Abstract
Background
Linear accelerator–based stereotactic radiosurgery delivered to cardiac arrhythmogenic foci could be a promising catheter‐free ablation modality. We tested the feasibility of in vivo atrioventricular (AV) node ablation in swine using stereotactic radiosurgery.
Methods and Results
Five Large White breed swine (weight 40–75 kg; 4 females) were studied. Single‐chamber St Jude pacemakers were implanted in each pig. The pigs were placed under general anesthesia, and coronary/cardiac computed tomography simulation scans were performed to localize the AV node. Cone beam computed tomography was used for target positioning. Stereotactic radiosurgery doses ranging from 35 to 40 Gy were delivered by a linear accelerator to the AV node, and the pigs were followed up with weekly pacemaker interrogations to observe for potential electrocardiographic changes. Once changes were observed, the pigs were euthanized, and pathology specimens of various tissues, including the AV node and tissues surrounding the AV node, were taken to study the effects of radiation. All 5 pigs had disturbances of AV conduction with progressive transition into complete heart block. Macroscopic inspection did not reveal damage to the myocardium, and pigs had preserved systolic function on echocardiography. Immunostaining revealed fibrosis in the target region of the AV node, whereas no fibrosis was detected in the nontargeted regions.
Conclusions
Catheter‐free radioablation using linear accelerator–based stereotactic radiosurgery is feasible in an intact swine model.
Keywords: arrhythmia, atrioventricular node, atrioventricular node ablation, noninvasive ablation, stereotactic radiosurgery
Subject Categories: Arrhythmias, Electrophysiology, Animal Models of Human Disease, Translational Studies, Computerized Tomography (CT)
Clinical Perspective
What Is New?
Real‐time computed tomography–guided target volume stereotactic noninvasive photon‐beam (x‐ray energy) radiosurgery ablation is feasible with electrical block and histopathologic fibrosis confirmed in targeted myocardium and without collateral damage.
This dose‐escalation study for cardiac radiosurgery in a porcine model is novel in that it demonstrated noninvasive ablation of the AV node with a 100% success rate, without complications, and with single‐fraction 35‐ to 40‐Gy doses of radiation.
There are ongoing US Food and Drug Administration‐approved clinical pilot studies of cardiac radioablation such as ENCORE‐VT (a phase I/II study of EP‐Guided Noninvasive Cardiac Radioablation for Treatment of Ventricular Tachycardia) (ClinicalTrials.gov Identifier: NCT02919618) and CyberHeart's Cardiac Arrhythmia Ablation Treatment: Patients With Refractory Ventricular Tachycardia/Fibrillation Stereotactic Radioablation for Catheter Ablation and Drug‐Refractory VT (ClinicalTrials.gov Identifier: NCT02661048).
What Are the Clinical Implications?
Noninvasive stereotactic cardiac arrhythmia radiosurgery ablation might have future clinical application in pulmonary vein isolation and scar‐related ventricular tachycardia ablation, especially with improved respiratory and cardiac motion‐tracking systems.
Cardiac arrhythmias are commonly encountered with various implications for morbidity, mortality, and medical care expenditure. Several modalities exist for the management of cardiac arrhythmias, including lifestyle changes, pharmacologic agents, and catheter ablation of cardiac arrhythmogenic foci. With improvements in procedural techniques and outcomes, catheter ablation is becoming a cornerstone for the management of cardiac arrhythmias.1
The field of radiation oncology has advanced tremendously from the conventional 2‐dimensional delivery system of the 1950s to the conventional 3‐dimensional delivery system of the 1980s to stereotactic radiosurgery (SRS) in the mid‐2000s coupled with image‐guided radiation therapy. This has resulted in increased precision and the ability to accurately target subcentimeter lesions, allowing linear accelerator–based SRS to become an important management modality for tumors in different organ systems such as the head and neck, lungs, and gastrointestinal tract while sparing nearby healthy tissues.2
Stereotactic radioablation using photon (x‐rays, γ‐energy) or particle (proton or carbon) therapy is a well‐established treatment paradigm in oncology adapted recently to the treatment of cardiac arrhythmias. Noninvasive ablation of arrhythmias is an ablative therapy delivered to the heart without entering the body with a physical instrument. Recently, several investigators have attempted to use ionizing radiation in animal experimental models to ablate different arrhythmogenic foci in animal hearts, with varying success rates. Radiosurgery with doses >32.5 Gy in the healthy pig heart have been shown to induce circumscribed scars.3 We performed an investigational feasibility study on atrioventricular (AV) node ablation using SRS and tested a dose escalation of photon beams for noninvasive ablation of the AV node in a live swine model.
Methods
Animals
A total of 5 Large White breed swine (4 females) ranging from 40 to 75 kg were studied sequentially. Initially, a single‐chamber pacemaker with an epicardial lead was implanted surgically. Two weeks following the surgical implantation of the pacemaker, the ablation procedure was performed, and the pigs were followed up thereafter with serial ECGs and pacemaker interrogations to assess for electrocardiographic changes. Once complete AV block was achieved, the pigs had cardiac echocardiographic assessments and were then euthanized. Tissue sections were taken from the AV node and organs near the AV node (heart, lungs, liver, and esophagus) and sent for histologic examination to determine the presence or absence of tissue damage. All animal experiments were approved by the Institutional Animal Care and Use Committee at the American University of Beirut in Lebanon.
Pacemaker Implantation
We performed ablation of the AV node in the study sample in an approach similar to that used in humans. In humans, before AV node ablation, a pacemaker is needed. Induction anesthesia was performed in a veterinary facility using an intramuscular 15 mg/kg dose of ketamine, and the pigs were transferred to a dedicated animal surgical unit. The pigs were then intubated and mechanically ventilated, and anesthesia was maintained using isoflurane. With the swine in the supine position and properly anesthetized, the chest and abdomen were scrubbed and draped. Proper antibiotic prophylaxis was given using a 200‐mg dose of long‐acting oxytetracycline. An area of myocardium with no coronary branches was identified for lead placement. The St Jude ventricular lead was screwed into place following the manufacturer's recommendations and was tested for threshold and resistance. The wire was then fixed to the fascial edges with silk sutures. The battery pocket was fashioned under the fat to the left of the midline in pig number 1 then under the muscle in subsequent animals. Hemostasis was achieved. The pericardium was left open, and the wound closed with nonabsorbable heavy suture for the fascia and skin. The pacemaker was programmed to a demand ventricular sensing and pacing mode, with a heart rate threshold of 50 beats per minute. The animals were then transferred to recover in the animal housing unit.
Atrioventricular Node Ablation
To give enough time for the animals to recover, the AV node ablation was performed 2 weeks after pacemaker implantation. During the ablation procedure, induction and maintenance anesthesia were achieved using ketamine. Induction anesthesia was achieved with a 10 mg/kg dose of ketamine, and anesthesia was maintained with subsequent appropriate dosing of ketamine. A half‐pipe–shaped body frame was used as a recipient to place the swine inside for a high degree of immobilization and reproducibility during simulation and treatment (Figure 1).
Figure 1.
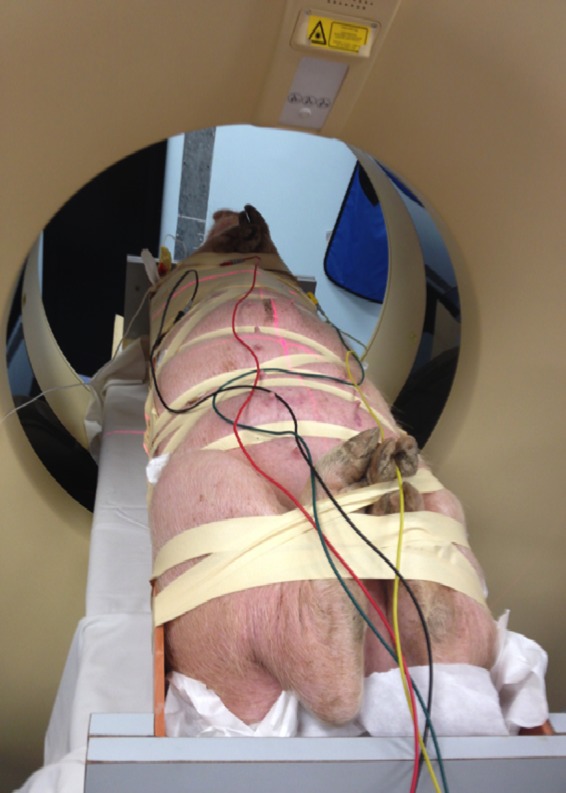
Pig under general anesthesia undergoing computed tomography scan to localize the atrioventricular node.
Simulation was done using a Siemens Somatom wide‐bore computed tomography (CT) simulator and flat table top. By use of wall‐mounted lasers, 3 metallic radiopaque markers (BBs) were placed at the level of the heart: 1 anterior BB was placed on the swine's skin and 2 lateral BBs were placed on the frame (to use a firm surface). Firm taping was used around the swine's body and the body frame to prevent possible swine movements despite anesthesia. The frame was leveled on simulator table top.
Electrodes were placed on the skin in the heart area to monitor heart rate and rhythm, and perform gated 4‐dimensional CT scan with contrast. Two CT sets were sent to the treatment‐planning system: diastolic phase and systolic phase, reconstructed at 1‐mm slice thickness and covering the heart area. The cardiologist and the radiation oncologist, in a process similar to that described by Sharma et al, performed AV node contouring and treatment planning.4 Contouring was done on both CT sets in the planning system, and gross target volume and an internal target volume were generated as the union of contours from both CT sets. Planning target volume was generated by adding a 5‐mm margin to the internal target volume (Figure 2).
Figure 2.
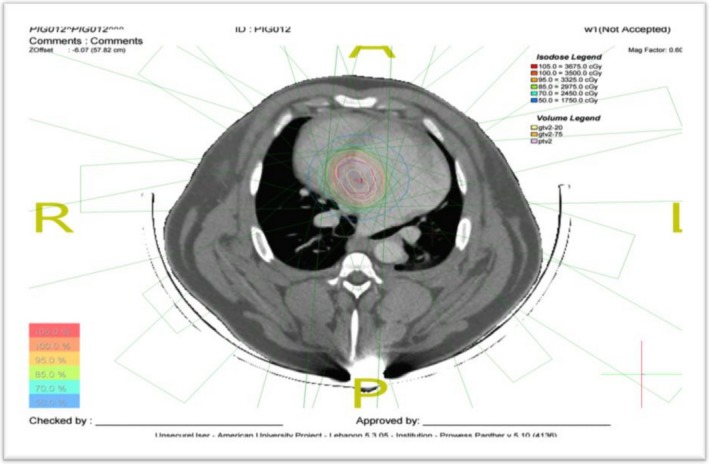
Computed tomography scan showing atrioventricular node location and radiation dose falloff.
Planning was done using Panther software (Prowess Inc, Concorde, CA) version 5.10. Nine coplanar beams were used 40 degrees apart, and a multileaf collimator with leaves 5 mm wide at isocenter to generate 6‐ or 15‐MV photon beams at 300 to 500 monitor units per minute. The field‐in‐field technique was used to make dose distribution more homogeneous. Dose calculation was done on either of the 2 CT sets.
The linear accelerator used for the SRS treatment was a Siemens Artiste. The swine was positioned on a linear accelerator couch using lasers and BBs. A cone beam computed tomography was performed before treatment and was compared to the reference CT from the treatment‐planning system. Automatic fusion was performed by Siemens Syngo (Malverne, PA) software, and correctional couch shifts were implemented to achieve millimeter accuracy in positioning. Once the swine was accurately positioned, the beam was turned on, with an average treatment time of about 15 minutes. A second cone beam computed tomography was performed after treatment for verification and to confirm absence of swine movement during treatment. Total procedure time from simulation start to end of treatment was about 3 to 4 hours. The first 3 pigs received a radiation dose of 35 Gy; the fourth and fifth pigs received a dose of 40 Gy.
Monitoring for Electrocardiographic Changes
Following the ablation procedure, pacemaker interrogation and ECGs were performed every week until permanent electrocardiographic changes were seen. Pigs were initially anesthetized using a 10 mg/kg intramuscular injection of ketamine. Interrogation and ECGs were performed weekly using a Merlin Patient Care System, a portable system that programs St Jude Medical's implantable devices. After the animals developed complete AV block, they had echocardiographic assessment and were sent for euthanization to obtain tissue samples for histology.
Histologic Studies
A histological study of the heart and surrounding organs (liver, lung, and esophagus) was carried out to explore possible alterations in tissue architecture.
Biopsy retrieval
After euthanization of the pig, its chest was opened with care to retrieve the pacemaker in proper condition. This was followed by biopsy from the left lobe of the liver, a lung biopsy from a region closest to the AV node, and an esophageal biopsy posterior to the heart. Next, the heart was removed, and biopsies from the AV node, the interventricular septum, and the right and left atria and ventricles were taken. Each biopsy was cut into 2 pieces, 1 for routine light microscopy was immersed in 10% formalin, and the other was kept in liquid nitrogen and then transferred to a freezer kept at −80°C for future molecular studies.
Processing of tissues for light microscopy
Five‐millimeter pieces from each of the biopsies were processed for routine microscopy according to standard methods. Five‐micrometer sections were stained with hematoxylin and eosin as well as periodic‐acid‐Schiff to depict the Purkinje cells. Microscopic views were retrieved on a screen, studied, and photographed for illustration.
Results
AV Node Ablation
All 5 pigs that were studied eventually developed complete AV block. The electrocardiographic progression of findings in pig 4 is shown (Figure 3). Given the fact that ECGs were being obtained once weekly, the exact day the pigs developed complete AV block can be anywhere within a 6‐day period, and therefore the data are presented as such (Table). In all pigs, there were no immediate findings after ablation. In 3 of the pigs, electrocardiographic changes were progressive. PR lengthening was seen at first, followed by higher‐degree AV blocks and eventually complete heart block. In the other 2 pigs, complete heart block occurred 1 week after a normal electrocardiographic pattern, so we did not see progressive change but rather a more abrupt AV conduction disturbance. The earliest AV blocks were seen in pigs 1 and 5, where complete heart blocks occurred 2 months after SRS. In the other 3 pigs, first‐degree followed by complete heart blocks occurred at a later time.
Figure 3.
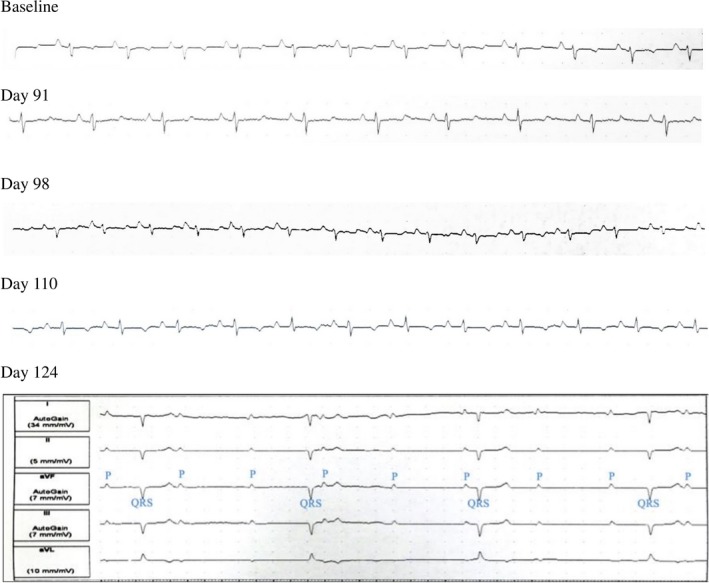
Progression of electrocardiographic changes in pig 4 and underlying rhythm during pacemaker interrogation showing complete atrioventricular dissociation (complete heart block) on day 124 postablation.
Table 1.
Dose of Radiation Targeted to Atrioventricular Node and Postoperative Days to Achieve Permanent Electrocardiographic Changes
| Pig Number | Radiation Dose (Gy) | Evidence of AV Block First Observed (Postoperative Days) | Complete Heart Block (Postoperative Days) |
|---|---|---|---|
| 1 | 35 | 66 to 72 | 66 to 72 |
| 2 | 35 | 102 to 108 | 109 to 115 |
| 3 | 35 | 199 to 205 | 229 to 235 |
| 4 | 40 | 104 to 110 | 118 to 124 |
| 5 | 40 | 55 to 61 | 55 to 61 |
AV indicates atrioventricular.
Pathologic Effects of Radiation
On gross examination of the heart, all pigs had a small scar surrounding the area of lead implantation around the left ventricular apex. The pathologic effects seen in the hearts of the animals were consistent with those of radiation exposure. Before euthanization, swine echocardiograms showed normal systolic function. The histologic sections of the AV node area showed severe architectural disruption, with loss of the smooth cellular organization usually seen in normal AV nodes, in addition to cellular necrosis and extensive fibrin deposition in the area. Sections from the surrounding tissues, however, including the liver, esophagus, and lungs, showed normal architecture with persistent cellular integrity, no evidence of cellular necrosis, and no evidence of fibrosis. Histologic sections taken from pig 4 are shown with fibrosis at the AV node and normal architecture of the surrounding ventricular septal tissue (Figures 4 and 5).
Figure 4.
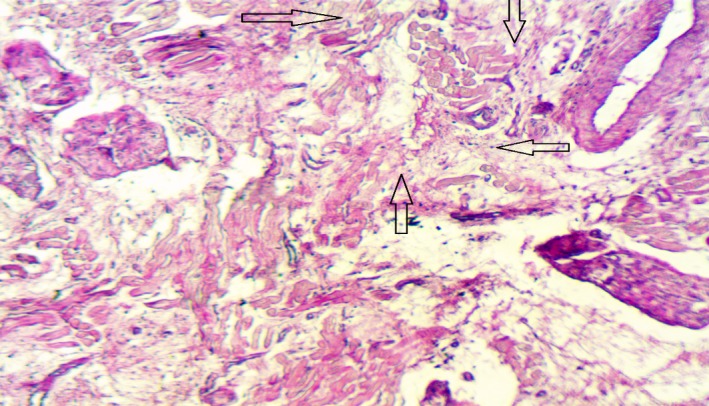
Fibrosis and loss of architecture in the atrioventricular node. The arrows point to areas of necrosis and loss of the specialized nodal cardiac muscle cells with fibrotic replacement (hematoxylin and eosin stained, magnification ×250).
Figure 5.
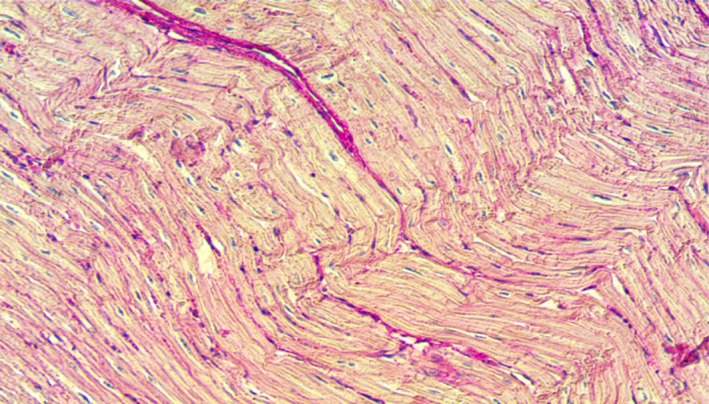
Normal histologic section of the ventricular septum.
Discussion
In this study we have demonstrated the feasibility of SRS for AV node ablation in an intact porcine model, and, to our knowledge, we are the third group to use radiation therapy for AV node ablation.4 Sharma et al attempted AV node ablation in 2 pigs: 1 procedure was successful with 70‐Gy ablation, whereas the other failed due to pacemaker infection.4 In our study we used doses of 35 to 40 Gy to successfully achieve complete heart blocks in 5 pigs. The pig in the Sharma et al study had AV node conduction changes earlier than 35 days after the procedure, which is likely related to the higher dose of radiation used. Our study is significant in that we demonstrated complete heart block in 5 pigs with a lower dose of radiation. Another group from the Mayo Clinic published their data recently in April 2017 on the external arrhythmia ablation using photon beams and achieved 86% success in achieving complete heart block in an intact animal model with a dose deescalation from 55 to 25 Gy.5 Our study was similarly conducted with a 100% success rate of complete heart block, albeit in a smaller sample size (5 versus 8), and complements their data showing the feasibility of achieving complete heart block with radiation.
In the swine in this study sample, AV conduction changes were seen at least 55 days after SRS. Two of the pigs developed abrupt complete heart blocks, whereas this change was seen gradually in the rest of the pigs. This suggests that the damaging effects that SRS has on AV nodal cells are not immediate. A higher dose of radiation may provide more immediate effects, but it is also important to avoid cardiotoxicity.
Because radiosurgery with doses >32.5 Gy in the healthy pig heart have been shown to induce circumscribed scars, and previous studies showed cardiotoxicity with doses >40 Gy, we decided to test a dose escalation of photon beams as noninvasive ablation of the AV node in a live porcine model starting with 35 Gy and not to exceed 40 Gy. We performed the investigational feasibility study on AV node ablation using SRS and had 5 successful procedures, with all 5 pigs showing complete AV block without complications (100% success). Not only did we demonstrate the feasibility of AV node ablation using SRS, but our results also establish the safety of this cardiac radioablation procedure, at least in pigs, with no radiation injury detected in nearby healthy tissues. The radiation dose falloff in SRS is around 10% to 15% per millimeter, with a resulting dose falloff of 50% at 2 to 5 mm, with only 0.05% of the dose remaining at 1 m from the target lesion.4, 6, 7 Therefore, SRS is associated with a sharp falloff in radiation dose. In addition, it is not associated with increased incidence of secondary malignancies, and it is thought that the high dose of radiation delivered by SRS results in cytotoxicity rather than mutagenicity. All this points to the short‐term as well as the long‐term safety and potential applicability of SRS outside the field of oncology.8, 9, 10 Because the use of SRS to the heart is a novel technique, long‐term data on cardiac complications including cardiac rupture or ventricular septal defect formation are lacking. The focused target area along with the sharp radiation dose falloff likely help in avoiding these complications. Additionally, histologic analysis of cardiac tissue surrounding the AV nodes showed completely normal architecture.
The question remains about the clinical applicability of our study. Catheter ablation for cardiac arrhythmias has become a central component for the treatment of different disorders, and in our study we used AV node ablation as a model to demonstrate the feasibility of achieving noninvasive ablation.
A study recently analyzed treatment planning for pulmonary vein isolation using CyberKnife in patients with atrial fibrillation11 and showed that CyberKnife could be used in up to 40% of patients for atrial fibrillation ablation. It is thought that with improved motion‐tracking systems and SRS technology, a larger percentage of patients can be eligible for the procedure.
Improved SRS and CT technology has allowed respiratory movements to be considered during the planning and implementation phases of the procedure and cardiac motion to be tracked with the use of intracardiac fiducial markers. These advances, along with improvements in imaging technology that allow localization of myocardial scars,12 make SRS a possible modality for ablation of ventricular tachycardia (VT) secondary to myocardial infarction or scarring. Two groups have already implemented SRS for VT ablation refractory to conventional therapy under local institutional review board–approved protocols for compassionate therapy. The first group used CyberKnife to ablate an arrhythmogenic lesion in the left ventricle of a patient with advanced dilated cardiomyopathy and recurrent VT and arrhythmic storms. This resulted in the reduction of PVCs from 9% to 10% to 1% to 3% in 10 days.13 The other group used stereotactic arrhythmia ablation protocol to ablate a VT substrate in a patient with long‐standing coronary artery disease, advanced heart failure, refractory VT, and several other comorbidities. The procedure was well tolerated, and there was a significant reduction in the number of VT episodes 9 months following the stereotactic arrhythmia ablation procedure. Eventually, the patient passed away from congestive heart failure progression, and recurrence of the VT.14
There were some limitations to our study. This was a feasibility study performed on a limited number of animals, even though all achieved the primary end point and showed promising results. Further studies on animals and eventually phase 1 human trials are warranted to further elucidate this novel approach to cardiac arrhythmias with respiratory motion tracking and cardiac motion compensation. However, this field of cardiac radioablation is moving rapidly with US Food and Drug Administration‐approved clinical pilot studies: ENCORE‐VT (Phase I/II Study of EP‐guided Noninvasive Cardiac Radioablation for Treatment of Ventricular Tachycardia) (ClinicalTrials.gov Identifier: NCT02919618) and CyberHeart's Cardiac Arrhythmia Ablation Treatment: Patients With Refractory Ventricular Tachycardia/Fibrillation Stereotactic Radioablation for Catheter Ablation and Drug‐Refractory VT (ClinicalTrials.gov Identifier: NCT02661048).
Our study design with pacemaker implantation in healthy swine followed by AV node ablation allowed us to demonstrate the feasibility and safety of SRS as a treatment modality for cardiac tissue ablation. We followed the pigs up until the day of complete AV block, after which the animals were euthanized. More studies are needed in the future to evaluate any potential long‐term cardiac adverse effects.
Conclusion
Catheter‐free ablation using stereotactic radiosurgery targeting specific sites determined by noninvasive cardiac anatomic mapping can produce cardiac lesions (without damage to nearby tissue) that can be used in the noninvasive treatment of cardiac arrhythmias.
Sources of Funding
This research was funded by the Farouk K. Jabre Biomedical Research Award (2014).
Disclosures
None.
(J Am Heart Assoc. 2017;6:e007193 DOI: 10.1161/JAHA.117.007193.)29079566
This article was handled independently by: N.A. Mark Estes III, MD, as a guest editor.
This project was 1 of the 3 clinical research finalists presented at the Heart Rhythm Society Young Investigator Award Session, May 10, 2017, in Chicago, IL.
Contributor Information
Marwan M. Refaat, Email: marwanrefaat@alumni.harvard.edu.
Bassem Youssef, Email: by04@aub.edu.lb.
References
- 1. Kneeland PP, Fang MC. Trends in catheter ablation for atrial fibrillation in the United States. J Hosp Med. 2009;4:E1–E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Le QT, Shirato H, Giaccia AJ, Koong AC. Emerging treatment paradigms in radiation oncology. Clin Cancer Res. 2015;21:3393–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blanck O, Bode F, Gebhard M, Hunold P, Brandt S, Bruder R, Grossherr M, Vonthein R, Rades D, Dunst J. Dose‐escalation study for cardiac radiosurgery in a porcine model. Int J Radiat Oncol Biol Phys. 2014;89:590–598. [DOI] [PubMed] [Google Scholar]
- 4. Sharma A, Wong D, Weidlich G, Fogarty T, Jack A, Sumanaweera T, Maguire P. Noninvasive stereotactic radiosurgery (CyberHeart) for creation of ablation lesions in the atrium. Heart Rhythm. 2010;7:802–810. [DOI] [PubMed] [Google Scholar]
- 5. Lehmann HI, Deisher AJ, Takami M, Kruse JJ, Song L, Anderson SE, Cusma JT, Parker KD, Johnson SB, Asirvatham SJ, Miller RC, Herman MG, Packer DL. External arrhythmia ablation using photon beams: ablation of the atrioventricular junction in an intact animal model. Circ Arrhythm Electrophysiol. 2017;10:e004304 DOI: 10.1161/CIRCEP.116.004304 [DOI] [PubMed] [Google Scholar]
- 6. Hong LX, Garg M, Lasala P, Kim M, Mah D, Chen CC, Yaparpalvi R, Mynampati D, Kuo HC, Guha C, Kalnicki S. Experience of micromultileaf collimator linear accelerator based single fraction stereotactic radiosurgery: tumor dose inhomogeneity, conformity, and dose fall off. Med Phys. 2011;38:1239–1247. [DOI] [PubMed] [Google Scholar]
- 7. Sahgal A, Ma L, Chang E, Shiu A, Larson DA, Laperriere N, Yin FF, Tsao M, Menard C, Basran P, Létourneau D, Heydarian M, Beachey D, Shukla V, Cusimano M, Hodaie M, Zadeh G, Bernstein M, Schwartz M. Advances in technology for intracranial stereotactic radiosurgery. Technol Cancer Res Treat. 2009;8:271–280. [DOI] [PubMed] [Google Scholar]
- 8. Patel TR, Chiang VL. Secondary neoplasms after stereotactic radiosurgery. World Neurosurg. 2014;8:594–599. [DOI] [PubMed] [Google Scholar]
- 9. Rahman M, Neal D, Baruch W, Bova FJ, Frentzen BH, Friedman WA. The risk of malignancy anywhere in the body after linear accelerator (LINAC) stereotactic radiosurgery. Stereotact Funct Neurosurg. 2014;92:323–333. [DOI] [PubMed] [Google Scholar]
- 10. Pollock BE, Link MJ, Stafford SL, Parney IF, Garces YI, Foote RL. The risk of radiation‐induced tumors or malignant transformation after single‐fraction intracranial radiosurgery: results based on a 25‐year experience. Int J Radiat Oncol Biol Phys. 2017;97:919–923. [DOI] [PubMed] [Google Scholar]
- 11. Blanck O, Ipsen S, Chan MK, Bauer R, Kerl M, Hunold P, Jacobi V, Bruder R, Schweikard A, Rades D, Vogl TJ, Kleine P, Bode F, Dunst J. Treatment planning considerations for robotic guided cardiac radiosurgery for atrial fibrillation. Cureus. 2016;8:e705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ipek EG, Nazarian S. Cardiac magnetic resonance for prediction of arrhythmogenic areas. Trends Cardiovasc Med. 2015;25:635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cvek J, Neuwirth R, Knybel L, Molenda L, Otahal B, Pindor J, Murávová M, Kodaj M, Fiala M, Branny M, Feltl D. Cardiac radiosurgery for malignant ventricular tachycardia. Cureus. 2014;6:e190. [Google Scholar]
- 14. Loo BW Jr, Soltys SG, Wang L, Lo A, Fahimian BP, Iagaru A, Norton L, Shan X, Gardner E, Fogarty T, Maguire P, Al‐Ahmad A, Zei P. Stereotactic ablative radiotherapy for the treatment of refractory cardiac ventricular arrhythmia. Circ Arrhythm Electrophysiol. 2015;8:748–750. [DOI] [PubMed] [Google Scholar]


