Abstract
Background
Loop diuretics are highly natriuretic but their short duration of action permits postdiuretic sodium retention, which limits salt loss unless dietary salt is severely restricted. We tested the hypothesis that a more prolonged duration of action would enhance salt loss.
Methods and Results
Ten healthy participants were crossed over between 20 mg of oral immediate‐release or extended‐release (ER) torsemide while consuming a fixed diet with 300 mmol·d−1 of Na+. Compared with immediate‐release, plasma torsemide after ER was 59% lower at 1 to 3 hours but 97% higher at 8 to 10 hours as a result of a >3‐fold prolongation of time to maximal plasma concentrations. The relationship of natriuresis to log torsemide excretion showed marked hysteresis, but participants spent twice as long with effective concentrations of torsemide after ER, thereby enhancing diuretic efficiency. Compared with immediate‐release, ER torsemide did not reduce creatinine clearance and increased fluid (1634±385 versus 728±445 mL, P<0.02) and Na+ output (98±15 versus 42±17 mmol, P<0.05) despite an 18% reduction in exposure. Neither formulation increased K+ excretion.
Conclusions
Torsemide ER prolongs urine drug levels, thereby increasing the time spent with effective drug concentrations, reduces postdiuretic Na+ retention, and moderates a fall in glomerular filtration rate. It caused significant Na+ loss even during very high salt intake. Thus, a short duration of action limits salt loss with loop diuretics. These conclusions warrant testing in subjects with edema and heart failure.
Keywords: diuretics, heart failure, kidney, sodium
Subject Categories: Basic Science Research, Translational Studies, Clinical Studies
Clinical Perspective
What Is New?
This crossover study in 10 normal volunteers consuming a high salt intake revealed that an extended‐release formulation of torsemide doubled the daily loss of sodium and fluid compared with the standard immediate‐release preparation.
What Are the Clinical Implications?
This is the first demonstration that a single dose of a loop diuretic can cause negative salt balance in persons with a very high salt intake.
Introduction
The prevalence of hypertension, congestive heart failure (CHF), and chronic kidney disease is increasing.1 Cardiovascular disease is the most common cause of death and disability worldwide,2 thereby encumbering a huge economic burden.3 Diuretics are the first line of treatment for these common conditions. The therapeutic effect of diuretics is dependent on a loss of body Na+ and fluid.4 Thus, their effects must be predictable if the burden of cardiovascular disease is to be reduced.
Glomerular filtration rate (GFR) is reduced in most subjects with edematous conditions, mandating the use of loop diuretics since these agents have the most potent acute pharmacological action of natriuresis and diuresis.
Despite their unrivaled acute natriuretic effectiveness, loop diuretics have been inadequate therapeutic agents. They are associated with several adverse effects including electrolyte and metabolic disturbances and reduction in GFR.5, 6, 7, 8, 9, 10 Furosemide causes little reduction in blood pressure (BP) in subjects with hypertension with preserved renal function.11, 12 Loop diuretics are usually preferred for subjects with chronic kidney disease. However, thiazides are usually preferred to treat subjects with hypertension if the GFR is not reduced. Furosemide has been shown to have poor and highly variable bioavailability that is worsened in decompensated CHF,13, 14 and this may account, at least in part, for the unpredictable effects of furosemide in treating subjects with CHF.14 Bumetanide is even more short acting. This has prompted suggestions that furosemide be replaced as the loop diuretic of choice by the more predictable torsemide, which is largely eliminated by the metabolism, has a high bioavailability even in subjects with CHF and chronic kidney disease, and causes little or no hypokalemia.7, 9
The short duration of action of 2 to 4 hours of all loop diuretics after oral dosing is a class defect that can lead to 3 problems. First, the urinary concentration of the loop diuretic resides within the therapeutic range for only a short period.10, 15 Second, the abrupt but short‐lived natriuresis leaves ≈20 hours for the kidney to regain the salt and water lost before the next daily dose.8, 12, 16, 17, 18, 19 These attributes account for the failure of furosemide and bumetanide to cause net Na+ loss over 1 to 3 days of once‐daily administration to normal subjects unless dietary salt is restricted to below 120 mmol·d−1.8, 16, 18 Third, the torrential acute diuresis (“Niagara effect”) can cause incontinence in subjects with impaired bladder control.20
We studied a novel extended‐release (ER) formulation of torsemide that delivers the drug into solution over 8 to 10 hours (Figure 1) to test the hypothesis that the inability of a single dose of a loop diuretic to deplete the body of salt and water during high salt intake is caused by a limited duration of action on the kidney. We compared the effects of 20 mg of oral torsemide as an immediate‐release (IR) or ER formulation given to 10 healthy volunteers consuming a fixed high‐salt diet containing 300 mmol Na+ daily. We measured pharmacokinetics, creatinine clearance (CCR), and patterns of electrolyte and water excretion after drug administration to assess the mechanisms of any differences.
Figure 1.
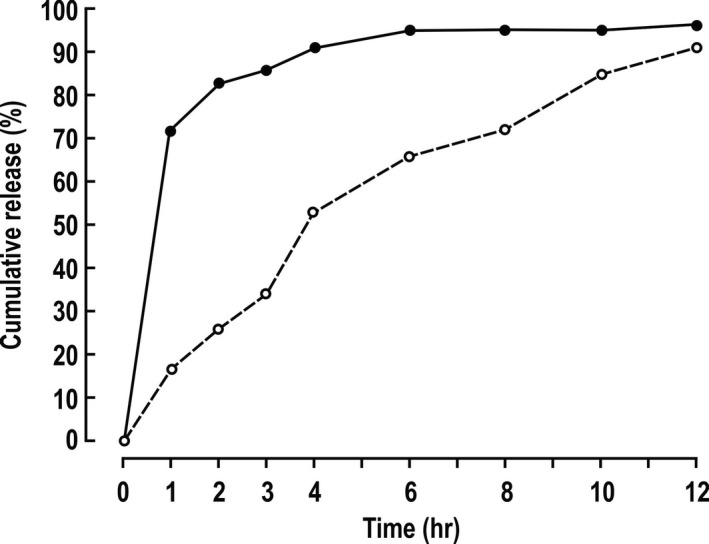
Torsemide dissolution in vitro. Mean values (n=2) for delivery of torsemide from 20 mg tablets into a stirred solution of 0.154 mol·L−1 NaCl at 37°C. Solid circles and continuous lines indicate immediate‐release torsemide; open circles and dashed lines, extended‐release torsemide.
Methods
Drug Formulation
This study compared torsemide IR (Demadex Rx) with a novel release (ER) formulation prepared by Sarfez, Inc as a matrix‐based ER formulation with specific ratios of hydroxypropyl methylcellulose to microcrystalline cellulose. The IR preparation delivered >80% of the drug into solution within 1 to 2 hours, whereas the ER formulation released this fraction over 10 to 12 hours (Figure 1).
Study Participants
Ten healthy volunteers, aged 21 to 45 years, were recruited. Each gave informed consent to participate. The study was passed by the LiLine Hospital ethics committee, Basaveshwara Nagar, Bangalore‐560079. It was assigned the Drug Controller General of India (equivalent of the US Foods Holding Corp) No. T‐BE‐296/13. Participants were not selected on the basis of sex or race/ethnic background. They had no significant medical history, were not taking medications, and had normal values for serum urea nitrogen, serum creatinine, plasma electrolytes, liver function tests, hemogram, and urinalysis. All participants had a BP <140/90 mm Hg. Their body weight ranged from 61.2 to 73.0 kg.
Trial Design
Each participant received both of the torsemide formulations in a randomized crossover design separated by a 3‐week washout period. The participants, the investigators, and those analyzing the results were unaware of the allocation to IR or ER formulation.
Subjects were preconsented, admitted to the study unit, and received fixed constant daily meals for 3 days containing 300 mmol of Na+ and 45 mmol of K+. Subjects remained in the metabolic department throughout the study period without visitors. Each meal was observed to ensure that all of the food was consumed. This provided strict control of Na+ and K+ intakes. Fluid was allowed ad libitum. BP and heart rate were measured using an automated device after 2 minutes of sitting. Subjects fasted for 12 hours prior to receiving the diuretic on day 3, and for 4 hours thereafter to allow for pharmacokinetic studies in the fasting state. To compensate for reduced salt intake (50 mmol of Na+) during the breakfast period, they received 233 mL of 0.154 mol·L−1 saline solution immediately before drug administration. During day 2 and 3 (the day before and the day of the diuretic), subjects collected a 24‐hour urine sample with additional recordings of timed excretion after the diuretic. Immediately before ingestion, and for 24 hours thereafter, blood and urine samples were taken at designated times. Aliquots of 2 mL of the urine were taken for analysis and the remainder added to a 24‐hour collection. Blood and urine were sampled at 30‐minute intervals for 3 hours after diuretic administration (0–3 hours), then hourly (3–4 hours), then 2 hourly (4–14 hours), and finally at 14 and 23 hours. At zero time, they received 20 mg of torsemide (IR or ER) with 330 mL of water. After completion of the study, subjects' BP and heart rate were recorded in the sitting position, and the subjects were then discharged.
Analyses
Urine samples were measured for volume and 1 mL aliquots taken for measurement of Na+, K+, and creatinine concentrations in an automated apparatus and 1 mL for torsemide concentration. Plasma and urine samples were extracted and analyzed for torsemide by a validated capillary zone electrophoresis method that recorded no signal in predrug samples of urine and plasma.21
Statistical Analysis
Mean±SEM data were calculated for each drug period in each individual. To test the hypothesis, within‐subject paired t tests were used to assess differences in 24‐hour excretion of Na+ and fluid after the IR versus ER preparations. The multiple P values reported for differences in parameters at different times after torsemide ER and IR were considered descriptive of the patterns of change observed after the 2 formulations. A P<0.05 was considered statistically significant.
Results
All 10 subjects completed both arms of the trial without any adverse effects.
The patterns of fluid, CCr, and Na+ and K+ excretion following diuretic administration are shown in Figure 2. The rate of fluid excretion and urinary excretion of sodium (UNaV) increased rapidly with the IR and ER preparations to a maximum at 1.0 to 1.5 hours, respectively (Figure 2A and 2C), and remained similar until 2.5 hours, after which the excretions were higher with the ER than IR formulations, until ≈12 hours when excretion was low with both formulations. The rate of fluid excretion and UNaV fell below prior levels by 4 hours after the IR but not until 12 hours after the ER administration. Potassium excretion increased sharply with both preparations and remained elevated for ≈4 hours (Figure 2D) but, after 12 hours, fell to levels mostly below the prior day in both groups. There was an initial sharp increase in CCr during the first 0.5 to 1 hour after administration of both IR and ER torsemide (Figure 2B), but this returned promptly to baseline and was reduced below baseline at 2 hours, where it largely remained thereafter.
Figure 2.
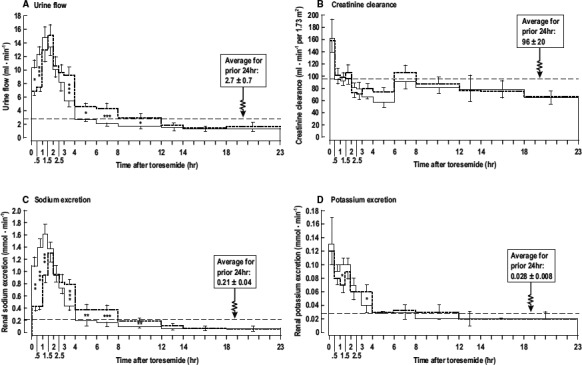
Fluid and electrolyte excretion and creatinine clearance after torsemide. Mean±SEM values (n=10 per group) comparing responses to 20 mg of torsemide immediate‐release (continuous lines) or extended‐release (dashed lines). A, urine flow; (B) creatinine clearance; (C) sodium excretion; (D) potassium excretion. The mean values for the previous 24 hours are indicated by the horizontal dotted lines. Comparing values at the same time points after dosing with immediate‐release torsemide or extended‐release torsemide: *P<0.05; **P<0.01; *** P<0.005.
The pharmacokinetic data are shown in the Table. Compared with IR, the peak serum concentration with ER was reduced by 69% and the overall area under the curve (AUC) was reduced by 18% to 21%. The time to reach peak serum concentration of ER was prolonged >3‐fold. There was a 59% reduction in AUC from 1 to 3 hours but a 97% increase in AUC from 8 to 10 hours.
Table 1.
Pharmocokinetic Parameters After Administration of Torsemide
| IR or ER Torsemide | |||
|---|---|---|---|
| Parameter | IR | ER | P Value |
| CMAX, ng·mL−1 | 2962±412 | 905±93 | <0.001 |
| AUC0‐t, h*·ng·mL−1 | 6493±688 | 5125±552 | <0.001 |
| AUCo‐inf, h*·ng·mL−1 | 6728±704 | 5543±565 | <0.001 |
| Tmax, h | 1.03±0.13 | 3.53±0.27 | <0.001 |
| AUC1–3, h*·ng·mL−1 | 2966±294 | 1225±161 | <0.001 |
| AUC8–10, h*·ng·mL−1 | 203±32 | 400±50 | <0.001 |
Mean±SEM values (n=10 per group). AUC indicates area under the curve; cmax, peak serum concentration; ER, extended‐release; IR, immediate‐release; Tmax, time to reach peak serum concentration.
The plasma torsemide concentrations rose rapidly after IR to peak within 1 hour, but the peak was delayed to ≈3.5 hours after ER (Figure 3A). Thereafter, the concentrations declined log‐linearly but were several‐fold higher after ER throughout the remainder of the day. Renal torsemide excretion followed a similar time course (Figure 3B). The relationship of increases in UNaV above basal values to the log of renal torsemide excretion (an index of natriuretic effect related to the delivery of drug to its active site) showed marked hysteresis (Figure 3C). There was a sharp rise in UNaV with log torsemide excretion during the initial ascending phase followed by an inflection and a much reduced natriuresis relative to torsemide excretion during the declining phase. Subjects spent twice as long in the early ascending phase after torsemide ER than IR. This resulted in a greater natriuretic efficiency as indexed by change in Na+ excreted per log torsemide excreted (Figure 3D).
Figure 3.
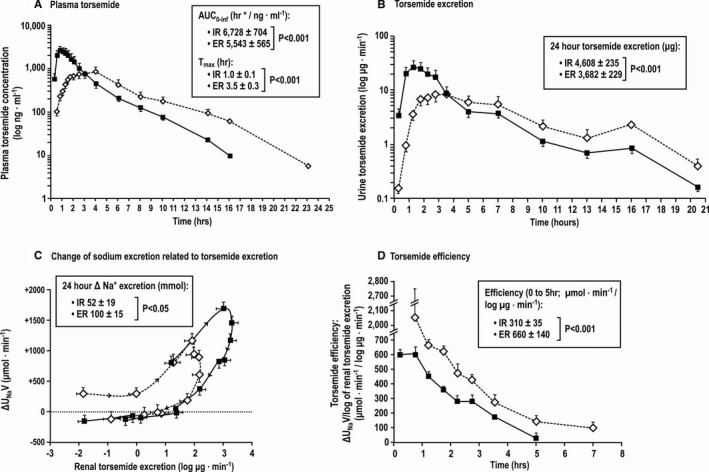
Torsemide kinetics and relationships to natriuresis. Mean (±SEM values) for plasma torsemide concentration (A), renal torsemide excretion (B), changes in sodium excretion related to renal torsemide excretion (log scale) (C), and torsemide natriuretic efficiency (D) comparing immediate‐release (IR) torsemide (solid circles) with extended‐release (ER) torsemide (open circles).
The mean changes for 24 hours after torsemide IR or ER, compared with the prior 24 hours, and the mean differences between the changes produced by the two formulations are shown in Figure 4. Fluid excretion was not significantly changed after IR but was increased after ER, resulting in a 2.2‐fold greater fluid loss of 906 mL (Figure 4A). The UNaV was increased after both IR and ER but was 2.2‐fold greater after ER (Figure 4B). The increase in UnaV did not differ whether torsemide ER or IR were given as first exposure or after the 3‐week washout period. The UnaV with torsemide ER on first and second exposure was +89±16 versus +106±18 and with torsemide IR was +48±18 versus +37±20 mmol·d−1. Neither drug changed 24‐hour potassium excretion (Figure 4C). The CCr was reduced by 25% following IR but was not significantly changed following ER (Figure 4D). Body weight was reduced only after the ER (Figure 4E). Neither IR nor ER changed mean BP, but there was a significant difference between the two with a small net fall of 4 mm Hg after ER versus IR (Figure 4F).
Figure 4.
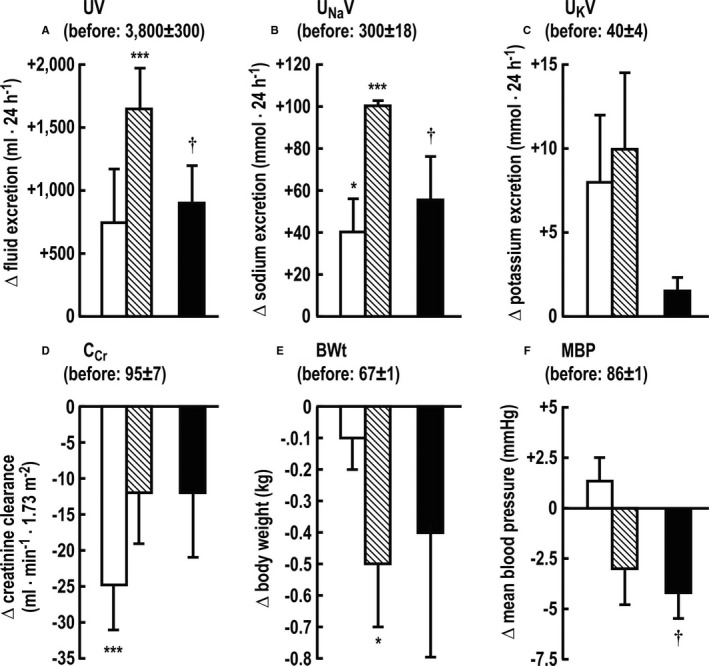
Excretion of fluid (UV; A), sodium (UNaV; B) and potassium (UKV; C); creatinine clearance (CCR; D); body weight (BWt; E); and mean blood pressure (MBP; F). Individual paired values for the changes in the 24 hours after torsemide from the prior 24 hours, comparing immediate‐release (IM) (open boxes) and extended‐release (ER) (crosshatched boxes) torsemide and differences (solid boxes) in the changes produced by torsemide ER vs IR. Significance of difference from prior, prediuretic day: *P<0.05; ***P<0.005. Significance of difference between changes with ER vs IR: † P<0.05.
Discussion
The main new findings from this study of healthy subjects are that a novel ER formulation of torsemide that delivers the drug into solution over 10 to 12 hours prolonged the natriuresis and diuresis and doubled the loss of fluid, body weight, and Na+ without a significant fall in GFR. The diuresis was followed by sustained renal fluid and Na+ retention, but this postdiuretic period was shortened after torsemide ER from ≈18 to ≈12 hours. Neither formulation led to a significant loss of potassium. The ER formulation prolonged the time to maximal plasma torsemide concentration by 3.5‐fold, with a corresponding reduction in torsemide AUC at 1 to 3 hours of 59%, but a doubling of AUC at 8 to 10 hours. The overall exposure to torsemide ER was reduced by 18%. The more gradual rise to peak renal torsemide excretion prolonged the time that the drug remained in the highly effective ascending phase of the hysteretic relationship between increase in urinary Na+ excretion above basal and log renal torsemide excretion, thereby increasing Na+ excreted per unit torsemide excreted (natriuretic efficiency). Thus, torsemide ER reduced postdiuretic Na+ reclamation, maintained GFR, and enhanced natriuretic efficiency that together resulted in greater Na+, fluid, and body weight loss after a single dose despite a somewhat reduced bioavailability.
The daily Na+ intake of 300 mmol matched prior studies with furosemide.8, 16 The IR formulation of torsemide (20 mg) did not induce fluid or weight loss and led to a modest net Na+ loss of 42 mmol. Similar studies with furosemide (40 mg) or bumetanide (1 mg) demonstrated no overall fluid, weight, or Na+ loss at 24 hours after dosing8, 16 despite similar initial large increases in Na+ excretion. The significant, albeit modest, Na+ loss with torsemide IR in this study may relate to the slightly increased duration of natriuresis of ≈4 hours compared with 2 to 3 hours after furosemide or bumetanide.
Two factors have been identified to account for the failure of loop diuretics to induce a consistent loss of Na+ and fluid without dietary salt restriction. First, Brater and colleagues15 related this to the limited time during which the urinary diuretic concentrations are within the 25% to 75% of maximal effective concentration (“most efficient” concentration). This period was increased from ≈2 hours after torsemide IR to ≈4 to 8 hours after torsemide ER (Figure 2). Second is a prolonged postdiuretic period of Na+ and fluid retention8, 16 that can fully offset even an intense initial short‐lived natriuresis unless dietary salt is restricted.8, 16 While some negative Na+ balance occurs with moderate (120 mmol·d−1) or severe (20 mmol·d−1) restriction of dietary Na+,18 this is difficult to achieve in clinical practice. This may account for the disappointing effects of loop diuretics as antihypertensive agents11 or as drugs to treat uncompensated CHF.9 The present findings that an ER formulation of torsemide induced fluid, body weight, and Na+ loss, despite a 300 mmol·d−1 Na+ intake, suggests that this may be the first loop diuretic formulation that does not require dietary salt restriction and monitoring of Na+ intake (from Na+ excretion) for efficacy.
Hypokalemia is a prominent adverse effect of furosemide, bumetanide, and thiazide diuretics. Torsemide does not routinely reduce serum potassium concentration (SK) and prevents thiazide‐induced K+ loss in rat models.22 Neither formulation of torsemide increased K+ excretion significantly in this study. The absence of kaliuresis after torsemide administration may relate to blockade of the mineralocorticosteroid receptors23 or reduction of aldosterone secretion.24 The absence of hypokalemia and the high and predictable bioavailability have led to the suggestion that torsemide be the preferred loop diuretic.7, 9, 13, 25 Indeed, subjects randomized to torsemide compared with furosemide for uncompensated CHF had a reduced rate of readmission for recurrent CHF, and a more cost‐effective treatment.25
The remarkable and rapid development of within‐dose tolerance may underlie the modest or indeed nonsignificant increase in Na+ excretion with a continuous intravenous infusion of a loop diuretic compared with an equivalent single oral dose.26, 27 Thus, intravenous infusion is not a reliable strategy to enhance natriuresis despite continuous delivery of the diuretic to its site of action.
Furosemide given to healthy subjects reduces 24‐hour CCr by ≈23%,8 which is comparable to the pattern observed after torsemide IR in this study. A fall in GFR compromises the fluid and salt‐depleting actions of the diuretic.28 The cause for the fall in GFR is unclear,5, 11 but the GFR was better preserved after ER torsemide in this study. Whether this will translate into better preservation of renal function during long‐term therapy with torsemide ER requires further study.
Previous studies have reported that a slow‐release formulation of furosemide has a somewhat improved antihypertensive efficacy29, 30, 31, 32, 33 but the low and variable bioavailability is worsened, which led to its discontinuation. Two ER formulations of torsemide have been developed. One was abandoned and the other improved antihypertensive efficacy only modestly and failed to improve fluid or electrolyte excretion likely because it prolonged the time to reach peak serum concentration only slightly.34, 35, 36
Study Limitations
We acknowledge some limitations of this study. First, participants were equilibrated to Na+ intake over 2 days, whereas we had previously used 3 days.8, 16, 17 However, during prolonged fixed levels of Na+, the individual 24‐hour Na+ excretion varies considerably.37, 38, 39 Importantly, the daily meals were identical during the two phases of the protocol. Therefore, differences in Na+ excretion with torsemide ER versus IR cannot be ascribed to differences in Na+ intake. Second, this study has a limited sample size of 10 participants. It should be followed up in a larger study of a group of subjects with edema.
Conclusions
An ER formulation of torsemide that increases drug delivery into solution from 2 to 12 hours doubled daily fluid and Na+ loss and mitigated significant reductions in GFR. Further studies over a more prolonged period in target patient populations will be required to test whether these short‐term beneficial effects in healthy subjects translate into enhanced therapeutic efficacy.
Author Contributions
The study was planned and overseen by Drs Shah and Wilcox and the data were analyzed and the first draft of the article was written by Dr Wilcox.
Sources of Funding
This work was supported in part by a Small Business Innovation Research Grant grant from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (#R43DK098856) to Sarfez Pharmaceuticals, Inc.
Disclosures
Dr Shah is the Founder and Director of Sarfez Inc and Dr Feig is the Chief Medical Officer. Dr Wilcox is the Chief Scientific Advisor of Sarfez Inc; Dr Pitt is a consultant and he and Dr Brater contributed to the study design and interpretation. Drs Wilcox and Shen have received no remuneration from the company.
(J Am Heart Assoc. 2017;6:e006135 DOI: 10.1161/JAHA.117.006135.)
References
- 1. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. [DOI] [PubMed] [Google Scholar]
- 2. Wang H, Dwyer‐Lindgren L, Lofgren KT, Rajaratnam JK, Marcus JR, Levin‐Rector A, Levitz CE, Lopez AD, Murray CJ. Age‐specific and sex‐specific mortality in 187 countries, 1970–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2071–2094. [DOI] [PubMed] [Google Scholar]
- 3. Chaker L, Falla A, van der Lee SJ, Muka T, Imo D, Jaspers L, Colpani V, Mendis S, Chowdhury R, Bramer WM, Pazoki R, Franco OH. The global impact of non‐communicable diseases on macro‐economic productivity: a systematic review. Eur J Epidemiol. 2015;30:357–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vasavada N, Agarwal R. Role of excess volume in the pathophysiology of hypertension in chronic kidney disease. Kidney Int. 2003;64:1772–1779. [DOI] [PubMed] [Google Scholar]
- 5. Wilcox CS. Metabolic and adverse effects of diuretics. Semin Nephrol. 1999;19:557–568. [PubMed] [Google Scholar]
- 6. Wilcox CS. New insights into diuretic use in patients with chronic renal disease. J Am Soc Nephrol. 2002;13:798–805. [DOI] [PubMed] [Google Scholar]
- 7. Wargo KA, Banta WM. A comprehensive review of the loop diuretics: should furosemide be first line? Ann Pharmacother. 2009;43:1836–1847. [DOI] [PubMed] [Google Scholar]
- 8. Wilcox CS, Mitch WE, Kelly RA, Skorecki K, Meyer TW, Friedman PA, Souney PF. Response of the kidney to furosemide. I. Effects of salt intake and renal compensation. J Lab Clin Med. 1983;102:450–458. [PubMed] [Google Scholar]
- 9. Pitt B, Nicklas J. Loop diuretics in patients with heart failure: time to change to torsemide? J Cardiovasc Pharmacol. 2009;53:435–437. [DOI] [PubMed] [Google Scholar]
- 10. Brater DC. Clinical pharmacology of loop diuretics. Drugs. 1991;41(suppl 3):14–22. [DOI] [PubMed] [Google Scholar]
- 11. Araoye MA, Chang MY, Khatri IM, Freis ED. Furosemide compared with hydrochlorothiazide. Long‐term treatment of hypertension. JAMA. 1978;240:1863–1866. [PubMed] [Google Scholar]
- 12. Loon NR, Wilcox CS, Unwin RJ. Mechanism of impaired natriuretic response to furosemide during prolonged therapy. Kidney Int. 1989;36:682–689. [DOI] [PubMed] [Google Scholar]
- 13. Sica DA. Pharmacotherapy in congestive heart failure: drug absorption in the management of congestive heart failure: loop diuretics. Congest Heart Fail. 2003;9:287–292. [DOI] [PubMed] [Google Scholar]
- 14. Vargo DL, Kramer WG, Black PK, Smith WB, Serpas T, Brater DC. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clin Pharmacol Ther. 1995;57:601–609. [DOI] [PubMed] [Google Scholar]
- 15. Ferguson JA, Sundblad KJ, Becker PK, Gorski JC, Rudy DW, Brater DC. Role of duration of diuretic effect in preventing sodium retention. Clin Pharmacol Ther. 1997;62:203–208. [DOI] [PubMed] [Google Scholar]
- 16. Kelly RA, Wilcox CS, Mitch WE, Meyer TW, Souney PF, Rayment CM, Friedman PA, Swartz SL. Response of the kidney to furosemide. II. Effect of captopril on sodium balance. Kidney Int. 1983;24:233–239. [DOI] [PubMed] [Google Scholar]
- 17. Wilcox CS, Guzman NJ, Mitch WE, Kelly RA, Maroni BJ, Souney PF, Rayment CM, Braun L, Colucci R, Loon NR. Na+, K+, and BP homeostasis in man during furosemide: effects of prazosin and captopril. Kidney Int. 1987;31:135–141. [DOI] [PubMed] [Google Scholar]
- 18. Wilcox CS, Loon NR, Ameer B, Limacher MC. Renal and hemodynamic responses to bumetanide in hypertension: effects of nitrendipine. Kidney Int. 1989;36:719–725. [DOI] [PubMed] [Google Scholar]
- 19. Almeshari K, Ahlstrom NG, Capraro FE, Wilcox CS. A volume‐independent component to postdiuretic sodium retention in humans. J Am Soc Nephrol. 1993;3:1878–1883. [DOI] [PubMed] [Google Scholar]
- 20. Tannenbaum C, Johnell K. Managing therapeutic competition in patients with heart failure, lower urinary tract symptoms and incontinence. Drugs Aging. 2014;31:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akesolo U, Gonzalez L, Jimenez RM, Alonso RM. Separation of the high‐ceiling diuretic torasemide and its metabolites by capillary zone electrophoresis with diode‐array detection. Electrophoresis. 2002;23:230–236. [DOI] [PubMed] [Google Scholar]
- 22. Knauf H, Mutschler E, Velazquez H, Giebisch G. Torasemide significantly reduces thiazide‐induced potassium and magnesium loss despite supra‐additive natriuresis. Eur J Clin Pharmacol. 2009;65:465–472. [DOI] [PubMed] [Google Scholar]
- 23. Tsutamoto T, Sakai H, Wada A, Ishikawa C, Ohno K, Fujii M, Yamamoto T, Takayama T, Dohke T, Horie M. Torasemide inhibits transcardiac extraction of aldosterone in patients with congestive heart failure. J Am Coll Cardiol. 2004;44:2252–2253. [DOI] [PubMed] [Google Scholar]
- 24. Goodfriend TL, Ball DL, Oelkers W, Bahr V. Torsemide inhibits aldosterone secretion in vitro. Life Sci. 1998;63:Pl45–Pl50. [DOI] [PubMed] [Google Scholar]
- 25. Stroupe KT, Forthofer MM, Brater DC, Murray MD. Healthcare costs of patients with heart failure treated with torasemide or furosemide. Pharmacoeconomics. 2000;17:429–440. [DOI] [PubMed] [Google Scholar]
- 26. Rudy DW, Voelker JR, Greene PK, Esparza FA, Brater DC. Loop diuretics for chronic renal insufficiency: a continuous infusion is more efficacious than bolus therapy. Ann Intern Med. 1991;115:360–366. [DOI] [PubMed] [Google Scholar]
- 27. van Meyel JJ, Smits P, Russel FG, Gerlag PG, Tan Y, Gribnau FW. Diuretic efficiency of furosemide during continuous administration versus bolus injection in healthy volunteers. Clin Pharmacol Ther. 1992;51:440–444. [DOI] [PubMed] [Google Scholar]
- 28. Ronco C, Di Lullo L. Cardiorenal syndrome. Heart Fail Clin. 2014;10:251–280. [DOI] [PubMed] [Google Scholar]
- 29. Hylander B, Danielson M, Eliasson K. Comparison of hydrochlorothiazide and slow release furosemide as adjuvant therapy to beta‐blockers in the treatment of moderate hypertension. Acta Med Scand. 1987;222:137–142. [DOI] [PubMed] [Google Scholar]
- 30. Jorgensen H, Anderssen N, Silsand T, Peterson LE. Long‐term treatment with slow‐release frusemide compared with thiazide treatment in arterial hypertension. J Int Med Res. 1989;17:552–559. [DOI] [PubMed] [Google Scholar]
- 31. Pehrsson SK. Multicentre comparison between slow‐release furosemide and bendroflumethiazide in congestive heart failure. Eur J Clin Pharmacol. 1985;28:235–239. [DOI] [PubMed] [Google Scholar]
- 32. Vermeulen A, Chadha DR. Diuretic effect of slow‐release furosemide in elderly patients. Eur J Clin Pharmacol. 1983;24:449–451. [DOI] [PubMed] [Google Scholar]
- 33. Wakelkamp M, Blechert A, Eriksson M, Gjellan K, Graffner C. The influence of frusemide formulation on diuretic effect and efficiency. Br J Clin Pharmacol. 1999;48:361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roca‐Cusachs A, Aracil‐Vilar J, Calvo‐Gomez C, Vaquer‐Perez JV, Laporta‐Crespo F, Rojas‐Serrano MJ, Guglietta A, Gropper S. Clinical effects of torasemide prolonged release in mild‐to‐moderate hypertension: a randomized noninferiority trial versus torasemide immediate release. Cardiovasc Ther. 2008;26:91–100. [DOI] [PubMed] [Google Scholar]
- 35. Barbanoj MJ, Ballester MR, Antonijoan RM, Puntes M, Gropper S, Santos B, Albet C, Guglietta A. A bioavailability/bioequivalence and pharmacokinetic study of two oral doses of torasemide (5 and 10 mg): prolonged‐release versus the conventional formulation. Clin Exp Pharmacol Physiol. 2009;36:469–477. [DOI] [PubMed] [Google Scholar]
- 36. Barbanoj MJ, Ballester MR, Antonijoan RM, Gich I, Pelagio P, Gropper S, Santos B, Guglietta A. Comparison of repeated‐dose pharmacokinetics of prolonged‐release and immediate‐release torasemide formulations in healthy young volunteers. Fundam Clin Pharmacol. 2009;23:115–125. [DOI] [PubMed] [Google Scholar]
- 37. Lerchl K, Rakova N, Dahlmann A, Rauh M, Goller U, Basner M, Dinges DF, Beck L, Agureev A, Larina I, Baranov V, Morukov B, Eckardt KU, Vassilieva G, Wabel P, Vienken J, Kirsch K, Johannes B, Krannich A, Luft FC, Titze J. Agreement between 24‐hour salt ingestion and sodium excretion in a controlled environment. Hypertension. 2015;66:850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Birukov A, Rakova N, Lerchl K, Engberink RH, Johannes B, Wabel P, Moissl U, Rauh M, Luft FC, Titze J. Ultra‐long‐term human salt balance studies reveal interrelations between sodium, potassium, and chloride intake and excretion. Am J Clin Nutr. 2016;104:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Titze J, Rakova N, Kopp C, Dahlmann A, Jantsch J, Luft FC. Balancing wobbles in the body sodium. Nephrol Dial Transplant. 2016;31:1078–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]


