Abstract
Background
Patients with hypertension with ECG left ventricular hypertrophy (LVH) have higher cardiovascular morbidity and mortality, but single ECG criteria may underestimate risk. Whether continued presence or new development of ECG LVH by 2 criteria can further concentrate risk during blood pressure lowering is unclear.
Methods and Results
Incident stroke, myocardial infarction, cardiovascular death, the composite of these outcomes, and all‐cause mortality were examined in relation to the presence of on‐treatment ECG LVH by Cornell product and/or Sokolow‐Lyon voltage during a mean of 4.8±0.9 years follow‐up in 9193 patients with hypertension randomized to losartan‐ or atenolol‐based regimens. Patients were categorized into 4 groups according to the presence or absence of ECG LVH by each criterion at baseline and yearly during the study. At baseline, LVH by both criteria was present in 960 patients (10.4%). Compared with the absence of ECG LVH by both criteria, persistence or development of ECG LVH by both criteria entered as a time‐varying covariate was associated with >3‐fold increased risks of events in multivariable Cox analyses adjusting for randomized treatment, baseline risk factors, and on‐treatment heart rate and systolic and diastolic blood pressures. Patients with ECG LVH by either Cornell product or Sokolow‐Lyon voltage had 45% to 140% higher risks of all end points.
Conclusions
Persistence or development of ECG LVH by both Cornell product and Sokolow‐Lyon voltage criteria during antihypertensive therapy is associated with markedly increased risks of cardiovascular end points and all‐cause mortality. Further study is indicated to determine whether additional therapy in these patients can reduce their risk.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00338260.
Keywords: ECG criteria, ECG, hypertension, hypertrophy, prognosis
Subject Categories: High Blood Pressure, Hypertension, Electrocardiology (ECG), Mortality/Survival
Clinical Perspective
What Is New?
The combination of 2 different ECG criteria for left ventricular hypertrophy can improve risk stratification compared with either criterion alone.
The persistence or development of ECG left ventricular hypertrophy by both Cornell product and Sokolow‐Lyon voltage was associated with >3‐fold increased risks of myocardial infarction, stroke, cardiovascular mortality, the composite end point of these 3 prior outcomes, and all‐cause mortality after adjusting for other known or suspected predictors of risk.
What Are the Clinical Implications?
The use of both Cornell product and Sokolow‐Lyon voltage together can aid clinicians in risk stratification of patients with hypertension.
These findings further suggest that patients with persistence or development of new left ventricular hypertrophy by both criteria might benefit from additional therapy aimed at regressing their left ventricular hypertrophy, but further study of this issue is needed.
Left ventricular hypertrophy (LVH) detected by 12‐lead ECG1, 2, 3 and by echocardiography4, 5, 6, 7, 8 are common manifestations of preclinical cardiovascular disease that strongly predict cardiovascular morbidity and mortality. Antihypertensive therapy aimed at reducing blood pressure (BP) can produce regression of LVH,3, 4, 9, 10, 11, 12, 13, 14, 15 and regression of ECG LVH and prevention of progression to LVH have been associated with a reduced risk of cardiovascular morbidity and mortality.2, 3, 12, 13, 16, 17, 18, 19, 20 Importantly, the improved prognosis with regression of ECG LVH is independent of reductions in BP during antihypertensive therapy.16, 17, 18, 19, 20 This increased risk associated with failure to regress LVH highlights the importance of better identifying patients who remain at residual risk despite aggressive BP lowering.16, 17, 18, 19, 20
The well‐recognized limited sensitivity of any one ECG LVH criterion as compared with imaging modalities has been put forward as a limitation of ECG‐dependent approaches to LVH diagnosis,21 despite findings that imaging and ECG methods appear to similarly track prognosis17, 21, 22 and may provide complimentary information.21, 23, 24, 25, 26, 27, 28 Based on the limited sensitivity of single ECG criteria, investigators have demonstrated that using both Cornell product (CP) and Sokolow‐Lyon voltage (SL) duration criteria together can increase sensitivity and population prevalence for detection of echocardiographic LVH, albeit with some loss of specificity29, 30 and that the presence of both of these criteria on ECG was associated with higher left ventricular (LV) mass index and a greater prevalence of echocardiographic LVH than either criterion alone or neither.31 The finding that different ECG LVH criteria identify different populations of patients at potentially increased risk16, 29, 30, 31 and the additive value of ECG and imaging‐based LVH for risk stratification21, 23, 24, 25, 26, 27, 28 suggests an opportunity to better track risk by combining 2 ECG LVH criteria with complimentary prognostic power. In this scenario, it might be predicted that the mutual absence of LVH by both criteria would be associated with the lowest risk, the presence of LVH by both criteria with the highest risk, and the presence of LVH by one or the other with intermediate risk. The LIFE (Losartan Intervention for Endpoint Reduction) study17, 18, 19, 20 recruited patients with ECG LVH by either CP and/or SL before enrollment, tracked the magnitude of ECG LVH by both criteria throughout follow‐up, and demonstrated that on‐treatment CP and SL criteria separately predicted cardiovascular risk but did not examine whether combining the 2 ECG LVH criteria could improve risk stratification. Therefore, the present post hoc analysis of data from the LIFE study was undertaken to examine whether the continued presence or new development of ECG LVH by both CP and SL was associated with increased risk of cardiovascular events and cardiovascular and all‐cause mortality compared with continued absence or regression of ECG LVH by both of these criteria, and whether the presence or development of LVH by either CP or SL is associated with intermediate elevation of risk.
Methods
Patients
The LIFE study17, 18, 19, 20, 32 enrolled 9193 patients with hypertension who had ECG LVH by CP33 and/or SL criteria34 on a screening ECG in a prospective, double‐blind randomized study that compared cardiovascular morbidity and mortality with use of losartan‐ as opposed to atenolol‐based treatment. The study was approved by all ethics committees concerned. As described in detail elsewhere,17, 18, 19, 20, 32 eligible patients for LIFE were men and women aged 55 to 80 years with previously untreated or treated essential hypertension with mean seated BP in the range of 160 to 200/95 to 115 mm Hg after 1 and 2 weeks on placebo who had not had a myocardial infarction (MI) or stroke within 6 months and did not require treatment with a β‐blocker, angiotensin‐converting enzyme inhibitor or angiotensin II type 1 receptor antagonist. Institutional review board approval was obtained and all participants gave informed written consent.
Treatment Regimens
Blinded treatment was begun with losartan 50 mg or atenolol 50 mg daily and matching placebo of the other agent, with a target BP of ≤140/90 mm Hg. During clinic visits at frequent intervals for the first 6 months and at 6‐month intervals thereafter, study therapy could be uptitrated by addition of hydrochlorothiazide 12.5 mg, followed by an increase in blinded losartan or atenolol to 100 mg daily. In patients whose BP was still not controlled, additional open‐label upward titration of hydrochlorothiazide and, if necessary, institution of therapy with a calcium channel blocker or additional other medications (excluding angiotensin II type 1 receptors or β‐blockers or angiotensin‐converting enzyme inhibitors) was added to the double‐blind treatment regimen.17, 18, 19, 20, 32
Electrocardiography
ECGs were obtained at study baseline, at 6 months, and at yearly follow‐up intervals until study termination or patient death. ECGs were interpreted at the Core Laboratory at Sahlgrenska University Hospital/Östra in Göteborg, Sweden, by experienced readers blinded to clinical information. QRS duration was measured to the nearest 4 ms and the QRS amplitudes to the nearest 0.5 mm (0.05 mV). The product of QRS duration times the CP combination (RaVL+SV3, with 6 mm added in women32, 33) >2440 mm·ms or SL (SV1+RV5/6) >38 mm were used to identify LVH.17, 18, 19, 20, 32, 33, 34 A sex adjustment of 6 mm, as opposed to the originally proposed 8 mm,17 was employed based on studies published when the LIFE study was getting started, suggesting that a higher threshold in women was necessary to maintain specificity.35, 36
End Point Determination
The LIFE study used a composite end point of cardiovascular death, nonfatal MI, or nonfatal stroke, according to previously defined criteria.32 These end points and the secondary end point of all‐cause mortality were ascertained and then verified by an expert end point committee who were blinded to ECG results when classifying possible morbid events.17, 18, 19, 20, 32
Statistical Analyses
Data management and analysis were performed with SPSS version 22 software (IBM). Data are presented as mean±SD for continuous variables and proportions for categorical variables. Patients were classified into 4 groups according to the presence or absence of ECG LVH by both CP and SL. Differences in prevalence between groups were compared using chi‐square analyses, and mean values of continuous variables were compared using 1‐way ANOVA.
Event rates in relation to the presence or absence of LVH by CP and SL at baseline were calculated from Kaplan–Meier survival estimates. The relation of the presence or absence of LVH by CP and/or SL to the risk of events was assessed using Cox proportional hazards models, with baseline and subsequent determinations of the presence or absence of LVH by CP and SL entered as time‐varying covariates.17, 18, 19, 20 Initial analyses were performed with CP and SL LVH as separate variables in univariate and multivariate Cox models and then with LVH classified by these variables into 4 groups, with the group with no LVH by either criterion serving as the reference group. Baseline risk factors and a treatment group indicator were included as standard covariates, and baseline and subsequent systolic and diastolic BPs and heart rate measurements were entered as time‐varying covariates. The 95% confidence interval (CI) of each hazard ratio (HR) was calculated from the estimated coefficients and their standard errors. The relationship of the combination of CP and SL LVH to the composite end point was further examined in relevant subgroups of the study population using the same multivariable Cox analysis as noted above and differences in the predictive value between subgroups tested by examining the interaction between the combined LVH variable and each subgroup variable in the overall population. For all tests, a 2‐tailed P<0.05 was required for statistical significance.
Results
At baseline, LVH by both criteria was present in 960 patients (10.4%). Baseline clinical and demographic characteristics of patients in relationship to the presence or absence of ECG LVH by CP and SL at study baseline are shown in Table 1. Patients across ECG LVH groups differed significantly with respect to age, sex, race, prevalent diabetes mellitus, history of ischemic heart disease, arrhythmia, stroke, peripheral vascular disease, smoking, prior antihypertensive treatment, body mass index, serum glucose, creatinine, total and high‐density lipoprotein cholesterol levels, and urine albumin/creatinine ratio. Compared with patients without LVH by either criterion, patients with ECG LVH by both criteria tended to be older, were less likely to be women, have diabetes mellitus, and received prior antihypertensive therapy. They were more likely to be black and a current smoker; have a history of ischemic heart disease, arrhythmia, stroke, and peripheral vascular disease; and had lower body mass index and serum glucose levels and higher serum creatinine and urine albumin/creatinine ratios.
Table 1.
Study Baseline Demographic and Clinical Characteristics in Relation to the Presence or Absence of ECG LVH by Both CP and SL at Baseline
| Variables | CP−/SL− (n=2023) | CP+/SL− (n=5220) | CP−/SL+ (n=990) | CP+/SL+ (n=960) | P Value |
|---|---|---|---|---|---|
| Age, y | 66.1±7.1 | 67.2±7.0 | 66.5±7.0 | 67.7±6.8 | <0.001 |
| Female sex, % | 56.7 | 58.4 | 32.5 | 46.0 | <0.001 |
| Black race, % | 5.3 | 4.0 | 13.6 | 8.5 | <0.001 |
| Diabetes mellitus, % | 11.6 | 14.5 | 10.1 | 10.4 | <0.001 |
| History of ischemic heart disease, % | 13.0 | 16.4 | 18.0 | 17.9 | <0.001 |
| History of myocardial infarction, % | 5.2 | 6.6 | 6.3 | 6.1 | 0.214 |
| History of arrhythmia, % | 5.2 | 7.0 | 8.0 | 9.8 | <0.001 |
| History of stroke, % | 3.6 | 4.3 | 5.7 | 5.2 | 0.031 |
| History of heart failure, % | 0.8 | 2.1 | 1.1 | 3.1 | <0.001 |
| History of peripheral vascular disease, % | 5.7 | 5.3 | 5.7 | 7.6 | 0.043 |
| Current smoker, % | 17.4 | 13.8 | 23.1 | 20.7 | <0.001 |
| Prior antihypertensive treatment, % | 71.8 | 73.4 | 67.9 | 70.9 | 0.003 |
| Randomized treatment (% losartan) | 50.5 | 50.0 | 49.2 | 50.8 | 0.870 |
| Body mass index, kg/m2 | 27.9±4.7 | 28.7±4.8 | 25.5±3.8 | 26.8±4.6 | <0.001 |
| Serum glucose, mmol/L | 5.90±2.03 | 6.14±2.31 | 5.80±1.99 | 5.87±2.02 | <0.001 |
| Total cholesterol, mmol/L | 6.03±1.12 | 6.08±1.13 | 5.85±1.08 | 6.05±1.15 | <0.001 |
| HDL cholesterol, mmol/L | 1.50±0.43 | 1.48±0.43 | 1.54±0.45 | 1.51±0.44 | <0.001 |
| Creatinine, μg/mmol/L | 85.4±19.7 | 85.8±19.4 | 92.3±23.0 | 90.7±21.0 | <0.001 |
| UACR, mg/mmol/L | 4.7±16.1 | 8.0±38.8 | 9.7±38.7 | 9.2±28.6 | <0.001 |
CP indicates Cornell product; HDL, high‐density lipoprotein; LVH, left ventricular hypertrophy; SL, Sokolow‐Lyon voltage; UACR, urine albumin/creatinine ratio.
BP and ECG LVH measurements at baseline and changes in these measurements between baseline and last in‐study determination in relation to the presence or absence of ECG LVH by CP and SL at study baseline are shown in Table 2. Patients across ECG LVH groups differed significantly with respect to all baseline BP and ECG measurements and for all changes in these measurements, with the exception of change in heart rate. Baseline levels of QRS duration, CP, and SL varied, as would be predicted by group definition. Changes in systolic and diastolic BPs were greatest in patients with ECG LVH by both criteria, while regression of ECG LVH during treatment was higher in groups defined by the presence of LVH by that criterion at baseline, and greatest among patients with ECG LVH by both criteria at study baseline.
Table 2.
Study Baseline and Change From Study Baseline to Last In‐Study Measurement of BP, ECG LVH, QRS Duration, and Heart Rate in Relation to the Presence or Absence of ECG LVH by Both CP and SL at Baseline
| Variables | CP−/SL− (n=2023) | CP+/SL− (n=5220) | CP−/SL+ (n=990) | CP+/SL+ (n=960) | P Value |
|---|---|---|---|---|---|
| Baseline measurements | |||||
| Systolic BP, mm Hg | 172±14 | 174±14 | 176±14 | 176±143 | <0.001 |
| Diastolic BP, mm Hg | 98±8 | 98±9 | 97±9 | 98±9 | <0.001 |
| CP, mm·ms | 2065±393 | 3212±897 | 1715±518 | 3454±1239 | <0.001 |
| SL, mm | 26.4±7.3 | 25.7±6.8 | 44.9±5.9 | 45.3±6.4 | <0.001 |
| QRS duration, ms | 92.8±11.5 | 105.7±19.5 | 96.6±9.4 | 103.8±18.5 | <0.001 |
| Heart rate, beats per min | 74.1±11.2 | 74.2±11.1 | 72.2±10.7 | 73.0±11.3 | <0.001 |
| Change from baseline to last measurement | |||||
| Systolic BP, mm Hg | −27.7±18.6 | −29.4±19.4 | −30.8±20.2 | −31.3±21.4 | <0.001 |
| Diastolic BP, mm Hg | −16.8±10.1 | −17.2±10.2 | −16.8±10.6 | −17.8±11.0 | <0.001 |
| CP, mm·ms | 13±659 | −270±852 | −13±790 | −428±1140 | <0.001 |
| SL, mm | −2.5±6.0 | −2.8±6.5 | −7.6±8.4 | −8.6±9.1 | <0.001 |
| QRS duration, ms | 2.7±11.5 | 1.5±12.3 | 1.4±13.1 | 1.4±13.2 | <0.001 |
| Heart rate, beats per min | −4.8±12.7 | −5.3±13.0 | −5.1±12.6 | −4.2±12.7 | 0.091 |
BP indicates blood pressure; CP, Cornell product; LVH, left ventricular hypertrophy; SL, Sokolow‐Lyon voltage.
During mean follow‐up of 4.8±0.9 years, MI occurred in 386 patients (4.2%); stroke in 541 patients (5.9%); cardiovascular death in 438 patients (4.8%); the LIFE composite end point of cardiovascular death, MI, or stroke in 1096 patients (11.9%); and all‐cause mortality in 814 patients (8.9%). Univariate and multivariable Cox analyses for the prediction of these end points by CP or SL LVH separately are shown in Table 3. Compared with the absence of LVH by each criterion, the presence of LVH by either SL or CP was associated with 39% to 128% increased unadjusted risks of these outcomes and from 14% to 69% increased adjusted risk of all outcomes in multivariable Cox models that adjusted for other risk factors.
Table 3.
Univariate and Mulitvariable Cox Models for the Prediction of Outcomes According to On‐Treatment LVH by Either CP or SL Treated as Time‐Dependent Covariates
| Outcomes | CP LVH | SL LVH | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Univariate | ||||||
| MI | 1.53 | 1.25–1.88 | <0.001 | 1.62 | 1.25–2.10 | <0.001 |
| Stroke | 1.44 | 1.21–1.71 | <0.001 | 2.28 | 1.87–2.10 | <0.001 |
| Cardiovascular death | 1.80 | 1.48–2.19 | <0.001 | 2.11 | 1.69–2.64 | <0.001 |
| Composite end point | 1.52 | 1.34–1.71 | <0.001 | 2.00 | 1.73–2.31 | <0.001 |
| All‐cause mortality | 1.39 | 1.21–1.60 | <0.001 | 2.05 | 1.74–2.43 | <0.001 |
| Multivariablea | ||||||
| MI | 1.28 | 1.05–1.49 | 0.014 | 1.33 | 1.14–1.55 | 0.009 |
| Stroke | 1.21 | 1.07–1.34 | 0.010 | 1.69 | 1.35–2.11 | <0.001 |
| Cardiovascular death | 1.37 | 1.11–1.70 | 0.004 | 1.53 | 1.19–1.98 | 0.001 |
| Composite end point | 1.17 | 1.03–1.34 | 0.021 | 1.50 | 1.27–1.76 | <0.001 |
| All‐cause mortality | 1.14 | 1.04–1.33 | 0.017 | 1.57 | 1.30–1.89 | <0.001 |
CI indicates confidence interval; CP, Cornell product; HR, hazard ratio; LVH, left ventricular hypertrophy; SL, Sokolow‐Lyon voltage.
Adjusted for randomized treatment, age, sex, prevalent diabetes mellitus, history of stroke, myocardial infarction (MI), ischemic heart disease, heart failure, peripheral vascular disease or prior antihypertensive treatment, baseline serum cholesterol, high‐density lipoprotein cholesterol, glucose and creatinine, and urine albumin/creatinine ratio treated as standard covariates, and on‐treatment heart rate and diastolic and systolic blood pressure treated as time‐varying covariates.
Compared with each LVH criterion alone (Table 3), the combination of CP and SL further concentrates the risk of all end points (Figures 1 and 2). Rates of each outcome in relation to the presence or absence of LVH by CP and SL at study baseline are shown in Figure 1. For all outcomes, event rates varied significantly across groups and were lowest in patients who did not have LVH, intermediate in patients with LVH by either CP or SL, and highest in patients with LVH by both criteria, with 2.5‐ to 3.5‐fold higher event rates among patients with LVH by both criteria than in those without ECG LVH by either.
Figure 1.
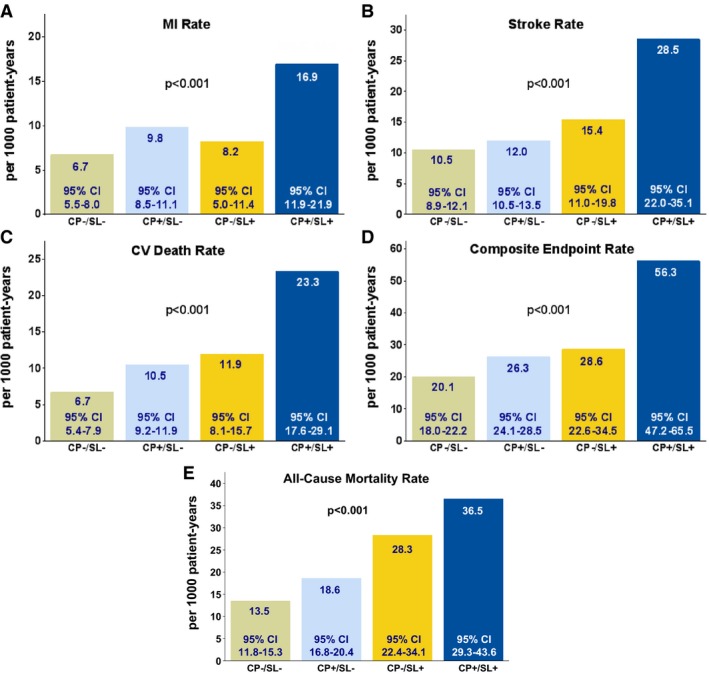
Rates of myocardial infarction (MI, Panel A), stroke (Panel B), cardiovascular death (Panel C), the composite end point (Panel D), and all‐cause mortality (Panel E) in relation to the presence or absence of ECG left ventricular hypertrophy by both Cornell product (CP) and Sokolow‐Lyon voltage (SL) at study baseline. CI indicates confidence interval; CV, cardiovascular.
Figure 2.

Risk of myocardial infarction (Panel A), stroke (Panel B), cardiovascular death (Panel C), the composite end point (Panel D), and all‐cause mortality (Panel E) in relation to the on‐treatment presence or absence of ECG left ventricular hypertrophy by both Cornell product (CP) and Sokolow‐Lyon voltage (SL) treated as time‐varying covariates in Cox analyses. *Adjusted for randomized treatment, age, sex, prevalent diabetes mellitus, history of stroke, myocardial infarction, ischemic heart disease, heart failure, peripheral vascular disease or prior antihypertensive treatment, baseline serum cholesterol, high‐density lipoprotein cholesterol, glucose and creatinine, and urine albumin/creatinine ratio treated as standard covariates, and on‐treatment heart rate and diastolic and systolic blood pressure treated as time‐varying covariates. CI indicates confidence interval.
The risk of cardiovascular morbidity and cardiovascular and all‐cause mortality in relation to the on‐treatment presence or absence of ECG LVH by both CP and SL treated as time‐varying covariates is shown in Figure 2. In univariate Cox analyses, the persistence or development of new ECG LVH was associated with significantly higher risks of all events: compared with the absence of LVH by both criteria, the presence of LVH by both CP and SL was associated with between 4.71‐ and 5.80‐fold increased risks of cardiovascular events, cardiovascular mortality and all‐cause mortality and the presence of LVH by one or the other criteria with intermediate increased risks of events. After controlling for randomized treatment with losartan or atenolol, age, sex, prevalent diabetes mellitus, history of stroke, MI, ischemic heart disease, heart failure, peripheral vascular disease or prior antihypertensive treatment, baseline serum cholesterol, high‐density lipoprotein cholesterol, glucose and creatinine, and urine albumin/creatinine ratio treated as standard covariates, and on‐treatment heart rate and diastolic and systolic BPs treated as time‐varying covariates, on‐treatment presence of ECG LVH by either criterion remained associated with between 45% and 140% increased risk of events and the presence of ECG LVH by both criteria was associated with a >3‐fold adjusted risk of all outcomes. There were no significant differences in the predictive value of the combination of CP and SL for the composite end point in subgroups of the LIFE study population stratified by sex, race, age, history of ischemic heart disease, diabetes mellitus, or randomized treatment allocation to losartan versus atenolol (Table 4).
Table 4.
Multivariable Cox Analyses to Assess the Predictive Value of the Combination of On‐Treatment CP and SL for the LIFE Study Composite End Point in Relevant Subgroups of the Study Population
| Subgroup | Composite End Point, No. | HR | 95% CI | P Value for Interaction |
|---|---|---|---|---|
| Sex | 0.190 | |||
| Female (n=4963) | 476 | |||
| CP+/SL− | 1.44 | 1.12–1.84 | ||
| CP−/SL+ | 1.62 | 1.02–2.57 | ||
| CP+/SL+ | 3.02 | 2.18–4.18 | ||
| Male (n=4230) | 620 | |||
| CP+/SL− | 1.44 | 1.17–1.78 | ||
| CP−/SL+ | 1.99 | 1.49–2.66 | ||
| CP+/SL+ | 3.13 | 2.41–4.06 | ||
| Race | 0.154 | |||
| White/other (n=8660) | 1021 | |||
| CP+/SL− | 1.44 | 1.22–1.70 | ||
| CP−/SL+ | 1.82 | 1.40–2.37 | ||
| CP+/SL+ | 3.20 | 2.60–3.95 | ||
| Black (n=533) | 75 | |||
| CP+/SL− | 1.69 | 0.84–3.40 | ||
| CP−/SL+ | 1.80 | 0.81–3.95 | ||
| CP+/SL+ | 1.87 | 0.79–4.42 | ||
| Age | 0.481 | |||
| <65 y (n=3489) | 250 | |||
| CP+/SL− | 1.52 | 1.11–2.08 | ||
| CP−/SL+ | 1.90 | 1.15–3.14 | ||
| CP+/SL+ | 4.60 | 3.08–6.88 | ||
| ≥65 y (n=5704) | 846 | |||
| CP+/SL− | 1.52 | 1.26–1.82 | ||
| CP−/SL+ | 2.03 | 1.53–2.69 | ||
| CP+/SL+ | 2.98 | 2.36–3.77 | ||
| History of ischemic heart disease | 0.969 | |||
| No (n=7724) | 802 | |||
| CP+/SL− | 1.46 | 1.22–1.75 | ||
| CP−/SL+ | 1.76 | 1.32–2.35 | ||
| CP+/SL+ | 3.06 | 2.42–3.87 | ||
| Yes (n=1469) | 294 | |||
| CP+/SL− | 1.48 | 1.04–2.10 | ||
| CP−/SL+ | 2.45 | 1.52–3.96 | ||
| CP+/SL+ | 3.50 | 2.32–5.27 | ||
| Diabetes mellitus | 0.140 | |||
| No (n=7998) | 854 | |||
| CP+/SL− | 1.45 | 1.21–1.73 | ||
| CP−/SL+ | 1.64 | 1.24–2.17 | ||
| CP+/SL+ | 3.02 | 2.40–3.79 | ||
| Yes (n=1195) | 242 | |||
| CP+/SL− | 1.53 | 1.05–2.21 | ||
| CP−/SL+ | 3.40 | 2.03–5.68 | ||
| CP+/SL+ | 3.44 | 2.17–5.45 | ||
| Randomized treatment | 0.209 | |||
| Atenolol (n=4558) | 588 | |||
| CP+/SL− | 1.36 | 1.09–1.70 | ||
| CP−/SL+ | 1.45 | 1.01–2.07 | ||
| CP+/SL+ | 2.62 | 1.98–3.47 | ||
| Losartan (n=4605) | ||||
| CP+/SL− | 508 | 1.55 | 1.23–1.96 | |
| CP−/SL+ | 2.44 | 1.74–3.41 | ||
| CP+/SL+ | 3.78 | 2.82–5.08 |
CI indicates confidence interval; CP, Cornell product; HR, hazard ratio; LIFE, Losartan Intervention For Endpoint Reduction; SL, Sokolow‐Lyon voltage.
Discussion
Previous work provides a conceptual framework for combining ECG LVH criteria to concentrate risk. A number of studies, including in the LIFE population, have demonstrated that serial assessment of either SL or CP can stratify risk of cardiovascular outcomes and cardiovascular and all‐cause mortality.16, 17, 18, 19, 20 However, none of these earlier studies analyzed whether combining these 2 criteria could further concentrate risk. Based on the limited sensitivity of single ECG LVH criteria, which utilize QRS voltage or voltage duration product measurements, several studies have demonstrated that defining ECG LVH by the presence of either increased CP or SL increased ECG sensitivity and population prevalence of LVH, but at the cost of lower specificity.29, 30 In analyses of baseline echocardiographic data from the LIFE study,31 persistence of ECG LVH by both CP and SL on ECGs performed at study baseline was associated with increased LV chamber volume and wall thickness, higher LV mass, and increased prevalences of echocardiographic LVH and abnormal LV geometry compared with the absence of ECG LVH by both criteria. Indeed, after adjusting for the possible effects of age, sex, race, baseline systolic BP, and body mass index on LV mass, the presence of ECG LVH by both CP and SL was associated with a >4‐fold increased odds of echocardiographic LVH.31 Paralleling outcome findings in the current study, the presence of ECG LVH by either CP or SL alone was associated with smaller increases in LV mass, abnormal LV geometry, and echocardiographic LVH.31
Further supporting the construct of using 2 different ECG LVH criteria together, combinations of ECG LVH with imaging‐derived LV mass determinations21, 28 or QT prolongation37 carry higher risk than either finding alone. In an analysis of 4748 participants in MESA (Multi‐Ethnic Study of Atherosclerosis),21 only the presence of LVH on both ECG (by either CP or SL) and cardiac MRI remained significantly associated with an increased risk of a composite end point of cardiovascular disease events after adjusting for other risk factors (HR, 1.77; 95% CI, 1.03–3.04), whereas neither LVH by ECG alone (HR, 1.20; 95% CI, 0.69–2.09) or on MRI alone (HR, 1.38; 95% CI, 0.98–1.96) were significantly associated with increased risk in their fully adjusted Cox model. Similarly, in patients with hypertension in the echocardiographic substudy of LIFE,28 the incidence of hospitalization for new‐onset heart failure was markedly higher in patients with LVH on both echocardiography and ECG (by either CP or SL) (4.9%) compared with patients with echocardiographic LVH only (2.2%), ECG LVH only (0.6%), or LVH on neither test (0%, P<0.01). After controlling for other heart failure risk factors in this population, the presence of LVH on both ECG and echocardiogram remained associated with a >3‐fold increased risk of new heart failure (HR, 3.60; 95% CI, 1.24–10.49). Lastly, among 7506 participants in NHANES III (US Third National Health and Nutrition Examination Survey), Soliman et al37 found that after adjusting for other risk factors, risk of all‐cause mortality was highest in the group with concomitant ECG LVH by CP and a prolonged heart rate–adjusted QT interval (HR, 1.63; 95% CI, 1.12–2.36) with intermediate adjusted mortality risks in those with isolated ECG LVH (HR, 1.48; 95% CI, 1.24–1.77) and those with an isolated long QT interval (HR, 1.27; 95% CI, 1.12–1.46).
The current study builds on the above findings and concepts and on the well‐established prognostic value of serial assessment of ECG LVH2, 3, 12, 13, 16, 17, 18, 19, 20 to demonstrate that the combination of 2 different ECG LVH criteria can dramatically concentrate risk in patients with hypertension undergoing treatment compared with either criterion alone. Taking advantage of the complimentary information provided by SL and CP criteria, these findings suggest that the persistence or development of ECG LVH by both criteria in the face of substantial BP lowering can identify patients who remain at >3‐fold increased adjusted risk of cardiovascular morbidity and cardiovascular or all‐cause mortality. In contrast, regression or absence of ECG by both criteria was associated with the lowest event rates over time, whereas persistence or development of LVH by either CP or SL, but not the other, was associated with intermediate risks of adverse outcomes (Figure 2). The high residual risk associated with persistence or development of ECG LVH by both criteria despite attaining similar levels of on‐treatment BP (Table 2) suggests that persistence of LVH by both criteria may identify a subgroup of patients with hypertension in whom more aggressive BP lowering could possibly improve their prognosis. However, the significantly increased risks of cardiovascular death, stroke, and LIFE study composite end point in patients with persistent LVH by CP criteria despite having average on‐treatment systolic BP ≤130 mm Hg38 raises the possibility that additional BP lowering in patients who do not adequately regress LVH may not improve prognosis. In contrast, Soliman et al39, 40 demonstrated that more intensive BP reduction was associated with greater LVH regression and lower rates of developing new LVH among patients with hypertension and diabetes mellitus in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) study39 and patients with diabetes mellitus in SPRINT (Systolic Blood Pressure Intervention Trial),40 but that the greater LVH regression in SPRINT did not appear to explain most of the reduction in cardiovascular events. Further study will be required to evaluate whether specifically targeting patients with persistent LVH to further reduce BP and produce regression of LVH can improve prognosis in this high‐risk subgroup of patients with hypertension.
Study Limitations
There are several limitations of the current study that warrant mention. First, this is a post hoc analysis of data from a randomized treatment trial that compared 2 different treatment approaches to BP reduction. Although determining the relationship of ECG LVH changes with treatment to outcome was a planned secondary analysis of the LIFE study,17 evaluation of the combination of SL and CP criteria was not a prespecified analysis. Because use of ECG LVH criteria to select patients for LIFE32 increased the baseline risk of the population, caution should be used in generalizing these findings to patients with hypertension at lower risk or those younger than 55 years. However, ECG LVH has demonstrated significant risk stratification in populations that have varied degrees of baseline risk and baseline prevalence of ECG LVH,1, 2, 3, 12, 13, 21, 23, 24, 25, 26, 27 suggesting that these findings are likely to apply in other, lower‐risk hypertensive populations.
Conclusions
These findings have important implications for the management of patients with hypertension. First, these observations suggest that serial assessment of both CP and SL can improve risk stratification in patients with hypertension during treatment. More intriguingly, the residual high risk associated with persistence or development of ECG LVH by both criteria suggest that these patients might benefit from additional therapy aimed at further lowering their BP and reducing their ECG LVH to reduce risk. Further study is clearly warranted to determine whether combining ECG LVH criteria similarly concentrates risk in other patient populations and under other treatment conditions and whether therapy targeted at regression of ECG LVH in patients with persistence of ECG LVH by both criteria can reduce risk or whether this risk marker identifies a subset of patients with hypertension whose prognosis is less likely to improve despite adequate BP reduction.
Disclosures
Ms Hille is employed by Merck & Co, Inc. Dr Kjeldsen has received modest honoraria from Abdi Ibrahim and MSD for lectures, and modest honoraria from Bayer and Takeda for lecturing and/or consulting. Dr Devereux has consulted for Merck & Co, Inc. Dr Okin has no disclosures.
(J Am Heart Assoc. 2017;6:e007564 DOI: 10.1161/JAHA.117.007564.)29151037
References
- 1. Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, Porcellati C. Prognostic value of a new electrocardiographic method for diagnosis of left ventricular hypertrophy. J Am Coll Cardiol. 1998;31:383–390. [DOI] [PubMed] [Google Scholar]
- 2. Levy D, Salomon M, D'Agostino RB, Belanger AJ, Kannel WB. Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation. 1994;90:1786–1793. [DOI] [PubMed] [Google Scholar]
- 3. Mathew J, Sleight P, Lonn E, Johnstone D, Pogue J, Yi Q, Bosch J, Sussex B, Probstfield J, Yusuf S; Heart Outcomes Prevention Evaluation (HOPE) Investigators . Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin‐converting enzyme inhibitor ramipril. Circulation. 2001;104:1615–1621. [DOI] [PubMed] [Google Scholar]
- 4. Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, Reboldi G, Porcellati C. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation. 1998;97:48–54. [DOI] [PubMed] [Google Scholar]
- 5. Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352. [DOI] [PubMed] [Google Scholar]
- 6. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. [DOI] [PubMed] [Google Scholar]
- 7. Liao Y, Cooper RS, McGee DL, Mensah GA, Ghali JK. The relative effects of left ventricular hypertrophy, coronary artery disease and ventricular dysfunction on survival among black adults. JAMA. 1995;273:1592–1597. [PubMed] [Google Scholar]
- 8. Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension. 2000;35:580–586. [DOI] [PubMed] [Google Scholar]
- 9. Dahlöf B, Pennert K, Hannson L. Reversal of left ventricular hypertrophy in hypertensive patients: a meta‐analysis of 109 treatment studies. Am J Hypertens. 1992;5:95–110. [DOI] [PubMed] [Google Scholar]
- 10. Schlaich MP, Schmieder RE. Left ventricular hypertrophy and its regression: pathophysiology and therapeutic approach: focus on treatment by antihypertensive agents. Am J Hypertens. 1998;11:1394–1404. [DOI] [PubMed] [Google Scholar]
- 11. Neaton JD, Grimm RH, Prineas RJ, Stamler J, Grandits GA, Elmer PJ, Cutler JA, Flack JM, Schoenberger JA, McDonald R; for the Treatment of Mild Hypertension Study group . Treatment of mild hypertension study: final results. JAMA. 1993;270:713–724. [PubMed] [Google Scholar]
- 12. Hypertension Detection and Follow‐up Program Cooperative Group . Five year findings of the hypertension detection and follow‐up program: prevention and reversal of left ventricular hypertrophy with antihypertensive drug therapy. Hypertension. 1985;7:105–112. [PubMed] [Google Scholar]
- 13. Prineas RJ, Rautaharju PM, Grandits G, Crow R; for the MRFIT Research Group . Independent risk for cardiovascular disease predicted by modified continuous score electrocardiographic criteria for 6‐year incidence and regression of left ventricular hypertrophy among clinically disease free men: 16‐year follow‐up for the multiple risk factor intervention trial. J Electrocardiol. 2001;34:91–101. [DOI] [PubMed] [Google Scholar]
- 14. Devereux RB, Palmieri V, Liu JE, Wachtell K, Bella JN, Boman K, Gerdts E, Nieminen MS, Papademetriou V, Dahlöf B. Progressive hypertrophy regression with sustained pressure reduction in hypertension: the Losartan Intervention For Endpoint Reduction study. J Hypertens. 2002;20:1445–1450. [DOI] [PubMed] [Google Scholar]
- 15. Okin PM, Devereux RB, Liu JE, Oikarinen L, Jern S, Kjeldsen SE, Julius S, Wachtell K, Nieminen MS, Dahlöf B. Regression of electrocardiographic left ventricular hypertrophy predicts regression of echocardiographic left ventricular mass: the LIFE Study. J Hum Hypertens. 2004;18:403–409. [DOI] [PubMed] [Google Scholar]
- 16. Salles GF, Cardoso CRL, Fiszman R, Muxfeldt E. Prognostic impact of baseline and serial changes in electrocardiographic left ventricular hypertrophy in resistant hypertension. Am Heart J. 2010;159:833–840. [DOI] [PubMed] [Google Scholar]
- 17. Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, Snapinn S, Harris KE, Aurup P, Edelman JM, Wedel H, Lindholm LH, Dahlöf B. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and prediction of major cardiovascular events: the LIFE Study. JAMA. 2004;292:2343–2349. [DOI] [PubMed] [Google Scholar]
- 18. Okin PM, Devereux RB, Harris KE, Jern S, Kjeldsen SE, Julius S, Edelman JM, Dahlöf B; for the LIFE Study Investigators . Reduction of electrocardiographic left ventricular hypertrophy is associated with decreased heart failure hospitalization in hypertensive patients. Ann Intern Med. 2007;147:311–319. [DOI] [PubMed] [Google Scholar]
- 19. Okin PM, Wachtell K, Devereux RB, Harris KE, Jern S, Kjeldsen SE, Julius S, Lindholm L, Nieminen MS, Edelman JM, Dahlöf B. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new‐onset atrial fibrillation: the LIFE Study. JAMA. 2006;296:1242–1248. [DOI] [PubMed] [Google Scholar]
- 20. Wachtell K, Okin PM, Olsen MH, Dahlöf B, Devereux RB, Ibsen H, Kjeldsen SE, Lindholm LH, Nieminen MS, Thygesen K. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and reduction in sudden cardiac death: the LIFE Study. Circulation. 2007;116:700–705. [DOI] [PubMed] [Google Scholar]
- 21. Bacharova L, Chen H, Estes EH, Mateasik A, Bluemke DA, Lima JAC, Bruke GL, Soliman EZ. Determinants of discrepancies in detection and comparison of the prognostic significance of left ventricular hypertrophy by electrocardiogram and cardiac magnetic resonance imaging. Am J Cardiol. 2015;115:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris K, Aurup P, Dahlöf B. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–2356. [DOI] [PubMed] [Google Scholar]
- 23. Verdecchia P, Angeli F, Cavallini C, Mazzotta G, Repaci S, Pede S, Borgioni C, Gentile G, Reboldi G. The voltage of R wave in lead aVL improves risk stratification in hypertensive patients without ECG left ventricular hypertrophy. J Hypertens. 2009;27:1697–1704. [DOI] [PubMed] [Google Scholar]
- 24. Narayanan K, Reinier K, Teodorescu C, Uy‐Evanado A, Chugh H, Gunson K, Jui J, Chugh SS. Electrocardiographic versus echocardiographic left ventricular hypertrophy and sudden cardiac arrest in the community. Heart Rhythm. 2014;11:1040–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cuspidi C, Facchetti R, Bombelli M, Sala C, Grassi G, Mancia G. Accuracy and prognostic significance of electrocardiographic markers of left ventricular hypertrophy in the general population: findings from the Pressioni Arteriose Monitorate E Loro Associazioni population. J Hypertens. 2014;32:921–928. [DOI] [PubMed] [Google Scholar]
- 26. Leigh JA, O'Neal WT, Soliman EZ. Electrocardiographic left ventricular hypertrophy as a predictor of cardiovascular disease independent of left ventricular anatomy in subjects ≥65 years. Am J Cardiol. 2016;117:1831–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patel N, O'Neal WT, Whalen SP, Soliman EZ. Electrocardiographic left ventricular hypertrophy predicts atrial fibrillation independent of left ventricular mass. Ann Noninvasive Electrocardiol. 2017;22:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gerdts E, Okin PM, Boman K, Wachtell K, Nieminen MS, Dahlöf B, Devereux RB. Association of heart failure hospitalizations with combined electrocardiography and echocardiography criteria for left ventricular hypertrophy. Am J Hypertens. 2012;25:678–683. [DOI] [PubMed] [Google Scholar]
- 29. Calderon A, Barrios V, Escobar C, Ferrer E, Barrios S, Gonzalez‐Pedel V, Montoro P, Navarro‐Cid J. Detection of left ventricular hypertrophy by different electrocardiographic criteria in clinical practice. Findings from the SARA study. Clin Exp Hypertens. 2010;32:145–153. [DOI] [PubMed] [Google Scholar]
- 30. Petersen SS, Pedersen LR, Pareek M, Nielsen ML, Diederichsen SZ, Leosdottir M, Nilsson PM, Diederichsen ACP, Olsen MH. Factors associated with diagnostic discrepancy for left ventricular hypertrophy between electrocardiography and echocardiography. Blood Press. 2017;26:54–63. [DOI] [PubMed] [Google Scholar]
- 31. Okin PM, Devereux RB, Jern S, Julius S, Kjeldsen SE, Dahlöf B; for the LIFE study investigators . Relation of echocardiographic left ventricular mass and hypertrophy to persistent electrocardiographic left ventricular hypertrophy in hypertensive patients: the LIFE Study. Am J Hypertens. 2001;14:775–782. [DOI] [PubMed] [Google Scholar]
- 32. Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe‐Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H; LIFE Study Group . Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 33. Okin PM, Roman MJ, Devereux RB, Kligfield P. Electrocardiographic identification of increased left ventricular mass by simple voltage‐duration products. J Am Coll Cardiol. 1995;25:417–423. [DOI] [PubMed] [Google Scholar]
- 34. Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–186. [DOI] [PubMed] [Google Scholar]
- 35. Schillaci G, Verdecchia P, Borgioni C, Ciucci A, Guerrieri M, Zampi I, Battistelli M, Bartoccini C, Porcellati C. Improved electrocardiographic diagnosis of echocardiographic left ventricular hypertrophy. Am J Cardiol. 1994;74:714–719. [DOI] [PubMed] [Google Scholar]
- 36. Norman JE, Levy D. Improved detection of echocardiographic left ventricular hypertrophy: a correlated database approach. J Am Coll Cardiol. 1995;26:1022–1029. [DOI] [PubMed] [Google Scholar]
- 37. Soliman EZ, Shah AJ, Boerkircher A, Li Y, Rautaharju P. Inter‐relationship between electrocardiographic left ventricular hypertrophy and QT prolongation as predictors of increased risk of mortality in the general population. Circ Arrhythm Electrophysiol. 2014;7:400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Okin PM, Hille DA, Kjeldsen SE, Dahlöf B, Devereux RB. Persistence of left ventricular hypertrophy is associated with increased cardiovascular morbidity and mortality in hypertensive patients with lower achieved systolic pressure during antihypertensive treatment. Blood Press. 2014;23:71–80. [DOI] [PubMed] [Google Scholar]
- 39. Soliman EZ, Byington RP, Bigger JT, Evans G, Okin PM, Goff DC Jr, Chen H. Effect of intensive blood pressure lowering on left ventricular hypertrophy in patients with diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Blood Pressure Trial. Hypertension. 2015;66:1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Soliman EZ, Ambrosius WT, Cushman WC, Zhang Z, Bates JT, Neyra JA, Carson TY, Tamariz L, Ghazi L, Cho ME, Shapiro BP, He J, Fine LJ, Lewis CE; for the SPRINT Research Study Group . Effect of intensive blood pressure lowering on left ventricular hypertrophy in patients with hypertension: SPRINT (Systolic Blood Pressure Intervention Trial). Circulation. 2017;136:440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]


