Abstract
Background
The optimal timing of coronary artery bypass grafting (CABG) in clinically stable patients with acute myocardial infarction who are unsuitable for percutaneous coronary intervention is unclear. We report our experience with early CABG in these patients.
Methods and Results
Between January 2001 and May 2015, 766 patients with ST‐segment–elevation myocardial infarction (STEMI, n=305) or non‐STEMI (NSTEMI, n=461) not including cardiogenic shock underwent CABG within 48 hours at our department. STEMI patients were younger than non‐STEMI patients (age 65 years [range: 58–72] versus 70 years [range: 62–75], P<0.001) with a lower EuroSCORE II (4.12 [range: 2.75–5.81] versus 4.58 [range: 2.80–7.74], P=0.009). STEMI patients had undergone preoperative percutaneous coronary intervention more often (20.3% versus 7.8%, P<0.001). Time to surgery was shorter in STEMI compared with non‐STEMI patients (5.0 hours [range: 3.2–8.8] versus 11.7 hours [range: 6.4–22.0], P<0.001). No significant differences concerning arterial graft use (93.8% versus 94.8%, P=0.540) or complete revascularization (87.5% versus 83.4%, P=0.121) were observed. The rate of strokes did not differ between the groups (2.0% versus 3.9%, P=0.134). Thirty‐day mortality was lower in STEMI patients (2.7% versus 6.6% P=0.018), especially when CABG was performed within 6 hours (1.8% versus 7.1%, P=0.041). Survival of STEMI and non‐STEMI patients was 94% versus 88% after 1 year (P<0.001), 87% versus 73% after 5 years (P<0.001), and 74% versus 57% after 10 years (P<0.001). Independent predictors of 30‐day and long‐term mortality included preoperatively increased lactate values, age, atrial fibrillation, and reduced left ventricular function.
Conclusions
Stable STEMI patients showed a lower rate of perioperative complications and better survival compared with non‐STEMI patients when CABG was performed within 48 hours.
Keywords: coronary artery bypass, myocardial infarction
Subject Categories: Myocardial Infarction, Cardiovascular Surgery, Cardiovascular Disease
Clinical Perspective
What Is New?
In this retrospective analysis, hemodynamically stable patients with ST‐segment–elevation myocardial infarction underwent early coronary artery bypass grafting with a significantly lower short‐ and long‐term mortality risk compared with patients with non–ST‐segment–elevation myocardial infarction.
What Are the Clinical Implications?
In contrast to current guideline recommendations, stable ST‐segment–elevation myocardial infarction patients with an indication for operative myocardial revascularization may safely undergo prompt coronary artery bypass grafting.
Introduction
About 20% to 30% of patients with acute myocardial infarction (AMI) are considered noneligible for percutaneous coronary intervention (PCI).1 The optimal timing of coronary artery bypass grafting (CABG) in this setting has remained a matter of discussion. On the one hand, delay of revascularization could result in recurrent myocardial infarction with irreversible loss of cardiac function. On the other hand, the increased rate of perioperative complications associated with CABG in this particular situation could mitigate any potential benefits. Reliable, contemporary data on this topic are rare. As summarized by Caceres et al in 2013, >50% of publications reporting on CABG in AMI patients date back to the 1990s.2 Mohr and his group published one of the most recent studies on this subject.3 They did not find any differences concerning the in‐hospital mortality of 758 patients with non–ST‐segment–elevation myocardial infarction (NSTEMI) when CABG was performed within 24 hours (6.0%), within 24 hours to 72 hours (4.7%), or within 21 days (5.1%). In a publication analyzing the CABG subgroup of the ACCOAST (Comparison of Prasugrel at the Time of PCI or as Pretreatment at the Time of Diagnosis in Patients with NSTEMI) study, 30‐day mortality varied between 3.9% if patients had undergone CABG within 3 days and 1.9% if operative revascularization was performed later on.4 Concerning the outcome of surgically treated STEMI patients, Khaladj et al observed a 15% mortality rate in STEMI patients undergoing emergency CABG within 6 hours after coronary angiography, whereas others reported all‐cause mortality rates between 8.9% (CABG after 24 hours) and 10.6% (CABG within 24 hours).5 To our knowledge, contemporary publications about operative revascularization in AMI usually include patients with cardiogenic shock (CS), as they compose up to 30% of the surgical AMI population. Although these patients are at the highest risk for surgery‐associated complications, they are also the group for which the benefit from timely revascularization has been demonstrated most convincingly.6 In contrast, the optimal timing of CABG in patients with STEMI or NSTEMI who do not suffer from CS has remained unclear. With this study, we intended to analyze the outcome of clinically stable patients with STEMI or NSTEMI who underwent early CABG in the acute phase of AMI. We hypothesized that this approach could be associated with fewer risks than generally assumed.
Methods
Data Source
Our department provides emergency surgical care 24 hours a day, 7 days a week to a catchment area that includes up to 9 hospitals and 1 practice scattered throughout the German federal state of Schleswig‐Holstein. Between January 2001 and May 2015, 766 consecutive patients (305 with STEMI, 461 with NSTEMI) underwent CABG within 48 hours. Retrospective data analysis was performed. Patients with combined procedures or with CS and/or resuscitated patients were excluded from this particular analysis. A patient was considered to be in CS if persistent hypotension (<90 mm Hg systolic blood pressure for at least 30 minutes) had been documented and/or a continuous infusion of catecholamines had been administered to maintain a systolic blood pressure >90 mm Hg at any time point traceable before surgery. General discrimination between STEMI and NSTEMI, if not clearly stated by the referring cardiologists, was made following current guideline recommendations.7, 8 If the time point of symptom onset was not comprehensible and the original, time‐stamped ECG was unavailable, we used the first significant increase in troponin levels in case of NSTEMI or the time point of coronary angiography in case of STEMI to determine the time between AMI and CABG. All aspects of information leading to the calculation of this time interval were checked as meticulously as possible to ensure a conclusive timeline. Patients or their surrogate decision makers provided written consent. In addition to 30‐day mortality, follow‐up data were obtained. Data were collected by contacting the respective patients by mail. For cases in which patients or relatives did not respond, we interrogated their general practitioner. If whereabouts remained still unknown, we contacted the public records office. The median follow‐up period of patients was 3.8 years (range: 0.1–14.5 years). Nine patients were lost during the follow‐up period (98.8% level of completeness). The study was approved by the institutional review committee.
Preoperative Management
Every case and time point of CABG was discussed with the referring cardiologist. Our department usually operates on AMI patients immediately after transfer from the initial treatment centers independent of hemodynamic stability, symptoms, or cardiac enzymes. This approach usually excludes patients who present with a definitely subsided myocardial infarction. CABG was performed under dual platelet therapy regardless of the P2Y12 inhibitor used.
Surgical Management
A standard median sternotomy and cardiopulmonary bypass with moderate hypothermic (34°C) cardiac arrest was performed in all but 1 patient. Myocardial arrest was obtained with cold blood cardioplegic solution applied antegrade via the ascending aorta. If insufficient myocardial protection by this approach was anticipated, antegrade cardioplegia was combined with retrograde administration via the coronary venous sinus. The choice of graft was left to the discretion of the surgeon in charge. If bleeding was not a concern, acetylsalicylic acid was administered orally starting on postoperative day 1.
Statistical Analyses
Nominal and ordinal data were described as absolute and relative frequencies and compared using the χ2 or Fisher exact test, if one of the expected values in the 2×2 table was <5. The interval and ratio data were tested for normal distribution by the Kolmogorov–Smirnov test. Normally distributed demographic and clinical patient data are presented as mean and standard deviation and compared using an unpaired t test. Not normally distributed data were described as median and 25th and 75th percentiles and compared using the Mann–Whitney U‐test. Preoperative parameters with a significant relation to 30‐day mortality in the univariate analyses were included in multiple logistic regression analysis to assess their relative impact (adjusted odds ratio), except for EuroSCORE II due to its collinearity with several variables. Covariates with significant univariate association with survival (log‐rank test) were included in Cox regression analysis to determine predictors for mid‐ and long‐term survival. None of the predictors violated the proportional hazards assumption.9 To assess the predictive abilities of the final models, R 2 and Somers's D were calculated and corrected for overfitting by internal validation methods, namely, bootstrap resampling with 100 replicates.10, 11 All tests were performed 2‐tailed at a significance level of 5%. Statistical analysis was conducted using SPSS version 15.0 (IBM Corp) and R version 3.3.2 (R Foundation for Statistical Computing), in particular, function validate from package rms.
Results
Preoperative Data
As shown in Table 1, patients with STEMI were significantly younger than those with NSTEMI (median age: 65 years [range: 58–72] versus 70 years [range: 62–75]; P<0.001). More patients with NSTEMI suffered from insulin‐dependent diabetes mellitus (n=18 [5.9%] versus n=48 [10.4%]; P=0.030) and impaired renal function preoperatively (n=31 [10.2%] versus n=92 [20.0%]; P<0.001). The EuroSCORE II was significantly higher in NSTEMI patients (4.12 [range: 2.75–5.81] versus 4.58 [range: 2.80–7.74]; P=0.009). Prehospital thrombolysis was performed more often in patients with STEMI (n=43 [14.1%] versus n=3 [0.7%]; P<0.001), as was intra‐aortic balloon pump implantation (n=33 [10.8%] versus n=29 [6.3%]; P=0.024). As demonstrated in Table 2, more STEMI patients had undergone interventional treatment before surgery (n=62 [20.3%] versus n=36 [7.8%]; P<0.001), and time from diagnosis to surgery was significantly shorter (5.0 hours [range: 3.2–8.8] versus 11.7 hours [range: 6.4–22.0]; P<0.001). NSTEMI patients were more often diagnosed with coronary 3‐vessel disease (n=243 [80.2%] versus n=397 [86.9%]; P=0.014; Table 2). Drug‐eluting stents (n=10 [3.3%] versus n=2 [0.4%]; P=0.002) and bare metal stents (n=8 [2.6%] versus n=3 [0.65%]; P=0.031) were more often implanted in these patients. Dual‐platelet therapy was applied more frequently in NSTEMI patients (n=73 [24.2%] versus n=142 [31.9%]; P=0.022). Glycoprotein IIb/IIIa antagonists were used more often in patients with STEMI (n=95 [31.5%] versus n=95 [20.9%]; P=0.001).
Table 1.
Patient Characteristics
| Parameter | STEMI (n=305) | NSTEMI (n=461) | P Value |
|---|---|---|---|
| Age, y | 65 (58–72) | 70 (62–75) | <0.001 |
| Sex (female) | 64 (21.0) | 116 (25.2) | 0.190 |
| BMI | 26.6 (24.6–29.4) | 27.2 (24.7–30.1) | 0.082 |
| IDDM | 18 (5.9) | 48 (10.4) | 0.030 |
| Arterial hypertension | 227 (74.4) | 373 (80.9) | 0.048 |
| Hyperlipidemia | 157 (51.5) | 228 (49.5) | 0.563 |
| Smoking | 121 (39.7) | 159 (34.5) | 0.144 |
| COPD | 23 (7.5) | 51 (11.1) | 0.133 |
| Peripheral arterial disease | 18 (5.9) | 56 (12.1) | 0.004 |
| Atrial fibrillation | 18 (5.9) | 48 (10.4) | 0.035 |
| LV function ≤50% | 120 (39.3) | 169 (36.7) | 0.493 |
| Renal impairment, (CC <50 mL/min/1.73 m²) | 31 (10.2) | 92 (20.0) | <0.001 |
| Renal replacement therapy | 2 (0.7) | 2 (0.4) | 0.651 |
| Severely reduced LV function (<30%) | 18 (5.9) | 21 (4.6) | 0.336 |
| EuroSCORE II (%) | 4.12 (2.75–5.81) | 4.58 (2.80–7.74) | 0.009 |
| Prehospital thrombolysis | 43 (14.1) | 3 (0.7) | <0.001 |
| IABP preoperatively | 33 (10.8) | 29 (6.3) | 0.024 |
| Intubated on arrival in the operating room | 2 (0.7) | 8 (1.7) | 0.330 |
| Continuous inotropic support | 2 (0.7) | 8 (1.7) | 0.330 |
| Time from diagnosis to surgery, h | 5.0 (3.2–8.8) | 11.7 (6.4–22.0) | <0.001 |
| Lactate, mmol/L | 1.20 (0.85–1.70) | 1.00 (0.80–1.40) | 0.001 |
| Lactate >2 mmol/L | 58 (19.0) | 46 (10.1) | 0.003 |
| Cerebrovascular event | 15 (4.9) | 29 (6.3) | 0.430 |
| Prior myocardial infarction | 32 (10.5) | 45 (9.8) | 0.746 |
| Prior cardiac surgery | 3 (1.0) | 8 (1.7) | 0.540 |
Values are expressed as count (percentage) or median (range). BMI indicates body mass index; CC, creatinine clearance; COPD, chronic obstructive pulmonary disease; IABP, intra‐aortic balloon pump; IDDM, insulin‐dependent diabetes mellitus; LV, left ventricular; NSTEMI, non–ST‐segment–elevation myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction.
Table 2.
Coronary Angiography
| Parameter | STEMI (n=305) | NSTEMI (n=461) | P Value |
|---|---|---|---|
| Coronary anatomy | |||
| 1‐VD | 13 (4.3) | 10 (2.2) | 0.098 |
| 2‐VD | 47 (15.5) | 49 (10.7) | 0.052 |
| 3‐VD | 243 (80.2) | 397 (86.9) | 0.014 |
| LM stenosis | 115 (38.1) | 180 (40.2) | 0.564 |
| LM thrombosis | 9 (3.0) | 8 (1.8) | 0.261 |
| LM dissection | 13 (4.3) | 14 (3.1) | 0.376 |
| PCI | 62 (20.3) | 36 (7.8) | <0.001 |
| Successful but incomplete revascularization | 17 (27.4) | 4 (11.1) | 0.080 |
| Failure | 28 (45.1) | 18 (50.0) | 0.679 |
| Complication | 17 (27.4) | 13 (36.1) | 0.375 |
| Stent implantation | |||
| DES | 10 (3.3) | 2 (0.4) | 0.002 |
| BMS | 8 (2.6) | 3 (0.65) | 0.031 |
| Other/unknown | 2 (0.7) | 0 (0) | 0.1572 |
| Medication | |||
| Acetylsalicylic acid and clopidogrel | 73 (24.2) | 142 (31.9) | 0.022 |
| Ticagrelor | 5 (1.7) | 21 (4.7) | 0.028 |
| GP IIb/IIIa antagonist | 95 (31.5) | 95 (20.9) | 0.001 |
Values are expressed as count (percentage). BMS indicates bare metal stent; DES, drug‐eluting stent; GP, glycoprotein; LM, left main; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; VD, vessel disease.
Intraoperative Data
Cross‐clamp times were significantly shorter in STEMI compared with NSTEMI patients (60 minutes [range: 47–74] versus 63 minutes [range: 49–79]; P=0.024; Table 3). Arterial grafts were used to a similar extent in STEMI (n=285 [93.8%]) and NSTEMI (n=437 [94.8%]; P=0.540) patients. The left internal thoracic artery was the most frequently utilized arterial graft, with proportionally more use in patients with NSTEMI (n=384 [83.5%]) compared with STEMI (n=237 [78.0%]), but this difference did not reach statistical significance (P=0.056). Complete revascularization was accomplished to a similar degree in both groups (STEMI: n=266 [87.5%] versus n=382 [83.4%]; P=0.121).
Table 3.
Intraoperative Details
| Parameter | STEMI (n=305) | NSTEMI (n=461) | P Value |
|---|---|---|---|
| Length of surgery | 212 (184–248) | 214 (187–247) | 0.419 |
| Bypass time | 101 (83–121) | 104 (85–127) | 0.122 |
| Cross‐clamp time | 60 (47–74) | 63 (49–79) | 0.024 |
| On‐pump CABG | 299 (98.0) | 447 (97.0) | 0.226 |
| Off‐pump CABG | 2 (0.7) | 2 (0.4) | 0.652 |
| Number of distal anastomoses | 3.0 (3.0–4.0) | 3.0 (3.0–4.0) | 0.160 |
| Arterial graft | 285 (93.8) | 437 (94.8) | 0.540 |
| LITA | 237 (78.0) | 384 (83.5) | 0.056 |
| Complete revascularization | 266 (87.5) | 382 (83.4) | 0.121 |
Values are expressed as count (percentage) or median (range). CABG indicates coronary artery bypass grafting; LITA, left internal thoracic artery; NSTEMI, non–ST‐segment–elevation myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction.
Postoperative Data
Patients with NSTEMI needed blood transfusions, defined as transfusion of >3 packed red blood cell units, more frequently than STEMI patients (n=122 [41.1%] versus n=232 [51.0%]; P=0.008; Table 4). The stroke rate was not different between STEMI patients (n=6 [2.0%]) and NSTEMI patients (n=18 [3.9%]; P=0.134). Peak levels of CK‐MB (creatine kinase‐MB) were higher in patients with STEMI (124.2 U/L [range: 64.1–261.9] versus 67.0 U/L [range: 43.6–115.5]; P<0.001). More STEMI than NSTEMI patients received intra‐aortic balloon pump implantation (n=7 [2.3%] versus n=1 [0.2%]; P=0.008).
Table 4.
Postoperative Details
| Parameter | STEMI (n=305) | NSTEMI (n=461) | P Value |
|---|---|---|---|
| Low cardiac output | 0 | 4 (0.9) | 0.156 |
| Sepsis | 10 (3.3) | 26 (5.7) | 0.135 |
| Ventilation >48 h | 71 (23.4) | 106 (23.0) | 0.901 |
| ICU >48 h | 155 (51.2) | 203 (44.2) | 0.061 |
| Rethoracotomy due to bleeding | 12 (4.0) | 14 (3.1) | 0.526 |
| Stroke | 6 (2.0) | 18 (3.9) | 0.134 |
| Renal replacement therapy | 16 (5.3) | 30 (6.6) | 0.470 |
| >3 U PRBC | 122 (41.1) | 232 (51.0) | 0.008 |
| >1 U TC | 70 (23.7) | 122 (26.7) | 0.362 |
| Myocardial infarction | 5 (1.7) | 5 (1.1) | 0.530 |
| Peak CK‐MB (U/L) | 124.2 (64.1–261.9) | 67.0 (43.6–115.5) | <0.001 |
| Ventricular arrhythmias | 16 (5.2) | 21 (4.6) | 0.241 |
| ECLS | 1 (0.3) | 4 (0.9) | 0.653 |
| IABP | 7 (2.3) | 1 (0.2) | 0.008 |
Values are expressed as count (percentage) or median (range). CK‐MB indicates creatine kinase‐MB; ECLS, extracorporeal life support; IABP, intra‐aortic‐balloon pump; ICU, intensive care unit; NSTEMI, non–ST‐segment–elevation myocardial infarction; PRBC, packed red blood cells; STEMI, ST‐segment–elevation myocardial infarction; TC, thrombocyte concentrate.
Outcome
Thirty‐day mortality (Table 5) was 2.7% in the STEMI group and 6.6% in the NSTEMI group (P=0.018). As shown in Figure 1, survival at 1 year was 94% in STEMI versus 88% in NSTEMI patients (P<0.001). Five‐year survival was 87% versus 73%, and 10‐year survival was 74% versus 57% (log‐rank P<0.001, Figure 1). NSTEMI patients aged >70 years at the time of CABG showed significantly reduced survival (62% versus 79%; log‐rank P=0.029; Figure 2A and 2B).
Table 5.
Outcome
| Parameter | STEMI | NSTEMI | P Value |
|---|---|---|---|
| Intraoperative mortality | 1 (0.3) | 1 (0.2) | 1.000 |
| 30‐d mortality | 8 (2.7) | 30 (6.6) | 0.018 |
| Time to surgery and 30‐d mortality | |||
| ≤6 h to surgery | 3 (1.8) | 7 (7.1) | 0.041 |
| 6–12 h to surgery | 4 (6.1) | 7 (5.5) | 1.000 |
| 12–48 h to surgery | 1 (1.8) | 16 (7.1) | 0.210 |
| NSTEMI <6 h (n=95) | NSTEMI ≥6 h (n=366) | ||
| Preoperative lactate, mmol/L | 1.15 (0.88–1.50) | 1.00 (0.88–1.30) | 0.019 |
| Lactate >2 mmol/L | 16 (16.9) | 30 (8.2) | 0.020 |
| Acetylsalicylic acid and clopidogrel | 14 (14.7) | 138 (37.7) | 0.007 |
| Preoperative IABP | 13 (13.7) | 16 (4.4) | 0.003 |
| STEMI <6 h (n=171) | STEMI ≥6 h (n=134) | ||
| CK‐MB U/L | 54 (19.5–101.5) | 78.5 (40.1–163.5) | 0.001 |
Values are expressed as count (percentage) or median (range). CK‐MB indicates creatine kinase‐MB; IABP, intra‐aortic‐balloon pump; NSTEMI, non–ST‐segment–elevation myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction.
Figure 1.
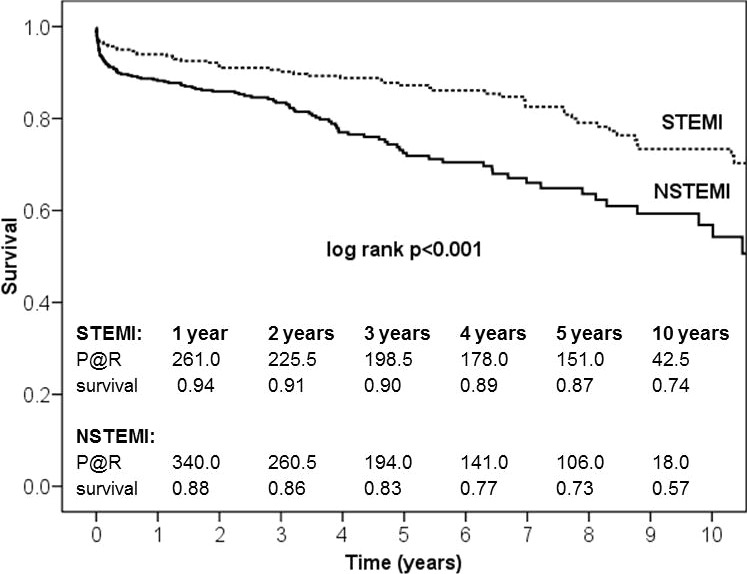
Ten‐year survival after acute myocardial infarction and coronary artery bypass. Kaplan–Meier survival curves for STEMI and NSTEMI patients showed significant survival differences. NSTEMI indicates non–ST‐segment–elevation myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction; P@R, patients at risk.
Figure 2.
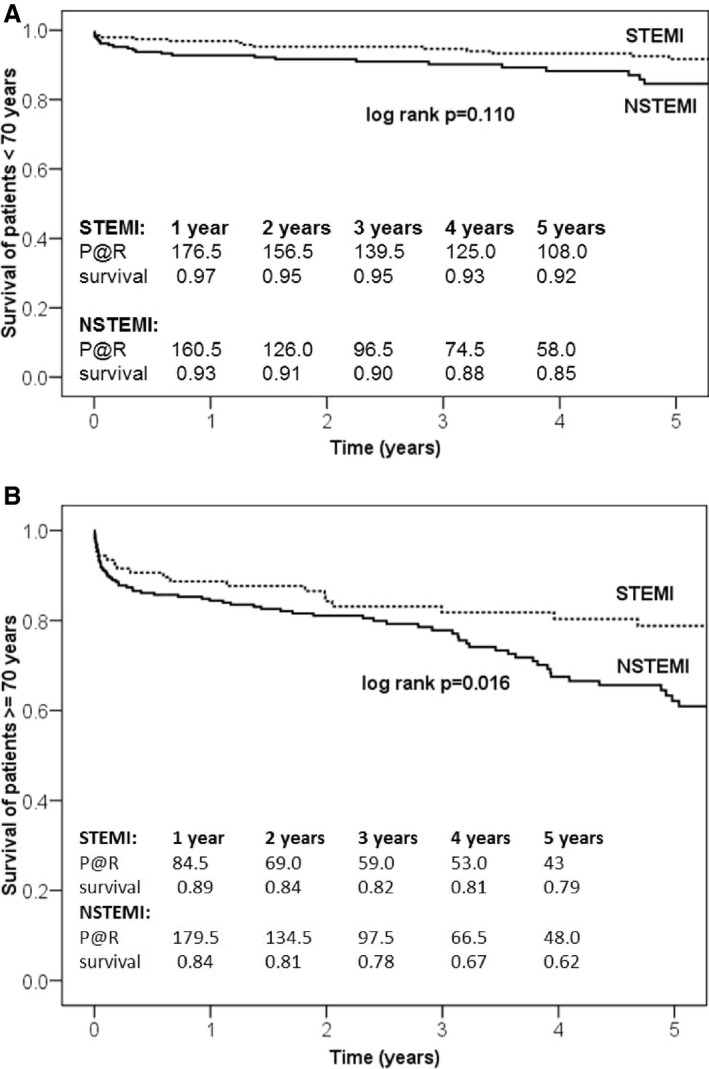
A, Five‐year survival of patients aged ≤70 y. B, Five‐year survival of patients aged >70 y. Kaplan–Meier survival curves for STEMI and NSTEMI patients showed an age‐dependent effect. NSTEMI indicates non–ST‐segment–elevation myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction; P@R, patients at risk.
Outcome and Timing of Surgery
Thirty‐day mortality was significantly increased in patients with NSTEMI patients who underwent surgery within 6 hours compared with STEMI patients (7.1% versus 1.8%; P=0.041). NSTEMI patients operated on within 6 hours less often received acetylsalicylic acid plus clopidogrel (P=0.007), had significantly higher lactate values (P=0.020), and underwent preoperative intra‐aortic balloon pump implantation (P=0.003) more often compared with NSTEMI patients operated on after 6 hours (Table 5). STEMI patients operated on within 6 hours showed significantly lower CK‐MB levels compared with STEMI patients who underwent CABG later on (P=0.001). No significant differences were observed within the STEMI (Figure 3A) and NSTEMI (Figure 3B) groups with regard to survival when time to surgery was further divided into patients who were operated on within 6, 6 to 12, or 12 to 48 hours.
Figure 3.
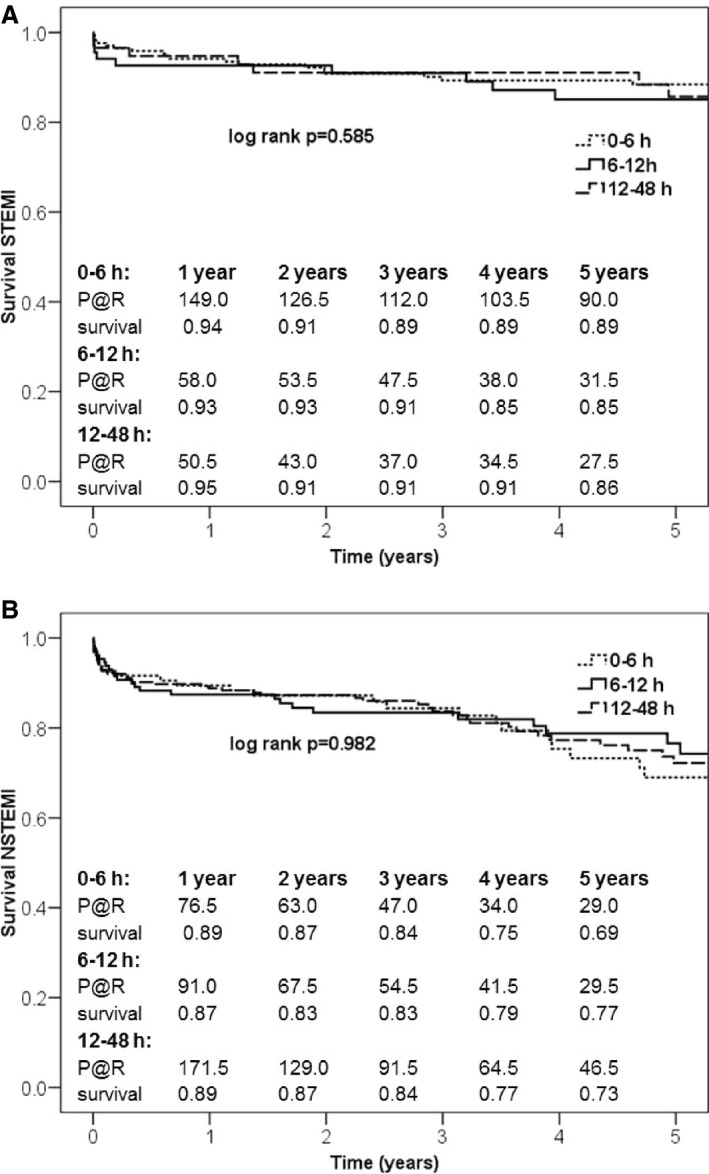
A, Five‐year survival of patients with STEMI analyzed by time to surgery. B, Five‐year survival of patients with NSTEMI analyzed by time to surgery. Kaplan–Meier survival curves for STEMI and NSTEMI patients showed no impact on survival within the groups after subdividing time to surgery. NSTEMI indicates non–ST‐segment–elevation myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction; P@R, patients at risk.
Predictors of 30‐day mortality
Independent risk factors for 30‐day mortality (Table 6) included preoperative atrial fibrillation (odds ratio [OR]: 4.290; 95% confidence interval [CI], 1.712–10.752; P=0.002), preoperative lactate >2 mmol/L (OR: 3.942; 95% CI, 1.550–10.023; P=0.004), peripheral arterial disease (OR: 3.848; 95% CI, 1.527–9.696; P=0.004), prior stroke (OR: 3.722; 95% CI 1.227–11.289; P=0.020), and reduced left ventricular function (≤50%; OR: 3.257; 95% CI, 1.398–7.586; P=0.006). Somers's D was 0.62 (bias corrected: 0.54), and R 2 was 0.21 (bias corrected: 0.14).
Table 6.
Predictors of 30‐Day Mortality
| Parameter | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Atrial fibrillation | 4.290 | 1.712–10.752 | 0.002 |
| Lactate >2 mmol/L | 3.942 | 1.550–10.023 | 0.004 |
| Peripheral arterial disease | 3.848 | 1.527–9.696 | 0.004 |
| Prior stroke | 3.722 | 1.227–11.289 | 0.020 |
| LV function ≤50% | 3.257 | 1.398–7.586 | 0.006 |
Included into model 1: age ≥70 y, preoperative lactate >2 mmol, creatinine clearance <50 mL/min/1.73 m2, chronic obstructive pulmonary disease, peripheral arterial disease, prior stroke, continuous inotropic support, atrial fibrillation, left main stenosis, LV function ≤50%, time from diagnosis to surgery <6 h. CI indicates confidence interval; LV, left ventricular.
Predictors of late mortality
Cox regression analysis revealed atrial fibrillation (hazard ratio [HR]: 2.184; 95% CI, 1.437–3.318; P<0.001), age >70 years (HR: 2.125; 95% CI, 1.426–3.166; P<0.001), peripheral arterial disease (HR: 2.106; 95% CI, 1.300–3.410; P=0.002), reduced left ventricular function (≤50%; HR: 1.983; 95% CI, 1.385–2.837; P<0.001), creatinine clearance <50 mL/min per 1.73 m² (HR: 1.955; 95% CI, 1.265–3.023; P=0.003), lactate >2 mmol/L (HR: 1.954; 95% CI, 1.270–3.008; P=0.002), and chronic obstructive pulmonary disease (HR: 1.640; 95% CI, 1.022–2.633; P=0.040) as independent predictors of late mortality (Table 7). Somers's D was 0.51 (bias corrected: 0.49), and R 2 was 0.18 (bias corrected: 0.16).
Table 7.
Predictors of Late Mortality
| Parameter | Hazard ratio | 95% CI | P Value |
|---|---|---|---|
| Atrial fibrillation | 2.184 | 1.437–3.318 | <0.001 |
| Age >70 y | 2.125 | 1.426–3.166 | <0.001 |
| Peripheral arterial disease | 2.106 | 1.300–3.410 | 0.002 |
| Reduced LV function ≤50% | 1.983 | 1.385–2.837 | <0.001 |
| CC <50 mL/min/1.73 m² | 1.955 | 1.265–3.023 | 0.003 |
| Lactate >2 mmol/L | 1.954 | 1.270–3.008 | 0.002 |
| COPD | 1.640 | 1.022–2.633 | 0.040 |
Included into model 1: age ≥70 y, preoperative lactate >2 mmol/L, CC <50 mL/min/1.73 m², COPD, peripheral arterial disease, prior stroke, continuous inotropic support, atrial fibrillation, non–ST‐segment–elevation myocardial infarction, left main stenosis, ejection fraction ≤50%, time from diagnosis to surgery <6 h. CC indicates creatinine clearance; CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Discussion
Low‐Risk STEMI Patients With Short Time to Decision for Surgery
Timing of operative revascularization for patients with AMI unsuitable for interventional treatment has been evaluated with conflicting results.12, 13 Consequently, only a minority of nonrevascularized patients undergo CABG, eventually.1, 14, 15 Our department offers early revascularization to all AMI patients. In the absence of CS, this approach was associated with superior short‐ and long‐term outcomes for STEMI patients compared with NSTEMI patients. STEMI patients were significantly younger, with lower risk profiles and shorter referral times, which indicated that the decision to recommend fast surgical treatment was made during or soon after coronary angiography.
Lower Stroke Rate and Fewer Bleeding Complications in STEMI Patients
Current guidelines emphasize that the risks associated with surgery in AMI will be maximal in the setting of STEMI.7 In particular, the increased likelihood of cerebrovascular events associated with CABG represents a major concern. It has become clear that the incidence of stroke per se is higher among patients with AMI, presumably due to the increased systemic inflammatory state.16 Although PCI‐focused AMI trials reported cerebrovascular event rates between ≈0.3% and 1%, stroke risks among AMI patients undergoing CABG range between 0.8% and 6%.3, 4, 5 We found a slightly although not statistically significant lower proportion of strokes in patients with STEMI compared with NSTEMI (2.0% versus 3.9%, P=0.134). The causes underlying perioperative strokes are multifactorial and thus difficult to modify.17 Nevertheless, cerebral protection devices currently under investigation during transcatheter aortic valve implantation could also provide a future possibility to reduce intraoperative thromboembolism.18, 19 In addition, intraoperative continuous near‐infrared spectroscopy measurements are an effective tool for the early detection of impaired cerebral tissue oxygenation.20 Although the impact of corrective steps on the neurological outcome of patients with low near‐infrared spectroscopy values and cardiac surgery has not been evaluated yet, it has become clear that intraoperative optimization of cerebral oxygenation leads to reduced release of markers of neurological injury.21
The fear of severe bleeding also influences risk assessment and time point selection of operative revascularization in AMI patients. In fact, 24% of STEMI and 31% of NSTEMI patients in our study received dual‐platelet inhibition (P=0.022) preoperatively. This finding was associated with a significantly lower transfusion rate in STEMI compared with NSTEMI patients (41% versus 51%, P=0.008). Given ambiguous trial results, however, transfusion indications after cardiac surgery have remained a matter of opinion and personal experience.22, 23 Consequently, this parameter has only limited value in the identification of severe bleeding events. In fact, we did not observe statistically significant differences in the rate of surgical reexploration due to bleeding between STEMI and NSTEMI patients (4% versus 3.1%, P=0.526). Neither transfusion nor rethoracotomy caused by bleeding was identified as a risk factor for mortality in this study.
Complex NSTEMI Patients With Delayed Surgery
As expected, NSTEMI patients were older and suffered from insulin‐dependent diabetes mellitus, arterial hypertension, impaired renal function, and multivessel coronary artery disease more frequently than patients with STEMI. In addition, time from diagnosis to surgery was significantly longer. This finding was probably due to several factors. First, the wider scope of discretion regarding the indication and time point of coronary angiography in NSTEMI patients naturally leads to delayed awareness of coronary artery disease severity.8 Although this circumstance apparently does not impair the outcome of most patients eligible for PCI, it may become a disadvantage for those in need of CABG. In addition, the decision to recommend surgery at all may have taken more time, considering the increased rate of perioperative risk factors in NSTEMI patients.
Outcome After Surgery Within 6 Hours
Overall, 56% of STEMI patients underwent surgery within 6 hours. Our collaborating cardiology partners obviously referred the majority of STEMI patients as quickly as possible if unsuitable for PCI. These patients demonstrated a lower short‐term mortality rate compared with NSTEMI patients. They were younger and had undergone PCI before CABG more often, which could have resulted in at least temporary improvement of myocardial perfusion. In contrast, only 20% of NSTEMI patients received surgery within 6 hours. These patients had particularly increased preoperative lactate levels compared with those NSTEMI patients with CABG at a later time point. In fact, lactate values were comparable to those of STEMI patients, who showed higher lactate levels during all other time frames except this one. We did not find any association among increased lactate and preoperative hypotension, use of catecholamines, or reduced left ventricular function. Nevertheless, the increased number of preoperative intra‐aortic balloon pump implantations in NSTEMI patients who were operated on early could indicate that impending cardiac failure was expected. Overall, NSTEMI patients—in contrast to STEMI patients—seemed to have been presented for rapid surgery particularly if deterioration was anticipated.
Outcome After 6 Hours
A comparison between preoperative parameters of STEMI patients who could have influenced the decision for or against early CABG revealed significant differences only as far as cardiac enzymes were concerned. STEMI patients operated on >6 hours later had higher CK‐MB levels, pointing to persisting or recurrent myocardial ischemia. NSTEMI patients operated on >6 hours later more often received dual‐platelet inhibition, but PCI rates among these groups did not differ. Consequently, this observation most likely indicates that time to surgery or time to decision was long enough to initiate dual‐platelet therapy, which has been shown to improve survival of patients with acute coronary syndromes, independent of the treatment strategy chosen.8
Lactate Levels for Identification of High‐Risk Patients
Increased lactate values have already been linked to mortality in patients with myocardial infarction treated by CABG but also after PCI.24, 25 Despite the exclusion of patients with clinically apparent CS, 19% of STEMI and 10% of NSTEMI patients still presented with preoperative lactate levels >2 mmol/L in this study. As stated, we could not find any association between this parameter and other indicators of impending CS. On the contrary, 43% of STEMI patients and 47% of NSTEMI patients with preoperative lactate levels >2 mmol/L were hypertensive—defined as a blood pressure >140/90 mm Hg—before undergoing surgery. Hypertension has already been linked to increased lactate that may be caused by obstruction of microvessels, with subsequently impaired tissue perfusion.26, 27 In addition, preoperatively sustained fasting periods with volume depletion also could have contributed to the rate of elevated lactate levels. Regardless of the underlying cause, NSTEMI patients with preoperatively elevated lactate levels seem to represent a particularly vulnerable cohort. This finding does not imply that CABG should be delayed in these patients. Instead, it facilitates the easy and fast identification of high‐risk NSTEMI patients. We feel that coronary angiography should not be delayed if increased lactate values are present, regardless of hemodynamic stability or symptoms. The therapeutic strategy—whether surgical, interventional, or conservative—may thus be determined before further clinical deterioration takes place.
Study Limitations
This study analyzed retrospective data from a single‐center experience with early CABG in AMI patients. It covers 14 years of cardiac care and thus also includes changes in the treatment of acute coronary syndromes over time. Consequently, any conclusions drawn from this work are limited by its design. Despite our various efforts to preclude all CS patients, we cannot completely rule out the possibility that a study participant with evolving CS indicated solely by increased lactate levels accidentally remained in this analysis. Nevertheless, we did not feel comfortable excluding patients based on this parameter alone because lactate elevations may be caused by several factors unrelated to CS, as discussed. Although our department offers operative revascularization to all AMI patients as a service 24 hours a day, 7 days a week, the initial decision to present a patient at a certain time point was made by our referring cardiologists. Consequently, the data presented also reflect local opinions about which patients might benefit from early operative revascularization.
Summary
Prospective data already demonstrated that conservative treatment is associated with poorer prognosis compared with urgent operative revascularization in patients with AMI and CS.6 Accordingly, guideline recommendations are clear regarding the approach in this clinical setting.7,8 Nevertheless, patients with CS are also at the highest risk for perioperative complications.6 It is unknown whether hemodynamically stable patients with STEMI or NSTEMI also suffer from increased perioperative risks. This has always been assumed, given the proinflammatory state of these patients. In fact, some previous surgical data indicated that delayed surgery may indeed result in better outcomes. However, it is unknown to what extent these data are biased by the possibility that AMI patients planned for CABG suffer from major adverse cardiovascular events while waiting for the initial infarction “to cool off” and thus are not included in any kind of risk evaluation. Furthermore, until recently, a majority of surgical publications on this topic did not differentiate between STEMI and NSTEMI, as many surgeons believed that differentiation did not matter.2 Subsequently, it was impossible to analyze the outcome of STEMI versus NSTEMI patients, although prospective PCI data have demonstrated substantial differences between these groups with regard to risk stratification, time frame for revascularization, and survival.7,8 Consequently, we feel that it is vital to extend the knowledge about timing of CABG in patients with STEMI and NSTEMI. Our institution offers early revascularization to all patients with AMI with no preselection of patients on our side. In particular, our data indicate that hemodynamically stable STEMI patients can safely undergo early operative revascularization. These findings do not support the guarded phrasing of current guideline recommendations. However, they mirror the commonly accepted conclusion drawn from multiple PCI‐driven, prospective studies: that early revascularization is beneficial in STEMI patients. In contrast, the surgical NSTEMI population described in this study consisted of older and sicker patients with consequently higher perioperative risk profiles for which the optimal treatment timing and goals were more difficult to determine. In contrast to the STEMI treatment recommendations, our NSTEMI data are largely in line with the conclusions outlined in the guidelines. These findings, however, also emphasize the need for a randomized controlled study that focuses on the questions of who and at which time point AMI patients considered unsuitable for PCI should undergo surgery; otherwise, we will not be able to tell whether the mortality rates and complications reported for early CABG in this scenario are low or high.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e005498 DOI: 10.1161/JAHA.117.005498.)
References
- 1. Dasari TW, Hamilton S, Chen AY, Wang TY, Peterson ED, de Lemos JA, Saucedo JF. Non‐eligibility for reperfusion therapy in patients presenting with ST‐segment elevation myocardial infarction: contemporary insights from the National Cardiovascular Data Registry (NCDR). Am Heart J. 2016;172:1–8. [DOI] [PubMed] [Google Scholar]
- 2. Caceres M, Weiman DS. Optimal timing of coronary artery bypass grafting in acute myocardial infarction. Ann Thorac Surg. 2013;95:365–372. [DOI] [PubMed] [Google Scholar]
- 3. Davierwala PM, Verevkin A, Leontyev S, Misfeld M, Borger MA, Mohr FW. Does timing of coronary artery bypass surgery affect early and long‐term outcomes in patients with non‐ST‐segment‐elevation myocardial infarction? Circulation. 2015;132:731–740. [DOI] [PubMed] [Google Scholar]
- 4. Dudek D, Dziewierz A, Widimsky P, Bolognese L, Goldstein P, Hamm C, Tanguay J‐F, LeNarz L, Miller DL, Brown E, Ten Berg J, Montalescot G. Impact of prasugrel pretreatment and timing of coronary artery bypass grafting on clinical outcomes of patients with non‐ST‐segment elevation myocardial infarction: from the A Comparison of Prasugrel at PCI or Time of Diagnosis of Non‐ST‐Elevation Myocardial Infarction (ACCOAST) study. Am Heart J. 2015;170:1025–1032. [DOI] [PubMed] [Google Scholar]
- 5. Khaladj N, Bobylev D, Peterss S, Guenther S, Pichlmaier M, Bagaev E, Martens A, Shrestha M, Haverich A, Hagl C. Immediate surgical coronary revascularisation in patients presenting with acute myocardial infarction. J Cardiothorac Surg. 2013;8:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hochman JS. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med. 1999;341:625–634. [DOI] [PubMed] [Google Scholar]
- 7. Steg PG, James SK, Atar D, Badano LP, Blomstrom‐Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez‐Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van't Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J. 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 8. Roffi M, Patrono C, Collet J‐P, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol C, Fitzsimons D, Halle M, Hamm C, Hildick‐Smith D, Huber K, Iliodromitis E, James S, Lewis BS, Lip GYH, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 9. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 10. Steyerberg EW, Harrell FE, Borsboom GJJM, Eijkemans MJC, Vergouwe Y, Habbema JDF. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. [DOI] [PubMed] [Google Scholar]
- 11. Harrell FE. Regression Modeling Strategies. 2015. 1‐501‐507. DOI: 10.1007/978-1-4757-3462-1. [DOI] [Google Scholar]
- 12. Anderson JL, Doty JR. Bypass surgery after non‐ST‐segment elevation myocardial infarction better early than late? JACC Cardiovasc Interv. 2010;3:428–430. [DOI] [PubMed] [Google Scholar]
- 13. Chedrawy EG, Massad MG. The role of surgery in ongoing infarction. Thorac Cardiovasc Surg. 2010;58:197–199. [DOI] [PubMed] [Google Scholar]
- 14. Sugiyama T, Hasegawa K, Kobayashi Y, Takahashi O, Fukui T, Tsugawa Y. Differential time trends of outcomes and costs of care for acute myocardial infarction hospitalizations by ST elevation and type of intervention in the United States, 2001–2011. J Am Heart Assoc. 2015;4:e001445 DOI: 10.1161/JAHA.114.001445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freisinger E, Fuerstenberg T, Malyar NM, Wellmann J, Keil U, Breithardt G, Reinecke H. German nationwide data on current trends and management of acute myocardial infarction: discrepancies between trials and real‐life. Eur Heart J. 2014;35:979–988. [DOI] [PubMed] [Google Scholar]
- 16. Hachet O, Guenancia C, Stamboul K, Daubail B, Richard C, Bejot Y, Yameogo V, Gudjoncik A, Cottin Y, Giroud M, Lorgis L. Frequency and predictors of stroke after acute myocardial infarction: specific aspects of in‐hospital and postdischarge events. Stroke. 2014;45:3514–3520. [DOI] [PubMed] [Google Scholar]
- 17. Sheth KN, Nourollahzadeh E. Neurologic complications of cardiac and vascular surgery. Handb Clin Neurol. 2017;141:573–592. [DOI] [PubMed] [Google Scholar]
- 18. Lansky AJ, Schofer J, Tchetche D, Stella P, Pietras CG, Parise H, Abrams K, Forrest JK, Cleman M, Reinohl J, Cuisset T, Blackman D, Bolotin G, Spitzer S, Kappert U, Gilard M, Modine T, Hildick‐Smith D, Haude M, Margolis P, Brickman AM, Voros S, Baumbach A. A prospective randomized evaluation of the TriGuard HDH embolic DEFLECTion device during transcatheter aortic valve implantation: results from the DEFLECT III trial. Eur Heart J. 2015;36:2070–2078. [DOI] [PubMed] [Google Scholar]
- 19. Haussig S, Mangner N, Dwyer MG, Lehmkuhl L, Lucke C, Woitek F, Holzhey DM, Mohr FW, Gutberlet M, Zivadinov R, Schuler G, Linke A. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN‐TAVI Randomized Clinical Trial. JAMA. 2016;316:592–601. [DOI] [PubMed] [Google Scholar]
- 20. Zheng F, Sheinberg R, Yee M‐S, Ono M, Zheng Y, Hogue CW. Cerebral near‐infrared spectroscopy monitoring and neurologic outcomes in adult cardiac surgery patients: a systematic review. Anesth Analg. 2013;116:663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harilall Y, Adam JK, Biccard BM, Reddi A. The effect of optimising cerebral tissue oxygen saturation on markers of neurological injury during coronary artery bypass graft surgery. Heart Lung Circ. 2014;23:68–74. [DOI] [PubMed] [Google Scholar]
- 22. Murphy GJ, Pike K, Rogers CA, Wordsworth S, Stokes EA, Angelini GD, Reeves BC. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. 2015;372:997–1008. [DOI] [PubMed] [Google Scholar]
- 23. Kilic A, Whitman GJR. Blood transfusions in cardiac surgery: indications, risks, and conservation strategies. Ann Thorac Surg. 2014;97:726–734. [DOI] [PubMed] [Google Scholar]
- 24. Davierwala PM, Leontyev S, Verevkin A, Rastan AJ, Mohr M, Bakhtiary F, Misfeld M, Mohr FW. Temporal trends in predictors of early and late mortality after emergency coronary artery bypass grafting for cardiogenic shock complicating acute myocardial infarction. Circulation. 2016;134:1224–1237. [DOI] [PubMed] [Google Scholar]
- 25. Lazzeri C, Valente S, Chiostri M, Gensini GF. Clinical significance of lactate in acute cardiac patients. World J Cardiol. 2015;7:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Demartini FE, Cannon PJ, Stason WB, Laragh JH. Lactic acid metabolism in hypertensive patients. Science. 1965;148:1482–1484. [DOI] [PubMed] [Google Scholar]
- 27. Juraschek SP, Bower JK, Selvin E, Shantha GPS, Hoogeveen RC, Ballantyne CM, Hunter Young J. Plasma lactate and incident hypertension in the atherosclerosis risk in communities study. Am J Hypertens. 2015;28:216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]


