Abstract
Background
Sepsis may adversely affect bleeding risk in anticoagulated patients with atrial fibrillation (AF), but the impact of warfarin treatment in such patients is poorly described. This registry‐based nationwide cohort study examined safety of oral anticoagulant treatment (OAC) in patients with preexisting AF who were hospitalized because of incident sepsis in the period 2000–2015.
Methods and Results
We identified 3030 AF patients who were warfarin users at the time of sepsis diagnosis, and we used inverse probability of treatment weighting to compare the rates of bleeding, thromboembolic events, and death within 90 days after sepsis diagnosis with a comparable cohort of 55721 patients without warfarin treatment and known AF. Weighted 90‐day bleeding rates were slightly higher among warfarin users compared with nonusers (0.14 versus 0.12 per 100 person‐years), yielding a weighted hazard ratio of 1.19 (95% confidence interval, 1.00–1.41). Thromboembolic event rates during the 90‐days after sepsis were marginally higher among warfarin users versus nonusers (0.04 versus 0.03; hazard ratio: 1.25, 95% confidence interval, 0.89–1.76), while the 90‐day all‐cause mortality was substantially lower among warfarin users (hazard ratio: 0.64, 95% confidence interval, 0.58–0.69). Various sensitivity analyses conducted to challenge the robustness these findings yielded results that were consistent with the main findings.
Conclusions
AF patients who are on warfarin therapy at sepsis diagnosis experienced an increase in bleeding rates within the 3 months following sepsis. Warfarin use was associated with lower mortality, despite virtually comparable thromboembolic event rates.
Keywords: anticoagulation, atrial fibrillation, cohort study, complication
Subject Categories: Atrial Fibrillation, Quality and Outcomes, Complications, Mortality/Survival
Clinical Perspective
What Is New?
In this nationwide cohort study, warfarin therapy in patients with preexisting atrial fibrillation during sepsis was associated with increased rates of bleeding complications, comparable rates of thromboembolic events, and significantly lower mortality during the 90‐days after sepsis compared with sepsis patients who were not warfarin users and had no known atrial fibrillation.
What Are the Clinical Implications?
Our results emphasize that cautious assessment of bleeding risk is warranted for patients with preexisting atrial fibrillation who are on continuous anticoagulant therapy after hospitalization with sepsis, but further research is needed to determine the optimal management of short‐ and long‐term anticoagulation strategies in patients with atrial fibrillation and sepsis.
Introduction
Sepsis is one of the most commonly encountered conditions among hospitalized patients and is associated with critical illness, high mortality, and healthcare costs.1 The clinical presentation of sepsis is diverse, but coagulation abnormalities are almost invariably present.1, 2, 3, 4 Consequently, sepsis is associated with high mortality that may result from multiple organ failure due to microvascular thrombosis or bleeding due to depletion of coagulation factors and platelets. Indeed, bleeding risk with antithrombotic therapy in severe sepsis patients remains a concern.5
Concerns about anticoagulation therapy in sepsis patients underscore the importance of assessing bleeding risk in patients whose home medications include oral anticoagulant (OAC) therapy. Two prior studies have indicated that anticoagulant therapy is associated with an increased risk of bleeding in AF patients during sepsis.5, 6 However, there is a lack of large‐scale, population‐based assessments of outcomes in sepsis patients with preexisting atrial fibrillation (AF) on continuous anticoagulant therapy. In a prior US study of Medicare beneficiaries, AF occurred in 25% of patients with hospitalized sepsis, of which 18% had preexisting AF.7
We linked nationwide health registries to identify all sepsis patients with preexisting AF who were on warfarin therapy before hospital admission with sepsis. We conducted a propensity‐weighted analysis comparing the risk of bleeding, thromboembolic events, and 90‐day mortality in this cohort with sepsis patients who were not warfarin users and who had no known AF.
Material and Methods
Setting and Data Sources
Our study was a propensity‐weighted analysis of administrative registry data covering the entire Danish population, encompassing 5 659 715 inhabitants (as of January 1, 2015) in 2000–2015. Denmark has a tax‐supported healthcare system providing free medical care and partial reimbursement of the costs of most prescribed medications, including warfarin. We identified all sepsis patients; their comorbidities; bleeding and thromboembolic outcomes in the Danish National Patient Register,8 which includes admission/discharge date; and discharge International Classification of Diseases diagnoses for >99% of somatic hospital admissions in Denmark since 1977. Warfarin exposure and concomitant medications was ascertained from the Danish National Prescription Registry,9 which holds purchase date, ATC (Anatomical Therapeutic Chemical) classification code, and package size for every prescription purchase in Denmark since 1994. Demographic information was obtained from the Danish Civil Registration System,10 which contains information on sex, date of birth, and vital and emigration status. We linked all data sources by means of the unique personal civil registration numbers assigned to all Danish residents since 1968.10 The study was approved by the Danish Data Protection Agency (2015‐57‐0001). Approval from an ethics committee is not required for anonymous registry‐based studies in Denmark. Data were provided by Statistics Denmark.
Study Population
We identified all patients hospitalized with an incident primary inpatient sepsis diagnosis between 2000 and 2015, excluding emergency department diagnoses and patients who had not been residents in Denmark for at least 1 year before sepsis diagnosis. We rationalized that patients with community‐onset sepsis would more likely be taking their usual home medications at the time of sepsis hospitalization. Accordingly, we restricted the study population to patients with no hospital contact within the 30 days preceding admission, as done in other studies.11
Comedication status and comorbidities at baseline were ascertained by medication claims within 1 year before index date and/or history of primary or secondary hospital discharge diagnoses (excluding emergency room diagnoses) since 1994. Table S1 provides information on all codes for diagnoses and medications. We further combined baseline information into CHA2DS2‐VASc stroke risk score12 to summarize the perceived stroke risk at baseline and a HAS‐BLED score13 as a measure of bleeding risk at baseline (see score definitions in Table S2). Owing to the lack of data on sepsis severity, the length of hospital stay (calculated as the date for sepsis admission/diagnosis until discharge) was used as a proxy, with longer stays reflecting more complicated (and severe) conditions.
Exposure
To avoid bias from inclusion of users of non–vitamin K OACs (NOACs), we excluded all patients who filled a prescription for NOACs within 365 days before admission. Within the study population of patients with incident sepsis, we then identified all patients who had redeemed a prescription for warfarin within 90 days before the date of admission with sepsis. We restricted the cohort of warfarin users to patients with a prior hospital diagnosis for nonvalvular AF to limit confounding from the underlying indication for warfarin therapy.
We similarly defined a comparison cohort consisting of sepsis patients who were warfarin nonusers (defined as no redemption of warfarin in a 365‐day period before date of sepsis diagnosis). Because AF patients not receiving warfarin or NOACs may represent selective channeling of treatments away from frail patients at increased risk of bleeding, we excluded patients with a prior hospital AF diagnosis; therefore, the comparison cohort comprised warfarin nonusers without AF.
To facilitate a balanced comparison of the 2 cohorts, we used an inverse probability of treatment weighted analysis with weights defined to obtain an estimate of the average treatment of the treated with the warfarin‐exposed cohort in focus. The weights were based on the propensity score for being an AF patient on warfarin at baseline, estimated using logistic regression with the following potential confounders used as treatment predictors: age (continuous), binary indicators for sex, calendar period, ischemic stroke, systemic embolism or transient ischemic attack, congestive heart failure, vascular disease, prior bleeding, hypertension, diabetes mellitus, cancer, chronic pulmonary disease, renal disease, hospital diagnosed pneumonia within the year before index, alcohol‐related disease, dementia, and depression, as well as recent prescriptions of digoxin, nonloop diuretics, beta blockers, calcium channel blockers, angiotensin‐converting enzyme inhibitors, clopidogrel, antidiabetic medications, and/or statins (see Table S1 for codes). Balances between the 2 exposure groups were evaluated by the standardized differences of all baseline covariates, using a threshold of 0.1 to indicate an imbalance.14
Outcomes
The primary study end points were bleeding complications and thromboembolic events (ischemic stroke, systemic embolism, transient ischemic attack) within 90 days of the date of sepsis hospitalization. Bleeding events included intracranial bleeding, gastrointestinal bleeding, and major clinically relevant bleeding in various anatomic positions. All‐cause mortality within 90 days of sepsis diagnosis was included as secondary end point. Hospital discharge diagnoses for all study outcomes were required to be primary in‐hospital codes, excluding emergency room and ambulatory diagnoses, to ensure higher validity of the outcomes. The coding accuracy of the selected outcomes was validated previously and found to be sufficiently accurate for epidemiological research.15
Statistical Analyses
We followed all study participants from the date of sepsis diagnosis until the outcome of interest, death, emigration, or end of the study, whichever occurred first. We examined baseline characteristics at the time of sepsis diagnosis according to exposure and estimated absolute standardized differences to determine the extent to which the inverse probability of treatment weighting balanced baseline characteristics (considering an absolute standardized difference ≥0.2 to be critical).16 We used time‐to‐event survival analyses to compare risk of end points according to warfarin exposure. Weighted incidence rates were calculated as number of events divided by person‐time, whereas cumulative incidence functions (by means of the Aalen–Johansen estimator), assuming death as a competing risk, were used to depict the risk of the primary outcomes, bleeding and thromboembolic events, within 90 days. Finally, we used weighted Cox proportional hazards models to compare event hazard rates according to warfarin exposure.
To challenge the robustness of our findings, we did 3 preplanned sensitivity analyses. First, we repeated all analyses using an alternative definition of the cohort of warfarin users (prescription redemption within 120, 60, and 30 days before sepsis diagnosis, respectively). Second, to quantify the impact of “prevalent user bias,”17 we restricted the warfarin cohort to new users of warfarin, defined as patients who redeemed their first‐ever prescription of warfarin within 90 days of the date of sepsis diagnosis. Third, because restriction to new users can guard against “healthy adherer bias” but may not eliminate healthy user bias, we also compared outcomes among warfarin users with those of propensity‐weighted warfarin nonusers who had redeemed prescriptions for other preventive medications (beta blockers, statins, calcium channel blockers, and/or angiotensin‐converting enzyme inhibitors) in an active comparator design.17
Analyses were conducted using Stata/MP version 14 (StataCorp LP).
Results
Figure 1 shows the assembly of the study population. After exclusions, the study population included 3030 sepsis patients with nonvalvular AF who were warfarin users and 55 721 sepsis patients who were warfarin nonusers (Table 1). Warfarin users were substantially older than nonusers (mean age: 78 versus 67 years) and had more comorbidities, in particular, heart failure, prior stroke, and vascular disease, resulting in a mean CHA2DS2‐VASc score 4.5 versus 2.7 in warfarin nonusers. After inverse probability of treatment weighting, all absolute standardized differences were <0.14, indicating that the weighted cohorts were comparable.
Figure 1.
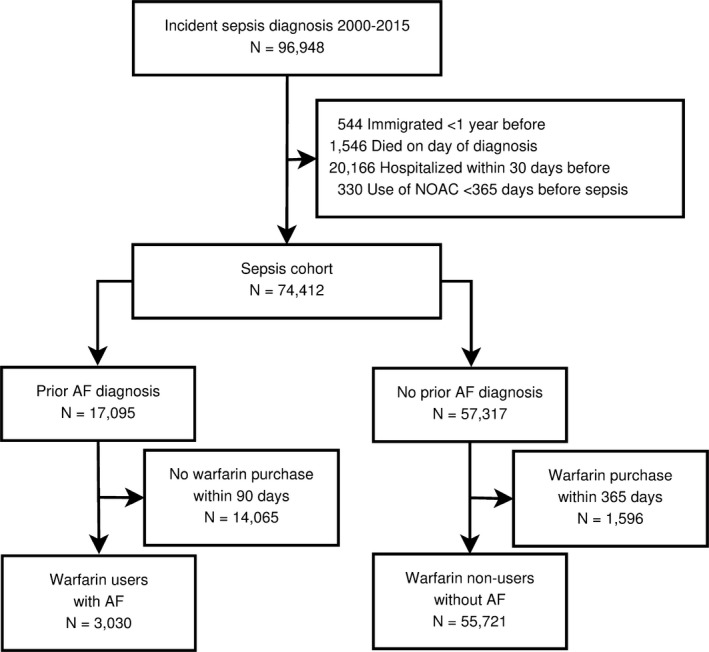
Patient inclusion flowchart. AF indicates atrial fibrillation; NOAC, non–vitamin K oral anticoagulant.
Table 1.
Descriptive Characteristics of Patients With Incident Sepsis in Denmark According to Use of OAC Therapy
| Characteristic | Warfarin Nonusers | Warfarin Users | Standardized Differencesa | |
|---|---|---|---|---|
| Before | After | |||
| Participants, n | 55 721 | 3030 | ||
| Female, % (n) | 47.0 (26 196) | 35.3 (1071) | 0.239 | 0.067 |
| Age, y, mean (SD) | 66.9 (20.4) | 78.2 (8.7) | 0.717 | 0.059 |
| Year of diagnosis, % (n) | ||||
| 2000–2003 | 14.4 (8017) | 6.4 (194) | 0.264 | 0.045 |
| 2004–2007 | 19.1 (10 670) | 15.9 (481) | 0.086 | 0.125 |
| 2008–2011 | 28.9 (16 127) | 30.5 (923) | 0.033 | 0.020 |
| 2012–2015 | 37.5 (20 907) | 47.3 (1432) | 0.198 | 0.139 |
| Days in hospital, mean (SD) | 19.2 (41.9) | 18.1 (21.9) | 0.033 | 0.015 |
| Severe sepsis, % (n) | 2.5 (1386) | 3.3 (100) | 0.049 | 0.072 |
| Comorbidity, % (n) | ||||
| Prior bleeding | 27.8 (15 489) | 47.1 (1426) | 0.406 | 0.026 |
| HAS‐BLED score, mean (SD) | 2.2 (1.5) | 3.2 (1.3) | 0.711 | 0.115 |
| CHA2DS2‐VASc score, mean (SD) | 2.7 (1.8) | 4.5 (1.6) | 1.040 | 0.080 |
| Prior stroke | 13.6 (7582) | 27.6 (836) | 0.351 | 0.016 |
| Heart failure or LVD | 26.0 (14 506) | 69.4 (2104) | 0.965 | 0.105 |
| Hypertension | 40.6 (22 604) | 80.3 (2434) | 0.890 | 0.048 |
| Vascular disease | 13.5 (7523) | 28.5 (865) | 0.376 | 0.067 |
| Renal dysfunction | 10.7 (5959) | 18.8 (569) | 0.230 | 0.033 |
| Diabetes mellitus | 17.2 (9586) | 29.5 (895) | 0.295 | 0.025 |
| Chronic pulmonary disease | 15.4 (8581) | 26.1 (792) | 0.267 | 0.037 |
| Cancer | 23.3 (13 002) | 24.9 (753) | 0.036 | 0.032 |
| Alcohol‐related disease | 8.8 (4918) | 4.0 (122) | 0.197 | 0.015 |
| Osteoporosis | 7.1 (3948) | 8.2 (247) | 0.040 | 0.004 |
| Dementia | 6.8 (3806) | 4.9 (147) | 0.085 | 0.017 |
| Depression | 25.2 (14 018) | 23.2 (702) | 0.047 | 0.009 |
| Pneumonia within previous 365 days | 19.1 (10 649) | 25.7 (780) | 0.160 | 0.019 |
| Ulcer disease | 7.5 (4164) | 8.8 (268) | 0.050 | 0.075 |
| Medications used, % (n) | ||||
| Digoxin | 1.7 (971) | 45.6 (1383) | 1.206 | 0.058 |
| Nonloop diuretics | 28.9 (16 117) | 45.0 (1365) | 0.339 | 0.020 |
| Loop diuretics | 23.3 (13 009) | 61.4 (1860) | 0.834 | 0.096 |
| Beta blocker | 18.3 (10 170) | 63.6 (1927) | 1.039 | 0.090 |
| Calcium channel blocker | 18.6 (10 352) | 32.6 (989) | 0.326 | 0.051 |
| Renin–angiotensin inhibitor | 28.8 (16 075) | 56.5 (1713) | 0.583 | 0.100 |
| Aspirin | 28.8 (16 052) | 32.1 (974) | 0.073 | 0.126 |
| Clopidogrel | 4.6 (2541) | 3.6 (110) | 0.047 | 0.013 |
| Statins | 23.0 (12 823) | 44.8 (1356) | 0.472 | 0.015 |
| NSAID | 27.8 (15 491) | 21.6 (655) | 0.144 | 0.081 |
| Systemic corticosteroids | 14.1 (7834) | 17.7 (536) | 0.100 | 0.008 |
| Proton pump inhibitors | 28.8 (16 053) | 33.1 (1003) | 0.093 | 0.007 |
LVD indicates left ventricular dysfunction; NSAID, nonsteroidal anti‐inflammatory drug; OAC, oral anticoagulant.
Standardized difference, before and after inverse probability of treatment weighting.
Figure 2 shows cumulative incidence curves of any bleeding complications and thromboembolic events 90 days after sepsis diagnosis for the crude and weighted populations. During the first 90 days after sepsis, crude bleeding rates were higher among patients on warfarin than among nonusers (0.14 versus 0.08; hazard ratio [HR]: 1.76; 95% confidence interval [CI], 1.56–1.99; Table 2, Figure 3). After propensity weighting, differences in 90‐day bleeding rates between warfarin users and nonusers were attenuated, although rates remained higher among warfarin users (0.14 versus 0.12; HR: 1.19; 95% CI, 1.00–1.41; Figure 3).
Figure 2.
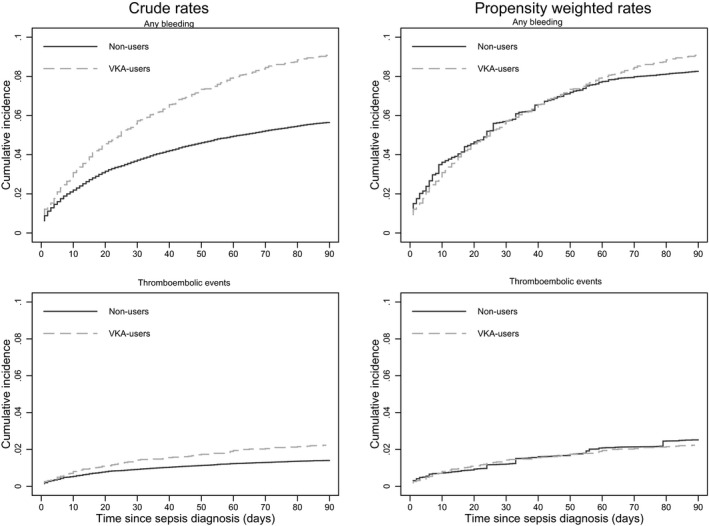
Cumulative incidence curves for the primary end points bleeding and thromboembolic events, according to use of oral anticoagulant therapy. VKA indicates Vitamin K antagonist.
Table 2.
Number of Events and Event Rates Within 90 Days After Sepsis Diagnosis According to Baseline Use of OAC Therapy
| Outcomes | Warfarin Nonusers Without AF | Warfarin Users With AF | ||||
|---|---|---|---|---|---|---|
| Events | Crude Rate | Weighted Rate | Events | Crude Rate | Weighted Rate | |
| Primary outcomes | ||||||
| Any bleeding event | 3067 | 0.08 | 0.12 | 282 | 0.14 | 0.14 |
| Thromboembolic events | 791 | 0.02 | 0.03 | 76 | 0.04 | 0.04 |
| Secondary outcome | ||||||
| All‐cause mortality | 13 484 | 0.33 | 0.66 | 847 | 0.40 | 0.40 |
AF indicates atrial fibrillation; OAC, oral anticoagulant.
Figure 3.
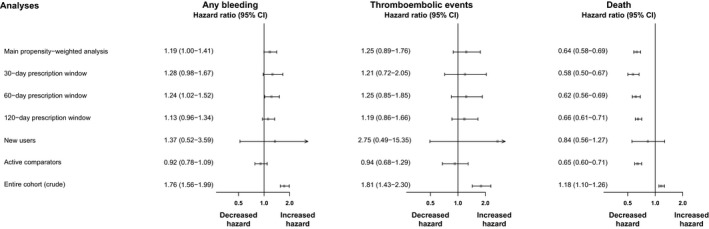
Hazard rates for the association between use of oral anticoagulant therapy and sepsis outcomes. CI indicates confidence interval.
Crude 90‐day rates of ischemic stroke or systemic thromboembolic events were low but higher in warfarin users than in nonusers (0.04 versus 0.02; HR: 1.81; 95% CI, 1.43–2.30; Figure 3). Weighted 90‐day rates were marginally higher among warfarin users versus nonusers (0.04 versus 0.03; HR: 1.25; 95% CI, 0.89–1.76), with the CI including unity. The 90‐day mortality rate was higher among warfarin users compared with nonusers before weighting (0.44 versus 0.33) but was significantly lower following propensity weighting (0.40 versus 0.66 [Table 2]), with a weighted HR of 0.64 (95% CI, 0.58–0.69).
Sensitivity Analyses
Sensitivity analyses using different time windows for ascertaining warfarin usage lead to similar conclusions as the main analyses (Figure 3). The sensitivity analyses restricted to new users of warfarin included 128 warfarin users in the weighted cohorts, and the modest sample size yielded low precision. Accordingly, the estimates were accompanied with very wide CIs. Compared with the main analyses, restriction to new users indicated slightly higher rates of bleeding and thromboembolic events among warfarin users compared with nonusers; however, the estimate of thromboembolic events should be interpreted with caution, given the very few events (only 5). Finally, restricting the comparison cohort to users of other preventive medications did not alter the conclusions of the main analyses. The finding of lower 90‐day mortality among warfarin users versus nonusers remained consistent across all sensitivity analyses (Figure 3).
Discussion
First, among AF patients with an incident sepsis diagnosis, we saw an increase in bleeding rates within 90 days of sepsis diagnosis compared with propensity‐weighted warfarin nonusers without AF. Second, concurrent rates of thromboembolic events were marginally higher among warfarin users versus nonusers. Third, mortality rates during the 90 days after sepsis were lower among AF patients who were taking warfarin compared with warfarin nonusers. These findings persisted through various sensitivity analyses conducted to challenge the robustness of our findings.
Prior studies have shown that patients with severe sepsis are at high risk of developing new‐onset AF,18, 19 which in turn can put them at a higher risk of stroke and death than patients with preexisting AF.20 Nevertheless, there is a paucity of studies on patients with preexisting AF who are taking continuous OACs. In a small study of 115 older adult patients with preexisting AF, Darwish et al5 observed no ischemic strokes and only 3 bleeding events in 35 patients with OACs versus none in patients without OACs. Recently, Walkey et al6 showed an increased risk of in‐hospital bleeding events in AF patients receiving parenteral anticoagulation during sepsis compared with propensity‐matched patients receiving no anticoagulation (8.6% versus 7.2%; relative risk: 1.21; 95% CI, 1.10–1.32). In this study, ≈80% of the patients had preexisting AF, in whom bleeding risk was lower than in patients with new‐onset AF (6.7% versus 12.6%).6 Nonetheless, it is difficult to determine whether this bleeding risk is substantial enough to warrant concern; some excess bleeding among warfarin users would be expected compared with warfarin nonusers. In line with our findings, Walkey et al6 reported comparable rates of thromboembolic events among AF patients with anticoagulation compared with matched comparisons with no anticoagulation.
Experimental studies have shown that coumarin derivatives may be able to blunt sepsis‐induced thrombin formation and disseminated intravascular coagulation.21 Nonetheless, although it would seem tempting to pursue a causal interpretation of the significantly lower mortality rates among warfarin users with AF, caution must be exercised because prevalent user bias could also explain the lower mortality.17 Our study included a substantial number of prevalent warfarin users, and longer term medication usage may be a surrogate for unmeasured factors associated with better prognosis.22 Nonetheless, a sensitivity analysis restricted to new users yielded results that were virtually consistent with the main findings, although CIs were too wide to permit reliable conclusions. Furthermore, restricting the comparison cohort to users of other preventive medications to avoid healthy adherer bias17 also yielded results consistent with the main findings. In addition, the lower mortality in warfarin users could stem from surveillance bias if physicians have a lower threshold for hospitalizing AF patients on warfarin than other patients presenting with signs and symptoms of sepsis. A conservative interpretation of our mortality estimates is that there is no markedly higher mortality among AF patients on warfarin therapy. This observation is clinically important because it suggests that AF patients who are on warfarin at the time of sepsis diagnosis are not at a higher risk of fatal bleeding.
At present, little is known regarding bleeding complications in AF patients using anticoagulation during sepsis. Current guidelines for management of AF do not address sepsis, despite the frequency with which these patients are encountered in clinical practice.7, 23, 24, 25 In the light of this lack, this study may provide support for a randomized trial of anticoagulation in severe sepsis accompanied by AF stratified by newly diagnosed versus prevalent AF.
Although our study has the strength of a large number of observations included in a relatively homogeneous population setting, it also has limitations. Because we used prescription redemption as a proxy for medication usage, some patients might not have been using warfarin therapy at the time of sepsis, for example, because of physician intervention. We lacked information about the international normalized ratio on admission and on how anticoagulation was managed during hospitalization; however, we restricted the study to patients with primary sepsis diagnoses and performed exclusions, which, at least heuristically, should ensure better specificity for community‐acquired sepsis, during which we suspect that patients would likely be taking their usual home medications at the time of admission. Moreover, warfarin has a long half‐life, and when therapy is discontinued, it takes ≈4 days for the international normalized ratio to reach 1.5 in almost all patients.26 We lacked clinical data on sepsis severity, presence of disseminated intravascular coagulation, and platelet counts; however, the mean length of hospital stay and the proportion of patients with severe sepsis or septic shock was comparable among warfarin users and nonusers. In addition, we included a number of frailty predictors in our propensity‐weighted analysis such as cancer, chronic lung disease, prior pneumonia, osteoporosis, dementia, and depression.27, 28 Another potential limitation is our reliance on administrative coding to identify patients with sepsis. According to prior validation studies, hospital diagnoses show good specificity but poor sensitivity for sepsis.29, 30 Finally, there is the possibility of residual and unmeasured confounding, including prevalent user bias and healthy adherer bias, which we addressed in various sensitivity analyses.
In conclusion, in this nationwide cohort study, warfarin therapy in patients with AF during sepsis was associated with increased rates of bleeding complications, comparable rates of thromboembolic events, and significantly lower mortality during the 90 days after sepsis. Nevertheless, in the absence of a randomized trial, we cannot establish whether our findings are attributable to a causal effect, residual confounding, or bias.
Sources of Funding
The Obel Family Foundation partly funded this research by an unrestricted grant. The sponsor had no role the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosures
Associate Professor Larsen has served as an investigator for Janssen Scientific Affairs, LLC and Boehringer Ingelheim, and has been on the speaker bureaus for Bayer, BMS/Pfizer, Roche Diagnostics, Boehringer Ingelheim and Takeda Pharma. Professor Lip has served as a consultant for Bayer/Janssen, BMS/Pfizer, Biotronik, Medtronic, Boehringer Ingelheim, Microlife, and Daiichi‐Sankyo and has been on the speakers bureau for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Micro‐ life, Roche, and Daiichi‐Sankyo. Senior statistician Skjøth has been consultant for Bayer. The remaining authors have no disclosures to report.
Supporting information
Table S1. Definitions on Comorbidity and Concomitant Medication According to the International Classification of Diseases, 10th Revision and ATC (Anatomical Therapeutic Chemical) Codes
Table S2. Risk Score Definitions
(J Am Heart Assoc. 2017;6:e007453 DOI: 10.1161/JAHA.117.007453.)29122810
References
- 1. Angus D, Van Der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. [DOI] [PubMed] [Google Scholar]
- 2. Levi M. Disseminated intravascular coagulation. Crit Care Med. 2007;35:2191–2195. [DOI] [PubMed] [Google Scholar]
- 3. Zeerleder S, Hack CE, Wuillemin WA. Disseminated intravascular coagulation in sepsis. Chest. 2005;128:2864–2875. [DOI] [PubMed] [Google Scholar]
- 4. Zarychanski R, Abou‐Setta AM, Kanji S, Turgeon AF, Kumar A, Houston DS, Rimmer E, Houston BL, McIntyre L, Fox‐Robichaud AE, Hébert P, Cook DJ, Fergusson DA; Canadian Critical Care Trials Group . The efficacy and safety of heparin in patients with sepsis: a systematic review and metaanalysis. Crit Care Med. 2015;43:511–518. [DOI] [PubMed] [Google Scholar]
- 5. Darwish OS, Strube S, Nguyen HM, Tanios MA. Challenges of anticoagulation for atrial fibrillation in patients with severe sepsis. Ann Pharmacother. 2013;47:1266–1271. [DOI] [PubMed] [Google Scholar]
- 6. Walkey AJ, Quinn EK, Winter MR, McManus DD, Benjamin EJ. Practice patterns and outcomes associated with use of anticoagulation among patients with atrial fibrillation during sepsis. JAMA Cardiol. 2016;1:682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walkey AJ, Greiner MA, Heckbert SR, Jensen PN, Piccini JP, Sinner MF, Curtis LH, Benjamin EJ. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165:949–955.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39:30–33. [DOI] [PubMed] [Google Scholar]
- 9. Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39:38–41. [DOI] [PubMed] [Google Scholar]
- 10. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549. [DOI] [PubMed] [Google Scholar]
- 11. Gradel KO, Nielsen SL, Pedersen C, Knudsen JD, Østergaard C, Arpi M, Jensen TG, Kolmos HJ, Schønheyder HC, Søgaard M, Lassen AT; Danish Collaborative Bacteraemia Network, Danish Observational Registry of Infectious Syndromes . No specific time window distinguishes between community‐, healthcare‐, and hospital‐acquired bacteremia, but they are prognostically robust. Infect Control Hosp Epidemiol. 2014;35:1474–1482. [DOI] [PubMed] [Google Scholar]
- 12. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 13. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 14. Austin PC. Some methods of propensity‐score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J. 2009;51:171–184. [DOI] [PubMed] [Google Scholar]
- 15. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Austin PC. A critical appraisal of propensity‐score matching in the medical literature between 1996 and 2003. Stat Med. 2008;27:2037–2049. [DOI] [PubMed] [Google Scholar]
- 17. Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med. 2011;26:546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meierhenrich R, Steinhilber E, Eggermann C, Weiss M, Voglic S, Bögelein D, Gauss A, Georgieff M, Stahl W. Incidence and prognostic impact of new‐onset atrial fibrillation in patients with septic shock: a prospective observational study. Crit Care. 2010;14:R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Annane D, Sébille V, Duboc D, Le Heuzey J‐Y, Sadoul N, Bouvier E, Bellissant E. Incidence and prognosis of sustained arrhythmias in critically ill patients. Am J Respir Crit Care Med. 2008;178:20–25. [DOI] [PubMed] [Google Scholar]
- 20. Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new‐onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306:2248–2254. DOI: 10.1001/jama.2011.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hollenstein U, Homoncik M, Knöbl P, Pernerstorfer T, Graggaber J, Eichler HG, Handler S, Jilma B. Acenocoumarol decreases tissue factor‐dependent coagulation during systemic inflammation in humans. Clin Pharmacol Ther. 2002;71:368–374. [DOI] [PubMed] [Google Scholar]
- 22. Ray WA. Evaluating medication effects outside of clinical trials: new‐user designs. Am J Epidemiol. 2003;158:915–920. [DOI] [PubMed] [Google Scholar]
- 23. Fonarow GC. Exploring the potential benefits and risks of anticoagulation for atrial fibrillation during hospitalization for sepsis. JAMA Cardiol. 2016;1:690–691. [DOI] [PubMed] [Google Scholar]
- 24. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; ACC/AHA Task Force Members . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H‐C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GYH, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 26. White RH, McKittrick T, Hutchinson R, Twitchell J. Temporary discontinuation of warfarin therapy: changes in the international normalized ratio. Ann Intern Med. 1995;122:40–42. [DOI] [PubMed] [Google Scholar]
- 27. Faurot KR, Jonsson Funk M, Pate V, Brookhart MA, Patrick A, Hanson LC, Castillo WC, Stürmer T. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf. 2015;24:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim DH, Schneeweiss S. Measuring frailty using claims data for pharmacoepidemiologic studies of mortality in older adults: evidence and recommendations. Pharmacoepidemiol Drug Saf. 2014;23:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang HE, Addis DR, Donnelly JP, Shapiro NI, Griffin RL, Safford MM, Baddley JW. Discharge diagnoses versus medical record review in the identification of community‐acquired sepsis. Crit Care. 2015;19:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jolley RJ, Sawka KJ, Yergens DW, Quan H, Jetté N, Doig CJ. Validity of administrative data in recording sepsis: a systematic review. Crit Care. 2015;19:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definitions on Comorbidity and Concomitant Medication According to the International Classification of Diseases, 10th Revision and ATC (Anatomical Therapeutic Chemical) Codes
Table S2. Risk Score Definitions


