Abstract
Background
In contrast to the effects of preterm birth, the extent to which shorter gestational age affects the cardiorespiratory fitness (CRF) levels of individuals who were born at term (ie, between 37 and 42 weeks) is largely unknown. The aim of this study was to examine whether life‐course CRF levels varied across different gestational ages within the at‐term range.
Methods and Results
The association between gestational age (in weeks) obtained from Child Health Services records and CRF, estimated from field and laboratory tests and expressed by maximal oxygen uptake level through adolescence to young adulthood, was examined in 791 participants in the Northern Ireland Young Hearts Study, all singletons born at term. Longitudinal data were analyzed with generalized estimating equations, accounting for important potential confounders. Mean levels of CRF were 45.6, 43.7, and 33.0 mL/kg per minute when participants were aged 12, 15, and 22 years, respectively. After adjustment for confounders, each week increase in gestational age was associated with 0.46 mL/kg per minute (95% confidence interval, 0.14–0.79) in CRF. Compared with individuals born full term (39–40 weeks, n=533) or late term (41–42 weeks, n=148), those who were born early term (37–38 weeks, n=110) had a higher incidence of poor CRF (risk ratio, 1.57; 95% confidence interval, 1.14–2.16). The changes in CRF through adolescence to young adulthood were similar across groups, with those born early term consistently displaying the lowest CRF.
Conclusions
These findings suggest that early‐term births within the at‐term range are linked to poorer CRF through adolescence to young adulthood, and may have important clinical and public health implications for policies about (avoidable) early‐term deliveries given their recent increasing trends.
Keywords: cardiorespiratory fitness, gestational age, longitudinal cohort study
Subject Categories: Epidemiology, Primary Prevention, Exercise, Risk Factors
Clinical Perspective
What Is New?
In singletons born at term (ie, at 37–42 weeks of gestation), each week increase in gestational age is associated with significantly higher levels of cardiopulmonary fitness through adolescence into young adulthood.
Compared with full‐ and late‐term births (39–42 weeks of gestation), early‐term births (37–38 weeks of gestation) have ≈57% higher risk of developing poor cardiopulmonary fitness levels during these age periods.
What Are the Clinical Implications?
Cardiovascular health risks associated with early‐term births are increasingly reported but remain poorly understood: associations with poorer life course cardiorespiratory fitness may explain, at least in part, these risks.
The present findings may, therefore, have important public health and clinical implications by helping to inform policies to deter current trends towards avoidable deliveries at lower gestational ages and thereby prevent poor cardiopulmonary fitness levels and related cardiovascular sequelae in new generations.
Introduction
Increases in preterm births1 and recent trends towards shorter gestational lengths within the at‐term period (ie, 37–42 weeks)2, 3 have revived the interest in the associations between gestational age and offspring health outcomes. Indeed, it is becoming increasingly evident that shorter gestation, even within the at‐term period, may lead to adverse health outcomes, such as neonatal and infant neurological, cognitive, and respiratory morbidity4, 5, 6, 7, 8, 9, 10, 11 and mortality.2, 12, 13, 14 Altogether, these findings suggest that infants born at term form a heterogeneous group among whom longer‐term health outcomes related to different gestational ages need to be investigated further.
One such important health outcome is cardiorespiratory fitness (CRF), a component of physical fitness that reflects the ability of the respiratory, circulatory, and muscular systems to supply oxygen to the exercising muscles during physical activity. CRF is a major determinant of metabolic and cardiovascular health during youth and later in life.15, 16, 17 Although it can be improved by postnatal experiences, particularly through regular moderate‐to‐vigorous intensity physical activity,15 there is evidence to suggest that gestational age may play a critical role as well.18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 However, most of the studies that have investigated this were confined to CRF comparisons between survivors of extremely or very preterm birth and term‐born controls,18, 19, 20, 21, 22, 23, 24, 26, 27, 28 often involving small sample sizes and insufficient control for confounding. In addition, all considered the term‐born comparator as a homogeneous group,18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 and CRF was often examined at 1 point in the individual's life only,18, 19, 20, 21, 22, 23, 24, 25, 26, 29 mainly childhood or adolescence,18, 19, 20, 22, 23, 24, 26 but rarely through28 and beyond27 these critical periods.
In view of these considerations, we examined data from participants in a longitudinal cohort study to ascertain the extent to which CRF levels, through adolescence into young adulthood, vary across different gestational lengths within the at‐term age range.
Methods
Study Design
The present study examines longitudinal data from participants in the Northern Ireland Young Hearts Project, a prospective cohort study that was designed to investigate the development of lifestyle and biological cardiovascular risk factors through adolescence to young adulthood.30, 31, 32, 33, 34 The study design and sampling procedures were described in detail elsewhere.30 Briefly, a 2‐stage cluster sampling procedure, with allowance for an expected 20% nonresponse rate, was used to select a 2% representative sample of boys and girls aged 12 and 15 years, considering the geographical spread and the different categories of school in Northern Ireland. One thousand fifteen students (509 aged 12 years and 506 aged 15 years) were enrolled into the study and were examined at the first wave (YH1) in 1989 to 1990 (78% response rate). The 12 year olds were reexamined with the same methods and procedures 3 years later (YH2; n=461, 91%). Between 1997 and 1999, all participants, then aged 20 to 25 years, were invited for a third evaluation (YH3); 48% of the original YH1 population, including 12 aged 12 years who did not participate in YH2, agreed to participate (n=489). Despite multiple efforts to relocate the participants, approximately half did not return for YH3. The reasons given were as follows: now living outside the region/too far to travel, busy with work/family commitments, and no longer interested.33 The representativeness of the Northern Ireland Young Hearts Project participants at the YH3 wave has been examined before.35 Specifically, dropouts tended to have higher blood pressure and adiposity levels and more often belonged to families of lower socioeconomic status (SES), but did not differ with regard to lifestyle risk factors (ie, physical activity, alcohol consumption, total energy intake, and intake of foods and nutrients) and CRF.35 The exact numbers of participants were 509 at the age of 12 years, 967 at the age of 15 years, and 489 at the age of 20 to 25 years (Figure 1A).
Figure 1.
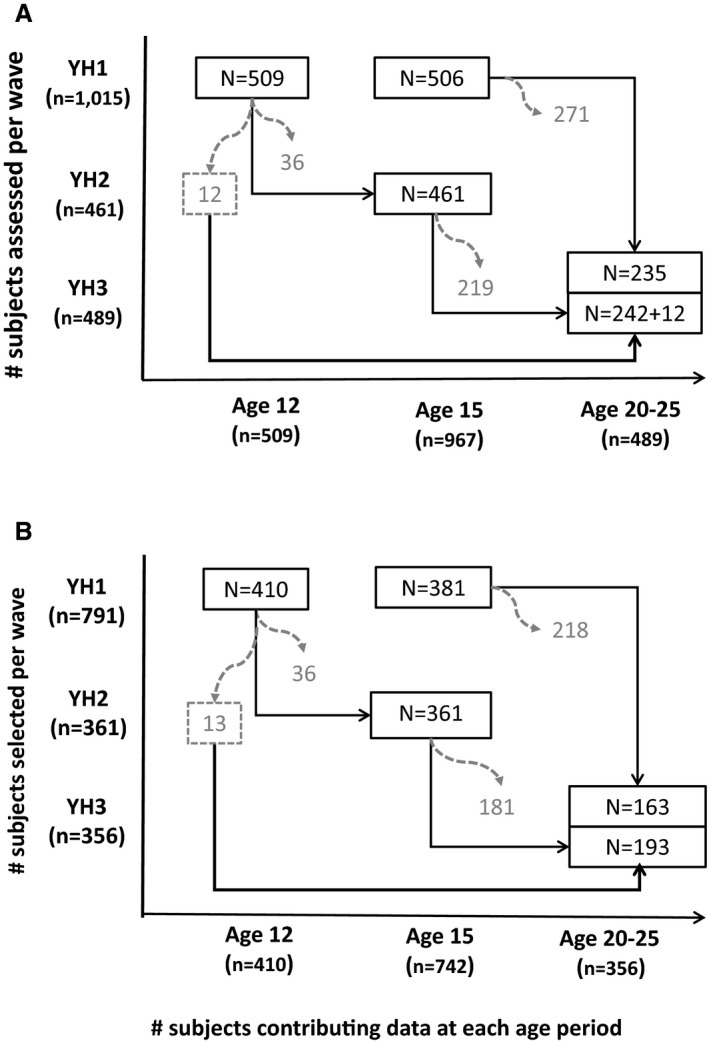
A, Numbers of participants in each wave of the Northern Ireland Young Hearts Project study, by age group. B, Number of participants selected for the present study participating in each wave of the study, by age group. YH1 indicates study first wave; YH2, study second wave; YH3, study third wave.
For each wave of the study, ethical approval was obtained from the Medical Research Ethical Committee of the Queen's University of Belfast, and written informed consent was obtained from all participants, and their parents or guardians during YH1 to YH2.
Sample Selection for the Present Study
At the start of the study (YH1), birth data from 935 participants were obtained from the Department of Health and Social Services (Northern Ireland), where the paper records collected since 1971 by the Child Health Services on all births to mothers normally resident in Northern Ireland were digitalized and managed. From these 935 participants, we excluded those with missing data on gestational age, mode of delivery, SES, breastfeeding duration, maternal age, maternal body mass index (BMI; in kilograms per meter squared), or maternal smoking habits (n=102). Participants were further excluded if they had an implausible combination of birth weight and gestational age (n=3), were born preterm (ie, <37 weeks of gestation, n=34), or missed CRF (n=3) or anthropometric (n=2) measurements at all ages. The present study thus includes 791 (410 aged 12 years and 381 aged 15 years) of the original 1015 Northern Ireland Young Hearts Project participants examined at YH1. These participants were all singletons born at term who had complete data on perinatal and maternal factors of interest and had at least 1 CRF and anthropometric assessment throughout the follow‐up period, contributing a total of 1508 observations across the 3 assessment waves. The exact numbers of these participants contributing data at each age period are shown in Figure 1B.
Gestational Age
Gestational age, to the nearest completed week from the first day of the last menstrual period, was retrieved from the Child Health Services records and used to group the study participants into 3 categories, according to current definitions36: early term (ET; 37–38 weeks), full term (FT; 39–40 weeks), and late term (LT; 41–42 weeks).
Other Perinatal Variables and Maternal Characteristics (Potential Confounders)
Maternal age (in years, calculated from birth date), mode of delivery (vaginal normal, vaginal instrumental [including forceps and vacuum], and cesarean), occupation of the main breadwinner in the children's family (used as indicator of SES), and birth weight (in grams) were also retrieved from the Child Health Services records.32 The occupations were recorded in 6 categories, according to the standard occupational classification of the Office and Population Censuses and Survey Statistics (ie, professionals, managerial and technical occupations, skilled nonmanual occupations, skilled manual occupations, partly skilled occupations, and unskilled occupations), and these were dichotomized as nonmanual (first 3) and manual (last 3).37 Birth weights were standardized for sex and gestational age by calculation of Z scores with the use of the UK1990 growth references provided by the British Child Growth Foundation.38 Breastfeeding duration (never, ≤3 months, and >3 months) and maternal height, weight (used to calculated maternal BMI), and smoking history (never, sporadic, and regular) were obtained from the parental questionnaire at the start of the study (YH1).34
Cardiorespiratory Fitness
When participants were aged 12 and 15 years (YH1 and YH2, respectively), CRF was determined by the 20‐m multistage shuttle run test,31, 32 a validated field test commonly used in epidemiological studies in children and adolescents worldwide.39, 40 In brief, the test required subjects to run back and forth between 2 lines set 20 m apart up to their volitional limit. Running pace was determined by audio signals, emitted from a prerecorded cassette tape, with the initial velocity being 8.5 km/h and increasing by 0.5 km/h every minute. A constant level of encouragement was given to the participants throughout the test, which was terminated when the subject failed to reach the end lines, concurrent with the audio signals, on 2 consecutive occasions. Scoring was first done by number of laps completed.32 These were converted to speed (in km/h) attained at the last completed stage of the test, following an algorithm that ensures comparability between 20‐m multistage shuttle run test protocols,39, 40 which was then used to estimate maximal oxygen uptake level (VO2max; in mL/kg per minute) according to the equations proposed by Leger et al.41
In young adulthood (YH3), CRF was measured using the physical work capacity at a heart rate (HR) of 170 beats per minute (PWC170) cycle‐ergometer test.33, 42 In brief, subjects were required to pedal at a steady pace (50–70 revolutions/min) for the duration of the test, which lasted ≈15 minutes. The workload was increased after each 3‐minute period until an HR of 170 beats per minute was achieved (assessed with a Polar Vantage HR monitor). Oxygen uptake was monitored throughout the test using an online respiratory gas analyzer (Quinton metabolic cart), and VO2max was predicted by extrapolation of VO2 at an HR of 170 beats per minute to the age‐adjusted estimated maximal HR.33, 42
Participants' levels of CRF were also categorized into poor versus normal, according to established age‐ and sex‐specific criterion‐reference standards for VO2max linked to poor cardiometabolic health (specifically, <41.8 mL/kg per minute [boys] and <34.6 mL/kg per minute [girls] during childhood/adolescence16 and <33 mL/kg per minute [men] and <24 mL/kg per minute [women] during young adulthood).43 Because VO2max is a physiological variable known to vary with body mass, expressing it in ratio with body mass does not account for the disproportionate increases in fat mass (FM) and fat‐free mass (FFM) with chronological age (and between sexes), leading to an artificial decrease in VO2max values during growth. Therefore, we have also estimated VO2max (in mL/min) per FFM and using theoretical (ie, in mL/min per kg0.67) and empirical (ie, based on the longitudinal data of the present study population, in mL/min per kg0.79) scaling factors for body mass derived from proportional allometric models.44, 45
Potential Mediators (Time‐Varying Covariates)
Maturity stage
Children and adolescents' maturity stage was assessed visually, by an experienced physician attached to the project, using Tanner stages for pubic hair development (set to stage V in all participants during young adulthood).30, 31, 32, 34 Accordingly, participants were categorized as prepubescent (stage I), pubescent (stages II–III), or post‐pubescent (stages IV–V).
Anthropometry
Throughout the longitudinal period, body weight was measured to the nearest 0.1 kg using an electronic scale, and standing height was measured to the nearest mm using a Harpenden portable stadiometer, while participants wore light indoor clothing and no shoes.30, 31, 34 BMI was calculated from these measures, and participants' weight status (underweight, normal, overweight, and obesity) was categorized according to BMI reference Z scores (during childhood/adolescence)46 or according to the following cutoff values (young adulthood): <18.5, 18.5 to 24.9, 25.0 to 29.9, and ≥30 kg/m2. Skinfold thicknesses (in mm) were measured with calipers at 4 body sites (triceps, biceps, subscapular, and suprailiac). The sum of these 4 skinfolds was used as a measure of total body adiposity31, 34 and to estimate percentage body fat,47 from which we derived FM as weight×%body fat and FFM as weight−FM (in kg).
Statistical Analyses
All data were analyzed with the use of the STATA version 14 software package. We used linear generalized estimating equations (GEEs) with an exchangeable correlation structure to analyze CRF (as a continuous variable) through adolescence to adulthood in relation to gestational age. GEE analyses account for the correlation of repeated and unequally numbered measures (ie, missing data) obtained over time in the same individuals. Gestational age was included in the GEE models as a continuous (in weeks) or categorical (treated as dummy with ET as referent group) variable. CRF was analyzed primarily in mL/min per kg but also relative to other expressions of body mass to allow appreciation of their impact (if any) and comparisons with other studies. We have also fitted GEE models to estimate the incidence risk ratio (RR; ie, the probability of having poor levels of CRF) associated with a 1‐week increase in gestational age or between FT or LT and ET groups. In these models, the Poisson distribution was assumed with a log link and robust error variance estimation.48 Tests for linear trends of (poor) CRF across categories of gestational age were conducted by using the mean values of gestational age for all the individuals included in each category as a continuous variable in the GEE models.
All analyses were first adjusted for participants' age at the time of the CRF assessment, sex, the product between participants' age and sex, birth weight Z scores, and cohort (model 1), and further for other perinatal (SES, mode of delivery, and breastfeeding duration) and maternal (age at child's birth, BMI, and smoking history) factors, as potential confounders (model 2). Participant's age at the time of the CRF assessment was treated as a categorical variable to model the nonlinear change of CRF over time. The product term between participants' age and sex was included in all models to accommodate the lower values of CRF in females than males across all ages as well as their different trajectories. Product terms between gestational age (in weeks or categories) and sex or cohort were also added to model 2 to examine whether the association between gestational age and CRF differed between the sexes or cohorts, but none were significant (P>0.202 for all these interaction terms). Therefore, all gestational age–CRF association estimates reported herein combine the data from males and females and participants from both the 12‐ and the 15‐year‐old cohorts. To examine whether the associations between gestational age and CRF were stable over the age periods covered by this study, interaction terms between gestational age and participants' age at follow‐up were also added to the models. The results of these analyses were used to illustrate the CRF trajectories from late childhood to young adulthood between different gestational age groups. Finally, to investigate the extent to which any associations between gestational age and (poor) CRF could be mediated by associations between gestational age and growth, maturation, and/or adiposity gain over the course of follow‐up, we added height, maturity level, and the sum of 4 skinfolds to model 2 described above (model 3).
Results
Perinatal and maternal characteristics of the participants included in the present study are shown in Table 1. Most participants were born FT (67.3%) or LT (18.7%), and 13.9% were born ET. Levels of CRF, body size, and adiposity during late childhood, adolescence, and young adulthood are shown in Table 2. Overall, when expressed in mL/min per kg or mL/min per kgFFM, CRF decreased slightly between childhood and adolescence but more sharply between adolescence and young adulthood. When expressed after allometric scaling, CRF increased slightly between childhood and adolescence, followed by a sharp decline thereafter. However, these trajectories and the mean CRF values at each age period differed by sex (Figure S1).
Table 1.
Perinatal Characteristics of the Study Sample (N=791)
| Variables | All (N=791) | Early Term (n=110) | Full Term (n=533) | Late Term (n=148) |
|---|---|---|---|---|
| Sex, % female | 52.0 | 54.5 | 51.4 | 52.0 |
| Gestational age, wks | 39.7 (1.0) | 37.7 (0.4) | 39.7 (0.4) | 41.1 (0.3) |
| Socioeconomic position, % manual | 27.3 | 31.8 | 26.3 | 27.7 |
| Birth weight, kg | 3.433 (0.478) | 3.233 (0.492) | 3.445 (0.473) | 3.532 (0.465) |
| Birth weight, Z scores | 0.02 (1.03) | 0.52 (1.12) | 0.03 (0.99) | −0.38 (0.89) |
| Mode of delivery, % | ||||
| Vaginal (normal) | 81.3 | 71.8 | 82.2 | 85.1 |
| Vaginal (assisted instrumental)a | 14.2 | 20.0 | 13.1 | 13.5 |
| Cesarean | 4.5 | 8.2 | 4.7 | 1.4 |
| Breastfeeding, % | ||||
| Never | 82.4 | 81.8 | 82.2 | 84.8 |
| ≤3 Mo | 11.1 | 12.7 | 10.7 | 11.5 |
| >3 Mo | 6.5 | 5.5 | 7.1 | 4.7 |
| Maternal age, y | 27.8 (5.6) | 27.6 (5.9) | 28.0 (5.6) | 27.1 (5.0) |
| Maternal BMI, kg/m2 | 24.6 (4.0) | 24.7 (4.3) | 24.5 (3.8) | 24.8 (4.1) |
| Normal weight, % | 65.0 | 62.7 | 65.9 | 65.5 |
| Overweight, % | 26.5 | 29.1 | 25.5 | 28.4 |
| Obese, % | 8.5 | 8.2 | 8.6 | 8.1 |
| Maternal smoking habits, % | ||||
| Non‐smoker | 63.5 | 67.3 | 63.8 | 59.5 |
| Sporadic smoker | 3.8 | 3.6 | 3.2 | 6.1 |
| Regular smoker | 32.7 | 29.1 | 33.0 | 34.5 |
Data are mean (SD) unless otherwise indicated. BMI indicates body mass index.
Including forceps or vacuum (ventouse).
Table 2.
CRF and Other Time‐Varying Covariates From Childhood to Young Adulthood
| Variables | Childhood (n=410)a | Adolescence (n=742)a | Young Adulthood (n=356)a |
|---|---|---|---|
| Sex, % female | 51.2 | 52.2 | 50.0 |
| Age, y | 12.5 (0.3) | 15.5 (0.3) | 22.4 (1.7) |
| VO2max, L/min | 1.96 (0.38) | 2.54 (0.58) | 2.31 (0.84) |
| VO2max, mL/min per kg | 45.6 (4.9) | 43.7 (6.9) | 33.0 (9.8) |
| VO2max, mL/min per kgFFM | 58.9 (4.7) | 56.8 (6.2) | 42.0 (9.5) |
| VO2max, mL/min per kg0.67 | 157 (16) | 167 (28) | 134 (41) |
| VO2max, mL/min per kg0.79 | 100 (10) | 102 (16) | 80 (24) |
| Poor CRF, %b | 8.1 | 14.3 | 31.2 |
| Height, cm | 150 (8) | 165 (8) | 171 (10) |
| Weight, kg | 43.3 (9.3) | 58.1 (9.9) | 69.7 (12.9) |
| Body mass index, kg/m2 | 19.0 (3.2) | 21.3 (3.1) | 23.7 (3.7) |
| Underweight, % | 9.8 | 5.0 | 2.2 |
| Normal weight, % | 73.4 | 79.5 | 68.3 |
| Overweight, % | 13.6 | 12.8 | 23.9 |
| Obese, % | 3.2 | 2.7 | 5.6 |
| Sum of 4 skinfolds, median (interquartile range), mm | 34.4 (27.6–49.5) | 38.6 (28.5–52.4) | 48.2 (35.6–59.6) |
| FFM, kg | 33.2 (5.6) | 44.5 (7.2) | 54.1 (10.9) |
| Maturity stage, %c | |||
| Prepubescent (stage I) | 48.7 | 1.0 | ··· |
| Pubescent (stages II–III) | 35.2 | 5.5 | ··· |
| Post‐pubescent (stages IV–V) | 16.1 | 93.5 | 100.0 |
Data are mean (SD) unless otherwise indicated. CRF indicates cardiorespiratory fitness; FFM, fat‐free mass; VO2max, maximal oxygen uptake (measure of CRF).
Numbers are exact number of individuals, from the 791 selected for the present study contributing data at the specific age period.
Poor CRF was defined on the basis of the following age‐ and sex‐specific cut points for VO2max (in mL/kg per minute): <41.8 (childhood and adolescence) and <33 (young adulthood) in males and <34.6 (childhood and adolescence) and <24 (young adulthood) in females.
According to Tanner stages for pubic hair development.
Gestational Age and CRF
Gestational age was significantly associated with CRF through adolescence to young adulthood, such that for each week increase in participants' gestational age, their CRF was 0.46 mL/min per kg (95% confidence interval [CI], 0.14–0.79) higher (model 1, Table 3). After adjustments for other perinatal and maternal factors, this association did not change: 0.46 mL/min per kg (95% CI, 0.14–0.78) (model 2, Table 3). When examined according to categories of gestational age, and after adjustments for all the potential confounders considered, individuals who were born FT or LT had increasingly higher levels of CFR than individuals who were born ET: 0.86 mL/min per kg (95% CI, −0.11 to 1.83) and 1.59 mL/min per kg (95% CI, 0.40–2.77), respectively (P=0.009 for linear trend, model 2, Table 3). Further adjustments for growth, maturation, and adiposity, as potential mediators, did not appreciably change our findings (model 3). A similar pattern of associations was observed when CRF was expressed in mL/min per kgFFM, mL/min per kg0.67, or mL/min per kg0.79 (Table 3), illustrating the lack of an association between gestational age and participants' body weight or FFM throughout the follow‐up (ß=−0.3 [95% CI, −0.9 to 0.4] kg and ß=−0.2 [95% CI, −0.5 to 0.3] kg, respectively, per week increase in gestational age).
Table 3.
Associations Between Gestational Age and Cardiopulmonary Fitness Through Adolescence to Young Adulthood (N=791)
| Main Outcome | Main Determinant | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CRF | Gestational Age | ß | 95% CI | P Value | ß | 95% CI | P Value | ß | 95% CI | P Value |
| mL/min per kg | Per week | 0.46 | 0.14 to 0.79 | 0.005 | 0.46 | 0.14 to0.78 | 0.004 | 0.41 | 0.13 to 0.68 | 0.004 |
| Per category | ||||||||||
| Early term | 0.00 | Ref. | ··· | 0.00 | Ref. | ··· | 0.00 | Ref. | ··· | |
| Full term | 0.87 | −0.11 to 1.86 | 0.082 | 0.86 | −0.11 to 1.83 | 0.082 | 0.62 | −0.22 to 1.45 | 0.147 | |
| Late term | 1.56 | 0.36 to 2.77 | 0.011 | 1.59 | 0.40 to 2.77 | 0.009 | 1.32 | 0.30 to 2.34 | 0.011 | |
| P value, linear trenda | 0.011 | 0.009 | 0.012 | |||||||
| mL/min per FFM | Per week | 0.54 | 0.19 to 0.89 | 0.003 | 0.54 | 0.19 to 0.88 | 0.002 | 0.53 | 0.19 to 0.88 | 0.003 |
| Per category | ||||||||||
| Early term | 0.00 | Ref. | ··· | 0.00 | Ref. | ··· | 0.00 | Ref. | ··· | |
| Full term | 0.88 | −0.18 to 1.95 | 0.105 | 0.88 | −0.18 to 1.93 | 0.104 | 0.86 | −0.20 to 1.91 | 0.111 | |
| Late term | 1.72 | 0.41 to 3.02 | 0.010 | 1.73 | 0.44 to 3.02 | 0.009 | 1.72 | 0.43 to 3.01 | 0.009 | |
| P value, linear trenda | 0.010 | 0.009 | 0.009 | |||||||
| mL/min per kg0.67 | Per week | 1.59 | 0.38 to 2.80 | 0.010 | 1.52 | 0.33 to 2.71 | 0.012 | 1.38 | 0.26 to 2.51 | 0.016 |
| Per category | ||||||||||
| Early term | 0.00 | Ref. | ··· | 0.00 | Ref. | ··· | ||||
| Full term | 2.94 | −0.74 to 6.62 | 0.117 | 2.77 | −0.85 to 6.40 | 0.134 | 1.88 | −1.55 to 5.31 | 0.282 | |
| Late term | 5.57 | 1.07 to 10.1 | 0.015 | 5.40 | 0.97 to 9.84 | 0.017 | 4.73 | 0.53 to 8.93 | 0.027 | |
| P value, linear trenda | 0.016 | 0.017 | 0.029 | |||||||
| mL/min per kg0.79 | Per week | 1.01 | 0.28 to 1.74 | 0.007 | 0.98 | 0.27 to 1.70 | 0.007 | 0.89 | 0.22 to 1.55 | 0.009 |
| Per category | ||||||||||
| Early term | 0.00 | Ref. | ··· | 0.00 | Ref. | ··· | 0.00 | Ref. | ··· | |
| Full term | 1.89 | −0.33 to 4.10 | 0.096 | 1.81 | −0.38 to 4.00 | 0.105 | 1.26 | −0.78 to 3.30 | 0.227 | |
| Late term | 3.49 | 0.77 to 6.21 | 0.012 | 3.45 | 0.77 to 6.12 | 0.012 | 2.97 | 0.47 to 5.47 | 0.020 | |
| P value, linear trenda | 0.012 | 0.012 | 0.021 | |||||||
Model 1, adjusted for cohort, age, sex, age×sex, and birth weight Z scores; model 2, model 1 plus adjustments for socioeconomic status, maternal age at child's birth, delivery mode, breastfeeding, maternal body mass index, and maternal smoking history; model 3, model 2 plus adjustment for height, maturity level, and total body fatness (sum of 4 skinfolds). CRF indicates cardiorespiratory fitness; FFM, fat‐free mass (in kg); Ref., reference; ß, mean difference in maximal oxygen uptake (in units indicated) throughout the longitudinal period per week increase in gestational age or between individuals who were born full (n=533) or late term (n=148) vs early term (n=110).
Mean gestational ages per gestational age category were 37.7 weeks (early term), 39.7 weeks (full term), and 41.1 weeks (late term).
Gestational Age and Incidence of Poor CRF
After adjustments for all confounders, each week increase in participants' gestational age was associated with a 14% relative risk reduction of poor CRF (model 2, Table 4). Notably, when comparing individuals according to categories of gestational age, those who were born FT or LT had similar lower risk estimates for poor CRF than those who were born ET: RR, 0.65 (95% CI, 0.47–0.89) and 0.59 (95% CI, 0.40–0.90), respectively. Conversely, compared with individuals born FT or LT (combined), individuals who were born ET had a 57% higher risk of poor CRF throughout adolescence to adulthood (RR, 1.57 [95% CI, 1.14–2.16]).
Table 4.
Associations Between Gestational Age and Incidence of Poor CRF Through Adolescence to Young Adulthood (N=791)a
| Main Determinant | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Gestational Age | RR | 95% CI | P Value | RR | 95% CI | P Value | RR | 95% CI | P Value |
| Per wk | 0.86 | 0.77–0.96 | 0.008 | 0.86 | 0.76–0.96 | 0.009 | 0.87 | 0.79–0.97 | 0.011 |
| Per category | |||||||||
| Early term | 1.00 | Ref. | ··· | 1.00 | Ref. | ··· | 1.00 | Ref. | ··· |
| Full term | 0.66 | 0.48–0.91 | 0.010 | 0.65 | 0.47–0.89 | 0.008 | 0.71 | 0.52–0.97 | 0.032 |
| Late term | 0.60 | 0.40–0.90 | 0.014 | 0.59 | 0.40–0.90 | 0.013 | 0.64 | 0.44–0.93 | 0.021 |
| P value, linear trendb | 0.012 | 0.011 | 0.021 | ||||||
Model 1, adjusted for cohort, age, sex, age×sex, and birth weight Z scores; model 2, model 1 plus adjustments for socioeconomic status, maternal age at child's birth, delivery mode, breastfeeding, maternal body mass index, and maternal smoking history; model 3, model 2 plus adjustment for height, maturity level, and total body fatness (sum of 4 skinfolds). Ref. indicates reference; RR, incident risk ratio of poor cardiorespiratory fitness through adolescence to young adulthood per week increase in gestational age or between individuals who were born full (n=533) or late term (n=148) vs early term (n=110); CRF indicates cardiorespiratory fitness.
Defined on the basis of the following age‐ and sex‐specific cut points for maximal oxygen uptake (in mL/kg per minute): <41.8 (childhood and adolescence) and <33 (young adulthood) in males and <34.6 (childhood and adolescence) and <24 (young adulthood) in females.
Mean gestational ages per gestational age category were 37.7 weeks (early term), 39.7 weeks (full term), and 41.1 weeks (late term).
Gestational Age and (Poor) CRF Trajectories
There were no significant interactions between gestational age (in weeks or categories) and participants' age at the time of CRF assessment (P>0.322 for all), indicating that the changes in CRF over time were similar across the different gestational ages, with those born ET displaying consistently lower levels of CRF (Figure 2A, when expressed in mL/kg per minute; Figure S2, when expressed in other units) or greater incidence of poor CRF (in percentage, Figure 2B) through adolescence to young adulthood.
Figure 2.
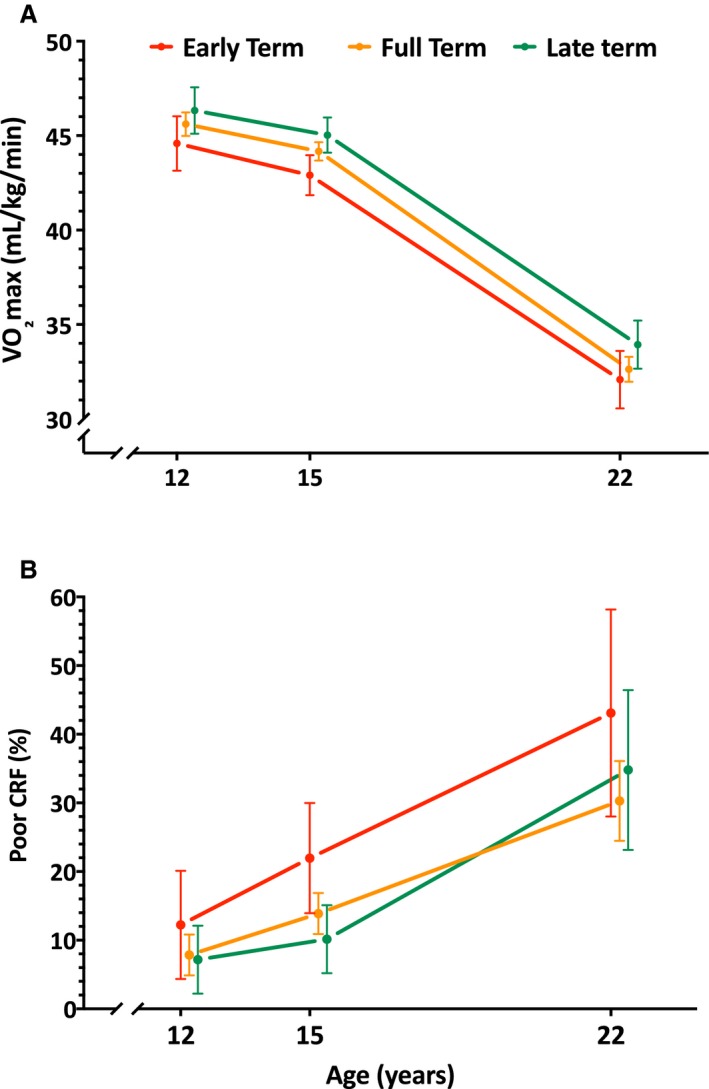
A, Trajectories of cardiorespiratory fitness (CRF), expressed by estimated maximal oxygen uptake (VO 2max; in mL/min per kg), through adolescence to young adulthood by categories of gestational age. B, Proportion of individuals with poor CRF (defined by age‐ and sex‐specific health‐related cutoff values of VO 2max) through adolescence to young adulthood, by categories of gestational age. Mean CRF levels or proportions with poor CRF at each age were estimated with generalized estimating equations models adjusted for sex, age×sex interactions, cohort, birth weight Z scores, socioeconomic status, delivery mode, breastfeeding, and maternal age, body mass index, and smoking. Error bars indicate 95% CIs.
Additional Analyses
To extract the public health impact of our estimates, we have also calculated the attributable fractions, and respective 95% CIs, among the participants exposed (AFe) and the overall study population (AFp) (Figure 3), according to the method recommended by Greenland and Drescher for cohort studies49 and implemented in STATA with the command punaf.50 Specifically, the AFe, indicating the fraction of poor CRF risk in the exposed population (ET) that would be eliminated if these people were to be shifted to the unexposed group (ie, FT or LT, combined) was 36.3% (95% CI, 12.5%–53.7%). The AFp, indicating how much of the poor CRF burden in the overall study population could be eliminated if ET births were eliminated from the population, was 6.9% (95% CI, 1.2%–12.2%). The AFs estimated herein refer to the time interval under analyses (ie, late childhood to young adulthood).
Figure 3.
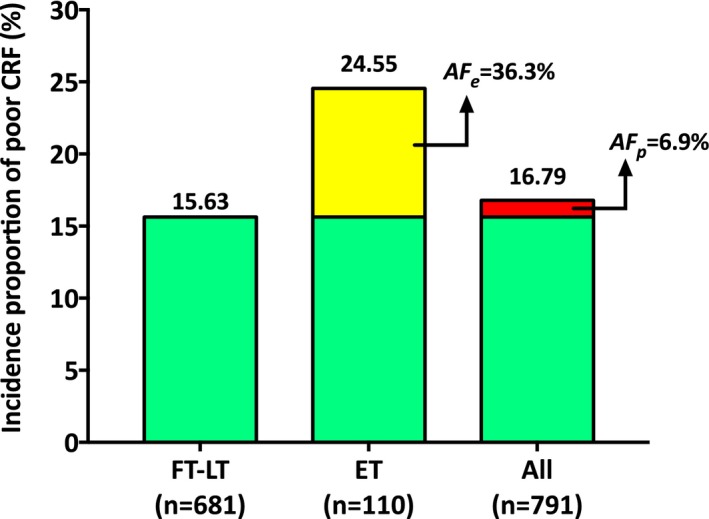
Incidence proportions of poor cardiorespiratory fitness (CRF) through adolescence to young adulthood, among participants who were born full term (FT), late term (LT), or early term (ET) and in the whole study population (All). Yellow portion of the bar depicting the incidence proportion of poor CRF among the ET group illustrates the magnitude of the attributable fraction among the exposed: AF e=[(24.55−15.63)/24.55]×100=36.3%. Likewise, the red portion of the bar depicting the incidence proportion of poor CRF among all participants illustrates the magnitude of the population attributable fraction: AF p=[(16.79−15.63)/16.79]×100=6.9%. Incidence proportions were adjusted for sex, participants' age at the time of CRF, age×sex interaction terms, cohort, birth weight Z scores, socioeconomic status, delivery mode, breastfeeding, and maternal age, body mass index, and smoking. The AFs were estimated according to the method recommended by Greenland and Drescher49 (implemented in STATA with the command punaf) and could be also approximated by the following equations: AF e=(RR−1)/RR and AF p=[(RR−1)/RR]×Pe, commonly used in cohort studies, where RR is the multivariable adjusted risk ratio of poor CRF for ET vs FT−LT (ie, 1.57) and Pe is the proportion of individuals with poor CRF who were born ET (or case fraction).
Discussion
Health risks associated with ET birth are increasingly reported but remain poorly understood. The present study, showing lower levels of CRF through adolescence into young adulthood in individuals who were born ET versus FT or LT, adds important information to this emerging field. Indeed, to the best of our knowledge, this is the first study that has examined variations in the levels of CRF across different gestational lengths within the at‐term range; it is also the first to have characterized these across different critical age periods in the life course.
Explanations for the protective and lifelong effects that longer gestational age seems to have on CRF align with the hypothesis that exposure of babies to the postnatal environment while still developmentally plastic may increase susceptibility to health impairments.51 Earlier births may interrupt normal development and lead to permanent changes of tissues and organs, such as fewer alveoli, lower capillary density, and a smaller vascular tree, which, in turn, may lead to long‐lasting impairments in maximal oxygen uptake and transport, muscular power, and motor coordination. Albeit confined to comparisons between individuals born extremely or very preterm versus at term (while considering the latter as a homogeneous group), previous studies have suggested that mild airway obstruction, gas trapping, and deficits in motor coordination may explain, at least in part, the lower CRF levels observed among those born prematurely.21, 22, 23 Differences in the postnatal experiences of children born preterm have also been advanced as a potential explanation. These may include nutritional supplementation and parental restrictions of offspring participation in physical activity because of perceptions of frailty. Accordingly, some studies have shown that individuals who were born (very) prematurely not only had a lower CRF but also reported lower participation in physical activities.20, 26 However, no such differences were observed when physical activity levels were measured objectively, despite significant differences in CRF.24 We deem the postnatal experience explanation less likely in the context of CRF differences between individuals who were all born at term. Possibly, genetic factors related to both gestational age and CRF may explain a significant portion of the associations observed in our study, although this needs to be further investigated. The fact that the associations remained stable across all ages, and that adjustments for growth, maturation, and adiposity, as potential mediators, did not appreciably change their strength, seems to lend some support to this hypothesis.
The importance of our findings is supported by the strong links between CRF and other cardiometabolic risk factors in youth and later in life, pathways that are likely to explain the beneficial effects of CRF on cardiovascular disease and mortality.15 Indeed, each 1 metabolic equivalent task (ie, 3.5 mL/kg per minute) lower level in CRF during young adulthood was associated with ≈13%, 8%, and 11% higher risk of hypertension, type 2 diabetes mellitus, and metabolic syndrome in middle age, respectively.52 A recent prospective study reported an 18% reduced risk of myocardial infarction per 1‐SD higher levels of CRF during adolescence.53 A meta‐analysis of prospective studies in mostly middle‐aged healthy men and women showed that a 1 metabolic equivalent task higher level in CRF was associated with 15% and 13% reductions in coronary heart disease/cardiovascular disease events and all‐cause mortality, respectively.54 By extrapolation, the extent to which our findings, showing a 0.46 mL/kg per minute difference per week increase in gestational age or a 1.59 mL/kg per minute difference between individuals born ET and LT (model 2, Table 3), translate to clinically relevant outcomes later in life remains disputable. By examining the associations between gestational age and the incidence of poor CRF defined by cut point levels that should raise red flags about the participants' cardiometabolic health status,16 we estimated a 14% relative risk reduction per week increase in gestational age within the at‐term range and a 57% relative risk increase between individuals who were born ET versus FT or LT (combined). However, exposures with low prevalence may have limited relevance for population health, even when they are strongly associated with the outcome of interest. Therefore, we have also translated our findings in terms of their relevance to public health by calculating AFs. These were 36.3% among the individuals born ET and 6.9% for the whole study population. Although statistically significant, the meaning of these figures may be better understood when compared with scenarios in which AFs are commonly estimated to inform public health policies. For instance, in a recent population‐based study from the United Kingdom, the AFs for having at least 3 hospital admissions between the ages of 9 months and 5 years and for limiting long‐standing illness at 5 years were 7.2% and 5.4%, respectively, in children born ET versus FT.11 In addition, although only 13.9% of the population included in our study were born ET (in 1974–1975 or 1977–1978), more recent birth data from Northern Ireland put this figure at a higher level (≈20% of all singletons born at term in 2014).55 The AFs reported in our study are, thus, likely underestimates of the current impact of ET on poor CRF. Still, apart from their magnitude, AFs only have translational value when the exposure of interest is causally related to the outcome and when the exposure is amenable to intervention. Causality of the gestational age–CRF association cannot be proved by means of randomized controlled trials and must, thus, be inferred on the basis of observational data. We confirmed essential criteria for an association to be deemed causal, such as plausibility, temporality, dose‐gradient, consistency, and coherency. Still, it remains that genetic factors can underlie both lower gestational age and poorer CRF and, therefore, their association may not be not causal. Furthermore, not all births occurring ET can or should be prevented (eg, if they are medically indicated or occur spontaneously). However, recent trend analyses have attributed the increases in ET births to increases in the rates of planned cesareans, and these do form an important potential modifiable portion.3
Our study has several limitations that need to be acknowledged. Gestational age was estimated according to the last menstrual period day method (the standard in the 1970s), which may have introduced measurement errors. However, there are no reasons to expect these should have differed by the participants' CRF levels and, thus, any error introduced was likely nondifferential. In the same line, accurate CRF estimation requires maximal exercise testing with direct measures of oxygen uptake. Although no such direct measures were obtained, the methods used in the Northern Ireland Young Hearts Project have been validated, also against each other,56 and are commonly used to characterize CRF and related health outcomes in large population‐based cohort studies. Switching to a new assessment method during young adulthood may have affected the shape of the CRF trajectory in the whole study population, most likely resulting in a less steep decline between adolescence and young adulthood than one would have observed had the 20‐m multistage shuttle run test been used at YH3. However, this does not affect the differences in CRF between gestational age groups at each period.
The study experienced considerable attrition of participants between adolescence and young adulthood. However, this raised little concern about the validity of the estimates reported because analyses of predictors of dropout showed that neither gestational age nor the baseline levels of CRF differed between those who remained in the study up to young adulthood and those who dropped out earlier (Table S1). Some of the covariates did differ (ie, SES, delivery mode, and adiposity), such that the group attending and providing data during young adulthood tended to have a healthier profile. If anything, these led to an underestimation of the estimates reported in our study. Indeed, sensitivity analyses excluding the young adulthood data from the analyses resulted in somewhat stronger associations (0.51 [0.21–0.81] mL/min per kg per week increase in gestational age and RR of 1.69 [1.14–2.51] for incidence of poor CRF in ET versus FT or LT [combined]). Data on the indication for cesarean deliveries (or labor inductions) were not available in the present study. It is, thus, possible that significant obstetric reasons may have led to cesarean deliveries (or labor inductions) before FT, and the poorer CRF observed in the ET group could, to some extent, reflect intrauterine compromise rather than prematurity itself.11 Indeed, cesarean deliveries occurred more often at shorter gestational ages (Table 1), but exclusion of cesarean deliveries (n=36) from the analyses did not materially affect our estimates (0.44 [0.11–0.76] mL/kg per minute per week increase in gestational age and RR of 1.53 [1.09–2.13] for incidence of poor CRF between ET and FT or LT [combined]).
Although we have adjusted our association estimates for a comprehensive set of potential confounders, some residual confounding can still affect them. Indeed, both maternal BMI and smoking history were obtained at the start of the study, when participants were aged 12 to 15 years, and do not directly reflect exposures to these maternal factors during or before pregnancy. Finally, our study was conducted in a cohort of white individuals, born in the 1970s in Northern Ireland, a region characterized by a high prevalence and incidence of cardiovascular disease in Europe. Caution may, thus, be warranted when extrapolating our findings to other regions/countries, ethnicities, and/or younger birth cohorts. Investigation of how current rates of ET affect offspring's CRF in younger cohorts and how these associations may be modified by recent improvement in breastfeeding rates (or deterioration in other postnatal feeding practices) may constitute an important future research agenda.
Conclusions
Among singletons who were born at term, increased gestational age is associated with higher levels of CRF through adolescence to young adulthood. From a public health perspective, these findings may have important implications and could help shape policies to deter current trends towards avoidable deliveries at lower gestational ages.
Sources of Funding
The Northern Ireland Young Hearts Study was supported by The British Heart Foundation.
Disclosures
None.
Supporting information
Table SI. Analyses of Predictors of Attrition in Young Adulthood Among the 791 Participants Selected From the NIYHP for the Present Study
Figure S1. Trajectories of cardiorespiratory fitness (CRF) through adolescence to young adulthood in the whole study population and by sex. A, Maximal oxygen uptake (VO2max; in mL/min per kg). B, VO2max in mL/min per fat‐free mass (FFM). C, VO2max in mL/min per kg0.67. D, VO2max in mL/min per kg0.79. Mean CRF levels at each age were estimated with generalized estimating equation models. Error bars indicate 95% CIs.
Figure S2. Trajectories of cardiorespiratory fitness (CRF) through adolescence to young adulthood by categories of gestational age. A, Maximal oxygen uptake (VO2max) expressed by VO2max in mL/min per fat‐free mass (FFM). B, VO2max in mL/min per kg0.67. C, VO2max in mL/min per kg0.79. Mean CRF levels or proportions with poor CRF at each age were estimated with generalized estimating equation models adjusted for sex, age and sex interactions, cohort, birth weight Z scores, socioeconomic status, delivery mode, breastfeeding, and maternal age, body mass index, and smoking. Error bars indicate 95% CIs.
(J Am Heart Assoc. 2017;6:e006467 DOI: 10.1161/JAHA.117.006467.)
The main results of this work were presented orally at the American Heart Association's Epidemiology and Prevention/Lifestyle and Cardiometabolic Health Scientific Sessions, March 7—10, Portland, OR.
References
- 1. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. [DOI] [PubMed] [Google Scholar]
- 2. Reddy, Bettegowda VR, Dias T, Yamada‐Kushnir T, Ko CW, Willinger M. Term pregnancy: a period of heterogeneous risk for infant mortality. Obstet Gynecol. 2011;117:1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nassar N, Schiff M, Roberts CL. Trends in the distribution of gestational age and contribution of planned births in New South Wales, Australia. PLoS One. 2013;8:e56238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Espel EV, Glynn LM, Sandman CA, Davis EP. Longer gestation among children born full term influences cognitive and motor development. PLoS One. 2014;9:e113758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghartey K, Coletta J, Lizarraga L, Murphy E, Ananth CV, Gyamfi‐Bannerman C. Neonatal respiratory morbidity in the early term delivery. Am J Obstet Gynecol. 2012;207:292.e291–294. [DOI] [PubMed] [Google Scholar]
- 6. Noble KG, Fifer WP, Rauh VA, Nomura Y, Andrews HF. Academic achievement varies with gestational age among children born at term. Pediatrics. 2012;130:e257–e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rose O, Blanco E, Martinez SM, Sim EK, Castillo M, Lozoff B, Vaucher YE, Gahagan S. Developmental scores at 1 year with increasing gestational age, 37‐41 weeks. Pediatrics. 2013;131:e1475–e1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Talge NM, Allswede DM, Holzman C. Gestational age at term, delivery circumstance, and their association with childhood attention deficit hyperactivity disorder symptoms. Paediatr Perinat Epidemiol. 2016;30:171–180. [DOI] [PubMed] [Google Scholar]
- 9. Yang S, Bergvall N, Cnattingius S, Kramer MS. Gestational age differences in health and development among young Swedish men born at term. Int J Epidemiol. 2010;39:1240–1249. [DOI] [PubMed] [Google Scholar]
- 10. Broekman BF, Wang C, Li Y, Rifkin‐Graboi A, Saw SM, Chong YS, Kwek K, Gluckman PD, Fortier MV, Meaney MJ, Qiu A; GUSTO Study Group . Gestational age and neonatal brain microstructure in term born infants: a birth cohort study. PLoS One. 2014;9:e115229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boyle EM, Poulsen G, Field DJ, Kurinczuk JJ, Wolke D, Alfirevic Z, Quigley MA. Effects of gestational age at birth on health outcomes at 3 and 5 years of age: population based cohort study. BMJ. 2012;344:e896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang X, Kramer MS. Variations in mortality and morbidity by gestational age among infants born at term. J Pediatr. 2009;154:358–362. [DOI] [PubMed] [Google Scholar]
- 13. Crump C, Sundquist K, Winkleby MA, Sundquist J. Early‐term birth (37‐38 weeks) and mortality in young adulthood. Epidemiology. 2013;24:270–276. [DOI] [PubMed] [Google Scholar]
- 14. Wu CS, Sun Y, Nohr EA, Olsen J. Trends in all‐cause mortality across gestational age in days for children born at term. PLoS One. 2015;10:e0144754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferreira I, Twisk JW. Physical activity, cardiorespiratory fitness, and cardiovascular health In: Armstrong N, van Mechelen W, eds. Oxford Textbook of Children's Sport and Exercise Medicine. Oxford, England: Oxford University Press; 2017:239–254. [Google Scholar]
- 16. Ruiz JR, Cavero‐Redondo I, Ortega FB, Welk GJ, Andersen LB, Martinez‐Vizcaino V. Cardiorespiratory fitness cut points to avoid cardiovascular disease risk in children and adolescents: what level of fitness should raise a red flag? A systematic review and meta‐analysis. Br J Sports Med. 2016;50:1451–1458. [DOI] [PubMed] [Google Scholar]
- 17. Ferreira I, Twisk JW, van Mechelen W, Kemper HC, Stehouwer CD. Development of fatness, fitness, and lifestyle from adolescence to the age of 36 years: determinants of the metabolic syndrome in young adults: the Amsterdam Growth and Health Longitudinal Study. Arch Intern Med. 2005;165:42–48. [DOI] [PubMed] [Google Scholar]
- 18. Falk B, Eliakim A, Dotan R, Liebermann DG, Regev R, Bar‐Or O. Birth weight and physical ability in 5‐ to 8‐yr‐old healthy children born prematurely. Med Sci Sports Exerc. 1997;29:1124–1130. [DOI] [PubMed] [Google Scholar]
- 19. Kilbride HW, Gelatt MC, Sabath RJ. Pulmonary function and exercise capacity for ELBW survivors in preadolescence: effect of neonatal chronic lung disease. J Pediatr. 2003;143:488–493. [DOI] [PubMed] [Google Scholar]
- 20. Rogers M, Fay TB, Whitfield MF, Tomlinson J, Grunau RE. Aerobic capacity, strength, flexibility, and activity level in unimpaired extremely low birth weight (<or=800 g) survivors at 17 years of age compared with term‐born control subjects. Pediatrics. 2005;116:e58–e65. [DOI] [PubMed] [Google Scholar]
- 21. Vrijlandt EJ, Gerritsen J, Boezen HM, Grevink RG, Duiverman EJ. Lung function and exercise capacity in young adults born prematurely. Am J Respir Crit Care Med. 2006;173:890–896. [DOI] [PubMed] [Google Scholar]
- 22. Smith LJ, van Asperen PP, McKay KO, Selvadurai H, Fitzgerald DA. Reduced exercise capacity in children born very preterm. Pediatrics. 2008;122:e287–e293. [DOI] [PubMed] [Google Scholar]
- 23. Burns YR, Danks M, O'Callaghan MJ, Gray PH, Cooper D, Poulsen L, Watter P. Motor coordination difficulties and physical fitness of extremely‐low‐birthweight children. Dev Med Child Neurol. 2009;51:136–142. [DOI] [PubMed] [Google Scholar]
- 24. Welsh L, Kirkby J, Lum S, Odendaal D, Marlow N, Derrick G, Stocks J; EPICure Study Group . The EPICure study: maximal exercise and physical activity in school children born extremely preterm. Thorax. 2010;65:165–172. [DOI] [PubMed] [Google Scholar]
- 25. Svedenkrans J, Henckel E, Kowalski J, Norman M, Bohlin K. Long‐term impact of preterm birth on exercise capacity in healthy young men: a national population‐based cohort study. PLoS One. 2013;8:e80869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Danks M, Burns YR, Gibbons K, Gray PH, O'Callaghan MJ, Poulsen L, Watter P. Fitness limitations in non‐disabled extremely low birthweight adolescents. J Paediatr Child Health. 2013;49:548–553. [DOI] [PubMed] [Google Scholar]
- 27. Clemm HH, Vollsaeter M, Roksund OD, Eide GE, Markestad T, Halvorsen T. Exercise capacity after extremely preterm birth: development from adolescence to adulthood. Ann Am Thorac Soc. 2014;11:537–545. [DOI] [PubMed] [Google Scholar]
- 28. Clemm HH, Vollsaeter M, Roksund OD, Markestad T, Halvorsen T. Adolescents who were born extremely preterm demonstrate modest decreases in exercise capacity. Acta Paediatr. 2015;104:1174–1181. [DOI] [PubMed] [Google Scholar]
- 29. Tikanmaki M, Tammelin T, Sipola‐Leppanen M, Kaseva N, Matinolli HM, Miettola S, Eriksson JG, Jarvelin MR, Vaarasmaki M, Kajantie E. Physical fitness in young adults born preterm. Pediatrics. 2016;137:e20151289. [DOI] [PubMed] [Google Scholar]
- 30. Boreham C, Savage JM, Primrose D, Cran G, Strain J. Coronary risk factors in schoolchildren. Arch Dis Child. 1993;68:182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boreham C, Twisk J, Murray L, Savage M, Strain JJ, Cran G. Fitness, fatness, and coronary heart disease risk in adolescents: the Northern Ireland Young Hearts Project. Med Sci Sports Exerc. 2001;33:270–274. [DOI] [PubMed] [Google Scholar]
- 32. Boreham CA, Murray L, Dedman D, Davey Smith G, Savage JM, Strain JJ. Birthweight and aerobic fitness in adolescents: the Northern Ireland Young Hearts Project. Public Health. 2001;115:373–379. [DOI] [PubMed] [Google Scholar]
- 33. Gallagher AM, Savage JM, Murray LJ, Davey Smith G, Young IS, Robson PJ, Neville CE, Cran G, Strain JJ, Boreham CA. A longitudinal study through adolescence to adulthood: the Young Hearts Project, Northern Ireland. Public Health. 2002;116:332–340. [DOI] [PubMed] [Google Scholar]
- 34. Holmes VA, Cardwell C, McKinley MC, Young IS, Murray LJ, Boreham CA, Woodside JV. Association between breast‐feeding and anthropometry and CVD risk factor status in adolescence and young adulthood: the Young Hearts Project, Northern Ireland. Public Health Nutr. 2010;13:771–778. [DOI] [PubMed] [Google Scholar]
- 35. van Lenthe FJ, Boreham CA, Twisk JW, Savage MJ, Murray L, Smith GD. What determines drop out in prospective studies of coronary heart disease risk factors between youth and young adulthood: the Young Hearts Study. J Epidemiol Community Health. 2001;55:681–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Spong CY. Defining “term” pregnancy: recommendations from the Defining “Term” Pregnancy Workgroup. JAMA. 2013;309:2445–2446. [DOI] [PubMed] [Google Scholar]
- 37. Van Lenthe FJ, Boreham CA, Twisk JW, Strain JJ, Savage JM, Smith GD. Socio‐economic position and coronary heart disease risk factors in youth: findings from the Young Hearts Project in Northern Ireland. Eur J Public Health. 2001;11:43–50. [DOI] [PubMed] [Google Scholar]
- 38. Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17:407–429. [PubMed] [Google Scholar]
- 39. Tomkinson GR, Lang JJ, Tremblay MS, Dale M, LeBlanc AG, Belanger K, Ortega FB, Leger L. International normative 20 m shuttle run values from 1 142 026 children and youth representing 50 countries [published online ahead of print May 20, 2016]. Br J Sports Med. DOI: 10.1136/bjsports-2016-095987. Available at: http://bjsm.bmj.com/content/early/2016/05/20/bjsports-2016-095987. Accessed July 20, 2017. [DOI] [PubMed] [Google Scholar]
- 40. Tomkinson GR, Leger LA, Olds TS, Cazorla G. Secular trends in the performance of children and adolescents (1980–2000): an analysis of 55 studies of the 20 m shuttle run test in 11 countries. Sports Med. 2003;33:285–300. [DOI] [PubMed] [Google Scholar]
- 41. Leger LA, Mercier D, Gadoury C, Lambert J. The multistage 20 metre shuttle run test for aerobic fitness. J Sports Sci. 1988;6:93–101. [DOI] [PubMed] [Google Scholar]
- 42. Boreham CA, Ferreira I, Twisk JW, Gallagher AM, Savage MJ, Murray LJ. Cardiorespiratory fitness, physical activity, and arterial stiffness: the Northern Ireland Young Hearts Project. Hypertension. 2004;44:721–726. [DOI] [PubMed] [Google Scholar]
- 43. Heywood V. The physical fitness specialist certification manual, The Cooper Institute for Aerobics Research, Dallas TX In: Heywood V, ed. Advance Fitness Assessment & Exercise Prescription. 2nd ed Leeds, England: Human Kinetics; 1998:48. [Google Scholar]
- 44. Armstrong N, Welsman JR. Peak oxygen uptake in relation to growth and maturation in 11‐ to 17‐year‐old humans. Eur J Appl Physiol. 2001;85:546–551. [DOI] [PubMed] [Google Scholar]
- 45. Nevill AM, Bate S, Holder RL. Modeling physiological and anthropometric variables known to vary with body size and other confounding variables. Am J Phys Anthropol. 2005;128(suppl S41):141–153. [DOI] [PubMed] [Google Scholar]
- 46. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. [DOI] [PubMed] [Google Scholar]
- 48. Yelland LN, Salter AB, Ryan P. Performance of the modified Poisson regression approach for estimating relative risks from clustered prospective data. Am J Epidemiol. 2011;174:984–992. [DOI] [PubMed] [Google Scholar]
- 49. Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics. 1993;49:865–872. [PubMed] [Google Scholar]
- 50. Newson RB. Attributable and unattributable risks and fractions and other scenario comparisons. Stata J. 2013;13:672–698. [Google Scholar]
- 51. Cooper R, Atherton K, Power C. Gestational age and risk factors for cardiovascular disease: evidence from the 1958 British birth cohort followed to mid‐life. Int J Epidemiol. 2009;38:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Carnethon MR, Gidding SS, Nehgme R, Sidney S, Jacobs DR Jr, Liu K. Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA. 2003;290:3092–3100. [DOI] [PubMed] [Google Scholar]
- 53. Hogstrom G, Nordstrom A, Nordstrom P. High aerobic fitness in late adolescence is associated with a reduced risk of myocardial infarction later in life: a nationwide cohort study in men. Eur Heart J. 2014;35:3133–3140. [DOI] [PubMed] [Google Scholar]
- 54. Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all‐cause mortality and cardiovascular events in healthy men and women: a meta‐analysis. JAMA. 2009;301:2024–2035. [DOI] [PubMed] [Google Scholar]
- 55. Children's Health in Northern Ireland . A statistical profile of births using data drawn from the Northern Ireland Child Health System, Northern Ireland Maternity System and Northern Ireland Statistics and Research Agency. Public Health Intelligence Unit. 2016. Available at: http://www.publichealth.hscni.net/sites/default/files/RUAG%20report%202015-16%20-%20Childrens%20Health%20in%20NI%20-%20FINAL%20REPORT%20-%20May%202016.pdf. Accessed July 20, 2017.
- 56. Boreham CA, Paliczka VJ, Nichols AK. A comparison of the PWC170 and 20‐MST tests of aerobic fitness in adolescent schoolchildren. J Sports Med Phys Fitness. 1990;30:19–23. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Analyses of Predictors of Attrition in Young Adulthood Among the 791 Participants Selected From the NIYHP for the Present Study
Figure S1. Trajectories of cardiorespiratory fitness (CRF) through adolescence to young adulthood in the whole study population and by sex. A, Maximal oxygen uptake (VO2max; in mL/min per kg). B, VO2max in mL/min per fat‐free mass (FFM). C, VO2max in mL/min per kg0.67. D, VO2max in mL/min per kg0.79. Mean CRF levels at each age were estimated with generalized estimating equation models. Error bars indicate 95% CIs.
Figure S2. Trajectories of cardiorespiratory fitness (CRF) through adolescence to young adulthood by categories of gestational age. A, Maximal oxygen uptake (VO2max) expressed by VO2max in mL/min per fat‐free mass (FFM). B, VO2max in mL/min per kg0.67. C, VO2max in mL/min per kg0.79. Mean CRF levels or proportions with poor CRF at each age were estimated with generalized estimating equation models adjusted for sex, age and sex interactions, cohort, birth weight Z scores, socioeconomic status, delivery mode, breastfeeding, and maternal age, body mass index, and smoking. Error bars indicate 95% CIs.


