Abstract
Background
Up to 40% of patients with transvenous implantable cardioverter‐defibrillator (ICD) experience lead‐associated complications and may suffer from high complication rates when lead extraction is indicated. Subcutaneous ICD may represent a feasible alternative; however, the efficacy of the subcutaneous ICD in the detection and treatment of ventricular arrhythmias in patients with hereditary arrhythmia syndromes has not been fully evaluated.
Methods and Results
Patients with primary hereditary arrhythmia syndromes who fulfilled indication for defibrillator placement were eligible for enrollment. Between 2010 and 2016, 62 consecutive patients with primary hereditary arrhythmia syndromes, without indication for antibradycardia therapy, were enrolled in the study. Mean follow‐up was 31.0±14.2 months. The study cohort comprised of 24 patients with Brugada syndrome, 17 with idiopathic ventricular fibrillation, 6 with long‐QT syndrome, 1 with short‐QT syndrome, 3 with catecholaminergic polymorphic ventricular tachycardia, 8 with hypertrophic cardiomyopathy, and 3 with arrhythmogenic right ventricular cardiomyopathy. Thirty‐nine patients were implanted for secondary prevention. Twenty‐two patients had a previous transvenous ICD implanted, but required revision because of infection or lead defects. A total of 20 spontaneous ventricular tachyarrhythmias requiring shock intervention occurred in 10 patients during follow‐up. All episodes were terminated within the first ICD shock delivery with 80 J. Two patients had inappropriate therapies caused by oversensing following an uneventful implantation. No pocket‐site infections and no premature revisions have occurred during follow‐up.
Conclusions
Our study supports the use of the subcutaneous ICD for both secondary and primary prevention of sudden cardiac death as a reliable alternative to the conventional transvenous ICD.
Keywords: Brugada syndrome, hypertrophic cardiomyopathy, implantable cardioverter‐defibrillator, sudden cardiac death, ventricular tachycardia arrhythmia
Subject Categories: Arrhythmias, Catheter Ablation and Implantable Cardioverter-Defibrillator, Sudden Cardiac Death, Ventricular Fibrillation
Clinical Perspective
What Is New?
Among patients with inherited arrhythmia syndromes, subcutaneous defibrillators showed promising results in detecting and treating ventricular tachyarrhythmias.
The rate of procedure‐related complications and inappropriate therapies was low, which may be explained (in part) by the less‐invasive operating technique, postoperative exercise testing, and continuous development of detection algorithms.
What Are the Clinical Implications?
Patients with inherited arrhythmia syndromes have a long life expectancy and ongoing arrhythmia risk, highlighting the potential risks and benefits of long‐term implantable cardioverter‐defibrillator therapy.
Subcutaneous implantable cardioverter‐defibrillators may be a reliable and durable alternative to the conventional transvenous implantable cardioverter‐defibrillator in prevention of sudden cardiac death in patients with inherited arrhythmia syndromes.
Introduction
The subcutaneous implantable cardioverter‐defibrillator (S‐ICD) is becoming an emerging alternative to transvenous ICD (TV‐ICD) systems. Although proven effective in the prevention of sudden cardiac death, TV‐ICD systems are still associated with considerable morbidity and acute and long‐term procedural risks.1, 2 Specifically, endocarditis, lead dislodgement, and lead failure with inappropriate therapies are only some of the possible relevant complications during long‐term ICD usage. The endovascular implanted ICD leads have been considered the weakest link in the TV‐ICD, with up to 20% annual lead failure rates for 8‐year‐old systems.2 Moreover, their extraction is a complex procedure requiring special technical equipment, and it is also associated with substantial comorbidity and mortality. The long‐term complications associated with intravascular ICD leads have been a rationale to develop the extravascular S‐ICD system. The S‐ICD (Boston Scientific, Natick, MA) senses, detects, and treats ventricular tachycardia/fibrillation (VT/VF), while remaining outside of the cardiac space and vasculature. The largest cohort for comparison to real‐world ICD patients is the pooled data from the IDE (Investigational Device Exemption) study and the EFFORTLESS (Evaluation of Factors Impacting Clinical Outcome and Cost Effectiveness of the S‐ICD) registry.3 On the basis of these findings, current evidence suggests that ideal candidates for the S‐ICD include young patients and those with limited vascular access, channelopathies, congenital heart disease, and past infection of TV‐ICD.
Young ICD indicated patients, such as survivors of sudden cardiac arrest attributed to primary VF or patients with inherited channelopathies are most likely to experience long‐term complications of ICD therapy, because of their mostly normal life expectancy and therefore longer‐than‐usual ICD requirement. Currently, evidence about the capabilities of the S‐ICD in detecting and terminating spontaneous tachyarrhythmias is scarce, and there might be some concern about shock efficacy for the termination of spontaneous VT/VF episodes.
Methods
Patient Selection
From November 2010 until August 2016, a total number of 190 S‐ICD implantations were performed at the University Medical Center Mannheim (Mannheim, Germany). Sixty‐two patients with hereditary arrhythmia syndromes were screened and included in this report. All patients provided written informed consent for participation. Approval for this analysis was obtained from the institutional review board of University Medical Center. The first patient was implanted in August 2011, the last in August 2016. ECG prescreening was performed in supine and standing positions. No screening failures occurred. All patients had normal sinus rhythm at baseline screening and no pacing indication.
Implantation Technique
All implantations were performed by a single operator. Implantation of the pulse generator was performed in the left anterior axillary line, over the sixth rib, beneath the latissimus dorsi muscle. The subcutaneous electrode was placed vertically in the subcutaneous tissue from the pulse generator to the xiphoid and then tunneled in a 90‐degree angle to the manubriosteral junction. The 2‐incision technique was applied in 17 patients. An intraoperative defibrillation test was routinely performed. In case of an ineffective conversion of VF with the first shock delivered at 65 J, reprogramming to reversed polarity and retesting was performed. Devices were programmed to a single (≥240 bpm) or dual zone (190–240 bpm) depending on the underlying diagnosis.
Exercise Screening
Fifty‐six patients (90%) underwent postoperative exercise screening to exclude oversensing of myopotentials. Exercise testing was performed in the upright position on a bicycle ergometer using standard World Health Organization protocols. Workload started at 50 W and increased by 25 W every 120 seconds until the patient was unable to maintain or tolerate the workload. With incremental heart rate increase, all sensing vectors were carefully observed and continuously monitored to determine the optimal discrimination of the QRS complexes and the T wave (R/T‐ratio). Furthermore, we screened for diaphragmatic or myopotential oversensing and evaluated sensing behavior during isometric exercises of the upper extremity. Testing was performed in different body positions, including prone, supine, left/right side, standing, and bending over.
Patient Follow‐up
Enrolled patients were followed quarterly. Device interrogations were performed at each scheduled visit, and additionally if a patient received shocks or if the clinical status of the patient changed.
Spontaneous Events and Therapy Characteristics
Spontaneous events were defined as episodes that triggered device activation with storage of electrograms. Only sustained episodes that led to a discharge of the device were counted as treated episodes. Nonsustained episodes were registered, but were not defined as clinical events. Time to therapy was defined from the onset of arrhythmia until a shock was delivered using electrograms stored by the S‐ICD system. First shock efficacy was defined as conversion of ventricular tachyarrhythmia before the start of a second charge.
Statistical Analysis
Baseline characteristics of patients are presented as percentages for dichotomous variables and mean±SD for continuous variables, unless otherwise indicated. Differences among continuous variables were assessed using Student t test or ANOVA after verification of normal data distribution. Categorical variables were assessed among groups by the chi‐square test or the Fisher exact test, as appropriate. Kaplan–Meier analyses were used to estimate the time to first event for appropriate and inappropriate shocks or complications (infections, electrode failures, and hospitalizations) caused by the S‐ICD. All tests were 2‐sided, and a P value of less than 0.05 was considered statistically significant. All calculations were generated using SPSS (version 22.0; SPSS, Inc, Chicago, IL).
Results
Patient Characteristics and Postoperative Assessment
From November 2010 until August 2016, a total of 190 S‐ICDs were implanted at our institution. Sixty‐two patients (41 male) were identified as patients with inherited arrhythmia syndromes (Table 1). Overall, there were 24 patients with Brugada syndrome, 17 with idiopathic fibrillation, 8 with genetically and clinically proven hypertrophic cardiomyopathy, 6 with long QT syndrome, 1 with short QT syndrome, 3 with catecholaminergic polymorphic VT, and 3 with arrhythmogenic right ventricular cardiomyopathy (Table 2). There were 22 patients (35%) that were previously implanted with TV‐ICD, but had indication for device removal/system change. Among those, 3 patients presented with symptoms of device‐related infection and were therefore explanted before the implantation of the S‐ICD. Nineteen patients had lead‐associated device malfunction. In 5 patients, device removal and lead extraction was performed before S‐ICD implantation, whereas the majority of TV‐ICD patients (n=17) had ≥2 abandoned right ventricular leads.
Table 1.
Demographic Data
| Patients included, n | 62 |
| Male, n (%) | 41 (66) |
| Height, cm | 175.6±8.6 |
| Weight, kg | 79.9±16.5 |
| Body mass index | 26.1±4.8 |
| Age at diagnosis, y | 35±13 |
| Age at implantation, y | 38±13 |
| Left ventricular ejection fraction, % | 58±6 |
| Diagnosis | |
| Brugada syndrome, n (%) | 24 (39) |
| Idiopathic ventricular fibrillation, n (%) | 17 (27) |
| Long‐QT syndrome, n (%) | 6 (10) |
| Short‐QT syndrome, n (%) | 1 (2) |
| Catecholaminergic polymorphic ventricular tachycardia, n (%) | 3 (5) |
| Hypertrophic cardiomyopathy, n (%) | 8 (13) |
| Arrhythmogenic right ventricular dysplasia, n (%) | 3 (5) |
| Secondary preventive ICD indication, n (%) | 39 (63) |
| History of atrial fibrillation, n (%) | 10 (16) |
| History of transvenous ICD placement, n (%) | 22 (35) |
| Infection related cause for S‐ICD implantation, n (%) | 3 (5) |
| Lead‐associated device malfunction, n (%) | 19 (31) |
| No. of patients with abandoned leads, n (%) | 17 (27) |
ICD indicates implantable cardioverter‐defibrillator; S‐ICD, subcutaneous implantable cardioverter‐defibrillator.
Table 2.
Clinical Data at Baseline
| Sinus rhythm at baseline, n (%) | 62 (100) |
| PR interval, ms | 162±42 |
| QRS duration, ms | 102±15 |
| QTc interval, ms | 426±28 |
| Fragmented QRS, n (%) | 18 (29) |
| Brugada type 1 ECG, n (%) | 10 (16) |
| T‐wave abnormalities, n (%) | 25 (40) |
| EP study performed, n (%) | 32 (52) |
| AH interval, ms | 111±31 |
| HV interval, ms | 48±8 |
| ERP AVN (S1 500 ms), ms | 280±70 |
| ERP ventricle (S1 500 ms), ms | 210±20 |
| Inducibility of ventricular arrhythmias, n (%) | 14 (23) |
AVN indicates atrioventricular node; EP, electrophysiology; ERP, effective refractory period.
All patients were eligible for the S‐ICD, with 46% having all 3 qualifying sensing vectors, 29% having 2 qualifying sensing vectors, and 25% having only 1 qualifying sensing vector. The primary sensing vector was the most compatible (82%), followed by the secondary vector (75%) and the alternate vector (65%). No screening failures occurred. Mean procedure duration was 49±13 minutes. Following successful implantation, VF was induced in all patients (50‐Hz stimulation between shock coil and generator) and successfully terminated by a 65‐J shock in 58 of 62 cases within the first therapy. Failure to terminate VF within the first shock could be resolved in 4 cases by changing the shock vector to reversed polarity (Table 3). No interaction between the abandoned right ventricular leads and first shock efficacy was observed.
Table 3.
Implant‐Procedure–Related Data
| Procedure duration, min | 50.0±13.4 |
| DFT shock impedance, Ohm | 77.6±25.5 |
| DFT first shock effective, n (%) | 58 (93) |
| Sensing vector | |
| Primary, n (%) | 28 (45) |
| Secondary, n (%) | 26 (42) |
| Alternate, n (%) | 8 (13) |
| Vector change based on postoperative exercise, n | 7 (12) |
DFT indicates defibrillation threshold.
Device Programming
Single‐zone programming with cutoff at >240 bpm was set up in 34 of 62 patients, whereas a dual‐zone programming was set up in the remainder of 28 patients (conditional zone 190 bpm, shock zone 240 bpm). Primary vector was programmed in 28 patients (45%), secondary vector in 26 (42%), and alternate vector in 8 (13%) patients. In 56 of 62 patients (90%), a postoperative exercise test was performed in order to determine the optimal sensing vector during daily life activity. In 7 patients, a change of the permanent sensing vector was programmed based on the information obtained during the exercise test at the discretion of the investigator. The main reason was a decreased R/T‐ratio during sinus tachycardia (Table 3).
Follow‐up Data
Demographic and clinical data are presented in Tables 1 and 2. Mean age at diagnosis was 35.8±12.4 years; mean age at implantation was 37.8±13.4 years. Mean follow‐up (FUP) was 35.6±16.2 months. FUP data of at least 12 months were available in 94% of included patients. There were 23 patients with primary (37%) and 39 with secondary ICD indication. In the subgroup of patients with secondary ICD indication, no stable monomorphic VT (<170 beats/min) was recorded. Ten patients had a history of clinical atrial fibrillation. Twenty‐two patients (35%) were previously implanted with a TV‐ICD. Of these patients, 12 had a history of inappropriate ICD therapy and 3 an indication for premature ICD revision.
Clinical Episodes of Ventricular Arrhythmias
There were 20 discrete spontaneous episodes of VT/VF recorded in 10 patients that required defibrillator therapy (Table 4). Nine of 10 patients that had ventricular tachyarrhythmia during FUP had a second prevention ICD indication. Mean arrhythmia cycle length was 220±33 ms. Mean time to therapy was 19.0±2.8 seconds. All 20 events were effectively converted within the first shock (100% first shock efficacy at 80 J). Twelve of 20 episodes were accompanied with syncopal symptoms and loss of consciousness. Six episodes were associated with arrhythmia prodromes (palpitations, presyncope) preceding the defibrillation, whereas 2 episodes occurred during sleep and did not cause symptoms before the ICD shock. There was no difference in arrhythmia cycle length among patients with single‐zone and dual‐zone programming (219±33 versus 229±34 ms; P=0.6). Similarly, time to therapy was not different among patients with single‐zone programming as compared to patients with dual‐zone programming (18.9±1.7 versus 19.3±3.4 seconds; P=0.7). Two patients that received appropriate therapy during FUP had abandoned right ventricular leads that, however, did not interfere with shock efficacy. There were no pocket‐site infections or lead failures that caused premature surgical revision of the pulse generator or the defibrillator lead. Table 5 displays the specific characteristics of patients with arrhythmic episodes, and Figure 1 graphically illustrates the frequency and cycle length of all spontaneous arrhythmias. Event‐free survival during follow‐up is depicted in Figure 2.
Table 4.
Follow‐up Data
| Follow‐up duration, mo | 31.0±14.2 |
| Appropriate ICD shocks, n | 20 |
| No. of patients with appropriate shocks, n (%) | 10 (16) |
| Cycle length of arrhythmia, ms | 222±33 |
| Time to shock, sec | 19.0±2.2 |
| First shock effective, n (%) | 20 (100) |
| Programmed therapy zones | |
| Single (>240 bpm), n (%) | 34 (55) |
| Dual (190–240 bpm/>240 bpm), n (%) | 28 (45) |
| Inappropriate ICD shocks, n | 4 |
| Number of patients with inappropriate shocks, n (%) | 2 (3) |
ICD indicates implantable cardioverter‐defibrillator.
Table 5.
Characteristics of Patients With Arrhythmic Episodes
| Episode | Patient No | Disease | CL (ms) | TTT (Second) |
|---|---|---|---|---|
| 1 | 1 | IVF | 220 | 18.6 |
| 2 | 2 | IVF | 200 | 18.0 |
| 3 | 2 | IVF | 210 | 20.8 |
| 4 | 2 | IVF | 200 | 19.6 |
| 5 | 3 | IVF | 260 | 14.6 |
| 6 | 4 | BRUGADA | 180 | 16.7 |
| 7 | 4 | BRUGADA | 180 | 17.5 |
| 8 | 4 | BRUGADA | 190 | 18.0 |
| 9 | 4 | BRUGADA | 210 | 17.0 |
| 10 | 4 | BRUGADA | 180 | 20.0 |
| 11 | 5 | BRUGADA | 310 | 23.0 |
| 12 | 6 | IVF | 200 | 19.4 |
| 13 | 7 | HCM | 270 | 23.8 |
| 14 | 8 | BRUGADA | 240 | 21.8 |
| 15 | 8 | BRUGADA | 230 | 20.2 |
| 16 | 8 | BRUGADA | 220 | 18.0 |
| 17 | 9 | CPVT | 230 | 18.2 |
| 18 | 9 | CPVT | 240 | 19.2 |
| 19 | 9 | CPVT | 240 | 17.8 |
| 20 | 10 | BRUGADA | 220 | 18.4 |
CL indicates cycle length; CPVT, catecholaminergic polymorphic ventricular tachycardia; HCM, hypertrophic cardiomyopathy; IVF, idiopathic ventricular fibrillation; TTT, time to therapy.
Figure 1.
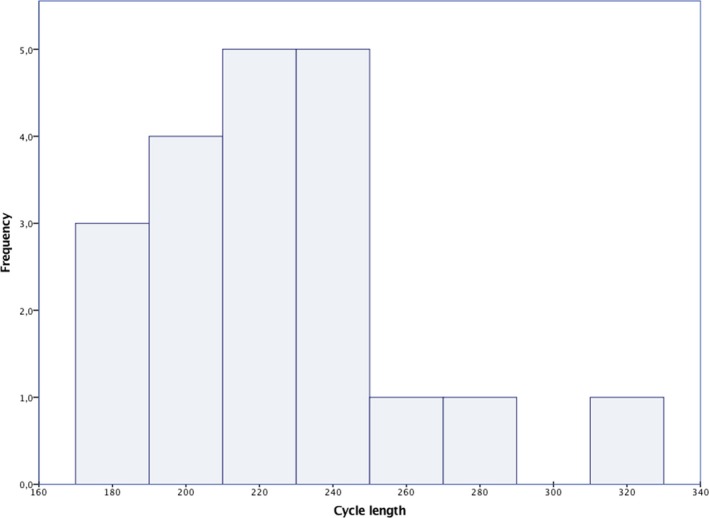
Illustration of all spontaneous episodes based on the cycle length and their frequency.
Figure 2.
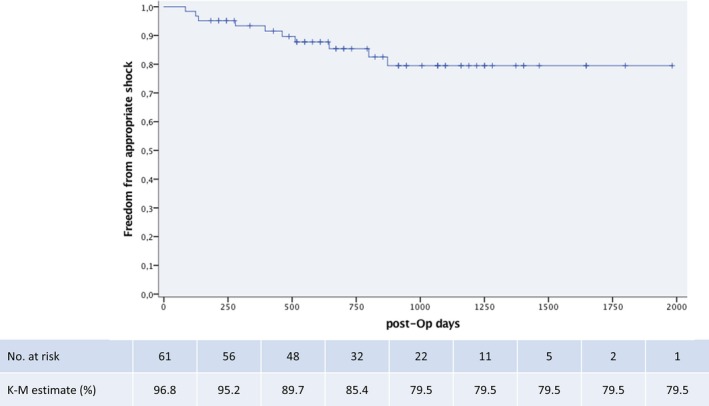
Kaplan–Meier curve showing proportion of patients with event‐free survival. K‐M indicates Kaplan–Meier; post‐Op, postoperative.
Inappropriate Shocks
Two of 62 patients (3.2%) experienced inappropriate shocks. In 1 patient, they occurred during physical effort 35 days after implantation. Interrogation of the S‐ICD showed oversensing of pectoral myopotentials (Figure 3), most likely caused by a suboptimal position of the defibrillator lead, placed toward the right side of the sternum (Figure 4). This was not evidenced by the time of the hospital discharge, because the postoperative exercise test described above was not routinely performed. Manual reprogramming of the sensing vector resolved the problem without the need for surgical revision. The patient remained free of symptoms for 35 months, until he developed VF while sleeping, and was adequately defibrillated by the S‐ICD. The second patient had 2 inappropriate therapies in the first 24 hours following uneventful implantation, most likely caused by air entrapment inside the impulse generator pocket. The issue resolved spontaneously without intervention.
Figure 3.
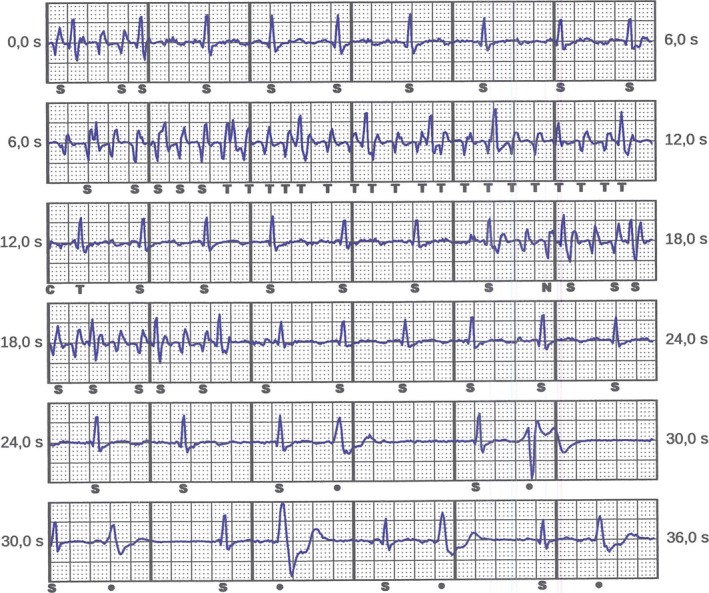
Detection of myopotentials triggering nonsustained oversensing (NSO) by the device.
Figure 4.
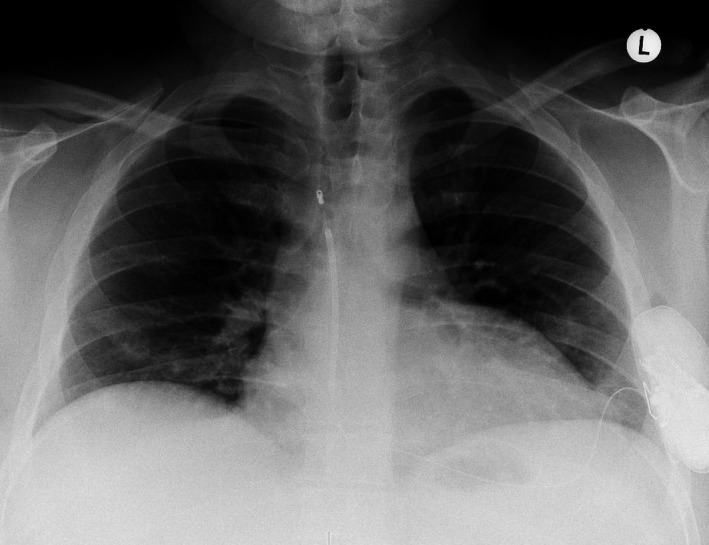
Chest X‐ray showing the final position of the pulse generator and the subcutaneous shock electrode. Upon detection of myopotentials, critical reevaluation identified a suboptimal position of the electrode placed in the right parasternal space.
Therapy Failures and Premature Revisions
No patient has died during FUP. Apart from reported syncopal episodes during VT/VF episodes, no patient reported syncopal episodes during FUP, which may be indicative of undersensed/untreated ventricular arrhythmias. No premature battery depletions or pocket‐site infections have occurred.
Discussion
Main Findings
We present long‐term single‐center FUP data of a prospectively followed cohort of patients with inherited arrhythmia syndromes. Our results demonstrate that the S‐ICD is safe and effective in terminating both induced and spontaneous VF and corroborate results reported by other researchers and larger groups of patients.3, 4 Our population was generally younger with a more‐preserved ejection fraction than those previously reported in prospective ICD trials, also commonly presenting with a rapid unstable VT/VF, rather than monomorphic VT. We included patients with a primary as well as secondary ICD indication. In the secondary prevention group, 9 patients experienced appropriate shocks during the FUP. On the other hand, 1 patient from the primary prevention group had appropriate therapies triggered by fast monomorphic and polymorphic VT.
Of the 321 subjects of the IDE trial who underwent attempted S‐ICD implantation, 7 (2.2%) did not receive the device as a result of incomplete or unsuccessful acute VF conversion testing.5 In contrast, we did not observe screening failures before implantation, despite varying ECG morphologies of the included patients (eg, ST‐segment elevation in Brugada patients, QRS splitting and T‐wave inversions in hypertrophic cardiomyopathy patients). All implantations were successfully performed, and in all patients, VF induction and defibrillation testing was successfully performed (94% first shock termination). These findings are in line with the results published by Bardy et al,6 in which induced VF was converted consecutively with 2 65‐J shocks in 58 of 59 patients (98%).
Clinical Implications and Eligibility for the S‐ICD
Owing to their unique ECG pattern and QRS/T wave ratio, Brugada patients may carry the risk of double counting (QRS/T wave) and inappropriate therapy. QRS morphology can be a challenge, for example, in type‐1 pattern, at the end of QRS there is ascending and quick slope followed by concave downsloping ST segment. Often, there is also a mismatch of QRS duration that is longer in V1 than in leads V5 and V6. Moreover, QRS can be fractionated and type 1 pattern can also be found in peripheral leads in up to 10% of the cases. These peculiarities can lead to double counting and risk of inappropriate shocks. In our study, all patients have passed the QRS morphology screening. Furthermore, the proportion of patients with 1 or more qualifying leads in our study population is comparable to that observed in previous series with different heart diseases.7, 8 Of note, hypertrophic cardiomyopathy was an independent predictor of screening failure in 1,7 but not in other, studies.8, 9 Indeed, the small number of hypertrophic cardiomyopathy patients enrolled in previous studies may explain the discrepancy in eligibility rates. Although we did not observe any screening failures, presence of T‐wave inversions,9 increased body weight7 and prolonged QRS duration8 seem to be associated with screening failure and ineligibility for the S‐ICD.
Appropriate Therapies and Real‐Life Defibrillation Efficacy
There were 20 appropriate therapies that occurred in 10 patients during the FUP. All episodes were successfully terminated within 1 shock at 80 J, yielding in a 100% first shock efficacy, which is comparable with studies with larger patient numbers.3 The majority of patients experienced hemodynamic effects, attributed to a long detection and treatment interval by the S‐ICD (average time to therapy, 19 seconds). Despite the fact that no patient was injured with loss of consciousness before therapy, the longer duration of time to therapy of the S‐ICD needs to be pointed out. In 1 patient, 3 subsequent episodes of VF have occurred within 6 hours, which can be classified as presence of electric storm. It is noteworthy that all episodes have been appropriately detected and adequately terminated by the S‐ICD. Other studies reported lower rates of first shock efficacy.10 Ineffective shock therapy is a life‐threatening situation, rarely noticed with current ICD systems, and can be sporadically observed in patients with ICD lead dislocation,11 hypertrophic cardiomyopathy,12 electrical storm,13 and Brugada syndrome.14 The multicenter study by Aydin et al10 reported outcome of 40 patients implanted in 3 German tertiary centers and based on their findings a first shock efficacy of 57%. Because we can only speculate about the wide discrepancy of the first shock efficacy, larger experience is required to provide confidence around these estimates.
Programming of Therapy Zones
We have programmed the conditional zone in 45% of the patients to 190 bpm (shock zone 240 bpm). The remainder of patients had a single shock zone, which was set to 240 bpm (shock box programming). It is well known that patients with channelopathies are more prone to fast polymorphic VT or VF, rather than slow monomorphic VT, and that programming to a single detection zone with a high VF cut‐off rate is safe and can reduce inappropriate ICD discharges.15 With respect to the preserved left ventricular ejection fraction in our patient population and the low‐risk of slow VT, our results demonstrate that, in all patients without relevant structural heart disease, single‐shock‐zone programming appears to be safe in detection of ventricular arrhythmias.
Inappropriate Therapies
Initially, T‐wave oversensing (TWOS) was considered a major drawback of the S‐ICD, causing a relevant proportion of inappropriate shocks. First S‐ICD studies reported up to 15% yearly rates of inappropriate shocks,4 with TWOS accounting for the vast majority of cases.5 The addition of a conditional tachycardia detection zone (2 zones versus 1 zone programming) significantly decreased the rate of inappropriate shocks attributed to supraventricular rhythms and TWOS.16 Recently, novel algorithms and filters (SMART pass) have been introduced in order to effectively reduce TWOS and myopotentials. In the EFFORTLESS trial, TWOS occurred in 58 of 317 episodes.17 In our cohort of 62 patients, we did not observe TWOS in any patient. There are several potential explanations of this finding. One explanation could be the low number of patients in comparison with the large EFFORTLESS registry. Another reason could be the vigorous exercise testing and adaptation of the sensing vector, based on the R/T ratio during tachycardia, which was routinely performed in the majority of patients after the implantation. Finally, we have programmed a single high‐rate‐therapy zone in the majority of our patients. Although in patients with structural heart disease this might not always be possible, it could have a considerable impact on inappropriate therapies and the occurrence of TWOS.
Additionally, Olde Nordkamp et al showed that programming the primary sensing vector was independently associated with a lower risk of inappropriate shocks.18 In our cohort, primary vector was programmed in the majority of patients (n=15). Interestingly, the only patient with inappropriate shocks had low amplitude oversensing, caused by myopotentials. After reprogramming to secondary vector and addition of software detection algorithms subsequently adopted, no further inappropriate therapies occurred. Three patients had clinical paroxysmal atrial fibrillation; however, no inappropriate shocks occurred.
One study has demonstrated the association between operator experience and the risk of inadequate therapy.4 Compared with our results, we can confirm that, in our cohort, the patient with inappropriate shocks was implanted at the early phase of the S‐ICD and before the introduction of algorithms for prevention of myopotential oversensing, which may also play an important role in the safety of the S‐ICD system.
Limitations
The present study was not a randomized trial. The major limitation is the lack of a control group. Therefore, a comparison of the S‐ICD with TV‐ICD is not possible. This study, however, was not intended to prove the concept of defibrillation in the prevention of sudden cardiac death, which has already been firmly established.19 This study intended to explore safety and efficacy of the S‐ICD in a prespecified cohort of patients, significantly different from those in major ICD trials.19, 20 The duration of the FUP does not take into account the possibility of dynamic progression of conduction disease, which might happen in unknown degrees over time depending on the patient and the substrate. However, with average FUP of nearly 3 years, safety and effectiveness goals with objective performance criteria were achieved, and a considerable number of spontaneous episodes were successfully treated.
Conclusion
The S‐ICD system is safe and effective for detecting and terminating life‐threatening ventricular arrhythmias in patients with inherited channelopathies. Our study supports the use of the S‐ICD for both secondary and primary prevention of sudden cardiac death as a reliable alternative to the conventional TV‐ICD.
Disclosures
Dr. Rudic has received honoraria from Boston Scientific. Dr. Kuschyk is a consultant for and has received grant support from Boston Scientific. All other authors have reported that they have no relationships relevant to the contents of this article to disclose.
(J Am Heart Assoc. 2017;6:e006265 DOI: 10.1161/JAHA.117.006265.)
References
- 1. Alter P, Waldhans S, Plachta E, Moosdorf R, Grimm W. Complications of implantable cardioverter defibrillator therapy in 440 consecutive patients. Pacing Clin Electrophysiol. 2005;28:926–932. [DOI] [PubMed] [Google Scholar]
- 2. Kleemann T, Becker T, Doenges K, Vater M, Senges J, Schneider S, Saggau W, Weisse U, Seidl K. Annual rate of transvenous defibrillation lead defects in implantable cardioverter‐defibrillators over a period of >10 years. Circulation. 2007;115:2474–2480. [DOI] [PubMed] [Google Scholar]
- 3. Burke MC, Gold MR, Knight BP, Barr CS, Theuns DA, Boersma LV, Knops RE, Weiss R, Leon AR, Herre JM, Husby M, Stein KM, Lambiase PD. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2‐year results from a pooled analysis of the IDE Study and EFFORTLESS Registry. J Am Coll Cardiol. 2015;65:1605–1615. [DOI] [PubMed] [Google Scholar]
- 4. Olde Nordkamp LR, Dabiri Abkenari L, Boersma LV, Maass AH, de Groot JR, van Oostrom AJ, Theuns DA, Jordaens LJ, Wilde AA, Knops RE. The entirely subcutaneous implantable cardioverter‐defibrillator: initial clinical experience in a large Dutch cohort. J Am Coll Cardiol. 2012;60:1933–1939. [DOI] [PubMed] [Google Scholar]
- 5. Weiss R, Knight BP, Gold MR, Leon AR, Herre JM, Hood M, Rashtian M, Kremers M, Crozier I, Lee KL, Smith W, Burke MC. Safety and efficacy of a totally subcutaneous implantable‐cardioverter defibrillator. Circulation. 2013;128:944–953. [DOI] [PubMed] [Google Scholar]
- 6. Bardy GH, Smith WM, Hood MA, Crozier IG, Melton IC, Jordaens L, Theuns D, Park RE, Wright DJ, Connelly DT, Fynn SP, Murgatroyd FD, Sperzel J, Neuzner J, Spitzer SG, Ardashev AV, Oduro A, Boersma L, Maass AH, Van Gelder IC, Wilde AA, van Dessel PF, Knops RE, Barr CS, Lupo P, Cappato R, Grace AA. An entirely subcutaneous implantable cardioverter‐defibrillator. N Engl J Med. 2010;363:36–44. [DOI] [PubMed] [Google Scholar]
- 7. Olde Nordkamp LR, Warnaars JL, Kooiman KM, de Groot JR, Rosenmoller BR, Wilde AA, Knops RE. Which patients are not suitable for a subcutaneous ICD: incidence and predictors of failed QRS‐T‐wave morphology screening. J Cardiovasc Electrophysiol. 2014;25:494–499. [DOI] [PubMed] [Google Scholar]
- 8. Randles DA, Hawkins NM, Shaw M, Patwala AY, Pettit SJ, Wright DJ. How many patients fulfil the surface electrocardiogram criteria for subcutaneous implantable cardioverter‐defibrillator implantation? Europace. 2014;16:1015–1021. [DOI] [PubMed] [Google Scholar]
- 9. Groh CA, Sharma S, Pelchovitz DJ, Bhave PD, Rhyner J, Verma N, Arora R, Chicos AB, Kim SS, Lin AC, Passman RS, Knight BP. Use of an electrocardiographic screening tool to determine candidacy for a subcutaneous implantable cardioverter‐defibrillator. Heart Rhythm. 2014;11:1361–1366. [DOI] [PubMed] [Google Scholar]
- 10. Aydin A, Hartel F, Schluter M, Butter C, Kobe J, Seifert M, Gosau N, Hoffmann B, Hoffmann M, Vettorazzi E, Wilke I, Wegscheider K, Reichenspurner H, Eckardt L, Steven D, Willems S. Shock efficacy of subcutaneous implantable cardioverter‐defibrillator for prevention of sudden cardiac death: initial multicenter experience. Circ Arrhythm Electrophysiol. 2012;5:913–919. [DOI] [PubMed] [Google Scholar]
- 11. Veltmann C, Borggrefe M, Schimpf R, Wolpert C. Fatal inappropriate ICD shock. J Cardiovasc Electrophysiol. 2007;18:326–328. [DOI] [PubMed] [Google Scholar]
- 12. Margos PN, Schomburg R, Kynast J, Khattab AA, Richardt G. Termination of ventricular tachycardia with antitachycardia pacing after ineffective shock therapy in an ICD recipient with hypertrophic cardiomyopathy. Indian Pacing Electrophysiol J. 2009;9:64–70. [PMC free article] [PubMed] [Google Scholar]
- 13. Brigadeau F, Kouakam C, Klug D, Marquie C, Duhamel A, Mizon‐Gerard F, Lacroix D, Kacet S. Clinical predictors and prognostic significance of electrical storm in patients with implantable cardioverter defibrillators. Eur Heart J. 2006;27:700–707. [DOI] [PubMed] [Google Scholar]
- 14. Watanabe H, Chinushi M, Sugiura H, Washizuka T, Komura S, Hosaka Y, Furushima H, Watanabe H, Hayashi J, Aizawa Y. Unsuccessful internal defibrillation in Brugada syndrome: focus on refractoriness and ventricular fibrillation cycle length. J Cardiovasc Electrophysiol. 2005;16:262–266. [DOI] [PubMed] [Google Scholar]
- 15. Veltmann C, Kuschyk J, Schimpf R, Streitner F, Schoene N, Borggrefe M, Wolpert C. Prevention of inappropriate ICD shocks in patients with Brugada syndrome. Clin Res Cardiol. 2010;99:37–44. [DOI] [PubMed] [Google Scholar]
- 16. Gold MR, Weiss R, Theuns DA, Smith W, Leon A, Knight BP, Carter N, Husby M, Burke MC. Use of a discrimination algorithm to reduce inappropriate shocks with a subcutaneous implantable cardioverter‐defibrillator. Heart Rhythm. 2014;11:1352–1358. [DOI] [PubMed] [Google Scholar]
- 17. Lambiase PD, Barr C, Theuns DA, Knops R, Neuzil P, Johansen JB, Hood M, Pedersen S, Kaab S, Murgatroyd F, Reeve HL, Carter N, Boersma L; EFFORTLESS Investigators . Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S‐ICD Registry. Eur Heart J. 2014;35:1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olde Nordkamp LR, Brouwer TF, Barr C, Theuns DA, Boersma LV, Johansen JB, Neuzil P, Wilde AA, Carter N, Husby M, Lambiase PD, Knops RE. Inappropriate shocks in the subcutaneous ICD: incidence, predictors and management. Int J Cardiol. 2015;195:126–133. [DOI] [PubMed] [Google Scholar]
- 19. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp‐Channing N, Davidson‐Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH; Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) Investigators . Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 20. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML; Multicenter Automatic Defibrillator Implantation Trial III . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. [DOI] [PubMed] [Google Scholar]


