Abstract
Background
One measurement of hs‐CRP (high‐sensitivity C‐reactive protein) is associated with increased risk of cardiovascular disease (CVD). The objective of this study was to characterize the association of cumulative exposure to increased hs‐CRP with incident cardiovascular events.
Methods and Results
We included 53 065 participants with hs‐CRP measured at 3 examinations in 2006, 2008, and 2010. Cumulative exposure to hs‐CRP was calculated as the weighted sum of the average hs‐CRP level for each time interval (level×time). Participants were classified into nonexposed group (hs‐CRP<3.0 mg/L in all 3 examinations), 1‐exposed group (hs‐CRP≥3.0 mg/L in 1 of the 3 examinations), 2‐exposed group (hs‐CRP≥3.0 mg/L in 2 of the 3 examinations), and 3‐exposed group (hs‐CRP≥3.0 mg/L in 3 examinations). Cox proportional hazards models were used to assess the association of cumulative hs‐CRP with incident CVD. The study showed a dose‐response pattern with risk of CVD and myocardial infarction as the number of years of exposure to hs‐CRP increases. Participants in the 3‐exposed group had significantly increased CVD risk with hazard ratio (95% confidence interval) of 1.38 (1.11–1.72), in comparison with 1.28 (1.07–1.52) for participants in the 2‐exposed group and 1.13 (0.97–1.31) for those in the 1‐exposed group (P<0.05); meanwhile, the similar and significant associations were also observed for myocardial infarction with respective hazard ratio (95% confidence interval) of 2.13 (1.42–3.18), 1.60 (1.12–2.27), and 1.57 (1.17–2.10). The associations between stroke and cumulative hs‐CRP were not statistically significant (P=0.360).
Conclusions
Cumulative exposure to hs‐CRP was dose dependently associated with a subsequent increased risk of CVD and myocardial infarction.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/. Unique identifier: ChiCTR‐TNC‐11001489.
Keywords: cardiovascular disease, cohort study, high sensitivity C‐reactive protein, incidence, risk factor
Subject Categories: Vascular Disease
Clinical Perspective
What Is New?
Our study confirms the association between high‐exposure levels of high‐sensitivity C‐reactive protein and myocardial infarction, with better predictive values than single‐shot measurements.
What Are the Clinical Implications?
In order to prevent the occurrence of chronic cardiovascular events, especially myocardial infarction, to avoid long‐grade inflammatory status has important clinical significance.
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality worldwide, making it important to improve the diagnostic and therapeutic capabilities, as well as the preventive strategies. Inflammation is essential to the initiation and progression of atherosclerosis,1, 2 the basic pathological process of most CVD.3, 4, 5 Owing to fact that changes in vascular inflammation can be hard to evaluate by current cardiac imaging methods, the role of circulating inflammatory biomarkers is increasing. Among the wide array of inflammatory biomarkers, hs‐CRP (high‐sensitivity C‐reactive protein) has been the most profoundly studied and received much more attention for its prospect as a cost‐effective and stable predictor for CVD screening and risk reclassification.6 Multiple prospective cohort studies, including the ARIC (Atherosclerosis Risk In Communities) Study, have reported that increased CRP (C‐reactive protein) levels were significantly associated with increased CVD event risk.7, 8, 9, 10, 11 Data from a stroke‐free, multiethnic, and community‐based cohort study, NOMAS (the Northern Manhattan Study), have suggested that participants with hs‐CRP >3 mg/L were at significantly increased risk of myocardial infarction (MI; adjusted hazard ratio [HR], 1.70; 95% confidence interval [CI], 1.04–2.77), but the ability of hs‐CRP to predict cerebrovascular events was not confirmed.12 It is noteworthy that previous analyses to investigate whether hs‐CRP really predicts future cardiovascular events were based on a single hs‐CRP measurement, and variability in hs‐CRP concentration remains unaccounted for.13, 14, 15 However, a single measurement of high hs‐CRP does not mean that the body state has sustained a high hs‐CRP for a long time. In addition, metabolic dysregulation, such as diabetes mellitus and obesity, dietary patterns, environmental pollutant burden, and potential inflammation, may lead to incidental high hs‐CRP concentration. Thus, a single high hs‐CRP may lead to incorrect classification of the risk assessment for CVD events.
Because single measurement of hs‐CRP may not reflect the cumulative burden and longitudinal variation associated with CVD risk, measurements of cumulative exposure that capture both the intensity and duration could more accurately estimate the effects of hs‐CRP.16 Serial elevated levels may be more prognostic than a single elevated measurement. For example, Doll and Hill first reported results of a large‐scale investigation undertaken in London and Wales to determine whether there was a significant association between carcinoma of the lung and high cumulative exposure to smoking.17, 18 Navar‐Boggan et al19 reported that cumulative exposure to hyperlipidemia increases the risk of coronary heart disease. Zemaitis et al,20 in a multiethnic cohort of subjects without diabetes mellitus, showed that cumulative exposure to elevated blood pressure may affect progression of urine albumin excretion. Therefore, to determine the association between cumulative exposure to hs‐CRPs and outcomes of CVD, the Kailuan study was initiated as a prospective cohort population study to investigate the risk factors and intervention strategies for CVD among community‐dwelling participants. Each participant underwent a comprehensive assessment of risk factors, including hs‐CRP and occurrence of cardiovascular events. Participants completed follow‐up assessment every 2 years.
Methods
Study Population
Participants enrolled in the Kailuan longitudinal cohort study were employees of the Kailuan Company residing in Kailuan community. Details of the design, objectives, recruitment, sampling, and quality‐control activities of the Kailuan study have been previously reported.21 Each participant underwent an interview of standardized questionnaires and clinical examinations in 11 hospitals responsible for health care of the community. Baseline data were collected from 2006 to 2007 with follow‐up health examinations conducted at sequential intervals of 2 years.
Of the 101 510 participants included in the baseline survey in 2006 to 2007, 53 065 were included in the final analysis as shown in Figure 1. All of the subjects gave informed consent to participate in this study. The study protocol was performed in accord with the guidelines of Helsinki Declaration and approved by the Ethics Committee of the Kailuan General Hospital (Tangshan, China). In the Kailuan study, we examined the association between hs‐CRP during 3 separate measurements and occurrence of CVD at the third examination among enrolled subjects at baseline.
Figure 1.
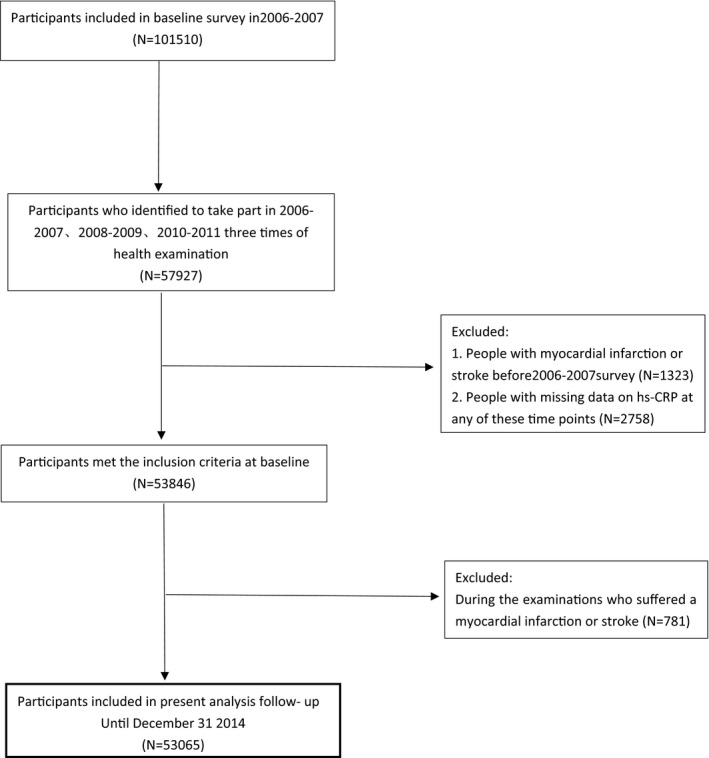
Study flow chart. hs‐CRP indicates high‐sensitivity C‐reactive protein.
Measurement of hs‐CRP
At each of the clinical examinations blood samples were drawn after an overnight fast. Serum levels of hs‐CRP were determined by an immunoturbidimetry assay (Kanto Chemical Co Inc, Tokyo, Japan), with a lower limit of detection of 0.1 mg/L. From 2006 to 2009, the Ministry of Health organized the proficiency testing program for the hs‐CRP measurement in our laboratory and all the values of (proficiency testing) were 100%. Precision was assessed by measuring the concentration of serum hs‐CRP twice a day with at least 2 hours of interval for 20 days in 2 common serum samples, yielded within‐run coefficient of variation of 6.53% and between‐run coefficient of variation of 4.78%. The interday and total coefficient of variations were 6.61% and 9.37%, respectively. Measuring errors were small. The reference intervals for hs‐CRP in our subjects that we established, using a HITACHI 7600 automated analyzer (Hitachi Ltd., Tokyo, Japan), was 0 to 5.0 mg/L.
Assessment of Potential Covariates
Self‐reported smoking and alcohol consumption was categorized as binary variables. Current smokers were defined as regular smoking at least 1 cigarette a day in the past 12 months. Subjects were classified as current drinkers if they reported average wine consumption of 100 mL or more a day for more than a year. Active physical activity was defined as regular exercise for at least 30 minutes at a time and more than 3 times a week. Hypertension was defined as systolic blood pressure (SBP) ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg or SBP/diastolic blood pressure <140/90 mm Hg but under active treatment of antihypertensive medication. Diabetes mellitus was defined as a fasting glucose level ≥7.0 mm/L or current use of antidiabetic medication with a fasting glucose level <7.0 mm/L. Hypercholesterolemia was defined as a total cholesterol ≥5.2 mmol/L, triglyceride ≥1.7 mmol/L, and low‐density lipoprotein ≥2.2 mmol/L or under active treatment of lipid‐lowering therapy.
Definitions of End Event
The cardiovascular events included myocardial infarction (MI) or any stroke (hemorrhagic and ischemic stroke). MI, including ST‐segment elevation myocardial infarction and non‐ST‐segment elevation myocardial infarction, was ascertained by the onset of angina pectoris, ischemic features in ECG, and rise in cardiac serum markers such as cardiac troponin T, cardiac troponin I, or creatine kinase‐MB.22, 23 Stroke was determined according to the diagnostic criteria from the World Health Organization combined with the use of computed tomography scans and nuclear magnetic resonance imaging technique.24 The follow‐up time interval was counted from the end of the third clinical examination in 2010 until December 31, 2014. CVD was determined according to a combination of the medical insurance system in Kailuan and 2 physicians’ diagnoses using information on clinical symptoms and signs from hospital records.
Cumulative Exposure to hs‐CRP
Cumulative exposure to hs‐CRP (cumhs‐CRP) was calculated as the weighted sum of the average hs‐CRP level for each: (hs‐CRP06+hs‐CRP08)/2×time06‐08+(hs‐CRP08+hs‐CRP10)/2×time08‐10, where hs‐CRP06, hs‐CRP08, and hs‐CRP10 indicate hs‐CRP at baseline, and time06‐08 and time08‐10 indicate the participant‐specific time intervals between consecutive examinations in years.
In addition to correlative exposure, we classified subject participants into 4 groups: the nonexposed group (hs‐CRP<3.0 mg/L in all 3 examinations); the 1‐exposed group (hs‐CRP≥3.0 mg/L in 1 of the 3 examinations); the 2‐exposed group (hs‐CRP≥3.0 mg/L in 2 of the 3 examinations); and the 3‐exposed group (hs‐CRP≥3.0 mg/L in all 3 examinations).
In Asians, the hs‐CRP cutoff of 3.0 mg/L was recommended for categorization into the high‐risk group.
Statistical Analyses
Analysis was performed using SPSS software (version 13.0; SPSS, Inc, Chicago, IL). Continuous variables were expressed as mean±SD when normally distributed. Differences among groups were assessed using 1‐way ANOVA, and post hoc comparisons were evaluated using the least significant difference or Dunnett t tests according to homogeneity of variance. However, hs‐CRP level and cumulative hs‐CRP were non‐normally distributed once they were logarithmically transformed or converted into quartiles, and subsequently assessed using 1‐way ANOVA. Categorical data are expressed as frequencies (percentages) and were compared by chi‐square test. The cumulative event rate of CVD was estimated using the life‐table method, and the differences between cumhs‐CRP exposed groups were compared using the log‐rank test. For the association between cumhs‐CRP and baseline hs‐CRP and incident CVD, the Cox proportional hazards model was used to calculate HR with 95% CI. A 2‐sided P<0.05 was considered statistically significant.
To evaluate the effects of acute inflammation (hs‐CRP≥10 mg/L), lipid‐lowering therapy, and treatment of antihypertensive medication on hs‐CRP, we performed sensitivity analysis by excluding these subjects and rerunning the analyses.
Results
General Characteristics
Baseline general characteristics of the 53 065 individuals selected for study are shown in Table 1. These 53 065 participants had an average age of 49.0±11.7 years, 40 433 were males (76.2%), and had an average of 3.96±0.48 years of follow‐up. We classified subjects into 4 groups according to the cumulative exposure to cumhs‐CRP: the nonexposed group; the 1‐exposed group; the 2‐exposed group; and the 3‐exposed group. The results (Table 1) showed that with increasing years of exposure to hs‐CRP, age, baseline SBP, baseline diastolic blood pressure, body mass index, fasting plasma glucose, total cholesterol, hs‐CRP in 2006, hs‐CRP in 2008, hs‐CRP in 2010, and cumulative hs‐CRP also increased significantly among different groups (P<0.001). There were significant differences in percentage of males, current smokers, current drinkers, physical activity, and the percentage of subjects under treatment of antihypertensive and antidiabetic medication (P<0.001). No significant differences were observed among groups in subjects under treatment of lipid‐lowering therapy (P=0.157). There were 48 445 individuals from the Kailuan cohort that were excluded from this analysis. A comparison of general characteristics of the 53 065 included in the 48 445 excluded individuals is given in Table S1. We report an estimate of effect size with every P value they report in Table 1 and Table S1. Using η2 (eta‐squared) estimates, the effect size is small in baseline, showing that these variables have very small influence. In addition, we categorized the hs‐CRP in 2006, hs‐CRP in 2008, and hs‐CRP in 2010 into quartiles (P<0.001) and reported the cumhs‐CRP by logarithmic transformations (Table 2).
Table 1.
Baseline General Characteristics of the 53 065 Individuals Selected for Study
| Variables | Total Sample (N=53 065) | Years of Exposure to cumhs‐CRP | η2 | P Value | |||
|---|---|---|---|---|---|---|---|
| Nonexposed (N=29 496) | 1‐Exposed (N=14 433) | 2‐Exposed (N=6147) | 3‐Exposed (N=2989) | ||||
| Male, n (%) | 40 433 (76.2) | 22 538 (76.4) | 11 770 (77.4) | 4629 (75.3) | 2096 (70.1) | 0.001 | <0.001 |
| Age, y | 49.00±11.73 | 47.47±11.34 | 48.86±11.68 | 53.36±11.69 | 55.79±11.15 | 0.044 | <0.001 |
| Baseline SBP, mm Hg | 128.41±19.79 | 126.48±18.97 | 128.92±19.79 | 133.36±21.09 | 134.74±21.53 | 0.019 | <0.001 |
| Baseline DBP, mm Hg | 82.64±11.35 | 81.72±11.04 | 83.16±11.45 | 84.82±11.85 | 84.72±11.87 | 0.010 | <0.001 |
| BMI, kg/m2 | 25.07±3.47 | 24.63±3.13 | 25.35±3.45 | 25.92±3.64 | 26.28±3.93 | 0.024 | <0.001 |
| FPG, mmol/L | 5.39±1.54 | 5.34±1.42 | 5.43±1.55 | 5.47±1.75 | 5.52±2.01 | 0.001 | <0.001 |
| TC, mmol/L | 4.93±1.14 | 4.88±1.14 | 4.94±1.15 | 5.02±1.07 | 5.09±1.07 | 0.003 | <0.001 |
| Current smokers, n (%) | 15 841 (29.9) | 9233 (31.3) | 4519 (31.3) | 1498 (24.4) | 591 (19.8) | 0.005 | <0.001 |
| Current drinkers, n (%) | 9729 (17.5) | 5552 (18.8) | 2545 (17.6) | 865 (14.1) | 317 (10.6) | 0.003 | <0.001 |
| Physical activity, n (%) | 7301 (13.8) | 4295 (14.6) | 1940 (13.4) | 761 (12.4) | 305 (10.2) | 0.001 | <0.001 |
| Hypertension, n (%) | 20 495 (38.6) | 10 016 (34.0) | 5825 (40.4) | 3073 (50.0) | 1581 (52.9) | 0.017 | <0.001 |
| Diabetes mellitus, n (%) | 4131 (7.8) | 1598 (6.6) | 1192 (8.3) | 640 (10.4) | 341 (11.4) | 0.003 | <0.001 |
| Hyperlipidemia, n (%) | 39 937 (75.3) | 22 002 (74.6) | 11 245 (77.9) | 4565 (74.3) | 2125 (71.1) | 0.002 | <0.001 |
| Antihypertensive medication, n (%) | 9364 (45.7) | 4313 (43.1) | 2681 (46.0) | 1513 (49.2) | 857 (54.2) | 0.003 | <0.001 |
| Antidiabetic medication, n (%) | 2697 (65.3) | 1159 (72.5) | 759 (63.7) | 486 (75.9) | 293 (85.9) | <0.001 | <0.001 |
| Lipid‐lowering medication, n (%) | 1169 (2.9) | 543 (2.5) | 325 (2.9) | 189 (4.1) | 112 (5.3) | <0.001 | 0.157 |
Continuous variables are presented as mean±SD; categorical variables are presented as numbers or percentages. Baseline DBP, diastolic blood pressure in 2006; Baseline SBP, systolic blood pressure in 2006; BMI, body mass index; cumhs‐CRP, cumulative high‐sensitivity C‐reactive protein; FPG, fasting plasma glucose; TC, total cholesterol.
Table 2.
hs‐CRP Level of Different Individuals Selected for Study
| Variables | Total Sample (N=101 510) | Years of Exposure to cumhs‐CRP | Exclude (N=48 445) | P Value | |||
|---|---|---|---|---|---|---|---|
| Nonexposed (N=29 496) | 1‐Exposed (N=14 433) | 2‐Exposed (N=6147) | 3‐Exposed (N=2989) | ||||
| 06hs‐CRP, mg/L | 0.76 [0.3, 2.16] | 0.46 [0.20, 0.97] | 1.05 [0.39, 2.83] | 4.00 [1.80, 7.60] | 7.00 [4.90–9.50] | 0.90 [0.33, 2.30] | <0.001 |
| 08hs‐CRP, mg/L | 1.60 [0.80, 3.30] | 1.00 [0.60, 1.61] | 2.70 [1.20, 4.10] | 4.50 [3.40, 6.50] | 5.60 [4.30, 8.70] | 1.60 [0.80, 3.30] | <0.001 |
| 10hs‐CRP, mg/L | 1.03 [0.50, 2.50] | 0.75 [0.37, 1.30] | 1.58 [0.70, 3.50] | 3.13 [1.11, 5.20] | 5.90 [4.20, 9.40] | 0.99 [0.30, 2.30] | <0.001 |
| Lgcumhs‐CRP | 081±0.43 | 0.52±0.27 | 1.06±0.27 | 1.31±0.22 | 1.48±0.22 | 0.43±0.01 | <0.001 |
06hs‐CRP indicates high‐sensitivity C‐reactive protein in 2006; 08hs‐CRP, high‐sensitivity C‐reactive protein in 2008; 10hs‐CRP, high‐sensitivity C‐reactive protein in 2010; cumhs‐CRP, cumulative high‐sensitivity C‐reactive protein; Lgcumhs‐CRP, The cumulative hs‐CRP needed to be log‐transformed.
The Cumulative Incidence of Cardiovascular Events Among Different Groups
Over the follow‐up period, there were 1057 new‐onset cardiovascular events, 273 MIs, 795 strokes, and 11 concurrent events. Strokes were 672 ischemic stroke, 136 hemorrhagic stroke, and 13 concurrent events. The cumulative incidence rate of cardiovascular events were elevated with the increasing years of exposure to cumhs‐CRP, and significant differences were observed in the total study population across different groups exposed to cumhs‐CRP by log‐rank test, as shown in Table 3 and Figure 2.
Table 3.
Incident of End Point Events of Study Population
| Variables | N=53 065 | Years of Exposure to cumhs‐CRP | P Value | |||
|---|---|---|---|---|---|---|
| Nonexposed (N=29 496) | 1‐Exposed (N=14 433) | 2‐Exposed (N=6147) | 3‐Exposed (N=2989) | |||
| CVD [n (%)] | 1057 (2.0) | 455 (1.5) | 299 (2.1) | 189 (3.1) | 114 (3.5) | <0.001 |
| MI [n (%)] | 273 (0.5) | 97 (0.3) | 88 (0.6) | 50 (0.8) | 38 (1.3) | <0.001 |
| Stroke [n (%)] | 795 (1.5) | 362 (1.2) | 216 (1.5) | 140 (2.3) | 77 (2.6) | <0.001 |
| Hemorrhagic stroke [n (%)] | 136 (0.3) | 57 (0.2) | 39 (0.3) | 29 (0.5) | 11 (0.4) | 0.001 |
| Ischemic stroke [n (%)] | 672 (1.3) | 309 (1.0) | 184 (1.3) | 113 (1.8) | 66 (2.2) | <0.001 |
cumhs‐CRP indicates cumulative high‐sensitivity C‐reactive protein; CVD, cardiovascular disease; MI, myocardial infarction.
Figure 2.
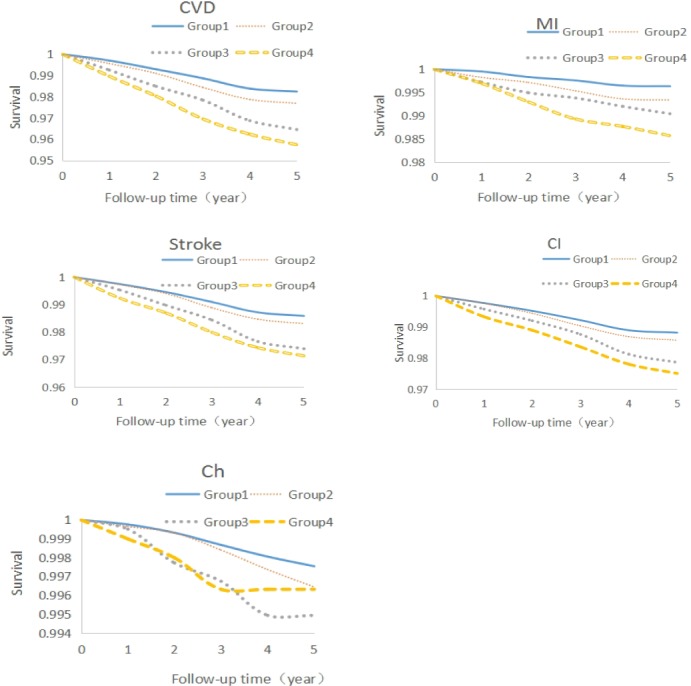
Survival curves of the total population. Ch indicates cerebral thrombosis; CI, cerebral hemorrhage; CVD, cardiovascular disease; Group 1, nonexposed group; Group 2, 1‐exposed group; Group 3, 2‐exposed group; Group 4, 3‐exposed group; MI, myocardial infarction.
The Cox Proportional Hazards Models of cumhs‐CRP for Cardiovascular Events
Cox proportional hazards models were used to determine whether cumulative exposure to hs‐CRP was associated with increasing risk of cardiovascular events. The nonexposed group was used as a reference group, and the independent variables were different cumulative exposure of hs‐CRP. The dependent variables included CVD, MI, stroke, ischemic stroke, and hemorrhagic stroke. HRs were first adjusted for age and sex (model 1). A subsequent analysis also adjusted for confounders, including baseline SBP, body mass index, fasting plasma glucose, total cholesterol, physical activity, smoking, drinking, and treatment of antihypertensive, antidiabetic, and lipid‐lowering medication (model 2). 1‐exposure to hs‐CRP was associated with CVD, MI, and stroke with respective HR (95% CI) of 1.13 (0.98–1.31), 1.58 (1.18–2.11), and 1.02 (0.86–1.21) in model 2. Similar associations were observed in the adjusted model 2 for 2‐exposure to hs‐CRP with respective HR (95% CI) of 1.29 (1.08–1.54), 1.63 (1.14–2.31), and 1.19 (0.97–1.45) and 3‐exposure to hs‐CRP with respective HR (95% CI) of 1.41 (1.14–1.75), 2.19 (1.48–3.25), and 1.19 (0.92–1.53). In a final analysis (model 3), we included baseline hs‐CRP. The results showed a dose‐response pattern with progressively increasing risk of CVD and MI as the number of years of exposure to hs‐CRP increases. Participants with the 3‐exposed group had significantly increased CVD risk with HR (95% CI) of 1.38 (1.11–1.72), in comparison with 1.28 (1.07–1.52) for participants with the 2‐exposed group and 1.13 (0.97–1.31) for those with the 1‐exposed group (P<0.05); meanwhile, similar and significant associations were also observed for MI with respective HR (95% CI) of 2.13 (1.42–3.18), 1.60 (1.12–2.27), and 1.57 (1.17–2.10). The associations between stroke and cumulative exposure to hs‐CRP were attenuated and not statistically significant (HR and 95% CI, 1.17 [0.90–1.52], 1.18 [0.96–1.45], and 1.02 [0.86–1.21]; P trend=0.360), as shown in Table 4.
Table 4.
Adjusted HRs and 95% CI of cumhs‐CRP for End Point Events by Cox Proportional Hazards Models
| Variables | aHR (95% CI) | ||||
|---|---|---|---|---|---|
| CVD | MI | Stroke | Hemorrhagic Stroke | ||
| Model 1b | |||||
| Nonexposed | |||||
| 1‐exposed | 1.22 (1.06–1.41) | 1.67 (1.26–2.25) | 1.11 (0.93–1.31) | 1.27 (0.85–1.91) | 1.10 (0.92–1.32) |
| 2‐exposed | 1.49 (1.25–1.77) | 1.84 (1.30–2.61) | 1.38 (1.13–1.68) | 1.87 (1.18–2.95) | 1.29 (1.04–1.60) |
| 3‐exposed | 1.69 (1.37–2.08) | 2.65 (1.80–3.89) | 1.41 (1.10–1.82) | 1.35 (0.70–2.61) | 1.40 (1.07–1.84) |
| P trend | <0.001 | <0.001 | 0.003 | 0.08 | 0.03 |
| Model 2c | |||||
| Nonexposed | |||||
| 1‐exposed | 1.13 (0.98–1.31) | 1.58 (1.18–2.11) | 1.02 (0.86–1.21) | 1.18 (0.79–1.78) | 1.01 (0.84–1.22) |
| 2‐exposed | 1.29 (1.08–1.54) | 1.63 (1.14–2.31) | 1.19 (0.97–1.45) | 1.62 (1.02–2.57) | 1.11 (0.89–1.39) |
| 3‐exposed | 1.41 (1.14–1.75) | 2.19 (1.48–3.25) | 1.19 (0.92–1.53) | 1.13 (0.58–2.21) | 1.17 (0.89–1.54) |
| P trend | 0.003 | <0.001 | 0.274 | 0.282 | 0.625 |
| cumhs‐CRP (+1 SD) | 1.05 (1.01–1.09) | 1.08 (1.02–1.13) | 1.04 (0.99–1.09) | 1.06 (0.97–1.15) | 1.03 (0.97–1.09) |
| Model 3d | |||||
| Nonexposed | |||||
| 1‐exposed | 1.13 (0.97–1.31) | 1.57 (1.17–2.10) | 1.02 (0.86–1.21) | 1.17 (0.77–1.76) | 1.02 (0.85–1.23) |
| 2‐exposed | 1.28 (1.07–1.52) | 1.60 (1.12–2.27) | 1.18 (0.96–1.45) | 1.55 (0.97–2.47) | 1.12 (0.89–1.41) |
| 3‐exposed | 1.38 (1.11–1.72) | 2.13 (1.42–3.18) | 1.17 (0.90–1.52) | 1.06 (0.54–2.08) | 1.19 (0.89–1.59) |
| P trend | 0.008 | 0.001 | 0.360 | 0.362 | 0.613 |
| 06hs‐CRP | 1.002 (0.997–1.007) | 1.003 (0.996–1.011) | 1.002 (0.995–1.008) | 1.007 (0.999–1.014) | 0.998 (0.987–1.010) |
| cumhs‐CRP (+1 SD) | 1.042 (0.996–1.092) | 1.068 (1.003–1.136) | 1.029 (0.970–1.093) | 1.003 (0.840–1.198) | 1.031 (0.968–1.099) |
06hs‐CRP indicates high‐sensitivity C‐reactive protein in 2006; CI, confidence interval. P trend, test of trend based on median value in each category for ordinal categorical variables; cumhs‐CRP, cumulative high‐sensitivity C‐reactive protein; CVD, cardiovascular disease; HR, hazard ratio; MI, myocardial infarction.
Compared with nonexposed group.
Model 1: adjusted for age and sex.
Model 2: adjusted for variables in Model 1 plus baseline SBP, BMI, FPG, TC, physical activity, smoking, drinking, and treatment of antihypertensive, antidiabetic, and lipid‐lowering medication.
Model 3: Model 2 plus 06hs‐CRP.
Cox proportional hazards models were also used to determine whether baseline hs‐CRP was associated with risk of cardiovascular events. To explore the role of baseline hs‐CRP in these analyses, secondary hierarchical analyses were also computed, including model 1, age and sex terms only, and model 2, model 1+baseline SBP, body mass index, fasting plasma glucose, total cholesterol, physical activity, smoking, drinking, and treatment of antihypertensive, antidiabetic, and lipid‐lowering medication. The results showed that a 1‐mg/L increase in baseline hs‐CRP was associated with elevated risk of CVD, MI, stroke, ischemic stroke, and hemorrhagic stroke with respective HR (95% CI) of 1.004 (1.001–1.008), 1.006 (1.001–1.011), 1.003 (0.998–1.009), 1.007 (1.001–1.014), and 1.001 (0.993–1.009), as shown in Table S2.
The Logistic Regression Model of Mean hs‐CRP and Cardiovascular Events
The logistic regression model was used to determine whether mean hs‐CRP was associated with risk of cardiovascular events. After adjusting for the same characteristics—age, sex, baseline SBP, body mass index, fasting plasma glucose, total cholesterol, physical activity, smoking, drinking, and treatment of antihypertensive, antidiabetic, and lipid‐lowering medication—the results showed that a 1‐mg/L increase in mean hs‐CRP was associated with elevated risk of CVD and MI with respective HR (95% CI) of 1.017 (1.003–1.032) and 1.025 (1.005–1.046). The associations between stroke mean hs‐CRP were attenuated and not statistically significant (HR and 95% CI, 1.013 [0.995–1.031], 1.014 [0.996–1.033], and 1.006 [0.956–1.058]; P=0.150; Table S3).
The Cox Proportional Hazards Models for Sensitivity Analysis
We performed a sensitivity analysis by rerunning the Cox proportional hazards models excluding individuals with acute inflammation (hs‐CRP≥10 mg/L at any examinations), lipid‐lowering therapy, or treatment with antihypertensive medication (Table 5). We excluded subjects with hs‐CRP≥10 mg/L during 3 examinations (model 4), under treatment of lipid‐lowering therapy (model 5), and antihypertensive (model 6) medication. Cox proportional hazards modeling was performed evaluating the impact of cumhs‐CRP and risk of CVD, MI, and stroke, adjusting for the same characteristics as in the previous analysis. Model 4 showed that the 3‐exposed group was significantly associated with CVD and MI with respective HR (95% CI) of 1.39 (1.04–1.86) and 2.28 (1.36–3.81). The same associations were also observed in models 5 and 6. Subjects with the 3‐exposed group had significantly increased CVD risk with HR (95% CI) of 1.40 (1.12–1.75) and MI risk with HR (95% CI) of 2.20 (1.47–3.29) in model 5, in comparison with 1.57 (1.17–2.10) for CVD and 2.17 (1.27–3.69) for MI in model 6. No significant associations between the 3‐exposed group and stroke were observed in these models.
Table 5.
Adjusted HRs and 95% CI of cumhs‐CRP for End Point Events by Cox Proportional Hazards Models (Sensitive Analyses)
| Variables | HR (95% CI) | ||||
|---|---|---|---|---|---|
| CVD | MI | Stroke | Hemorrhagic Stroke | Ischemic Stroke | |
| Model 4 | |||||
| Nonexposed | |||||
| 1‐exposed | 1.11 (0.95–1.30) | 1.50 (1.01–2.05) | 1.01 (0.85–1.22) | 1.12 (0.73–1.74) | 1.01 (0.83–1.23) |
| 2‐exposed | 1.29 (1.06–1.58) | 1.69 (1.14–2.52) | 1.18 (0.93–1.49) | 1.37 (0.79–2.39) | 1.13 (0.88–1.46) |
| 3‐exposed | 1.39 (1.04–1.86) | 2.28 (1.36–3.81) | 1.17 (0.83–1.66) | 0.66 (0.20–2.12) | 1.23 (0.85–1.77) |
| P trend | 0.015 | 0.002 | 0.410 | 0.547 | 0.507 |
| cumhs‐CRP (+1 SD) | 1.38 (1.14–1.66) | 2.12 (1.51–2.99) | 1.17 (0.94–1.46) | 1.10 (0.63–1.92) | 1.16 (0.91–1.48) |
| Model 5 | |||||
| Nonexposed | |||||
| 1‐exposed | 1.14 (0.98–1.32) | 1.60 (1.20–2.15) | 1.02 (0.86–1.22) | 1.07 (0.90–1.63) | 1.03 (0.86–1.25) |
| 2‐exposed | 1.26 (1.05–1.51) | 1.54 (1.07–2.21) | 1.17 (0.95–1.44) | 1.56 (0.97–2.49) | 1.11 (0.88–1.40) |
| 3‐exposed | 1.40 (1.12–1.75) | 2.20 (1.47–3.29) | 1.17 (0.89–1.53) | 0.99 (0.49–2.00) | 1.20 (0.90–1.62) |
| P trend | 0.009 | <0.001 | 0.403 | 0.278 | 0.623 |
| cumhs‐CRP (+1 SD) | 1.04 (0.99–1.09) | 1.07 (1.00–1.14) | 1.03 (0.96–1.09) | 0.96 (0.78–1.19) | 1.03 (0.97–1.10) |
| Model 6 | |||||
| Nonexposed | |||||
| 1‐exposed | 1.10 (0.91–1.34) | 1.41 (0.97–2.03) | 1.01 (0.80–1.27) | 1.28 (0.73–2.26) | 1.00 (0.78–1.28) |
| 2‐exposed | 1.25 (0.98–1.59) | 1.37 (0.85–2.21) | 1.18 (0.89–1.57) | 1.79 (0.94–3.42) | 1.09 (0.78–1.51) |
| 3‐exposed | 1.57 (1.17–2.10) | 2.17 (1.27–3.69) | 1.34 (0.94–1.91) | 1.55 (0.65–3.69) | 1.35 (0.90–2.02) |
| P trend | 0.020 | 0.032 | 0.313 | 0.338 | 0.517 |
| cumhs‐CRP (+1 SD) | 1.06 (1.00–1.13) | 1.07 (0.97–1.18) | 1.06 (0.99–1.13) | 1.04 (0.87–1.26) | 1.06 (0.98–1.14) |
P trend: test of trend based on median value in each category for ordinal categorical variables. Model 4: Model 3 excluding the subjects with hs‐CRP≥10 mg/L during 3 examinations. Model 5: Model 4 excluding subjects under treatment of lipid‐lowering medication. Model 6: Model 5 excluding subjects under treatment of antihypertensive medication. CI indicates confidence interval; cumhs‐CRP, cumulative high‐sensitivity C‐reactive protein; CVD, cardiovascular disease; HR, hazard ratio; MI, myocardial infarction.
Discussion
In this study, we found that increased exposure to high hs‐CRP was significantly associated with elevated incidence rate of CVD, MI, and stroke (ischemic stroke and hemorrhagic stroke). Compared with the nonexposed group, the 3‐exposed group was associated with an increased incidence rate of CVD, MI, and stroke by 2%, 1%, and 1.4%, respectively. Meanwhile, log‐rank tests suggested that there were significant differences of incidence rate across different groups in the total study population (P<0.05).
Sustained high exposure to hs‐CRP was identified as a risk factor for CVD. In comparison with the nonexposed group, 3‐exposed to elevated cumhs‐CRP was associated with a 38% and 13% increased risk of CVD and MI, which were consistent with the results from Laaksonen et al25 for the association between variability of hs‐CRP and risk of MI. More important, not only does cumhs‐CRP increase future risk of CVD, but also the length of exposure to elevated cumhs‐CRP affects future CVD risk in a dose‐responsive manner, because extended exposure to higher hs‐CRP (4 years) significantly (P<0.05) increased risk for future CVD and MI compared with exposure to high hs‐CRP for only 1 or 2 years. Laaksonen et al25 also reported that sustained elevated hs‐CRP was associated with higher risk of CVD compared with sustained low/moderate hs‐CRP. However, previous studies have been reliant on a single time point by which to assess hs‐CRP level, which may have occurred several decades preceding the event and is therefore likely to yield biased estimates of the association. Moreover, there has been no consideration of how hs‐CRP level varies within individuals over time and the subsequent impact that this would have on the cumulative exposure to hs‐CRP level and future risk of CVD. Thus, serial elevated levels may be more prognostic because it reflects less risk of misclassification.
Furthermore, although there were significant associations between baseline hs‐CRP and future CVD, the significant associations disappeared after inducing cumhs‐CRP in the Cox proportional hazards models, which indicated a stronger role for cumhs‐CRP in predicting the risk of future CVD. Given that hs‐CRP rises with a dynamic range and its level is affected by metabolic dysregulation, such as diabetes mellitus and obesity, environments, and potential inflammation,26, 27, 28, 29 analyses from 1 measurement of CRP in serum samples were less likely to represent “true” associations between inflammatory biomarkers and CVD. Thus, the Centers for Disease Control and Prevention and the American Heart Association recommend jointly that 2 measurements of hs‐CRP with a 2‐week interval may decrease variability among individuals and increase the stability of measurement.26, 30
It is noteworthy that the cumulative incidence rate of stroke tended to increase as the length of exposure to higher cumhs‐CRP elevated. However, higher cumhs‐CRP failed to predict the risk of stroke in the present study regardless of ischemic or hemorrhagic stroke. The analysis of data from the NOMAS12 consistently did not support the ability of CRP to predict stroke, which suggested the effects of CRP on risk of stroke was attenuated and not significant after adjusting for other risk factors (HR, 1.20; 95% CI, 0.78–1.86). No associations between hs‐CRP and stroke have been confirmed from other evidence and remain to be investigated.
To eliminate the influence of acute inflammation and infectious diseases, sensitive analyses were performed in model 4 by excluding subjects with hs‐CRP ≥10 mg/L and similar results were obtained. In addition, considering the results from the ASCOT (Anglo‐Scandinavian Cardiac Outcomes Trial) trial6 that lipid‐lowering medications, such as statin, may reduce the relative risk of CVD predicted by hs‐CRP, model 5 was performed to find that incidence rate of CVD and MI elevated 40% and 120%, respectively, compared with previous results whereas the rate of stroke did not increase significantly, which were in accord with the results of the ASCOT trial. Similar results were shown when model 6 was performed, with the incidence rate of CVD and MI increasing 57% and 117%, respectively.
The association between cumhs‐CRP and risk of CVD suggested by epidemiological and observational studies may be explained by how inflammation affects endothelial dysfunction and multiple stages of intravascular plaque agglomeration, triggering atherosclerosis and subsequently a series of harmful and ischemic complications.31 Systemic inflammation can also cause imbalance of the fibrinolytic system,32 both of which are characteristics of atherosclerosis progression. Furthermore, some studies have reported that hs‐CRP participates in innate immunity and acquired immune process, which indicates the dual role of hs‐CRP for future CVD as not only a marker, but also an activator.33 Another explanation is that aggravating inflammation may be a marker of some basic pathological change. As time goes on, the deterioration of the inflammation may be closely associated with CVD.27, 33, 34 Nevertheless, these possible mechanisms are still under research.
The strengths of this study relate to the large sample size of subjects to find the significant association of sustained and increased hs‐CRP with elevated risk of CVD, especially MI. Many multiple, large meta‐analyses have confirmed the observational association between hs‐CRP levels and CV events, such as Wolfgang K's article: High‐sensitivity C‐reactive protein and atherosclerotic disease, from improved risk prediction to risk‐guided therapy about the association between the hs‐CRP and CV events. Mario Di's review about the role of CRP in cerebrovascular disease had also proceeded the meta‐analyses about the association between the hs‐CRP and CVD. They proposed the correlation between the hs‐CRP and cardiovascular event: The higher levels of CRP increased the risk of CVD events. The present study suggests that public health interventions, including taking efforts to avoid inflammatory diseases, prevent inflammatory biomarkers such as hs‐CRP from increasing for generally healthy middle‐aged individuals, and taking effective methods to cure the inflammatory diseases as soon as possible and controlling the level of hs‐CRP as much as possible for individuals suffering from inflammatory diseases, should be taken into consideration to decrease the risk of CVD and improve quality of life. From this study, we also found that the repeated measurement data can be collected, collated, and fully utilized, which may greatly promote the development of a new era of medical research.
This study has several limitations. First, the relatively short follow‐up period averaged 3.96 years, which may not be sufficient to make the end point events completely occur. In the stroke event, although the incidence of stroke in the study population was higher than the incidence of MI, high levels of cumhs‐CRP exposure had no predictive value for stroke events. It remains to be seen whether cum hs‐CRP levels have an impact on stroke events. Second, there remains residual confounding in need of adjustment, such as diseases and medication that can increase CRP. Third, we do not have a trajectory of rising or falling CRP to influence the association. It would add to the message of the report and possibly point toward a mechanism for the effect. Moreover, possible mechanisms are still pending further study to be confirmed. Last, but not least, the present study recruited only northlanders and cannot be generalized to all populations.
Conclusion
In conclusion, we found that cumulative measurements of hs‐CRP are better than single measurement for identification of subjects at higher risk of CVD and MI and risk prediction of disease development.
Disclosures
None.
Supporting information
Table S1. Comparison of Baseline General Characteristics of the 53 065 Individuals Selected for Study and the Remainder of the Kailuan Cohort
Table S2. Adjusted HRs and 95% CI of Baseline hs‐CRP for End Point Events by Cox Proportional Hazards Models
Table S3. Adjusted ORs and 95% CI of Mean hs‐CRP for End Point Events by Logistic Regression Model
Acknowledgments
The authors thank the participants of the Kailuan study. The authors would also express their appreciation for the help from the laboratory staff of the Department of Cardiology of Kailuan Hospital.
(J Am Heart Assoc. 2017;6:e005610 DOI: 10.1161/JAHA.117.005610.)
Contributor Information
Shouling Wu, Email: Drwusl@163.com.
Shike Hou, Email: huoshikedr@126.com.
References
- 1. Strandberg TE, Tilvis RS. C‐reactive protein, cardiovascular risk factors, and mortality in a prospective study in the elderly. Arterioscler Thromb Vasc Biol. 2000;20:1057–1060. [DOI] [PubMed] [Google Scholar]
- 2. Linden F, Domschke G, Erbel C, Akhavanpoor M, Katus HA, Gleissner CA. Inflammatory therapeutic targets in coronary atherosclerosis‐from molecular biology to clinical application. Front Physiol. 2014;5:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mora S, Musunuru K, Blumenthal RS. The clinical utility of high‐sensitivity C‐reactive proteinin cardiovascular disease and the potential implication of JUPITER on current practice guidelines. Clin Chem. 2009;55:219–228. [DOI] [PubMed] [Google Scholar]
- 4. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. [DOI] [PubMed] [Google Scholar]
- 5. Libby P, Ridker PM, Hansson GK; Leducq Transatlantic Network on Atherothrombosis . Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koenig W. High‐sensitivity C‐reactive protein and atherosclerotic disease: from improved riskprediction to risk‐guided therapy. Int J Cardiol. 2013;168:5126–5134. [DOI] [PubMed] [Google Scholar]
- 7. Hermida J, Lopez FL, Montes R, Matsushita K, Astor BC, Alonso A. Usefulness of high‐sensitivity C‐reactive protein to predict mortality in patients with atrial fibrillation (From the Atherosclerosis Risk in Communities [ARIC] Study). Am J Cardiol. 2012;109:95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. C‐reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. [DOI] [PubMed] [Google Scholar]
- 9. Shah T, Casas JP, Cooper JA, Tzoulaki I, Sofat R, McCormack V, Smeeth L, Deanfield JE, Lowe GD, Rumley A, Fowkes FG, Humphries SE, Hingorani AD. Critical appraisal of CRP measurement for the prediction of coronary heart disease events: new data and systematic review of 31 prospective cohorts. Int J Epidemiol. 2009;38:217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Chambless LE, Myerson M, Wu KK, Sharrett AR, Boerwinkle E. Lipoprotein‐associated phospholipase A2, high‐sensitivity C‐reactive protein, and risk for incident ischemic stroke in middle‐aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Arch Intern Med. 2005;165:2479–2484. [DOI] [PubMed] [Google Scholar]
- 11. Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, Sharrett AR. Lipoprotein‐associated phospholipase A2, high‐sensitivity C‐reactive protein, and risk for incident coronary heart disease in middle‐aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2004;109:837–842. [DOI] [PubMed] [Google Scholar]
- 12. Di Napoli M, Elkind MS, Godoy DA, Singh P, Papa F, Popa‐Wagner A. Role of C‐reactive protein in cerebrovascular disease: a critical review. Expert Rev Cardiovasc Ther. 2011;9:1565–1584. [DOI] [PubMed] [Google Scholar]
- 13. Pan HC, Sheu WH, Lee WJ, Lee WL, Liao YC, Wang KY, Lee IT, Wang JS, Liang KW. Coronary severity score and C‐reactive protein predict major adversecardiovascular events in patients with stable coronary artery disease (from the Taichung CAD study). Clin Chim Acta. 2015;445:93–100. [DOI] [PubMed] [Google Scholar]
- 14. Sabatine MS, Morrow DA, Jablonski KA, Rice MM, Warnica JW, Domanski MJ, Hsia J, Gersh BJ, Rifai N, Ridker PM, Pfeffer MA, Braunwald E; PEACE Investigators . Prognostic significance of the Centers for Disease Control/American Heart Association high‐sensitivity C‐reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528–1536. [DOI] [PubMed] [Google Scholar]
- 15. Ndrepepa G, Braun S, Tada T, Guerra E, Schunkert H, Laugwitz KL, Kastrati A. Comparative prognostic value of low‐density lipoprotein cholesterol and C‐reactive protein in patients with stable coronary artery disease treated with percutaneous coronary intervention and chronic statin therapy. Cardiovasc Revasc Med. 2014;15:131–136. [DOI] [PubMed] [Google Scholar]
- 16. Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer R, Coker LH, Sidney S. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014;129:1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doll R, Hill AB. Smoking and carcinoma of the lung; preliminary report. Br Med J. 1950;2:739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Doll R, Hill AB. A study of the aetiology of carcinoma of the lung. Br Med J. 1952;2:1271–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Navar‐Boggan AM, Peterson ED, D'Agostino RB Sr, Neely B, Sniderman AD, Pencina MJ. Hyperlipidemia in early adulthood increases long‐term risk of coronary heart disease. Circulation. 2015;131:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zemaitis P, Liu K, Jacobs DR Jr, Cushman M, Durazo‐Arvizu R, Shoham D, Palmas W, Cooper R, Kramer H. Cumulative systolic BP and changes in urine albuminto‐creatinine ratios in nondiabetic participants of the multi‐ethnic study of atherosclerosis. Clin J Am Soc Nephrol. 2014;9:1922–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu SL, Huang ZR, Yang XC, Zhou Y, Wang A, Chen L, Zhao H, Ruan C, Wu Y, Xin A, Li K, Jin C, Cai J. Prevalence of ideal cardiovascular health and its relationship with the 4‐year cardiovascular events in a northern Chinese industrial city. Circ Cardiovasc Qual Outcomes. 2012;5:487–493. [DOI] [PubMed] [Google Scholar]
- 22. American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions , O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis‐Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. [DOI] [PubMed] [Google Scholar]
- 23. Writing Committee Members , January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC Jr, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; ACC/AHA Task Force Members , Anderson AL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Creager MA, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen W‐K, Stevenson WG, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stroke—1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20:1407–1431. [DOI] [PubMed] [Google Scholar]
- 25. Laaksonen DE, Niskanen L, Nyyssönen K, Punnonen K, Tuomainen TP, Salonen JT. C‐reactive protein in the prediction of cardiovascular and overall mortality in middle‐aged men: a population based cohort study. Eur Heart J. 2005;26:1783–1789. [DOI] [PubMed] [Google Scholar]
- 26. Parrinello CM, Lutsey PL, Ballantyne CM, Folsom AR, Pankow JS, Selvin E. Six‐year change in high‐sensitivity C‐reactive protein and risk of diabetes, cardiovascular disease, and mortality. Am Heart J. 2015;170:380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DeGoma EM, French B, Dunbar RL, Allison MA, Mohler ER III, Budoff MJ. Intraindividual variability of C‐reactive protein: the Multi‐Ethnic Study of Atherosclerosis. Atherosclerosis. 2012;224:274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bower JK, Lazo M, Juraschek SP, Selvin E. Within‐person variability in high‐sensitivity C‐reactive protein. Arch Intern Med. 2012;172:1519–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu S, Li Y, Jin C, Yang P, Li D, Li H, Shen C. Intra‐individual variability of high‐sensitivity C‐reactive protein in Chinese general population. Int J Cardiol. 2012;157:75–79. [DOI] [PubMed] [Google Scholar]
- 30. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr, Taubert K, Tracy RP, Vinicor F; Centers for Disease Control and Prevention; American Heart Association . Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for health care professionals from the Centers for Disease Control and prevention and the American Heart Association. Circulation. 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 31. Hingorani AD, Cross J, Kharbanda RK, Mullen MJ, Bhagat K, Taylor M, Donald AE, Palacios M, Griffin GE, Deanfield JE, MacAllister RJ, Vallance P. Acute systemic inflammation impairs endothelium‐dependent dilatation in humans. Circulation. 2000;102:994–999. [DOI] [PubMed] [Google Scholar]
- 32. Meltzer ME, Doggen CJ, de Groot PG, Rosendaal FR, Lisman T. Plasma levels of fibrinolytic proteins and the risk of myocardial infarction in men. Blood. 2010;116:529–536. [DOI] [PubMed] [Google Scholar]
- 33. Metti AL, Yaffe K, Boudreau RM, Simonsick EM, Carnahan RM, Satterfield S, Harris TB, Ayonayon HN, Rosano C, Cauley JA; Health ABC Study . Trajectories of inflammatory markers and cognitive decline over 10 years. Neurobiol Aging. 2014;35:2785–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koenig W, Sund M, Frolich M, Lowel H, Hutchinson WL, Pepys MB. Refinement of the association of serum C‐reactive protein concentration and coronary heart disease risk by correction for within‐subject variation over time. The MONICA Augsburgh Studies, 1984 and 1987. Am J Epidemiol. 2003;158:357–364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of Baseline General Characteristics of the 53 065 Individuals Selected for Study and the Remainder of the Kailuan Cohort
Table S2. Adjusted HRs and 95% CI of Baseline hs‐CRP for End Point Events by Cox Proportional Hazards Models
Table S3. Adjusted ORs and 95% CI of Mean hs‐CRP for End Point Events by Logistic Regression Model


