Abstract
Background
CD4+ T helper (Th) cells, including Th1, Th2, and Th17 cells, play critical roles in angiotensin II–induced hypertension. Th22 cells, a novel subset of Th cells, take part in cardiovascular diseases by producing IL‐22 (interleukin 22). This study aimed to investigate whether IL‐22 is involved in hypertension.
Methods and Results
Th22 cells and IL‐22 levels were detected in angiotensin II–infused mice, and the results showed that Th22 cells and IL‐22 levels significantly increased. To determine the effect of Th22/IL‐22 on blood pressure regulation, angiotensin II–infused mice were treated with recombinant mouse IL‐22, an anti–IL‐22 neutralizing monoclonal antibody, or control. Treatment with recombinant IL‐22 resulted in increased blood pressure, amplified inflammatory responses, and aggravated endothelial dysfunction, whereas the anti–IL‐22 neutralizing monoclonal antibody decreased blood pressure, reduced inflammatory responses, and attenuated endothelial dysfunction. To determine whether the STAT3 (signal transducer and activator of transcription 3) pathway mediates the effect of IL‐22 on blood pressure regulation, the special STAT3 pathway inhibitor S31‐201 was administered to mice treated with recombinant IL‐22. S31‐201 treatment significantly ameliorated the IL‐22 effects of increased blood pressure and endothelial dysfunction. In addition, serum IL‐22 levels were significantly increased in hypertensive patients compared with healthy persons. Correlation analysis showed a positive correlation between IL‐22 levels and blood pressure.
Conclusions
IL‐22 amplifies the inflammatory response, induces endothelial dysfunction and promotes blood pressure elevation in angiotensin II–induced hypertensive mice. The STAT3 pathway mediates the effect of IL‐22 on hypertension. Blocking IL‐22 may be a novel therapeutic strategy to prevent and treat hypertension.
Keywords: angiotensin II, endothelial dysfunction, hypertension, inflammation, interleukin
Subject Categories: Hypertension, Atherosclerosis, Coronary Artery Disease
Clinical Perspective
What Is New?
Previous studies had demonstrated that the subset of CD4+ T cells, including Th1 and Th17, participated in hypertension.
In this study, we found that as a new subset of CD4+ T cells, Th22 cells released IL‐22 (interleukin 22). IL‐22 binds to the receptors of endothelial cells and activated the STAT3 (signal transducer and activator of transcription 3) pathway, amplified inflammatory response, and aggravated endothelial dysfunction and hypertension in angiotensin II–treated mice.
What Are the Clinical Implications?
This discovery suggests the relationship between CD4+ T cells and hypertension. In clinical hypertension, many patients experience cardiac fibrosis, ischemic cardiomyopathy, and even chronic heart failure.
Although our results may suggest a new idea for the treatment of clinical hypertension, the role of IL‐22 in these clinical complications of hypertension is unclear. Further study of IL‐22 and eventually larger randomized studies are needed to determine the safety and efficacy of potential IL‐22–related antihypertensive drugs.
Hypertension is a common clinical disorder characterized by increased systolic blood pressure (SBP) and/or diastolic blood pressure (BP).1 Hypertension is a major modifiable risk factor for cardiovascular disease and leads to serious cardio–cerebrovascular complications, including heart failure, acute myocardial infarction, and stroke, which account for ≈30% of all deaths worldwide.2, 3
CD4+ T helper (Th) cells are critical in the angiotensin II–induced hypertension model. Th cells can be functionally subdivided into 3 types according to their cytokine secretion profiles: Th1, Th2, and Th17 cells, which mainly secrete IFN‐γ (interferon γ), IL‐4, and IL‐17, respectively. Both IFN‐γ and IL‐17 deficiency significantly reduce elevated BP in angiotensin II–induced hypertension models.4, 5 IL‐4 deficiency significantly accelerates cardiac fibrotic remodeling in angiotensin II–induced hypertrophy, although it has no effect on BP.6
Th22 cells, the fourth subpopulation of Th cells, were recently identified in 2009.7, 8, 9 Data from clinical and experimental investigations have demonstrated that Th22 cells take part in inflammatory and autoimmune diseases such as atherosclerosis,10 myocarditis,11, 12 rheumatoid arthritis,13 and systemic lupus erythematosus14 by secreting IL‐22, which is a proinflammatory cytokine that belongs to the IL‐10 cytokine family. The IL‐10 cytokine family comprises 6 members, including IL‐10, IL‐19, IL‐20, IL‐22, IL‐24, and IL‐26.15 IL‐10 is an anti‐inflammatory cytokine, and its antihypertensive role has been identified in numerous studies.16, 17, 18 IL‐19 is an anti‐inflammatory cytokine, whereas IL‐20 is a proinflammatory cytokine. Although the protective role of IL‐19 and the atherogenic role of IL‐20 have been identified,19, 20, 21 the roles of IL‐19 and IL‐20 in hypertension remain unknown. IL‐24 is secreted by both immune and nonimmune cells. Lee et al found that the expression of IL‐24 decreased in spontaneously hypertensive rats but increased after treatment with antihypertensive drugs.22 In addition, IL‐24 treatment attenuated the expression of vascular inflammation and hypertension‐related genes in mouse vascular smooth muscle cells, indicating that IL‐24 is a novel therapeutic target for hypertension. IL‐26 is a proinflammatory cytokine that is mainly secreted by Th17 cells. Corvaisier et al found that IL‐26 induces proinflammatory cytokine secretion and upregulates Th17 cell generation.23 These results suggest that IL‐26 may play a pathogenic role in hypertension by upregulating Th17‐type responses and promoting the production of proinflammatory cytokines. IL‐22 is secreted by numerous types of cells including activated Th1 cells, Th17 cells, Th22 cells, natural killer cells, natural killer T cells and lymphoid tissue‐inducer cells, and Th22 cells are the critical source of IL‐22 during the later stages of inflammation.24, 25 IL‐22 is upregulated in many chronic inflammatory and autoimmune diseases, and the exact role of IL‐22 appears to depend on specific inflammatory microenvironments: A protective role of IL‐22 was found in a myocarditis model,11, 12 whereas a pathogenic role of IL‐22 has been demonstrated in atherosclerosis.10 Whether IL‐22 plays a role in hypertension remains unknown. This study aimed to investigate the effect of IL‐22 on angiotensin II–induced hypertension.
Materials and Methods
Animals and Animal Model
Male C57BL/6J mice (26.5–28.5 g; HFK Bioscience, Beijing, China) aged 10 to 12 weeks were housed in a pathogen‐free mouse room (12 hours light/12 hours dark; temperature 22–24°C) at Renmin Hospital, Wuhan University, and received water ad libitum by the Animal Care Facility Service. A mouse model of hypertension was first established by angiotensin II infusion, and Th22/IL‐22 levels were observed in weeks 1, 2, and 4 (n=8 for each group). Saline‐infused mice served as a baseline (n=8). In addition, angiotensin II–induced hypertension mice were treated with vehicle (50 μL, n=8), recombinant mouse IL‐22 (rIL‐22; 15 μg/kg, n=8), mouse anti–IL‐22 neutralizing monoclonal antibody (anti–IL‐22 mAb; 1.25 μg, n=8)26 or an equivalent amount of isotype IgG (n=8). Mice infused with saline and treated with vehicle served as controls (n=8). Finally, angiotensin II–induced hypertensive mice were treated with S31‐201 (2.5 mg/kg),27 rIL‐22 plus S31‐201, or vehicle and rIL‐22 (n=8 for each group). All experimental procedures were performed in accordance with the institutional guidelines of the animal care and use committee of Renmin Hospital, Wuhan University, and this study was approved by the ethics committee of the People's Hospital of Guangxi Zhuang Autonomous Region (Nanning, China) and Renmin Hospital of Wuhan University (Wuhan, China). Institutional review board approval was also obtained.
Chronic Angiotensin II Infusion
Osmotic minipumps (Alzet model 2001/2002/2004) were implanted subcutaneously in the nape of the neck after anesthesia induction with isoflurane (2%). Angiotensin II was infused at a rate of 750 ng/(min×kg), as described.28
Flow Cytometry Analyses
Mouse splenic lymphocytes were isolated and resuspended in RPMI 1640 complete culture medium at a density of ≈5×106 cells/mL. Then, 400 μL cell suspensions were stimulated with 2 μL/mL cell stimulation cocktail composed of 40.5 μmol/L phorbol myristate acetate, 670 μmol/L ionomycin, 5.5 μmol/L brefeldin, and 1 μmol/L monensin in an environment with 5% CO2 at 37°C for 4 hours. Cells were collected and strained with phycoerythrin Cye‐7–conjugated anti–mouse CD4. Fixation and permeabilization were necessary before staining with fluorescein isothiocyanate anti–mouse IFN‐γ, phycoerythrin‐labeled anti–IL‐17A and allophycocyanin‐labeled anti–IL‐22. Isotype controls were included for compensation and to confirm antibody specificity. Th22 cells were defined as CD4+IFN‐γ−IL‐17−IL‐22+. Cell stimulation cocktail and all flow antibodies were purchased from eBioscience and stained according to the manufacturer's instructions.
Enzyme‐Linked Immunosorbent Assay
Serum was obtained after the samples were centrifugalized for 15 minutes at 4000 g. The serum levels of IL‐22, IL‐1β, IL‐6, IL‐17, TNF‐α (tumor necrosis factor α), IFN‐γ, ICAM (intercellular adhesion molecule), and MCP‐1 (monocyte chemoattractant protein‐1) chemokine in mice and IL‐22 in humans were determined by ELISA kits (eBioscience), according to the manufacturer's instructions. All samples were measured in duplicate.
BP Measurement
The SBP and the heart rate of the mice were detected via a carotid catheter‐calibrated tail‐cuff system (CODA, Kent Scientific) before and after the mice were treated, as described previously.28, 29, 30
Quantitative Polymerase Chain Reaction
The total mRNA was extracted with TRIZOL Reagent W, and cDNA was synthesized from 2 mg total mRNA using oligo(dT) primers and a reverse transcription kit according to the manufacturer's instructions. Polymerase chain reaction amplifications were performed using LightCycler 480 SYBR Green Master Mix (Roche Diagnostics). The relative mRNA expression levels of IL‐1β, IL‐6, IL‐17, TNF‐α, IFN‐γ, ICAM, and MCP‐1 were investigated, the results were normalized against the expression levels of GAPDH (glyceraldehyde‐3‐phosphate dehydrogenase). The reverse transcription quantitative polymerase chain reaction primer sequences are shown in Table 1.
Table 1.
RT‐PCR Primers Used
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| IL‐1β | GGGCCTCAAAGGAAAGAATC | TACCAGTTGGGGAACTCTGC |
| IL‐6 | AGTTGCCTTCTTGGGACTGA | TCCACGATTTCCCAGAGAAC |
| IL‐17 | TCCAGAAGGCCCTCAGACTA | AGCATCTTCTCGACCCTGAA |
| IFN‐γ | ACTGGCAAAAGGATGGTGAC | TGAGCTCATTGAATGCTTGG |
| TNF‐α | CCCAGGGACCTCTCTCTAATC | ATGGGCTACAGGCTTGTCACT |
| MCP‐1 | CTTCTGTGCCTGCTGCTCAT | CGGAGTTTGGGTTTGCTTGTC |
| ICAM‐1 | TTGGGCATAGAGACCCCGTT | GCACATTGCTCAGTTCATACACC |
| GAPDH | AACTTTGGCATTGTGGAAGG | CACATTGGGGGTAGGAACAC |
RT‐PCR indicates reverse transcription polymerase chain reaction.
Vascular Ring Experiments
To measure vascular reactivity, parts of the aorta were placed in cold physiological salt solution.31 The aortic rings were cut into 3 to 4 mm sections and were connected to an isometric force transducer in a custom‐designed 15‐mL organ chamber filled with 37°C cold physiological salt solution and bubbled with 95% O2 and 5% CO2. Parts of the vascular rings were treated with indomethacin (10 μmol/L, 60 minutes). Concentration–force curves were obtained in a half‐log, cumulative fashion to acetylcholine and sodium nitroprusside following contraction to an EC70 concentration (1 mmol/L) of phenylephrine.
Western Blot Analysis
The total protein of the thoracic aorta was extracted and detected with the BCA Protein Assay Kit (Thermo Fisher Scientific). Approximately 20 μg total protein was separated by electrophoresis on 8% Laemmli sodium dodecyl sulfate polyacrylamide gels, and after electrophoresis, they were transferred to Immobilon‐FL PVDF membranes (Millipore). The membranes were blocked with 5% nonfat milk, and the membranes were then incubated with anti‐STAT3, anti–phosphorylated STAT3, anti‐eNOS (anti–endothelial nitric oxide), anti–phosphorylated eNOS threonine 495 (Thr495), and anti‐GAPDH antibodies at 4°C overnight, followed by incubation with the secondary antibody at room temperature for 1 hour. The blots were scanned using a 2‐color infrared imaging system (Odyssey; LI‐COR Biosciences).
Collection of Human Blood Samples
Fasting venous peripheral blood samples from healthy persons (n=24) and newly diagnosed hypertensive patients (n=46) were collected at the People's Hospital of Guangxi Zhuang Autonomous Region and Renmin Hospital, Wuhan University, from September 2014 to October 2015. The blood samples were used to measure IL‐22 levels by ELISA (eBioscience). All patients provided informed consent. This research protocol was approved by the People's Hospital of Guangxi Zhuang Autonomous Region and the Wuhan University Ethics Committee for the Protection of Human Subjects. Institutional review board approval was also obtained. The clinical data of the healthy persons and patients are listed in Table 2, which shows unadjusted analyses.
Table 2.
Clinical Data of the Control Participants and Hypertensive Patients
| Characteristic | Control | Hypertension | P Value |
|---|---|---|---|
| Age, y | 53.2±13.8 | 57.1±9.7 | 0.17 |
| Sex (male/female) | 14/10 | 23/23 | 0.51 |
| Smoking, n (%) | 8 (33.3) | 17 (36.9) | 0.76 |
| Drinking, n (%) | 7 (29.2) | 14 (30.4) | 0.91 |
| BMI >25, n (%) | 12 (50) | 24 (52.2) | 0.86 |
| Family story, n (%) | 8 (33.3) | 19 (41.3) | 0.52 |
| Hyperlipidemia, n (%) | 5 (20.9) | 9 (19.6) | 0.90 |
| Diabetes mellitus, n (%) | 4 (16.7) | 6 (13.9) | 0.73 |
| Atherosclerosis, n (%) | 6 (25) | 11 (23.9) | 0.92 |
| SBP, mm Hg | 112.8±11.4 | 161±13.7 | <0.01 |
| DBP, mm Hg | (65.3, 75.0) | (85.9, 102.0) | <0.01 |
| TC, mmol/L | (4.0, 4.7) | (4.3, 4.8) | 0.48 |
| TG, mmol/L | (1.2, 1.9) | (1.2, 1.6) | 0.52 |
| HDL, mmol/L | 1.5±0.4 | 1.5±0.4 | 0.98 |
| LDL, mmol/L | 2.4±0.5 | 2.3±0.6 | 0.66 |
| Glucose, mmol/L | (5.2, 6.7) | (4.9, 6.3) | 0.17 |
| Hemoglobin A1c, % | (5.1, 6.2) | (5.2, 6.2) | 0.32 |
| BMI, kg/m2 | 24.6±3.2 | 24.8±2.7 | 0.77 |
| Medications | |||
| ASA, n (%) | 6 (25) | 13 (28.3) | 0.77 |
| Statin, n (%) | 10 (41.7) | 15 (32.6) | 0.45 |
| Oral hypoglycemic, n (%) | 4 (16.7) | 6 (13) | 0.73 |
| Insulin, n (%) | 1 (4.2) | 2 (4.3) | 0.97 |
ASA indicates aspirin; BMI, body mass index; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SBP, systolic blood pressure; TC, total cholesterol; TG, total triglycerides.
Statistical Analyses
The SPSS version 18.0 (IBM Corp) was used for data analysis. Data from animal experiments are expressed as mean±SD. Differences between 2 groups were compared by Student t tests. Differences between multiple groups were compared by 1‐way ANOVA, followed by Tukey's multiple comparisons test. For the human experiments, data with a normal distribution were expressed as mean±SD. Differences between 2 groups were compared by Student t tests, whereas data with abnormal distribution were expressed as lower quartile and upper quartile and were compared by the Mann–Whitney U test; categorical variables are presented as counts (percentages). The Spearman correlation was used to calculate correlations between SBP, diastolic BP, and serum IL‐22 levels. Multiple regression tests were used to analyze the associations between serum IL‐22 and risk factors for hypertension in human subjects. P<0.05 was considered significant and was the threshold used to reject the null hypothesis.
Results
Chronic Angiotensin II Infusion Increased Th22/IL‐22 Levels in Mice
Compared with baseline levels, the percentage of Th22 cells significantly increased by week 1, continued to increase by week 2, reached a peak, and then was continuously maintained at high levels through the end of 4 week (Figure 1A and 1B). Similar results were obtained for serum IL‐22 levels (Figure 1C).
Figure 1.
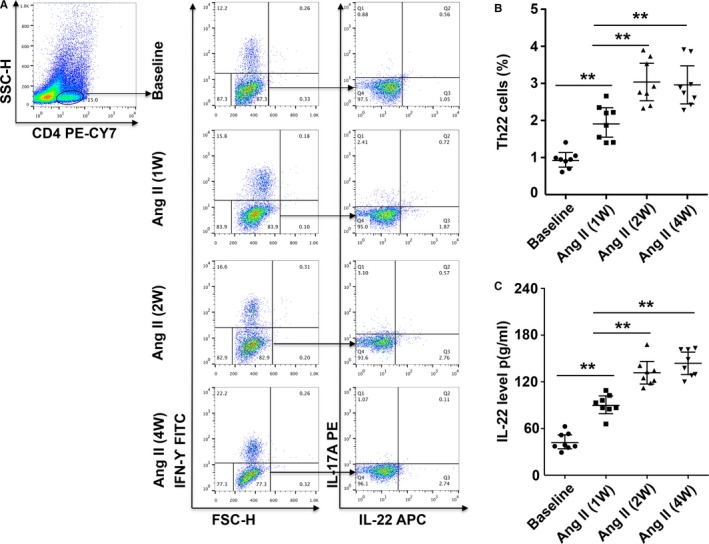
Th22 cells and IL‐22 levels in mice chronically infused with Ang II. A, Th22 cells were identified among CD4+ T cells based on their expression of CD4+IFN‐γ−IL‐17−IL‐22+. B, Th22 cell percentages at baseline and after Ang II treatment in mice. C, Serum IL‐22 levels measured by ELISA at baseline and after Ang II treatment in mice. n=8 for each group. **P<0.01. Ang II indicates angiotensin II; IFN‐γ, interferon γ; IL‐22, interleukin 22; Th22, T helper 22 cells.
IL‐22 Increased Angiotensin II–Induced Hypertension
At baseline, no differences were observed in SBP among the 5 groups (Figure 2A). Results similar to the changes in the percentage of Th22 cells and serum IL‐22 were found for SBP with chronic angiotensin II infusion, and treatment with rIL‐22 increased angiotensin II–induced hypertension (week 4 SBP: angiotensin II and rIL‐22 group: 179±7 mm Hg; angiotensin II group: 155±4 mm Hg; Figure 2A). Mouse anti–IL‐22 monoclonal antibody (mAb) partly prevented BP elevation (week 4 SBP: angiotensin II and anti–IL‐22 mAb group: 132±4 mm Hg; angiotensin II group: 155±4 mm Hg; Figure 2A). No difference in SBP was found between the angiotensin II group and the angiotensin II with IgG group. In addition, there were no significant differences in heart rate among the 5 groups (Figure 2B).
Figure 2.
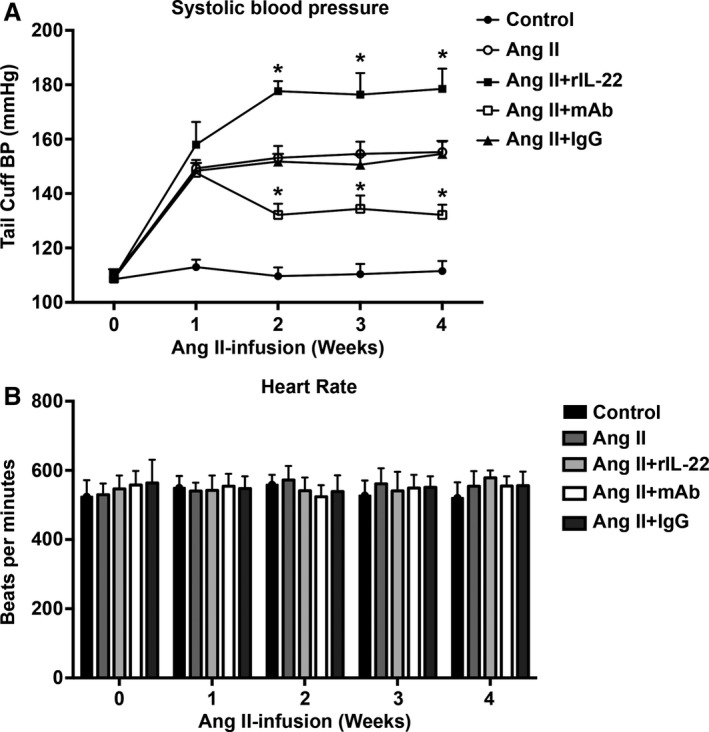
Blood pressure and heart rate of angiotensin II–infused mice. A, Systolic blood pressure measurements obtained via a tail‐cuff at different time points for each group. B, Heart rate of the 5 groups. Data are expressed as the mean±SD. n=8 for each group. *P<0.05 vs Ang II–treated group. Ang II indicates angiotensin II; mAb, neutralizing monoclonal antibody; rIL‐22, recombinant mouse IL‐22.
IL‐22 Aggravated Both Systemic and Local Inflammation
To examine the effect of IL‐22 on systemic inflammation, we tested the circulatory cytokine levels of the 5 groups. Compared with the angiotensin II group, the angiotensin II and rIL‐22 group had higher levels of IL‐1β, IL‐6, IL‐17A, TNF‐α, IFN‐γ, ICAM, and MCP‐1 (Figure 3A). Opposite results were found for the angiotensin II and anti–IL‐22 mAb group compared with the angiotensin II group (Figure 3A), and no significant difference was found for the angiotensin II and IgG group. In addition, we analyzed the mRNA expression of these cytokines in the aortas of the mice. Compared with the angiotensin II group, higher mRNA levels of IL‐1β, IL‐6, IL‐17A, TNF‐α, IFN‐γ, ICAM, and MCP‐1 were found in the angiotensin II and rIL‐22 group (Figure 3B), lower mRNA levels of these cytokines were found in the angiotensin II and anti–IL‐22 mAb group (Figure 3B), and isotype IgG had no effect on mRNA expression.
Figure 3.
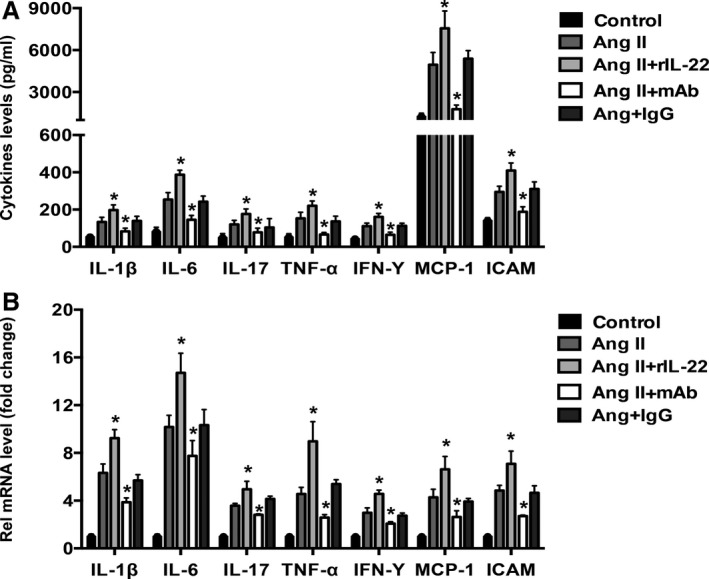
Serum cytokine and mRNA expression levels in the aortas for the 5 groups. A, The levels of serum cytokines related to hypertension or endothelia function, including IL‐1β, IL‐6, IL‐17A, TNF‐α, IFN‐γ, ICAM and MCP‐1, in the 5 groups. n=8 for each group. B, The mRNA expression of these cytokines in the aortas of the 5 groups. n=3 for each group. *P<0.05 vs Ang II–treated group. Ang II indicates angiotensin II; ICAM, intercellular cell adhesion molecule; IFN‐γ, interferon γ; IL, interleukin; MCP‐1, monocyte chemoattractant protein 1; TNF‐α, tumor necrosis factor α.
IL‐22 Aggravated Angiotensin II–Induced Vascular Dysfunction
We next tested the endothelium‐dependent and ‐independent relaxation responses of endothelium‐intact aortas isolated from mice that had been treated for 4 weeks. Angiotensin II significantly reduced acetylcholine‐induced relaxation responses, increased phenylephrine‐induced contractions, and mildly decreased sodium nitroprusside‐induced relaxation responses (Figure 4A through 4D), as reported previously; therefore, angiotensin II–induced hypertension was associated with endothelial dysfunction. The effect of angiotensin II on endothelial dysfunction was aggravated by rIL‐22, prevented by anti–IL‐22 mAb, and was not affected by isotype IgG (Figure 4A through 4C). To investigate the effect of the inflammatory response on endothelial function, parts of the vascular rings were treated with indomethacin, revealing that indomethacin prevented the effect of IL‐22 on angiotensin II–induced vascular dysfunction (Figure 4D).
Figure 4.
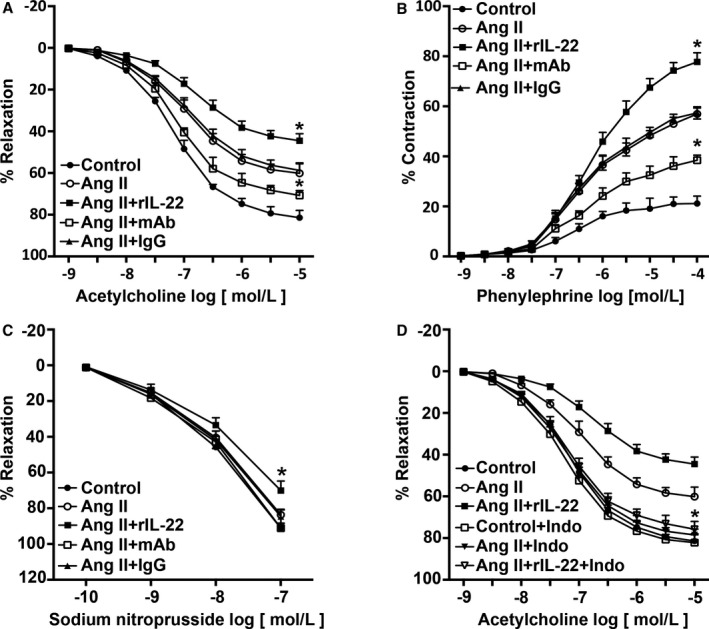
Effect of IL‐22 on Ang II–induced vascular dysfunction. A, Effect of IL‐22 on relaxation responses to acetylcholine were measured in endothelial‐intact aortas isolated from each group. B, Effect of IL‐22 on contraction responses to phenylephrine were measured in endothelial‐intact aortas isolated from each group. C, Effect of IL‐22 on relaxation responses to sodium nitroprusside were measured in endothelial‐intact aortas isolated from each group. D, Effect of indomethacin on relaxation responses to ACH. n=5 to 6 for each group. *P<0.05 vs Ang II–treated group. Ang II indicates angiotensin II; IL‐22, interleukin 22; Indo, indomethacin; mAb, neutralizing monoclonal antibody; rIL‐22, recombinant mouse interleukin 22.
The STAT3 Pathway Mediated the Effect of IL‐22
STAT3 and eNOS Thr495 phosphorylation was measured by Western blot in mouse aortas, and the results demonstrated that angiotensin II significantly increased phosphorylation of the STAT3 pathway and eNOS Thr495. This effect could be further increased by rIL‐22 and partly prevented by anti–IL‐22 mAb, and no significant difference was observed for treatment with isotype IgG (Figure 5A and 5B).
Figure 5.
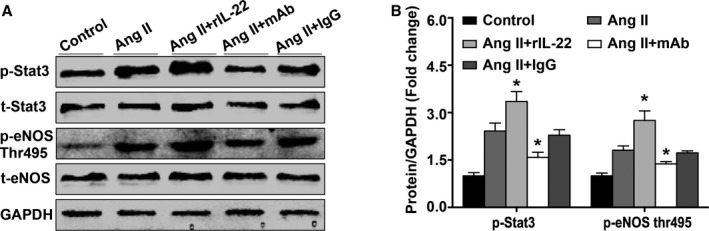
Effect of IL‐22 on the phosphorylation of STAT3 and eNOS Thr495. A and B, Representative images and quantification p‐STAT3, t‐STAT3, p‐eNOS Thr495, t‐eNOS, and GAPDH were measured by western blot in the aortic vascular of each group. n=2 to 3 for each group. *P<0.05 vs Ang II–treated group. Ang II indicates angiotensin II; eNOS, endothelial nitric oxide synthase; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; mAb, neutralizing monoclonal antibody; p, phosphorylated; rIL‐22, recombinant mouse interleukin 22; STAT3, signal transducer and activator of transcription 3; t, total; Thr, threonine.
To determine whether the STAT3 pathway plays a critical role in the effect of IL‐22 on BP and endothelial function, the special STAT3 pathway inhibitor S31‐201 was used in rIL‐22‐treated mice. Treatment with S31‐201 prevented the IL‐22–induced increase in angiotensin II–induced hypertension (week 4 SBP: angiotensin II, rIL‐22, and S31‐201 group: 110±3 mm Hg; angiotensin II and rIL‐22 group: 179±4 mm Hg; angiotensin II and S31‐201 group 111±4 mm Hg; Figure 6A). S31‐201–treated mice also exhibited decreased STAT3 phosphorylation (Figure 6B). In addition, the effect of IL‐22 on angiotensin II–induced vascular dysfunction was prevented by S31‐201 (Figure 6C through 6E).
Figure 6.
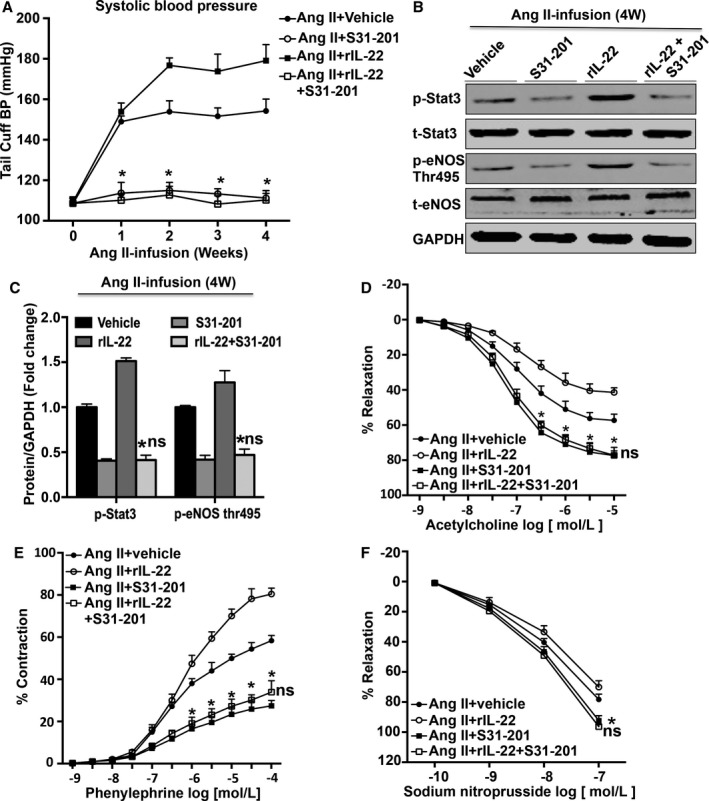
Effect of S31‐201 on blood pressure and vascular dysfunction. A, systolic blood pressure measurements obtained via a tail‐cuff at different time points for each group, n=8 for each group. B, Representative images and (C) densitometry quantification of each protein, n=3 for each group. D, Effect of S31‐201 on relaxation responses to acetylcholine were measured in endothelial‐intact aortas isolated from each group. n=5 to 6 for each group. E, Effect of S31‐201 on contraction responses to phenylephrine were measured in endothelial‐intact aortas isolated from each group. n=5 to 6 for each group. F, Effect of S31‐201 on relaxation responses to sodium nitroprusside were measured in endothelial‐intact aortas isolated from each group, n=5 to 6 for each group. *P<0.05 vs Ang II and rIL‐22–treated group. ns P>0.05 vs angiotensin II and S31‐201–treated group. Ang II indicates angiotensin II; eNOS, endothelial nitric oxide synthase; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; mAb, neutralizing monoclonal antibody; p, phosphorylated; rIL‐22, recombinant mouse interleukin 22; STAT3, signal transducer and activator of transcription 3; t, total; Thr, threonine.
Serum IL‐22 Levels Increased in Hypertensive Patients
Serum IL‐22 levels were significantly increased in hypertensive patients compared with control participants (Figure 7A). Correlation analysis revealed that the level of IL‐22 was positively correlated with SBP and diastolic BP in the hypertensive patients (Figure 7B and 7C). Furthermore, multivariate analysis taking into account the major risk factors for hypertension revealed higher levels of IL‐22 in participants who smoked, whereas other risk factors, including male sex, older age, obesity, smoking, drinking, and family history had no significant effect on serum IL‐22 levels. The serum levels of each group are listed in Table 3 (adjusted model).
Figure 7.
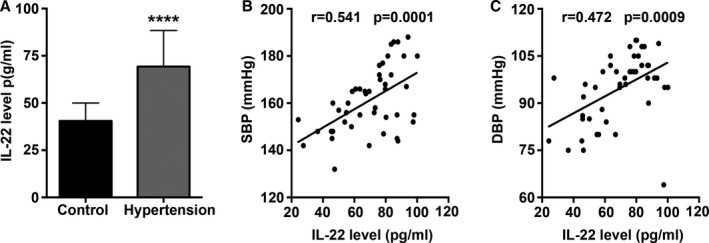
Serum IL‐22 protein levels in hypertensive patients. A, Serum IL‐22 levels measured by ELISA in control participants and hypertensive patients. B, Correlation analysis of SBP and IL‐22 levels in hypertensive patients. C, Correlation analysis of DBP and IL‐22 levels in hypertensive patients. ****P<0.0001 vs control group. DBP indicates diastolic blood pressure; IL‐22, interleukin 22; SBP, systolic blood pressure.
Table 3.
Serum IL‐22 Levels in Patients With Concomitant Risk Factors for Hypertension (R 2=0.444, P<0.0001)
| Factors | β | 95% CI | P Value |
|---|---|---|---|
| Hypertension | 0.637 | 0.451–0.824 | 0.000 |
| Male sex | 0.020 | −0.217 to 0.257 | 0.865 |
| Age | 0.002 | −0.183 to 0.188 | 0.981 |
| Smoking | 0.329 | 0.091–0.567 | 0.008 |
| Drinking | −0.023 | −0.216 to 0.170 | 0.812 |
| BMI | −0.125 | −0.310 to 0.060 | 0.182 |
| Family history | −0.001 | −0.186 to 0.184 | 0.992 |
| Cholesterol | −0.065 | −0.262 to 0.131 | 0.509 |
| LDL‐C | 0.040 | −0.154 to 0.233 | 0.682 |
| Glucose | −0.045 | −0.236 to 0.145 | 0.635 |
BMI indicates body mass index; CI, confidence interval; IL‐22, interleukin 22; LDL, low‐density lipoprotein.
Discussion
Previous studies have revealed that angiotensin II not only promotes T‐cell proliferation but also regulates Th1, Th2 and Th17 cell activities in vivo, thereby playing vital roles in angiotensin II–induced hypertension and target organ damage. Shao et al found that exogenous angiotensin II directly promoted the production of IFN‐γ and inhibited the production of IL‐4 in cultured rat T lymphocytes, which is associated with the onset of kidney injury.32 Moreover, using a mouse atherosclerosis model, Mazzolai et al reported that endogenous angiotensin II elicited a Th1 response, which is widely identified as a type of T lymphocyte with a proatherosclerotic role, and led to unstable atherosclerotic plaques.33 Taken together, these studies demonstrated that the imbalance between Th1 and Th2 cells induced by angiotensin II is associated with elevated BP and hypertensive complications. Numerous investigations have focused on Th17 cells and hypertension as well as its complications over the course of a decade. The Th17 response undoubtedly plays a pathogenic role in hypertension.4, 34, 35 Madhur et al found that increased Th17 cell and IL‐17 levels were accompanied by elevated BP in angiotensin II–infused mice, whereas IL‐17 deficiency resulted in reduced BP and reversed vascular dysfunction.5 IL‐17 even significantly induced increased BP in wild‐type mice not treated with angiotensin II.34 Interestingly, Madhur and Itani et al found that angiotensin II directly promoted IL‐17 production in cultured mouse T lymphocytes but had no effect on IL‐17 production in cultured human T lymphocytes.4, 35
In the present study, we investigated the role of Th22/IL‐22 in an angiotensin II–induced hypertension model. We first measured the levels of Th22/IL‐22 in angiotensin II–infused mice and controls. The results showed that 2 weeks of angiotensin II treatment significantly increased Th22 cells and IL‐22 levels compared with controls, and these effects were maintained until the end of the experiment. In fact, the changes in Th22 cells and IL‐22 levels were consistent with the BP values of the angiotensin II–treated mice. Consequently, these results revealed that Th22/IL‐22 responses are upregulated by angiotensin II in hypertension.
To determine whether IL‐22 plays a role in the effect of angiotensin II on BP regulation, we used rIL‐22 to enhance or anti–IL‐22 mAb to block the effects of IL‐22 in angiotensin II–treated mice. The resulted showed that rIL‐22 treatment further increased BP up to 179 mm Hg, whereas anti‐IL‐22 mAb induced a sharp decrease in BP to as low as 132 mm Hg, although this value was still higher than that of the control group. These findings suggest a direct effect of IL‐22 on BP regulation in angiotensin II–induced hypertensive mice.
Inflammation plays a critical role in hypertension. Many inflammatory mediators, including IFN‐γ, IL‐17, IL‐1β, IL‐6, TNF‐α, and chemokine MCP‐1, significantly promote the development of hypertension and target organ damage in hypertension models.4, 5, 35, 36, 37, 38, 39, 40 Accumulating evidence has demonstrated that IL‐22 increases the expression of IL‐1β, IL‐6, IL‐17, and MCP‐1, suggesting that IL‐22 might be involved in BP regulation by inducing the production of these inflammatory mediators. Therefore, we measured whether local and systemic inflammatory responses were regulated by IL‐22. We found that rIL‐22 increased the production of these inflammatory mediators in the angiotensin II–induced hypertension model, whereas anti–IL‐22 mAb significantly decreased the levels of inflammatory mediators. Taken together, these results suggest that IL‐22 may be involved in hypertension by amplifying the inflammatory response.
Accumulating evidence has demonstrated that endothelial dysfunction promotes BP elevation and serves as a bridge between angiotensin II and hypertension.18, 41, 42, 43 In the present study, we investigated whether IL‐22 plays a role in regulating endothelial function. The results showed that rIL‐22 further promoted the effect of angiotensin II on endothelium‐dependent and ‐independent relaxation responses, whereas anti–IL‐22 mAb reversed the effect of angiotensin II. Moreover, eNOS Thr495 phosphorylation is crucial for eNOS activity and nitric oxide release, thereby playing a role in endothelial function and BP homeostasis.44, 45 Consequently, the activity of eNOS Thr495 phosphorylation is important for endothelial function. In the present study, we found that rIL‐22 significantly upregulated eNOS Thr495 phosphorylation, whereas anti–IL‐22 mAb significantly downregulated eNOS Thr495 phosphorylation; therefore, IL‐22 might elevate BP by impairing endothelial function.
It is well known that excessive activation of the STAT3 pathway is critical in angiotensin II–induced inflammatory responses and oxidative stress, which result in hypertension.27, 46 Evidence from animal experimental studies has shown that after binding to the heterodimeric receptor complex IL‐10R β chain and IL‐22R, IL‐22 activates the JAK/STAT pathway predominantly via STAT3.12, 47, 48 Consequently, we investigated whether the STAT3 pathway mediated the effect of IL‐22 on hypertension. First, we found that STAT3 phosphorylation is upregulated by rIL‐22 and inhibited by anti–IL‐22 mAb, indicating that the activity of STAT3 is associated with IL‐22 levels. Second, to determine whether STAT3 plays a critical role in the effects of IL‐22 on BP and endothelial function, mice were treated with the STAT3 inhibitor S3I‐201, which selectively inhibits STAT3 phosphorylation, dimerization, and STAT3‐dependent gene transcription. The results showed that S3I‐201 treatment significantly inhibited STAT3 phosphorylation and led to a reduction in BP. Taken together, STAT3 mediates the effects of IL‐22 on endothelial function and hypertension.
To extend our results to human hypertension, we examined the serum levels of IL‐22 in normotensive and newly diagnosed hypertensive patients. The serum levels of IL‐22 were significantly higher in hypertensive patients than in normotensive participants. In hypertensive patients, the serum levels of IL‐22 were positively correlated with SBP and diastolic BP. These results suggest a tight relationship between IL‐22 and human hypertension; however, the sample used was too small, and other types of hypertension, such as white‐coat syndrome, poorly controlled hypertension, and well‐controlled hypertension, were not considered.
In summary, the results of our study are the first to demonstrate that the Th22/IL‐22 response is upregulated by angiotensin II treatment and that IL‐22 promotes BP elevation in angiotensin II–induced hypertension. Although the exact mechanisms of IL‐22 on hypertension have not been fully clarified, amplifying inflammatory response and inducing endothelial dysfunction may mediate the effect of IL‐22 on hypertension. Consequently, blocking the effects of Th22/IL‐22 could be a therapeutic option for preventing and treating hypertension.
Conclusions
This study revealed that binding of IL‐22, IL‐22R1, and IL‐10R2 could further activate the STAT3 pathway and aggravate angiotensin II–induced endothelial dysfunction and hypertension, whereas neutralization of IL‐22 had an opposite biological function. IL‐22 is a new target for hypertension.
Sources of Funding
This work was supported by the National Natural Science Foundation of China (No. 81360055, 81170208, 81460081, and 81560085).
Disclosures
None.
Acknowledgments
The authors are particularly grateful to Tao Zeng for his expert assistance in analysis of the clinical experimental data of this article.
(J Am Heart Assoc. 2017;6:e005875 DOI: 10.1161/JAHA.117.005875.)
Contributor Information
Yingzhong Lin, Email: yingzhonglin@126.com.
Jun Wan, Email: wanjun1963@126.com.
References
- 1. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JJ, Jones DW, Materson BJ, Oparil S, Wright JJ, Roccella EJ. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 2. Gaziano TA. Cardiovascular disease in the developing world and its cost‐effective management. Circulation. 2005;112:3547–3553. [DOI] [PubMed] [Google Scholar]
- 3. Pearson TA. Cardiovascular disease in developing countries: myths, realities, and opportunities. Cardiovasc Drugs Ther. 1999;13:95–104. [DOI] [PubMed] [Google Scholar]
- 4. Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, Delpire E, Harrison DG, McDonough AA. Renal transporter activation during angiotensin‐II hypertension is blunted in interferon‐gamma‐/‐ and interleukin‐17A‐/‐ mice. Hypertension. 2015;65:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II‐induced hypertension and vascular dysfunction. Hypertension. 2015;55:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peng H, Sarwar Z, Yang XP, Peterson EL, Xu J, Janic B, Rhaleb N, Carretero OA, Rhaleb NE. Profibrotic role for interleukin‐4 in cardiac remodeling and dysfunction. Hypertension. 2015;66:582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)‐17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. [DOI] [PubMed] [Google Scholar]
- 8. Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin‐homing memory T cells. Nat Immunol. 2009;10:857–863. [DOI] [PubMed] [Google Scholar]
- 9. Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl‐Hoffmann C, Behrendt H, Durham SR, Schmidt‐Weber CB, Cavani A. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rattik S, Hultman K, Rauch U, Soderberg I, Sundius L, Ljungcrantz I, Hultgardh‐Nilsson A, Wigren M, Bjorkbacka H, Fredrikson GN, Nilsson J. IL‐22 affects smooth muscle cell phenotype and plaque formation in apolipoprotein E knockout mice. Atherosclerosis. 2015;242:506–514. [DOI] [PubMed] [Google Scholar]
- 11. Kong Q, Wu W, Yang F, Liu Y, Xue Y, Gao M, Lai W, Pan X, Yan Y, Pang Y, Deng Y. Increased expressions of IL‐22 and Th22 cells in the coxsackievirus B3‐induced mice acute viral myocarditis. Virol J. 2012;9:232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kong Q, Xue Y, Wu W, Yang F, Liu Y, Gao M, Lai W, Pan X. IL‐22 exacerbates the severity of CVB3‐induced acute viral myocarditis in IL‐17A‐deficient mice. Mol Med Rep. 2013;7:1329–1335. [DOI] [PubMed] [Google Scholar]
- 13. Zhao M, Li Y, Xiao W. Anti‐apoptotic effect of interleukin‐22 on fibroblast‐like synoviocytes in patients with rheumatoid arthritis is mediated via the signal transducer and activator of transcription 3 signaling pathway. Int J Rheum Dis. 2016;20:214–224. [DOI] [PubMed] [Google Scholar]
- 14. Azizi G, Simhag A, El RN, Mirshafiey A. Th22 cells contribution in immunopathogenesis of rheumatic diseases. Iran J Allergy Asthma Immunol. 2015;14:246–254. [PubMed] [Google Scholar]
- 15. Conti P, Kempuraj D, Frydas S, Kandere K, Boucher W, Letourneau R, Madhappan B, Sagimoto K, Christodoulou S, Theoharides TC. IL‐10 subfamily members: IL‐19, IL‐20, IL‐22, IL‐24 and IL‐26. Immunol Lett. 2003;88:171–174. [DOI] [PubMed] [Google Scholar]
- 16. Chatterjee P, Chiasson VL, Kopriva SE, Young KJ, Chatterjee V, Jones KA, Mitchell BM. Interleukin 10 deficiency exacerbates toll‐like receptor 3‐induced preeclampsia‐like symptoms in mice. Hypertension. 2011;58:489–496. [DOI] [PubMed] [Google Scholar]
- 17. Kim HY, Cha HJ, Kim HS. CCL5 upregulates IL‐10 expression and partially mediates the antihypertensive effects of IL‐10 in the vascular smooth muscle cells of spontaneously hypertensive rats. Hypertens Res. 2015;38:666–674. [DOI] [PubMed] [Google Scholar]
- 18. South S, Chiasson VL, Mitchell BM. Interleukin‐10 reduces inflammation, endothelial dysfunction, and blood pressure in hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R713–R719. [DOI] [PubMed] [Google Scholar]
- 19. Ellison S, Gabunia K, Kelemen SE, England RN, Scalia R, Richards JM, Orr AW, Traylor JG Jr, Rogers T, Cornwell W, Berglund LM, Goncalves I, Gomez MF, Autieri MV. Attenuation of experimental atherosclerosis by interleukin‐19. Arterioscler Thromb Vasc Biol. 2013;33:2316–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gabunia K, Ellison S, Kelemen S, Kako F, Cornwell WD, Rogers TJ, Datta PK, Ouimet M, Moore KJ, Autieri MV. IL‐19 halts progression of atherosclerotic plaque, polarizes, and increases cholesterol uptake and efflux in macrophages. Am J Pathol. 2016;186:1361–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen WY, Cheng BC, Jiang MJ, Hsieh MY, Chang MS. IL‐20 is expressed in atherosclerosis plaques and promotes atherosclerosis in apolipoprotein E‐deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2090–2095. [DOI] [PubMed] [Google Scholar]
- 22. Lee KM, Kang HA, Ko CB, Oh EH, Park M, Lee HY, Choi HR, Yun CH, Jung WW, Oh JW, Kang HS. Differential gene expression profiles in spontaneously hypertensive rats induced by administration of enalapril and nifedipine. Int J Mol Med. 2013;31:179–187. [DOI] [PubMed] [Google Scholar]
- 23. Corvaisier M, Delneste Y, Jeanvoine H, Preisser L, Blanchard S, Garo E, Hoppe E, Barré B, Audran M, Bouvard B, Saint‐André JP, Jeannin P. IL‐26 is overexpressed in rheumatoid arthritis and induces proinflammatory cytokine production and Th17 cell generation. PLoS Biol. 2012;10:e1001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dudakov JA, Hanash AM, van den Brink MR. Interleukin‐22: immunobiology and pathology. Annu Rev Immunol. 2015;33:747–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Basu R, O'Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, Hatton RD, Weaver CT. Th22 cells are an important source of IL‐22 for host protection against enteropathogenic bacteria. Immunity. 2012;37:1061–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liang M, Wang J, Chu H, Zhu X, He H, Liu Q, Qiu J, Zhou X, Guan M, Xue Y, Chen X, Zou H. Interleukin‐22 inhibits bleomycin‐induced pulmonary fibrosis. Mediators Inflamm. 2013;8:209179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson AW, Kinzenbaw DA, Modrick ML, Faraci FM. Small‐molecule inhibitors of signal transducer and activator of transcription 3 protect against angiotensin II‐induced vascular dysfunction and hypertension. Hypertension. 2013;61:437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou CC, Ahmad S, Mi T, Xia L, Abbasi S, Hewett PW, Sun C, Ahmed A, Kellems RE, Xia Y. Angiotensin II induces soluble fms‐Like tyrosine kinase‐1 release via calcineurin signaling pathway in pregnancy. Circ Res. 2007;100:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou CC, Irani RA, Zhang Y, Blackwell SC, Mi T, Wen J, Shelat H, Geng YJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody‐mediated tumor necrosis factor‐alpha induction contributes to increased soluble endoglin production in preeclampsia. Circulation. 2010;121:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre‐eclampsia in pregnant mice. Nat Med. 2008;14:855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Long C, Cook LG, Hamilton SL, Wu GY, Mitchell BM. FK506 binding protein 12/12.6 depletion increases endothelial nitric oxide synthase threonine 495 phosphorylation and blood pressure. Hypertension. 2007;49:569–576. [DOI] [PubMed] [Google Scholar]
- 32. Shao J, Nangaku M, Miyata T, Inagi R, Yamada K, Kurokawa K, Fujita T. Imbalance of T‐cell subsets in angiotensin II‐infused hypertensive rats with kidney injury. Hypertension. 2003;42:31–38. [DOI] [PubMed] [Google Scholar]
- 33. Mazzolai L, Duchosal MA, Korber M, Bouzourene K, Aubert JF, Hao H, Vallet V, Brunner HR, Nussberger J, Gabbiani G, Hayoz D. Endogenous angiotensin II induces atherosclerotic plaque vulnerability and elicits a Th1 response in ApoE‐/‐ mice. Hypertension. 2004;44:277–282. [DOI] [PubMed] [Google Scholar]
- 34. Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM. Interleukin‐17 causes Rho‐kinase‐mediated endothelial dysfunction and hypertension. Cardiovasc Res. 2013;97:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Itani HA, McMaster WJ, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, Konior A, Prejbisz A, Januszewicz A, Norlander AE, Chen W, Bonami RH, Marshall AF, Poffenberger G, Weyand CM, Madhur MS, Moore DJ, Harrison DG, Guzik TJ. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension. 2016;68:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Campese VM, Ye S, Zhong H. Downregulation of neuronal nitric oxide synthase and interleukin‐1beta mediates angiotensin II‐dependent stimulation of sympathetic nerve activity. Hypertension. 2002;39:519–524. [DOI] [PubMed] [Google Scholar]
- 37. Brands MW, Banes‐Berceli AK, Inscho EW, Al‐Azawi H, Allen AJ, Labazi H. Interleukin 6 knockout prevents angiotensin II hypertension: role of renal vasoconstriction and janus kinase 2/signal transducer and activator of transcription 3 activation. Hypertension. 2010;56:879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang J, Patel MB, Griffiths R, Mao A, Song YS, Karlovich NS, Sparks MA, Jin H, Wu M, Lin EE, Crowley SD. Tumor necrosis factor‐alpha produced in the kidney contributes to angiotensin II‐dependent hypertension. Hypertension. 2014;64:1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ishibashi M, Hiasa K, Zhao Q, Inoue S, Ohtani K, Kitamoto S, Tsuchihashi M, Sugaya T, Charo IF, Kura S, Tsuzuki T, Ishibashi T, Takeshita A, Egashira K. Critical role of monocyte chemoattractant protein‐1 receptor CCR2 on monocytes in hypertension‐induced vascular inflammation and remodeling. Circ Res. 2004;94:1203–1210. [DOI] [PubMed] [Google Scholar]
- 40. Takahashi M, Suzuki E, Takeda R, Oba S, Nishimatsu H, Kimura K, Nagano T, Nagai R, Hirata Y. Angiotensin II and tumor necrosis factor‐alpha synergistically promote monocyte chemoattractant protein‐1 expression: roles of NF‐kappaB, p38, and reactive oxygen species. Am J Physiol Heart Circ Physiol. 2008;294:H2879–H2888. [DOI] [PubMed] [Google Scholar]
- 41. Craige SM, Kroller‐Schon S, Li C, Kant S, Cai S, Chen K, Contractor MM, Pei Y, Schulz E, Keaney JJ. PGC‐1alpha dictates endothelial function through regulation of eNOS expression. Sci Rep. 2016;6:38210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boegehold MA, Drenjancevic I, Lombard JH. Salt, angiotensin II, superoxide, and endothelial function. Compr Physiol. 2015;6:215–254. [DOI] [PubMed] [Google Scholar]
- 43. Hernanz R, Martinez‐Revelles S, Palacios R, Martin A, Cachofeiro V, Aguado A, Garcia‐Redondo L, Barrus MT, de Batista PR, Briones AM, Salaices M, Alonso MJ. Toll‐like receptor 4 contributes to vascular remodelling and endothelial dysfunction in angiotensin II‐induced hypertension. Br J Pharmacol. 2015;172:3159–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Long C, Cook LG, Wu GY, Mitchell BM. Removal of FKBP12/12.6 from endothelial ryanodine receptors leads to an intracellular calcium leak and endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2007;27:1580–1586. [DOI] [PubMed] [Google Scholar]
- 45. Cook LG, Chiasson VL, Long C, Wu GY, Mitchell BM. Tacrolimus reduces nitric oxide synthase function by binding to FKBP rather than by its calcineurin effect. Kidney Int. 2009;75:719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li Y, Kinzenbaw DA, Modrick ML, Pewe LL, Faraci FM. Context‐dependent effects of SOCS3 in angiotensin II‐induced vascular dysfunction and hypertension in mice: mechanisms and role of bone marrow‐derived cells. Am J Physiol Heart Circ Physiol. 2016;311:H146–H156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC. Interleukin‐22 (IL‐22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL‐10. J Biol Chem. 2002;277:33676–33682. [DOI] [PubMed] [Google Scholar]
- 48. Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL‐22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. [DOI] [PubMed] [Google Scholar]


