Abstract
Background
Duchenne muscular dystrophy (DMD) is frequently complicated by development of a cardiomyopathy. Despite significant medical advances provided to DMD patients over the past 2 decades, there remains a group of DMD patients who die prematurely. The current study sought to identify a set of prognostic factors that portend a worse outcome among adult DMD patients.
Methods and Results
A retrospective cohort of 43 consecutive patients was followed in the adult UT Southwestern Neuromuscular Cardiomyopathy Clinic. Clinical data were abstracted from the electronic medical record to generate baseline characteristics. The population was stratified by survival to time of analysis and compared with characteristics associated with death. The DMD population was in the early 20s, with median follow‐up times over 2 years. All the patients had developed a cardiomyopathy, with the majority of the patients on angiotensin‐converting enzyme inhibitors (86%) and steroids (56%), but few other guideline‐directed heart failure medications. Comparison between the nonsurviving and surviving cohorts found several poor prognostic factors, including lower body mass index (17.3 [14.8–19.3] versus 25.8 [20.8–29.1] kg/m2, P<0.01), alanine aminotransferase levels (26 [18–42] versus 53 [37–81] units/L, P=0.001), maximum inspiratory pressures (13 [0–30] versus 33 [25–40] cmH2O, P=0.03), and elevated cardiac biomarkers (N‐terminal pro‐brain natriuretic peptide: 288 [72–1632] versus 35 [21–135] pg/mL, P=0.03].
Conclusions
The findings demonstrate a DMD population with a high burden of cardiomyopathy. The nonsurviving cohort was comparatively underweight, and had worse respiratory profiles and elevated cardiac biomarkers. Collectively, these factors highlight a high‐risk cardiovascular population with a worse prognosis.
Keywords: cardiac biomarkers, cardiomyopathy, Duchenne muscular dystrophy, heart failure therapy, prognostic factors
Subject Categories: Cardiomyopathy, Heart Failure, Biomarkers, Prognosis
Clinical Perspective
What Is New?
In 2017, most Duchenne muscular dystrophy (DMD) patients live into young adulthood, with the majority of these patients developing a cardiomyopathy.
The majority of young adult DMD patients are on angiotensin‐converting enzyme inhibitors and steroids, but few other guideline‐directed heart failure medications.
The current study identified several important factors that are associated with death, including being underweight, and having poor respiratory profiles and elevated cardiac biomarkers.
These factors highlight a high‐risk DMD population with a worse cardiovascular and overall prognosis.
What Are the Clinical Implications?
The growing adult DMD population is a continuing challenge for the adult cardiology community, and studies investigating this groups’ specific clinical characteristics are vital to delivering appropriate care to this high‐risk population.
The current study is an initial step toward recognizing the high‐risk subset of patients within the DMD population, allowing for more intensive and tailored approaches to therapy.
Future studies will expand upon the current findings with prospective, multicenter models to create predictive models and investigate novel approaches to treating DMD‐associated cardiomyopathy.
Ultimately, these future studies will provide better cardiovascular care to this emerging and vulnerable population.
Duchenne muscular dystrophy (DMD) is an X‐linked disorder that affects ≈1 in 3500 to 5000 males born in the United States.1 Traditionally, DMD is a condition primarily affecting children. As recently as 2 decades ago, the life expectancy for this condition was less than 20 years of age. However, over the late 1980s and early 1990s, improvements in home ventilator care and spinal surgery pushed the average age of mortality into the mid to late 20s.2, 3 As a result, the disease course has extended out of the realm of pediatrics and into the adult healthcare system. This improvement in early mortality has led to a change in the mode of death. Whereas before pulmonary complications were the primary driver of mortality, currently the development of a cardiomyopathy is the most prevalent cause of death.
The development of a cardiomyopathy is a well‐defined consequence of DMD. Cardiac abnormalities become noticeable very early in the course of the disease, and by the time patients are 18 years old, the majority will have developed a cardiomyopathy.4 Given the high burden of cardiomyopathy in this population, focused cardiovascular care is now recommended as part of a multidisciplinary approach to DMD treatment.5 Currently, DMD patients are treated in a similar manner to those patients with traditional nonischemic cardiomyopathy.6, 7 Standard guideline‐directed heart failure medical therapy such as angiotensin‐converting enzyme inhibitors,8, 9, 10, 11 angiotensin II receptor blockers,12 β‐blockers,10, 13, 14 and mineralocorticoid receptor antagonists15 have all demonstrated various levels of efficacy at treating DMD‐associated cardiomyopathy. In addition, many of these patients receive steroid therapy, which has been suggested to improve both overall survival and delay progression in the cardiomyopathy.16, 17, 18 In recent years, there has been some very limited data on heart transplantation and durable mechanical circulatory support in DMD patients with severe cardiomyopathy, though overall these types of therapies remain reserved for a minority of patients.19, 20, 21 However, most of these data have been obtained from the pediatric population and, until recently, care of this cohort of patients has been under the purview of pediatric cardiologists. Furthermore, natural history data suggest that the development of a cardiomyopathy is not adequately diagnosed and treated. In 1 study, only 50% of DMD patients over the age of 18 with evidence of cardiomyopathy were on heart failure medications.22 As this population ages, adult cardiologists, especially heart failure–trained cardiologists, will need to become more familiar with these patients and be able to recognize those at high risk for poor outcomes.
The University of Texas Southwestern Medical Center has a robust adult Neuromuscular Cardiomyopathy Clinic and has a National Institutes of Health–funded Wellstone Muscular Dystrophy Cooperative Research Center with a particular emphasis on identifying a therapeutic treatment for DMD. Adult DMD patients are followed and managed by a single heart failure/Ventricular Assist Device/transplant‐trained cardiologist with specific expertise in neuromuscular‐associated cardiomyopathy. In the current study, the aim was to identify poor prognostic factors in DMD patients recently referred to this clinic to receive specialized cardiovascular care. We hypothesized that nonsurviving DMD patients have a set of prognostic factors that portend a worse outcome as compared with the surviving DMD patients. The identification of poor prognostic factors will enable the ability to differentiate DMD patients who are at higher risk of death.
Methods
The current study was undertaken under the auspices of an approved Institutional Review Board protocol and handled in accordance with the University of Texas Southwestern Medical Center's Institutional Guidelines. Since this study was a retrospective cohort study, the requirement for informed patient consent was waived. A retrospective chart review was undertaken on 43 consecutive DMD patients who were followed in the UT Southwestern Neuromuscular Cardiomyopathy Clinic and who had their initial clinic visit between January 1, 2006 and June 30, 2016. These patients were identified using administrative diagnostic codes for DMD supplemented with chart‐based verification by a cardiologist and prior genetic testing from the childhood records. Patients who had at least 6 months of follow‐up in the Clinic were included in the analysis.
All DMD patients who transition from the pediatric clinics to the adult clinics within the UT Southwestern Health Care system are referred to the Adult Neuromuscular Cardiomyopathy Clinic. Prior to the transition, the cardiovascular care of these DMD patients was not standardized. However, once these DMD patients transited to the Adult Neuromuscular Cardiomyopathy Clinic, they all underwent a comprehensive cardiopulmonary assessment utilizing a standard protocol under the care of an American Board of Internal Medicine board‐certified heart failure/transplant cardiologist.
Demographic, laboratory, echocardiographic, electrocardiographic (via a 12‐lead ECG and a 24‐hour Holter monitor), medication, and pulmonary function data obtained at the time of the initial clinic visit were abstracted from the medical records. Echocardiographic data were collected during the course of routine medical care. All images were interpreted by board‐certified cardiologists in accordance with guidelines set by the American Society of Echocardiography.23 The left ventricular ejection fraction was determined by the biplane method of discs in the apical 4‐chamber views. Wall thickness and chamber dimensions were measured in the parasternal long‐axis views. Data from comprehensive pulmonary function testing, which included the maximal inspiratory pressure (MIP) and maximal expiratory pressure, were also collected during the course of routine medical care. The pulmonary function tests were read by board‐certified pulmonologists in accordance with guidelines set by the American Thoracic Society.24 Patients were stratified by whether or not they survived to the time of the analysis. Data were compared between the nonsurvivor and survivor cohorts to assess for differences between the 2 groups.
Hospitalizations during the follow‐up period were catalogued as cardiac, pulmonary, or noncardiopulmonary. Hospitalizations were considered cardiac if the primary reason for hospitalization was decompensated heart failure, arrhythmia, or chest pain/acute coronary syndrome. Pulmonary hospitalizations included viral or bacterial pneumonias and acute respiratory failure. The cause of death in the nonsurvivors was abstracted from the chart and classified as either cardiac or pulmonary. Sudden death was considered a cardiac cause, given that most sudden death in this age group is considered cardiac in nature.25, 26
In order to account for the possibility of age differences between the nonsurvivors and survivors, an age‐matched subgroup analysis was undertaken. Nonsurviving patients were age‐matched in a 1:2 ratio with surviving patients. Each unique survivor was included one‐time only. One nonsurvivor patient was removed for inability to age‐match because of very advanced age compared with the rest of the population. Seven nonsurvivors and 14 survivors were compared across all parameters.
Statistical Methods
Statistical comparisons for continuous variables were done by Mann–Whitney U testing. Proportions were compared by the Fischer exact test. P<0.05 were considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Baseline Characteristics
Forty‐three patients with the diagnosis of DMD were identified, all of whom were confirmed via genetic testing. Median follow‐up time in the clinic for the whole group was 28 months (interquartile range: 13–52). Median age of the population on entering the clinic was 21 years old (21–24), while median age at the time of analysis was 24 years (21–27). Demographic, comorbidity, and medication data obtained at the initial clinic visit are presented in Table 1, while the baseline characteristics are displayed in Table 2. The majority of the population was white (56% white, 33% Hispanic, 7% Asian, and 4% black). All the DMD patients had developed a cardiomyopathy, with over half of the patients demonstrating evidence of ventricular ectopy. The vast majority of the DMD patients had significant scoliosis (88%). There were low rates of significant comorbidities such as hypertension (2%), type 2 diabetes mellitus (2%), and hyperlipidemia (23%). There was no chronic renal disease in this population, with a median creatinine of 0.13 mg/dL (0.08–0.20). The overall population had a normal median body mass index (BMI: 24.0 kg/m2 [19.2–28.3]) and mean arterial pressure (83 mm Hg [78–90]). Median heart rate (96 bpm [82–108]) was close to the upper limit of the accepted normal range.27, 28 Most patients were on angiotensin‐converting enzyme inhibitors or angiotensin II receptor blocker therapy (86%) on arrival to the clinic, but only about a third of patients were receiving β‐blockers (37%). Oral steroid therapy was present in just over half of the patients (56%). There was limited use of mineralocorticoid receptor antagonist and diuretic therapy (7% and 5%, respectively). The proportion of patients on guideline‐directed medical therapy increased by the end of the analysis period, especially in regard to β‐blocker and mineralocorticoid receptor antagonist therapy (Table 3). Nearly half of all the patients had some degree of elevation in the hepatic enzymes (44%), with a median aspartate aminotransferase of 38 units/L (32–49) and median alanine aminotransferase of 46 units/L (34–71). Finally, the blood count was normal among the DMD patients (median white blood cell count: 9.3×109 cells/L [6.9–11.7], median hematocrit: 43% [42–46], and median platelet count: 246×109 cells/L [212–319]).
Table 1.
Demographics, Comorbidities, and Medications
| Parameters | Total (n=43) | Nonsurvivors (n=8) | Survivors (n=35) | P Value |
|---|---|---|---|---|
| Sex, % | ||||
| Male | 100 | 100 | 100 | |
| Race, % | ||||
| White | 56 | 50 | 57 | |
| Hispanic | 33 | 25 | 34 | |
| Asian | 7 | 12 | 6 | |
| Black | 4 | 13 | 3 | |
| Comorbidities, % | ||||
| Cardiomyopathy | 100 | 100 | 100 | >0.99 |
| Chronic renal disease | 0 | 0 | 0 | N/A |
| Coronary artery disease | 0 | 0 | 0 | N/A |
| Diabetes mellitus (Type 2) | 2 | 0 | 3 | <0.01 |
| Hyperlipidemia | 23 | 13 | 26 | 0.66 |
| Hypertension | 2 | 13 | 0 | 0.19 |
| Restrictive lung disease | 100 | 100 | 100 | >0.99 |
| Scoliosis | 88 | 100 | 86 | 0.56 |
| Transaminitis | 44 | 0 | 54 | <0.01 |
| Ventilator use | 79 | 88 | 77 | 0.66 |
| Ventricular ectopy (as assessed by 24‐h Holters and/or AICD interrogation) | 58 | 63 | 57 | >0.99 |
| Medications, % | ||||
| ACE‐I/ARB | 86 | 88 | 86 | 0.69 |
| β‐Blocker | 37 | 50 | 34 | 0.32 |
| Digoxin | 40 | 38 | 40 | 0.61 |
| Diuretic | 5 | 13 | 3 | 0.34 |
| Mineralocorticoid receptor antagonist | 7 | 13 | 6 | 0.47 |
| Steroid | 56 | 25 | 63 | 0.06 |
ACE‐I indicates angiotensin‐converting enzyme inhibitors; AICD, automatic implantable cardioverter defibrillator; ARB, angiotensin II receptor blockers; N/A, not applicable.
Table 2.
Baseline Characteristics
| Parameters | Total (n=43) | Nonsurvivors (n=8) | Survivors (n=35) | P Value |
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| Age at clinic entry, y | 21 (21–24) | 24 (22–29) | 21 (20–22) | 0.01 |
| Age at time of analysis | 24 (21–27) | 26 (24–31) | 23 (21–27) | 0.03 |
| Mo followed in clinic | 28 (13–52) | 28 (24–42) | 33 (11–55) | 0.96 |
| Height, m | 1.68 (1.60–1.75) | 1.72 (1.67–1.82) | 1.68 (1.60–1.72) | 0.22 |
| Weight, kg | 67.1 (54.1–81.6) | 48.3 (42.7–61.0) | 69.4 (60.0–81.6) | 0.01 |
| BMI, kg/m2 | 24.0 (19.2–28.3) | 17.3 (14.8–19.3) | 25.8 (20.8–29.1) | <0.01 |
| Heart rate, bpm | 96 (82–108) | 104 (82–114) | 95 (82–107) | 0.57 |
| Systolic blood pressure, mm Hg | 115 (104–124) | 108 (102–116) | 116 (105–124) | 0.24 |
| Diastolic blood pressure, mm Hg | 67 (64–75) | 69 (65–79) | 67 (64–75) | 0.85 |
| Mean arterial blood pressure, mm Hg | 83 (78–90) | 82 (74–91) | 83 (79–90) | 0.66 |
| Pulse pressure, mm Hg | 45 (37–53) | 40 (34–46) | 47 (39–54) | 0.08 |
| Laboratory parameters | ||||
| Sodium, mEq/L | 139 (136–140) | 137 (134–140) | 139 (136–140) | 0.12 |
| Potassium, mEq/L | 4.1 (4.0–4.3) | 4.1 (3.9–4.3) | 4.1 (4.0–4.3) | 0.54 |
| Chloride, mEq/L | 100 (98–102) | 101 (95–102) | 100 (98–102) | 0.97 |
| Bicarbonate, mEq/L | 26 (23–27) | 25 (24–27) | 26 (23–27) | 0.59 |
| Blood urea nitrogen, mg/dL | 10 (8–11) | 10 (7–12) | 10 (8–11) | 0.74 |
| Creatinine, mg/dL | 0.13 (0.08–0.20) | 0.14 (0.08–0.18) | 0.13 (0.08–0.20) | 0.79 |
| Albumin, mg/dL | 4.5 (4.1–4.8) | 4.5 (4.2–4.7) | 4.5 (4.1–4.8) | 0.85 |
| Total protein, g/dL | 7.4 (7.2–8.0) | 7.4 (7.1–7.8) | 7.5 (7.2–8.0) | 0.71 |
| AST, units/L | 38 (32–49) | 33 (19–46) | 39 (34–52) | 0.12 |
| ALT, units/L | 46 (34–71) | 26 (18–42) | 53 (37–81) | <0.01 |
| Alkaline phosphatase, units/L | 66 (53–83) | 66 (56–79) | 66 (53–89) | 0.81 |
| Total bilirubin, mg/dL | 0.5 (0.4–0.7) | 0.5 (0.4–0.9) | 0.5 (0.4–0.7) | 0.89 |
| White blood cell count, ×109 cells/L | 9.3 (6.9–11.7) | 8.7 (6.9–11.1) | 9.8 (6.9–11.7) | 0.65 |
| Hemoglobin, g/dL | 14.4 (13.8–15.1) | 14.5 (13.4–15.8) | 14.4 (13.8–15.0) | 0.73 |
| Hematocrit, % | 43 (42–46) | 44 (40–47) | 43 (42–46) | 0.99 |
| Platelets, ×109 cells/L | 246 (212–319) | 238 (195–251) | 250 (212–326) | 0.33 |
| Total cholesterol, mg/dL | 154 (132–178) | 145 (129–164) | 157 (132–182) | 0.47 |
| LDL, mg/dL | 80 (67–107) | 72 (66–80) | 83 (69–107) | 0.42 |
| HDL, mg/dL | 50 (38–59) | 61 (34–70) | 50 (38–58) | 0.61 |
| Non‐HDL cholesterol, mg/dL | 101 (85–129) | 86 (75–94) | 108 (89–130) | 0.25 |
| Triglycerides, mg/dL | 95 (60–138) | 66 (47–80) | 98 (60–144) | 0.12 |
| Hemoglobin A1C, % | 5.1 (4.9–5.5) | 4.9 (4.7–5.1) | 5.2 (4.9–5.5) | 0.06 |
| Thyroid‐stimulating hormone, units/mL | 1.7 (1.2–2.5) | 2.0 (1.4–2.8) | 1.5 (1.0–2.4) | 0.28 |
| Free T4, ng/dL | 1.5 (1.4–1.6) | 1.6 (1.4–1.8) | 1.5 (1.4–1.6) | 0.21 |
| Uric acid, mg/dL | 5.4 (4.8–6.1) | 5.0 (3.7–9.0) | 5.5 (4.8–6.1) | 0.47 |
| CK, units/L | 531 (379–784) | 523 (215–830) | 544 (384–784) | 0.71 |
| CK‐MB, units/L | 17 (11–23) | 12 (7–24) | 17 (12–21) | 0.63 |
| CK‐MB index | 3.0 (2.4–3.4) | 2.9 (2.4–3.4) | 3.0 (2.3–3.5) | 0.83 |
| NT‐proBNP, pg/mL | 59 (21–221) | 288 (72–1632) | 35 (21–135) | 0.03 |
| Pulmonary function parameters | ||||
| FEV1 | 0.95 (0.63–1.69) | 0.38 (0.35–1.69) | 0.97 (0.69–1.73) | 0.09 |
| FEV1% predicted | 22 (16–41) | 8 (8–41) | 23 (16–41) | 0.10 |
| FVC | 1.24 (0.70–1.90) | 0.40 (0.37–1.90) | 1.28 (0.79–1.90) | 0.08 |
| FVC% predicted | 21 (15–39) | 7 (7–42) | 24 (16–38) | 0.12 |
| FEV1/FVC, % | 89 (83–95) | 97 (94–99) | 88 (83–95) | 0.04 |
| Maximum expiratory pressure, cmH2O | 26 (15–35) | 13 (0–30) | 26 (16–37) | 0.13 |
| Maximum inspiratory pressure, cmH2O | 30 (24–52) | 13 (0–30) | 33 (25–40) | 0.03 |
| Electrocardiographic parameters | ||||
| PR, ms | 126 (119–136) | 128 (120–143) | 124 (119–136.0) | 0.55 |
| QRS, ms | 91 (85–100) | 93 (81–111) | 91 (86–100) | >0.99 |
| QT, ms | 350 (340–359) | 353 (302–365) | 350 (342–356) | 0.95 |
| QTc, ms | 427 (404–443) | 439 (420–450) | 425 (399–441) | 0.25 |
| Echocardiographic parameters | ||||
| Ejection fraction, % | 35 (24–52) | 38 (31–43) | 30 (23–52) | >0.99 |
| IVSD, cm | 0.80 (0.66–0.87) | 0.78 (0.64–0.88) | 0.80 (0.66–0.87) | 0.92 |
| LVPWD, cm | 0.81 (0.66–0.90) | 0.76 (0.62–0.89) | 0.81 (0.70–0.90) | 0.56 |
| LVESD, cm | 3.2 (2.7–4.1) | 3.2 (2.8–4.1) | 3.2 (2.7–4.1) | 0.98 |
| LVEDD, cm | 4.5 (3.9–5.3) | 4.0 (3.6–4.9) | 4.7 (4.1–5.3) | 0.34 |
| Left atrial diameter, cm | 3.1 (2.6–3.7) | 3.2 (3.1–3.5) | 3.0 (2.6–3.9) | 0.92 |
| Aortic root diameter, cm | 2.5 (2.4–2.7) | 2.5 (2.4–2.6) | 2.5 (2.2–2.8) | 0.71 |
ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; bpm, beats per minute; CK, creatinine kinase; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HDL, high‐density lipoprotein; IQR, interquartile range; IVSD, intraventricular septal dimension; LDL, low‐density lipoprotein; LVEDD, left ventricular end‐diastolic dimension; LVESD, left ventricular end‐systolic dimension; LVPWD, left ventricular posterior wall dimension; MB, muscle‐brain isoenzyme; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Table 3.
Use of Guideline‐Directed Heart Failure Medications
| Medication | Total (n=43) | Nonsurvivors (n=8) | Survivors (n=35) | |||
|---|---|---|---|---|---|---|
| Initial | End | Initial | End | Initial | End | |
| β‐Blocker, % | 37 | 74 | 50 | 88 | 34 | 71 |
| ACE‐I or ARB, % | 86 | 98 | 88 | 100 | 86 | 97 |
| Mineralocorticoid receptor antagonist, % | 7 | 47 | 13 | 38 | 6 | 49 |
ACE‐I indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blockers.
Every patient included in the analysis underwent comprehensive pulmonary function testing, which was consistent with restrictive lung disease, and the majority were using home ventilator therapy (79%). The percent forced expiratory volume in 1 s to forced vital capacity was elevated, consistent with restrictive lung disease (median 89% [83–95]). In addition, the maximal expiratory pressure and MIP, which reflect the strength of the diaphragm, were low (Table 2 and Figure 1: 26 [15–35] and 30 cmH2O [24–52], respectively).
Figure 1.
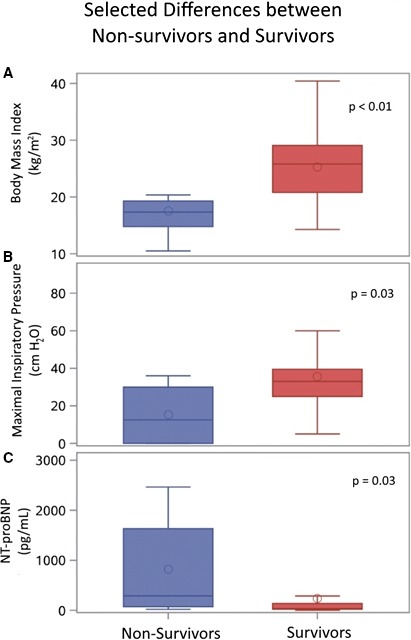
Box‐and‐whisker plot comparisons between nonsurvivors (n=8) and survivors (n=35) by (A) body mass index, (B) maximal inspiratory pressure, and (C) NT‐proBNP. NT‐proBNP indicates N‐terminal pro‐brain natriuretic peptide.
The ejection fraction, as assessed by echocardiography, was moderately depressed, with a median ejection fraction in the group of 35% (24–52). Left ventricular cavity dimensions showed a median left ventricular end‐diastolic diameter of 4.5 cm (3.9–5.3). Only 16% of patients had an automatic implantable cardioverter‐defibrillator in place, with 9% of patients having combined biventricular pacemaker/automatic implantable cardioverter‐defibrillator devices. While 58% of patients had some evidence of ventricular ectopy (0.5–1.5% of the total recorded beats) on either Holter monitoring or automatic implantable cardioverter‐defibrillator interrogation, no patient had sustained ventricular tachycardia or ventricular fibrillation. With respect to cardiac biomarkers, the median N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) for the evaluated group was in the normal range (median 59 pg/dL [21–221]). Creatinine kinase levels were elevated (median 531 ng/dL [379–784]). Cardiac troponin values were detected at low levels in a minority of patients (40%). Electrocardiographic intervals were within normal limits, with a median PR interval of 126 ms (119–136), median QRS duration of 91 ms (85–100), and median QTc interval of 427 ms (404–443).
Just under half of the total population was hospitalized or had an emergency room visit at least once (19/43 or 44%), with 50 total events during the course of follow‐up (1 event per 2.43 patient years). There were nearly equal proportions of cardiac (12/50 or 24%) and pulmonary (11/50 or 22%) hospitalizations or emergency room visits; the remaining admissions were for noncardiopulmonary complaints, primarily gastrointestinal or musculoskeletal issues.
Nonsurvivors Versus Survivors
Eight of the 43 patients died after establishing care within the clinic. Five of these patients died suddenly in their sleep, 1 died of heart failure in the hospital, and 2 patients died of sepsis secondary to pneumonia (Table 4). Upon entry into the clinic, the nonsurviving cohort was older as compared with the surviving cohort (median age 24 [22–29] versus 21 [20–22] years, nonsurvivors versus survivors, P=0.01). Both cohorts had a similar amount of follow‐up time in the clinic (28 [24–42] versus 33 [11–58] months, nonsurvivors versus survivors, P=0.96). The median age of death among the nonsurviving cohort was 26 years old (24–31). Comorbidities were similar in both subgroups, except the surviving cohort had a higher proportion of patients with elevated hepatic enzymes (0% versus 54%, nonsurvivors versus survivors, P<0.01). Medical therapy before establishing care in the clinic was similar in both cohorts, with no significant difference in proportions of patients on angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers, β‐blockers, digoxin, and mineralocorticoid receptor antagonists, though steroid use trended towards significance (25% versus 63%, nonsurvivors versus survivors, P=0.06). Guideline‐directed medical therapy use increased in both groups by the end of the analysis period (Table 3). There was no difference in the percentage of any hospitalizations (50% versus 43%, nonsurvivors versus survivors, P>0.99); however, there was a trend towards the nonsurvivors being more likely to be hospitalized for cardiopulmonary reasons (50% versus 17%, nonsurvivors versus survivors, P=0.07) (Figure 2). Baseline vital signs on entering the clinic were similar between the 2 cohorts. The nonsurviving cohort had significantly lower body weights and BMIs on arrival at the clinic when compared with the surviving cohort (Table 2 and Figure 1: 48.3 [42.7–61.0] versus 69.4 [60.0–81.6] kg, P=0.01 and 17.33 [14.8–19.3] versus 25.8 [20.8–29.1] kg/m2, P<0.01; nonsurvivors versus survivors, respectively). In addition, a higher proportion of the patients within the nonsurviving cohort were underweight (BMI <18 kg/m2) (75% versus 11%, nonsurvivors versus survivors, P<0.01).
Table 4.
Cause of Death
| Patient | NT‐proBNP (pg/mL) Within 3 Mo of Death | Cardiopulmonary Hospitalization Within 3 Mo of Death? | Presence of a BiV/AICD or AICD | Cause of Death | Cardiac or Pulmonary |
|---|---|---|---|---|---|
| 1 | 2632 | No | Yes | Sudden death | Cardiac |
| 2 | 34 313 | Yes | Yes | Heart failure | Cardiac |
| 3 | 109 | Yes | No | Pneumonia | Pulmonary |
| 4 | 85 | No | No | Sudden death | Cardiac |
| 5 | 423 | No | No | Sudden death | Cardiac |
| 6 | 23 976 | Yes | No | Pneumonia | Pulmonary |
| 7 | 633 | No | No | Sudden death | Cardiac |
| 8 | None recorded | No | No | Sudden death | Cardiac |
AICD indicates automatic implantable cardioverter defibrillator; BiV/AICD, combined biventricular pacemaker with an automatic implantable cardioverter defibrillator; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Figure 2.
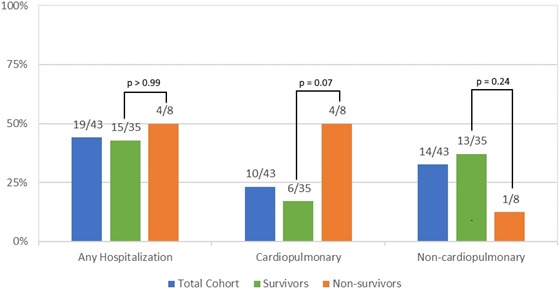
Percentage of the total cohort (n=43), survivors (n=35), and nonsurvivors (n=8) hospitalized for any reason, cardiopulmonary causes, or noncardiopulmonary causes.
A larger but not statistically significant proportion of patients in the nonsurviving cohort had ejection fractions <50% as compared with the surviving cohort (87% versus 59%, nonsurvivors versus survivors, P=0.15). The groups had similar baseline ejection fractions on echocardiography (38% [31–43] versus 30% [23–52.0], nonsurvivors versus survivors, P>0.99). However, NT‐proBNP values were significantly higher in the nonsurviving cohort (Table 2 and Figure 1: 288 [72–1632] versus 34.5 [21–135] pg/mL, nonsurvivors versus survivors, P=0.03). The creatinine kinase levels were similar in the nonsurviving and surviving groups (median 523 units/L [215–830] versus 544 units/L [384–784], nonsurvivors versus survivors, P=0.71). A higher proportion of patients had elevated troponins (either troponin T or I) in the nonsurviving group, though this analysis did not achieve statistical significance (57% versus 37%, nonsurvivors versus survivors, P=0.41). Blood count values including white blood cell count, hemoglobin, hematocrit, and platelet counts were similar between the 2 subgroups. Most of the comprehensive metabolic panel values were similar in both subgroups (Table 2). The alanine aminotransferase levels were significantly lower in the nonsurviving cohort as compared with the surviving cohort (26 units/L [18–42] versus 53 units/L [37–81], nonsurvivors versus survivors, P<0.01), with a trend towards lower aspartate aminotransferase values (33 units/L [19–46] versus 39 units/L [34–52], nonsurvivors versus survivors, P=0.12). Though all the patients had restrictive lung disease as defined by the American Thoracic Society criteria, the degree of respiratory muscle weakness seen in the nonsurviving cohort was significantly worse, with lower MIP (13 [0–30] versus 33.0 [25–40] cmH2O, nonsurvivors versus survivors, P=0.03) and a trend towards worse maximal expiratory pressure as compared with the surviving cohort (13 [0–30] versus 26 [16–37] cmH2O, nonsurvivors versus survivors, P=0.13).
Finally, in order to account for the difference in age between the nonsurvivor and survivor cohorts at the time of entry into the clinic, a univariate subgroup analysis was undertaken (Table 5). In this analysis, the BMI remained significantly lower in the nonsurvivors patients compared with the surviving patients (17.2 [13.5–18.2] versus 23.5 [19.5–29.1] kg/m2, nonsurvivors versus survivors, P=0.01). The nonsurvivors had lower systolic blood pressures (107 [101–110] versus 117 [110–127] mm Hg, nonsurvivors versus survivors, P=0.03), while the NT‐proBNP remained significantly higher in the nonsurviving group (665 [72–1632] versus 46 [19–79] pg/mL, nonsurvivors versus survivor, P=0.04). The MIP was no longer significantly lower in the nonsurviving group when they were age matched to survivors (13 [0–30] versus 33 [25–35] cmH2O, nonsurvivors versus survivors, P=0.10).
Table 5.
Baseline Characteristics for Age‐Matched Subgroup Analysis
| Nonsurvivors (n=7) | Survivors (n=14) | P Value | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Age at clinic entry, y | 23 (22–26) | 23 (22–25) | >0.99 |
| Age at time of analysis | 25 (24–28) | 27 (25–30) | 0.28 |
| Mo followed in clinic | 27 (22–34) | 54 (39–70) | 0.01 |
| Height, m | 1.68 (1.65–1.80) | 1.70 (1.65–1.75) | 0.82 |
| Weight, kg | 47.6 (39.9–60.8) | 68.3 (60.0–81.6) | 0.02 |
| BMI, kg/m2 | 17.2 (13.5–18.2) | 23.5 (19.5–29.1) | 0.01 |
| Heart rate, bpm | 106 (76–117) | 83 (74–85) | 0.08 |
| Systolic blood pressure, mm Hg | 107 (101–110) | 117 (110–127) | 0.03 |
| Diastolic blood pressure, mm Hg | 65 (64–79) | 72 (65–80) | 0.39 |
| Mean arterial blood pressure, mm Hg | 79 (71–89) | 87 (82–93) | 0.14 |
| AST, units/L | 34 (21–50) | 37 (32–42) | 0.61 |
| ALT, units/L | 30 (19–47) | 43 (36–58) | 0.06 |
| Hemoglobin, g/dL | 14.0 (12.8–15.8) | 14.4 (13.9–15.0) | 0.87 |
| Hematocrit, % | 42 (39–47) | 43 (42–45) | 0.81 |
| Platelets, ×109 cells/μL | 234 (156–255) | 263 (188–319) | 0.45 |
| CK, units/L | 537 (255–882) | 447 (307–464) | 0.26 |
| CK‐MB, units/L | 17 (7–24) | 13 (11–14) | 0.51 |
| NT‐proBNP, pg/mL | 665 (72–1632) | 46 (19–79) | 0.04 |
| FEV1% predicted | 8 (8–41) | 17 (12–53) | 0.19 |
| FVC% predicted | 7 (7–42) | 17 (14–50) | 0.09 |
| FEV1/FVC, % | 97 (94–99) | 83 (67–89) | 0.03 |
| Maximum expiratory pressure, cmH2O | 13 (0–30) | 18 (10–45) | 0.38 |
| Maximum inspiratory pressure, cmH2O | 13 (0–30) | 33 (25–35) | 0.10 |
| Ejection fraction, % | 35 (28–45) | 47 (27–52) | 0.62 |
ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; bpm, beats per minute; CK, creatinine kinase; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IQR, interquartile range; MB, muscle‐brain isoenzyme; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Discussion
As the DMD population survives longer because of improvements in respiratory and spinal care, adult cardiologists, specifically heart failure cardiologists, will be providing care to an increasing number of these patients. As such, it is important to begin to define the phenotype of adult DMD patients and what factors should receive particular attention when evaluating a DMD patient in the clinic. To the best of our knowledge, the current study is the first to specifically describe the clinical characteristics of adult DMD patients (age >18 years) and describe factors that may be associated with higher risk of death within adult DMD patients.
Baseline Characteristics of Adult DMD Patients
The baseline characteristics provide a framework of the types of patients that an adult cardiologist who delivers care to this vulnerable population can expect to encounter. A standard clinic population will comprise patients mostly in their early 20s with a high degree of pulmonary disease and ventilator use, ideally managed in a multidisciplinary clinic in collaboration with a pulmonologist specializing in the care of patients with neuromuscular disorders. The described clinic population had medication regimens that vary, with lower penetrance of initial β‐blocker use as compared with other, more traditional nonischemic cardiomyopathy populations. There was relatively little use of diuretics in this population. Steroid therapy was commonly used, the benefit of which has been demonstrated in previous trials,16, 17, 18 making it important for a cardiologist managing DMD patients to be familiar with the dosing regimens prescribed for this indication.29 In keeping with the majority of cardiomyopathy clinics around the country, the practice of this particular clinic was to aggressively initiate and uptitrate guideline‐directed medical therapy for heart failure. Achieving target dosages of these medications was less successful, though in general this is a common occurrence in the management of heart failure patients.30
Cardiac function should be closely monitored and the data suggest that one should expect high rates of cardiac dysfunction. However, the degree of cardiac dysfunction may be subtle. In our study, cardiac function was measured by echocardiography; however, cardiac magnetic resonance imaging has proven to be a more sensitive method for assessing cardiac dysfunction in this patient population.31 Obtaining a cardiac magnetic resonance imaging scan will provide a better assessment of the degree of cardiomyopathy and may provide better guidance regarding therapy. Our findings did not show dilated left ventricles for the group as a whole. This is an interesting finding and it may be because of the fact that the mode of pathological cardiac remodeling in DMD patients is distinct from patients with traditional nonischemic cardiomyopathy. Automatic implantable cardioverter‐defibrillators in the group were implanted as primary prevention of sudden death in patients with depressed ejection fractions <35% for >3 months on optimal medical therapy. This approach is standard practice in heart failure management, and in our practice we consider implantation of automatic implantable cardioverter defibrillators for primary prevention after the patient has been on maximally tolerated guideline‐directed medical therapy for at least 3 months.32
Finally, laboratory data will reveal several important findings. As a whole, the clinic population had elevated total creatinine kinase levels and relatively high rates of transaminitis, which is consistent with prior data noting elevated hepatic enzymes in the DMD population.33, 34 Given the lack of significant liver dysfunction seen in prior DMD studies despite elevated hepatic enzymes, 1 possible explanation is that the elevated transaminases are markers of active muscle breakdown, in a similar fashion to the observed elevated total creatinine kinase levels.34
Differences Between Nonsurvivors and Survivors
The results identify several potential adverse prognostic indicators in the adult DMD population. First, though rates of ventilator use were similar between the nonsurviving and surviving groups, the nonsurviving group had significantly worse diaphragmatic muscle strength, evident by the lower MIP. This finding was not surprising, given that respiratory muscle dysfunction is a known consequence of DMD.35
Second, there was a significant difference in the NT‐proBNP concentration between the nonsurviving and surviving cohorts. NT‐proBNP is a well‐known marker of cardiac dysfunction and portends a worse prognosis.36 In this clinic population, the nonsurvivors had a significantly higher NT‐proBNP at baseline than did those who survived. Prior work has shown that a common mode of death among the pediatric DMD population is cardiac death,1, 6 and the current data further complement these studies. In our study, there was a trend towards a higher proportion of the nonsurvivors having at least 1 cardiopulmonary hospitalization. In addition, most of the nonsurvivors had elevated NT‐proBNPs recorded within 3 months before their death and 6 of the 8 patients died of presumed cardiac causes. In spite of 88% of the nonsurviving DMD patients being on β‐blockers at the time of death, 5 of the 8 deaths occurred during their sleep, presumably because of sudden cardiac death.
Third and perhaps most strikingly, the nonsurviving cohort had significantly lower weights and BMIs. These findings were not driven by a few outliers, as the nonsurviving cohort had a significantly higher proportion of patients who were underweight on arrival to the clinic. These observations have not been previously reported in the literature to the best of our knowledge. In other disease states, including patients with advanced end‐stage cardiomyopathy, being underweight and having low muscle mass are considered to be poor prognostic indicators.37, 38, 39, 40 In these cases, low body weights with decreased BMIs were thought to be markers for frailty and end‐stage disease. The current study is the first to show such an association in DMD patients. In the DMD population, this may be a sign of more extensive muscle wasting and advanced heart failure. Our nonsurviving cohort had significantly lower alanine aminotransferase values. Interestingly, all the cases of elevated hepatic enzymes were found within the surviving DMD population. As mentioned above, the hepatic enzymes are often elevated in the DMD population, which is thought to be related to muscle breakdown. The lack of elevated hepatic enzymes in the nonsurviving cohort could indicate end‐stage disease, given that the muscle wasting process had run its course with less wasting, though this observation remains speculative.
Finally, given there was a significant difference in age on arrival to the clinic between the nonsurvivors and survivors, a subgroup analysis was undertaken to examine age‐matched subgroups to determine whether the differences noted above still held when age of entry into the clinic was taken into account. The age‐matched subgroup analysis revealed that the nonsurvivors had lower systolic blood pressure, while the differences in BMI and NT‐proBNP remained statistically significant. On the other hand, MIP and alanine aminotransferase were no longer significantly higher in the survivor cohort.
Our findings suggest that there is a subset of patients who are at higher risk for poor outcomes, which may be marked by low BMI, worse cardiac status, and poor respiratory function. The lower BMI could be a manifestation of the overall disease state, with lower BMIs indicating more advanced stage disease. We believe this argues for earlier initiation of guideline‐directed heart failure therapy beyond angiotensin‐converting enzyme inhibitors, including starting β‐blockers and mineralocorticoid antagonists and implantation of automatic implantable cardioverter defibrillators. A more aggressive and early use of standard guideline‐directed heart failure therapy may prevent decline of cardiac function, help slow progression to end‐stage disease, and ultimately decrease the risk of sudden death in this patient population. In addition, an evaluation of a DMD patient with high‐risk features on initial presentation should involve a transparent and open discussion with the patient and his family regarding the goals of care to ensure a clear understanding of overall prognosis. Collectively, this approach calls upon close coordinated efforts between the pediatric and adult care teams in order to fully optimize clinical outcomes among DMD patients.
The current study has several strengths. First, the clinic is managed by a single heart failure/VAD/transplant trained cardiologist with special expertise in managing patients affected by a variety of neuromuscular diseases, which adds a level of standardization to the care of the patients studied. Second, the clinic serves as a continuum of care from the Pediatric MDA Clinic within the Children's Medical Center of Dallas, a well‐regarded Center for the management of neuromuscular disorders of childhood. However, this study should be interpreted in light of several limitations. First, this is a single‐center experience. While the clinic cohort is relatively large for a DMD‐specific population, the practice patterns may be somewhat different compared with other centers, which may limit the generalizability of the findings. There were several findings that trend in the direction of being significant, such as the proportion of deceased patients with elevated troponins, narrower pulse pressures in the nonsurviving cohort, and higher aspartate aminotransferase values in the surviving cohort. Overall, the small sample size may have increased the chance of a type II error, leading to false negative results. While we believe that associations found have biological and clinical validity, multiple testing leading to false positive results is a possible limitation. In addition, the total number of deaths is too small to allow for advanced multivariable modeling with Cox regression to obtain proportional hazards. Another limitation remains the possibility of survivor bias. Although we per se cannot account for this bias in the current study, in 2017 the number of DMD patients who die of advanced DMD‐associated cardiomyopathy in their teenage years is believed to be low. Finally, given that this is a retrospective cross‐sectional study, the results are not meant to imply causal associations, and should be interpreted as exploratory analyses.
The growing adult DMD population is a continuing challenge for the adult cardiology community, and studies investigating this groups’ specific clinical characteristics are vital to delivering appropriate care to this high‐risk population. The current study identified several important factors that are associated with death and is an initial step toward recognizing the high‐risk subset of patients within this population, allowing for more intensive and tailored approaches to therapy. Collectively, the data also raise the question of whether implantation of automatic implantable cardioverter defibrillators in DMD patients should be pursued concomitantly while guideline‐directed medical therapy is being pursued. Future studies will expand upon the current findings with prospective, multicenter models to create predictive models and investigate novel approaches to treating DMD‐associated cardiomyopathy. Ultimately these future studies will provide better cardiovascular care to this emerging and vulnerable population with a high burden of developing a cardiomyopathy.
Sources of Funding
This study was supported by grants awarded to Drs. Drazner (James M. Wooten Chair in Cardiology provided by the UT Southwestern Medical Center), Khan (Wellstone Clinical Research Fellowship Award), and Mammen (National Institutes of Health Research Grants [R01‐HL102478 and U54‐AR068791]).
Disclosures
Dr. Mammen is Co‐Chair of the AHA Innovative Research Grant Committee (Basic Science 2), a member of the California Institute of Regenerative Medicine Grant Review Committee, a consultant and site Principal Investigator for the OAR/D‐OAR Registry (CareDx Inc), a consultant and recipient of a research grant from PhaseBio, and a consultant for Catabasis and HeartWare. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2017;6:e006340. DOI: 10.1161/JAHA.117.006340.)
References
- 1. Emery AEH. The muscular dystrophies. Lancet. 2002;359:687–695. [DOI] [PubMed] [Google Scholar]
- 2. Eagle M, Baudouin SV, Chandler C, Giddings DR, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord. 2002;12:926–929. [DOI] [PubMed] [Google Scholar]
- 3. Passamano L, Taglia A, Palladino A, Viggiano E, D'Ambrosio P, Scutifero M, Rosaria Cecio M, Torre V, De Luca F, Picillo E, Paciello O, Piluso G, Nigro G, Politano L. Improvement of survival in Duchenne muscular dystrophy: retrospective analysis of 835 patients. Acta Myol. 2012;31:121–125. [PMC free article] [PubMed] [Google Scholar]
- 4. Nigro G, Comi LI, Politano L, Bain RJ. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol. 1990;26:271–277. [DOI] [PubMed] [Google Scholar]
- 5. Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, Kaul A, Kinnett K, McDonald C, Pandya S, Poysky J, Shapiro F, Tomezsko J, Constantin C. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol. 2010;9:177–189. [DOI] [PubMed] [Google Scholar]
- 6. McNally EM, Kaltman JR, Benson DW, Canter CE, Cripe LH, Duan D, Finder JD, Hoffman EP, Judge DP, Kertesz N, Kinnett K, Kirsch R, Metzger JM, Pearson GD, Rafael‐Fortney JA, Raman SV, Spurney CF, Targum SL, Wagner KR, Markham LW. Contemporary cardiac issues in Duchenne muscular dystrophy. Circulation. 2015;131:1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, Kaul A, Kinnett K, McDonald C, Pandya S, Poysky J, Shapiro F, Tomezsko J, Constantin C. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93. [DOI] [PubMed] [Google Scholar]
- 8. Duboc D, Meune C, Lerebours G, Devaux J‐Y, Vaksmann G, Bécane H‐M. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol. 2005;45:855–857. [DOI] [PubMed] [Google Scholar]
- 9. Duboc D, Meune C, Pierre B, Wahbi K, Eymard B, Toutain A, Berard C, Vaksmann G, Weber S, Bécane H‐M. Perindopril preventive treatment on mortality in Duchenne muscular dystrophy: 10 years’ follow‐up. Am Heart J. 2007;154:596–602. [DOI] [PubMed] [Google Scholar]
- 10. Viollet L, Thrush PT, Flanigan KM, Mendell JR, Allen HD. Effects of angiotensin‐converting enzyme inhibitors and/or beta blockers on the cardiomyopathy in Duchenne muscular dystrophy. Am J Cardiol. 2012;110:98–102. [DOI] [PubMed] [Google Scholar]
- 11. Silva MC, Magalhães TA, Meira Z, Rassi C, Andrade A, Gutierrez PS, Azevedo CF, Gurgel‐Giannetti J, Vainzof M, Zatz M, Kalil‐Filho R, Rochitte CE. Myocardial fibrosis progression in Duchenne and Becker muscular dystrophy: a randomized clinical trial. JAMA Cardiol. 2017;2:190–199. [DOI] [PubMed] [Google Scholar]
- 12. Allen HD, Flanigan KM, Thrush PT, Dvorchik I, Yin H, Canter C, Connolly AM, Parrish M, McDonald CM, Braunlin E, Colan SD, Day J, Darras B, Mendell JR. A randomized, double‐blind trial of lisinopril and losartan for the treatment of cardiomyopathy in Duchenne muscular dystrophy. PLoS Curr. 2013. Edition 1. doi: 10.1371/currents.md.2cc69a1dae4be7dfe2bcb420024ea865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kajimoto H, Ishigaki K, Okumura K, Tomimatsu H, Nakazawa M, Saito K, Osawa M, Nakanishi T. Beta‐blocker therapy for cardiac dysfunction in patients with muscular dystrophy. Circ J. 2006;70:991–994. [DOI] [PubMed] [Google Scholar]
- 14. Matsumura T, Tamura T, Kuru S, Kikuchi Y, Kawai M. Carvedilol can prevent cardiac events in Duchenne muscular dystrophy. Intern Med. 2010;49:1357–1363. [DOI] [PubMed] [Google Scholar]
- 15. Raman SV, Hor KN, Mazur W, Halnon NJ, Kissel JT, He X, Tran T, Smart S, McCarthy B, Taylor MD, Jefferies JL, Rafael‐Fortney JA, Lowe J, Roble SL, Cripe LH. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: a randomised, double‐blind, placebo‐controlled trial. Lancet Neurol. 2015;14:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mendell JR, Moxley RT, Griggs RC, Brooke MH, Fenichel GM, Miller JP, King W, Signore L, Pandya S, Florence J, Schierbecker J, Robison J, Kaiser K, Mandel S, Arfken C, Gilder B. Randomized, double‐blind six‐month trial of prednisone in Duchenne's muscular dystrophy. N Engl J Med. 1989;320:1592–1597. [DOI] [PubMed] [Google Scholar]
- 17. Schram G, Fournier A, Leduc H, Dahdah N, Therien J, Vanasse M, Khairy P. All‐cause mortality and cardiovascular outcomes with prophylactic steroid therapy in Duchenne muscular dystrophy. J Am Coll Cardiol. 2013;61:948–954. [DOI] [PubMed] [Google Scholar]
- 18. Dec GW. Steroid therapy effectively delays Duchenne's cardiomyopathy. J Am Coll Cardiol. 2013;61:955–956. [DOI] [PubMed] [Google Scholar]
- 19. Wu RS, Gupta S, Brown RN, Yancy CW, Wald JW, Kaiser P, Kirklin NM, Patel PC, Markham DW, Drazner MH, Garry DJ, Mammen PPA. Clinical outcomes after cardiac transplantation in muscular dystrophy patients. J Heart Lung Transplant. 2010;29:432–438. [DOI] [PubMed] [Google Scholar]
- 20. Ryan TD, Jefferies JL, Sawnani H, Wong BL, Gardner A, Del Corral M, Lorts A, Morales DLS. Implantation of the HeartMate II and HeartWare left ventricular assist devices in patients with Duchenne muscular dystrophy: lessons learned from the first applications. ASAIO J. 2014;60:246–248. [DOI] [PubMed] [Google Scholar]
- 21. Stoller D, Araj F, Amin A, Fitzsimmons C, Morlend R, Thibodeau JT, Ramaciotti C, Drazner MH, Meyer DM, Mammen PPA. Implantation of a left ventricular assist device to provide long term support for end‐stage Duchenne muscular dystrophy‐associated cardiomyopathy. ESC Heart Fail. 2017;4:379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spurney C, Shimizu R, Hache LP, Kolski H, Gordish‐Dressman H, Clemens PR; CINRG Investigators . CINRG Duchenne Natural History Study demonstrates insufficient diagnosis and treatment of cardiomyopathy in Duchenne muscular dystrophy. Muscle Nerve. 2014;50:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 24. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. [DOI] [PubMed] [Google Scholar]
- 25. Eckart RE, Shry EA, Burke AP, McNear JA, Appel DA, Castillo‐Rojas LM, Avedissian L, Pearse LA, Potter RN, Tremaine L, Gentlesk PJ, Huffer L, Reich SS, Stevenson WG. Sudden death in young adults: an autopsy‐based series of a population undergoing active surveillance. J Am Coll Cardiol. 2011;58:1254–1261. [DOI] [PubMed] [Google Scholar]
- 26. Puranik R, Chow CK, Duflou JA, Kilborn MJ, McGuire MA. Sudden death in the young. Heart Rhythm. 2005;2:1277–1282. [DOI] [PubMed] [Google Scholar]
- 27. Mason JW, Ramseth DJ, Chanter DO, Moon TE, Goodman DB, Mendzelevski B. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol. 2007;40:228–234. [DOI] [PubMed] [Google Scholar]
- 28. Aladin AI, Whelton SP, Al‐Mallah MH, Blaha MJ, Keteyian SJ, Juraschek SP, Rubin J, Brawner CA, Michos ED. Relation of resting heart rate to risk for all‐cause mortality by gender after considering exercise capacity (the Henry Ford exercise testing project). Am J Cardiol. 2014;114:1701–1706. [DOI] [PubMed] [Google Scholar]
- 29. Matthews E, Brassington R, Kuntzer T, Jichi F, Manzur AY. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2016;(5):CD003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gheorghiade M, Albert NM, Curtis AB, Thomas Heywood J, McBride ML, Inge PJ, Mehra MR, O'Connor CM, Reynolds D, Walsh MN, Yancy CW, Fonarow GC. Medication dosing in outpatients with heart failure after implementation of a practice‐based performance improvement intervention: findings from IMPROVE HF. Congest Heart Fail. 2012;18:9–17. [DOI] [PubMed] [Google Scholar]
- 31. Silva MC, Meira ZMA, Gurgel Giannetti J, da Silva MM, Oliveira Campos AF, de Melo Barbosa M, Filho GMS, de Aguiar Ferreira R, Zatz M, Rochitte CE. Myocardial delayed enhancement by magnetic resonance imaging in patients with muscular dystrophy. J Am Coll Cardiol. 2007;49:1874–1879. [DOI] [PubMed] [Google Scholar]
- 32. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation and American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 33. Zhu Y, Zhang H, Sun Y, Li Y, Deng L, Wen X, Wang H, Zhang C. Serum enzyme profiles differentiate five types of muscular dystrophy. Dis Markers. 2015;2015:543282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McMillan HJ, Gregas M, Darras BT, Kang PB. Serum transaminase levels in boys with Duchenne and Becker muscular dystrophy. Pediatrics. 2011;127:e132. [DOI] [PubMed] [Google Scholar]
- 35. LoMauro A, D'Angelo MG, Aliverti A. Assessment and management of respiratory function in patients with Duchenne muscular dystrophy: current and emerging options. Ther Clin Risk Manag. 2015;11:1475–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Santaguida PL, Don‐Wauchope AC, Oremus M, McKelvie R, Ali U, Hill SA, Balion C, Booth RA, Brown JA, Bustamam A, Sohel N, Raina P. BNP and NT‐proBNP as prognostic markers in persons with acute decompensated heart failure: a systematic review. Heart Fail Rev. 2014;19:453–470. [DOI] [PubMed] [Google Scholar]
- 37. Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb‐Peploe KM, Harrington D, Kox WJ, Poole‐Wilson PA, Coats AJS. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. [DOI] [PubMed] [Google Scholar]
- 38. Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102. [DOI] [PubMed] [Google Scholar]
- 39. Lavie CJ, De Schutter A, Alpert MA, Mehra MR, Milani RV, Ventura HO. Obesity paradox, cachexia, frailty, and heart failure. Heart Fail Clin. 2014;10:319–326. [DOI] [PubMed] [Google Scholar]
- 40. DiBello JR, Miller R, Khandker R, Bourgeois N, Galwey N, Clark RV. Association between low muscle mass, functional limitations and hospitalisation in heart failure: NHANES 1999–2004. Age Ageing. 2015;44:948–954. [DOI] [PubMed] [Google Scholar]


