Abstract
Background
In obstructive hypertrophic cardiomyopathy patients with preserved left ventricular (LV) ejection fraction, we sought to determine whether LV global longitudinal strain (LV‐GLS) provided incremental prognostic utility.
Methods and Results
We studied 1019 patients with documented hypertrophic cardiomyopathy (mean age, 50±12 years; 63% men) evaluated at our center between 2001 and 2011. We excluded age <18 years, maximal LV outflow tract gradient <30 mm Hg, bundle branch block or atrial fibrillation, past pacemaker/cardiac surgery, including myectomy/alcohol ablation, and obstructive coronary artery disease. Average resting LV‐GLS was measured offline on 2‐, 3‐, 4‐chamber views using Velocity Vector Imaging (Siemens, Malvern, PA). Outcome was a composite of cardiac death and appropriate internal defibrillator (implantable cardioverter defibrillator) discharge. Maximal LV thickness, LV ejection fraction, indexed left atrial dimension, rest and maximal LV outflow tract gradient, and LV‐GLS were 2.0±0.2 cm, 62±4%, 2.2±4 cm/m2, 52±42 mm Hg, 103±36 mm Hg, and −13.6±4%. During 9.4±3 years of follow‐up, 668 (66%), 166 (16%), and 122 (20%), respectively, had myectomy, atrial fibrillation, and implantable cardioverter defibrillator implantation, whereas 69 (7%) had composite events (62 cardiac deaths). Multivariable competing risk regression analysis revealed that higher age (subhazard ratio, 1.04 [1.02–1.07]), AF during follow‐up (subhazard ratio, 1.39 [1.11–1.69]), and worsening LV‐GLS (subhazard ratio, 1.11 [1.05–1.22]) were associated with worse outcomes, whereas myectomy (subhazard ratio, 0.44 [0.25–0.72]) was associated with improved outcomes (all P<0.01). Sixty‐one percent of events occurred in patients with LV‐GLS worse than median (−13.7%).
Conclusions
In obstructive hypertrophic cardiomyopathy patients with preserved LV ejection fraction, abnormal LV‐GLS was independently associated with higher events, whereas myectomy was associated with improved outcomes.
Keywords: hypertrophic cardiomyopathy, outcome, strain
Subject Categories: Hypertrophy, Echocardiography, Mortality/Survival
Clinical Perspective
What Is New?
In a large group of obstructive hypertrophic cardiomyopathy patients with preserved left ventricular (LV) ejection fraction, abnormal LV‐global longitudinal strain is independently associated with higher events, whereas myectomy is associated with improved outcomes.
What Are the Clinical Implications?
We could potentially identify “at‐risk” obstructive hypertrophic cardiomyopathy patients with an abnormal LV‐global longitudinal strain who may potentially benefit from an earlier surgery given that the cohort with LV‐global longitudinal strain worse than median who had not undergone myectomy had significantly worse outcomes.
Additionally, a small proportion of hypertrophic cardiomyopathy patients with severely reduced LV‐global longitudinal strain (worse than ≈ −7%) appear to decline clinically, despite myectomy, and may eventually warrant additional risk stratification and, in some instances, transplantation.
Introduction
Hypertrophic cardiomyopathy (HCM) is a common inherited cardiomyopathy with a varied phenotypic expression ranging from asymptomatic to congestive heart failure (CHF) to death, which occurs in ≈0.5%/year.1, 2 Left ventricular (LV) outflow tract (LVOT) obstruction, observed in ≈70% HCM patients, results in reduced exercise capacity, which, if uncorrected, can progress to CHF and death.3, 4, 5 In such patients, surgical myectomy provides long‐term relief of LVOT obstruction and freedom from recurrent symptoms.6, 7, 8, 9, 10 Because of ambiguity in patients' perception of their symptoms, we utilize a combination of symptoms and assessment of exercise capacity (to elicit latent symptoms/LVOT gradient) to determine surgical timing in obstructive HCM patients with an improvement in long‐term survival in the operated patients versus those managed conservatively.11
According to the current guidelines, especially in patients with significant mitral regurgitation and aortic stenosis, there are recommendations to offer earlier surgery in selected “at‐risk” individuals, preceding onset of symptoms or LV dysfunction.12 This has resulted in an increased impetus to identify predisposed individuals earlier. Recent data have suggested that LV global longitudinal strain (LV‐GLS) provides quantitative assessment of LV contractile function, which is more sensitive than LV ejection fraction (LVEF).13 In HCM, LV‐GLS is associated with histopathological changes, in vitro myocardial performance, and myocardial fibrosis.14, 15, 16 Although it is intuitive to think that LV‐GLS could potentially offer incremental utility in HCM patients, prognostic data regarding the role of LV‐GLS in HCM patients are limited.17, 18, 19, 20, 21, 22 We sought to study whether LV‐GLS provides incremental prognostic utility for long‐term events in patients with obstructive HCM.
Methods
Study Population
This observational study consisted of 1019 consecutive patients, aged ≥18 years with obstructive HCM, who underwent a clinical evaluation at our tertiary care center between 2001 and 2011. They constitute a part of an ongoing institutional review board–approved registry with waiver of individual informed consent. Of the original data set of 2483 patients, we excluded the following patients: (1) patients with concomitant ≥moderate aortic/mitral stenosis (n=162); (2) LVEF <50% (n=38); (3) maximal (including provokable) LVOT obstruction <30 mm Hg (n=407); (4) apical HCM (n=62); (5) past alcohol ablation or myectomy (n=51); (6) bundle branch block or atrial fibrillation (AF; n=220); (7) past pacemaker+defibrillator (n=190); and (8) documented obstructive coronary artery disease (n=235). Given that symptomatic patients with severe apical hypertrophy and nonobstructive HCM have a different pathophysiological course and management, we restricted our analysis only to obstructive HCM patients. Because myectomy significantly impacts outcomes,6, 7, 8, 9, 10, 11 it would not be appropriate to compare outcomes in a group of obstructed HCM patients who had a myectomy versus those who never needed one (because of lack of LVOT obstruction). Forty‐six patients who underwent alcohol septal ablation during follow‐up were excluded because it was performed in individuals who were turned down for surgery because of prohibitive noncardiac comorbidities. In addition, 53 patients with unreliable LV‐GLS data were not included in the analysis. The diagnosis of HCM was made by experienced cardiologists, based on typical features, with ventricular myocardial hypertrophy (LV wall thickness ≥15 mm) occurring in absence of any other disease responsible for hypertrophy.1, 2 Additionally, in patients with borderline LV wall thickness (≈15 mm), presence of resting/provocable LVOT obstruction (LVOT gradient ≥30 mm Hg) also aided in the diagnosis.1, 2 No patients had uncontrolled hypertension or the characteristic sigmoid‐shaped septum with dynamic LVOT obstruction commonly observed on echocardiography with hypertensive heart disease.23, 24
Data were entered prospectively in the electronic medical records at the time of initial visit and subsequently manually extracted. We recorded major sudden cardiac death risk factors (0, 1, and ≥2), along with disease modifiers.1 Nonsustained ventricular tachycardia (VT) and abnormal blood pressure response to exercise (with severe LVOT obstruction) were not considered major risk factors.1 In addition, based on the 2014 European Society of Cardiology (ESC) guidelines, we calculated % 5‐year risk of sudden cardiac death (ESC risk score) using the previously described formula.2 Maximal LVOT gradient was recorded as the highest number obtained either at rest or provocation (but excluding the maximal gradient obtained on exercise stress echocardiography). Complete data on nonsustained VT were obtained as follows: 77% from Holter monitors performed at our institution and 22% from Holter monitoring performed at referring institutions within 1 year of their initial evaluation at our institution.
Imaging
All patients underwent comprehensive echocardiograms using commercially available equipment (Philips, Bothell, WA; General Electric, Waukesha, WI; and Siemens, Malvern, PA). Maximal end‐diastolic LV wall thickness (maximum recorded dimension of the LV on 2‐dimensional images), LV dimensions, and left atrial area were measured according to guidelines.25 Resting LVOT peak velocity was measured by continuous‐wave Doppler echocardiography, and pressure gradient was estimated by using simplified Bernoulli equation. Care was taken to avoid contamination of LVOT waveform by mitral regurgitation if present. In patients with resting LVOT gradients <30 mm Hg, provocative maneuvers, including Valsalva and amyl nitrite, were used. In patients with low resting gradients and/or New York Heart Association Class I and II symptoms, we performed exercise treadmill echocardiography to assess exercise impairment, blood pressure response, and maximal postexercise LVOT gradient.26 Abnormal blood pressure response, defined as a 20 mm Hg drop in systolic blood pressure at peak stress or lack of augmentation at peak stress, was recorded.1, 2 In patients with resting peak LVOT gradient >100 mm Hg, provocative maneuvers were not performed. Degree of resting mitral regurgitation was assessed (none‐severe), using multiple criteria.27 Grades of resting diastolic function were assigned, based upon multiple criteria (E to A ratio, left atrial area, and LVEF).28 Tissue Doppler data were incorporated where available. In an event of missing data, stored images were retrieved and the measurements were recorded.
Left Ventricular Global Longitudinal Strain
LV‐GLS measurements were obtained from baseline resting transthoracic echocardiograms using gray‐scale images recorded in apical 2‐, 3‐, and 4‐chamber views. LV‐GLS was analyzed offline using Velocity Vector Imaging (VVI; Siemens Medical Solutions, Mountain View, CA).14, 16, 24 All raw data were stored in DICOM format without compression. The frame rate was ≥30 frames/sec. After manual definition of the LV endocardial border, the endocardium was automatically tracked throughout the cardiac cycle. Global LV strain was obtained by averaging all segmental strain values from all 3 apical views. Peak global strain was defined as the peak negative value on the strain curve during the entire cardiac cycle. All measurements were made offline by investigators blinded to all clinical and demographic information (A.T.R., J.B.). Measurements were performed and averaged over 3 cardiac cycles. Because the reported LV‐GLS values are negative, a lower absolute number represented a worse value than higher (Figure 1).
Figure 1.
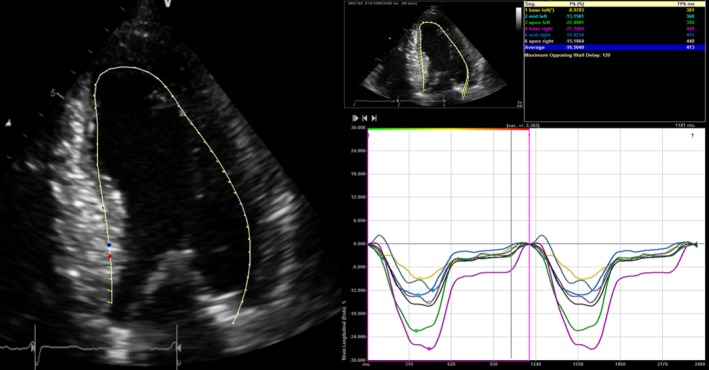
An example of left ventricular global longitudinal strain measurement from the current study.
Surgery for LVOT Obstruction
In patients who had undergone surgery to relieve LVOT obstruction, date and type of surgical procedures performed were recorded: (1) myectomy and (2) myectomy+mitral valve repair/replacement. Because of exclusion of patients with obstructive coronary disease, no patient needed concomitant coronary artery bypass grafting. Details of surgical techniques have been described previously.7, 10, 29 The indications for surgery were as follows: New York Heart Association Class III/IV or intractable angina; exertional syncope with severe (>50 mm Hg) LVOT obstruction; and impaired exercise capacity. Patients deemed in New York Heart Association Class I had additional reasons (ie, exertional syncope with severe LVOT obstruction or impaired exercise capacity) to recommend surgery. The need for surgery was ascertained after a consensus between experienced cardiologists and cardiothoracic surgeons.
Outcomes Assessment
Duration of follow‐up ranged between initial office visit and event/last office follow‐up. We ascertained events by phone call follow‐up to the patient/family member. Death notification was confirmed by observation of death certificate or verified with a family member. When phone call follow‐up was not feasible, we queried state and nationally available databases. The last query was performed in May 2016. Cause of death was ascertained as cardiac death, noncardiac death, or unknown after review of records and/or discussion with family. In addition, we recorded successful resuscitation from cardiac arrest or appropriate internal cardioverter defibrillator (ICD) shocks (with defibrillation threshold of >200 beats on electrogram reviews). For the composite primary end point, we included cardiac death (not including documented noncardiac death attributed to cancer, liver failure, and primary respiratory or neurological issues) and appropriate ICD discharge. Patients with an unknown cause of death were included as part of the composite primary outcome, because these patients died suddenly and none of them had a documented noncardiac comorbidity to explain their death, as previously described.9 In addition, we also recorded a secondary outcome of all‐cause mortality and appropriate ICD discharge. Arrhythmias, occurring during follow‐up (atrial fibrillation [AF], VT, and nonsustained VT) were recorded based on history, ECGs, Holter monitoring, and telemetry performed at our institution or locally during follow‐up. In the subgroup that underwent surgery, AF occurring within the first 30 days postoperatively was not included in the analysis.
Statistical Analysis
Continuous variables are expressed as mean±SD and/or median (with interquartile range) and compared using ANOVA (normal distribution) or Mann–Whitney U test (non‐normal distribution), as appropriate. Categorical data are expressed as percentage and compared using chi‐square. Variability of LV‐GLS was assessed by the 2 observers (A.T.R., J.B.) in 9 subjects, with each observer measuring each sample 2 times, for a total of 36 measurements. Subsequently, a 2‐way ANOVA was performed in order to calculate intra‐ and interobserver standard error of measurements (SEMintra, SEMinter). SEMintra expresses the random error by a typical observer, whereas SEMinter is a measurement of the variation caused when there are 2 different observers. SEMinter calculations were performed assuming a random‐effects model. Because longer‐term primary composite events and noncardiac death were competing risks, multivariable (adjusting for relevant variables associated with outcomes in HCM patients) survival analysis was performed by competing risk regression analysis using the Fine–Gray proportional subhazards model, and subdistribution hazard ratios were calculated, along with 95% confidence intervals.30, 31 Similarly, to assess for the association of various predictors with longer‐term secondary composite outcomes, multivariable Cox proportional hazards analysis was utilized and hazard ratio with 95% confidence interval were calculated. In the operative group, only AF noted beyond >30 days was utilized for survival analysis. Additionally, Kaplan–Meier curves were generated to determine the cumulative proportion of patients with events as a function over time and compared using log‐rank statistic. The discriminative ability of survival models for longer‐term composite primary events were compared using c‐statistic. Categorical net reclassification improvement (NRI) was utilized to determine significant reclassification of risk for composite primary events (noncardiac deaths were censored at the time of event). We evaluated the relationship between LV‐GLS and risk of composite primary events using a parametric multiphase hazard model. To assess the possible nonlinear relationship between LV‐GLS and risk of a primary composite event, covariate LV‐GLS was modeled as a quadratic spline with 5 knots at 10th, 25th, 50th, 75th, and 90th percentile values of LV‐GLS. Statistical analysis was performed using SPSS (version 11.5; SPSS Inc, Chicago, IL), SAS (version 9.4; SAS Institute Inc, Cary, NC), and R software (3.0.3; R foundation for Statistical Computing, Vienna, Austria). A P value of <0.05 was considered significant.
Results
The clinical and echocardiographic data are shown in Tables 1 and 2. A high proportion (75%) of patients did not have a major sudden cardiac death risk factor. Similarly, the mean ESC risk score was low (4.1±0.7) and 65% patients belonged to the “low‐risk” category. The median resting LV‐GLS in was (−13.7% [−16.2%, −11.0%]), and only 47 (5%) patients had an LV‐GLS value better than −19.7% (considered to be the “normal” cutoff32). Forty‐four (4%) patients had severely reduced LV‐GLS at < −7%, with no difference between surgical and nonsurgical groups (33 [5%] versus 11 [3%]; P=0.12). The intra‐ and interobserver variability of LV‐GLS measurements were as follows: SEMintra was 0.8% whereas SEMinter was 1.3%. Six‐hundred twenty‐seven patients underwent metabolic exercise stress echocardiography with a mean peak oxygen consumption of 21 mL/kg/min, and only 41% patients achieved >85% age‐sex predicted metabolic equivalents of task. At a median time of 37 [8, 118] days following initial evaluation at our center, 668 (66%) underwent surgical relief of LVOT obstruction.
Table 1.
Baseline Demographic and Clinical Data of the Study Population (n=1019)
| Variable | Total Sample (N=1019) |
|---|---|
| Age, y | 50±12 |
| Male sex | 640 (63%) |
| Hypertension | 454 (45%) |
| Diabetes mellitus | 120 (12%) |
| Family history of HCM | 172 (17%) |
| Family history of sudden cardiac death | 110 (11%) |
| History of sudden cardiac death | 6 (0.6%) |
| Unexplained syncope | 146 (14%) |
| History of nonsustained ventricular tachycardia | 112 (11%) |
| Beta‐blockers | 746 (73%) |
| Calcium‐channel blocker | 316 (31%) |
| Disopyramide | 42 (4%) |
| Angina | 143 (14%) |
| NYHA Class | |
| I | 296 (29%) |
| II | 526 (52%) |
| III/IV | 197 (19%) |
| Major HCM risk factors | |
| None | 766 (75%) |
| 1 | 225 (22%) |
| 2 or more | 28 (3%) |
| 5‐y ESC risk score (%) | 4.1±0.7 |
| ESC risk score categories | |
| Low (<4%) | 663 (65%) |
| Intermediate (4–6%) | 194 (19%) |
| High (>6%) | 162 (16%) |
ESC indicates European Society of Cardiology; HCM, hypertrophic cardiomyopathy; NYHA, New York Heart Association.
Table 2.
Baseline Echocardiographic Variables for the Study Population (n=1019)
| Variable | Total (N=1019) |
|---|---|
| Left ventricular ejection fraction, % | 62±4 |
| Indexed left ventricular end‐diastolic dimension, cm/m2 | 2.02±0.3 |
| Indexed left ventricular end‐systolic dimension, cm/m2 | 1.2±0.3 |
| Maximal left ventricular thickness, cm | 2.0±0.2 |
| Indexed left atrial dimensions, cm/m2 | 2.2±0.4 |
| Diastolic dysfunction | |
| Abnormal relaxation | 937 (92%) |
| Pseudonormal | 71 (7%) |
| Restrictive physiology | 11 (1%) |
| Resting mitral regurgitation | |
| None | 43 (4%) |
| I to II+ | 881 (87%) |
| ≥III+ | 95 (9%) |
| Resting LVOT gradient, mm Hg | 52±42 |
| Resting LVOG gradient >30 mm Hg | 718 (70%) |
| Maximal LVOT gradient, mm Hg | 103±36 |
| LV‐GLS, % | −13.6±4 |
| LV‐GLS | |
| Better than median (≥ −13.7%) | 510 (50%) |
| Worse than median (<13.7%) | 509 (50%) |
| Metabolic exercise echocardiography | 627 (62%) |
| >85% Age‐sex predicted METsa | 255 (41%) |
| Maximum METs achieveda | 6.6±2 |
| Peak oxygen consumption, mL/kg/mina | 21±6 |
| Abnormal BP response to exercisea | 8 (1%) |
BP indicates blood pressure; LV‐GLS, left ventricular global longitudinal strain; LVOT, left ventricular outflow tract; METs, metabolic equivalents of task.
Data based upon the subgroup that underwent cardiopulmonary stress echocardiography.
Outcomes
Mean total duration of follow‐up was 9.4±3 years (median, 9.4 [interquartile range, 6.9, 11.9 years]). Surgical details, along with differences in relevant characteristics, separated based on surgery versus no surgery, are shown in Data S1 and Table S1.
During this time frame, there were a total of 72 deaths (62 cardiac and 10 noncardiac deaths). A total of 69 patients (10%) met the composite primary end point (62 cardiac deaths and 7 appropriate ICD discharge), whereas 79 met the secondary composite end point (all‐cause mortality and appropriate ICD discharge). No patient needed a heart transplantation or had a stroke‐related death. In patients who developed multiple end points, time to first event was utilized as an event time cutoff. There were no deaths within 30 days (or preceding hospital discharge) following myectomy. In addition, 24 (2%) patients had a stroke, with no difference between surgical and nonsurgical subgroups (1.9% versus 2.7%; P=0.22). Also, there were 10 patients in the nonsurgical group that progressed to New York Heart Association Class III and IV during follow‐up, but did not have LVOT obstruction >50 mm Hg to justify surgical myectomy.
Multivariable survival analysis for the primary composite outcome, using the competing risk assumptions, are shown in Table 3. When LV‐GLS was added to clinical model 1 (standard major risk factors+age+sex+LVOT gradient+new AF during follow‐up), the categorical NRI was 0.28 (0.22–0.34; P<0.001). Further addition of myectomy increased the categorical NRI to 0.38 (0.26–0.51; P<0.01). The c‐statistic for clinical model 1 (as above) was 0.59 (0.51–0.73; P=0.04). Further addition of LV‐GLS and myectomy increased the c‐statistic to 0.69 (0.58–0.81) and 0.78 (0.64–0.89), respectively (both P<0.001). Similarly, when LV‐GLS was added to clinical model 2 (ESC risk score+new AF during follow‐up), the categorical NRI was 0.31 (0.24–0.37; P<0.001). Further addition of myectomy increased the categorical NRI to 0.40 (0.25–0.53; P<0.01). The c‐statistic for clinical model 2 (as above) was 0.61 (0.52–0.75; P=0.04). Further addition of LV‐GLS and myectomy increased the c‐statistic to 0.70 (0.57–0.82) and 0.77 (0.63–0.90), respectively (both P<0.001).
Table 3.
Multivariable Competing Risk Regression Analysis in the Study Population (n=1019) Based on the Fine–Gray Proportional Subhazards Model (Primary Composite Events=69; Noncardiac Deaths=10)
| Subhazard Ratio | P Value | |
|---|---|---|
| (A) Model 1 (with standard major ACC/AHA SCD risk factors included in analysis) | ||
| Age, y | 1.04 [1.02–1.07] | <0.001 |
| Atrial fibrillation during follow‐up | 1.39 [1.11–1.69] | <0.001 |
| LV‐GLS (for every % worsening) | 1.11 [1.05–1.22] | <0.001 |
| Surgical myectomy | 0.44 [0.25–0.72] | <0.01 |
| Following potential additional predictors were considered for analysis, but were not significant: standard ACC/AHA major SCD risk factors (none, 1, ≥2), sex, maximal LVOT gradient, medical therapy | ||
| (B) Model 2 (with ESC risk score included in analysis) | ||
| Atrial fibrillation during follow‐up | 1.47 [1.17–2.21] | <0.001 |
| LV‐GLS (for every % worsening) | 1.13 [1.08–1.22] | <0.001 |
| Surgical myectomy | 0.42 [0.22–0.64] | <0.01 |
| Following potential additional predictors were considered for analysis, but were not significant: ESC risk score, sex, medical therapy | ||
ACC/AHA indicates American College of Cardiology and American Heart Association; ESC, European Society of Cardiology; LV‐GLS, left ventricular global longitudinal strain; LVOT, left ventricular outflow tract; SCD, sudden cardiac death.
The breakdown of composite primary events in patients, separated of LV‐GLS better or worse than median, were significantly different as follows: 42 of 509 (8%) for LV‐GLS worse than median and 27 of 501 (5%) for LV‐GLS better than median (P=0.03). Kaplan–Meier curves, separated into these 2 categories, are shown in Figure 2. Of note, 61% of composite primary events occurred in patients with LV‐GLS worse than median. The breakdown of composite primary events in patients that underwent surgery versus not was as follows: 34 of 668 (5%) versus 35 of 351 (10%; P=0.003). Kaplan–Meier curves, separated based on surgery versus not, are shown in Figure 3.
Figure 2.
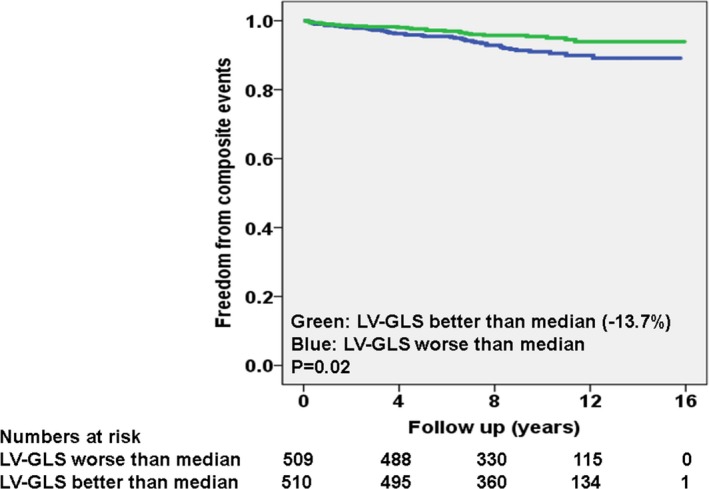
Kaplan–Meier curves for primary composite event, separated on the basis of left ventricular global longitudinal strain (LV‐GLS) better or worse than median.
Figure 3.
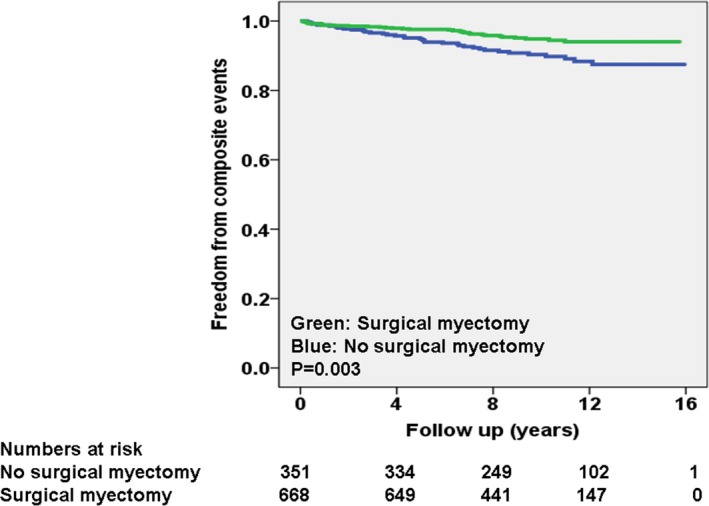
Kaplan–Meier curves for primary composite event, separated on the basis of myectomy vs not during follow‐up.
In order to understand the interplay surgery and abnormal LV‐GLS, we created 4 subgroups. The breakdown of composite primary events in these 4 subgroups were significantly different, as follows (P<0.01): (1) surgery and LV‐GLS better than median (14 of 322 [4%]); (2) no surgery and LV‐GLS better than median (14 of 189 [7%]); (3) surgery and LV‐GLS worse than median (20 of 346 [6%]); and (4) no surgery and LV‐GLS worse than median (21 of 162 [13%]). Kaplan–Meier curves, separated based on these 4 subgroups, are shown in Figure 4.
Figure 4.
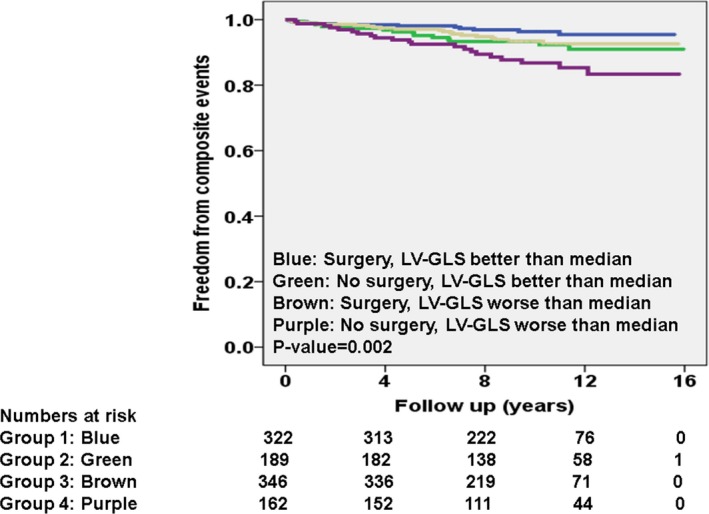
Kaplan–Meier curves for primary composite event, separated into 4 subgroups on the basis of left ventricular global longitudinal strain (LV‐GLS) better or worse than median and myectomy vs not during follow‐up.
Finally, the data on 5‐year hazard (for composite primary events) using quadratic spline with 5 knots, for the study population as a whole, as well as divided into surgical and nonsurgical groups, are shown in Figure 5A and 5B. Patients with LV‐GLS better than −14% had excellent 5‐year event‐free survival. However, the risk of events continuously increased when LV‐GLS worsened below −14%, with an exponential increase when LV‐GLS worsened below ≈ −7% (Figure 5A). These findings were similar in both surgical and nonsurgical patients (Figure 5B).
Figure 5.
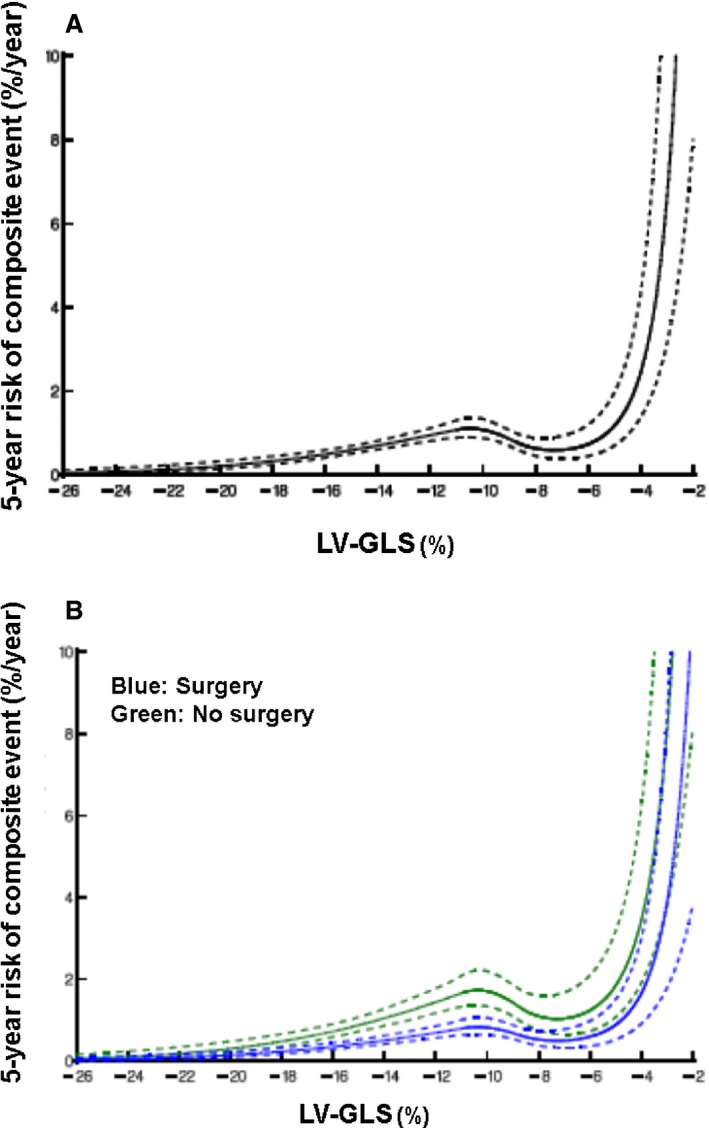
Nomogram of estimated risk of primary composites at 5 years for resting left ventricular global longitudinal strain (LV‐GLS) in (A) the overall cohort and (B) stratified based on myectomy vs not. Solid line represents the 5‐year parametric estimates of instantaneous risk of event. Solid lines are enclosed by a 68% confidence interval (dotted lines).
In addition, we performed multivariable Cox proportional hazard survival analysis for the secondary composite outcomes. The results were similar and are shown in Tables S2 and S3.
Discussion
In the current study of adult obstructive HCM patients with preserved LVEF managed at a high‐volume tertiary referral center, we demonstrate that LV‐GLS provides incremental prognostic utility for the long‐term events of appropriate ICD discharge and cardiac death. LV‐GLS also improved risk classification in these patients, independent of other known prognostic variables like American College of Cardiology and American Heart Association major risk factors and the ESC risk score (because these comprise of factors known to be associated with outcomes in HCM patients). Additionally, myectomy was associated with improved long‐term survival, as described.6, 7, 8, 9, 10, 11 Furthermore, obstructive HCM patients with resting LV‐GLS values worse than median who did not undergo surgery had significantly worse survival during longer‐term follow‐up. It appears that patients with LV‐GLS better than −14% had excellent 5‐year event‐free survival. However, the risk of events continuously increased when LV‐GLS worsened below −14%, with an exponential increase when LV‐GLS worsened below ≈ −7%, irrespective of surgery.
This was a “lower‐risk” population with the absence of major HCM‐related risk factors in 75% and a low ESC 5‐year risk score for 65%. This is likely attributed to our inclusion and exclusion criteria because we wanted to study a homogeneous study population to avoid significant confounding. We excluded nonobstructed HCM patients because the natural history and treatment considerations are very different from those that are not obstructed. The subgroup of nonobstructed patients includes a heterogeneous spectrum of patients including the ones that are (1) in the early stages with mild HCM and truly nonobstructive, (2) late‐stage burnt‐out HCM, and (3) apical HCM. Additionally, because of potential impact on LV‐GLS measurements, patients with bundle branch block, AF, or who had previously undergone myectomy/alcohol septal ablation were excluded. The exclusion of patients with past pacemaker/ICD placement inherently excludes patients with HCM who had these devices placed because they are at high risk for sudden cardiac death based on current recommendations. Additionally, patients with coronary artery disease were excluded because they are at higher risk compared with HCM patients without coronary artery disease. Correspondingly and not unexpectedly, over the long‐term follow‐up, the number of composite events (appropriate ICD discharge and cardiac death) were lower than what we have previously reported.10, 11
The assertion that subtle LV dysfunction may occur in patients before any reduction in LVEF was reiterated in the current study. The hallmark of HCM, at a histological level, is presence of myocyte disarray/hypertrophy, coronary arteriole dysplasia, and fibrosis,33 and these findings are likely mediated through abnormal genetic predispositions. These characteristics may result in premature LV hypertrophy independent of LVOT obstruction and increased afterload and can occur in the presence of preserved LV systolic function. Indeed, unlike severe AS or hypertensive heart disease of the elderly, where LV hypertrophy and LV‐GLS abnormalities are compensatory and occur at an older age, such changes are observed at a much earlier age in HCM.24, 34 In HCM, it has been recognized that some of these histopathological findings are associated with abnormalities in LV‐GLS, independent of LVOT obstruction, preceding the onset of overt LV systolic dysfunction. In a previous report, we demonstrated a significant association between preoperatively measured basal septal strain rate (at the site of the myocardium removed during myectomy) and myocyte disarray, hypertrophy, and coronary arteriole dysplasia observed on histopathological analysis of the myectomy tissue.14 In another proof‐of‐concept study, we demonstrated a significant association between basal septal strain rate and in vitro myocardial performance (resting tension and developed tension) of the same segment removed during myectomy.16 It appears that myocyte disarray, a hallmark of HCM histopathology, may predispose to worsening regional LV mechanics, preceding overt LV systolic dysfunction. Indeed past similar efforts in transgenic mice have demonstrated an association between myocardial fibrosis, strain, and actual myocardial performance.16 In addition to direct histopathological analysis quantifying myocardial fibrosis, we demonstrated that the identification of myocardial fibrosis noninvasively using late‐gadolinium enhancement on cardiac magnetic resonance imaging also significantly correlate with measurements of LV‐GLS.15
However, the prognostic data on LV‐GLS in HCM are sparse.17, 18, 19, 20, 21, 22 Two recent studies (24 and 32 patients each) have demonstrated an association between LV‐GLS and nonsustained ventricular tachycardia.17, 18 In a report of 44 patients, LV‐GLS was found to be attenuated in patients with a major cardiac event.19 In another study, LV‐GLS was an independent predictor of ICD therapy.20 Two studies (400 and 472 patients each) reported incremental prognostic utility of LV‐GLS in HCM; however, these studies included additional end points and the duration of follow‐up was relatively short at 3 to 4 years. One study included CHF, stroke, myocardial infarction, and need for transplantation whereas the other study included CHF admissions as part of the composite end point.21, 22
Another important point that needs to be recognized is that there is ambiguity with regard to the definition of what constitutes “normal” LV‐GLS. Based on a meta‐analysis, the cutoff for normal LV‐GLS value is −19.7%.32 The cutoff, observed in the current study, was also significantly lower than the normal LV‐GLS values (−17.3%) reported in a study of healthy individuals, free of cardiovascular disease, where only VVI software was utilized (similar to the current study).35 That study also demonstrated that LV‐GLS values obtained using VVI software (similar to our study) were similar for different echocardiography vendors.35 On the other hand, another study has demonstrated that whereas there is a very strong correlation between LV‐GLS values obtained using different strain analysis packages in the same individuals, the absolute mean values vary between −18% and −21.5% (with a mean of −20% for the Siemens strain software).36 However, that study included patients with a wide spectrum of LV systolic function, rather than healthy individuals free of cardiovascular disease. It appears that LV‐GLS seems to be load dependent and is significantly lower (even with preserved LVEF) in patients with severely increased afterload (eg, severe aortic stenosis), similar to obstructive HCM.32, 34, 37 However, despite similar median LV‐GLS values, survival in aortic stenosis patients is much worse than in HCM patients, potentially suggesting that an “acquired” abnormality, like in severe aortic stenosis,34 is significantly worse than a genetically mediated disease like HCM.
Based on the results of the current study, abnormal LV‐GLS may be useful to identify “at risk” obstructive HCM patients who may benefit from earlier myectomy given that the cohort with LV‐GLS worse than median who had not undergone myectomy had significantly worse outcomes. Additionally, a small proportion of HCM patients with severely reduced LV‐GLS (worse than ≈ −7%) appear to decline clinically despite surgical relief of LVOT obstruction and may eventually warrant additional risk stratification and, in some instances, transplantation. The current findings are hypothesis generating and need to be prospectively validated, in the form of a multicenter trial.
Limitations
This was a retrospective, observational study from a single tertiary center, which could have potential selection bias. It is possible that the rate of arrhythmias (especially silent AF or VT) are under‐reported in this analysis because of lack of use of implantable loop recorders and other continuous monitoring devices. However, most recommendations in HCM are based upon similar retrospective, observational studies and consensus opinions. The results of all basic testing were available to all clinicians at the time of decision making, introducing further bias. However, LV‐GLS was measured retrospectively and the results were not available for clinical decision making. Although the current data suggest that patients with LV‐GLS worse than median who do not undergo surgery were associated with the highest event rate, the study is potentially underpowered to make conclusive assertions about subgroup analyses. A large proportion of the cohort underwent surgical relief of LVOT obstruction, consistent with the referral pattern at our high‐volume tertiary care center. However, this allowed us to test the hypothesis of whether surgery does modulate hard outcomes. There is a potential that VVI Strain analysis was performed at low frame rates in some cases, which could potentially reduce accuracy of the analysis. We also report all‐cause mortality in addition to cardiac mortality, because it is more objective and unbiased than cardiac mortality. Because development of CHF is not a hard end point, it was not included in the analysis.
Conclusions
In a large group of adult obstructive HCM patients with preserved LVEF, we demonstrate that LV‐GLS provides incremental prognostic utility for long‐term adverse events. Long‐term outcomes were significantly worse in the subgroup of obstructive HCM patients who had resting LV‐GLS worse than median who did not undergo surgical myectomy. These findings need further validation.
Sources of Funding
The study was supported, in part, by a gift from Reginald and Jamie Baxter Family to Dr Desai.
Disclosures
No financial conflicts for any authors or external funding utilized. Dr Desai is supported by the Haslam Family endowed chair in cardiovascular medicine and is a consultant for Myocardia, Inc.
Supporting information
Data S1.
Table S1. Relevant Baseline and Imaging Characteristics of Study Sample, Separated on Basis of Surgery vs Not (n=1019)
Tables S2 and S3. Multivariable Cox Proportional Hazard Analysis of the Study Population for the Secondary Composite End Point of All‐Cause Mortality and Appropriate ICD Discharge (Total n=1019; Number of Events n=79)
Acknowledgment
We acknowledge Penny Houghtaling, PhD, Department of Quantitative Health Sciences, for her statistical support for quadratic spline analysis.
(J Am Heart Assoc. 2017;6:e006514 DOI: 10.1161/JAHA.117.006514.)
References
- 1. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e783–e831. [DOI] [PubMed] [Google Scholar]
- 2. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 3. Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F, Maron BJ. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348:295–303. [DOI] [PubMed] [Google Scholar]
- 4. Elliott PM, Gimeno JR, Tome MT, Shah J, Ward D, Thaman R, Mogensen J, McKenna WJ. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur Heart J. 2006;27:1933–1941. [DOI] [PubMed] [Google Scholar]
- 5. Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, Nistri S, Cecchi F, Udelson JE, Maron BJ. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232–2239. [DOI] [PubMed] [Google Scholar]
- 6. Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S, Gersh BJ, Ackerman MJ, McCully RB, Dearani JA, Schaff HV, Danielson GK, Tajik AJ, Nishimura RA. Long‐term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:470–476. [DOI] [PubMed] [Google Scholar]
- 7. Smedira NG, Lytle BW, Lever HM, Rajeswaran J, Krishnaswamy G, Kaple RK, Dolney DO, Blackstone EH. Current effectiveness and risks of isolated septal myectomy for hypertrophic obstructive cardiomyopathy. Ann Thorac Surg. 2008;85:127–133. [DOI] [PubMed] [Google Scholar]
- 8. Woo A, Williams WG, Choi R, Wigle ED, Rozenblyum E, Fedwick K, Siu S, Ralph‐Edwards A, Rakowski H. Clinical and echocardiographic determinants of long‐term survival after surgical myectomy in obstructive hypertrophic cardiomyopathy. Circulation. 2005;111:2033–2041. [DOI] [PubMed] [Google Scholar]
- 9. Ball W, Ivanov J, Rakowski H, Wigle ED, Linghorne M, Ralph‐Edwards A, Williams WG, Schwartz L, Guttman A, Woo A. Long‐term survival in patients with resting obstructive hypertrophic cardiomyopathy comparison of conservative versus invasive treatment. J Am Coll Cardiol. 2011;58:2313–2321. [DOI] [PubMed] [Google Scholar]
- 10. Desai MY, Bhonsale A, Smedira NG, Naji P, Thamilarasan M, Lytle BW, Lever HM. Predictors of long‐term outcomes in symptomatic hypertrophic obstructive cardiomyopathy patients undergoing surgical relief of left ventricular outflow tract obstruction. Circulation. 2013;128:209–216. [DOI] [PubMed] [Google Scholar]
- 11. Desai MY, Smedira NG, Bhonsale A, Thamilarasan M, Lytle BW, Lever HM. Symptom assessment and exercise impairment in surgical decision making in hypertrophic obstructive cardiomyopathy: relationship to outcomes. J Thorac Cardiovasc Surg. 2015;150:928–935.e921. [DOI] [PubMed] [Google Scholar]
- 12. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2438–2488. [DOI] [PubMed] [Google Scholar]
- 13. Marwick TH, Leano RL, Brown J, Sun JP, Hoffmann R, Lysyansky P, Becker M, Thomas JD. Myocardial strain measurement with 2‐dimensional speckle‐tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging. 2009;2:80–84. [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi T, Popovic Z, Bhonsale A, Smedira NG, Tan C, Rodriguez ER, Thamilarasan M, Lytle BW, Lever HM, Desai MY. Association between septal strain rate and histopathology in symptomatic hypertrophic cardiomyopathy patients undergoing septal myectomy. Am Heart J. 2013;166:503–511. [DOI] [PubMed] [Google Scholar]
- 15. Popovic ZB, Kwon DH, Mishra M, Buakhamsri A, Greenberg NL, Thamilarasan M, Flamm SD, Thomas JD, Lever HM, Desai MY. Association between regional ventricular function and myocardial fibrosis in hypertrophic cardiomyopathy assessed by speckle tracking echocardiography and delayed hyperenhancement magnetic resonance imaging. J Am Soc Echocardiogr. 2008;21:1299–1305. [DOI] [PubMed] [Google Scholar]
- 16. Dhillon A, Sweet W, Popovic ZB, Smedira NG, Thamilarasan M, Lytle BW, Tan C, Starling RC, Lever HM, Moravec CS, Desai MY. Association of noninvasively measured left ventricular mechanics with in vitro muscle contractile performance: a prospective study in hypertrophic cardiomyopathy patients. J Am Heart Assoc. 2014;3:e001269. doi: 10.1161/JAHA.114.001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Di Salvo G, Pacileo G, Limongelli G, Baldini L, Rea A, Verrengia M, D'Andrea A, Russo MG, Calabro R. Non sustained ventricular tachycardia in hypertrophic cardiomyopathy and new ultrasonic derived parameters. J Am Soc Echocardiogr. 2010;23:581–590. [DOI] [PubMed] [Google Scholar]
- 18. Correia E, Rodrigues B, Santos LF, Moreira D, Gama P, Cabral C, Santos O. Longitudinal left ventricular strain in hypertrophic cardiomyopathy: correlation with nonsustained ventricular tachycardia. Echocardiography. 2011;28:709–714. [DOI] [PubMed] [Google Scholar]
- 19. Funabashi N, Takaoka H, Horie S, Ozawa K, Takahashi M, Yajima R, Saito M, Fujiwara K, Tani A, Kamata T, Kanaeda A, Uehara M, Kataoka A, Kobayashi Y. Risk stratification using myocardial peak longitudinal‐strain on speckle‐tracking transthoracic‐echocardiogram to predict major adverse cardiac events in non ischemic hypertrophic‐cardiomyopathy subjects confirmed by MDCT. Int J Cardiol. 2013;168:4586–4589. [DOI] [PubMed] [Google Scholar]
- 20. Debonnaire P, Thijssen J, Leong DP, Joyce E, Katsanos S, Hoogslag GE, Schalij MJ, Atsma DE, Bax JJ, Delgado V, Marsan NA. Global longitudinal strain and left atrial volume index improve prediction of appropriate implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy patients. Int J Cardiovasc Imaging. 2014;30:549–558. [DOI] [PubMed] [Google Scholar]
- 21. Reant P, Mirabel M, Lloyd G, Peyrou J, Lopez Ayala JM, Dickie S, Bulluck H, Captur G, Rosmini S, Guttmann O, Demetrescu C, Pantazis A, Tome‐Esteban M, Moon JC, Lafitte S, McKenna WJ. Global longitudinal strain is associated with heart failure outcomes in hypertrophic cardiomyopathy. Heart. 2016;102:741–747. [DOI] [PubMed] [Google Scholar]
- 22. Liu H, Pozios I, Haileselassie B, Nowbar A, Sorensen L, Phillip S, Lu D, Ventoulis I, Luo H, Araham R, Abraham TP. Role of global longtitudinal strain in predicting outcomes in hypertrophic cardiomyopathy. Am J Cardiol. 2017;120:670–675. [DOI] [PubMed] [Google Scholar]
- 23. Lever HM, Karam RF, Currie PJ, Healy BP. Hypertrophic cardiomyopathy in the elderly. Distinctions from the young based on cardiac shape. Circulation. 1989;79:580–589. [DOI] [PubMed] [Google Scholar]
- 24. Kobayashi T, Dhillon A, Popovic Z, Bhonsale A, Smedira NG, Thamilarasan M, Lytle BW, Lever HM, Desai MY. Differences in global and regional left ventricular myocardial mechanics in various morphologic subtypes of patients with obstructive hypertrophic cardiomyopathy referred for ventricular septal myotomy/myectomy. Am J Cardiol. 2014;113:1879–1885. [DOI] [PubMed] [Google Scholar]
- 25. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 26. Desai MY, Bhonsale A, Patel P, Naji P, Smedira NG, Thamilarasan M, Lytle BW, Lever HM. Exercise echocardiography in asymptomatic HCM: exercise capacity, and not LV outflow tract gradient predicts long‐term outcomes. JACC Cardiovasc Imaging. 2014;7:26–36. [DOI] [PubMed] [Google Scholar]
- 27. Zoghbi WA, Enriquez‐Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. [DOI] [PubMed] [Google Scholar]
- 28. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. [DOI] [PubMed] [Google Scholar]
- 29. Kwon DH, Smedira NG, Thamilarasan M, Lytle BW, Lever H, Desai MY. Characteristics and surgical outcomes of symptomatic patients with hypertrophic cardiomyopathy with abnormal papillary muscle morphology undergoing papillary muscle reorientation. J Thorac Cardiovasc Surg. 2010;140:317–324. [DOI] [PubMed] [Google Scholar]
- 30. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 31. Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–387. [DOI] [PubMed] [Google Scholar]
- 32. Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH. Normal ranges of left ventricular strain: a meta‐analysis. J Am Soc Echocardiogr. 2013;26:185–191. [DOI] [PubMed] [Google Scholar]
- 33. Varnava AM, Elliott PM, Sharma S, McKenna WJ, Davies MJ. Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease. Heart. 2000;84:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kusunose K, Goodman A, Parikh R, Barr T, Agarwal S, Popovic ZB, Grimm RA, Griffin BP, Desai MY. Incremental prognostic value of left ventricular global longitudinal strain in patients with aortic stenosis and preserved ejection fraction. Circ Cardiovasc Imaging. 2014;7:938–945. [DOI] [PubMed] [Google Scholar]
- 35. Fine NM, Shah AA, Han IY, Yu Y, Hsiao JF, Koshino Y, Saleh HK, Miller FA Jr, Oh JK, Pellikka PA, Villarraga HR. Left and right ventricular strain and strain rate measurement in normal adults using velocity vector imaging: an assessment of reference values and intersystem agreement. Int J Cardiovasc Imaging. 2013;29:571–580. [DOI] [PubMed] [Google Scholar]
- 36. Farsalinos KE, Daraban AM, Unlu S, Thomas JD, Badano LP, Voigt JU. Head‐to‐head comparison of global longitudinal strain measurements among nine different vendors: the EACVI/ASE Inter‐Vendor Comparison Study. J Am Soc Echocardiogr. 2015;28:1171–1181.e1172. [DOI] [PubMed] [Google Scholar]
- 37. Carasso S, Yang H, Woo A, Vannan MA, Jamorski M, Wigle ED, Rakowski H. Systolic myocardial mechanics in hypertrophic cardiomyopathy: novel concepts and implications for clinical status. J Am Soc Echocardiogr. 2008;21:675–683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Table S1. Relevant Baseline and Imaging Characteristics of Study Sample, Separated on Basis of Surgery vs Not (n=1019)
Tables S2 and S3. Multivariable Cox Proportional Hazard Analysis of the Study Population for the Secondary Composite End Point of All‐Cause Mortality and Appropriate ICD Discharge (Total n=1019; Number of Events n=79)


