Abstract
Background
The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors evolocumab and alirocumab substantially reduce low‐density lipoprotein cholesterol (LDL‐C) when added to statin therapy in patients who need additional LDL‐C reduction.
Methods and Results
We conducted a systematic review and network meta‐analysis of randomized trials of lipid‐lowering therapies from database inception through August 2016 (45 058 records retrieved). We found 69 trials of lipid‐lowering therapies that enrolled patients requiring further LDL‐C reduction while on maximally tolerated medium‐ or high‐intensity statin, of which 15 could be relevant for inclusion in LDL‐C reduction networks with evolocumab, alirocumab, ezetimibe, and placebo as treatment arms. PCSK9 inhibitors significantly reduced LDL‐C by 54% to 74% versus placebo and 26% to 46% versus ezetimibe. There were significant treatment differences for evolocumab 140 mg every 2 weeks at the mean of weeks 10 and 12 versus placebo (−74.1%; 95% credible interval −79.81% to −68.58%), alirocumab 75 mg (−20.03%; 95% credible interval −27.32% to −12.96%), and alirocumab 150 mg (−13.63%; 95% credible interval −22.43% to −5.33%) at ≥12 weeks. Treatment differences were similar in direction and magnitude for PCSK9 inhibitor monthly dosing. Adverse events were similar between PCSK9 inhibitors and control. Rates of adverse events were similar between PCSK9 inhibitors versus placebo or ezetimibe.
Conclusions
PCSK9 inhibitors added to medium‐ to high‐intensity statin therapy significantly reduce LDL‐C in patients requiring further LDL‐C reduction. The network meta‐analysis showed a significant treatment difference in LDL‐C reduction for evolocumab versus alirocumab.
Keywords: alirocumab, evidence‐based medicine, evolocumab, ezetimibe, lipids, low‐density lipoprotein cholesterol, meta‐analysis, proprotein convertase subtilisin/kexin type 9 inhibitor, statin therapy
Subject Categories: Lipids and Cholesterol, Cardiovascular Disease, Meta Analysis
Clinical Perspective
What Is New?
Patients who need additional lowering of low‐density lipoprotein‐cholesterol (LDL‐C) despite statin therapy may benefit from additional lipid‐lowering therapy such as evolocumab or alirocumab (proprotein convertase subtilisin/kexin type 9 inhibitors [PCSK9]).
A systematic literature review found 74 total studies that explored LDL‐C lowering in patients receiving statin background therapy; of these, 15 were used to conduct a network meta‐analysis of evolocumab, alirocumab, and ezetimibe.
A network meta‐analysis found that evolocumab 140 mg every 2 weeks reduced LDL‐C by 74% versus placebo and 46% versus ezetimibe; alirocumab 75 mg every 2 weeks, 54% and 26%; alirocumab 150 mg every 2 weeks, 60% and 32%; evolocumab 420 mg every month, 72% and 48%; and alirocumab 300 mg every month, 52% and 28%.
What Are the Clinical Implications?
Studies of PCSK9 inhibitors in a range of populations and risk profiles have consistently showed a substantial relative reduction in LDL‐C additional to that provided by statins—often more than 60%, as shown in the present analysis.
Such incremental LDL‐C reduction can allow patients with high unmet need (eg, those at very high cardiovascular risk) to achieve LDL‐C levels below target, which is expected to reduce their residual risk of cardiovascular events.
Lowering low‐density lipoprotein cholesterol (LDL‐C) levels with statins reduces the risk of atherosclerotic cardiovascular disease (CVD).1, 2, 3, 4, 5, 6 The IMPROVE‐IT trial7 substantiates that LDL‐C reduction with nonstatin therapy further reduces risk of CVD, although the absolute reduction in cardiovascular events was small because of modest LDL‐C lowering with ezetimibe on top of a statin.8 There remains, however, a population of high‐risk patients who have elevated LDL‐C despite statin therapy and who have residual risk of cardiovascular events and mortality.9 As a result, there is an unmet need for new therapies to provide this high‐risk population with incremental LDL‐C reduction beyond that which can be achieved by statins and other oral lipid‐lowering therapies. Moreover, there is evidence that the lower LDL‐C achieved provides further risk reduction.10, 11
Produced mostly in the liver, proprotein convertase subtilisin/kexin type 9 (PCSK9) in plasma binds to hepatic LDL receptors on the cell surface and targets them for degradation, thereby decreasing the number of LDL receptors and increasing LDL‐C levels. PCSK9 was identified as a target when people with variants that upregulated or downregulated this protein led to, respectively, greater and lesser risk of cardiovascular events.6 The PCSK9 inhibitors evolocumab and alirocumab were recently approved for LDL‐C reduction when added to maximally tolerated statin therapy.
To date there are no head‐to‐head studies comparing the LDL‐C–lowering capacity of PCSK9 inhibitors to each other. In the absence of such trials indirect treatment comparisons and network meta‐analyses based on a robust systematic literature review can inform evidence‐based healthcare decision making.12 Within network meta‐analyses, indirect treatment comparison allows the comparison of 2 therapies that share a common comparator,13 whereas mixed treatment comparison allows a combination of direct and indirect evidence.14, 15
Systematic reviews with subsequent meta‐analyses have been conducted using clinical studies of PCSK9 inhibitors.16, 17, 18, 19, 20 However, such studies have either pooled PCSK9 inhibitors together as a class16, 17, 18, 19 or provided pooled efficacy estimates for evolocumab versus control and alirocumab versus control without making any formal indirect comparisons.20 Finally, none of the meta‐analyses specifically focused on patients whose hypercholesterolemia was not controlled with statin therapy alone, the primary populations for which evolocumab and alirocumab are indicated.21, 22, 23, 24
We therefore conducted a systematic review and network meta‐analysis to compare LDL‐C reduction with evolocumab to other lipid‐lowering therapies (including alirocumab) in patients receiving statin background therapy.
Methods
Objectives, Study Selection, Quality Assessment, and Data Abstraction
We conducted this systematic review and network meta‐analysis with a target population of patients with hypercholesterolemia whose condition is not adequately controlled according to European lipid goals25 with moderate‐ to high‐intensity statin background therapy and who remain at risk of cardiovascular events. The therapies (ie, interventions) we assessed were evolocumab and other pharmacologic agents for the management of hypercholesterolemia. The control for each therapy was placebo (ie, background statin therapy alone) and all other therapies that share a common comparator. The efficacy outcomes of interest were percentage change from baseline in LDL‐C, high‐density lipoprotein cholesterol (HDL‐C), non‐HDL‐C, apolipoprotein B (ApoB), and lipoprotein (a) [Lp(a)] and cardiovascular events (not the focus of this article owing to a lack of available data for analysis). The safety outcomes of interest were any adverse event (AE), treatment‐related AE, and serious AE.
The systematic review adhered to methods published by the Centre for Reviews and Dissemination26 and the Cochrane Collaboration.27 Randomized studies were included if they enrolled adults (≥18 years) with primary familial or nonfamilial hypercholesterolemia who were candidates for evolocumab or other pharmacological lipid‐lowering therapies added to statins. Only studies with ≥12 weeks of follow‐up and ≥10 patients per group were included. Studies were excluded if they included patients with organ transplantations, infectious diseases such as HIV/AIDS, New York Heart Association grade III‐IV heart failure, or stage 4 or 5 renal dysfunction. Studies were excluded if patients were only receiving a low‐intensity statin as background, as were those that solely studied statin therapy. Only doses and frequencies that are marketed in the United States or European Union or investigated in phase 3 studies were included.
We searched MEDLINE, Embase, the Cochrane Databases of Systematic Reviews and Controlled Trials CENTRAL, the Database of Abstracts of Reviews of Effects, and the Health Technology Assessment Database from inception to August 2016. The search strategy was limited where possible to randomized studies and those in humans but was not limited by date or language. We searched clinical trial registries and conference abstracts, presentations or posters, in order to identify unpublished studies. For studies sponsored by Amgen, the sponsor of the evolocumab clinical trial program, we used both publications and clinical study reports. Keywords for the searches included the hypercholesterolemia disease state and all therapies used to modify atherogenic lipids (see Data S1). For quality assurance, the Embase search strategy was peer‐reviewed by a second information specialist using the Canadian Agency for Drugs and Technologies in Health peer review checklist.28
Two independent reviewers screened titles and abstracts to exclude records that obviously did not meet inclusion criteria; 2 reviewers then obtained and independently screened full texts for inclusion in the systematic review.
Data were extracted by 1 reviewer and independently checked for errors by another reviewer. The same process was used to assess the methodological quality of all included studies using the Cochrane Collaboration Risk of Bias Assessment Tool.27 Throughout the screening and data extraction process, discrepancies between reviewers were resolved through discussion or by consulting a third reviewer.
Data Synthesis and Analysis
Networks were created including studies that provided sufficient data for synthesis and with the aim of ensuring as much homogeneity as possible (eg, based on study design and clinical characteristics). All available data from the included studies were incorporated except data from patients in evolocumab studies with no statin use before enrollment (30% of LDL‐C Assessment w/PCSK9 MonoclonaL Antibody Inhibition Combined with Statin ThErapy – 2 (LAPLACE‐2))29 and patients assigned to diet alone based on their cardiovascular risk (12% of Durable Effect of PCSK9 antibody CompARed wiTh placEbo Study (DESCARTES)).30
We conducted meta‐analyses only if the underlying studies were considered to be statistically and clinically homogenous. We assessed statistical heterogeneity with the chi‐squared test (P<0.10 was considered significant for heterogeneity) and the I2 value and by visual inspection of the forest plots. We could not assess publication bias because there were not enough studies in each direct meta‐analysis to generate a funnel plot. Stata (StataCorp; College Station, TX) version 13.1 was used to conduct direct meta‐analyses using random effects models. To explore the robustness of results, sensitivity analyses were performed by excluding specific studies (eg, if they were associated with heterogeneity in direct meta‐analyses, or unique populations such those enrolled in studies conducted in Japan) or by relaxing inclusion criteria and including additional studies.
The network meta‐analysis was conducted using Bayesian models31 in WinBUGS (MRC Biostatistics Unit; Cambridge, UK) version 1.4.3. We estimated the mean treatment difference or risk ratio for each comparison after an initial burn‐in of 40 000 Markov chain Monte Carlo simulations, followed by a further 40 000 simulations. Two chains were used. We used noninformative normal priors (mean 0, variance 10 000) for treatment effects and a noninformative uniform prior (interval 0, 5) to estimate the between‐study standard deviation. We assessed convergence and autocorrelation by monitoring the trace and autocorrelation plots in WinBUGS. None of the models showed any problems with convergence. We obtained the median estimate of the mean difference or risk ratio from the posterior distribution and reported it with the 2.5% and 97.5% estimates of the distribution (the 95% credible interval [CrI]). We assessed model fit using residual deviance and the deviance information criterion. All analyses used random‐effects models and the treatment effect from each study (ie, mean difference, rather than the mean and standard error for each group).
Within the network meta‐analysis, we reviewed assumptions of homogeneity based on the I2 statistic from the direct meta‐analyses, similarity using the baseline characteristics and designs of the included studies, and consistency using the IFPLOT command in Stata in comparisons with both direct and indirect comparisons. We conducted sensitivity analyses to explore any heterogeneity by excluding individual studies or those in different populations. We also conducted sensitivity analyses combining both evolocumab dosing groups and including studies with all background therapies.
We excluded on a post hoc basis nodes in the networks that included fenofibrate or anacetrapib from this article. The anacetrapib arm was excluded because this cholesterylester transfer protein inhibitor's cardiovascular outcomes trial is ongoing, and all of the prior trials in this drug class have been neutral or negative in risk reduction.32 Moreover, a recent meta‐analysis of lipid‐lowering therapy found that therapies that upregulated LDL receptor function were linearly associated with reductions in cardiovascular events per 1 mmol/L reduction in LDL‐C. This relationship was less consistent with fibrates and cholesterylester transfer protein inhibitors, and statin‐era trials in particular were negative or neutral in reducing cardiovascular events.5 We also excluded bococizumab after Pfizer announced they were halting clinical and commercial development of this PCSK9 inhibitor.33 Pfizer noted in its press release that studies of bococizumab showed reduced efficacy over time and more injection‐site reactions than evolocumab and alirocumab.33
Evolocumab can be administered as 140 mg every 2 weeks (Q2W) or 420 mg monthly (QM), and we generated separate networks for each dosing option. The co–primary end points for most evolocumab studies were the percentage change in LDL‐C from baseline to the mean of 10 and 12 weeks and to week 12. The co–primary end point of 10 and 12 weeks allows a better representation of the efficacy of evolocumab across the dosing period, particularly for monthly administration, and is included in international prescribing information. To be concise, this analysis for evolocumab (140 mg Q2W) is the focus of the main text and sensitivity analysis. Key results for week 12 are reported in Figure S1. Because data from some comparator studies were available only for follow‐up of longer than 12 weeks (eg, up to 78 weeks), we analyzed values using evolocumab at the mean of 10 and 12 weeks or at week 12 versus comparators at ≥12 weeks. In practice, week‐12 data were available for percentage reduction in LDL‐C but less consistently for other lipid end points.
If the outcome was not available at week 12, we used the nearest time point after week 12. For alirocumab studies, in which dose titration is often employed, we specifically aimed to analyze patients who were taking only 75 mg Q2W, only 150 mg Q2W, or only 300 mg QM.
Results
Figure 1 displays the systematic review flow diagram. The systematic review found 45 058 unique records, of which 44 318 were excluded based on the title and abstract. The full papers of the 740 remaining records were assessed for eligibility, and 502 were excluded with reasons, leaving 238 records reporting 74 studies (studies and records included and excluded are displayed in Data S2). These 74 studies had study data available, and 69 of them focused on a population requiring further LDL‐C reduction while on maximally tolerated statin therapy. The remaining 5 studies were in statin‐intolerant patients. Table S1 displays population characteristics of the studies.
Figure 1.
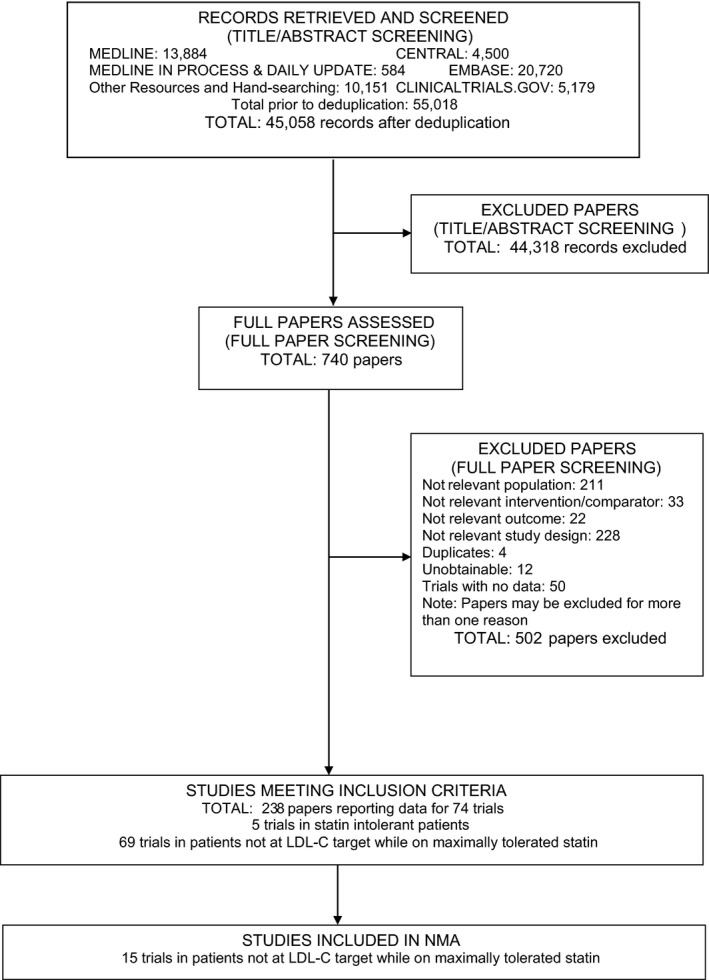
Study flow diagram of the systematic review. Articles in the “Excluded Papers” stage could be excluded for ≥1 reason. The network meta‐analysis of statin‐intolerant patients yielded a small sample size and did not include pending results of a phase 3 study of evolocumab in this population. LDL ‐ C indicates low‐density lipoprotein cholesterol; NMA, network meta‐analysis.
A total of 54 studies were excluded from all LDL‐C networks (Table S2), and 15 in which patients predominantly received moderate‐ or high‐intensity statin background therapy were included in the primary networks (ie, those most closely aligned with the research question) (Table 1, Figure 2).29, 30, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 We created separate networks for comparing evolocumab to other lipid‐lowering therapies by dosing regimen: 140 mg Q2W or 420 mg QM (Figure 2). Both networks included placebo and ezetimibe (10 mg daily). The evolocumab 140 mg Q2W network also included alirocumab 75 mg and 150 mg Q2W; the evolocumab 420 mg QM network included alirocumab 300 mg QM.
Table 1.
Specific Details About Studies Included in Main Q2W or QM Network
| Study Name | Follow‐Up, Weeks | Age, ya | Investigational Drug and Dose | Control | Type HC | CVD Risk Status | FH Status | Type 2 Diabetes Mellitus Status | Obesity Status | Background Therapy |
|---|---|---|---|---|---|---|---|---|---|---|
| DESCARTES30 | 52 | 55.9 (10.8)b | EvoMab 420 mg QM | Placebo | Primary or secondary HC | With or without CVD or equivalent | NR/unclear | With and without | All | Diet through 80 mg atorvastatin+ezetimibe |
| LAPLACE‐TIMI 5734 | 12 | 62.0 (55.0‐67.0) | EvoMab 70, 105, or 140 mg Q2W; 280, 350, or 420 mg QM | Placebo | Primary HC | Without prior CVD | NR/unclear | With and without | Overweight | Statin±ezetimibe at physician discretion |
| LAPLACE‐229 | 12 | 59.6 (9.9)b | EvoMab 140 mg Q2W; 420 mg QM | Placebo | Mixed dyslipidemia | NR/unclear | NR/unclear | With and without | Overweight | Moderate to high dose atorvastatin or rosuvastatin, moderate dose simvastatin |
| YUKAWA‐135 | 12 | 61.5 (9.7) | EvoMab 70 or 140 mg Q2W; 280 or 420 mg QM | Placebo | Primary or secondary HC | With or without CVD or equivalent | NR/unclear | With and without | Overweight | Statin as prescribed by physician |
| YUKAWA‐236 | 12 | 62 (11)b | EvoMab 140 mg Q2W; 420 mg QM | Placebo | Primary or secondary HC | With or without CVD or equivalent | HoFH and HeFH eligible | With and without | NR/unclear | 20 mg atorvastatin (intensive dose for Japanese population) |
| McKenney 201237 | 12 | 56.7 (10.0) | AliMab 50, 100, 150, or 200 mg Q2W; 300 mg QM | Placebo | Primary HC | NR/unclear | NR/unclear | With and without | Overweight | 10, 20, 40 mg atorvastatin |
| ODYSSEY CHOICE I41 | 56 | 60.7 (9.1)c | AliMab 75 mg Q2W or 300 mg QM | Placebo | Primary HC | Moderate‐ to very‐high‐ risk, no CVD | HoFH excluded | With and without | Normal, overweight, and obese | Maximally‐tolerated atorvastatin, rosuvastatin, or simvastatin |
| ODYSSEY COMBO I40 | 52 | 63.0 (9.5)d | AliMab 75 mg Q2W | Placebo | Primary or secondary HC | With or without CVD or equivalent | No FH patients | With and without | NR/unclear | Maximally tolerated statin with/without other lipid‐lowering therapy |
| ODYSSEY COMBO II38 | 104 | 61.7 (9.4)d | AliMab 75 mg Q2W | Ezetimibe | Primary or secondary HC | With or without CVD or equivalent | NR/unclear | NR/unclear | NR/unclear | Stable maximally tolerated statin therapy |
| ODYSSEY HIGH FH39 | 78 | 49.8 (14.2)d | AliMab 150 mg Q2W | Placebo | HeFH only | NR/unclear | HeFH only | NR/unclear | NR/unclear | Maximally tolerated statin with/without other lipid‐lowering therapy |
| ODYSSEY JAPAN45 | 24 | 60.3 (9.7)d | AliMab 75 mg Q2W | Placebo | NR/unclear | With or without CVD | NR/unclear | NR/unclear | NR/unclear | Stable lipid lowering therapy |
| ODYSSEY LONG TERM46 | 78 | 60.4 (10.4) | AliMab 150 mg Q2W | Placebo | Primary HC | With or without CVD or equivalent | HeFH included | NR/unclear | NR/unclear | Maximally tolerated statin with/without other lipid‐lowering therapy |
| ODYSSEY OPTIONS I42 | 24 | 64.2 (10.4)e | AliMab 75 mg Q2W | Placebo, ezetimibe | Primary or secondary HC | CVD or equivalent | Non‐FH or HeFH | With and without | NR/unclear | Statins according to study group assignment |
| ODYSSEY OPTIONS II43 | 24 | 57.9 (8.9)f | AliMab 75 mg Q2W | Placebo, ezetimibe | Primary or secondary HC | CVD or equivalent | Non‐FH or HeFH | NR/unclear | NR/unclear | Statins according to study group assignment |
| Masana 200544 | 48 | 61 (28‐83)g | Ezetimibe | Placebo | Primary or secondary HC | With or without CVD or equivalent | NR/unclear | With and without | Overweight | Up to 80 mg simvastatin |
CVD indicates cardiovascular disease; EvoMab, evolocumab; FH, familial hypercholesterolemia; HC, hypercholesterolemia; HeFH, heterozygous familial hypercholesterolemia; HoFH, homozygous familial hypercholesterolemia; NR, not reported; Q2W, every 2 weeks; QM, monthly.
Values are mean (standard deviation) or median (interquartile range). Mean age for all patients given unless unavailable, in which case the intervention group was used (marked with footnote). There was no indication in the references that ages were statistically different between groups.
All evolocumab patients.
Alirocumab 75 mg Q2W taking statins.
All alirocumab patients.
Alirocumab 75/150 mg Q2W+atorvastatin 40 mg.
Alirocumab 75/150 mg Q2W+rosuvastatin 20 mg.
All ezetimibe patients. Values in parentheses represent the range of ages observed.
Figure 2.
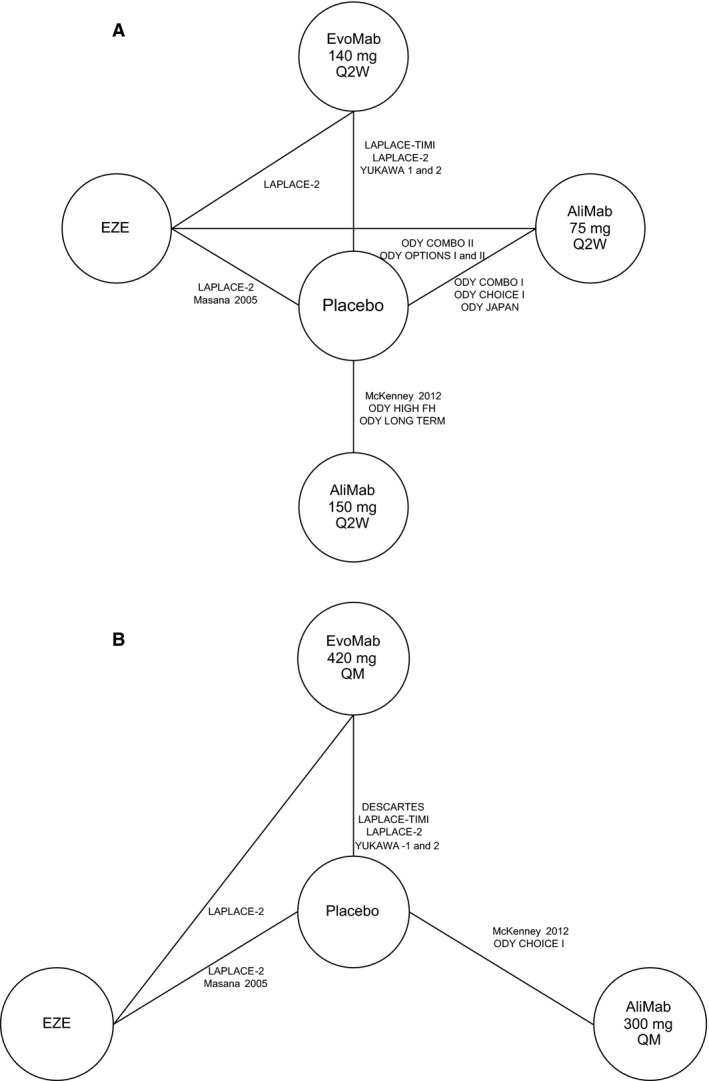
Network of available connections for comparing change in LDL‐C. A, Evolocumab 140 mg Q2W (every 2 weeks). B, Evolocumab 420 mg QM (every month). Lines between boxes denote direct comparisons. AliMab indicates alirocumab; EvoMab, evolocumab; EZE, ezetimibe; LDL‐C, low‐density lipoprotein cholesterol; ODY, ODYSSEY.
There were 4 studies of evolocumab29, 34, 35, 36 LDL‐C Assessment w/PCSK9 MonoclonaL Antibody Inhibition Combined with Statin ThErapy – Thrombolysis In Myocardial Infarction – 57 (LAPLACE‐TIMI‐57), LAPLACE‐2, StudY of LDL‐Cholesterol Reduction Using a Monoclonal PCSK9 Antibody in Japanese Patients With Advanced Cardiovascular Risk – 1 (YUKAWA‐1), and YUKAWA‐2) in both networks, all of which were 12 weeks in duration. There was 1 additional study of evolocumab (DESCARTES30) in the 420 mg QM network that was 52 weeks in duration. All studies compared evolocumab to placebo, and 1 study29 (LAPLACE‐2) also included a comparison with ezetimibe.
In total, there were 9 studies of alirocumab37, 38, 40, 41, 45, 46, 47, 48 in the Q2W network (McKenney 2012 and ODYSSEY COMBO I and II, OPTIONS I and II, CHOICE I, JAPAN, HIGH FH, and LONG‐TERM), of which 2 (McKenney 2012 and CHOICE I) were included in the QM network.37, 41, 48 Alirocumab studies were 12 to 104 weeks in duration. All studies reported 12‐ and 24‐week data except 1 that reported 24‐week data only (in the network meta‐analyses, the 12‐week data were used except for the study in which it was not available). The alirocumab 75‐ and 150‐mg Q2W doses were included as separate therapies in the Q2W network, and the 300‐mg QM dose was included in the QM network. Six studies compared alirocumab to placebo, and 3 studies38, 47 (ODYSSEY COMBO II and ODYSSEY OPTIONS I and II) compared alirocumab 75 mg Q2W to ezetimibe.
Finally, there was 1 eligible study44 comparing ezetimibe to placebo (Masana 2005).
Risk of bias was assessed by judging how well all included studies reported across 8 domains of the Cochrane Risk of Bias Assessment Tool (Figure S2). In this article we focus on those studies that were included in the primary analysis LDL‐C networks. All evolocumab studies29, 30, 34, 35, 36 had low risk of bias across all criteria. The risk of bias in 5 alirocumab studies37, 38, 40, 41, 46, 47, 48, 49 was unclear in at least 1 area. The most common reason for an unclear risk of bias was insufficient reporting of allocation of concealment and randomization methods.
Lipid‐Lowering Efficacy of Evolocumab Compared to Other Therapies
Direct head‐to‐head comparisons are displayed in Figure S3.
Treatment differences between lipid‐lowering therapies for the percentage reduction in LDL‐C from baseline are displayed in Figure 3 for evolocumab at the mean of weeks 10 and 12 versus comparators at ≥12 weeks and in Figure S3 for evolocumab at week 12 versus comparators at ≥12 weeks. All treatment differences between evolocumab 140 mg, alirocumab 75 mg, alirocumab 150 mg, or ezetimibe and placebo were statistically significant.
Figure 3.
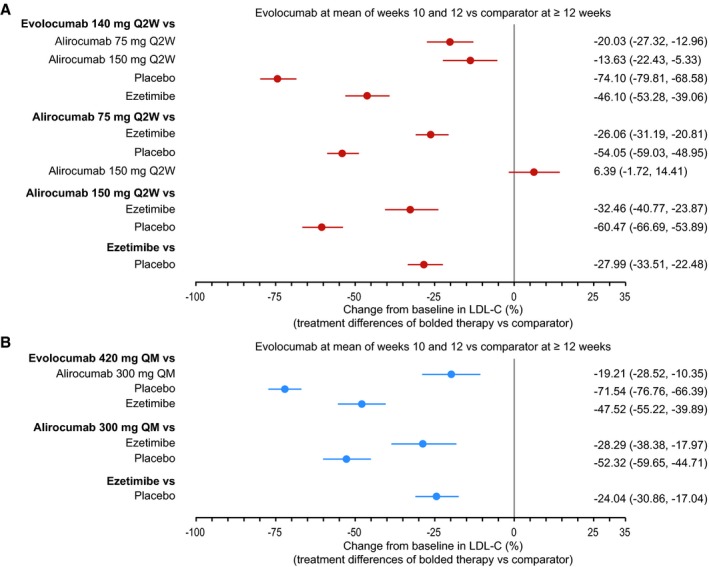
Treatment difference in percentage LDL‐C change (95% credible interval) in response to evolocumab 140 mg Q2W network (A) or evolocumab 420 mg QM network (B): evolocumab at the mean of weeks 10 and 12 vs comparator at ≥12 weeks. LDL‐C indicates low‐density lipoprotein cholesterol; Q2W, every 2 weeks; QM, every month.
Among PCSK9 inhibitors, evolocumab had a greater LDL‐C reduction than alirocumab. For evolocumab 140 mg Q2W at the mean of weeks 10 and 12 versus comparators at ≥12 weeks, the treatment difference versus alirocumab 75 mg was −20.03% (95% CrI −27.32% to −12.96%) and −13.63% (95% CrI −22.43% to −5.33%) compared with alirocumab 150 mg. The treatment difference between evolocumab 420 mg QM and alirocumab 300 mg QM was −19.21% (95% CrI −28.52% to −10.35%) for evolocumab at the mean of weeks 10 and 12 and comparators at ≥12 weeks. Treatment differences were similar for evolocumab at week 12 versus comparators at ≥12 weeks (Figure S1).
We also conducted a post hoc analysis of evolocumab 140 mg Q2W and 420 mg QM combined as 1 treatment arm at the mean of weeks 10 and 12 versus alirocumab 75 mg (−18.32%, 95% CrI −24.30% to −12.40%) or 150 mg (−11.06%, 95% CrI −18.72% to −3.73%) Q2W at ≥12 weeks (Figures S4A and S5A). Another post hoc analysis included all studies that met inclusion criteria, regardless of the background therapy (eg, ezetimibe, other lipid‐lowering therapies, low‐intensity/no statin) (Figures S4B and S5B): evolocumab 140 mg Q2W at the mean of weeks 10 and 12 versus alirocumab at weeks ≥12 was −16.76% (95% CrI, −22.54% to −11.02%) for 75 mg Q2W and −9.88% (95% CrI, −17.60% to −2.29%) for 150 mg Q2W.
Direct meta‐analyses suggested that high statistical heterogeneity (I2≥70%) was observed for some comparisons. This was investigated using sensitivity analyses (excluding studies conducted in Japan35, 36, 45 [YUKAWA‐1, YUKAWA‐2, and ODYSSEY‐JAPAN], and also ODYSSEY HIGH FH39). Sensitivity analyses of direct head‐to‐head comparisons did not substantially change the results but did reduce the statistical heterogeneity (Figure S3). In the network meta‐analysis, moreover, we performed several sensitivity analyses excluding studies conducted in Japan35, 36, 45 or ODYSSEY HIGH FH,39 all of which drove heterogeneity (Figure S6). In general, the conclusions of these sensitivity analyses with regard to percentage LDL‐C reduction were consistent in direction and statistical significance with the main analyses, although the magnitudes changed slightly.
Networks were developed for other lipid end points (Figures S4C through S4E). Network meta‐analysis of HDL‐C results demonstrated a moderate increase from baseline associated with evolocumab and alirocumab compared with placebo or ezetimibe. Network meta‐analysis results for non‐HDL‐C were similar in direction and magnitude to LDL‐C results; the same was true of the results for ApoB and Lp(a), although the networks were smaller for these comparisons (Figure S7).
Safety
There were no statistically significant differences in the risk of any, treatment‐related, or serious AEs between evolocumab, alirocumab, or ezetimibe and placebo except for the QM doses of evolocumab and alirocumab (Table 2). Evolocumab 420 mg and alirocumab 300 mg QM resulted in risk ratios of treatment‐related AEs of 1.47 (95% confidence interval 1.03–2.09) and 1.17 (95% confidence interval 1.01–1.35) compared with placebo. There were, however, very few treatment‐related AEs, and none was considered serious.
Table 2.
Risk Ratio (95% CI) for Occurrence of Any AE, Treatment‐Related AE, and Serious AE
| Comparison | Any AE | Treatment‐Related AE | Serious AE |
|---|---|---|---|
| Evolocumab 140 mg Q2W vs placebo | 1.10 (0.93‐1.29) | 1.10 (0.42‐2.85) | 0.96 (0.44‐2.09) |
| Evolocumab 420 mg QM vs placebo | 1.03 (0.91‐1.18) | 1.47 (1.03‐2.09) | 0.91 (0.38‐2.16) |
| Alirocumab 75 mg Q2W vs placebo | 1.06 (0.92‐1.22) | 1.25 (0.87‐1.81) | 1.00 (0.74‐1.34) |
| Alirocumab 150 mg Q2W vs placebo | 1.25 (0.76‐2.08) | NR | 1.05 (0.40‐2.75) |
| Alirocumab 300 mg QM vs placebo | 1.26 (0.89‐1.79) | 1.17 (1.01‐1.35) | 1.03 (0.07‐15.78) |
| Ezetimibe vs placebo | 1.04 (0.89‐1.21) | 1.17 (0.68‐2.00) | 0.77 (0.44‐1.36) |
AE indicates adverse event; CI, confidence interval; NR, not reported; Q2W, every 2 weeks; QM, monthly.
Discussion
Our systematic review of lipid‐lowering therapies added to medium‐ to high‐intensity statin therapy and subsequent network meta‐analysis confirms the substantial LDL‐C reductions of PCSK9 inhibitors versus placebo or ezetimibe in individual trials. Among the PCSK9 inhibitors, evolocumab appeared to have a greater reduction than alirocumab (75 mg Q2W, ≈20%; 150 mg Q2W, ≈10%; 300 mg QM, ≈20%). These treatment differences were directionally consistent in the various analyses we conducted (ie, exclusion of studies leading to heterogeneity, variation of dosing amount and interval, broader background therapy spectrum). There was also some evidence of proportional treatment differences between evolocumab and other therapies in HDL‐C, non‐HDL‐C, ApoB, and Lp(a). The incidence of AEs was similar between individual therapies and placebo except for significantly higher treatment‐related AEs for evolocumab and alirocumab QM versus placebo.
Our work provides information on PCSK9 inhibitors and ezetimibe added to statin therapy in those requiring further LDL‐C reduction. The trials generally evaluated patients either with CVD or at high risk of a CVD event, which is the expected target population for PCSK9 inhibitors both now and after cardiovascular outcomes trials for these medications are completed.50, 51 We also characterized the reductions observed for PCSK9 inhibitors in other parameters including non‐HDL‐C and Lp(a). Non‐HDL‐C is emerging as a meaningful measure of CVD event risk,52 and Lp(a) is associated with CVD event risk but is not reduced by statins.53, 54
Finally, we analyzed dose‐specific LDL‐C reductions between PCSK9 inhibitors, which have not been a focus of published meta‐analyses.16, 17, 18 The classwide reduction of LDL‐C with PCSK9 inhibitors observed in these meta‐analyses16, 17, 18 is consistent with what we observed in the comparison of individual PCSK9 inhibitors versus placebo or ezetimibe.
In our network meta‐analysis we found evidence of a significantly greater reduction of evolocumab versus alirocumab. The treatment difference of evolocumab 140 mg Q2W versus alirocumab 75 mg was larger than the comparison to alirocumab 150 mg. The treatment difference between evolocumab 420 mg QM versus alirocumab 300 mg QM reflected the fact that more of the study drug was administered to patients treated with evolocumab than to those treated with alirocumab. The treatment difference between evolocumab and alirocumab was directionally consistent in the various analyses we conducted. Our approach differed from that of other meta‐analyses by analyzing evolocumab and alirocumab separately. Lipinski and colleagues17 and Li and colleagues16 analyzed PCSK9 inhibitors as a class. Navarese and colleagues' meta‐analysis18 likewise considered the class in the primary analysis but suggests, in a secondary analysis, that there was a significantly greater reduction in LDL‐C with evolocumab versus placebo than alirocumab versus placebo.
We studied other atherogenic lipids when the data were available, and the direction and magnitude of treatment differences between the lipid‐lowering therapies were in line with those for LDL‐C. HDL‐C was increased modestly with evolocumab compared with other therapies, but the difference was not always significant. Non‐HDL‐C and ApoB were reduced, as expected, in line with LDL‐C. Lp(a) was reduced by ≈38% with evolocumab versus placebo, and there was a modest treatment difference favoring evolocumab versus alirocumab. This estimated modest ≈9% to 14% difference in Lp(a) is of uncertain clinical significance. Other meta‐analyses found similar results in the PCSK9 class as a whole.16, 17, 18
There are limitations to this review. In terms of comparing the LDL‐C–lowering capacity of PCSK9 inhibitors to each other, to date there have been no such head‐to‐head studies that would be the best way to remove any potential residual confounders. Thus, this review is limited by the quantity and quality of the data available from the included clinical trials. Additionally, because 75 and 150 mg Q2W were not studied in a parallel‐group trial, FDA review55 concluded that there is lack of availability of a well‐characterized estimate of the treatment effect for each dose. Another limitation of our analysis is that most of the studies included in the networks were relatively short‐term (mostly 12 and 24 weeks). Longer‐term follow‐up studies of evolocumab and alirocumab have not shown evidence of loss of efficacy or increased rates of AEs.30, 46, 50, 56
Conclusions
Based on network meta‐analyses, the PCSK9 inhibitors evolocumab and alirocumab were associated with reductions in LDL‐C of 54% to 74% versus placebo and 26% to 46% versus ezetimibe in patients not adequately controlled by statins alone. Recognizing the limitations of indirect comparison, our synthesis of the available data shows a greater reduction with evolocumab in LDL‐C versus alirocumab 75 mg Q2W with evidence also suggesting more intense LDL‐C reduction versus alirocumab 150 mg Q2W. There was some evidence to suggest that evolocumab may also significantly increase HDL‐C and decrease non‐HDL‐C, ApoB, and Lp(a) levels in comparison to alirocumab and other treatments. Further research is needed into the effects of evolocumab and alirocumab on the risk of cardiovascular events.
Author Contributions
All authors contributed to the scope and content of the article before the outline was composed, and all authors approved the final draft for submission.
Sources of Funding
Amgen sponsored the systematic review and network meta‐analysis, which was conducted by Kleijnen Systematic Reviews, Ltd under contract to Amgen. This systematic review and network meta‐analysis was sponsored by Amgen Inc, as were all studies of evolocumab included in the analysis.
Disclosures
Toth has received consulting and speakers' bureau fees from Amarin, Amgen, Kowa, Merck, Regeneron, and Sanofi and consulting fees from Gemfire. Sattar has received consulting fees from Amgen and has presented at an Amgen‐ and Sanofi‐sponsored symposium. Stroes has participated in Amgen, Sanofi, and Pfizer clinical trials; received consulting fees from Amgen, Sanofi, Merck, Novartis, Cerenis, and Ionis; has nonremunerative positions of influence at Ionis and Chiesi; and is on the speakers' bureau for Medcon Europe. Worthy, Deshpande, Forbes, and Ross are employees of Kleijnen Systematic Reviews Ltd. Kleijnen is owner and director of Kleijnen Systematic Reviews Ltd. Worth, Bray, Bridges, and Gandra are employees and stockholders of Amgen Inc. Cheng is a stockholder and former employee of Amgen Inc. Dent is an employee and stockholder of Esperion Therapeutics Inc and a stockholder and former employee of Amgen Inc.
Supporting information
Data S1. Full Search Strategy.
Data S2. Trials Included/Excluded, Full Paper Selection Stage.
Table S1. Methodology and Characteristics of Included Studies
Table S2. Studies Retrieved by the Systematic Review but Excluded From the Network Meta‐Analysis Shaded rows are included in a sensitivity analysis but not in the main.
Figure S1. Treatment difference in percentage LDL‐C (95% credible interval) change from baseline, evolocumab 140 mg Q2W (A) or evolocumab 420 mg QM (B) at week 12 vs comparator at week 12 analyses.
Figure S2. Risk of bias assessed in 69 trials.
Figure S3. Direct meta‐analyses of LDL‐C reduction.
Figure S4. Network for comparing other lipids with evolocumab 140 mg Q2W vs other therapies. AliMab indicates alirocumab; ApoB, apolipoprotein B; EvoMab, evolocumab; EZE, ezetimibe; HDL‐C, high‐density lipoprotein‐cholesterol; Lp(a), lipoprotein(a); non‐HDL‐C, nonhigh‐density lipoprotein‐cholesterol; Q2W, every 2 weeks; QM, every month. Study acronym definitions are available in the source references.
Figure S5. Treatment difference in percentage LDL‐C (95% credible interval) change A, Evolocumab 140 mg Q2W and 420 mg every month combined at the mean of weeks 10 and 12 vs comparator at ≥12 weeks. B, Evolocumab 140 mg Q2W at the mean of weeks 10 and 12 vs comparator at ≥12 weeks with any background therapy.
Figure S6. Sensitivity analysis: treatment difference in percentage LDL‐C (95% credible interval) change from baseline, evolocumab 140 mg Q2W at the mean of weeks 10 and 12 vs comparator at ≥12 weeks (A) excluding Japan studies; (B) ODYSSEY HIGH FH. Evolocumab 420 mg every 4 weeks at weeks 10 and 12 vs comparator at ≥12 weeks (C) excluding studies conducted in Japan.
Figure S7. Treatment difference in percentage (95% credible interval) change from baseline, evolocumab 140 mg Q2W at the mean of weeks 10 and 12 vs comparator at ≥12 weeks: (A) HDL‐C; (B) non‐HDL‐C; (C) ApoB; (D) Lp(a).
Acknowledgments
We thank Tim Peoples, MA, ELS, CMPP, of Amgen Inc for writing assistance and Kim Reid and Adrian Hernandez of Kleijnen Systematic Reviews, Ltd for data extraction and quality assessment.
(J Am Heart Assoc. 2017;6:e005367 DOI: 10.1161/JAHA.116.005367.)
References
- 1. Cholesterol Treatment Trialists Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cholesterol Treatment Trialists Collaborators . Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 3. Cholesterol Treatment Trialists Collaboration . Efficacy and safety of LDL‐lowering therapy among men and women: meta‐analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. [DOI] [PubMed] [Google Scholar]
- 4. Cholesterol Treatment Trialists Collaboration . The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta‐analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association between lowering LDL‐C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta‐analysis. JAMA. 2016;316:1289–1297. [DOI] [PubMed] [Google Scholar]
- 6. Blom DJ, Dent R, Castro RC, Toth PP. PCSK9 inhibition in the management of hyperlipidemia: focus on evolocumab. Vasc Health Risk Manag. 2016;12:185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM; for the IMPROVE‐IT Investigators . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 8. Catapano AL, Ference BA. IMPROVE‐IT and genetics reaffirm the causal role of LDL in cardiovascular disease. Atherosclerosis. 2015;241:498–501. [DOI] [PubMed] [Google Scholar]
- 9. Aggarwal J, Patel J, Yu J, Stern K, Menzin J, Harrison DH. LDL‐C goal achievement after adding or switching to ezetimibe in patients with clinical atherosclerotic cardiovascular disease or probable HeFH [in press]. J Manag Care Spec Pharm. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amarenco P, Pedersen TR, LaRosa JC, Waters DD, DeMicco DA, Simes RJ, Keech AC, Colquhoun D, Hitman GA, Betteridge DJ, Clearfield MB, Downs JR, Colhoun HM, Gotto AM Jr, Ridker PM, Grundy SM, Kastelein JJ. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta‐analysis of statin trials. J Am Coll Cardiol. 2014;64:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK; Treating to New Targets (TNT) Investigators . Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. [DOI] [PubMed] [Google Scholar]
- 12. Murad MH, Montori VM, Ioannidis JP, Jaeschke R, Devereaux PJ, Prasad K, Neumann I, Carrasco‐Labra A, Agoritsas T, Hatala R, Meade MO, Wyer P, Cook DJ, Guyatt G. How to read a systematic review and meta‐analysis and apply the results to patient care: users' guides to the medical literature. JAMA. 2014;312:171–179. [DOI] [PubMed] [Google Scholar]
- 13. Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta‐analysis of randomized controlled trials. Med Decis Making. 2013;33:607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoaglin DC, Hawkins N, Jansen JP, Scott DA, Itzler R, Cappelleri JC, Boersma C, Thompson D, Larholt KM, Diaz M, Barrett A. Conducting indirect‐treatment‐comparison and network‐meta‐analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health. 2011;14:429–437. [DOI] [PubMed] [Google Scholar]
- 15. Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, Lee K, Boersma C, Annemans L, Cappelleri JC. Interpreting indirect treatment comparisons and network meta‐analysis for health‐care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14:417–428. [DOI] [PubMed] [Google Scholar]
- 16. Li C, Lin L, Zhang W, Zhou L, Wang H, Luo X, Luo H, Cai Y, Zeng C. Efficiency and safety of proprotein convertase subtilisin/kexin 9 monoclonal antibody on hypercholesterolemia: a meta‐analysis of 20 randomized controlled trials. J Am Heart Assoc. 2015;4:e001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lipinski MJ, Benedetto U, Escarcega RO, Biondi‐Zoccai G, Lhermusier T, Baker NC, Torguson R, Brewer HB Jr, Waksman R. The impact of proprotein convertase subtilisin‐kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta‐analysis. Eur Heart J. 2016;37:536–545. [DOI] [PubMed] [Google Scholar]
- 18. Navarese EP, Kolodziejczak M, Schulze V, Gurbel PA, Tantry U, Lin Y, Brockmeyer M, Kandzari DE, Kubica JM, D'Agostino RB Sr, Kubica J, Volpe M, Agewall S, Kereiakes DJ, Kelm M. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta‐analysis. Ann Intern Med. 2015;163:40–51. [DOI] [PubMed] [Google Scholar]
- 19. Peng W, Peng W, Qian Z, Ke Z, Yi L, Jian Z, Chongrong Q, Qiang F. Therapeutic efficacy of PCSK9 monoclonal antibodies in statin‐nonresponsive patients with hypercholesterolemia and dyslipidemia: a systematic review and meta‐analysis. Int J Cardiol. 2016;222:119–129. [DOI] [PubMed] [Google Scholar]
- 20. Zhang XL, Zhu QQ, Zhu L, Chen JZ, Chen QH, Li GN, Xie J, Kang LN, Xu B. Safety and efficacy of anti‐PCSK9 antibodies: a meta‐analysis of 25 randomized, controlled trials. BMC Med. 2015;13:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanofi‐Aventis US LLC . Praluent® prescribing information [Internet]. Updated April 2017. Available at: http://products.sanofi.us/praluent/praluent.pdf. Accessed May 23, 2017.
- 22. Amgen Inc . Repatha® (evolocumab) prescribing information [Internet]. Updated July 2016. Available at: http://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/repatha/repatha_pi_hcp_english.ashx. Accessed May 23, 2017.
- 23. Sanofi‐Aventis Groupe . Praluent® summary of product characteristics [Internet]. Updated December 9, 2016. Available at: https://www.medicines.org.uk/emc/medicine/30956. Accessed May 23, 2017.
- 24. Amgen Europe B.V . Repatha® (evolocumab) summary of product characteristics [Internet]. Updated February 24, 2017. Available at: https://www.medicines.org.uk/emc/medicine/30628. Accessed May 23, 2017.
- 25. Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D. ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32:1769–1818. [DOI] [PubMed] [Google Scholar]
- 26. Centre for Reviews and Dissemination (CRD) . Systematic Reviews: CRD's Guidance for Undertaking Reviews in Health Care [Internet]. 3rd ed Updated January 2009. Available at: http://www.york.ac.uk/inst/crd/SysRev/!SSL!/WebHelp/SysRev3.htm. Accessed May 23, 2017. [Google Scholar]
- 27. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Internet]. 2011 Updated March 2011. Available at: http://handbook.cochrane.org/. Accessed May 23, 2017. [Google Scholar]
- 28. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. [DOI] [PubMed] [Google Scholar]
- 29. Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, Somaratne R, Legg JC, Nelson P, Scott R, Wasserman SM, Weiss R; for the LAPLACE‐2 Investigators . Effect of evolocumab or ezetimibe added to moderate‐ or high‐intensity statin therapy on LDL‐C lowering in patients with hypercholesterolemia: the LAPLACE‐2 randomized clinical trial. JAMA. 2014;311:1870–1882. [DOI] [PubMed] [Google Scholar]
- 30. Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, Ceska R, Roth E, Koren MJ, Ballantyne CM, Monsalvo ML, Tsirtsonis K, Kim JB, Scott R, Wasserman SM, Stein EA; for the DESCARTES Investigators . A 52‐week placebo‐controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809–1819. [DOI] [PubMed] [Google Scholar]
- 31. Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124. [DOI] [PubMed] [Google Scholar]
- 32. Di Bartolo B, Takata K, Duong M, Nicholls SJ. CETP inhibition in CVD prevention: an actual appraisal. Curr Cardiol Rep. 2016;18:43. [DOI] [PubMed] [Google Scholar]
- 33. Pfizer Inc . Pfizer discontinues global development of bococizumab, its investigational PCSK9 inhibitor [press release]. Updated November 1, 2016. Available at: http://www.pfizer.com/news/press-release/press-release-detail/pfizer_discontinues_global_development_of_bococizumab_its_investigational_pcsk9_inhibitor. Accessed May 23, 2017.
- 34. Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, Liu T, Mohanavelu S, Hoffman EB, McDonald ST, Abrahamsen TE, Wasserman SM, Scott R, Sabatine MS; for the LAPLACE‐2 Investigators . Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE‐TIMI 57): a randomised, placebo‐controlled, dose‐ranging, phase 2 study. Lancet. 2012;380:2007–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hirayama A, Honarpour N, Yoshida M, Yamashita S, Huang F, Wasserman SM, Teramoto T. Effects of evolocumab (AMG 145), a monoclonal antibody to PCSK9, in hypercholesterolemic, statin‐treated Japanese patients at high cardiovascular risk—primary results from the phase 2 YUKAWA study. Circ J. 2014;78:1073–1082. [DOI] [PubMed] [Google Scholar]
- 36. Kiyosue A, Honarpour N, Kurtz C, Xue A, Wasserman SM, Hirayama A. A phase 3 study of evolocumab (AMG 145) in statin‐treated Japanese patients at high cardiovascular risk. Am J Cardiol. 2016;117:40–47. [DOI] [PubMed] [Google Scholar]
- 37. McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59:2344–2353. [DOI] [PubMed] [Google Scholar]
- 38. Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM; for the ODYSSEY COMBO II Investigators . Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J. 2015;36:1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ginsberg HN, Rader DJ, Raal FJ, Guyton JR, Baccara‐Dinet MT, Lorenzato C, Pordy R, Stroes E. Efficacy and safety of alirocumab in patients with heterozygous familial hypercholesterolemia and LDL‐C of 160 mg/dl or higher. Cardiovasc Drugs Ther. 2016;30:473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kereiakes DJ, Robinson JG, Cannon CP, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J. 2015;169:906–915.e913. [DOI] [PubMed] [Google Scholar]
- 41. Roth EM, Rader D, Moriarty PM, Bergeron J, Langslet G, Baccara‐Dinet M, Zhao J, Manvelian G. A randomized phase 3 trial evaluating alirocumab every four weeks dosing as add‐on to statin or as monotherapy: ODYSSEY CHOICE I. Presented at IAS‐ISA 2015 Congress, Amsterdam, The Netherlands; May 16‐23, 2015.
- 42. Bays H, Gaudet D, Weiss R, Ruiz JL, Watts GF, Gouni‐Berthold I, Robinson J, Zhao J, Hanotin C, Donahue S. Alirocumab as add‐on to atorvastatin versus other lipid treatment strategies: ODYSSEY OPTIONS I randomized trial. J Clin Endocrinol Metab. 2015;100:3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Farnier M, Jones P, Severance R, Averna M, Steinhagen‐Thiessen E, Colhoun HM, Du Y, Hanotin C, Donahue S. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular‐risk patients: the ODYSSEY OPTIONS II randomized trial. Atherosclerosis. 2016;244:138–146. [DOI] [PubMed] [Google Scholar]
- 44. Masana L, Mata P, Gagne C, Sirah W, Cho M, Johnson‐Levonas AO, Meehan A, Troxell JK, Gumbiner B; Ezetimibe Study Group . Long‐term safety and tolerability profiles and lipid‐modifying efficacy of ezetimibe coadministered with ongoing simvastatin treatment: a multicenter, randomized, double‐blind, placebo‐controlled, 48‐week extension study. Clin Ther. 2005;27:174–184. [DOI] [PubMed] [Google Scholar]
- 45. Teramoto T, Kobayashi M, Tasaki H, Yagyu H, Higashikata T, Takagi Y, Uno K, Baccara‐Dinet MT, Nohara A. Efficacy and safety of alirocumab in Japanese patients with heterozygous familial hypercholesterolemia or at high cardiovascular risk with hypercholesterolemia not adequately controlled with statins: ODYSSEY JAPAN Randomized Controlled Trial. Circ J. 2016;80:1980–1987. [DOI] [PubMed] [Google Scholar]
- 46. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ; for the ODYSSEY LONG TERM Investigators . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. [DOI] [PubMed] [Google Scholar]
- 47. Bays H, Farnier M, Gaudet D, Weiss R, Lima Ruiz J, Watts GF, Gouni‐Berthold I, Robinson JG, Jones PH, Severance R, Averna M, Steinhagen‐Thiessen E, Colhoun HM, Zhao J, Du Y, Hanotin C, Donahue S. Efficacy and safety of combining alirocumab with atorvastatin or rosuvastatin versus statin intensification or adding ezetimibe in high cardiovascular risk patients: ODYSSEY OPTIONS I and II. Circulation. 2014;130:2118. [Google Scholar]
- 48. Regeneron Pharmaceuticals Inc., Sanofi . Study to evaluate the efficacy and safety of an every four weeks treatment regimen of alirocumab (REGN727/SAR236553) in patients with primary hypercholesterolemia (ODYSSEY CHOICE 1). NCT01926782. ClinicalTrials.gov. Updated January 30, 2017. Available at: http://ClinicalTrials.gov/show/NCT01926782. Accessed June 5, 2017.
- 49. Sanofi . Efficacy and safety evaluation of alirocumab in patients with heterozygous familial hypercholesterolemia or high cardiovascular risk patients with hypercholesterolemia on lipid modifying therapy. NCT02107898. ClinicalTrials.gov. Updated September 27, 2016. Available at: http://clinicaltrials.gov/show/NCT02107898. Accessed June 5, 2017.
- 50. Sabatine MS, Giugliano RP, Keech A, Honarpour N, Wang H, Liu T, Wasserman SM, Scott R, Sever PS, Pedersen TR. Rationale and design of the Further cardiovascular OUtcomes Research with PCSK9 Inhibition in subjects with Elevated Risk trial. Am Heart J. 2016;173:94–101. [DOI] [PubMed] [Google Scholar]
- 51. Schwartz GG, Bessac L, Berdan LG, Bhatt DL, Bittner V, Diaz R, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Mahaffey KW, Moryusef A, Pordy R, Roe MT, Rorick T, Sasiela WJ, Shirodaria C, Szarek M, Tamby JF, Tricoci P, White H, Zeiher A, Steg PG. Effect of alirocumab, a monoclonal antibody to PCSK9, on long‐term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY OUTCOMES trial. Am Heart J. 2014;168:682–689. [DOI] [PubMed] [Google Scholar]
- 52. JBS Board . Joint British Societies' consensus recommendations for the prevention of cardiovascular disease (JBS3). Heart. 2014;100(suppl 2):ii1–ii67. [DOI] [PubMed] [Google Scholar]
- 53. Boekholdt SM, Arsenault BJ, Mora S, Pedersen TR, LaRosa JC, Nestel PJ, Simes RJ, Durrington P, Hitman GA, Welch KM, DeMicco DA, Zwinderman AH, Clearfield MB, Downs JR, Tonkin AM, Colhoun HM, Gotto AM Jr, Ridker PM, Kastelein JJ. Association of LDL cholesterol, non‐HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta‐analysis. JAMA. 2012;307:1302–1309. [DOI] [PubMed] [Google Scholar]
- 54. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FD, Lochen ML, Lollgen H, Marques‐Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WM; Authors/Task Force Members . 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. US Food & Drug Administration . Praluent® (alirocumab) summary review. Application number 125559Orig1s000. Updated July 24, 2015. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/125559Orig1s000SumR.pdf. Accessed May 23, 2017.
- 56. Jones PH, Bays HE, Chaudhari U, Pordy R, Lorenzato C, Miller K, Robinson JG. Safety of alirocumab (a PCSK9 monoclonal antibody) from 14 randomized trials. Am J Cardiol. 2016;118:1805–1811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Full Search Strategy.
Data S2. Trials Included/Excluded, Full Paper Selection Stage.
Table S1. Methodology and Characteristics of Included Studies
Table S2. Studies Retrieved by the Systematic Review but Excluded From the Network Meta‐Analysis Shaded rows are included in a sensitivity analysis but not in the main.
Figure S1. Treatment difference in percentage LDL‐C (95% credible interval) change from baseline, evolocumab 140 mg Q2W (A) or evolocumab 420 mg QM (B) at week 12 vs comparator at week 12 analyses.
Figure S2. Risk of bias assessed in 69 trials.
Figure S3. Direct meta‐analyses of LDL‐C reduction.
Figure S4. Network for comparing other lipids with evolocumab 140 mg Q2W vs other therapies. AliMab indicates alirocumab; ApoB, apolipoprotein B; EvoMab, evolocumab; EZE, ezetimibe; HDL‐C, high‐density lipoprotein‐cholesterol; Lp(a), lipoprotein(a); non‐HDL‐C, nonhigh‐density lipoprotein‐cholesterol; Q2W, every 2 weeks; QM, every month. Study acronym definitions are available in the source references.
Figure S5. Treatment difference in percentage LDL‐C (95% credible interval) change A, Evolocumab 140 mg Q2W and 420 mg every month combined at the mean of weeks 10 and 12 vs comparator at ≥12 weeks. B, Evolocumab 140 mg Q2W at the mean of weeks 10 and 12 vs comparator at ≥12 weeks with any background therapy.
Figure S6. Sensitivity analysis: treatment difference in percentage LDL‐C (95% credible interval) change from baseline, evolocumab 140 mg Q2W at the mean of weeks 10 and 12 vs comparator at ≥12 weeks (A) excluding Japan studies; (B) ODYSSEY HIGH FH. Evolocumab 420 mg every 4 weeks at weeks 10 and 12 vs comparator at ≥12 weeks (C) excluding studies conducted in Japan.
Figure S7. Treatment difference in percentage (95% credible interval) change from baseline, evolocumab 140 mg Q2W at the mean of weeks 10 and 12 vs comparator at ≥12 weeks: (A) HDL‐C; (B) non‐HDL‐C; (C) ApoB; (D) Lp(a).


