Abstract
Background
Evaluation of cardiovascular prognosis in patients with stable coronary heart disease is based on clinical characteristics and biomarkers indicating dysglycemia, dyslipidemia, renal dysfunction, and possibly cardiac dysfunction. Inflammation plays a key role in atherosclerosis, but the association between inflammatory biomarkers and clinical outcomes is less studied in this population.
Methods and Results
Overall, 15 828 patients with coronary heart disease in the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) trial were randomized to treatment with darapladib or placebo and observed for a median of 3.7 years. In 14 611 patients, levels of interleukin‐6 (IL‐6) and high‐sensitivity C‐reactive protein were measured in plasma samples: median levels were 2.1 (interquartile range, 1.4–3.2) ng/L and 1.3 (interquartile range, 0.6–3.1) mg/L, respectively. Associations between continuous levels or quartile groups and adjudicated outcomes were evaluated by spline graphs and Cox regression adjusted for clinical factors and cardiovascular biomarkers. IL‐6 was associated with increased risk of major adverse cardiovascular events (quartile 4 versus quartile 1 hazard ratio [HR], 1.60; 95% confidence interval [CI], 1.30–1.97; P<0.0001); cardiovascular death (HR, 2.15; 95% CI, 1.53–3.04; P<0.0001); myocardial infarction (HR, 1.53; 95% CI, 1.14–2.04; P<0.05); all‐cause mortality (HR, 2.11; 95% CI, 1.62–2.76; P<0.0001); and risk of hospitalization for heart failure (HR, 2.28; 95% CI, 1.34–3.89; P<0.001). Cancer death was doubled in the highest IL‐6 quartile group (HR, 2.34; 95% CI, 1.20–4.53; P<0.05). High‐sensitivity C‐reactive protein was associated with both cardiovascular and non‐cardiovascular events in the unadjusted model, but these did not remain after multivariable adjustments.
Conclusions
IL‐6, an upstream inflammatory marker, was independently associated with the risk of major adverse cardiovascular events, cardiovascular and all‐cause mortality, myocardial infarction, heart failure, and cancer mortality in patients with stable coronary heart disease. IL‐6 might reflect a pathophysiological process involved in the development of these events.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00799903.
Keywords: coronary disease, C‐reactive protein, inflammation, interleukin‐6, white blood cells
Subject Categories: Biomarkers, Inflammation, Chronic Ischemic Heart Disease, Coronary Artery Disease
Clinical Perspective
What Is New?
This study demonstrates that interleukin‐6 (IL‐6), an upstream inflammatory marker, was independently associated with the risk of major coronary events, cardiovascular and all‐cause mortality, myocardial infarction, heart failure, and cancer mortality in patients with stable coronary heart disease, which indicates a potential pathophysiological association.
What Are the Clinical Implications?
Because IL‐6 is strongly associated with clinical events, anti‐inflammatory drugs targeting IL‐6 may be a potential future target for the treatment of stable coronary heart disease.
Inflammation plays a key role in the initiation and progression of atherosclerotic disease.1 Interleukin‐6 (IL‐6) is considered an upstream inflammatory cytokine that plays a central role as a mediator propagating the inflammatory response and is essential to the initiation and progression of the atherosclerotic process.2 Upstream IL‐6 leads to the hepatic production of downstream acute‐phase reactant C‐reactive protein (CRP). Several inflammatory biomarkers, such as IL‐6, have been associated with and predicted the risk of future cardiovascular events,3 supporting the inflammation hypothesis. The association between these markers, including the proximal mediator IL‐6 and high‐sensitivity CRP (hs‐CRP), and different events has been demonstrated in both healthy individuals4, 5 and patients with acute coronary syndrome.6 Assessment of the risk of events among patients with stable coronary heart disease (CHD) is mainly based on clinical characteristics and biomarkers indicating dysglycemia, dyslipidemia, renal dysfunction, and possibly inflammatory biomarkers, such as white blood cell (WBC) counts.7 WBC count, in previous studies, has been associated with cardiovascular mortality.8 The extent to which IL‐6 and hs‐CRP are associated with cardiovascular or non‐cardiovascular events in this population is less well known.9
The STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) trial was a large global study that randomized 15 828 patients with stable chronic coronary artery disease to evaluate the efficacy and safety of darapladib, 160 mg (an inhibitor of lipoprotein‐associated phospholipase A2 [Lp‐PLA2]), or placebo, added to optimal standard of care. The median follow‐up was 3.7 years. The results have been presented previously.10
The aim of this substudy was to assess the independent association between the levels of biomarkers of inflammation, hs‐CRP and IL‐6, to the risk of cardiovascular death, myocardial infarction (MI), stroke, hospitalization for heart failure, or cancer.
Methods
Study Design
The study design has been previously presented.11 The study was approved by national regulatory authorities and by local ethics committees or institutional review boards, according to local regulations, and all patients gave informed consent. In summary, patients with stable CHD, defined as prior MI, prior coronary revascularization, or multivessel CHD confirmed by coronary angiography, were eligible. In addition, patients had to meet at least 1 of the following cardiovascular risk criteria: aged ≥60 years, diabetes mellitus requiring pharmacotherapy, high‐density lipoprotein cholesterol level <1.03 mmol/L, current or previous smoker (defined as ≥5 cigarettes per day on average), significant renal dysfunction (estimated glomerular filtration rate ≥30 and <60 mL/min per 1.73 m2 or urine albumin/creatinine ratio ≥30 mg albumin/g creatinine), or polyvascular disease (CHD and cerebrovascular disease or CHD and peripheral arterial disease). The primary end point of major adverse coronary events (MACEs) was the composite of cardiovascular death, MI, or stroke. One of the secondary end points was major coronary events consisting of CHD death, MI, and urgent coronary revascularization for myocardial ischemia. A blinded clinical events committee adjudicated all selected efficacy end points, using prespecified criteria. The event definitions and main results of the study have been presented elsewhere.10
Biochemical Methods
Venous blood samples were obtained at randomization before the start of study drug treatment. All tubes, EDTA for hs‐CRP and citrate for IL‐6, were centrifuged within 30 minutes at 2000g for 10 minutes in room temperature and then frozen at −20°C or colder. Long‐term storage was at −70°C or colder. Levels of IL‐6 and hs‐CRP were measured in 14 611 and in 14 406 patients, respectively. Data on WBC counts were available in 15 272 individuals. Plasma concentrations of high sensitive IL‐6 were analyzed using an ELISA technique. Plasma concentrations of hs‐CRP were analyzed using a particle‐enhanced immunonephelometry assay, CardioPhase hs‐CRP. The levels of high‐sensitivity cardiac troponin‐T, NT‐proBNP (N‐terminal pro B‐type natriuretic peptide), growth differentiation factor 15 (precommercial assay), and cystatin C were determined in EDTA plasma by electrochemiluminescence immunoassays, using a Cobas Analytics e601, performed at the Uppsala Clinical Research Center Laboratory at Uppsala University (Uppsala, Sweden). Lp‐PLA2 activity was measured in an automated enzyme assay system (PLAC Test for Lp‐PLA2 Activity).
Statistical Analysis
All outcomes were analyzed with IL‐6, hs‐CRP, and WBC count, as both categorical variables based on quartile groups and continuous predictors. For the analyses based on quartile groups, several adjusted Cox proportional hazard models (models 1–3 shown below) were used. The hazard ratio (HR) and 95% confidence interval (CI) were calculated, using the group with the lowest biomarker levels as reference. Kaplan‐Meier estimates of the cumulative risk to first occurrence of an event were calculated and plotted by biomarker quartile groups. For the analysis based on continuous IL‐6 and hs‐CRP, a Cox proportional hazards model was used, with the continuous biomarker as a restricted cubic spline. All analyses were performed using observed cases without imputation of missing data.
Multivariable models were performed in 4 steps. A basic model included the biomarker under consideration and randomized treatment. The first model (model 1) added clinical background characteristics (age, sex, race group, diabetes mellitus, hypertension, blood pressure, smoking, body mass index, renal function, prior MI, prior percutaneous coronary intervention or coronary artery bypass graft surgery, multivessel coronary artery disease, and polyvascular disease). The second model (model 2) added standard biomarkers, such as hemoglobin, estimated glomerular filtration rate (according to the Chronic Kidney Disease Epidemiology Collaboration), low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and triglycerides to the previous model. Last, in the third fully adjusted model (model 3), we added the following other biomarkers of prognostic importance: high‐sensitivity cardiac troponin‐T, NT‐proBNP, cystatin‐C, hs‐CRP, IL‐6, WBC counts, growth differentiation factor 15, and Lp‐PLA2. When analyzing 1 inflammatory marker (IL‐6, hs‐CRP, or WBC count), the other 2 were entered into the model.
Results
IL‐6 and CRP Levels
The median IL‐6 level was 2.1 ng/L. The baseline characteristics by quartile groups of IL‐6 are shown in Table 1. Most clinical factors were associated with higher IL‐6 levels, such as age, region, white race, body mass index, smoking, hypertension, renal dysfunction, multivessel disease, and polyvascular disease (Table 2). The strongest independently associated variables of increased IL‐6 levels were region and smoking (Table 2).
Table 1.
Summary of Demographic and Baseline Characteristics by Baseline Quartile Groups of IL‐6 and hs‐CRP
| Characteristics | IL‐6 | hs‐CRP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <1.4 ng/L (n=3148) | 1.4–2.1 ng/L (n=3952) | 2.1–3.2 ng/L (n=3742) | ≥3.2 ng/L (n=3769) | P Value | <0.6 mg/L (n=2872) | 0.6–1.3 mg/L (n=3987) | 1.3–3.1 mg/L (n=3864) | ≥3.1 mg/L (n=3683) | P Value | |
| Age at randomization, y | 62.8 (9.2) | 64.2 (9.1) | 64.8 (9.3) | 65.8 (9.5) | <0.0001 | 64.8 (9.0) | 64.8 (9.2) | 64.3 (9.4) | 63.8 (9.5) | <0.0001 |
| Male sex, n (%) | 2601 (82.6) | 3284 (83.1) | 3006 (80.3) | 3020 (80.1) | 0.0006 | 2427 (84.5) | 3310 (83.0) | 3161 (81.8) | 2844 (77.2) | <0.0001 |
| Race, n (%) | <0.0001 | <0.0001 | ||||||||
| White | 2326 (73.9) | 3231 (81.8) | 3129 (83.6) | 3107 (82.4) | 1924 (67.0) | 3158 (79.2) | 3201 (82.8) | 3065 (83.2) | ||
| Black | 38 (1.2) | 85 (2.2) | 80 (2.1) | 113 (3.0) | 32 (1.1) | 62 (1.6) | 72 (1.9) | 138 (3.7) | ||
| Central/South/South East Asian | 253 (8.0) | 253 (6.4) | 263 (7.0) | 288 (7.6) | 263 (9.2) | 264 (6.6) | 250 (6.5) | 215 (5.8) | ||
| East Asian/Japanese | 470 (14.9) | 286 (7.2) | 206 (5.5) | 178 (4.7) | 602 (21.0) | 408 (10.2) | 256 (6.6) | 192 (5.2) | ||
| Other | 61 (1.9) | 97 (2.5) | 64 (1.7) | 83 (2.2) | 51 (1.8) | 95 (2.4) | 85 (2.2) | 73 (2.0) | ||
| Geographic region, n (%) | <0.0001 | <0.0001 | ||||||||
| Asia/Pacific | 818 (26.0) | 645 (16.3) | 580 (15.5) | 538 (14.3) | 943 (32.8) | 800 (20.1) | 615 (15.9) | 478 (13.0) | ||
| Eastern Europe | 668 (21.2) | 979 (24.8) | 953 (25.5) | 850 (22.6) | 469 (16.3) | 865 (21.7) | 1003 (26.0) | 1006 (27.3) | ||
| North America | 709 (22.5) | 1050 (26.6) | 998 (26.7) | 1082 (28.7) | 669 (23.3) | 1069 (26.8) | 977 (25.3) | 1046 (28.4) | ||
| South America | 113 (3.6) | 208 (5.3) | 240 (6.4) | 314 (8.3) | 127 (4.4) | 229 (5.7) | 220 (5.7) | 232 (6.3) | ||
| Western Europe | 840 (26.7) | 1070 (27.1) | 971 (25.9) | 985 (26.1) | 664 (23.1) | 1024 (25.7) | 1049 (27.1) | 921 (25.0) | ||
| BMI, kg/m2 | 27.5 (4.1) | 28.8 (4.6) | 29.7 (5.1) | 30.0 (5.7) | <0.0001 | 27.0 (4.2) | 28.5 (4.5) | 29.5 (4.9) | 30.5 (5.6) | <0.0001 |
| Weight, kg | 79.5 (15.3) | 83.7 (16.4) | 85.9 (17.5) | 86.6 (19.6) | <0.0001 | 78.1 (15.5) | 82.5 (15.9) | 85.8 (17.7) | 88.0 (19.0) | <0.0001 |
| Current smoker, n (%) | 490 (15.6) | 673 (17.0) | 696 (18.6) | 794 (21.1) | <0.0001 | 407 (14.2) | 600 (15.0) | 754 (19.5) | 857 (23.3) | <0.0001 |
| Hypertension, n (%) | 2066 (65.6) | 2806 (71.0) | 2742 (73.3) | 2838 (75.3) | <0.0001 | 1904 (66.3) | 2749 (68.9) | 2799 (72.4) | 2813 (76.4) | <0.0001 |
| Diabetes mellitus, n (%) | 1032 (32.8) | 1470 (37.2) | 1483 (39.6) | 1666 (44.2) | <0.0001 | 1014 (35.3) | 1444 (36.2) | 1451 (37.6) | 1640 (44.5) | <0.0001 |
| Renal dysfunction, n (%) | 632 (20.1) | 1046 (26.5) | 1220 (32.6) | 1505 (39.9) | <0.0001 | 688 (24.0) | 1094 (27.4) | 1198 (31.0) | 1354 (36.8) | <0.0001 |
| Prior MI, n (%) | 1823 (57.9) | 2376 (60.1) | 2243 (59.9) | 2209 (58.6) | 0.1752 | 1649 (57.4) | 2309 (57.9) | 2280 (59.0) | 2180 (59.2) | 0.3826 |
| Multivessel CHD, n (%) | 392 (12.5) | 545 (13.8) | 533 (14.2) | 604 (16.0) | 0.0003 | 363 (12.6) | 537 (13.5) | 527 (13.6) | 565 (15.3) | 0.0116 |
| Polyvascular disease, n (%) | 309 (9.8) | 559 (14.1) | 645 (17.2) | 712 (18.9) | <0.0001 | 313 (10.9) | 524 (13.1) | 619 (16.0) | 723 (19.6) | <0.0001 |
| Prior PCI/CABG surgery, n (%) | 2386 (75.8) | 2969 (75.1) | 2787 (74.5) | 2752 (73.0) | 0.0472 | 2205 (76.8) | 3042 (76.3) | 2835 (73.4) | 2702 (73.4) | 0.0003 |
| Systolic BP, mm Hg | 130.6 (15.8) | 132.1 (16.1) | 132.2 (16.5) | 131.7 (17.2) | <0.0001 | 130.4 (16.0) | 131.8 (16.2) | 132.2 (16.5) | 132.0 (16.8) | <0.0001 |
| Diastolic BP, mm Hg | 78.8 (10.0) | 79.0 (10.2) | 79.0 (10.3) | 77.9 (10.7) | <0.0001 | 77.6 (10.1) | 78.8 (10.3) | 79.3 (10.3) | 79.1 (10.4) | <0.0001 |
| hs‐CRP, mg/L | 1.1 (1.7) | 1.6 (1.9) | 2.7 (3.3) | 6.5 (11.7) | <0.0001 | |||||
| cTnT‐hs, ng/L | 9.1 (6.8) | 11.3 (13.1) | 12.6 (16.2) | 16.3 (25.2) | <0.0001 | 10.4 (8.2) | 11.5 (9.9) | 12.5 (17.3) | 14.8 (28.1) | <0.0001 |
| NT‐proBNP, ng/L | 217.8 (311.1) | 284.5 (409.1) | 350.9 (495.5) | 604.0 (1344.4) | <0.0001 | 285.3 (506.5) | 307.2 (526.3) | 362.3 (701.5) | 525.9 (1228.9) | <0.0001 |
| Cystatin C, mg/L | 1.0 (0.2) | 1.0 (0.2) | 1.1 (0.3) | 1.2 (0.4) | <0.0001 | 1.0 (0.3) | 1.0 (0.3) | 1.1 (0.3) | 1.1 (0.3) | <0.0001 |
| GDF‐15, ng/L | 1248 (819) | 1433 (948) | 1586 (1022) | 1958 (1549) | <0.0001 | 1433.2 (974.3) | 1477.2 (1059.2) | 1543.9 (1085.7) | 1794.2 (1382.8) | <0.0001 |
| Lp‐PLA2 activity, μmol/min per L | 169.5 (46.9) | 175.5 (46.9) | 177.1 (47.6) | 180.0 (49.3) | <0.0001 | 167.6 (47.6) | 173.4 (45.4) | 179.9 (47.6) | 180.8 (49.8) | <0.0001 |
BMI indicates body mass index; BP, blood pressure; CABG, coronary artery bypass graft; CHD, coronary heart disease; cTnT‐hs, high‐sensitivity cardiac troponin‐T; GDF‐15, growth differentiation factor 15; hs‐CRP, high‐sensitivity C‐reactive protein; IL‐6, interleukin‐6; Lp‐PLA2, lipoprotein‐associated phospholipase A2; MI, myocardial infarction; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; and PCI, percutaneous coronary intervention.
Table 2.
Multivariate Analyses of Factors Associated With IL‐6 and hs‐CRP Levels
| Background Characteristic | IL‐6 | hs‐CRP | ||||
|---|---|---|---|---|---|---|
| Relative Increase | 95% CI | P Value | Relative Increase | 95% CI | P Value | |
| Female vs male | 1.02 | 0.97–1.07 | 0.5023 | 1.21 | 1.15–1.27 | <0.0001 |
| Eastern Europe vs North America | 0.98 | 0.93–1.03 | 0.4229 | 1.17 | 1.12–1.24 | <0.0001 |
| Western Europe vs North America | 0.97 | 0.92–1.02 | 0.2385 | 1.09 | 1.03–1.14 | 0.0017 |
| South America vs North America | 1.27 | 1.15–1.40 | <0.0001 | 1.20 | 1.10– 1.31 | <0.0001 |
| Asia/Pacific vs North America | 0.93 | 0.88–0.99 | 0.0135 | 0.81 | 0.76–0.86 | <0.0001 |
| Diagnosis of hypertension | 1.02 | 0.98–1.06 | 0.3776 | 1.06 | 1.02–1.11 | 0.0061 |
| Previous MI | 1.03 | 0.99–1.07 | 0.1968 | 1.01 | 0.97–1.06 | 0.4928 |
| Previous PCI or CABG surgery | 0.99 | 0.95–1.04 | 0.7455 | 0.95 | 0.91–1.00 | 0.0331 |
| Multivessel CHD | 1.09 | 1.03–1.15 | 0.0020 | 1.09 | 1.04–1.15 | 0.0013 |
| Diabetes mellitus | 1.03 | 0.99–1.07 | 0.2044 | 1.01 | 0.97–1.05 | 0.6371 |
| Former smoker vs never smoked | 1.07 | 1.03–1.12 | 0.0014 | 1.12 | 1.08–1.17 | <0.0001 |
| Current smoker vs never smoked | 1.23 | 1.16–1.31 | <0.0001 | 1.46 | 1.38–1.55 | <0.0001 |
| Polyvascular disease | 1.14 | 1.08–1.20 | <0.0001 | 1.23 | 1.17–1.30 | <0.0001 |
| Significant renal dysfunction | 1.17 | 1.13–1.22 | <0.0001 | 1.25 | 1.20–1.30 | <0.0001 |
| Age, 10‐y increase | 1.09 | 1.07–1.12 | <0.0001 | 0.99 | 0.97–1.01 | 0.4672 |
| BMI, 1‐U increase | 1.02 | 1.02–1.02 | <0.0001 | 1.05 | 1.04–1.05 | <0.0001 |
Multivariable adjustments for randomized treatment, age, systolic blood pressure, BMI, sex, history of hypertension, geographic region for final reporting, prior MI, prior coronary revascularization (PCI or CABG surgery), prior multivessel CHD, baseline diabetes mellitus, smoking, polyvascular disease, and significant renal dysfunction (model 1). BMI indicates body mass index; CABG, coronary artery bypass graft; CHD, coronary heart disease; CI, confidence interval; hs‐CRP, high‐sensitivity C‐reactive protein; IL‐6, interleukin‐6; MI, myocardial infarction; and PCI, percutaneous coronary intervention.
The median level of hs‐CRP was 1.3 mg/L. Patient characteristics at baseline by quartile groups of hs‐CRP are shown in Table 1. Higher levels of hs‐CRP were associated with female sex, body mass index, region, white race, smoking, hypertension, renal dysfunction, polyvascular disease, and multivessel coronary disease (Table 2). High hs‐CRP was also associated with higher levels of low‐density lipoprotein cholesterol, triglycerides, and WBC count; lower levels of high‐density lipoprotein cholesterol; and more frequent use of secondary prevention drugs, such as β blockers and angiotensin‐converting enzyme inhibitors, but less use of aspirin and statins (data not shown). The strongest independently associated variables of increased hs‐CRP levels were smoking, renal dysfunction, and female sex (Table 2).
IL‐6 Levels and Outcomes
The unadjusted association between quartile groups of IL‐6 and risk of MACE is presented as Kaplan‐Meier (KM) plots in Figure 1A, showing a graded increase in risk in the higher quartile groups. Figure 2A illustrates restricted cubic spline plots for continuous levels of IL‐6 and risk of MACE, cardiovascular death, and hospitalization for heart failure. With IL‐6 levels >1.5 ng/L, the risk of cardiovascular death and MACE started to increase to an almost 4‐fold difference among those with the highest values. The C‐indexes for the risk of individual clinical outcomes, when adding IL‐6 (to model 1), are shown in Table 3. There is an average increase in C‐index of 2% to 3%, such as a C‐index change from 0.636 (95% CI, 0.620–0.652) to 0.654 (95% CI, 0.639–0.669) for the risk of MACE and from 0.764 (95% CI, 0.738–0.789) to 0.793 (95% CI, 0.768–0.817) for heart failure. Figure 3A through 3C illustrate the Forest plots, with the HR for the lowest quartile group as reference, with different adjustment levels. In the fully adjusted model (Figure 3C), which also included standard biomarkers (hs‐CRP, growth differentiation factor 15, and Lp‐PLA2 activity), the HRs for the risk of various end points are shown. The HR for MACE in the highest quartile group compared with the lowest was 1.59 (95% CI, 1.29–1.97; P<0.0001) and the corresponding HR for cardiovascular death was 2.16 (95% CI, 1.52–3.06; P<0.0001). High IL‐6 levels were also associated with the risk of MI (HR, 1.55; 95% CI, 1.16–2.09; P<0.05) and all‐cause mortality (HR, 2.04; 95% CI, 1.56–2.68; P<0.0001). In addition, IL‐6 was predictive of the risk of hospitalization for HF, with an HR of 2.37 (95% CI, 1.34–4.18; P<0.001). Of interest, the risks of non‐cardiovascular death and specifically cancer deaths were more than doubled in the highest quartile. There was no statistically significant association between IL‐6 and risk of stroke.
Figure 1.
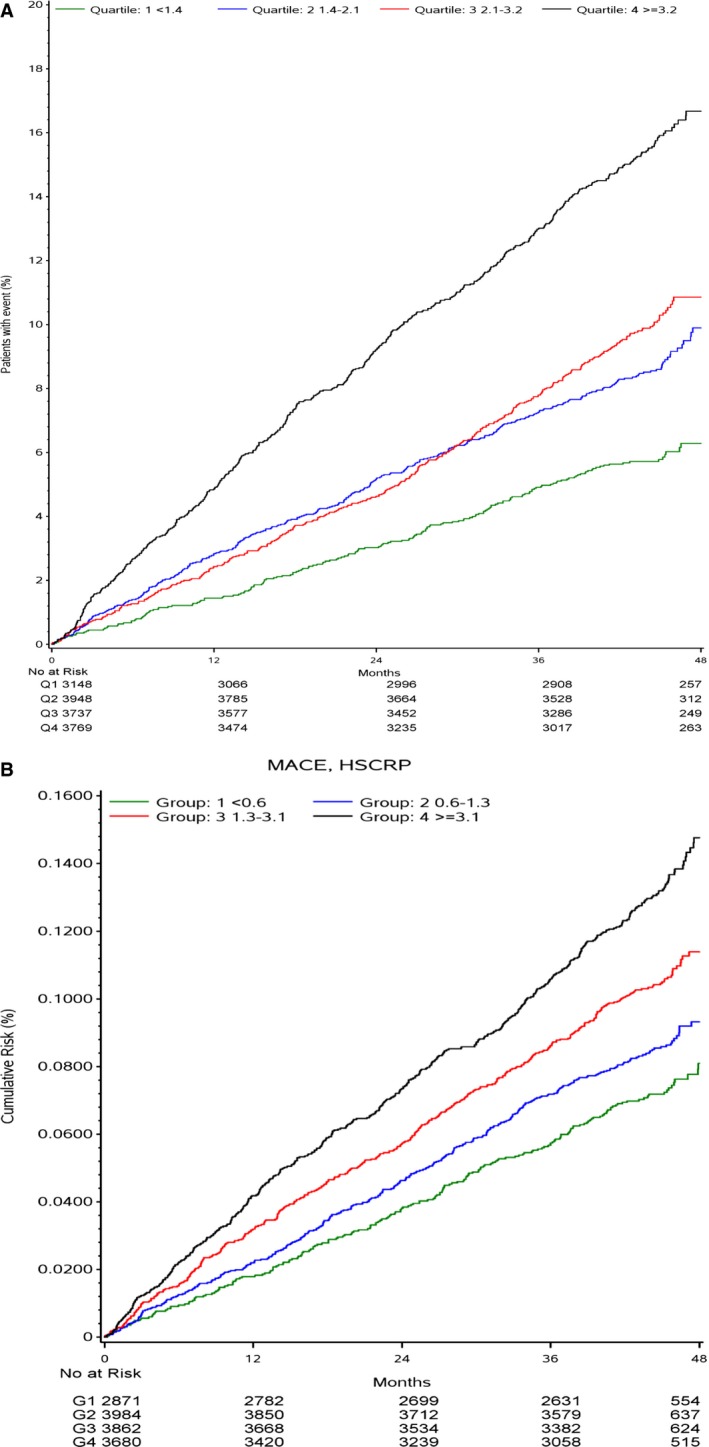
A, Kaplan‐Meier curves for major adverse cardiovascular event (MACE) by interleukin‐6 quartile (Q) groups. B, Kaplan‐Meier curves for MACE by high‐sensitivity C‐reactive protein (hs‐CRP) Q groups.
Figure 2.
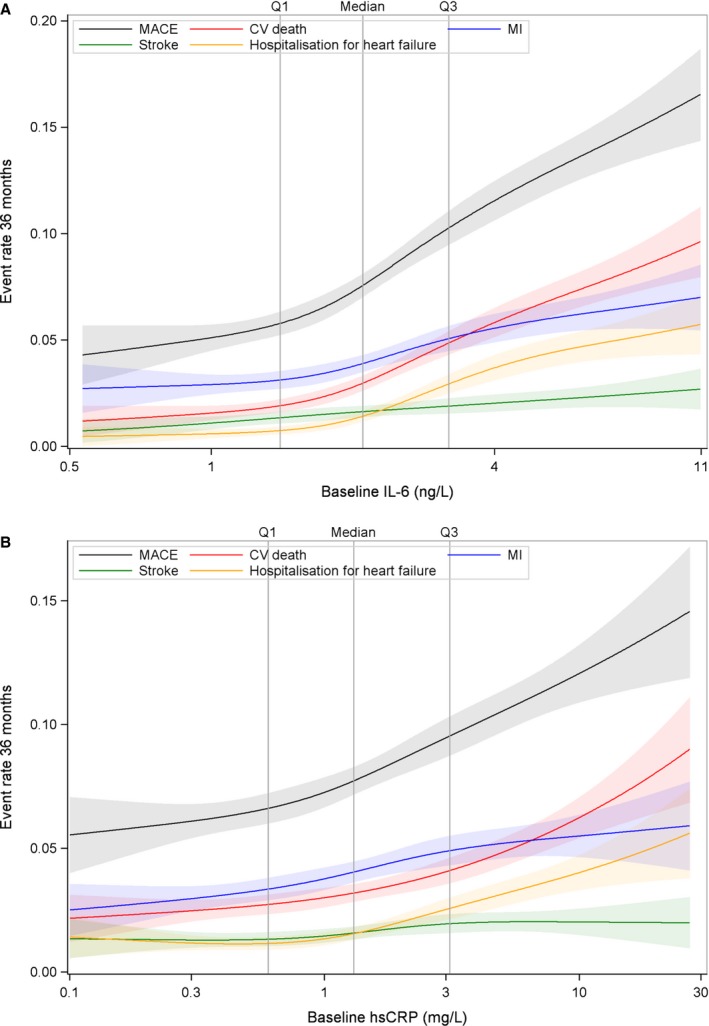
A, Spline plots for major adverse cardiovascular event (MACE), cardiovascular (CV) death, myocardial infarction (MI), heart failure, and stroke by quartile (Q) groups of interleukin‐6 (IL‐6). B, Spline plots for MACE, CV death, MI, heart failure, and stroke by Q groups of high‐sensitivity C‐reactive protein (hs‐CRP).
Table 3.
C‐Indexes for Adding IL‐6 by Categories and Various Clinical Outcomes
| Outcome | Model | C‐Index (95% CI) |
|---|---|---|
| MACE | Model 1 | 0.636 (0.620–0.652) |
| Model 1+IL‐6 | 0.654 (0.639–0.669) | |
| MCE | Model 1 | 0.624 (0.608–0.640) |
| Model 1+IL‐6 | 0.639 (0.623–0.655) | |
| Cardiovascular death | Model 1 | 0.731 (0.710–0.753) |
| Model 1+IL‐6 | 0.755 (0.735–0.775) | |
| MI | Model 1 | 0.632 (0.610–0.654) |
| Model 1+IL‐6 | 0.641 (0.618–0.663) | |
| Stroke | Model 1 | 0.649 (0.614–0.684) |
| Model 1+IL‐6 | 0.657 (0.622–0.692) | |
| Heart failure | Model 1 | 0.764 (0.738–0.789) |
| Model 1+IL‐6 | 0.793 (0.768–0.817) | |
| Total death | Model 1 | 0.711 (0.694–0.728) |
| Model 1+IL‐6 | 0.739 (0.723–0.755) | |
| Cancer death | Model 1 | 0.708 (0.668–0.747) |
| Model 1+IL‐6 | 0.742 (0.704–0.780) |
CI indicates confidence interval; IL‐6, interleukin‐6; MACE, major adverse cardiovascular event; MCE, major coronary event; MI, myocardial infarction.
Figure 3.
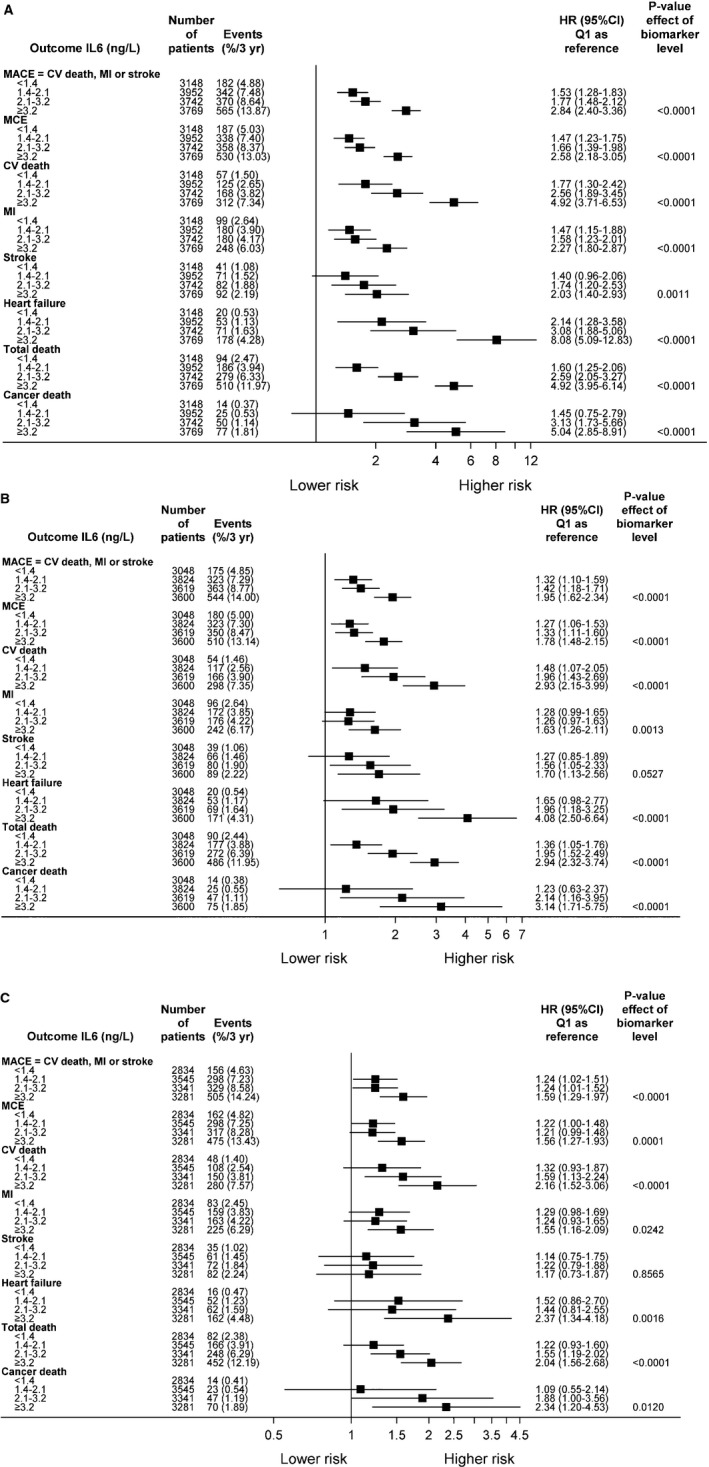
A, The impact of interleukin‐6 (IL‐6) by baseline quartile (Q) groups on outcome (unadjusted analyses). B, The impact of IL‐6 by baseline Q groups on outcome. Adjustments were made for randomized treatment, age, systolic blood pressure (BP), body mass index (BMI), sex, history of hypertension, geographic region for final reporting, prior myocardial infarction (MI), prior coronary revascularization (percutaneous coronary intervention [PCI] or coronary artery bypass graft [CABG] surgery), prior multivessel coronary heart disease (CHD), baseline diabetes mellitus, smoking, polyvascular disease, and significant renal dysfunction. C, The impact of IL‐6 by Q groups at baseline on outcome. Adjustments were made for all variables in model B + hemoglobin, white blood cell count, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglycerides, estimated glomerular filtration rate (according to the Chronic Kidney Disease Epidemiology Collaboration), high‐sensitivity cardiac troponin‐T, NT‐proBNP (Nterminal pro B‐type natriuretic peptide), high‐sensitivity C‐reactive protein, cystatin C, growth differentiation factor 15, and lipoprotein‐associated phospholipase A2 activity. CI indicates confidence interval; CV, cardiovascular; HR, hazard ratio; MACE, major adverse cardiovascular event; and MCE, major coronary event.
hs‐CRP Level and Outcomes
The unadjusted association between quartile groups of hs‐CRP and risk of MACE is presented as KM plots in Figure 1B, showing a graded increase in risk with higher quartile groups.
In Figure 2B, restricted cubic spline plots (unadjusted) for continuous levels of CRP and the risk of MACE, cardiovascular death, MI, and stroke are depicted. The steepest curves were seen for MACE, mainly driven by an increased risk for cardiovascular death.
In the unadjusted analysis, Forest plots on cardiovascular outcomes by quartile groups of baseline hs‐CRP (quartile 4 versus quartile 1) were gradually associated with increased risk of MACE (HR, 1.89; 95% CI, 1.61–2.22; P<0.0001), cardiovascular death (HR, 2.16; 95% CI, 1.70–2.75; P<0.0001), stroke (HR, 1.76; 95% CI, 1.21–2.55; P<0.05), hospitalization for heart failure (HR, 3.51; 95% CI, 2.4–5.09; P<0.0001), non‐cardiovascular mortality (HR, 2.46; 95% CI, 2.03–2.98; P<0.0001), and cancer deaths (HR, 3.01; 95% CI, 1.79–5.08; P<0.0001) over time, with increasing levels in upper hs‐CRP quartiles (Figure 4A). However, the associations were slightly attenuated in model 2 (Figure 4B) and in the fully adjusted model 3. As indicated in Figure 4C, these associations were strongly completely attenuated and hs‐CRP was no longer significantly associated with any of the cardiovascular (MACE [HR, 0.95; 95% CI, 0.78–1.17; P=0.84] or cardiovascular death [HR, 0.77; 95% CI, 0.57–1.04; P=0.40]) or non‐cardiovascular (cancer death [HR, 1.58; 95% CI, 0.85–2.93; P=0.47]) outcomes.
Figure 4.
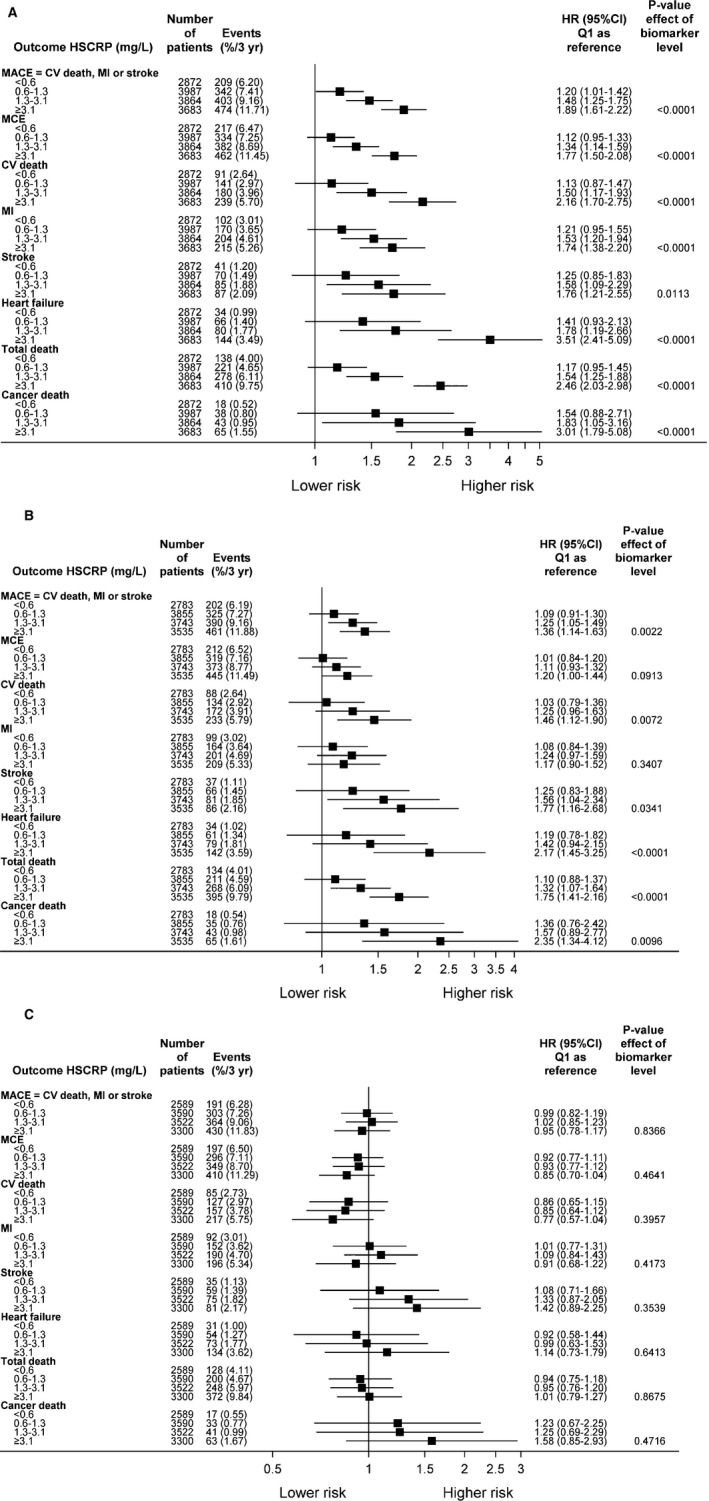
A, The impact of high‐sensitivity C‐reactive protein (hs‐CRP) by baseline quartile (Q) groups on outcome (unadjusted analyses). B, The impact of hs‐CRP by baseline Q groups on outcome. Adjustments were made for randomized treatment, age, systolic blood pressure (BP), body mass index (BMI), sex, history of hypertension, geographic region for final reporting, prior myocardial infarction (MI), prior coronary revascularization (percutaneous coronary intervention [PCI] or coronary artery bypass graft [CABG] surgery), prior multivessel coronary heart disease (CHD), baseline diabetes mellitus, smoking, polyvascular disease, and significant renal dysfunction. C, The impact of hs‐CRP by baseline Q groups on outcome. Adjustments were made for all variables in model B + hemoglobin, white blood cell count, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglycerides, estimated glomerular filtration rate (according to the Chronic Kidney Disease Epidemiology Collaboration), high‐sensitivity cardiac troponin‐T, NT‐proBNP (N‐terminal pro B‐type natriuretic peptide), interleukin‐6, cystatin C, growth differentiation factor 15, and lipoprotein‐associated phospholipase A2 activity. CI indicates confidence interval; CV, cardiovascular; HR, hazard ratio; MACE, major adverse cardiovascular event; and MCE, major coronary event.
WBC Count
Similar analyses as above were performed with WBC counts, both unadjusted and after multivariable adjustments. Tables S1 and S2 show baseline demographics and predictors of levels of WBC count. WBC counts were most strongly associated with smoking, region, and polyvascular disease (Table S2). WBC counts were associated with increased rate of MACE by increasing quartile groups (Figure S1) and were seen as a continuous variable in spline plots for the separate end points (Figure S2). WBC counts were associated with the risk of MACE (HR, 1.34; 95% CI, 1.12–1.60; P<0.05), major coronary events (HR, 1.28; 95% CI, 1.07–1.53; P<0.05), cardiovascular death (HR, 1.49; 95% CI, 1.13–1.96; P<0.05), and all‐cause mortality (HR, 1.42; 95% CI, 1.15–1.75; P<0.05) in the fully adjusted model (quartile 4 versus quartile 1) (Figure S3). No significant associations to the individual events MI, stroke, or non‐cardiovascular deaths were observed.
Discussion
The role of inflammation as a mechanism involved in the development of CHD is well established, although the importance of the many different pathways is more poorly understood.1 We have, in the present study, evaluated the independent prognostic associations between 3 of the most important systemic inflammatory markers (IL‐6, hs‐CRP, and WBC count) and the risk of cardiovascular and non‐cardiovascular outcomes in a large prospective study of patients with stable CHD. The main findings were that IL‐6 was strongly associated with the risk of MACE, MI, cardiovascular death, total death, and hospitalization for heart failure after multivariable adjustments for conventional risk factors and standard and specific biomarkers, including CRP, growth differentiation factor 15, and Lp‐PLA2 activity. This was corroborated by a higher C‐index when adding IL‐6 to the model. hs‐CRP was significantly associated with the risk of MACE, cardiovascular death, and hospitalization for heart failure only in unadjusted models, which were no longer significant after adjusting for clinical variables and biomarkers. Finally, WBC count remained associated with MACE, cardiovascular death, and major coronary events after multivariable adjustments.
These findings underline the importance of inflammation as an important mechanistic pathway for the risk of future clinical outcomes. Also, there is a potential for developing new treatments targeting inflammation.
We found an interesting positive association between IL‐6 and the risk of MI. This has been shown previously in a large study in stable patients with a previous MI or unstable angina.8 In a recent meta‐analysis on healthy individuals, IL‐6 was associated with an adjusted HR of 1.25 (95% CI, 1.19–1.32) for the risk of MI.3 In a previous smaller study on patients with unstable angina after percutaneous coronary intervention, IL‐6, but not hs‐CRP, was associated with recurrent MI.12 Our results, thus, extend the prognostic importance of IL‐6 in healthy individuals, patients with atrial fibrillation,13 patients with acute coronary syndromes or patients after cardiac arrest,14 and those with stable CHD. The results show the association with cardiovascular events, which, to our knowledge, is the largest prospective study on this population. Interestingly, in a recent small study on patients with CHD, IL‐6 (compared with CRP) was more strongly associated with presence of thin‐cap fibroatheroma. The fibroatheroma was detected by optical coherence tomography during percutaneous coronary intervention,15 showing a potential mechanistic link to rupture‐prone plaques. Of interest, the risk of hospitalization for heart failure was significantly increased among patients with the highest IL‐6 levels, a finding that, to our awareness, has not been shown previously in a population with stable CHD. IL‐6 has predicted poor prognosis as a single risk marker or, as in a recent study on multimarker models,16 among patients with established heart failure. There are mechanistic hypotheses on how IL‐6 could cause cardiovascular death and hospitalization for heart failure. Short‐term IL‐6 elevations may be a protective response to an acute MI, whereas heart failure leads to long‐term IL‐6 production, which may have a negative causal role.17
Of interest, IL‐6 was not only associated with the risk of cardiovascular events (MACE, MI, and cardiovascular death) but also with all‐cause mortality and death from cancer. The explanations for these associations are not clearly understood. The associations to these parameters and to hospitalization for heart failure were stronger than those to major coronary events and MI, suggesting that inflammation is related to more diverse determinants of health than atherosclerosis alone. Chronic diseases, such as cancer or rheumatic diseases, may lead to long‐term signaling of IL‐6 that may be deleterious.17 No associations to stroke were found, which is consistent with other studies.
Three important markers of inflammation were compared in this analysis; IL‐6 seemed to completely blunt the predictive power of CRP, whereas WBC count remained an intermediate strong predictor. IL‐6, an upstream inflammatory marker, is considered to orchestrate the inflammatory response in atherosclerosis.2, 18 The IL‐6 effects may be mediated by downstream inflammatory proteins, such as CRP. There is still a debate about the role of CRP and whether it is directly involved in the atherosclerotic process or more simply a marker of risk. CRP has been suggested to increase synthesis of matrix metalloproteinases, thereby increasing collagen degradation and activation of the complement system.19 The other biomarkers, troponin T and NT‐proBNP, also seemed to strongly attenuate the association of hs‐CRP to cardiovascular outcome. This highlights the importance of other biomarkers, reflecting other physiologic mechanisms that seem to attenuate the association between CRP and prognosis.
The observed independent associations between IL‐6 and cardiovascular outcomes cannot be interpreted as if these relationships are causal, although there are indications in this direction. The presence of a polymorphism in the IL‐6 receptor was associated with a graded decrease in CRP and fibrinogen and possibly with lowering the risk of coronary artery disease in 1 study.20 This may be supported by a previous prospective study of patients with chronic kidney disease in whom the functional polymorphism 2174 G/C in the promoter of the IL‐6 gene was associated with history of cardiovascular disease and predicted the risk for future cardiovascular events.21
Causality remains to be evaluated in interventional studies. There are a few studies evaluating IL‐6 receptor inhibitors, a potential treatment target, of which 1 drug is tocilizumab, which was tested in patients with rheumatoid arthritis. However, there were significant safety concerns with elevations of both low‐density lipoprotein cholesterol and total cholesterol levels22; IL‐6 blockers are not suitable for prevention of atherosclerosis. Other anti‐inflammatory drugs, like canakinumab, are being tested in patients at high risk after MI.23 Similar polymorphisms for CRP, known to affect the levels of CRP, have been studied using mendelian randomization. However, these were not shown to be associated with an increased risk of ischemic heart disease.24
There are strengths and limitations with the current study. The major strengths are the long‐term, large, global, prospective approach in patients with stable CHD, with complete data on well‐defined and adjudicated events. This provided reliable results about the clinical outcomes during follow‐up. The study is an observational comparison, based on levels of IL‐6, hs‐CRP, and WBC count and outcomes. However, despite efforts to adjust for baseline differences between the quartile groups, residual confounding cannot be excluded.
Conclusion
In this long‐term prospective study on patients with stable CHD with optimal medical treatment, the inflammatory biomarker IL‐6, but not CRP, was independently associated with the risk of cardiovascular death, MACE, MI, hospitalization for heart failure, and all‐cause mortality. Multivariable adjustments for clinical parameters and cardiac, renal, and other inflammatory biomarkers were made. Also, WBC count carried independent prognostic information on the risk of cardiovascular events. These findings underline the importance of inflammatory activity as an important pathway for future cardiovascular fatal and nonfatal events and for non‐cardiovascular mortality in patients with stable CHD.
Sources of Funding
The STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) study was funded by GlaxoSmithKline. Roche Diagnostics (Rotkreuz, Switzerland) supported the research by providing the growth differentiation factor 15 assay free of charge.
Disclosures
Held reports an institutional research grant and speaker's bureau from AstraZeneca; and institutional research grants from Bristol‐Myers Squibb Merck & Co, GlaxoSmithKline, and Roche. White reports research grants and personal fees from GlaxoSmithKline; research grants and advisory board member for AstraZeneca; and research grants from Sanofi‐Aventis, Eli Lilly, National Institutes of Health, Merck Sharp & Dohme, George Institute, Omthera Pharmaceuticals, Pfizer New Zealand, Intarcia Therapeutics Inc., Elsai Inc., Dal‐GenE, and Daiichi‐Sankyo Pharma Development. Stewart reports grants and nonfinancial support from GlaxoSmithKline. Budaj reports investigator and consulting fees and honoraria for lectures from AstraZeneca, Sanofi‐Aventis, Bristol Myers Squibb/Pfizer, Novartis, and GlaxoSmithKline; and investigator fees from Boehringer Ingelheim and Eisai. Cannon reports research grants and consulting fees from Arisaph, AstraZeneca, Bristol‐Myers Squibb, Boehringer Ingelheim, GlaxoSmithKline, Merck, and Takeda; research grants from Janssen; and consulting fees from Alnylam, Amgen, Boehringer Ingelheim/Lilly, Kowa, Lipimedix, Pfizer, Regeneron, and Sanofi. Hochman reports travel reimbursement from GlaxoSmithKline; and support for drug distribution related to the ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) Trial from AstraZeneca. Koenig reports lecture and consultancy fees from Novartis, Amgen, and AstraZeneca; lecture fees from Actavis and Berlin‐Chemie; consultancy fees from GlaxoSmithKline, The Medicines Company, Pfizer, Merck Sharpe & Dohme, and Kowa; and research grants from Roche Diagnostics, Abbott, Singulex, and Beckmann. Siegbahn reports institutional research grants from AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb/Pfizer, and GlaxoSmithKline. Steg reports personal fees from GlaxoSmithKline, Amarin, Bayer, Boehringer Ingelheim, Bristol‐Myers‐Squibb, Daiichi‐Sankyo, Eli Lilly, Merck‐Sharpe‐Dohme, Novartis, Pfizer, The Medicines Company, CLS‐Behring, and Janssen; grants, personal fees, and other from Sanofi and Servier; and personal fees and other from AstraZeneca. Soffer reports employee and stock ownership of GlaxoSmithKline. Weaver has nothing to report. Östlund reports an institutional research grant from GlaxoSmithKline. Wallentin reports institutional research grants, consultancy fees, lecture fees, and travel support from Bristol‐Myers Squibb/Pfizer, AstraZeneca, GlaxoSmithKline, and Boehringer Ingelheim; institutional research grants from Merck & Co and Roche; consultancy fees from Abbott; and 2 patents involving growth differentiation factor 15.
Supporting information
Table S1. Summary of Demographic and Baseline Characteristics by Baseline Quartile Groups of WBC Count*
Table S2. Multivariate Analyses of Factors Associated With WBC Count*
Figure S1. Kaplan‐Meier curves for major adverse cardiovascular event (MACE) by baseline white blood cell (WBC) quartile (Q) groups.
Figure S2. Spline plots for major adverse cardiovascular event (MACE), cardiovascular (CV) death, myocardial infarction (MI), heart failure, and stroke by baseline quartile groups of white blood cell (WBC) count.
Figure S3. The impact of white blood cell (WBC) count on outcomes by baseline quartile (Q) groups.
Appendix S1. List of STABILITY Investigators.
Acknowledgments
Editorial support was provided by Emma Sandberg and Vendela Roos (Uppsala Clinical Research Center, Uppsala, Sweden).
(J Am Heart Assoc. 2017;6:e005077 DOI: 10.1161/JAHA.116.005077.)
References
- 1. Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hartman J, Frishman WH. Inflammation and atherosclerosis: a review of the role of interleukin‐6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev. 2014;22:147–151. [DOI] [PubMed] [Google Scholar]
- 3. Kaptoge S, Seshasai SR, Gao P, Freitag DF, Butterworth AS, Borglykke A, Di Angelantonio E, Gudnason V, Rumley A, Lowe GD, Jorgensen T, Danesh J. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta‐analysis. Eur Heart J. 2014;35:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. C‐reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. [DOI] [PubMed] [Google Scholar]
- 5. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. [DOI] [PubMed] [Google Scholar]
- 6. Biasucci LM, Liuzzo G, Grillo RL, Caligiuri G, Rebuzzi AG, Buffon A, Summaria F, Ginnetti F, Fadda G, Maseri A. Elevated levels of C‐reactive protein at discharge in patients with unstable angina predict recurrent instability. Circulation. 1999;99:855–860. [DOI] [PubMed] [Google Scholar]
- 7. Kahan T, Forslund L, Held C, Bjorkander I, Billing E, Eriksson SV, Nasman P, Rehnqvist N, Hjemdahl P. Risk prediction in stable angina pectoris. Eur J Clin Invest. 2013;43:141–151. [DOI] [PubMed] [Google Scholar]
- 8. Stewart RA, White HD, Kirby AC, Heritier SR, Simes RJ, Nestel PJ, West MJ, Colquhoun DM, Tonkin AM; Long‐Term Intervention With Pravastatin in Ischemic Disease (LIPID) Study Investigators . White blood cell count predicts reduction in coronary heart disease mortality with pravastatin. Circulation. 2005;111:1756–1762. [DOI] [PubMed] [Google Scholar]
- 9. Haverkate F, Thompson SG, Pyke SD, Gallimore JR, Pepys MB; European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Production of C‐reactive protein and risk of coronary events in stable and unstable angina. Lancet. 1997;349:462–466. [DOI] [PubMed] [Google Scholar]
- 10. STABILITY Investigators , White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, Budaj A, Harrington RA, Steg PG, Ardissino D, Armstrong PW, Avezum A, Aylward PE, Bryce A, Chen H, Chen MF, Corbalan R, Dalby AJ, Danchin N, De Winter RJ, Denchev S, Diaz R, Elisaf M, Flather MD, Goudev AR, Granger CB, Grinfeld L, Hochman JS, Husted S, Kim HS, Koenig W, Linhart A, Lonn E, Lopez‐Sendon J, Manolis AJ, Mohler ER III, Nicolau JC, Pais P, Parkhomenko A, Pedersen TR, Pella D, Ramos‐Corrales MA, Ruda M, Sereg M, Siddique S, Sinnaeve P, Smith P, Sritara P, Swart HP, Sy RG, Teramoto T, Tse HF, Watson D, Weaver WD, Weiss R, Viigimaa M, Vinereanu D, Zhu J, Cannon CP, Wallentin L. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370:1702–1711. [DOI] [PubMed] [Google Scholar]
- 11. White H, Held C, Stewart R, Watson D, Harrington R, Budaj A, Steg PG, Cannon CP, Krug‐Gourley S, Wittes J, Trivedi T, Tarka E, Wallentin L. Study design and rationale for the clinical outcomes of the STABILITY Trial (STabilization of Atherosclerotic plaque By Initiation of darapLadIb TherapY) comparing darapladib versus placebo in patients with coronary heart disease. Am Heart J. 2010;160:655–661. [DOI] [PubMed] [Google Scholar]
- 12. Chen SL, Liu Y, Lin L, Ye F, Zhang JJ, Tian NL, Zhang JX, Hu ZY, Xu T, Li L, Xu B, Latif F, Nguyen T. Interleukin‐6, but not C‐reactive protein, predicts the occurrence of cardiovascular events after drug‐eluting stent for unstable angina. J Interv Cardiol. 2014;27:142–154. [DOI] [PubMed] [Google Scholar]
- 13. Hijazi Z, Aulin J, Andersson U, Alexander JH, Gersh B, Granger CB, Hanna M, Horowitz J, Hylek EM, Lopes RD, Siegbahn A, Wallentin L; ARISTOTLE Investigators . Biomarkers of inflammation and risk of cardiovascular events in anticoagulated patients with atrial fibrillation. Heart. 2016;102:508–517. [DOI] [PubMed] [Google Scholar]
- 14. Bro‐Jeppesen J, Kjaergaard J, Stammet P, Wise MP, Hovdenes J, Aneman A, Horn J, Devaux Y, Erlinge D, Gasche Y, Wanscher M, Cronberg T, Friberg H, Wetterslev J, Pellis T, Kuiper M, Nielsen N, Hassager C; TTM‐Trial Investigators . Predictive value of interleukin‐6 in post‐cardiac arrest patients treated with targeted temperature management at 33 degrees C or 36 degrees C. Resuscitation. 2015;98:1–8. [DOI] [PubMed] [Google Scholar]
- 15. Koyama K, Yoneyama K, Mitarai T, Ishibashi Y, Takahashi E, Kongoji K, Harada T, Akashi YJ. Association between inflammatory biomarkers and thin‐cap fibroatheroma detected by optical coherence tomography in patients with coronary heart disease. Arch Med Sci. 2015;11:505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Demissei BG, Cleland JG, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison B, Givertz MM, Bloomfield DM, Dittrich H, van der Meer P, van Veldhuisen DJ, Hillege HL, Voors AA. Optimizing clinical use of biomarkers in high‐risk acute heart failure patients. Eur J Heart Fail. 2016;18:269–280. [DOI] [PubMed] [Google Scholar]
- 17. Fontes JA, Rose NR, Cihakova D. The varying faces of IL‐6: from cardiac protection to cardiac failure. Cytokine. 2015;74:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ridker PM, Luscher TF. Anti‐inflammatory therapies for cardiovascular disease. Eur Heart J. 2014;35:1782–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bisoendial RJ, Boekholdt SM, Vergeer M, Stroes ES, Kastelein JJ. C‐reactive protein is a mediator of cardiovascular disease. Eur Heart J. 2010;31:2087–2091. [DOI] [PubMed] [Google Scholar]
- 20. IL6 Genetics Consortium Emerging Risk Factors Collaboration , Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, Gao P, Saleheen D, Rendon A, Nelson CP, Braund PS, Hall AS, Chasman DI, Tybjaerg‐Hansen A, Chambers JC, Benjamin EJ, Franks PW, Clarke R, Wilde AA, Trip MD, Steri M, Witteman JC, Qi L, van der Schoot CE, de Faire U, Erdmann J, Stringham HM, Koenig W, Rader DJ, Melzer D, Reich D, Psaty BM, Kleber ME, Panagiotakos DB, Willeit J, Wennberg P, Woodward M, Adamovic S, Rimm EB, Meade TW, Gillum RF, Shaffer JA, Hofman A, Onat A, Sundstrom J, Wassertheil‐Smoller S, Mellstrom D, Gallacher J, Cushman M, Tracy RP, Kauhanen J, Karlsson M, Salonen JT, Wilhelmsen L, Amouyel P, Cantin B, Best LG, Ben‐Shlomo Y, Manson JE, Davey‐Smith G, de Bakker PI, O'Donnell CJ, Wilson JF, Wilson AG, Assimes TL, Jansson JO, Ohlsson C, Tivesten A, Ljunggren O, Reilly MP, Hamsten A, Ingelsson E, Cambien F, Hung J, Thomas GN, Boehnke M, Schunkert H, Asselbergs FW, Kastelein JJ, Gudnason V, Salomaa V, Harris TB, Kooner JS, Allin KH, Nordestgaard BG, Hopewell JC, Goodall AH, Ridker PM, Holm H, Watkins H, Ouwehand WH, Samani NJ, Kaptoge S, Di Angelantonio E, Harari O, Danesh J. Interleukin‐6 receptor pathways in coronary heart disease: a collaborative meta‐analysis of 82 studies. Lancet. 2012;379:1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spoto B, Mattace‐Raso F, Sijbrands E, Leonardis D, Testa A, Pisano A, Pizzini P, Cutrupi S, Parlongo RM, D'Arrigo G, Tripepi G, Mallamaci F, Zoccali C. Association of IL‐6 and a functional polymorphism in the IL‐6 gene with cardiovascular events in patients with CKD. Clin J Am Soc Nephrol. 2015;10:232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gabay C, Emery P, van Vollenhoven R, Dikranian A, Alten R, Pavelka K, Klearman M, Musselman D, Agarwal S, Green J, Kavanaugh A; ADACTA Study Investigators . Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double‐blind, controlled phase 4 trial. Lancet. 2013;381:1541–1550. [DOI] [PubMed] [Google Scholar]
- 23. Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin‐1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti‐inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J. 2011;162:597–605. [DOI] [PubMed] [Google Scholar]
- 24. Zacho J, Tybjaerg‐Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C‐reactive protein and ischemic vascular disease. N Engl J Med. 2008;359:1897–1908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of Demographic and Baseline Characteristics by Baseline Quartile Groups of WBC Count*
Table S2. Multivariate Analyses of Factors Associated With WBC Count*
Figure S1. Kaplan‐Meier curves for major adverse cardiovascular event (MACE) by baseline white blood cell (WBC) quartile (Q) groups.
Figure S2. Spline plots for major adverse cardiovascular event (MACE), cardiovascular (CV) death, myocardial infarction (MI), heart failure, and stroke by baseline quartile groups of white blood cell (WBC) count.
Figure S3. The impact of white blood cell (WBC) count on outcomes by baseline quartile (Q) groups.
Appendix S1. List of STABILITY Investigators.


