 25 Hypertension and diabetes mellitus were defined using 1 hospitalization discharge code in any position or 2 outpatient claims in ≤2 years. Congestive heart failure, peripheral artery disease, myocardial infarction, stroke, and transient ischemic attack were defined using 1 hospitalization discharge code in any position.24 Chronic kidney disease was defined using hospitalization discharge codes in any position or dialysis codes (V45.1, V56, 39.95, 54.98, Z99.2, Z49) in 1 hospitalization or 1 outpatient claim.25 Dialysis‐related hospitalizations were not counted as kidney failure if a concurrent acute kidney injury code (584) was present. We graphed the temporal trends of oral anticoagulation prescriptions for all patients and stratified by high‐risk (CHADS2 ≥2 or age ≥75 years), moderate‐risk (CHADS2=1 or age 65–74 years), and low‐risk (CHADS2=0 or age <65 years) groups. Risk was determined at entry into the study. We also graphed the temporal trends in the rates of ischemic stroke, hemorrhagic stroke, and death per person‐years. The temporal trends for drug prescription and stroke and death outcomes are adjusted for age and sex only.
Table 1.
Comorbidities and Outcomes Case Definitions Using ICD‐9 and ICD‐10
| Comorbidities | ICD‐9 | ICD‐10 |
|---|---|---|
| Congestive heart failure | 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 425.4 to 425.9, 428.x | I09.9, I11.0, I13.0, I13.2, I25.5, I42.0, I42.5 to I42.9, I43.x, I50.x, P29.0 |
| Hypertension | 401 to 405 | I10, I11–I13, I15 |
| Diabetes mellitus | 250 | E10.0, E10.1, E10.6, E10.8, E10.9, E11.0, E11.1, E11.6, E11.8, E11.9, E12.0, E12.1, E12.6, E12.8, E12.9, E13.0, E13.1, E13.6, E13.8, E13.9, E14.0, E14.1, E14.6, E14.8, E14.9 E10.2–E10.5, E10.7, E11.2–E11.5, E11.7, E12.2–E12.5, E12.7, E13.2 to E13.5, E13.7, E14.2–E14.5, E14.7 |
| Myocardial infarction | 410.x, 412.x | I21.x, I22.x, I25.2 |
| Peripheral vascular disease | 093.0, 437.3, 440.x, 441.x, 443.1 to 443.9, 47.1, 557.1, 557.9, V43.4 | I70.x, I71.x, I73.1, I73.8, I73.9, I77.1, I79.0, I79.2, K55.1, K55.8, K55.9, Z95.8, Z95.9 |
| Ischemic stroke or transient ischemic attack | 362.3, 433.x1, 434.x1, 436, 435.x | H34.1, H34.2, I63.x, I64.x, G45.x (except G45.4) |
| Chronic kidney disease | V45.1, V56, 39.95, 54.98, V42.0, 55.6, 996.81, 585.6, 586, 403.01, 403.91 | Z99.2, Z49, Z45.2, Z94.0, N18.5, N18.6, N19, I12.0 |
| Outcomes | ICD‐9 | ICD‐10 |
| Ischemic stroke | N/A | H34.1, H34.2, I63, I64 |
| Hemorrhagic stroke | N/A | I60, I61 |
| Subdural hemorrhage | N/A | I62, S06.5a |
| Gastrointestinal hemorrhage | N/A | K25, K26, K27, K28, K29, K92.0 |
ICD‐9 indicates International Classification of Diseases, Ninth Revision; ICD‐10 indicates International Classification of Diseases, Tenth Revision; N/A, not applicable.
S06.5 in any diagnostic position because it is an injury code.
All analyses were conducted using SAS 9.4 (SAS Institute Inc), and graphs were created using Excel 2013 (Microsoft Office). This study received approval from the University of Calgary institutional review board for research, and a waiver of consent was granted (REB16−1859).
Results
Among 34 965 patients with new diagnosis of nonvalvular AF, there were 19 579 (56.0%) male patients, the median age was 73.0 (interquartile range: 62.1–81.9), and 9628 (27.5%) patients were never anticoagulated during follow‐up. Table 2 shows the baseline characteristics by anticoagulation status and type. At study censor date (occurrence of a primary outcome or end of study), 16 077 (46.0%) patients were not anticoagulated, 9292 (26.6%) patients were on warfarin, 3156 (9.0%) were on dabigatran, 1786 (5.1%) were on apixaban, and 4654 (13.3%) were on rivaroxaban. Among the 25 337 patients who received anticoagulation, 6844 (27.0%) patients made at least 1 switch from warfarin to a DOAC during the study, and 569 (2.2%) patients switched from a DOAC to warfarin. The person‐year contributions at study censor date are presented in Table 3.
Table 2.
Baseline Characteristics by Anticoagulation Status and Type
| Ever Anticoagulated | Never Anticoagulated | All | |||
|---|---|---|---|---|---|
| Ever on DOAC (n=12 581) | Ever on Warfarin (n=19 267) | Warfarin and DOAC (n=6511)a | n=9628 | N=34 965 | |
| Age, y, median (IQR) | 71.9 (63.2–80.1) | 75.1 (65.5–82.3) | 73.6 (65.2–80.7) | 69.1 (55.4–82.7) | 73.0 (62.1–81.9) |
| Male, n (%) | 7273 (57.8) | 10 696 (55.5) | 3608 (55.4) | 5212 (54.1) | 19 573 (56.0) |
| CHF, n (%) | 542 (4.3) | 1189 (6.2) | 333 (5.1) | 390 (4.1) | 1788 (5.1) |
| Hypertension | 6832 (54.3) | 10 227 (53.1) | 3569 (54.8) | 4216 (43.8) | 17 706 (50.6) |
| Diabetes mellitus | 3321 (26.4) | 5551 (28.8) | 1842 (28.3) | 1965 (20.4) | 8995 (25.7) |
| Ischemic stroke or TIA, n (%) | 1405 (11.2) | 2441 (12.7) | 792 (12.2) | 884 (9.2) | 3938 (11.3) |
| PAD, n (%) | 1272 (10.1) | 2697 (14.0) | 748 (11.5) | 1018 (10.6) | 4239 (12.1) |
| AMI, n (%) | 1083 (8.6) | 2228 (11.6) | 629 (9.7) | 830 (8.6) | 3512 (10.0) |
| CKD, n (%) | 320 (2.5) | 1018 (5.3) | 212 (3.3) | 474 (4.9) | 1600 (4.6) |
| CHADS2, n (%) | |||||
| 0 | 2528 (20.1) | 3187 (16.5) | 1092 (16.8) | 3250 (33.8) | 7873 (22.5) |
| 1 | 4413 (35.1) | 6266 (32.5) | 2254 (34.6) | 2803 (29.1) | 11 228 (32.1) |
| 2 | 3573 (28.4) | 5987 (31.1) | 1988 (30.5) | 2122 (22.0) | 9694 (27.7) |
| 3 | 1319 (10.5) | 2347 (12.2) | 780 (12.0) | 867 (9.0) | 3783 (10.8) |
| 4 | 594 (4.7) | 1159 (6.0) | 343 (5.3) | 438 (4.5) | 1848 (5.3) |
| 5 | 145 (1.2) | 302 (1.6) | 79 (1.2) | 140 (1.5) | 508 (1.5) |
| 6 | 9 (0.1) | 19 (0.1) | 5 (0.1) | 8 (0.1) | 31 (0.1) |
| Median CHADS2 (IQR) | 1.9 (1.1–2.7) | 2.0 (1.3–2.8) | 2.0 (1.2–2.8) | 1.6 (0.7–2.6) | 1.9 (1.1–2.7) |
| Median CHADS2‐VASc (IQR) | 3.2 (2.1–4.3) | 3.5 (2.4–4.6) | 3.4 (2.3–4.4) | 2.8 (1.4–4.2) | 3.3 (2.0–4.4) |
| Ever on Alberta Blue Cross public insurance, n (%) | 8041 (63.9) | 14 624 (75.9) | 5343 (82.1) | Not applicable | 17 322 (49.5) |
AMI indicates acute myocardial infarction; CHADS2, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, history of cerebral ischemia; CHA2DS2‐VASc: 1 point for each congestive heart failure, hypertension, age 65–74 years, diabetes mellitus, vascular disease (myocardial infarction or peripheral artery disease), female sex, and 2 points for each history of cerebral ischemia and age ≥75 years; CHF, congestive heart failure; CKD, chronic kidney disease; DOAC, direct oral anticoagulant; IQR, interquartile range; PAD, peripheral artery disease; TIA: transient ischemic attack.
Filled prescription for warfarin and DOAC during follow‐up, not on both therapies simultaneously. Categories are not mutually exclusive because a patient could have been exposed to different anticoagulation status and types during the follow‐up time.
Table 3.
Person‐Year Contribution at Study Censor Date (Occurrence of a Primary Outcome or End of Study)
| Treatment type | Person‐Years |
|---|---|
| No anticoagulant | 42 751 |
| Warfarin | 37 812 |
| Direct oral anticoagulant | 20 321 |
The age–sex adjusted event rates with 95% CIs are presented in Table 4, as well as the multivariable hazard ratios (HRs) and 95% CIs. Considering anticoagulation as a time‐varying exposure variable, patients on DOACs were less likely to suffer the composite outcome of all stroke and death compared with warfarin (HR: 0.90; 95% CI, 0.83–0.97). Patients treated with oral anticoagulation were less likely to suffer an ischemic stroke compared with those without anticoagulation, but there was no additional reduction in ischemic stroke risk associated with DOACs compared with warfarin (HR: 1.12; 95% CI, 0.94–1.34). DOACs were associated with less hemorrhagic stroke compared with warfarin (HR: 0.60; 95% CI, 0.40–0.91). Myocardial infarction occurrence was similar in all groups except for a slight decrease in the warfarin group compared with no anticoagulation, but the CI approached the null. For the safety outcomes, warfarin, but not DOACs, was associated with increased subdural hemorrhage (HR: 1.70; 95% CI, 1.27–2.29). For gastrointestinal hemorrhages, we only present age–sex standardized event rates because the event rate ratios changed with time, and the absolute number of events was too small to present HR estimates stratified by time. The sensitivity analysis with Alberta Blue Cross public insurance flag in the multivariable analysis did not change the direction of the effects (Table 5).
Table 4.
Adjusted Event Rates and HRs (95% CIs)
| Outcome | Event Rate (95% CI)a | HR (95% CI)b | ||||
|---|---|---|---|---|---|---|
| Warfarin | DOAC | No A/C | Warfarin Over No A/C | DOAC Over No A/C | DOAC Over Warfarin | |
| Stroke, deaths, myocardial infarction | ||||||
| All stroke and death | 65.3 (62.8–67.9) | 50.4 (47.3–62.8) | 146.0 (142.0–150.1) | 0.42 (0.40–0.44)c | 0.38 (0.35–0.40)c | 0.90 (0.83–0.97)c |
| Ischemic stroke | 9.9 (9.0–11.0) | 9.8 (8.5–11.3) | 13.7 (12.5–15.0) | 0.68 (0.60–0.78)c | 0.77 (0.65–0.91)c | 1.12 (0.94–1.34) |
| Hemorrhagic stroke | 2.6 (2.1–3.2) | 1.5 (1.0–2.2) | 1.1 (0.8–1.5) | 2.35 (1.63–3.38)c | 1.41 (0.88–2.26) | 0.60 (0.40–0.91)c |
| Death | 56.6 (54.4–59.0) | 42.0 (39.2–44.9) | 138.0 (134.1–141.9) | 0.39 (0.37–0.41)c | 0.33 (0.31–0.36)c | 0.86 (0.79–0.93)c |
| Myocardial infarction | 8.0 (6.8–9.3) | 7.9 (6.8–9.3) | 8.8 (7.9–9.9) | 0.85 (0.73–0.99)c | 1.01 (0.83–1.22) | 1.19 (0.97–1.44) |
| Hemorrhagic complications | ||||||
| GI hemorrhage | 8.0 (7.2–9.0) | 6.9 (5.8–8.1) | 8.8 (7.8–9.8) | N/A | N/A | N/A |
| Subdural hemorrhage | 3.2 [2.6, 3.8] | 1.8 [1.3, 2.5] | 2.0 [1.5, 2.5] | 1.70 [1.27, 2.29]c | 1.01 [0.68, 1.51] | 0.60 [0.41, 0.86]c |
A/C indicates anticoagulant; CI, confidence interval; DOAC, direct oral anticoagulant; GI, gastrointestinal; HR, hazard ratio; N/A, not applicable.
Event rates (1000 person‐years) are adjusted for age and sex.
HRs (95% CIs) are adjusted for adjusted for age (continuous), sex, congestive heart failure, hypertension, diabetes mellitus, prior transient ischemic attack or ischemic stroke, prior acute myocardial infarction, peripheral artery disease, and chronic kidney disease.
Statistically significant (p<0.05)
Table 5.
Sensitivity Analysis With Adjustment for Alberta Blue Cross Public Insurance Flag in Multivariable Analysis
| Outcome | Event rate (95% CI)a | HR (95% CI)b | |
|---|---|---|---|
| Warfarin | DOAC | DOAC Over Warfarin | |
| Stroke, deaths, myocardial infarction | |||
| All stroke and death | 65.3 (62.8–67.9) | 50.4 (47.3–53.6) | 0.84 (0.77–0.90)c |
| Acute ischemic stroke | 10.7 (9.6–11.8) | 10.2 (8.8–11.9) | 1.05 (0.87–1.26) |
| Hemorrhagic stroke | 2.8 (2.3–3.5) | 1.7 (1.1–2.4) | 0.56 (0.37–0.86)c |
| Death | 61.2 (58.8–63.8) | 47.4 (44.2–50.9) | 0.80 (0.74–0.88)c |
| Myocardial infarction | 8.6 (7.7–9.7) | 7.9 (6.7–9.2) | 1.0 (0.85–1.29) |
| Hemorrhagic complications | |||
| GI hemorrhage | 8.5 (7.6–9.6) | 7.2 (6.1–8.7) | N/A |
| Subdural hemorrhage | 3.5 (3.0–4.2) | 1.8 (1.3–2.6) | 0.53 (0.36–0.78)c |
CI indicates confidence interval; DOAC, direct oral anticoagulant; GI, gastrointestinal; HR, hazard ratio; N/A, not applicable.
Event rates (1000 person‐years) are adjusted for age, sex, and Alberta Blue Cross flag.
HRs (95% CIs) are adjusted for adjusted for age (continuous), sex, congestive heart failure, hypertension, diabetes mellitus, prior transient ischemic attack or ischemic stroke, prior acute myocardial infarction, peripheral artery disease, chronic kidney disease, and Alberta Blue Cross flag.
Statistically significant (p<0.05)
Figure 1 shows the temporal trends in occurrence rates of ischemic stroke, hemorrhagic stroke, and death as well as the prescription patterns of oral anticoagulation for the full cohort. Temporal trends for the outcomes of interest were stable. During the study follow‐up period, the use of DOACs increased rapidly, whereas the use of warfarin declined so that the total proportion of patients on oral anticoagulation remained stable. When stratified by risk, the use of DOACs increased most steeply in the high‐ and moderate‐risk groups (Figure 2). Prescriptions for DOACs have not yet surpassed that for warfarin in the high‐risk group. Temporal trends in prescription patterns remained stable for all oral anticoagulation types, regardless of risk group (Figure 3).
Figure 1.
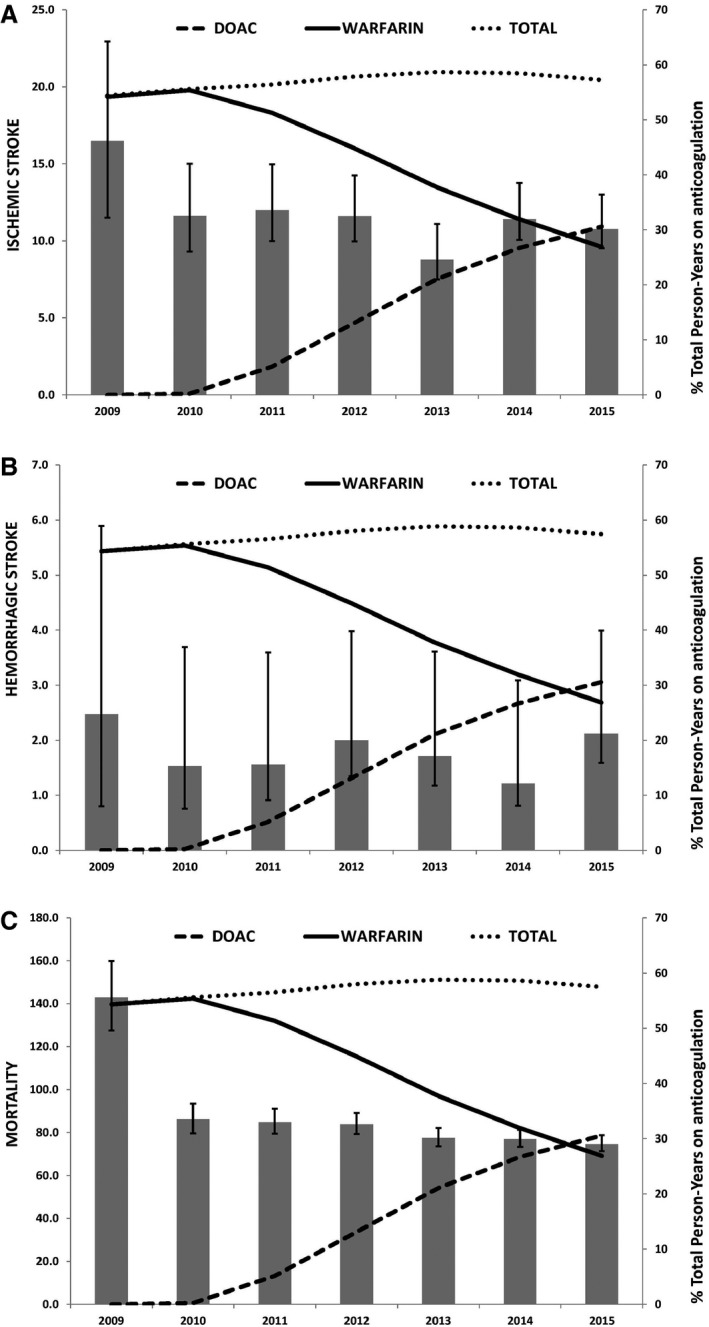
Temporal trends of oral anticoagulation prescription and occurrence of ischemic stroke (A), hemorrhagic stroke (B), and death (C). Age–sex adjusted rates per 1000 person‐years. In 2009, the first year of the study, the occurrence of outcomes was high and likely artificially inflated because only patients with incident atrial fibrillation (AF) were included as opposed to the following years in which a combination of incident and prevalent AF patients were followed. Incident AF is often diagnosed in the context of a stroke or other medical condition, leading to higher apparent risk of stroke or death in the immediate period after diagnosis. DOAC indicates direct oral anticoagulant.
Figure 2.
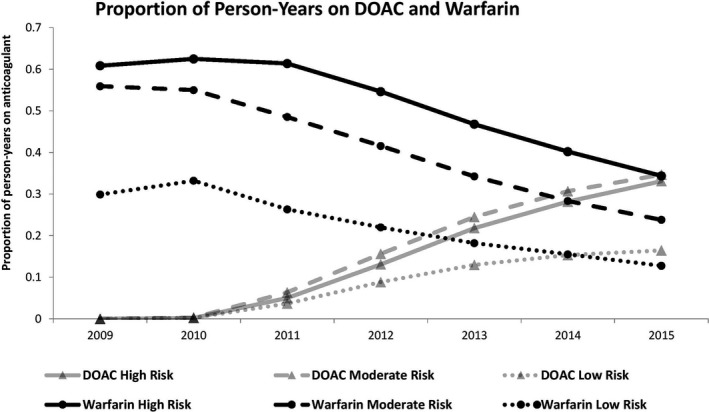
Temporal trends of DOAC and warfarin prescriptions stratified by high risk (CHADS 2 ≥2 or age ≥75 years), moderate risk (CHADS 2=1 or age 65–74 years), or low risk (CHADS 2=0 or age <65 years). CHADS 2 indicates congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack; DOAC, direct oral anticoagulant.
Figure 3.
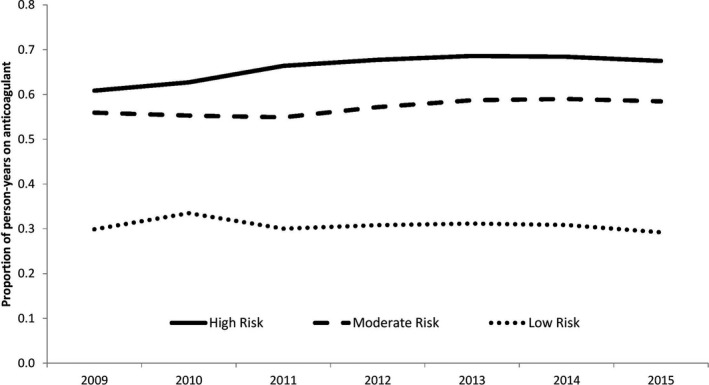
Temporal trends of all oral anticoagulation prescriptions stratified by high risk (CHADS 2 ≥2 or age ≥75 years), moderate risk (CHADS 2=1 or age 65–74 years), or low risk (CHADS 2=0 or age <65 years). CHADS 2 indicates congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack; DOAC, direct oral anticoagulant.
Discussions
This study of ≈35 000 nonvalvular AF patients from a complete population shows that treatment with DOACs is associated with reduced risk for a combined end point of all‐cause stroke or death compared with warfarin, even after adjustment for baseline differences. Consistent with the pivotal clinical trials comparing warfarin and DOACs,3, 4, 5, 6 meta‐analysis of clinical trials data,8 and other population‐based analyses,12, 14, 15 the protective effect of DOACs in our study is driven by lower rates of hemorrhagic stroke and death. DOAC treatment is not associated with increased risk of myocardial infarction. Only warfarin, and not DOACs, is associated with increased subdural hemorrhage. We confirm that treatment with an oral anticoagulant is associated with less ischemic stroke compared with no anticoagulation. In real‐world clinical practice, our findings suggest DOACs are simply safer than warfarin.
Although we show a reduction in risk of death among DOAC users, our data do not fully explain the reasons for the observed decrease in mortality. The ischemic stroke and myocardial infarction risks are similar between warfarin and DOACs. Although there were fewer hemorrhagic strokes in the DOAC group, the absolute number of events was low (n=106, n=31, and n=42 in the warfarin, DOAC, and no anticoagulation groups, respectively). Neither the reduction in hemorrhagic stroke nor the reduction in ischemic stroke fully accounts for the reduction in mortality. Although residual confounding may be a partial explanation, additional contributors to a reduction in mortality could be a reduction in the severity of stroke. If stroke severity per event is reduced in the DOAC group, then the risk of death will fall. This is an important hypothesis to test in future studies.
The real‐world prescription patterns for oral anticoagulants show that since the approval of DOACs in Canada in October 2010, DOACs have been fully adopted into clinical practice. Earlier studies found a moderate uptake of DOACs in the United States26 and Canada,27 but we demonstrated that in 2015, DOAC use surpassed that of warfarin. Importantly, these trends highlight a greater challenge: The total proportion of AF patients on anticoagulation has remained relatively stable, even in the high‐risk category that includes patients aged ≥75 years or with a CHADS2 score ≥2. Not surprisingly, the incidence rates of ischemic stroke, hemorrhagic stroke, and mortality did not significantly change throughout the study period. Although rates of anticoagulation were shown to be rising 15 to 20 years ago with an associated decrease in ischemic stroke,28, 29 our results are consistent with recent studies showing a plateau in anticoagulation rates and stroke incidence.19 Two recent US studies found a slight increase in anticoagulation rates since the introduction of DOACs.30, 31 These studies, however, used data from a US national registry of cardiovascular care practices, which may favor enrollment of highly motivated patients under specialist care, and the generalizability of these results to the population may be limited. Our findings suggest that the increasing use of DOACs is not yet closing the gap between scientific evidence and clinical practice in the general population. Stroke prevention remains suboptimal because anticoagulation is routinely underused.32, 33 It is important to explore and address patient preference and physician perception of the risk–benefit balance, particularly because evidence from this and other studies confirms the greater safety of DOACs.34, 35 Intervention trials based on education, measurement, and feedback and electronic alert systems aiming to improve anticoagulation rates are relevant and currently under way.36, 37
Our study has several strengths, including the analysis of a complete population and a long duration of follow‐up. In addition, we studied all DOACs currently available in Canada (dabigatran, rivaroxaban, apixaban), and we treated anticoagulation exposure as a time‐varying variable to reflect real‐world treatment patterns. Nevertheless, our study has limitations. Given the relatively small number of DOACs in each category, we could neither study the effects of the individual DOACs nor differing doses. Acetylsalicylic acid is available over the counter and could not be reliably assessed. Although we carefully considered baseline characteristics for risk adjustment, including drug insurance status, unmeasured patient, clinician, and health‐system factors associated with the selection of an oral anticoagulation regimen may result in residual confounding. The temporal trends for stroke and death outcomes also need to be interpreted with more caution because they are adjusted only for age and sex. Our study is vulnerable to limitations inherent to the use of administrative data. Because the Pharmaceutical Information Network contains data only on dispensed drugs, we could not assess for primary nonadherence. We could not link with laboratory information (including international normalized ratio, such that we could not estimate the time in the therapeutic range) and could not adjudicate outcome events. However, we used validated case definitions to identify comorbid disease and outcomes, and the use of administrative data allowed for the study of a complete population over a long period of time.
Conclusions
The results of this contemporary comparative effectiveness study on DOACs, warfarin, and no anticoagulation are expected to aid physicians in choosing the most effective and safe oral anticoagulant in routine clinical practice. Because medication reimbursement for DOACs is still lacking in Canada, the results of our study may be used as support to improve the accessibility to DOACs. Overall rates of anticoagulation, stroke, and death are unchanged despite having more choices of oral anticoagulation. Prospective studies evaluating and intervening on the barriers of oral anticoagulation in AF continue to be needed.
Disclosures
Hill received research support from Medtronic LLC, Bayer Canada, and Boehringer Ingelheim. Wilton consulted for Arca Biopharma. The other authors have no disclosures to declare.
(J Am Heart Assoc. 2017;6:e007129 DOI: 10.1161/JAHA.117.007129.)29080863
References
- 1. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 2. Yu AYX, Malo S, Wilton S, Parkash R, Svenson LW, Hill MD. Anticoagulation and population risk of stroke and death in incident atrial fibrillation: a population‐based cohort study. CMAJ Open. 2016;4:E1–E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; Re‐LY Steering Committee and I . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 4. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; Investigators EA‐T . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 5. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; Committees A Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 6. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; Investigators RA . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 7. Lip GY, Mitchell SA, Liu X, Liu LZ, Phatak H, Kachroo S, Batson S. Relative efficacy and safety of non‐vitamin k oral anticoagulants for non‐valvular atrial fibrillation: network meta‐analysis comparing apixaban, dabigatran, rivaroxaban and edoxaban in three patient subgroups. Int J Cardiol. 2016;204:88–94. [DOI] [PubMed] [Google Scholar]
- 8. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 9. Camm AJ, Amarenco P, Haas S, Hess S, Kirchhof P, Kuhls S, van Eickels M, Turpie AG. XANTUS: a real‐world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J. 2016;37:1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steinberg BA, Holmes DN, Piccini JP, Ansell J, Chang P, Fonarow GC, Gersh B, Mahaffey KW, Kowey PR, Ezekowitz MD, Singer DE, Thomas L, Peterson ED, Hylek EM. Early adoption of dabigatran and its dosing in us patients with atrial fibrillation: results from the outcomes registry for better informed treatment of atrial fibrillation. J Am Heart Assoc. 2013;2:e000535 DOI: 10.1161/JAHA.113.000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mekaj YH, Mekaj AY, Duci SB, Miftari EI. New oral anticoagulants: their advantages and disadvantages compared with vitamin k antagonists in the prevention and treatment of patients with thromboembolic events. Ther Clin Risk Manag. 2015;11:967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Larsen TB, Skjoth F, Nielsen PB, Kjaeldgaard JN, Lip GY. Comparative effectiveness and safety of non‐vitamin k antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yao X, Abraham NS, Sangaralingham LR, Bellolio MF, McBane RD, Shah ND, Noseworthy PA. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc. 2016;5:e003725 DOI: 10.1161/JAHA.116.003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Avgil Tsadok M, Jackevicius CA, Rahme E, Humphries KH, Pilote L. Sex differences in dabigatran use, safety, and effectiveness in a population‐based cohort of patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2015;8:593–599. [DOI] [PubMed] [Google Scholar]
- 15. Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, Sheu TC, Mott K, Goulding MR, Houstoun M, MaCurdy TE, Worrall C, Kelman JA. Cardiovascular, bleeding, and mortality risks in elderly medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–164. [DOI] [PubMed] [Google Scholar]
- 16. Sorensen R, Gislason G, Torp‐Pedersen C, Olesen JB, Fosbol EL, Hvidtfeldt MW, Karasoy D, Lamberts M, Charlot M, Kober L, Weeke P, Lip GY, Hansen ML. Dabigatran use in Danish atrial fibrillation patients in 2011: a nationwide study. BMJ Open. 2013;3:e002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton‐Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Seward JB, Bailey KR, Iwasaka T, Tsang TS. Time trends of ischemic stroke incidence and mortality in patients diagnosed with first atrial fibrillation in 1980 to 2000: report of a community‐based study. Stroke. 2005;36:2362–2366. [DOI] [PubMed] [Google Scholar]
- 19. Chamberlain AM, Brown RD Jr, Alonso A, Gersh BJ, Killian JM, Weston SA, Roger VL. No decline in the risk of stroke following incident atrial fibrillation since 2000 in the community: a concerning trend. J Am Heart Assoc. 2016;5:e003408 DOI: 10.1161/JAHA.116.003408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36:1776–1781. [DOI] [PubMed] [Google Scholar]
- 22. Ghali WA, Quan H, Brant R, van Melle G, Norris CM, Faris PD, Galbraith PD, Knudtson ML; Investigators A . Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA. 2001;286:1494–1497. [DOI] [PubMed] [Google Scholar]
- 23. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 24. Tonelli M, Wiebe N, Fortin M, Guthrie B, Hemmelgarn BR, James MT, Klarenbach SW, Lewanczuk R, Manns BJ, Ronksley P, Sargious P, Straus S, Quan H; Alberta Kidney Disease N . Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Mak. 2015;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rebholz CM, Coresh J, Ballew SH, McMahon B, Whelton SP, Selvin E, Grams ME. Kidney failure and ESRD in the atherosclerosis risk in communities (ARIC) study: comparing ascertainment of treated and untreated kidney failure in a cohort study. Am J Kidney Dis. 2015;66:231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patel PA, Zhao X, Fonarow GC, Lytle BL, Smith EE, Xian Y, Bhatt DL, Peterson ED, Schwamm LH, Hernandez AF. Novel oral anticoagulant use among patients with atrial fibrillation hospitalized with ischemic stroke or transient ischemic attack. Circ Cardiovasc Qual Outcomes. 2015;8:383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu Y, Holbrook AM, Simpson CS, Dowlatshahi D, Johnson AP. Prescribing patterns of novel oral anticoagulants following regulatory approval for atrial fibrillation in Ontario, Canada: a population‐based descriptive analysis. CMAJ Open. 2013;1:E115–E119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shroff GR, Solid CA, Herzog CA. Temporal trends in ischemic stroke and anticoagulation therapy among medicare patients with atrial fibrillation: a 15‐year perspective (1992–2007). JAMA Intern Med. 2013;173:159–160. [DOI] [PubMed] [Google Scholar]
- 29. Pilote L, Eisenberg MJ, Essebag V, Tu JV, Humphries KH, Leung Yinko SS, Behlouli H, Guo H, Jackevicius CA. Temporal trends in medication use and outcomes in atrial fibrillation. Can J Cardiol. 2013;29:1241–1248. [DOI] [PubMed] [Google Scholar]
- 30. Katz DF, Maddox TM, Turakhia M, Gehi A, O'Brien EC, Lubitz SA, Turchin A, Doros G, Lei L, Varosy P, Marzec L, Hsu JC. Contemporary trends in oral anticoagulant prescription in atrial fibrillation patients at low to moderate risk of stroke after guideline‐recommended change in use of the CHADS2 to the CHA2DS2‐VASc score for thromboembolic risk assessment: analysis from the national cardiovascular data registry's outpatient practice innovation and clinical excellence atrial fibrillation registry. Circ Cardiovasc Qual Outcomes. 2017;10:e003476. [DOI] [PubMed] [Google Scholar]
- 31. Marzec LN, Wang J, Shah ND, Chan PS, Ting HH, Gosch KL, Hsu JC, Maddox TM. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. 2017;69:2475–2484. [DOI] [PubMed] [Google Scholar]
- 32. Gladstone DJ, Bui E, Fang J, Laupacis A, Lindsay MP, Tu JV, Silver FL, Kapral MK. Potentially preventable strokes in high‐risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke. 2009;40:235–240. [DOI] [PubMed] [Google Scholar]
- 33. Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638–645.e4. [DOI] [PubMed] [Google Scholar]
- 34. Gattellari M, Worthington J, Zwar N, Middleton S. Barriers to the use of anticoagulation for nonvalvular atrial fibrillation: a representative survey of Australian family physicians. Stroke. 2008;39:227–230. [DOI] [PubMed] [Google Scholar]
- 35. MacLean S, Mulla S, Akl EA, Jankowski M, Vandvik PO, Ebrahim S, McLeod S, Bhatnagar N, Guyatt GH. Patient and preferences in decision making for antithrombotic therapy: a systematic review: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e1S–e23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Silbernagel G, Spirk D, Hager A, Baumgartner I, Kucher N. Electronic alert system for improving stroke prevention among hospitalized oral‐anticoagulation‐naive patients with atrial fibrillation: a randomized trial. J Am Heart Assoc. 2016;5:e003776 DOI: 10.1161/JAHA.116.003776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rao MP, Ciobanu AO, Lopes RD, Fox KA, Xian Y, Pokorney SD, Al‐Khalidi HR, Jiang J, Kamath DY, Berwanger O, Xavier D, Bahit CM, Tajer C, Vinereanu D, Huo Y, Granger CB. A clustered randomized trial to IMProve treatment with AntiCoagulanTS in patients with Atrial Fibrillation (IMPACT‐AF): design and rationale. Am Heart J. 2016;176:107–113. [DOI] [PubMed] [Google Scholar]


