Abstract
Background
The impact of coronary artery disease (CAD) on outcomes after transcatheter aortic valve replacement (TAVR) is understudied. Literature on the prognostic role of CAD in the survival of patients undergoing TAVR shows conflicting results. This meta‐analysis aims to investigate how CAD impacts patient survival following TAVR.
Methods and Results
We completed a comprehensive literature search of Embase, MEDLINE, and the Cochrane Library, and included studies reporting outcome of TAVR based on CAD status of patients for the analysis. From the initial 1631 citations, 15 studies reporting on 8013 patients were analyzed using a random‐effects model. Of the 8013 patients undergoing TAVR, with a median age of 81.3 years (79–85.1 years), 46.6% (40–55.7) were men and 3899 (48.7%) had CAD (ranging from 30.8% to 78.2% in various studies). Overall, 3121 SAPIEN/SAPIEN XT/SAPIEN 3 (39.6%) and 4763 CoreValve (60.4%) prostheses were implanted, with transfemoral access being the most frequently used approach for the implantation (76.1%). Our analysis showed no significant difference between patients with and without CAD for all‐cause mortality at 30 days post TAVR, with a cumulative odds ratio of 1.07 (95% confidence interval, 0.82–1.40; P=0.62). However, there was a significant increase in all‐cause mortality at 1 year in the CAD group compared with patients without CAD, with a cumulative odds ratio of 1.21 (95% confidence interval, 1.07–1.36; P=0.002).
Conclusions
Even though coexisting CAD does not impact 30‐day mortality, it does have an impact on 1‐year mortality in patients undergoing TAVR. Our results highlight a need to revisit the revascularization strategies for concomitant CAD in patients with TAVR.
Keywords: coronary artery disease, meta‐analysis, revascularization, transcatheter aortic valve implantation, transcatheter aortic valve replacement
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation, Coronary Artery Disease, Percutaneous Coronary Intervention
Clinical Perspective
What Is New?
Even though prevalence of coronary artery disease (CAD) in patients undergoing transcatheter aortic valve replacement (TAVR) ranges from 40% to 70%, literature regarding the impact of CAD on TAVR outcomes yields conflicting results.
Coexisting CAD is a negative prognostic indicator with regard to survival of patients undergoing TAVR at 1‐year follow‐up.
There were no significant differences between patients with and without CAD for mortality and other Valve Academic Research Consortium outcomes at 30‐day follow‐up after TAVR.
Studying the impact of CAD on patient outcomes after TAVR is hindered by nonuniform CAD definitions and infrequent reporting on anatomical and functional assessment of CAD in the published literature.
What Are the Clinical Implications?
Since CAD has a negative effect on prognosis at 1‐year follow‐up, it is important to revisit the revascularization strategies that could be implemented.
Randomized controlled trials are needed to determine the role of routine revascularization and optimal timing of revascularization in patients with significant CAD undergoing TAVR.
It is important to find the appropriate management of concomitant CAD as the TAVR population expands to include younger and lower‐risk individuals.
Introduction
Aortic stenosis (AS) and coronary artery disease (CAD) frequently exist together.1 The coexistence of these 2 entities can be attributed to their similar risk factors and pathophysiology.2, 3, 4 The prevalence of CAD in severe AS ranges from 30% to 50%,5, 6, 7, 8 and increases with age.9
The presence of CAD has been identified as a negative prognostic indicator for patients undergoing surgical aortic valve replacement (SAVR).10, 11, 12 It is known that addition of coronary artery bypass grafting to SAVR increases the perioperative mortality in patients undergoing SAVR,13, 14, 15, 16 but studies have also reported improved short‐ and long‐term survival in patients with AS and CAD who underwent combined SAVR and coronary artery bypass grafting, compared with those who underwent isolated SAVR.17, 18 Based on these findings, the current guidelines for primary aortic valve disease requiring surgical intervention recommend coronary artery bypass grafting in all patients with significant stenoses at the time of SAVR.19
Since the “first‐in‐man” transcatheter aortic valve replacement (TAVR) in 2002,20 TAVR has emerged as a reasonable alternative in patients with severe AS who are inoperable or at high risk for SAVR.21, 22 Currently, data are emerging on intermediate‐risk patients as well.23 Because the patients selected to undergo TAVR are typically elderly with multiple comorbid conditions, the prevalence of CAD among them is generally high. Major randomized controlled trials (RCTs) and registries have demonstrated that the prevalence of CAD in patients undergoing TAVR can range from 40% to 70% or more.21, 22, 23, 24, 25, 26, 27
The impact of CAD on TAVR outcomes is unclear, as the major RCTs of TAVR, PARTNER (Placement of Aortic Transcatheter Valve Trial) I,21 and PARTNER II23 excluded patients with untreated clinically significant CAD requiring revascularization and patients with complex CAD (unprotected left main coronary artery or syntax score >32), respectively. Identifying the optimum revascularization strategy for patients with TAVR who have concomitant CAD is an area of ongoing debate. The few published studies evaluating the impact of CAD on TAVR outcomes and long‐term survival show conflicting results.28, 29, 30, 31 The 2 published meta‐analyses32, 33 that have attempted to identify the role of CAD in patients undergoing TAVR showed that CAD did not significantly alter the outcomes of TAVR. However, those analyses were limited by small numbers of studies and patients.
Therefore, although the high prevalence of CAD in the TAVR patient population is well documented, the data about the impact of CAD on TAVR outcomes are conflicting and unclear. The present meta‐analysis aims to investigate the impact of CAD on short‐term outcomes and follow‐up survival after TAVR.
Methods
Search Strategy
A systematic review of published literature was conducted following PRISMA (Preferred Reporting Items for Systematic reviews and Meta‐Analyses) guidelines.34 A computerized search of all publications in the MEDLINE, Embase, and Cochrane Central databases were searched. Relevant MeSH headings and variations of the words “TAVR,” “coronary artery disease,” and “revascularization” were used. Citations were screened at the title and abstract level and limited to English language and human patients. Full text along with online supplements reporting TAVR outcomes (at least all‐cause mortality) based on CAD status of patients were retrieved, and the bibliographies of the relevant articles were searched manually to identify any additional pertinent studies. This was last assessed as up to date on May 15, 2017.
Study Selection
Two independent reviewers initially screened all possible articles for inclusion at the title and/or abstract level, with the disagreement resolved by consensus. If potentially eligible, the complete article was then reviewed according to the following selection criteria.
Inclusion criteria were:
Studies that reported primary outcome (all‐cause mortality at 30 days and 1 year after TAVR) based on the CAD status of patients.
Studies that reported secondary end points at 30 days and/or 1 year after TAVR.
Exclusion criteria were:
Duplicate of publication/overlap of patients.
Outcomes of the interest (all‐cause mortality and/or Valve Academic Research Consortium [VARC] end points following TAVR based on the CAD status of patients) was not clearly reported or was impossible to calculate from published results.
Conference presentations, case reports, reviews, and editorials.
Studies in languages other than English.
Study End Points
The primary outcomes of interest for our study were all‐cause mortality at 30 days and 1 year after TAVR. Secondary outcomes included: (1) cardiovascular mortality at 30 days, (2) myocardial infarction at 30 days, (3) stroke at 30 days, (4) major bleeding at 30 days, (5) vascular complications at 30 days, (6) cardiovascular mortality at 1 year, (7) myocardial infarction at 1 year, and (8) stroke at 1 year. Standard definitions, as described by the VARC35, 36 were accepted for all of the outcomes after TAVR. Articles that clearly described their own definition of outcomes were also included. Definitions for CAD used by the included studies are mentioned in Table 1.
Table 1.
Study Characteristics
| Author | Year | Design | Country | Total, No. | CAD vs No CAD Comparison |
|---|---|---|---|---|---|
| Dewey et al28 | 2010 | Retrospective review of data records | 12 Centers in North America and Europe | 171 | Present |
| Gasparetto et al29 | 2013 | Prospective single‐center registry | Italy | 191 | Present |
| Gautier et al30 | 2011 | Retrospective review of data records | France | 145 | Present |
| Khawaja et al38 | 2015 | Retrospective review of data records | England | 271 | Present |
| Linke et al39 | 2014 | Prospective multicenter study | 44 Centers in 12 countries | 1001 | Absent; details were extracted |
| Mancio et al31 | 2015 | Prospective single‐center registry | Portugal | 91 | Present |
| Masson et al40 | 2010 | Retrospective review of single‐center registry | Canada | 136 | Present |
| Muñoz‐García et al41 | 2013 | Retrospective review of multicenter registry | 43 Centers in 9 European and Ibero‐American countries | 1220 | Absent; details were extracted |
| Panico et al42 | 2012 | Prospective single‐center registry | Italy | 118 | Absent; details were extracted |
| Paradis et al43 | 2017 | Retrospective review of data records | North America | 377 | Present |
| Rodés‐Cabau et al44 | 2010 | Retrospective review of multicenter national registry | 6 Centers in Canada | 339 | Absent; Details were extracted |
| Snow et al45 | 2015 | Retrospective review of national registry | 31 Centers in the United Kingdom | 2562 | Present |
| Stefanini et al46 | 2014 | Prospective single‐center registry | Switzerland | 445 | Present |
| Ussia et al47 | 2013 | Prospective multicenter database | 14 Centers in Italy | 659 | Present |
| Zivelonghi et al37 | 2017 | Retrospective review of data records | Italy | 287 | Present |
CAD indicates coronary artery disease.
Data Abstraction and Individual Study Quality Appraisal
All data were extracted from article text, tables, figures, and supplementary material. Data on study population, design of study, demographics, procedural details, and outcomes were collected. Two reviewers (K.S. and K.B.) independently conducted the literature searches, study eligibility assessment, and data extraction. Any discrepancies were resolved following discussion and consensus.
Two authors (K.S. and K.B.) independently assessed the risk of bias of included studies using the standardized Newcastle‐Ottawa Scale (Table S1). This validated instrument for appraising observational studies measures the risk of bias in 8 categories: representativeness of the exposed cohort (S1); selection of the nonexposed cohort (S2); ascertainment of exposure (S3); demonstration that the outcome of interest was not present at the start of the study (S4); comparability (C1 and C2); assessment of outcome (E1); whether follow‐up long enough for outcomes to occur (E2); and adequacy of follow‐up of cohorts (E3). Sensitivity analysis was performed for the primary outcomes.
Statistical Analysis
Data extracted from the studies were tabulated and analyzed using the “metan” package in STATA 13 (Statacorp). Pooled odds ratios were calculated using a Dersimonian‐Laird random‐effects model, with inverse variance weights for included studies. Continuous variables were analyzed using a weighted mean and compared with a Student t test. Statistical significance was set at P=0.05 (2‐tailed). Heterogeneity was assessed by I 2 test. Heterogeneity was considered low if I 2 <25% and significant if I 2 >75%. To address publication bias, we used 2 methods: (1) visual inspection of the funnel plots (Figures S1 and S2) and (2) Egger's test. We also performed sensitivity analysis (Figures S3 and S4) to evaluate how removal of each study impacts overall outcome.
Results
Study Selection
A total of 1631 records were identified in the preliminary search: 1286 from Embase and 335 from MEDLINE. After elimination of duplicates, 1326 records were screened by title and abstract. This excluded 1235 reports. The other 91 articles were retrieved and full texts were reviewed. After applying inclusion and exclusion criteria to the full text articles, 72 articles were removed, while the full text screening and 4 articles were removed during data extraction. Finally, 15 publications were selected for the present study.28, 29, 30, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 The study selection process is presented in Figure 1. According to the PRISMA statement.34
Figure 1.
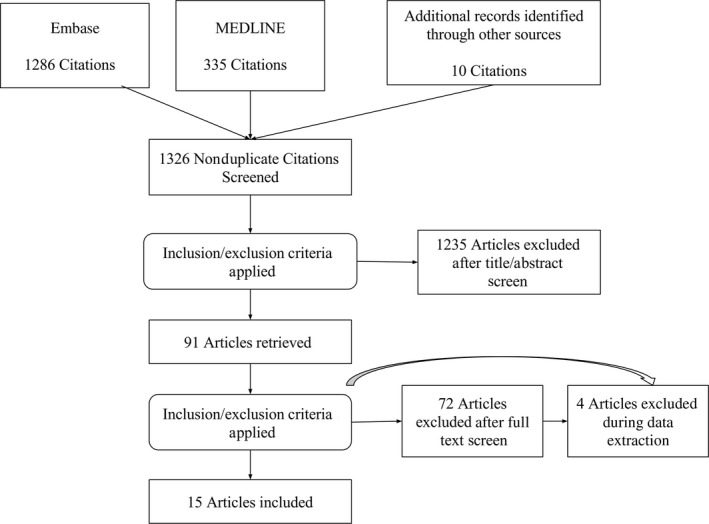
Flow diagram of study selection according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta‐Analyses) statement.
Study Characteristics
Among the included studies, the majority (9 studies) were retrospective in design. All included studies were published between 2010 and 2017. The CAD status–based comparisons were present in 11 studies, and CAD status–based outcome details were extracted in the remaining 4 studies39, 41, 42, 44 The details of the included studies such as study design, year of publication, country of origin, and sample size are given in Table 1.
Baseline Characteristics and Procedural Details
Table 2 shows the baseline characteristics of patients in the included studies. A total of 8013 patients who underwent TAVR were included, with a median age of 81.3 years (79–85.1 years), and 46.6% (40–55.7%) were men. The mean logistic EuroSCORE (European system for cardiac operative risk evaluation) was >20% in 10 studies (among 13 studies that reported it). The mean Society of Thoracic Surgeons risk score was >5% in all 9 studies that reported it. Of the total of 8013 patients, 3899 (48.7%) had CAD (ranging from 30.8% to 78.2% in various studies). Even though there were slight variations in the definition of CAD among the included studies, the majority used prior coronary artery bypass grafting/percutaneous coronary intervention (PCI) or >70% stenosis (>50% for left main) as the indicator of CAD status.
Table 2.
Baseline Features of Patients and CAD Definition Used in Included Studies
| Study | Mean Age, y | Men, % | STS (Mean) | EuroSCORE (Mean) | CAD, % | CAD Definition |
|---|---|---|---|---|---|---|
| Dewey et al28 | 83.8 | 49.1 | 12.06 | 30.86 | 49.1 | Prior CABG/PCI |
| Gasparetto et al29 | 80.5 | 42.4 | NA | 21.4 | 59.2 | Prior CABG/PCI and/or presence of any coronary stenosis of at least 50% |
| Gautier et al30 | 82 | 52.7 | 16 | 28 | 57.2 | Prior CABG/PCI or >70% stenosis (>50% for left main) |
| Khawaja et al38 | 82.5 | 55.7 | 6.14 | 21.46 | 34.3 | >70% stenosis (>50% for left main) |
| Linke et al39 | 81.1 | 49 | 5.3 | 16 | 57.8 | Not specified |
| Mancio et al31 | 79 | 52 | 6 | NA | 50.5 | Prior CABG/PCI and/or presence of any coronary stenosis of at least 50% |
| Masson et al40 | 85.1 | 50.7 | 9.1 | 21 | 30.8 | Prior CABG/PCI or >50% stenosis (extent was assessed by DMJS) |
| Muñoz‐García et al41 | 80.7 | 45.3 | NA | 17.8 | 36.1 | Not specified |
| Panico et al42 | 82.5 | 46.6 | NA | 25.8 | 51.7 | Not specified |
| Paradis et al43 | 82.5 | 51.9 | 8.5 | 25.4 | 78.2 | >50% stenosis in vessels >1.5 mm in diameter |
| Rodés‐Cabau et al44 | 81 | 44.8 | 9.8 | NA | 69 | Not specified |
| Snow et al45 | 81.3 | 46.3 | NA | 18.06 | 45.7 | >50% stenosis of the left main or 3 main coronaries or their major epicardial branches |
| Stefanini et al46 | 82.5 | 44 | 6.9 | 23.4 | 64.5 | >50% stenosis in vessels ≥1.5 mm in diameter |
| Ussia et al47 | 81.2 | 40 | NA | 23.1 | 38.1 | Prior CABG/PCI |
| Zivelonghi et al37 | 81.2 | 43.2 | NA | 28.6 | 42.9 | >50% Stenosis |
CABG indicates coronary artery bypass grafting; CAD, coronary artery disease; DMJS, Duke Myocardial Jeopardy Score; EuroSCORE, European system for cardiac operative risk evaluation; NA, not available; PCI, percutaneous coronary intervention; STS, the Society of Thoracic Surgeons risk score.
Apart from Gautier et al30 all of the studies reported the procedural details of TAVR such as different valve types and the approaches used. Overall, 3121 Edwards SAPIEN/SAPIEN XT/SAPIEN 3 (39.6%) and 4763 Medtronic CoreValve (60.4%) prostheses were implanted. A total of 6000 (76.1%) prostheses were implanted via the transfemoral approach, and the remainder were implanted via the transapical/transaortic/trans‐subclavian route, with transaortic delivery being the least followed approach among the 3 procedures. Ten of 15 studies reported the revascularization strategies used for patients with CAD. Of the total 4676 patients in those 10 studies, 2399 (51.3%) had CAD, and among those 2399 patients with CAD, 524 patients (21.8%) underwent PCI prior to TAVR. Table 3 shows the TAVR procedural details of the included studies.
Table 3.
Procedural (TAVR) and Revascularization Details of Included Studies
| Study | Transfemoral | Transapical | Trans‐Subclavian | Transaortic | Edwards SAPIEN | Core Valve | PCI (Staged/Concomitant) Prior to TAVR, No. (%) |
|---|---|---|---|---|---|---|---|
| Dewey et al28 | 136 | 35 | 0 | 0 | 171 | 0 | 0/84 (0) |
| Gasparetto et al29 | 128 | 58 | 5 | 0 | 104 | 87 | 39/113 (34.5) |
| Gautier et al30 | NA | NA | NA | NA | NA | NA | 11/83 (13.3) |
| Khawaja et al38 | 124 | 96 | 0 | 51 | 271 | 0 | 25/93 (26.9) |
| Linke et al39 | 880 | 0 | 95 | 21 | 0 | 996 | Not specified |
| Mancio et al31 | 87 | 4 | 0 | 0 | 12 | 79 | 13/46 (28.3) |
| Masson et al40 | 93 | 43 | 0 | 0 | 0 | 136 | 15/104 (14.4) |
| Muñoz‐García et al41 | 1155 | 0 | 65 | 0 | 0 | 1220 | Not specified |
| Panico et al42 | 116 | 0 | 2 | 0 | 82 | 36 | Not specified |
| Paradis et al43 | 182 | 195 | 0 | 0 | 377 | 0 | 54/295 (18.3) |
| Rodés‐Cabau et al44 | 167 | 172 | 0 | 0 | 339 | 0 | Not specified |
| Snow et al45 | 1749 | NA | NA | NA | 1345 | 1243 | 172/1171 (14.7) |
| Stefanini et al46 | 348 | 92 | 5 | 0 | 202 | 240 | 139/287 (48.4) |
| Ussia et al47 | 595 | 0 | 64 | 0 | 0 | 659 | Not specified |
| Zivelonghi et al37 | 240 | 44 | 3 | 0 | 218 | 67 | 56/123 (45.5) |
NA indicates not available; PCI, percutaneous coronary intervention; TAVR, transcatheter aortic valve replacement.
Outcomes
The 30‐day all‐cause mortality was reported in 13 studies, and 1‐year all‐cause mortality was reported in all of the studies except Mancio et al31 (reported 30‐day and 2‐year outcomes only) and Zivelonghi et al37 (30‐day outcomes only). Six studies29, 30, 31, 37, 46, 47 reported 30‐day VARC end points (cardiovascular death, myocardial infarction, stroke, major bleeding, and vascular complications) based on the CAD status of patients, whereas VARC end points (cardiovascular death, myocardial infarction, and stroke) at 1 year based on the CAD status of patients were present in only 3 studies.29, 43, 46
While the median 30‐day all‐cause mortality for CAD patients post TAVR was 7.1% (ranging from 4.4% to 13.1%), that of patients post TAVR without CAD was 5.9% (1.2–14.5%). The median 1‐year all‐cause mortality for patients with CAD post TAVR was 19.75% (14.5–35.7%) and that of patients without CAD was 17.75% (14.6–25.8%).
As shown in Figure 2, our analysis showed no statistically significant difference for all‐cause mortality at 30 days between patients with and without CAD, with a cumulative odds ratio of 1.07 (95% confidence interval, 0.82–1.40; P=0.62). However, there was a statistically significant increase in all‐cause mortality at 1 year in the CAD cohort compared with the patients without CAD (Figure 3), with a cumulative odds ratio of 1.21 (95% confidence interval, 1.07–1.36; P=0.002). No statistically significant difference was found among patients with and without CAD in the analysis of studies, which reported other VARC end points in separate cohorts (CAD versus no CAD) at 30 days and 1 year (Figures 4, 5 through 6).
Figure 2.
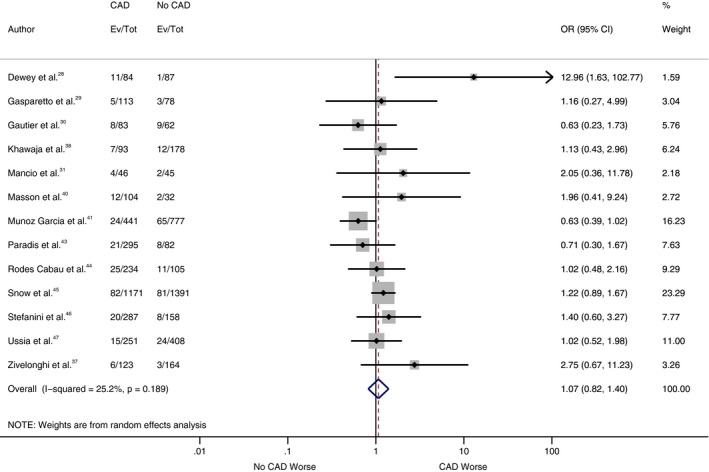
Forest plot comparing risk of 30‐day all‐cause mortality between patients with and without coronary artery disease (CAD). The diamond indicates the overall summary estimate for the analysis. The center of the diamond represents the point estimate and the width represents 95% confidence interval (CI). OR indicates odds ratio.
Figure 3.
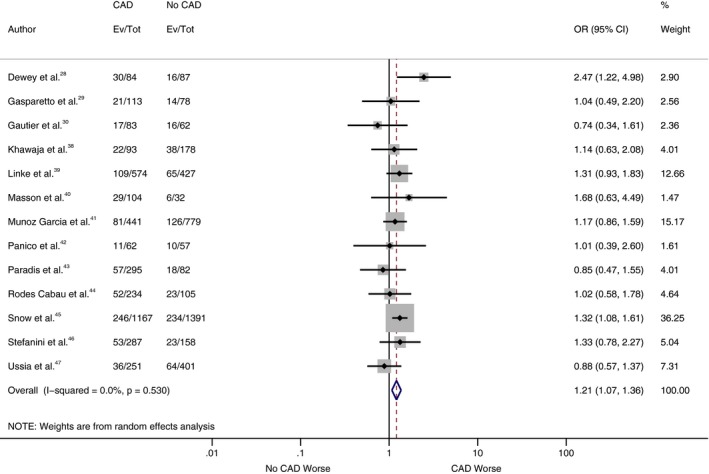
Forest plot comparing risk of all‐cause mortality at 1 year between patients with and without coronary artery disease (CAD). The diamond indicates the overall summary estimate for the analysis. The center of the diamond represents the point estimate and the width represents 95% confidence interval (CI). OR indicates odds ratio.
Figure 4.
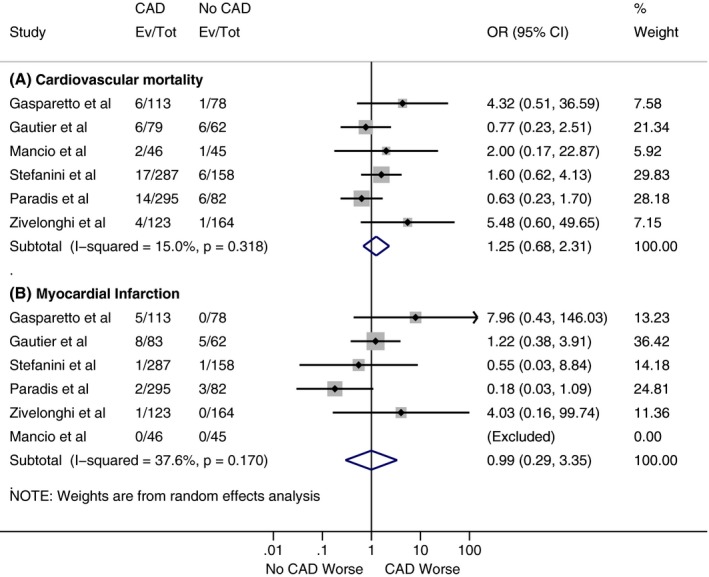
Forest plots comparing risk of (A) 30‐day cardiovascular mortality and (B) 30‐day myocardial infarction between patients with and without coronary artery disease (CAD). The diamond indicates the overall summary estimate for the analysis. The center of the diamond represents the point estimate and the width represents 95% confidence interval. OR indicates odds ratio.
Figure 5.
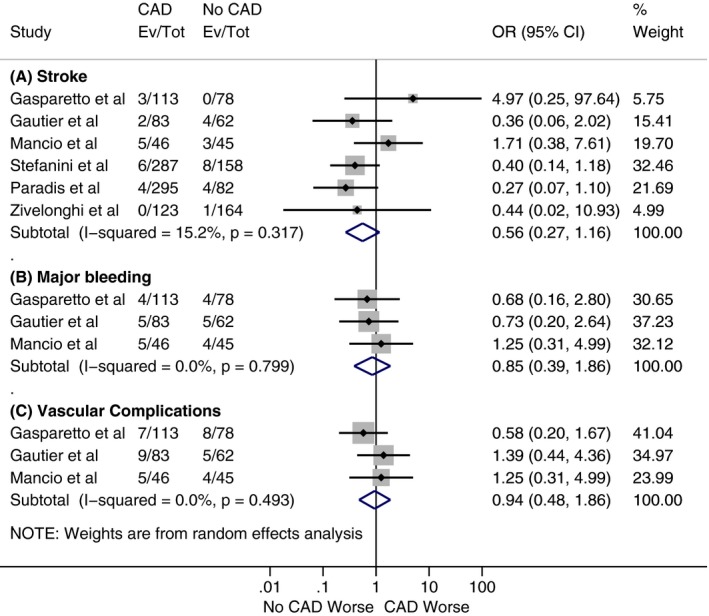
Forest plots comparing (A) 30‐day risk of stroke, (B) 30‐day risk of major bleeding, and (C) 30‐day risk of vascular complications between patients with and without coronary artery disease (CAD). The diamond indicates the overall summary estimate for the analysis. The center of the diamond represents the point estimate and the width represents 95% confidence interval (CI). OR indicates odds ratio.
Figure 6.
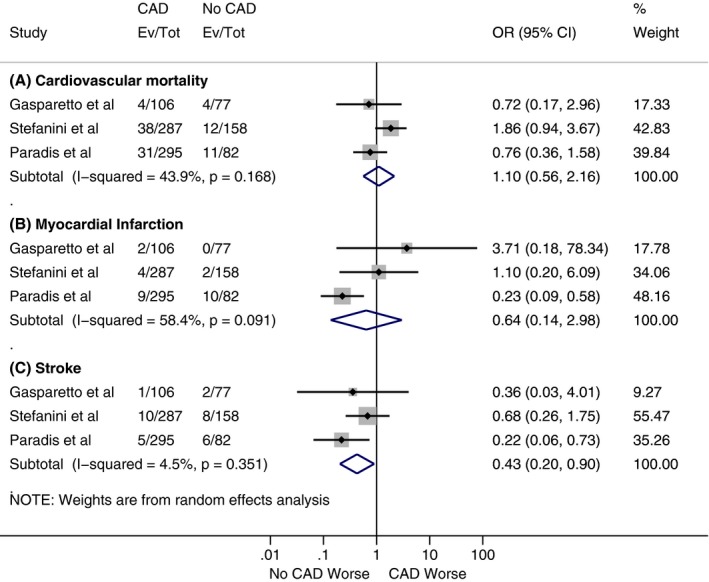
Forest plots comparing (A) 1‐year risk of cardiovascular mortality, (B) 1‐year risk of myocardial infarction, and (C) 1‐year risk of stroke between patients with and without coronary artery disease (CAD). The diamond indicates the overall summary estimate for the analysis. The center of the diamond represents the point estimate and the width represents 95% confidence interval (CI). OR indicates odds ratio.
Publication Bias
Publication bias in the primary outcome of interest (all‐cause mortality at 30 days and 1 year) was initially evaluated graphically with funnel plots (Figures S1 and S2), which showed some asymmetry in both outcomes. Egger's regression asymmetry was applied for quantitative assessment and generated P values of 0.21 (30‐day all‐cause mortality) and 0.44 (1‐year all‐cause mortality), indicating that conclusions were not altered because of publication bias.
Discussion
To our knowledge, this is the first meta‐analysis with more than 5000 patients to examine the impact of CAD on TAVR outcomes. This meta‐analysis has 2 main findings. First, although there was no significant difference for all‐cause mortality at 30 days, there was a significant increase in all‐cause mortality at 1 year in the CAD group, compared with patients without CAD. Second, procedural complications including cardiovascular mortality, myocardial infarction, stroke, bleeding, and vascular complications are no different based on CAD status. Notably, we identified important limitations of the data, which are nonuniformity in the CAD definitions used in RCTs, registries, and large observational studies and absence of stratified TAVR outcomes based on the CAD status of patients in RCTs and major registries.
The first published study on the impact of CAD in TAVR outcomes was by Dewey et al28 in 2010. They reported the outcomes on 171 patients based on CAD status and found that presence of CAD was the most significant factor associated with 30‐day mortality with an odds ratio of 10.1%. Since then, most other studies have failed to show any statistically significant difference between the cohorts for 30‐day mortality. With 1‐year follow‐up, reports of mortality varied widely. Dewey et al28 and Mancio et al31 found significantly higher mortality in the CAD cohort. On the contrary, many other studies did not show any statistically significant difference between the groups. One approach was to classify patients with CAD according to Syntax scores. Based on this stratification, Stefanini et al46 and Khawaja et al38 showed that it is the complexity and severity of CAD (higher Syntax scores) that have more prognostic implications in the TAVR outcomes rather than the mere presence of CAD. While both studies showed higher mortality at 1 year, only Khawaja et al demonstrated higher mortality at 30 days as well. These results were condensed into a meta‐analysis by Taha et al,33 which showed that a residual or baseline Syntax score ≤10 does not affect the clinical outcomes after TAVR. D'Ascenzo et al32 were the first to perform a pooled analysis on the prognostic role of CAD in TAVR outcomes. Their analysis revealed that even though CAD represented a common amnestic finding in patients undergoing TAVR, it does not affect the midterm outcomes after TAVR procedures.
The benefits of revascularization and the impact of nonrevascularized myocardium in TAVR outcomes is an ongoing debate. Kleczynski et al48 showed that incomplete coronary revascularization may be an independent predictor of all‐cause mortality after TAVR; however, on the contrary, Van Meighem et al49 state that complete revascularization is not a prerequisite for success in current TAVR practice. Even though Goel et al50 showed that PCI can be performed in patients with severe symptomatic AS and CAD without an increased risk of short‐term mortality, considerable uncertainty persists regarding the timing of revascularization (concomitant versus staged). This meta‐analysis highlights the need for further randomized studies to find the optimal revascularization strategy for CAD in TAVR candidates and to ascertain the impact of nonrevascularized myocardium in TAVR outcomes. The results of the ongoing ACTIVATION (Percutaneous Coronary Intervention Prior to Transcatheter Aortic Valve Implantation) RCT51 (PCI prior to transcatheter aortic valve implantation) in this regard are anxiously awaited.
Limitations
Even though our large meta‐analysis is able to provide some indications regarding the impact of CAD on TAVR outcomes, there are a few limitations to our analysis. First, this was a meta‐analysis performed on study‐level data and it lacks individual patient‐level data. Second, there was nonuniformity of CAD definitions among the included studies. Third, only limited studies reported all of the VARC or secondary end points at 30 days (6 studies) and 1 year (3 studies) based on the CAD status of the patients and, hence, results from these analyses should be extrapolated with caution. In addition, we do not have sufficient granular data to study other details such as how the impact of syntax scores might vary or how unprotected left main coronary artery disease (and/or ostial disease of the left anterior descending coronary artery) might impact the outcomes separately compared with other types of CAD.
Conclusions
The present meta‐analysis of 15 studies comparing mortality in patients with and without CAD undergoing TAVR did not find a statistically significant difference in mortality at 30 days. However, there was a significant increase in mortality at 1 year in the patients with CAD undergoing TAVR, compared with patients who did not have concomitant CAD. There are a few directions we can take from here. First, since CAD has a prognostic role in the survival of patients, it is important to revisit revascularization strategies that could be implemented. However, data are limited on the safety and efficacy of revascularization in these patients and further prospective research is needed to validate these conclusions. Second, there is need for additional randomized studies to investigate the impact of pre‐TAVR PCI in the outcomes after TAVR. Third, more data are needed to determine whether a staged or simultaneous PCI prior to TAVR is preferable. Finally, if the studies show favorable results with staged PCI, further studies will be required to elucidate the optimal time gap between PCI and TAVR.
Disclosures
None.
Supporting information
Table S1. Assessment of Study Quality Using the Newcastle Ottawa Scale
Figure S1. Funnel plot for all‐cause mortality at 30 days after transcatheter aortic valve replacement (TAVR).
Figure S2. Funnel plot for all‐cause mortality at 1 year after transcatheter aortic valve replacement (TAVR).
Figure S3. Sensitivity analysis for all‐cause mortality at 30 days.
Figure S4. Sensitivity analysis for all‐cause mortality at 1 year.
(J Am Heart Assoc. 2017;6:e006092 DOI: 10.1161/JAHA.117.006092.)
References
- 1. Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic‐valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. [DOI] [PubMed] [Google Scholar]
- 2. Carabello BA, Paulus WJ. Aortic stenosis. Lancet. 2009;373:956–966. [DOI] [PubMed] [Google Scholar]
- 3. Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of “degenerative” valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. [DOI] [PubMed] [Google Scholar]
- 4. Otto CM. Calcific aortic stenosis—time to look more closely at the valve. N Engl J Med. 2008;359:1395–1398. [DOI] [PubMed] [Google Scholar]
- 5. Exadactylos N, Sugrue DD, Oakley CM. Prevalence of coronary artery disease in patients with isolated aortic valve stenosis. Br Heart J. 1984;51:121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vandeplas A, Willems JL, Piessens J, De Geest H. Frequency of angina pectoris and coronary artery disease in severe isolated valvular aortic stenosis. Am J Cardiol. 1988;62:117–120. [DOI] [PubMed] [Google Scholar]
- 7. Rapp AH, Hillis LD, Lange RA, Cigarroa JE. Prevalence of coronary artery disease in patients with aortic stenosis with and without angina pectoris. Am J Cardiol. 2001;87:1216–1217; A7. [DOI] [PubMed] [Google Scholar]
- 8. Ortlepp JR, Schmitz F, Bozoglu T, Hanrath P, Hoffmann R. Cardiovascular risk factors in patients with aortic stenosis predict prevalence of coronary artery disease but not of aortic stenosis: an angiographic pair matched case‐control study. Heart. 2003;89:1019–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lombard JT, Selzer A. Valvular aortic stenosis. A clinical and hemodynamic profile of patients. Ann Intern Med. 1987;106:292–298. [DOI] [PubMed] [Google Scholar]
- 10. Beach JM, Mihaljevic T, Svensson LG, Rajeswaran J, Marwick T, Griffin B, Johnston DR, Sabik JF, Blackstone EH. Coronary artery disease and outcomes of aortic valve replacement for severe aortic stenosis. J Am Coll Cardiol. 2013;61:837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Likosky DS, Sorensen MJ, Dacey LJ, Baribeau YR, Leavitt BJ, DiScipio AW, Hernandez F, Cochran RP, Quinn R, Helm RE, Charlesworth DC, Clough RA, Malenka DJ, Sisto DA, Sardella G, Olmstead EM, Ross CS, O'Connor GT. Long‐term survival of the very elderly undergoing aortic valve surgery. Circulation. 2009;120:S127–S133. [DOI] [PubMed] [Google Scholar]
- 12. Tjang YS, van Hees Y, Körfer R, Grobbee DE, van der Heijden GJMG. Predictors of mortality after aortic valve replacement. Eur J Cardiothorac Surg. 2007;32:469–474. [DOI] [PubMed] [Google Scholar]
- 13. Edwards FH, Peterson ED, Coombs LP, DeLong ER, Jamieson WR, Shroyer ALW, Grover FL. Prediction of operative mortality after valve replacement surgery. J Am Coll Cardiol. 2001;37:885–892. [DOI] [PubMed] [Google Scholar]
- 14. Hannan EL, Wu C, Bennett EV, Carlson RE, Culliford AT, Gold JP, Higgins RSD, Smith CR, Jones RH. Risk index for predicting in‐hospital mortality for cardiac valve surgery. Ann Thorac Surg. 2007;83:921–929. [DOI] [PubMed] [Google Scholar]
- 15. Nowicki ER, Birkmeyer NJO, Weintraub RW, Leavitt BJ, Sanders JH, Dacey LJ, Clough RA, Quinn RD, Charlesworth DC, Sisto DA, Uhlig PN, Olmstead EM, O'Connor GT; Northern New England Cardiovascular Disease Study Group and the Center for Evaluative Clinical Sciences DMS . Multivariable prediction of in‐hospital mortality associated with aortic and mitral valve surgery in Northern New England. Ann Thorac Surg. 2004;77:1966–1977. [DOI] [PubMed] [Google Scholar]
- 16. Kuduvalli M, Grayson AD, Au J, Grotte G, Bridgewater B, Fabri BM. A multi‐centre additive and logistic risk model for in‐hospital mortality following aortic valve replacement. Eur J Cardiothorac Surg. 2007;31:607–613. [DOI] [PubMed] [Google Scholar]
- 17. Lytle BW, Cosgrove DM, Goormastic M, Loop FD. Aortic valve replacement and coronary bypass grafting for patients with aortic stenosis and coronary artery disease: early and late results. Eur Heart J. 1988;9(suppl E):143–147. [DOI] [PubMed] [Google Scholar]
- 18. Lund O, Nielsen TT, Pilegaard HK, Magnussen K, Knudsen MA. The influence of coronary artery disease and bypass grafting on early and late survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg. 1990;100:327–337. [PubMed] [Google Scholar]
- 19. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, Thomas JD, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Creager MA, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen W‐K, Stevenson WG, Yancy CW. 2014 AHA/ACC guideline for the management of patients with valvular heart disease. J Thorac Cardiovasc Surg. 2014;148:e1–e132. [DOI] [PubMed] [Google Scholar]
- 20. Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, Derumeaux G, Anselme F, Laborde F, Leon MB. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106:3006–3008. [DOI] [PubMed] [Google Scholar]
- 21. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 22. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ; PARTNER Trial Investigators . Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 23. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 24. Abdel‐Wahab M, Mehilli J, Frerker C, Neumann F‐J, Kurz T, Tölg R, Zachow D, Guerra E, Massberg S, Schäfer U, El‐Mawardy M, Richardt G. Comparison of balloon‐expandable vs self‐expandable valves in patients undergoing transcatheter aortic valve replacement. JAMA. 2014;311:1503. [DOI] [PubMed] [Google Scholar]
- 25. Hamm CW, Mollmann H, Holzhey D, Beckmann A, Veit C, Figulla H‐R, Cremer J, Kuck K‐H, Lange R, Zahn R, Sack S, Schuler G, Walther T, Beyersdorf F, Bohm M, Heusch G, Funkat A‐K, Meinertz T, Neumann T, Papoutsis K, Schneider S, Welz A, Mohr FW. The German Aortic Valve Registry (GARY): in‐hospital outcome. Eur Heart J. 2014;35:1588–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomas M, Schymik G, Walther T, Himbert D, Lefèvre T, Treede H, Eggebrecht H, Rubino P, Michev I, Lange R, Anderson WN, Wendler O. Thirty‐day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: a European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2010;122:62–69. [DOI] [PubMed] [Google Scholar]
- 27. Eltchaninoff H, Prat A, Gilard M, Leguerrier A, Blanchard D, Fournial G, Iung B, Donzeau‐Gouge P, Tribouilloy C, Debrux JL, Pavie A, Gueret P. Transcatheter aortic valve implantation: early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J. 2011;32:191–197. [DOI] [PubMed] [Google Scholar]
- 28. Dewey TM, Brown DL, Herbert MA, Culica D, Smith CR, Leon MB, Svensson LG, Tuzcu M, Webb JG, Cribier A, Mack MJ. Effect of concomitant coronary artery disease on procedural and late outcomes of transcatheter aortic valve implantation. Ann Thorac Surg. 2010;89:758–767. [DOI] [PubMed] [Google Scholar]
- 29. Gasparetto V, Fraccaro C, Tarantini G, Buja P, D'Onofrio A, Yzeiraj E, Pittarello D, Isabella G, Gerosa G, Iliceto S, Napodano M. Safety and effectiveness of a selective strategy for coronary artery revascularization before transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2013;81:376–383. [DOI] [PubMed] [Google Scholar]
- 30. Gautier M, Pepin M, Himbert D, Ducrocq G, Iung B, Dilly M‐P, Attias D, Nataf P, Vahanian A. Impact of coronary artery disease on indications for transcatheter aortic valve implantation and on procedural outcomes. EuroIntervention. 2011;7:549–555. [DOI] [PubMed] [Google Scholar]
- 31. Mancio J, Fontes‐Carvalho R, Oliveira M, Caeiro D, Braga P, Bettencourt N, Ribeiro VG. Coronary artery disease and symptomatic severe aortic valve stenosis: clinical outcomes after transcatheter aortic valve implantation. Front Cardiovasc Med. 2015;2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. D'Ascenzo F, Conrotto F, Giordana F, Moretti C, D'Amico M, Salizzoni S, Omedè P, La Torre M, Thomas M, Khawaja Z, Hildick‐Smith D, Ussia G, Barbanti M, Tamburino C, Webb J, Schnabel RB, Seiffert M, Wilde S, Treede H, Gasparetto V, Napodano M, Tarantini G, Presbitero P, Mennuni M, Rossi ML, Gasparini M, Biondi Zoccai G, Lupo M, Rinaldi M, Gaita F, Marra S. Mid‐term prognostic value of coronary artery disease in patients undergoing transcatheter aortic valve implantation: a meta‐analysis of adjusted observational results. Int J Cardiol. 2013;168:2528–2532. [DOI] [PubMed] [Google Scholar]
- 33. Taha S, Moretti C, D'Ascenzo F, Van Mieghem NM, Omedè P, Montefusco A, Ghany MA, Fouaad D, Demitry S, Zoccai GB, Gaita F. Impact of residual coronary artery disease on patients undergoing TAVI: a meta‐analysis of adjusted observational studies. Int J Cardiol. 2015;181:77–80. [DOI] [PubMed] [Google Scholar]
- 34. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, Krucoff MW, Mack M, Mehran R, Miller C, Morel M‐A, Petersen J, Popma JJ, Takkenberg JJM, Vahanian A, van Es G‐A, Vranckx P, Webb JG, Windecker S, Serruys PW. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart J. 2011;32:205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es G‐A, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés‐Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. [DOI] [PubMed] [Google Scholar]
- 37. Zivelonghi C, Lunardi M, Pesarini G, Scarsini R, Piccoli A, Ferrero V, Gottin L, Milano A, Faggian G, Vassanelli C, Ribichini F. Coronary artery disease in patients undergoing transcatheter aortic valve implantation. A single center registry on prevalence, management and immediate clinical impact. Cor Vasa. 2017;59:e23–e28. [Google Scholar]
- 38. Khawaja MZ, Asrress KN, Haran H, Arri S, Nadra I, Bolter K, Wilson K, Clack L, Hancock J, Young CP, Bapat V, Thomas M, Redwood S. The effect of coronary artery disease defined by quantitative coronary angiography and SYNTAX score upon outcome after transcatheter aortic valve implantation (TAVI) using the Edwards bioprosthesis. EuroIntervention. 2015;11:450–455. [DOI] [PubMed] [Google Scholar]
- 39. Linke A, Wenaweser P, Gerckens U, Tamburino C, Bosmans J, Bleiziffer S, Blackman D, Schafer U, Muller R, Sievert H, Sondergaard L, Klugmann S, Hoffmann R, Tchetche D, Colombo A, Legrand VM, Bedogni F, LePrince P, Schuler G, Mazzitelli D, Eftychiou C, Frerker C, Boekstegers P, Windecker S, Mohr F‐W, Woitek F, Lange R, Bauernschmitt R, Brecker S. Treatment of aortic stenosis with a self‐expanding transcatheter valve: the International Multi‐centre ADVANCE Study. Eur Heart J. 2014;35:2672–2684. [DOI] [PubMed] [Google Scholar]
- 40. Masson J‐B, Lee M, Boone RH, Al Ali A, Al Bugami S, Hamburger J, John Mancini GB, Ye J, Cheung A, Humphries KH, Wood D, Nietlispach F, Webb JG. Impact of coronary artery disease on outcomes after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2010;76:165–173. [DOI] [PubMed] [Google Scholar]
- 41. Muñoz‐García AJ, Del Valle R, Trillo‐Nouche R, Elízaga J, Gimeno F, Hernández‐Antolín R, Teles R, De Gama Ribeiro V, Molina E, Cequier Á, Urbano‐Carrillo C, Cruz‐González I, Payaslian M, Patricio L, Sztejfman M, Íñiguez A, Rodríguez V, Scuteri A, Caorsi C, López‐Otero D, Avanzas P, Alonso‐Briales JH, Hernández‐García JM, Morís C. The Ibero‐American transcatheter aortic valve implantation registry with the CoreValve prosthesis. Early and long‐term results. Int J Cardiol. 2013;169:359–365. [DOI] [PubMed] [Google Scholar]
- 42. Panico C, Pagnotta P, Mennuni M, Corrada E, Barbaro C, Rossi M, Lisignoli L, Zavalloni V, Parenti D, Belli G, Gasparini G, Presbitero P. Predictors of mortality in patients undergoing percutaneous aortic valve implantation. Minerva Cardioangiol. 2012;60:561–571. [PubMed] [Google Scholar]
- 43. Paradis J, White JM, Généreux P, Urena M, Doshi D, Nazif T, Hahn R, George I, Khalique O, Harjai K, Lasalle L, Labbé BM, DeLarochellière R, Doyle D, Dumont É, Mohammadi S, Leon MB, Rodés‐Cabau J, Kodali S. Impact of coronary artery disease severity assessed with the SYNTAX Score on outcomes following transcatheter aortic valve replacement. J Am Heart Assoc. 2017;6:e005070 DOI: 10.1161/JAHA.116.005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rodés‐Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, Osten M, Natarajan MK, Velianou JL, Martucci G, DeVarennes B, Chisholm R, Peterson MD, Lichtenstein SV, Nietlispach F, Doyle D, DeLarochellière R, Teoh K, Chu V, Dancea A, Lachapelle K, Cheema A, Latter D, Horlick E. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk. J Am Coll Cardiol. 2010;55:1080–1090. [DOI] [PubMed] [Google Scholar]
- 45. Snow TM, Ludman P, Banya W, DeBelder M, MacCarthy PM, Davies SW, Di Mario C, Moat NE. Management of concomitant coronary artery disease in patients undergoing transcatheter aortic valve implantation: the United Kingdom TAVI Registry. Int J Cardiol. 2015;199:253–260. [DOI] [PubMed] [Google Scholar]
- 46. Stefanini GG, Stortecky S, Cao D, Rat‐Wirtzler J, O'Sullivan CJ, Gloekler S, Buellesfeld L, Khattab AA, Nietlispach F, Pilgrim T, Huber C, Carrel T, Meier B, Jüni P, Wenaweser P, Windecker S. Coronary artery disease severity and aortic stenosis: clinical outcomes according to SYNTAX score in patients undergoing transcatheter aortic valve implantation. Eur Heart J. 2014;35:2530–2540. [DOI] [PubMed] [Google Scholar]
- 47. Ussia GP, Barbanti M, Colombo A, Tarantini G, Petronio AS, Ettori F, Ramondo A, Santoro G, Klugmann S, Bedogni F, Antoniucci D, Maisano F, Marzocchi A, Poli A, De Carlo M, Fiorina C, De Marco F, Napodano M, Violini R, Bortone AS, Tamburino C. Impact of coronary artery disease in elderly patients undergoing transcatheter aortic valve implantation: insight from the Italian CoreValve Registry. Int J Cardiol. 2013;167:943–950. [DOI] [PubMed] [Google Scholar]
- 48. Kleczynski P, Dziewierz A, Bagienski M, Rzeszutko L, Sorysz D, Trebacz J, Sobczynski R, Tomala M, Gackowski A, Dudek D. Impact of coronary artery disease burden on 12‐month mortality of patients after transcatheter aortic valve implantation. J Interv Cardiol. 2016;29:375–381. [DOI] [PubMed] [Google Scholar]
- 49. Van Mieghem NM, van der Boon RM, Faqiri E, Diletti R, Schultz C, van Geuns R‐J, Serruys PW, Kappetein A‐P, van Domburg RT, de Jaegere PP. Complete revascularization is not a prerequisite for success in current transcatheter aortic valve implantation practice. JACC Cardiovasc Interv. 2013;6:867–875. [DOI] [PubMed] [Google Scholar]
- 50. Goel SS, Agarwal S, Tuzcu EM, Ellis SG, Svensson LG, Zaman T, Bajaj N, Joseph L, Patel NS, Aksoy O, Stewart WJ, Griffin BP, Kapadia SR. Percutaneous coronary intervention in patients with severe aortic stenosis: implications for transcatheter aortic valve replacement. Circulation. 2012;125:1005–1013. [DOI] [PubMed] [Google Scholar]
- 51. Khawaja MZ, Wang D, Pocock S, Redwood SR, Thomas MR. The percutaneous coronary intervention prior to transcatheter aortic valve implantation (ACTIVATION) trial: study protocol for a randomized controlled trial. Trials. 2014;15:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Assessment of Study Quality Using the Newcastle Ottawa Scale
Figure S1. Funnel plot for all‐cause mortality at 30 days after transcatheter aortic valve replacement (TAVR).
Figure S2. Funnel plot for all‐cause mortality at 1 year after transcatheter aortic valve replacement (TAVR).
Figure S3. Sensitivity analysis for all‐cause mortality at 30 days.
Figure S4. Sensitivity analysis for all‐cause mortality at 1 year.


