Abstract
Background
Few data exist on the association between strength training and mortality rates. We sought to examine the association between strength training and all‐cause, cardiovascular disease, and cancer mortality.
Methods and Results
Beginning in 2001 to 2005, 28 879 women throughout the United States (average baseline age, 62.2 years) from the Women's Health Study who were free of cardiovascular disease, diabetes mellitus, and cancer reported their physical activities, including strength training. During follow‐up (average, 12.0 years) through 2015, investigators documented 3055 deaths (411 from cardiovascular disease and 748 from cancer). After adjusting for covariables, including aerobic activity, time in strength training showed a quadratic association with all‐cause mortality (P=0.36 for linear trend; P<0.001 for quadratic trend); hazard ratios across 5 categories of strength training (0, 1–19, 20–59, 60–149, and ≥150 min/wk) were 1.0 (referent), 0.73 (95% confidence interval, 0.65–0.82), 0.71 (0.62–0.82), 0.81 (0.67–0.97), and 1.10 (0.77–1.56), respectively. A significant quadratic association was also observed for cardiovascular disease death (P=0.007) but not cancer death (P=0.41). Spline models also indicated a J‐shaped nonlinear association for all‐cause mortality (P=0.020); the point estimates of hazard ratios were <1.00 for 1 to 145 min/wk of strength training, compared with 0 min/wk, whereas hazard ratios were >1.00 for ≥146 min/wk of strength training. However, confidence intervals were wide at higher levels of strength training.
Conclusions
Time in strength training showed a J‐shaped association with all‐cause mortality in older women. A moderate amount of time in strength training seemed beneficial for longevity, independent of aerobic activity; however, any potential risk with more time (≈≥150 min/wk) should be further investigated.
Keywords: exercise, longevity, longitudinal cohort study, muscle‐strengthening activity, weight training
Subject Categories: Exercise, Epidemiology
Clinical Perspective
What Is New?
A moderate amount (≈1–145 min/wk) of strength training was associated with lower risk of all‐cause mortality compared with 0 min/wk, independent of aerobic activity.
On the basis of a small number of deaths, women doing ≥150 min/wk of strength training did not have lower mortality risk, but instead may have similar or higher risk compared with women not doing any strength training.
A quadratic association was also observed for deaths from cardiovascular disease, but not cancer death.
When considered jointly, women doing both any strength training and ≥150 min/wk of aerobic activity had the lowest mortality risk.
What Are the Clinical Implications?
A moderate amount (≈1–145 min/wk) of strength training may benefit longevity, regardless of participation in aerobic physical activity.
As the American Heart Association and the current federal physical activity guidelines recommend, doing both aerobic physical activity and strength training (muscle‐strengthening activity) is beneficial for health.
Current physical activity (PA) guidelines recommend that adults do muscle‐strengthening activity (resistance or strength training) for ≥2 days a week in addition to aerobic activity for health benefits.1, 2 However, compared with well‐established effects of aerobic activity, the effect of strength training on preventing premature death has been little investigated.3, 4 Strength training prevents age‐related loss of muscle mass and bone, and it enhances functional health, especially later in life.5 Development and maintenance of metabolically active lean muscle mass is important for enhancing glucose metabolism.6 Short‐term randomized trials showed that strength training reduced weight and improved biomarkers of type 2 diabetes mellitus and cardiovascular disease (CVD) risk, including blood lipids.7, 8 However, a systematic review of multiple randomized trials also indicated that high‐intensity strength training increased arterial stiffness in young adults, indicating potential risk of CVD.9 Hypertension and prehypertension also are significantly more common in NFL players, who regularly perform strenuous strength trainings, than the general US population.10 Although randomized trials showed that strength training reduced blood pressure, those beneficial effects were not observed in hypertensive populations.11, 12 In addition, harm (adverse effects) from the training interventions were not reported in many studies, and long‐term complications or mortality is not known.8
There is little research directly examining the longitudinal associations of strength training with incident type 2 diabetes mellitus and CVD risk, and studies have reported inconsistent results.13, 14, 15, 16 Several cohort studies revealed that muscle strength was inversely associated with mortality.17, 18, 19 However, muscle strength is likely affected by genetic factors20 as well, and these data cannot directly inform recommendations on what people should do. Thus, further research on strength training is warranted to generate evidence to inform recommendations. Few studies have examined prospective associations of strength training with mortality, indicating mixed results.3, 4, 16 However, these previous analyses used only a dichotomous variable (yes/no participation) or analyzed mortality risk as a secondary outcome and, thus, performed no detailed analysis on the dose‐response relationship. Any dose‐response (eg, quadratic) relationship between muscle‐strengthening activity and mortality risk remains unclear. Given the potential for high‐intensity strength training to increase arterial stiffness and blood pressure, we were interested particularly in any adverse effects at high levels of participation.
Therefore, this study aimed to examine prospectively the association between strength training and mortality from all causes, CVD, and cancer in women, and to evaluate the shape of any dose‐response. Our prespecified hypothesis was that a moderate amount of time in strength training is associated with greater longevity, compared with no such activity, and that the dose‐response is nonlinear, with potentially adverse associations seen at higher amounts of strength training.
Methods
Study Participants
We analyzed data from the Women's Health Study, a completed randomized trial examining low‐dose aspirin and vitamin E for the prevention of CVD and cancer among 39 876 healthy women, conducted from 1992 to 2004.21, 22, 23 Women completed health questionnaires every 6 months during the first year and annually thereafter. After the scheduled conclusion of the trial, women were followed up in an observational study. For this study, the 37 162 women who returned the 96‐month questionnaire (when strength training was first ascertained) were eligible. We excluded 3969 women with missing information on PA then, and 4314 women diagnosed as having CVD (myocardial infarction, stroke, percutaneous transluminal coronary angioplasty, or coronary artery bypass grafting), cancer, or diabetes mellitus before the 96‐month questionnaire, leaving 28 879 women. This study protocol was approved by the Institutional Review Board of Brigham and Women's Hospital (Boston, MA). Participants provided consent to participate.
Patient Involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in the design and implementation of the study. Participants can read about research findings from the Women's Health Study via the study website, and selected findings are highlighted in periodic newsletters.
Assessment of PA
On the 96‐month health questionnaire (baseline), women reported their walking pace, flights of stairs climbed, and time spent per week in various leisure time activities or groups of activities. A strength training question was also included (Figure S1), “During the past month, what was your approximate time per week spent at each of the following recreational activities? Weight lifting/strength training.” This PA questionnaire is based on the College Alumni Health Study questionnaire,24 and has been shown to be reliable and valid.25 In women, the 2‐year test‐retest correlation was 0.59, and when compared with activity recalls, PA estimates yielded a correlation of 0.79.25 PA was then updated on the 120‐, 144‐, 168‐, 204‐, and 228‐month follow‐up questionnaires.
Women were categorized a priori on the basis of minutes per week spent in strength training and in other PA, with categories defined to obtain as even a distribution of cases as possible. Aerobic moderate‐to‐vigorous PA (MVPA), defined as at least 3 metabolic equivalents of intensity,26 included jogging, running, tennis/squash/racquetball, walking, bicycling, aerobic exercise/aerobic dance/exercise machines, lap swimming, stair climbing, and other aerobic activities. Total PA included aerobic MVPA, strength training, and lower‐intensity activities. The 5 categories were as follows: 0, 1 to 19, 20 to 59, 60 to 149, and ≥150 min/wk of participation.
Assessment of Covariates
Baseline information was collected on age, race, education, height, weight, smoking habits, hypertension, high cholesterol, menopausal status, hormone use, physical examination for screening in the past year (ie, health checkup), and parental history of myocardial infarction or cancer by a self‐report questionnaire. Dietary habits (alcohol, energy, saturated fat, fiber, and fruit and vegetable intake) were assessed using a semiquantitative food questionnaire.27 Weight, smoking habits, hormone use, alcohol intake, hypertension, and high cholesterol were updated from the 120‐, 144‐, 168‐, 204‐, and 228‐month follow‐up questionnaires. Diet information was updated only on the 120‐month questionnaire. Reported diagnoses of CVD, diabetes mellitus, and cancer were confirmed using medical records.
Mortality Surveillance
Study participants were followed up for mortality (all‐cause, CVD, and cancer mortality) from the date women returned the 96‐month questionnaire through December 31, 2015. Family members or postal authorities reported most deaths. Medical records and/or death certificates were obtained to confirm causes of these deaths; only these deaths with confirmed causes were analyzed for cause‐specific analysis. All events were adjudicated according to predefined criteria by an end point committee of physicians.21 Other deaths were ascertained using the National Death Index. Mortality follow‐up is >99% complete. The confirmation process for the causes of deaths took time by nature and caused the discrepancy between numbers of death from all causes and those from CVD and cancer.
Statistical Analyses
Participant characteristics were described by min/wk of strength training. To test for a linear trend in baseline covariates by strength training, we used linear regression models for continuous variables and Cochran‐Armitage trend test for categorical variables. For strength training, aerobic MVPA, and total PA, we calculated hazard ratios (HRs) and 95% confidence intervals (95% CIs) comparing the rates of all‐cause, CVD, and cancer deaths across categories of time spent per week in each activity type using Cox proportional hazard models. Nonfatal events (eg, CVD incidence) were not analyzed in this study. The covariables were selected a priori and based on known factors potentially confounding the associations. In separate models, linear and quadratic trends were tested by using continuous variables of PAs. We adjusted for age and trial randomization in model 1; we additionally adjusted for race, education, postmenopausal status, hormone use, smoking status, parental history of myocardial infarction or cancer, alcohol intake, energy intake, saturated fat intake, fiber intake, fruit and vegetable intake, physical examination for screening, and time per week spent in aerobic MVPA (for strength training and vice versa) in model 2; and further adjusted for body mass index (BMI), calculated as kilograms (weight) per meter squared (height), and incidence of hypertension, high cholesterol, CVD, diabetes mellitus, and cancer before and during follow‐up in model 3. We used cumulative‐average updated (ie, time‐varying cumulative average) value of PA in the main analyses.28 Additional analyses using simple updated activity (the most recent value) and baseline value were also performed.
A continuous association between strength training and all‐cause mortality was further estimated without assuming linearity by fitting restricted cubic spline models,29 with the knots corresponding to the 50th, 75th, and 90th percentiles of weekly times of strength training within doers (ie, 39.5, 90, and 150 min/wk) and the reference value of 0 min/wk. We chose these 3 knots to place them evenly throughout the range of strength training (0–480 min/wk) considering its right skewed distribution and the actual values of percentiles. Both the 10th and 25th percentiles were 10 min/wk among doers. Because adding many knots in a narrow range or sparse area would not give useful information and may produce an unstable curve, we did not place additional knots in the <39.5 or >150 min/wk ranges. Covariates included were the same as those in the fully adjusted model (model 3).
We tested if the association of strength training with all‐cause mortality differed by age (<60 or ≥60 years) or BMI (<25, 25–<30, or ≥30 kg/m2). For the subgroup and cause‐specific mortality analyses, 3 categories of strength training were used because of small numbers of outcomes in the cells (0, 1–59, and ≥60 min/wk). We tested for interaction using model fit statistics (−2 log likelihood) with and without interaction terms and χ2 test.
To examine the joint association of strength training and aerobic activity, we compared the rates of all‐cause deaths across combinations of participation in strength training and aerobic activities using the cut points of ≥150 min/wk of aerobic activity and any (>0 min/wk of) strength training.
We used a complete‐case analysis in multivariable models without imputation for missing data. No model excluded >708 participants (2.5%) because of missing data. The proportional hazards assumption was examined for all baseline‐variable models using interaction terms with logarithm of follow‐up time and found not to be violated (P>0.05).
We also performed a 2‐year lag analysis for all time‐varying and baseline‐exposure analyses to minimize the possibility of reverse causation. For baseline‐exposure analysis, we excluded 155 deaths that occurred during the first 2 years of follow‐up. Analyses were performed using SAS version 9.4.
Results
Participant characteristics by categories of time spent in strength training are shown in Table 1. At baseline, 6100 women (21.1%) engaged in some strength training and 2894 women (10.0%) spent at least 60 min/wk in strength training, whereas 13 702 women (47.5%) participated in at least 150 minutes of aerobic MVPA per week. On average, women were 62.2 years old (SD, 6.8 years) with a BMI of 26.8 kg/m2 (SD, 5.3 kg/m2). Strength training at baseline was inversely associated with age, BMI, postmenopausal status, smoking, hypertension, high cholesterol, and intake of saturated fat. Strength training at baseline was positively associated with education; hormone use; intakes of alcohol, fiber, fruits, and vegetables; screening; aerobic MVPA; and total PA time.
Table 1.
Baseline Characteristics of Participants by Time Spent Strength Training: Women's Health Study
| Characteristics | Strength Training, Min/Wk | P Value for Linear Trend | ||||
|---|---|---|---|---|---|---|
| 0 | 1–19 | 20–59 | 60–149 | ≥150 | ||
| (n=22 779) | (n=1640) | (n=1566) | (n=1859) | (n=1035) | ||
| Age, mean (SD), y | 62.4 (6.9) | 61.7 (6.5) | 61.4 (6.2) | 61.3 (6.2) | 61.5 (6.3) | <0.001 |
| White race, N (%) | 21 533 (95.3) | 1547 (94.9) | 1488 (96.0) | 1778 (96.3) | 984 (95.7) | 0.052 |
| Education, bachelor's degree or higher, N (%) | 9182 (50.0) | 877 (54.4) | 902 (58.5) | 1061 (58.0) | 562 (55.5) | <0.001 |
| BMI, mean (SD), kg/m2 | 27.1 (5.4) | 25.7 (4.7) | 25.3 (4.2) | 25.3 (4.4) | 25.1 (4.4) | <0.001 |
| Postmenopausal, N (%) | 20 595 (90.4) | 1482 (90.4) | 1407 (89.9) | 1651 (88.8) | 930 (89.9) | 0.048 |
| Current hormone therapy, N (%)a | 12 526 (60.8) | 989 (66.7) | 963 (68.4) | 1105 (67.0) | 618 (66.5) | <0.001 |
| Current smoking, N (%) | 2177 (9.6) | 72 (4.4) | 55 (3.5) | 83 (4.5) | 45 (4.4) | <0.001 |
| Parental history of MI, N (%) | 3191 (14.2) | 218 (13.4) | 212 (13.7) | 257 (14.0) | 152 (15.0) | 0.97 |
| Parental history of cancer, N (%) | 4028 (17.7) | 323 (19.7) | 238 (15.2) | 325 (17.5) | 183 (17.7) | 0.47 |
| Hypertension, N (%) | 10 525 (46.2) | 580 (35.4) | 543 (34.7) | 681 (36.6) | 369 (35.7) | <0.001 |
| High cholesterol, N (%) | 11 796 (51.8) | 783 (47.7) | 719 (45.9) | 841 (45.2) | 459 (44.4) | <0.001 |
| Alcohol intake, mean (SD), g/d | 4.2 (8.4) | 4.8 (8.1) | 5.0 (7.8) | 5.5 (8.4) | 5.4 (8.2) | <0.001 |
| Energy intake, mean (SD), kcal/d | 1723 (533) | 1757 (517) | 1758 (491) | 1736 (514) | 1727 (529) | 0.057 |
| Saturated fat intake, mean (SD), g/d | 20.0 (8.2) | 19.3 (7.5) | 18.8 (7.1) | 18.2 (7.4) | 17.7 (7.4) | <0.001 |
| Fiber intake, mean (SD), g/d | 18.5 (8.0) | 20.0 (8.1) | 20.2 (8.0) | 20.9 (8.7) | 21.4 (9.4) | <0.001 |
| Fruits and vegetables, mean (SD), servings/d | 5.9 (3.5) | 6.3 (3.7) | 6.5 (3.3) | 6.8 (3.4) | 7.2 (3.9) | <0.001 |
| Physical examination for screening, N (%) | 13 849 (61.3) | 1077 (66.1) | 1046 (67.0) | 1229 (66.4) | 677 (65.9) | <0.001 |
| Aerobic MVPA, median (IQR), min/wkb | 90 (12–242) | 140 (51–269) | 187 (100–317) | 252 (152–446) | 364 (202–600) | <0.001 |
| Total PA, median (IQR), min/wkc | 97 (14–292) | 168 (74–314) | 257 (168–403) | 372 (252–552) | 607 (437–943) | <0.001 |
BMI indicates body mass index (calculated as kilograms [weight] per meter squared [height]); IQR, interquartile range; MI, myocardial infarction; MVPA, moderate‐to‐vigorous PA; and PA, physical activity.
Among postmenopausal women (n=26 065).
Aerobic MVPA was defined as activities requiring an intensity of at least 3 metabolic equivalents and included nonslow walking, jogging, running, bicycling, tennis, aerobic exercises, lap swimming, other aerobic activities, and stair climbing.
Total PA additionally included lower‐intensity activities and strength training.
During an average follow‐up of 12.0 years (SD, 3.2 years) and 346 843 person‐years of observation, 3055 deaths (10.6%) occurred, 411 from CVD and 748 from cancer. There were 603 confirmed non‐CVD, noncancer deaths; for the remaining 1293 deaths, the ascertainment of cause of death is ongoing per study protocol. Among those who died during the follow‐up, there was no significant difference in strength training participation between those whose death adjudication was complete (mean [SD], 12 [46] min/wk; n=1762) and those whose adjudication was ongoing (mean [SD], 10 [39] min/wk; n=1293) (P=0.35). Unadjusted cumulative incidence curves of deaths from all causes, CVD, and cancer by baseline weekly time of strength training can be found in Figure S2. In the multivariable analysis, the association between time spent in strength training and all‐cause mortality showed a quadratic trend (P<0.001 for quadratic trend) (Table 2). Women with a moderate amount (1–149 min/wk) of strength training had 19% to 29% lower risks of mortality compared with those who did not participate in strength training. However, women with the highest amount (≥150 min/wk) of strength training did not have lower mortality risk (HR [95% CI], 1.10 [0.77–1.56]). In contrast, women performing ≥150 min/wk of aerobic MVPA or total PA had the lowest mortality risk across their respective categories of activity.
Table 2.
Relative Risks of All‐Cause Mortality With Strength Training and Other Physical Activities: Women's Health Study
| Variable | Physical Activities, Min/Wk | P Value for Linear Trend | P Value for Quadratic Trend | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1–19 | 20–59 | 60–149 | ≥150 | |||
| Strength training | |||||||
| Cases (person‐years) | 2599 (271 749) | 130 (20 224) | 107 (19 319) | 133 (23 035) | 86 (12 515) | ||
| Model 1a | Reference | 0.64 (0.58–0.71) | 0.59 (0.51–0.67) | 0.60 (0.51–0.72) | 0.85 (0.61–1.18) | <0.001 | <0.001 |
| Model 2b | Reference | 0.75 (0.67–0.84) | 0.76 (0.66–0.87) | 0.87 (0.72–1.04) | 1.20 (0.84–1.72) | 0.84 | <0.001 |
| Model 3c | Reference | 0.73 (0.65–0.82) | 0.71 (0.62–0.82) | 0.81 (0.67–0.97) | 1.10 (0.77–1.56) | 0.36 | <0.001 |
| Aerobic MVPAd | |||||||
| Cases (person‐years) | 415 (22 691) | 684 (61 696) | 286 (36 714) | 404 (58 702) | 1266 (167 040) | ||
| Model 1a | Reference | 0.58 (0.49–0.68) | 0.45 (0.38–0.52) | 0.32 (0.27–0.37) | 0.25 (0.22–0.30) | <0.001 | <0.001 |
| Model 2b | Reference | 0.65 (0.55–0.76) | 0.55 (0.46–0.65) | 0.41 (0.35–0.49) | 0.34 (0.29–0.40) | <0.001 | <0.001 |
| Model 3c | Reference | 0.67 (0.56–0.79) | 0.56 (0.47–0.66) | 0.41 (0.35–0.49) | 0.36 (0.31–0.43) | <0.001 | <0.001 |
| Total PAe | |||||||
| Cases (person‐years) | 352 (18 873) | 607 (54 139) | 296 (34 860) | 427 (56 610) | 1373 (182 361) | ||
| Model 1a | Reference | 0.59 (0.49–0.70) | 0.45 (0.37–0.53) | 0.35 (0.29–0.41) | 0.25 (0.21–0.29) | <0.001 | <0.001 |
| Model 2b | Reference | 0.65 (0.54–0.78) | 0.53 (0.44–0.64) | 0.43 (0.36–0.51) | 0.31 (0.26–0.37) | <0.001 | <0.001 |
| Model 3c | Reference | 0.67 (0.56–0.81) | 0.54 (0.45–0.66) | 0.43 (0.36–0.52) | 0.32 (0.27–0.38) | <0.001 | <0.001 |
All values are hazard ratios (95% confidence intervals) based on cumulative average models unless otherwise stated. Cases (person‐years) are shown on the basis of the categories of baseline physical activities. MVPA indicates moderate‐to‐vigorous PA; and PA, physical activity.
Model 1 is adjusted for age and trial randomization.
Model 2 is further adjusted for race, education, postmenopausal status, hormone use, smoking status, parental history of myocardial infarction or cancer, alcohol intake, energy intake, saturated fat intake, fiber intake, fruit and vegetable intake, physical examination for screening, and time per week spent in aerobic MVPA (for strength training and vice versa).
Model 3 is further adjusted for body mass index and incidence of hypertension, high cholesterol, cardiovascular diseases, diabetes mellitus, and cancer before and during follow‐up.
Aerobic MVPA was defined as activities requiring an intensity of at least 3 metabolic equivalents and included nonslow walking, jogging, running, bicycling, tennis, aerobic exercises, lap swimming, other aerobic activities, and stair climbing. Aerobic MVPA was analyzed in the same model with strength training simultaneously.
Total PA additionally included strength training and lower‐intensity activities.
Spline models indicated a J‐shaped nonlinear association between strength training and all‐cause mortality (P=0.020). The point estimates of HRs for 1 to 145 min/wk of strength training were <1.00, compared with 0 min/wk, whereas HRs of >1.00 were seen with ≥146 min/wk of strength training, with wide CIs at higher levels of activity (Figure). The lowest HR (0.87) was observed at 82 min/wk of strength training.
Figure 1.
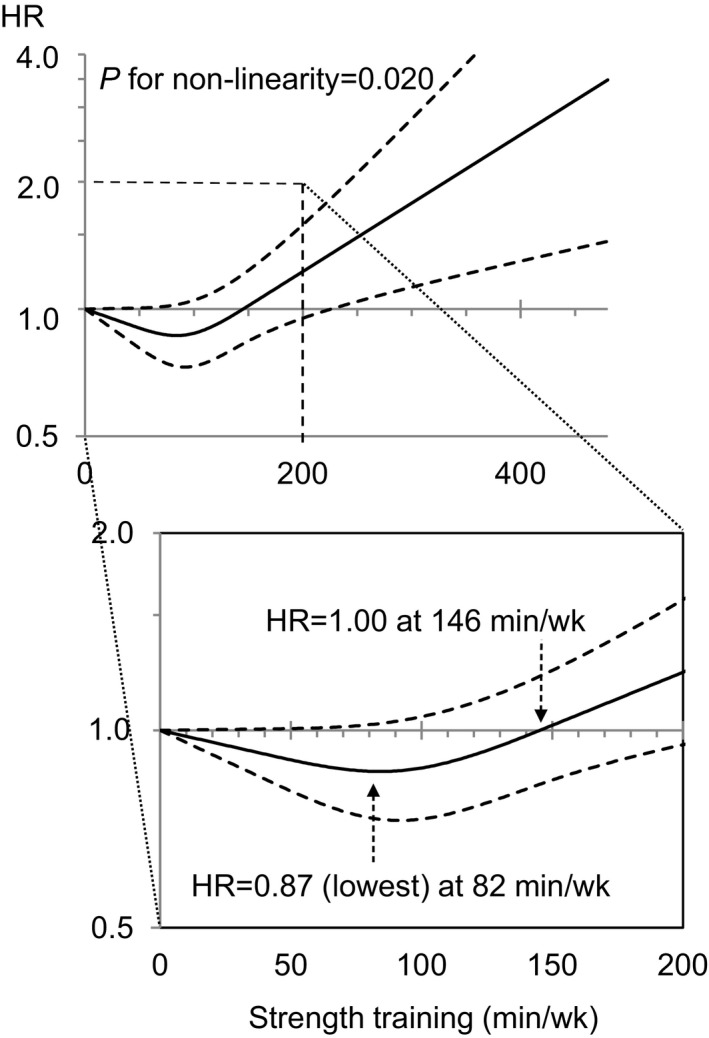
Strength training and all‐cause mortality risk. The solid line presents the hazard ratio (HR) of the restricted cubic spline model adjusted for aerobic activity and other potential confounders, with the knots specified at 50th, 75th, and 90th percentiles of weekly times of strength training among women who performed such activity (ie, 39.5, 90, and 150 min/wk, respectively), and the reference value was set at 0 min/wk. The dashed lines show 95% confidence intervals.
Table 3 shows the results of the cause‐specific mortality analysis. A significant quadratic association was observed for CVD death (P=0.007 for quadratic) but not for cancer death (P=0.41 for quadratic). In subgroup analyses, significant quadratic associations were observed in older (≥60 years old) but not younger women, and nonobese women but not obese women; however, none of those interactions were significant (P≥0.056) (Table S1).
Table 3.
Relative Risks of Death From CVD and Cancer With Strength Training: Women's Health Study
| Outcome | Strength Training, Min/Wk | P Value for Linear Trend | P Value for Quadratic Trend | ||
|---|---|---|---|---|---|
| 0 | 1–59 | ≥60 | |||
| CVD death | |||||
| Cases (person‐years) | 361 (271 749) | 31 (39 544) | 19 (35 550) | ||
| Model 1a | Reference | 0.59 (0.46–0.76) | 0.43 (0.26–0.72) | 0.003 | <0.001 |
| Model 2b | Reference | 0.71 (0.55–0.93) | 0.67 (0.39–1.14) | 0.35 | 0.007 |
| Model 3c | Reference | 0.65 (0.50–0.85) | 0.72 (0.42–1.22) | 0.33 | 0.007 |
| Cancer death | |||||
| Cases (person‐years) | 607 (271 749) | 66 (39 544) | 75 (35 550) | ||
| Model 1a | Reference | 0.80 (0.68–0.95) | 0.82 (0.62–1.09) | 0.45 | 0.061 |
| Model 2b | Reference | 0.88 (0.73–1.05) | 0.99 (0.73–1.33) | 0.28 | 0.65 |
| Model 3c | Reference | 0.87 (0.73–1.05) | 0.92 (0.68–1.24) | 0.88 | 0.41 |
All values are hazard ratios (95% confidence intervals) based on cumulative average models unless otherwise stated. Cases (person‐years) are shown on the basis of the categories of baseline physical activity. CVD indicates cardiovascular disease.
Model 1 is adjusted for age and trial randomization.
Model 2 is further adjusted for postmenopausal status, hormone use, smoking status, parental history of myocardial infarction or cancer, alcohol intake, energy intake, saturated fat intake, fiber intake, fruit and vegetable intake, and time per week spent in aerobic moderate‐to‐vigorous physical activity.
Model 3 is further adjusted for body mass index and incidence of hypertension, high cholesterol, CVDs, diabetes mellitus, and cancer before and during follow‐up.
Table 4 shows HRs of mortality for the joint categories of aerobic MVPA and strength training. Compared with women performing <150 min/wk of aerobic MVPA and no strength training, women with either ≥150 min/wk aerobic MVPA alone or any strength training (>0 min/wk) alone had lower all‐cause mortality risks (29% and 26%, respectively), whereas women with both ≥150 min/wk of aerobic MVPA and any strength training had the lowest mortality risk (HR [95% CI], 0.54 [0.47–0.61]). Similar associations were observed for CVD mortality. Interactions between aerobic MVPA and strength training were not significant (P≥0.23).
Table 4.
Relative Risks of Mortality According to Joint Categories of Aerobic and Strength Training: Women's Health Study
| Outcome | Aerobic MVPA <150 Min/Wk and No Strength Training | Aerobic MVPA ≥150 Min/Wk and No Strength Training | Aerobic MVPA <150 Min/Wk and Any Strength Training | Aerobic MVPA ≥150 Min/Wk and Any Strength Training | P Value for Interaction |
|---|---|---|---|---|---|
| All‐cause death | |||||
| Cases (person‐years) | 1620 (156 331) | 979 (115 418) | 169 (23 472) | 287 (51 622) | |
| HR (95% Cl) | Reference | 0.71 (0.64–0.79) | 0.74 (0.65–0.85) | 0.54 (0.47–0.61) | 0.33 |
| CVD death | |||||
| Cases (person‐years) | 228 (156 331) | 133 (115 418) | 17 (23 472) | 33 (51 622) | |
| HR (95% Cl) | Reference | 0.74 (0.56–0.98) | 0.75 (0.53–1.07) | 0.43 (0.29–0.63) | 0.23 |
| Cancer death | |||||
| Cases (person‐years) | 348 (156 331) | 259 (115 418) | 36 (23 472) | 105 (51 622) | |
| HR (95% Cl) | Reference | 0.97 (0.79–1.19) | 0.91 (0.70–1.18) | 0.93 (0.74–1.17) | 0.45 |
All groups are mutually exclusive. HRs (95% CIs) are calculated on the basis of cumulative average values. Cases (person‐years) are shown on the basis of the categories of baseline physical activities. Multivariable model is adjusted for age, race, education, postmenopausal status, hormone use, smoking status, parental history of myocardial infarction or cancer, alcohol intake, energy intake, saturated fat intake, fiber intake, fruit and vegetable intake, physical examination for screening, body mass index, incidence of hypertension, high cholesterol, CVDs, diabetes mellitus, and cancer before and during follow‐up, and trial randomization. CI indicates confidence interval; CVD, cardiovascular disease; HR, hazard ratio; and MVPA, moderate‐to‐vigorous physical activity.
Sensitivity analyses with simple updated and baseline PA values yielded similar results for all‐cause mortality (Table S2). The lag analysis, which excluded all deaths occurring within 2 years, also did not materially change the results. For example, in the lag analysis of cumulative‐average updated value model, time in strength training showed a quadratic association with all‐cause mortality (P=0.77 for linear trend; P<0.001 for quadratic trend). HRs across 5 categories of strength training (0, 1–19, 20–59, 60–149, and ≥150 min/wk) were 1.0 (referent), 0.74 (95% CI, 0.66–0.84), 0.75 (0.65–0.87), 0.91 (0.76–1.08), and 1.19 (0.86–1.64), respectively.
Discussion
Time in strength training showed a J‐shaped association with all‐cause mortality in older women. Women who performed a moderate amount (≈<150 min/wk) of strength training had a lower risk of mortality, compared with those did not. However, on the basis of a small number of deaths (86 cases), ≈≥150 min/wk of strength training appeared not to be associated with lower mortality risk, but instead may be associated with similar or higher risk, compared with 0 min/wk.
When considered jointly with aerobic MVPA, doing any strength training and <150 min/wk of aerobic MVPA was associated with 26% lower risk of all‐cause mortality; doing any strength training and ≥150 min/wk of aerobic MVPA, 46% lower risk. Previous studies with dichotomous analysis also showed lower risk of mortality associated with any4 or ≥2 d/wk3 of strength training after adjusting for aerobic activity. A recent study using the National Health Interview Survey data showed that older adults doing guideline‐concordant strength training (ie, ≥2 d/wk) had 46% lower odds of all‐cause mortality than those who did not.3 Similarly, a cohort study in cancer survivors showed 33% lower risk of mortality in those performing free weights or weight training at least 1 d/wk, compared with those did not.4 However, none of these studies examined the dose‐response relationship. Another cohort study in men, which primarily focused on the risk of type 2 diabetes mellitus, showed that men performing weight training 1 to 59, 60 to 149, and ≥150 min/wk had multivariable‐adjusted HRs of 0.88, 1.04, and 1.11 (P=0.38 for trend) for all‐cause mortality, respectively, compared with men reporting no weight training.16 Although the previous study did not test statistically (and not mention about), the results implied the quadratic association as similar to the current findings. Because both the optimal doses and (if any) potential harmful doses of PA have drawn scientific and clinical attention,30 this study provides important insight into the questions. In this study, the magnitude of the association (ie, HRs) obtained from different models (categorical versus spline; cumulative‐average versus baseline value) differed slightly. All showed similar quadratic association with the lowest mortality risk observed at a moderate amount of strength training and similar or higher risk at the highest amount of strength training, compared with none. Specific optimal and (if any) harmful doses should be further investigated in future studies.
More time in aerobic MVPA was associated with lower mortality risk. Therefore, the observed J‐shaped association of strength training and mortality risk was not considered as a result of confounding by aerobic MVPA. In a pooled analysis of 6 cohort studies, there was no evidence of harm, even at ≥10 times the recommended minimum (150 min/wk) of leisure‐time (mostly aerobic) PA on longevity.31
Possible mechanisms underlying beneficial effects of strength training on mortality risk include increased muscle mass and muscle strength, improved physical functioning,5, 17, 18 improved glucose metabolism,6 weight loss or maintenance, and improved other CVD risks (eg, blood lipids).7, 8 Our full model with the adjustment of BMI, incident hypertension, high cholesterol, CVD, diabetes mellitus, and cancer during follow‐up showed that the quadratic associations between strength training and mortality risks were still statistically significant after adjusting for those potential intermediate variables in the pathway between strength training and mortality risk. Some other mechanisms, including improved physical functioning, as noted above, might play a role as well in the pathway of the observed association, especially lower mortality risk among women with a moderate amount of strength training.
On the other hand, the potential risk of increased arterial stiffness leading to subsequent CVD events may partly explain the observed higher risk of mortality in those with the largest amounts of strength training.9 The results of a cohort study examining the association between weight training and risk of incident CVD in men were suggestive of a quadratic trend, with the highest CVD risk (HR [95% CI], 1.24 [0.73–2.09]) observed in those with the highest amount of weight training (≥5.0 h/wk).15 Similarly, a recent analysis of the Women's Health Study showed that the risk of incident CVD was not significantly different between those with 0 and ≥120 min/wk of strength training, although significant risk reductions of incident type 2 diabetes mellitus and CVD were observed with any participation in strength training, versus none.32 During high‐intensity resistance exercise, blood pressure increases to as high as ≈345/245 mm Hg.33 However, over the longer term, a small but significant blood pressure lowering effect of strength training (3–4 mm Hg reduction after ≥4 weeks of training) has been shown, except in hypertensive populations.11, 12 Heavy strength training increases plasma norepinephrine levels (ie, sympathetic nervous system activity),34 and the pressure load induced by the training may lead to a mild form of cardiac hypertrophy.35 In our study, the full model with the adjustment of incident hypertension, CVD, and other diseases during follow‐up showed less evident increased mortality risk (HR, 1.10) among women with ≥150 min/wk of strength training than their risk before these adjustments (HR, 1.20), indicating their role in the pathway of the link between the highest amount of strength training and higher mortality risk.
The results of the cause‐specific analysis showed a significant quadratic association for CVD death but not cancer death. This should be interpreted with caution because the number of death causes was small, resulting in low statistical power. Further study with a larger number of outcomes is needed to investigate the associations with cause‐specific mortalities and possible effect modifications by age or obesity status.
Important strengths of this study include its prospective design with >10 years of follow‐up, large overall sample size, detailed analyses on dose‐response relationship, and repeated measures of exposures. Our study also has several potential limitations. First, the observational nature of this study does not allow us to establish causation. However, in the absence of randomized controlled trials assessing the independent effect of strength training on mortality, this study represents an advance in quantifying the detailed dose‐response between strength training and mortality. Furthermore, such trials are likely infeasible (because of compliance and cost) for investigating mortality over a long time period. Second, information on PA was self‐reported and subject to response and recall bias. However, there is no standard method to objectively measure strength training in a free‐living environment.36 No clear definition of strength training was provided in the questionnaire other than asking about “weight lifting/strength training.” Participants may thus have had different interpretations of the types and intensities of activities that counted. Information on types, intensity, frequency (d/wk), or repetitions of strength training was not evaluated, either. Thus, we were unable to directly examine the effect of the level recommended by current guidelines (≥2 d/wk).1, 2 In addition to the total volume (weekly time), the intensity and the type (“dynamic” versus “static or isometric”) of strength training should also be investigated. Third, although plausible potential mechanisms exist for the observed findings, we did not investigate them directly. Fourth, residual confounding might partly explain the findings because potential confounders were self‐reported. Some HRs (ie, the magnitude of associations) might be underestimated or overestimated and, thus, should be interpreted carefully. Finally, participants were older, primarily white, and of higher socioeconomic status. Thus, the generalizability of the present findings may be limited, although previous analyses of objectively assessed PA data from Women's Health Study participants showed similar levels of activity and sedentary behavior to a comparably aged US national sample.37, 38 The baseline (2001–2005) prevalence of strength training in the present study (21.1%) was also similar to that in the National Health Interview Survey (23.6% in 2003).39
Conclusions
Time in strength training showed a J‐shaped association with all‐cause mortality in older women. Strength training, when performed in a moderate amount (≈≤150 min/wk), was associated with a lower mortality risk. However, on the basis of a small number of deaths, ≈≥150 min/wk of strength training appeared not associated with lower mortality risk, but similar or possibly higher mortality rates, compared with 0 min/wk. A significant quadratic association was also observed for CVD death but not cancer death. Further study with larger samples is warranted to confirm the shape of dose‐response observed in the present study.
Sources of Funding
This research was supported by research grants CA047988, CA182913, HL043851, HL080467, and HL099355 from the National Institutes of Health. Kamada was supported by the Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad and Young Scientists and the Sasakawa Sports Foundation. Shiroma was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging. The funding bodies did not have a role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication. Kamada and Lee had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
None.
Supporting information
Table S1. Relative Risks of All‐Cause Mortality With Strength Training by Subgroup, Women's Health Study (2001–2015)
Table S2. Relative Risks of All‐Cause Mortality With Strength Training (Simple‐Updated and Baseline‐Value Models), Women's Health Study (2001–2015)
Figure S1. Physical Activity Questionnaire, Women's Health Study.
Figure S2. Unadjusted cumulative incidence of deaths from all‐cause (A), cardiovascular diseases (B), and cancer (C) by baseline weekly time of strength training, Women's Health Study (2001–2015).
Acknowledgments
We thank the staff of the Women's Health Study (Brigham and Women's Hospital), particularly M. Vinayaga Moorthy, PhD, and Chunying Li, MPH. None of the people named were compensated.
(J Am Heart Assoc. 2017;6:e007677 DOI: 10.1161/JAHA.117.007677.)29089346
References
- 1. World Health Organization (WHO) . Global recommendations on physical activity for health. 2010. Available at: http://www.who.int/dietphysicalactivity/publications/9789241599979/en/index.html. Accessed January 5, 2017. [PubMed]
- 2. US Department of Health and Human Services . 2008 Physical activity guidelines for Americans. 2008. Available at: http://www.health.gov/paguidelines/guidelines/default.aspx. Accessed January 5, 2017.
- 3. Kraschnewski JL, Sciamanna CN, Poger JM, Rovniak LS, Lehman EB, Cooper AB, Ballentine NH, Ciccolo JT. Is strength training associated with mortality benefits? A 15 year cohort study of US older adults. Prev Med. 2016;87:121–127. [DOI] [PubMed] [Google Scholar]
- 4. Hardee JP, Porter RR, Sui X, Archer E, Lee IM, Lavie CJ, Blair SN. The effect of resistance exercise on all‐cause mortality in cancer survivors. Mayo Clin Proc. 2014;89:1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda‐Sceppa C. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1094–1105. [DOI] [PubMed] [Google Scholar]
- 6. Ivy JL, Zderic TW, Fogt DL. Prevention and treatment of non‐insulin‐dependent diabetes mellitus. Exerc Sport Sci Rev. 1999;27:1–35. [PubMed] [Google Scholar]
- 7. Umpierre D, Ribeiro PA, Schaan BD, Ribeiro JP. Volume of supervised exercise training impacts glycaemic control in patients with type 2 diabetes: a systematic review with meta‐regression analysis. Diabetologia. 2013;56:242–251. [DOI] [PubMed] [Google Scholar]
- 8. Yang Z, Scott CA, Mao C, Tang J, Farmer AJ. Resistance exercise versus aerobic exercise for type 2 diabetes: a systematic review and meta‐analysis. Sports Med. 2014;44:487–499. [DOI] [PubMed] [Google Scholar]
- 9. Miyachi M. Effects of resistance training on arterial stiffness: a meta‐analysis. Br J Sports Med. 2013;47:393–396. [DOI] [PubMed] [Google Scholar]
- 10. Tucker AM, Vogel RA, Lincoln AE, Dunn RE, Ahrensfield DC, Allen TW, Castle LW, Heyer RA, Pellman EJ, Strollo PJ, Wilson PW, Yates AP. Prevalence of cardiovascular disease risk factors among National Football League players. JAMA. 2009;301:2111–2119. [DOI] [PubMed] [Google Scholar]
- 11. Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta‐analysis. J Am Heart Assoc. 2013;2:e004473 DOI: 10.1161/JAHA.112.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cornelissen VA, Fagard RH, Coeckelberghs E, Vanhees L. Impact of resistance training on blood pressure and other cardiovascular risk factors: a meta‐analysis of randomized, controlled trials. Hypertension. 2011;58:950–958. [DOI] [PubMed] [Google Scholar]
- 13. Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288:1994–2000. [DOI] [PubMed] [Google Scholar]
- 14. Grontved A, Pan A, Mekary RA, Stampfer M, Willett WC, Manson JE, Hu FB. Muscle‐strengthening and conditioning activities and risk of type 2 diabetes: a prospective study in two cohorts of US women. PLoS Med. 2014;11:e1001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chomistek AK, Cook NR, Flint AJ, Rimm EB. Vigorous‐intensity leisure‐time physical activity and risk of major chronic disease in men. Med Sci Sports Exerc. 2012;44:1898–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grøntved A, Rimm EB, Willett WC, Andersen LB, Hu FB. A prospective study of weight training and risk of type 2 diabetes mellitus in men. Arch Intern Med. 2012;172:1306–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leong DP, Teo KK, Rangarajan S, Lopez‐Jaramillo P, Avezum A Jr, Orlandini A, Seron P, Ahmed SH, Rosengren A, Kelishadi R, Rahman O, Swaminathan S, Iqbal R, Gupta R, Lear SA, Oguz A, Yusoff K, Zatonska K, Chifamba J, Igumbor E, Mohan V, Anjana RM, Gu H, Li W, Yusuf S; Prospective Urban Rural Epidemiology (PURE) Study Investigators . Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386:266–273. [DOI] [PubMed] [Google Scholar]
- 18. Ruiz JR, Sui X, Lobelo F, Morrow JR Jr, Jackson AW, Sjostrom M, Blair SN. Association between muscular strength and mortality in men: prospective cohort study. BMJ. 2008;337:a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sasaki H, Kasagi F, Yamada M, Fujita S. Grip strength predicts cause‐specific mortality in middle‐aged and elderly persons. Am J Med. 2007;120:337–342. [DOI] [PubMed] [Google Scholar]
- 20. Beunen G, Thomis M. Gene driven power athletes? Genetic variation in muscular strength and power. Br J Sports Med. 2006;40:822–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low‐dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. [DOI] [PubMed] [Google Scholar]
- 22. Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Low‐dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. [DOI] [PubMed] [Google Scholar]
- 23. Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. [DOI] [PubMed] [Google Scholar]
- 24. Ainsworth BE, Leon AS, Richardson MT, Jacobs DR, Paffenbarger RS Jr. Accuracy of the college alumnus Physical Activity Questionnaire. J Clin Epidemiol. 1993;46:1403–1411. [DOI] [PubMed] [Google Scholar]
- 25. Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self‐administered Physical Activity Questionnaire. Int J Epidemiol. 1994;23:991–999. [DOI] [PubMed] [Google Scholar]
- 26. Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Buchner D, Ettinger W, Heath GW, King AC, Kriska A, Leon AS, King AC, Marcus BH, Morris J, Paffenbarger RS Jr, Patrick K, Pollock ML, Rippe JM, Sallis J, Wilmore JH. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. [DOI] [PubMed] [Google Scholar]
- 27. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 28. Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–540. [DOI] [PubMed] [Google Scholar]
- 29. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. [DOI] [PubMed] [Google Scholar]
- 30. Eijsvogels TM, Thompson PD. Exercise is medicine: at any dose? JAMA. 2015;314:1915–1916. [DOI] [PubMed] [Google Scholar]
- 31. Arem H, Moore SC, Patel A, Hartge P, Berrington de Gonzalez A, Visvanathan K, Campbell PT, Freedman M, Weiderpass E, Adami HO, Linet MS, Lee IM, Matthews CE. Leisure time physical activity and mortality: a detailed pooled analysis of the dose‐response relationship. JAMA Intern Med. 2015;175:959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shiroma EJ, Cook NR, Manson JE, Moorthy MV, Buring JE, Rimm EB, Lee IM. Strength training and the risk of type 2 diabetes and cardiovascular disease. Med Sci Sports Exerc. 2017;49:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palatini P, Mos L, Munari L, Valle F, Del Torre M, Rossi A, Varotto L, Macor F, Martina S, Pessina AC. Blood pressure changes during heavy‐resistance exercise. J Hypertens Suppl. 1989;7:S72–S73. [DOI] [PubMed] [Google Scholar]
- 34. Pratley R, Nicklas B, Rubin M, Miller J, Smith A, Smith M, Hurley B, Goldberg A. Strength training increases resting metabolic rate and norepinephrine levels in healthy 50‐ to 65‐yr‐old men. J Appl Physiol (1985). 1994;76:133–137. [DOI] [PubMed] [Google Scholar]
- 35. Longhurst JC, Stebbins CL. The power athlete. Cardiol Clin. 1997;15:413–429. [DOI] [PubMed] [Google Scholar]
- 36. Conger SA, Guo J, Fulkerson SM, Pedigo L, Chen H, Bassett DR. Objective assessment of strength training exercises using a wrist‐worn accelerometer. Med Sci Sports Exerc. 2016;48:1847–1855. [DOI] [PubMed] [Google Scholar]
- 37. Shiroma EJ, Freedson PS, Trost SG, Lee IM. Patterns of accelerometer‐assessed sedentary behavior in older women. JAMA. 2013;310:2562–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee IM, Shiroma EJ. Using accelerometers to measure physical activity in large‐scale epidemiological studies: issues and challenges. Br J Sports Med. 2014;48:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Centers for Disease Control and Prevention. National Health Interview Survey . http://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm. Accessed June 7, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Relative Risks of All‐Cause Mortality With Strength Training by Subgroup, Women's Health Study (2001–2015)
Table S2. Relative Risks of All‐Cause Mortality With Strength Training (Simple‐Updated and Baseline‐Value Models), Women's Health Study (2001–2015)
Figure S1. Physical Activity Questionnaire, Women's Health Study.
Figure S2. Unadjusted cumulative incidence of deaths from all‐cause (A), cardiovascular diseases (B), and cancer (C) by baseline weekly time of strength training, Women's Health Study (2001–2015).


