Abstract
Background
Higher vegetable intake is consistently associated with lower atherosclerotic vascular disease (ASVD) events. However, the components responsible and mechanisms involved are uncertain. Nonnutritive phytochemicals may be involved. The objective of this study was to investigate the associations of total vegetable intake and types of vegetables grouped according to phytochemical constituents with ASVD mortality.
Methods and Results
The cohort consisted of 1226 Australian women aged 70 years and older without clinical ASVD or diabetes mellitus at baseline (1998). Vegetable intakes were calculated per serving (75 g/d) and were also classified into prespecified types relating to phytochemical constituents. ASVD‐related deaths were ascertained from linked mortality data. During 15 years (15 947 person‐years) of follow‐up, 238 ASVD‐related deaths occurred. A 1‐serving increment of vegetable intake was associated with a 20% lower hazard of ASVD‐related death (multivariable‐adjusted hazard ratio, 0.80; 95% confidence interval, 0.69–0.94 [P=0.005]). In multivariable‐adjusted models for vegetable types, cruciferous (per 10‐g/d increase: hazard ratio, 0.87; 95% confidence interval, 0.81–0.94 [P<0.001]) and allium (per 5‐g/d increase: hazard ratio, 0.82; 95% confidence interval, 0.73–0.94 [P=0.003]) vegetables were inversely associated with ASVD‐related deaths. The inclusion of other vegetable types, as well as lifestyle and cardiovascular risk factors, did not alter these associations. Yellow/orange/red (P=0.463), leafy green (P=0.063), and legume (P=0.379) vegetables were not significant.
Conclusions
Consistent with current evidence, higher cruciferous and allium vegetable intakes were associated with a lower risk of ASVD mortality. In addition, cruciferous and allium vegetables are recognized to be a good source of several nonnutritive phytochemicals such as organosulfur compounds.
Clinical Trial Registration
URL: http://www.anzctr.org.au. Unique identifier: ACTRN12617000640303.
Keywords: allium, atherosclerosis, atherosclerotic vascular disease, cardiovascular events, cruciferous, diet, follow‐up study, observational studies, vegetables
Subject Categories: Diet and Nutrition, Cardiovascular Disease, Epidemiology, Women, Vascular Disease
Clinical Perspective
What Is New?
In a cohort of older Australian women, higher intakes of cruciferous and allium vegetables were independently associated with lower risk of atherosclerotic vascular disease mortality.
What Are the Clinical Implications?
Cruciferous and allium vegetables are recognized to be a good source of several nonnutritive phytochemicals such as organosulfur compounds.
If future studies support our findings, dietary guidelines promoting increased vegetable intake could highlight the importance of cruciferous and allium vegetables for vascular health.
Introduction
Atherosclerotic vascular disease (ASVD) is the most common cause of death worldwide.1 Higher vegetable intake has been consistently associated with lower risk of ASVD.2 Results from these studies have provided the basis for guideline recommendations around the world to increase vegetable intake.3, 4, 5 In recent years, further analysis of the role of specific vegetable and fruit species has been undertaken to identify potentially beneficial varieties and thus to allow consideration of the potential nutrient and phytochemical effects on health outcomes. A recent report of the beneficial effects of certain vegetables and fruits on the risks of developing hypertension is an excellent example of the approach required to advance our knowledge of the role of specific vegetable and fruit species on preventing disease.6
Vegetables contain many important nutrients and phytochemicals that may be cardioprotective.7 For example, recent meta‐analyses have identified the beneficial effects on health outcomes of dietary fiber8 and the micronutrients potassium9 and magnesium.10 In addition, specific groups of phytochemicals such as organosulfur compounds, carotenoids, nitrogen‐containing compounds, and polyphenols have been postulated to have additional benefits towards cardiovascular health.11, 12, 13, 14 Thus, the aim of this study was to investigate the associations of total vegetable intake as well as types of vegetables grouped according to phytochemical constituents with ASVD mortality over 15 years of follow‐up in a cohort of older adult women.
Methods
Ethics Statement
The PLSAW (Perth Longitudinal Study of Aging in Women) was approved by the University of Western Australia's human ethics committee and complied with the Declaration of Helsinki. Written informed consents were obtained from all participants. Human ethics approval for the use of linked data for the project was provided by the Western Australian Department of Health Human Research Ethics Committee (project #2009/24).
Study Population
The population of this prospective cohort study consisted of participants in the PLSAW. This study originated in 1998 as the CAIFOS (Calcium Intake Fracture Outcome Study). The CAIFOS was a 5‐year, double‐blind, randomized, placebo‐controlled trial of daily calcium supplementation (1.2 g calcium carbonate) to prevent fractures.15 Women aged 70 years and older (n=1500) were recruited from the Western Australian general population by mail using the Electoral Roll, which is maintained for all Australian citizens enrolled to vote in Western Australia. After the completion of the CAIFOS, participants were enrolled in a 10‐year extension follow‐up study. In total, there were 15 years of follow‐up (PLSAW).
Eligibility criteria included available data on all exposure and outcome variables. Dietary data were available for 1485 of 1500 (99.0%) participants at baseline. Participants (17 of 1485 [1.1%]) with implausible energy intakes (<2100 kJ [500 kcal] or >14 700 kJ [3500 kcal]) were excluded. Further exclusion of participants with prevalent ASVD (n=152), diabetes mellitus (n=69), or both (n=21) at baseline resulted in 1226 of 1500 (81.7%) participants being included for the analysis of this study. Prevalent ASVD and/or diabetes mellitus at baseline was an a priori exclusion criteria because participants with a clinical diagnosis of ASVD or diabetes mellitus may have resulted in altered dietary intake and thereby attenuated the association of interest.
History of ASVD at baseline (1998) was determined from principal hospital discharge diagnosis codes from 1980 to 1998 using the Hospital Morbidity Data Collection, linked via the Western Australian Data Linkage System. Diagnosis codes were defined using the Manual of the International Statistical Classification of Diseases, Injuries and Causes of Death, 9th Revision (ICD‐9)16 and the Australian version of the International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM).17 ASVD diagnosis codes included ischemic heart disease (ICD‐9/ICD‐9‐CM codes 410–414); heart failure (ICD‐9/ICD‐9‐CM code 428); cerebrovascular disease, excluding hemorrhage (ICD‐9/ICD‐9‐CM codes 433–438); and peripheral arterial disease (ICD‐9/ICD‐9‐CM codes 440–444). Baseline history of diabetes mellitus was determined from medication use, which was coded (T89001–T90009) using the International Classification of Primary Care—Plus (ICPC‐Plus) method.18 This coding methodology allows aggregation of different terms for similar pathologic entities as defined by the ICD‐10 coding system.
ASVD Mortality Assessment
Any death relating to ASVD was the primary outcome of the study. Coded multiple causes of death data over a 15‐year period were retrieved from linked mortality data via the Western Australian Data Linkage System. Multiple causes of death data included the underlying cause of death and all associated causes of death listed on the death certificate. The causes of death were obtained from the coded death certificate using information in parts 1 and 2 of the death certificate or all diagnosis text fields from the death certificate when coded deaths were not yet available. ASVD deaths were defined using diagnosis codes from the ICD‐9‐CM 16 and the International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Australian Modification (ICD‐10‐AM).19 ASVD‐related death diagnosis codes included deaths attributed to ischemic heart disease (ICD‐9‐CM codes 410–414 and ICD‐10‐AM codes I20–I25); heart failure (ICD‐9‐CM code 428 and ICD‐10‐AM code I50); cerebrovascular disease, excluding hemorrhage (ICD‐9‐CM codes 433–438 and ICD‐10‐AM codes I63‐I69, G45.9); and peripheral arterial disease (ICD‐9‐CM codes 440–444 and ICD‐10‐AM codes I70–I74).
Dietary Intake Assessment
A self‐administered, semiquantitative food frequency questionnaire developed by the Cancer Council of Victoria was used to assess dietary intake at baseline (1998), 5 years (2003), and 7 years (2005).20 This questionnaire was designed to assess the usual frequency of dietary intakes over a 12‐month period with 10 frequency response options ranging from “never” to “3 or more times per day” and has been validated using 7‐day weighed food records.21 Portion size was calculated using photographs of scaled portions of different food types. Energy (kJ/d) and nutrient intakes were calculated by the Cancer Council of Victoria using the NUTTAB 95 food composition database22 and were supplemented by other data where necessary. Individual food items were calculated in grams per day by the Cancer Council of Victoria. Participants were supervised by a research assistant while completing the food frequency questionnaire. Food models and food charts as well as measuring cups and measuring spoons were provided to ensure the accuracy of reported food consumption.
Total vegetable intake
Total vegetable intake was calculated in servings per day. Vegetable servings were based on the 2013 Australian Dietary Guidelines of 1 serving of vegetables being equivalent to 75 g.4 Servings per day were calculated as a continuous variable and then categorized as a discrete variable (<2 servings, 2 to <3 servings, ≥3 servings). Vegetable intake was assessed using 24 vegetable items and did not include “potatoes, roasted or fried, including hot chips” as hot chips are not recommended as part of a healthy diet.4 “Potatoes cooked without fat” were included.
Vegetable type
Classification of vegetable types were based on the 2013 Australian Dietary Guidelines4 and modified to include the following subgroups of vegetables relating to phytochemical profiles: cruciferous vegetables (cabbage, brussels sprouts, cauliflower, and broccoli)—source of organosulfur compounds11; allium vegetables (onion, leek, and garlic)—source of organosulfur compounds11; yellow/orange/red vegetables (tomato, capsicum, beetroot, carrot, and pumpkin)—source of carotenoids such as lycopene and beta carotene12; leafy green vegetables (lettuce and other salad greens, celery, silver beet, and spinach)—source of nitrogen‐containing compounds such as nitrate13; and legumes (peas, greens beans, bean sprouts and alfalfa sprouts, baked beans, soy beans, soy bean curd and tofu, and other beans)—source of polyphenolic compounds such as isoflavonoids and saponins.14
Nutrient‐Rich Foods Index
We assessed overall diet quality using the Nutrient‐Rich Foods Index.23 This index was calculated using the Nutrient Reference Values for Australia and New Zealand based on adult women older than 70 years24 and has been previously described.25
Baseline Demographic and Clinical Assessment
Body mass index (BMI) (kg/m2) was calculated using body weight (kg) and height (m), which were assessed while participants were wearing light clothes without socks and shoes. Body weight was measured using digital scales to the nearest 0.1 kg and height was assessed using a wall‐mounted stadiometer to the nearest 0.1 cm. Physical activity was assessed by asking participants about participation in sport, recreation, and/or regular physical activities undertaken in the past 3 months before their baseline visit.26 The level of activity (kJ/d) was calculated using a validated method applying the type of activity, time engaged in the activity, and body weight.27, 28 Alcohol (g/d) intake was assessed along with other dietary intakes using the validated food frequency questionnaire developed by the Cancer Council of Victoria as described above. Smoking history was collected using a questionnaire and was coded as nonsmoker or ex‐smoker/current smoker if they had smoked >1 cigarette per day for longer than 3 months at any time in their life. Socioeconomic status was calculated using the Socioeconomic Indexes for Areas developed by the Australian Bureau of Statistics.29 The index ranked residential postcodes according to relative socioeconomic advantage and disadvantage. Participants were coded into 6 groups from the top 10% most highly disadvantaged to the top 10% least disadvantaged.29
Participants were asked to provide a detailed medical history and list of medications, which were verified by their general practitioner where possible. The ICPC‐Plus method was used to code medication use.18 Medication use included antihypertensive, statin, and low‐dose aspirin medications. These medications were used to adjust for ASVD risk factors such as hypertension and dyslipidemia. Baseline serum creatinine was analyzed in 1106 of 1226 (90.2%) participants using an isotope dilution mass spectrometry traceable Jaffe kinetic assay for creatinine on a Hitachi 917 analyzer (Roche Diagnostics GmbH). Estimated glomerular filtration rate was then calculated using the Chronic Kidney Disease Epidemiology Collaboration equation30 and was added to the multivariable‐adjusted models, as this has been shown to predict ASVD in this cohort.31 Total cholesterol, high‐density lipoprotein cholesterol, and triglyceride concentrations were analyzed in 895 of 1226 (73.0%) participants using a Hitachi 917 auto analyzer (Roche Diagnostics). Low‐density lipoprotein cholesterol was calculated in 888 of 1226 (72.4%) participants using the Friedewald method.32
Framingham 10‐Year General Cardiovascular Risk Scores
The Framingham risk score (FRS) was calculated using BMI data in 1188 participants. The FRS included age, sex, previous diabetes mellitus, BMI, current smoking status, and treated (prescribed antihypertensive medications) or untreated systolic blood pressure using the equation and estimated regression coefficients developed by D'Agostino and others.33
These scores were confirmed using the online calculator developed by D'Agostino and Pencina based on the publication by D'Agostino and others.33
Statistical Analysis
An analytical protocol was produced before analysis began. Statistical significance was set at a 2‐sided type 1 error rate of P<0.05. All data were analyzed using IBM SPSS Statistics for Windows, version 21.0 (IBM Corporation) and SAS software, version 9.4 (SAS Institute Inc). Descriptive statistics of normally distributed continuous variables were expressed as mean±SD; non‐normally distributed continuous variables (physical activity, alcohol intake, allium vegetable intake, nut intake, fish intake, processed meat intake, and red meat intake) were expressed as median and interquartile range; and categorical variables were expressed as number and proportion (percentage). Differences in baseline characteristics among vegetable serving categories were tested using 1‐way ANOVA for normally distributed continuous variables and the Kruskal–Wallis test for non‐normally distributed continuous variables. Chi‐square test for independence was used to test for differences in baseline characteristics among vegetable serving categories for categorical variables.
The primary outcome of the study was any death relating to ASVD. Complete follow‐up of death records was available for all participants who remained in Western Australia, which was likely to be almost all participants given their age. The follow‐up period for each participant was calculated in days from their baseline visit until their last day of follow‐up, which was their date of death or 15 years of complete follow‐up. Cox proportional hazards modeling was used to analyze the associations between vegetable variables and ASVD mortality. Total vegetable intake was analyzed per serving (75 g/d) and then further categorized into 3 groups (<2 servings, 2 to <3 servings, and ≥3 servings). For vegetable types, intakes of cruciferous, yellow/orange/red, leafy green, and legumes varieties were analyzed per 10 g/d. Intakes of allium were analyzed per 5 g/d. Three models of adjustment were used for the Cox proportional hazards modeling. These models included unadjusted, age‐ and energy‐adjusted, and multivariable‐adjusted models. The multivariable‐adjusted models included age, BMI, level of physical activity, alcohol intake, energy intake, Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate (entered as continuous variables), smoking history, socioeconomic status, the CAIFOS supplementation group of calcium versus placebo, antihypertensive medication, statin medication, and low‐dose aspirin (entered as categorical variables). Covariates were selected on an a priori basis as these covariates are known risk factors for ASVD. We also tested for evidence of linear trends across categories of vegetable servings using the median value for each category as a continuous variable in separate Cox proportional hazards models. Cox proportional hazards assumptions were tested using log‐log plots, which were shown to be parallel, indicating that proportional hazards assumptions were not violated.
Sensitivity analyses
Reverse causality bias was explored in multivariable‐adjusted analyses, excluding all‐cause deaths occurring within the first 24 months, for total vegetable intake (per 75 g/d) and intakes of cruciferous (per 10 g/d), allium (per 5 g/d), and leafy green (per 10 g/d) vegetables.
Since higher vegetable intake may be considered a surrogate marker of a healthier diet, we further adjusted for diet quality using the Nutrient‐Rich Foods Index in multivariable‐adjusted models for total vegetable intake (per 75 g/d) and intakes of cruciferous (per 10 g/d) and allium (per 5 g/d) vegetables. We also considered the impact of possible dietary confounders by including the estimated daily intakes of total fruit (g/d), fish (g/d), nuts (g/d), fiber (g/d), potassium (mg/d), magnesium (mg/d), beta carotene (μg/d), and saturated fat (g/d).34 These dietary confounders were entered separately as continuous variables in multivariable‐adjusted Cox proportional hazards models on a variable‐by‐variable basis for total vegetable intake (per 75 g/d) and intakes of cruciferous (per 10 g/d) and allium (per 5 g/d) vegetables.
Subgroups of ASVD‐related mortality, including ischemic heart disease and ischemic cerebrovascular disease mortality, were investigated in multivariable‐adjusted models for total vegetable intake and intakes of cruciferous (per 10 g/d) and allium (per 5 g/d) vegetables. The FRS was used in multivariable‐adjusted models to adjust for the estimated 10‐year risk of developing cardiovascular disease for total vegetable intake (per 75 g/d) and intakes of cruciferous (per 10 g/d) and allium (per 5 g/d) vegetables. The multivariable‐adjusted models included level of physical activity, alcohol intake, socioeconomic status, the CAIFOS supplementation group of calcium versus placebo, statin use, low‐dose aspirin use, Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate, and energy intake. Age, BMI, antihypertensive medication, and smoking history were not included in this model because the FRS takes into account the participants’ age, BMI, untreated systolic blood pressure, and current smoking status.
The relationship between cruciferous, allium, and total vegetables were investigated using Spearman rank order correlation (ρ). To examine whether the individual associations of cruciferous and allium vegetables were not caused by collinearity and were independent of total vegetable intake, a forward stepwise Cox proportional hazards model with all multivariable‐adjusted variables as well as total vegetable intake and intakes of cruciferous (per 10 g/d) and allium (per 5 g/d) vegetables was tested.
One‐way repeated measures ANOVA was used to test for differences between intakes of cruciferous, allium, and total vegetables at baseline (1998), 5 years (2003), and 7 years (2005). The average of the 3 time points (baseline and 5 and 7 years) for cruciferous, allium, and total vegetables were used separately in multivariable‐adjusted Cox proportional hazards models to account for the change in intake.
Results
Participants and Their Characteristics
The flow diagram for participant selection is shown in Figure 1. In the 15 947 person‐years of follow‐up, there were 238 of 1226 (19.4%) ASVD‐related deaths, of which 128 of 1226 (10.4%) were caused by ischemic heart disease and 92 of 1226 (7.5%) were caused by ischemic cerebrovascular disease. Participant demographics, medication use, and biochemical analyses presented according to all 1226 study participants and by categories of vegetable servings are presented in Table 1. Nutrient and food intakes are presented in Table 2. Body weight (kg) (Table 1) and all dietary intakes (Table 2) were significantly different among vegetable serving categories (P<0.05 for all). Participants who consumed more vegetable servings also consumed more of each vegetable type (P<0.001) (Table 2). The mean vegetable intake was 196.5 (SD, 78.8) g/d and vegetable servings was 2.6 (SD, 1.0) for all participants. The mean intake (from highest to lowest) of vegetable types was: yellow/orange/red vegetables 52.4 (SD, 27.6) g/d; cruciferous vegetables 32.3 (SD, 22.0) g/d; legumes 27.0 (SD, 18.7) g/d; and leafy green vegetables 18.8 (SD, 12.1) g/d. The median intake for allium vegetables was 6.2 (interquartile range, 2.9–10.6) g/d (Table 2). The percentage contribution of vegetable types, including potatoes, from all vegetables consumed (g/d) are presented in Figure 2.
Figure 1.

Participant flow diagram. ASVD indicates atherosclerotic vascular disease.
Table 1.
Baseline Characteristics of All Study Participants and by Categories of Vegetable Servingsa
| Participant Demographics | All Participants (N=1226) | <2 Servings (n=355) | 2 to <3 Servings (n=486) | ≥3 Servings (n=385) | P Value |
|---|---|---|---|---|---|
| Age, y | 75.1±2.7 | 75.2±2.7 | 75.0±2.6 | 75.0±2.6 | 0.636 |
| BMI, kg/m2 | 27.0±4.6 | 26.7±4.7 | 27.0±4.3 | 27.2±4.8 | 0.334 |
| Body weight, kg | 68.1±12.1 | 66.8±11.8 | 68.2±11.9 | 69.2±12.5 | 0.029 |
| Physical activity, median (IQR), kJ/d | 460.5 (101.7–860.8) | 419.9 (0.0–860.9) | 472.7 (163.7–884.0) | 491.3 (187.1–854.6) | 0.248 |
| Alcohol intake, median (IQR), g/d, | 2.1 (0.3–10.4) | 2.0 (0.3–9.8) | 2.0 (0.3–10.1) | 2.3 (0.3–11.3) | 0.929 |
| Smoking history, No. (%)b | 441 (36.0) | 125 (35.3) | 186 (38.7) | 130 (33.9) | 0.327 |
| Socioeconomic statusc | 0.789 | ||||
| Top 10% most highly disadvantaged, No. (%) | 41 (3.3) | 11 (3.1) | 17 (3.5) | 13 (3.4) | |
| Highly disadvantaged, No. (%) | 146 (11.9) | 45 (12.7) | 54 (11.2) | 47 (12.4) | |
| Moderate‐highly disadvantaged, No. (%) | 194 (15.8) | 54 (15.3) | 81 (16.8) | 59 (15.6) | |
| Low‐moderately disadvantaged, No. (%) | 185 (15.1) | 59 (16.7) | 63 (13.0) | 63 (16.6) | |
| Low disadvantage, No. (%) | 255 (20.8) | 69 (19.5) | 100 (20.7) | 86 (22.7) | |
| Top 10% least disadvantaged, No. (%) | 394 (32.1) | 115 (32.6) | 168 (34.8) | 111 (29.3) | |
| Treatment with calcium supplements, No. (%) | 641 (52.3) | 167 (47.0) | 261 (53.8) | 213 (55.3) | 0.055 |
| Framingham risk score, %d | 20.6±9.1 | 20.2±9.1 | 20.7±9.2 | 20.9±9.0 | 0.561 |
| Medication use | |||||
| Antihypertensive medication, No. (%) | 493 (40.2) | 135 (38.0) | 195 (40.1) | 163 (42.3) | 0.489 |
| Statin medication, No. (%) | 184 (15.0) | 53 (14.9) | 73 (15.0) | 58 (15.1) | 0.999 |
| Low‐dose aspirin, No. (%) | 193 (15.7) | 66 (18.6) | 78 (16.0) | 49 (12.7) | 0.089 |
| Biochemical analyses | |||||
| CKD‐EPI eGFR, mL/min per 1.73 m2 e | 67.6±13.0 | 67.4±13.3 | 67.2±12.3 | 68.1±13.5 | 0.677 |
| Total cholesterol, mmol/Lf | 5.9±1.1 | 6.0±1.1 | 6.0±1.0 | 5.8±1.1 | 0.265 |
| HDL cholesterol, mmol/Lf | 1.5±0.4 | 1.5±0.4 | 1.5±0.4 | 1.4±0.4 | 0.125 |
| LDL cholesterol, mmol/Lg | 3.7±1.0 | 3.8±1.0 | 3.8±0.9 | 3.7±1.0 | 0.425 |
| Triglycerides, mmol/Lf | 1.5±0.7 | 1.5±0.7 | 1.5±0.7 | 1.6±0.7 | 0.511 |
BMI indicates body mass index; CKD‐EPI eGFR, Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate; HDL, high‐density lipoprotein; IQR, interquartile range; LDL, low‐density lipoprotein.
Vegetable servings were calculated based on the 2013 Australian Dietary Guidelines of a vegetable serving equal to 75 g/d. P values are a comparison between groups using ANOVA, Kruskal–Wallis test, and chi‐square test where appropriate. Values are presented as mean±SD unless otherwise indicated.
Measured in 1218 participants.
Measured in 1215 participants.
Measured in 1188 participants.
Measured in 1106 participants.
Measured in 895 participants.
Measured in 888 participants.
Table 2.
Dietary Intakes of All Study Participants and by Categories of Vegetable Servingsa
| All Participants (N=1226) | <2 Servings (n=355) | 2 to <3 Servings (n=486) | ≥3 Servings (n=385) | P Value | |
|---|---|---|---|---|---|
| Dietary intakes | |||||
| Vegetable servings, g/d | 2.6±1.0 | 1.5±0.4 | 2.5±0.3 | 3.8±0.8 | |
| Vegetables, g/d | 196.5±78.8 | 111.7±29.4 | 186.2±21.3 | 287.5±58.2 | <0.001 |
| Cruciferous vegetables, g/d | 32.3±22.0 | 18.9±12.8 | 31.2±18.1 | 46.0±25.1 | <0.001 |
| Allium vegetables, median (IQR), g/d | 6.2 (2.9–10.6) | 3.5 (1.7–6.2) | 6.2 (3.4–10.1) | 10.0 (5.9–15.5) | <0.001 |
| Yellow/orange/red vegetables, g/d | 52.4±27.6 | 30.1±14.7 | 50.9±18.3 | 74.8±29.2 | <0.001 |
| Leafy green vegetables, g/d | 18.8±12.1 | 13.3±9.4 | 19.2±11.1 | 23.4±13.4 | <0.001 |
| Legumes, g/d | 27.0±18.7 | 16.5±9.9 | 26.2±14.8 | 37.6±23.2 | <0.001 |
| Nutrient‐Rich Foods Index | 75.2±24.4 | 78.7±22.4 | 77.9±24.3 | 68.4±24.8 | <0.001 |
| Energy, kJ/d | 7146.5±2091.9 | 6225.9±1757.7 | 7021.0±1949.9 | 8153.7±2118.6 | <0.001 |
| Total fat, g/d | 64.7±23.3 | 57.4±20.5 | 63.9±22.6 | 72.4±24.4 | <0.001 |
| Saturated fat, g/d | 25.8±11.2 | 23.9±10.5 | 25.3±10.9 | 28.1±11.8 | <0.001 |
| Monounsaturated fat, g/d | 22.5±8.7 | 19.4±7.2 | 22.3±8.3 | 25.6±9.3 | <0.001 |
| Polyunsaturated fat, g/d | 10.6±4.8 | 9.2±4.2 | 10.6±4.6 | 12.0±5.1 | <0.001 |
| Omega 3 fatty acids, g/d | 1.3±0.6 | 1.1±0.5 | 1.3±0.6 | 1.6±0.7 | <0.001 |
| Dietary cholesterol, mg/d | 238.6±99.6 | 214.7±90.4 | 236.0±94.8 | 264.0±107.6 | <0.001 |
| Protein, g/d | 79.5±26.4 | 66.8±20.8 | 78.3±25.0 | 92.6±26.6 | <0.001 |
| Carbohydrate, g/d | 191.1±58.1 | 165.1±48.6 | 186.6±52.5 | 220.7±59.8 | <0.001 |
| Sugar, g/d | 92.1±31.9 | 81.1±28.3 | 89.6±28.8 | 105.4±34.1 | <0.001 |
| Fiber, g/d | 22.8±7.8 | 17.7±5.3 | 22.4±6.3 | 28.0±8.1 | <0.001 |
| Potassium, mg/d | 2948.9±844.9 | 2368.6±625.1 | 2882.7±674.2 | 3567.6±801.3 | <0.001 |
| Magnesium, mg/d | 298.9±93.0 | 247.9±70.3 | 293.7±81.9 | 352.5±96.1 | <0.001 |
| Beta carotene, μg/d | 2747.9±1260.0 | 1676.3±578.9 | 2682.9±847.1 | 3818.2±1278.2 | <0.001 |
| Fruit, g/d | 256.1±131.5 | 206.3±111.4 | 255.6±116.5 | 302.7±148.8 | <0.001 |
| Nuts, median (IQR), g/d | 0.6 (0.2–2.7) | 0.3 (0.1–2.0) | 0.5 (0.2–2.2) | 1.1 (0.3–4.5) | <0.001 |
| Fish, median (IQR), g/d | 19.3 (9.3–35.7) | 14.5 (6.8–27.0) | 19.3 (8.7–35.0) | 25.4 (13.4–49.3) | <0.001 |
| Red meat intake, median (IQR), g/d | 42.2 (23.6–69.5) | 31.3 (16.4–48.3) | 42.5 (25.3–65.2) | 58.3 (33.1–92.4) | <0.001 |
| Processed meat intake, median (IQR), g/d | 10.4 (4.9–20.6) | 9.6 (3.8–18.2) | 11.2 (5.0–21.7) | 10.8 (5.1–22.4) | 0.011 |
IQR indicates interquartile range.
Vegetable servings were calculated based on the 2013 Australian Dietary Guidelines of a vegetable serving equal to 75 g/d. P values are a comparison between groups using ANOVA and Kruskal–Wallis test where appropriate. Values are presented as mean±SD unless otherwise indicated. Cruciferous vegetables included cabbage, brussels sprouts, cauliflower, and broccoli. Allium vegetables included onion, leek, and garlic. Yellow/orange/red vegetables included tomato, capsicum, beetroot, carrot, and pumpkin. Leafy green vegetables included lettuce and other salad greens, celery, silver beet, and spinach. Legumes included peas, greens beans, bean sprouts and alfalfa sprouts, baked beans, soy beans, soy bean curd and tofu, and other beans.
Figure 2.
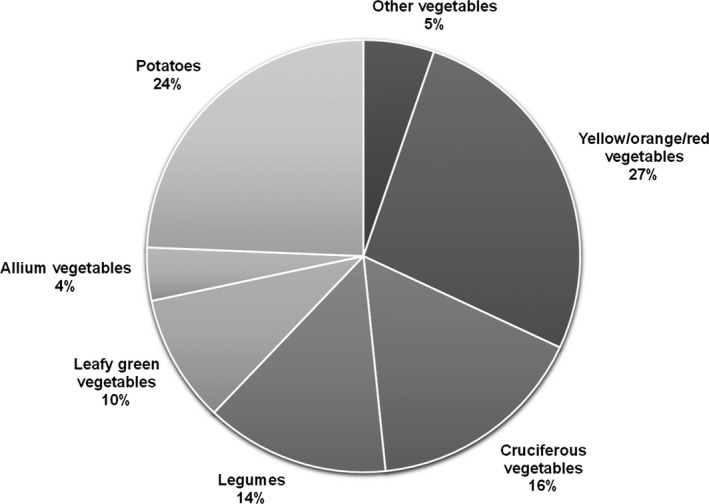
Percentage contribution of vegetable types from total vegetables (g/d) consumed. Cruciferous vegetables included cabbage, brussels sprouts, cauliflower, and broccoli. Allium vegetables included onion, leek, and garlic. Yellow/orange/red vegetables included tomato, capsicum, beetroot, carrot, and pumpkin. Leafy green vegetables included lettuce and other salad greens, celery, silver beet, and spinach. Legumes included peas, greens beans, bean sprouts and alfalfa sprouts, baked beans, soy beans, soy bean curd and tofu, and other beans.
Total Vegetable Intake and ASVD Mortality
The association of total vegetable intake with ASVD mortality was statistically significant in unadjusted, age‐ and energy‐adjusted, and multivariable‐adjusted models (P<0.01 for all models) (Table 3). Multivariable‐adjusted cumulative survival curves for ASVD mortality according to vegetable servings are presented in Figure 3. The survival benefit of higher vegetable intakes diverged after 60 months.
Table 3.
Association of Total Vegetable Intake and by Serving Categories With ASVD Mortalitya
| All Participants (N=1226) | P Valueb | <2 Servings (n=355) | 2 to <3 Servings (n=486) | ≥3 Servings (n=385) | P for Trendc | |
|---|---|---|---|---|---|---|
| Median vegetable servings, No. | 1.5 | 2.5 | 3.6 | |||
| ASVD | ||||||
| Deaths, No. (%) | 238 (19.4) | 83 (23.4) | 98 (20.2) | 57 (14.8) | ||
| Unadjusted | 0.80 (0.70–0.92) | 0.001 | 1.00 (Referent) | 0.80 (0.60–1.08) | 0.58 (0.42–0.82) | 0.002 |
| Age‐ and energy‐adjusted | 0.82 (0.71–0.94) | 0.005 | 1.00 (Referent) | 0.84 (0.62–1.13) | 0.61 (0.43–0.87) | 0.007 |
| Multivariable‐adjustedd | 0.80 (0.69–0.94) | 0.005 | 1.00 (Referent) | 0.78 (0.57–1.08) | 0.57 (0.38–0.83) | 0.004 |
ASVD indicates atherosclerotic vascular disease.
Results are presented as hazard ratios (95% confidence intervals) using Cox proportional hazards modeling. Vegetable servings were calculated based on the 2013 Australian Dietary Guidelines of a vegetable serving equal to 75 g/d.
P values are for vegetable servings (per 75 g/d) entered as a continuous variable.
P values are a trend test using the median values of each vegetable serving category in Cox proportional hazards models.
Multivariable‐adjusted model included age, body mass index, physical activity, alcohol intake, smoking history, socioeconomic status, calcium supplementation group, antihypertensive medication, statin medication, low‐dose aspirin, Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate, and energy intake.
Figure 3.
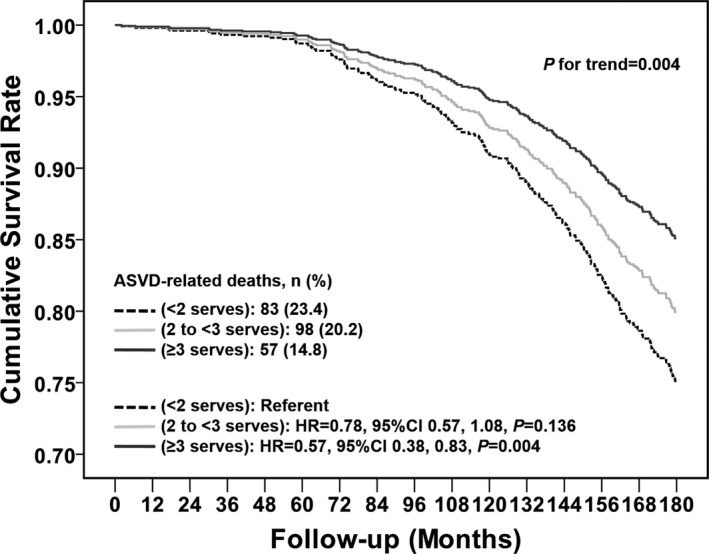
Multivariable‐adjusted cumulative survival curves for atherosclerotic vascular disease (ASVD) mortality according to vegetable serving categories. The multivariable‐adjusted model included age, body mass index, physical activity, alcohol intake, smoking history, socioeconomic status, calcium supplementation group, antihypertensive medication, statin medication, low‐dose aspirin, Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate, and energy intake. CI indicates confidence interval; HR, hazard ratio.
To avoid the potential of reverse causality, participants who died in the first 24 months were excluded. Total vegetable intake remained a statistically significant predictor of ASVD death (per 75‐g/d increase: hazard ratio [HR], 0.81; 95% confidence interval [CI], 0.70–0.95 [P=0.008]).
Additional adjustment for the Nutrient‐Rich Foods Index attenuated the association of total vegetable intake (per 75‐g/d increase: HR, 0.86; 95% CI, 0.73–1.02 [P=0.076]). Separate analyses that adjusted for the individual dietary factors, total fruit, fish, nuts, and beta carotene, did not change the association between total vegetable intake and ASVD mortality (Table 4). Adjustment for potassium, magnesium, fiber, and saturated fat attenuated the association (Table 4).
Table 4.
Multivariable‐Adjusted Hazard Ratios and 95% Confidence Intervals for ASVD‐Related Mortality for Cruciferous and Total Vegetable Intake With Additional Adjustments for Individual Dietary Confoundersa
| All Participants (N=1226) | P Value | |
|---|---|---|
| Cruciferous vegetables | ||
| Multivariable‐adjustedb plus total fruit, g/d | 0.87 (0.81–0.94) | <0.001 |
| Multivariable‐adjusted plus fish, g/d | 0.87 (0.81–0.94) | <0.001 |
| Multivariable‐adjusted plus nuts, g/d | 0.87 (0.81–0.94) | <0.001 |
| Multivariable‐adjusted plus fiber, g/d | 0.88 (0.82–0.95) | 0.001 |
| Multivariable‐adjusted plus potassium, mg/d | 0.89 (0.83–0.96) | 0.003 |
| Multivariable‐adjusted plus magnesium, mg/d | 0.89 (0.82–0.96) | 0.002 |
| Multivariable‐adjusted plus beta carotene, μg/d | 0.87 (0.81–0.94) | 0.001 |
| Multivariable‐adjusted plus saturated fat, g/d | 0.89 (0.82–0.96) | 0.002 |
| Allium vegetables | ||
| Multivariable‐adjusted plus total fruit, g/d | 0.83 (0.73–0.94) | 0.003 |
| Multivariable‐adjusted plus fish, g/d | 0.82 (0.73–0.94) | 0.003 |
| Multivariable‐adjusted plus nuts, g/d | 0.83 (0.73–0.94) | 0.004 |
| Multivariable‐adjusted plus fiber, g/d | 0.84 (0.74–0.96) | 0.010 |
| Multivariable‐adjusted plus potassium, mg/d | 0.85 (0.75–0.96) | 0.011 |
| Multivariable‐adjusted plus magnesium, mg/d | 0.84 (0.74–0.95) | 0.006 |
| Multivariable‐adjusted plus beta carotene, μg/d | 0.83 (0.73–0.95) | 0.007 |
| Multivariable‐adjusted plus saturated fat, g/d | 0.85 (0.75–0.96) | 0.009 |
| Total vegetables | ||
| Multivariable‐adjusted plus total fruit, g/d | 0.81 (0.67–0.94) | 0.006 |
| Multivariable‐adjusted plus fish, g/d | 0.81 (0.69–0.94) | 0.006 |
| Multivariable‐adjusted plus nuts, g/d | 0.81 (0.70–0.94) | 0.006 |
| Multivariable‐adjusted plus fiber, g/d | 0.84 (0.71–1.00) | 0.051 |
| Multivariable‐adjusted plus potassium, mg/d | 0.88 (0.74–1.06) | 0.183 |
| Multivariable‐adjusted plus magnesium, mg/d | 0.86 (0.73–1.01) | 0.059 |
| Multivariable‐adjusted plus beta carotene, μg/d | 0.78 (0.63–0.95) | 0.015 |
| Multivariable‐adjusted plus saturated fat, g/d | 0.86 (0.73–1.01) | 0.057 |
Results are presented as hazard ratios (95% confidence intervals) using multivariable‐adjusted Cox proportional hazards modeling with additional adjustment for dietary confounders per 10 g/d for cruciferous vegetables, per 5 g/d for allium vegetables, and per 75 g/d for total vegetables.
The multivariable‐adjusted model included age, body mass index, physical activity, alcohol intake, smoking history, socioeconomic status, calcium supplementation group, antihypertensive medication, statin medication, low‐dose aspirin, Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate, and energy intake. Cruciferous vegetables included cabbage, brussels sprouts, cauliflower, and broccoli. Allium vegetables included onion, leek, and garlic.
Total vegetable intake was inversely associated with ischemic heart disease mortality with borderline significance (P=0.050). Ischemic cerebrovascular disease mortality did not reach significance (P=0.074) (Table 5).
Table 5.
Multivariable‐Adjusted Hazard Ratios and 95% Confidence Intervals for the Association of Intakes of Cruciferous, Allium, and Total Vegetables With Ischemic Heart Disease Mortality and Ischemic Cerebrovascular Disease Mortalitya
| All Participants (N=1226) | P Value | |
|---|---|---|
| Ischemic heart disease mortality, No. (%) | 128 (10.4) | |
| Cruciferous vegetables | 0.83 (0.75–0.92) | <0.001 |
| Allium vegetables | 0.82 (0.70–0.97) | 0.022 |
| Total vegetables | 0.82 (0.67–1.00) | 0.050 |
| Ischemic cerebrovascular disease mortality, No. (%) | 92 (7.5) | |
| Cruciferous vegetables | 0.94 (0.84–1.05) | 0.299 |
| Allium vegetables | 0.75 (0.60–0.93) | 0.011 |
| Total vegetables | 0.80 (0.62–1.02) | 0.074 |
Results are presented as hazard ratios (95% confidence intervals) using multivariable‐adjusted Cox proportional hazards modeling per 10 g/d for cruciferous vegetables, per 5 g/d for allium vegetables, and per 75 g/d for total vegetables. Cruciferous vegetables included cabbage, brussels sprouts, cauliflower, and broccoli. Allium vegetables included onion, leek, and garlic. The multivariable‐adjusted model included age, body mass index, physical activity, alcohol intake, smoking history, socioeconomic status, calcium supplementation group, antihypertensive medication, statin medication, low‐dose aspirin, Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate, and energy intake.
Vegetable Types and ASVD Mortality
The associations between intakes of vegetable types and ASVD mortality are presented in Table 6. Higher intakes of cruciferous and allium vegetables were associated with lower ASVD mortality in unadjusted, age‐ and energy‐adjusted, and multivariable‐adjusted models (P<0.01 for all). These associations were also independent of other vegetable intakes (P<0.05 for both cruciferous and allium). Higher intakes of leafy green vegetables were associated with lower ASVD mortality in the unadjusted model (P=0.048) but not in the age‐ and energy‐adjusted (P=0.055) and multivariable‐adjusted (P=0.063) models. Likewise, higher intakes of yellow/orange/red vegetables were associated with lower ASVD mortality in the unadjusted model (P=0.040) but not in the adjusted models (P>0.05). Lastly, intakes of legumes were not associated with ASVD mortality in any models (P>0.05).
Table 6.
The Association Between Intakes of Specific Types of Vegetables and ASVDa
| All Participants (N=1226) | P Value | |
|---|---|---|
| Cruciferous vegetables | ||
| Unadjusted | 0.88 (0.83–0.94) | <0.001 |
| Age‐ and energy‐adjusted | 0.88 (0.82–0.94) | <0.001 |
| Multivariable‐adjustedb | 0.87 (0.81–0.94) | <0.001 |
| Multivariable‐adjusted plus noncruciferous vegetables | 0.88 (0.81–0.95) | 0.001 |
| Allium vegetables | ||
| Unadjusted | 0.82 (0.74–0.92) | 0.001 |
| Age‐ and energy‐adjusted | 0.84 (0.75–0.95) | 0.005 |
| Multivariable‐adjusted | 0.82 (0.73–0.94) | 0.003 |
| Multivariable‐adjusted plus nonallium vegetables | 0.85 (0.75–0.97) | 0.017 |
| Yellow/orange/red vegetables | ||
| Unadjusted | 0.95 (0.90–1.00) | 0.040 |
| Age‐ and energy‐adjusted | 0.96 (0.91–1.01) | 0.114 |
| Multivariable‐adjusted | 0.98 (0.93–1.04) | 0.463 |
| Multivariable‐adjusted plus non‐yellow/orang/red vegetables | 1.02 (0.96–1.08) | 0.511 |
| Leafy green vegetables | ||
| Unadjusted | 0.89 (0.80–1.00) | 0.048 |
| Age and energy‐adjusted | 0.90 (0.80–1.00) | 0.055 |
| Multivariable‐adjusted | 0.89 (0.79–1.01) | 0.063 |
| Multivariable‐adjusted plus nonleafy green vegetables | 0.91 (0.80–1.03) | 0.127 |
| Legumes | ||
| Unadjusted | 0.97 (0.90–1.04) | 0.394 |
| Age‐ and energy‐adjusted | 0.98 (0.91–1.05) | 0.511 |
| Multivariable‐adjusted | 0.97 (0.90–1.04) | 0.379 |
| Multivariable‐adjusted plus nonlegumes | 0.99 (0.92–1.07) | 0.793 |
ASVD indicates atherosclerotic vascular disease.
Results are presented as hazard ratios (95% confidence intervals) using Cox proportional hazards modeling per 5 g/d for allium vegetables and per 10 g/d for all other vegetable types. Cruciferous vegetables included cabbage, brussels sprouts, cauliflower, and broccoli. Allium vegetables included onion, leek, and garlic. Yellow/orange/red vegetables included tomato, capsicum, beetroot, carrot, and pumpkin. Leafy green vegetables included lettuce and other salad greens, celery, silver beet, and spinach. Legumes included peas, greens beans, bean sprouts and alfalfa sprouts, baked beans, soy beans, soy bean curd and tofu, and other beans.
Multivariable‐adjusted models included age, body mass index, physical activity, alcohol intake, smoking history, socioeconomic status, calcium supplementation group, antihypertensive medication, statin medication, low‐dose aspirin, Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate, and energy intake.
When excluding participants who died in the first 24 months of the study, cruciferous (per 10 g/d) and allium (per 5 g/d) vegetables remained inversely associated with ASVD mortality, with a multivariable‐adjusted HR of 0.87 (95% CI, 0.80–0.93) and 0.83 (95% CI, 0.73–0.94) (P<0.01 for both). Although not significant in the initial multivariable‐adjusted analysis, intakes of leafy green vegetables (per 10 g/d) were significant after excluding all deaths that occurred in the first 24 months (multivariable‐adjusted HR, 0.88; 95% CI, 0.78–1.00 [P=0.047]). The median intake for leafy green vegetables for participants excluded in the first 24 months was 12.1 (interquartile range, 3.9–25.8) g/d, whereas for the cohort excluding these participants it was 17.1 (interquartile range, 9.8–25.8) g/d.
Additional adjustment for the Nutrient‐Rich Foods Index did not alter the significant associations of cruciferous (per 10‐g/d increase: HR, 0.89; 95% CI, 0.82–0.95 [P=0.002]) and allium (per 5‐g/d increase: HR, 0.84; 95% CI, 0.74–0.95 [P=0.006]) vegetable intakes. Separate analyses that adjusted for individual dietary factors (total fruit, fish, nuts, fiber, potassium, magnesium, beta carotene, and saturated fat) did not change the associations of both cruciferous and allium vegetable intakes with ASVD mortality (Table 4).
Cruciferous and allium vegetable intakes were inversely associated with ischemic heart disease mortality (P<0.05 for both), and allium vegetable intake was inversely associated with ischemic cerebrovascular disease mortality (P=0.011) (Table 5).
Additional Sensitivity Analyses
Separate analyses that adjusted for FRS did not change the interpretation of the associations between cruciferous (per 10‐g/d increase: HR, 0.88; 95% CI, 0.82–0.95 [P=0.001]), allium (per 5‐g/d increase: HR, 0.81; 95% CI, 0.71–0.92 [P=0.001]), and total vegetables (per 75‐g/d increase: HR, 0.81; 95% CI, 0.69–0.94 [P=0.006]) and ASVD mortality.
There was a weak positive correlation between intakes of cruciferous and allium vegetables (Spearman's ρ=0.12, P<0.001) and a moderate positive correlation between intakes of allium and total vegetables (Spearman's ρ=0.44, P<0.001) and intakes of cruciferous and total vegetables (Spearman's ρ=0.51, P<0.001). In a forward stepwise Cox proportional hazards model, which included all multivariable‐adjusted variables and intakes of cruciferous, allium, and total vegetables, age (P<0.001), BMI (P=0.016), antihypertensive medication (P<0.001), cruciferous vegetables (P<0.001), and allium vegetables (P=0.014) were independent predictors of ASVD mortality (Table 7).
Table 7.
Hazard Ratios and 95% Confidence Intervals for the Best Predictive Model for ASVD Mortality Including Multivariable‐Adjusted Variables and Intakes of Cruciferous, Allium, and Total Vegetablesa
| All Participants (N=1226) | P Value | |
|---|---|---|
| Age, y | 1.17 (1.11–1.23) | <0.001 |
| BMI, kg/m2 | 1.04 (1.01–1.07) | 0.016 |
| Antihypertensive medication (yes/no) | 1.64 (1.24–2.16) | <0.001 |
| Cruciferous vegetable intake, per 10 g/d | 0.88 (0.82–0.94) | <0.001 |
| Allium vegetable intake, per 5 g/d | 0.86 (0.76–0.97) | 0.014 |
ASVD indicates atherosclerotic vascular disease.
Results are presented as hazard ratios (95% confidence interval) using forward stepwise Cox proportional hazards modeling. Cruciferous vegetables included cabbage, brussels sprouts, cauliflower, and broccoli. Allium vegetables included onion, leek, and garlic. Forward stepwise Cox proportional hazards model included age, body mass index (BMI), physical activity, alcohol intake, smoking history, socioeconomic status, calcium supplementation group, antihypertensive medication, statin medication, low‐dose aspirin, Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate, and energy intake, and intakes of cruciferous, allium, and total vegetables.
The mean (SD) of cruciferous, allium, and total vegetables at baseline (1998) and 5 years (2003), and 7 years (2005) are presented in Table 8. A significant effect for time was observed for intakes of cruciferous, allium, and total vegetables using 1‐way repeated measures ANOVA (Wilks Λ P<0.01 for all). To account for this change, the average was individually calculated across all 3 time points (baseline and 5 and 7 years) for cruciferous, allium, and total vegetable intakes. These average values were then entered separately in multivariable‐adjusted Cox proportional hazards models for ASVD mortality. This did not substantively alter the interpretation of the associations between baseline values and ASVD‐related mortality (Table 9).
Table 8.
Mean (SD) for Intakes of Cruciferous, Allium, and Total Vegetables at Baseline and at 5 Years (2003) and 7 Years (2005)a
| Mean | SD | |
|---|---|---|
| Cruciferous vegetables, g/d | ||
| Baselineb | 32.3 | 22.0 |
| 5 y (2003)c | 32.2 | 23.0 |
| 7 y (2005)d | 30.0 | 21.3 |
| Allium vegetables, g/d | ||
| Baseline | 7.8 | 6.7 |
| 5 y (2003) | 6.5 | 5.5 |
| 7 y (2005) | 6.0 | 5.5 |
| Total vegetables, g/d | ||
| Baseline | 196.5 | 78.9 |
| 5 y (2003) | 176.3 | 73.6 |
| 7 y (2005) | 169.3 | 68.6 |
Cruciferous vegetables included cabbage, brussels sprouts, cauliflower, and broccoli. Allium vegetables included onion, leek, and garlic.
N=1226
N=1023
N=852
Table 9.
Multivariable‐Adjusted Hazard Ratios and 95% Confidence Intervals for ASVD‐Related Mortality for Intakes of Cruciferous, Allium, and Total Vegetables Averaged Across Baseline, 5 Years (2003) and 7 Years (2005)a
| All Participants (N=1226) | P Value | |
|---|---|---|
| Cruciferous vegetables | 0.83 (0.76–0.91) | <0.001 |
| Allium vegetables | 0.83 (0.71–0.97) | 0.017 |
| Total vegetables | 0.74 (0.61–0.89) | 0.002 |
ASVD indicates atherosclerotic vascular disease.
Results are presented as hazard ratios (95% confidence intervals) using multivariable‐adjusted Cox proportional hazards modeling per 10 g/d for cruciferous vegetables, per 5 g/d for allium vegetables, and per 75 g/d for total vegetables. The multivariable‐adjusted model included age, body mass index, physical activity, alcohol intake, smoking history, socioeconomic status, calcium supplementation group, antihypertensive medication, statin medication, low‐dose aspirin, Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate, and energy intake. Cruciferous vegetables included cabbage, brussels sprouts, cauliflower, and broccoli. Allium vegetables included onion, leek, and garlic.
Discussion
This study demonstrates that total vegetable intake was inversely associated with ASVD mortality, supporting current guideline recommendations to increase vegetable consumption.3, 4, 5 The major new finding is that both cruciferous and allium vegetables were inversely associated with ASVD mortality. These associations remained after extensive adjustment for other vegetable intakes as well as lifestyle and cardiovascular risk factors. In a forward stepwise model with cruciferous, allium, and total vegetable intake, cruciferous and allium vegetables were both independent predictors of ASVD mortality. Total vegetable intake was not considered an independent predictor possibly because of the moderate correlations of allium and cruciferous vegetables with total vegetable intakes. This should not be misinterpreted to indicate a lack of importance for total vegetable intake. If replicated in other studies, these data further emphasize the importance of cruciferous and allium vegetable intakes in reducing ASVD.
Emerging evidence suggests a protective role for cruciferous vegetables in cardiovascular health.35, 36, 37, 38 Evidence also suggests allium vegetables such as onions to be protective.39, 40 However, this relationship is inconsistent for garlic.40 The results of some population studies and associated meta‐analyses reporting cruciferous and allium vegetable consumption have not always identified an association, possibly because of type II error.41 Cruciferous and allium vegetables contain a variety of nutritive and nonnutritive components that may benefit cardiovascular health. We studied the potential effects of nutritive components such as potassium9 and magnesium10 on the relationships between cruciferous and allium vegetables and ASVD mortality. We found no evidence of change in efficacy. Organosulfur compounds, a nonnutritive component of both cruciferous and allium vegetables, may have benefits.11
Organosulfur compounds are organic sulfur‐containing compounds that have the ability to be hydrogen sulfide (H2S) donors.42 H2S is a gasotransmitter that plays an important role in the regulation of vasodilation, angiogenesis, inflammation, oxidative stress, and apoptosis.43 Organosulfur compounds found abundantly in cruciferous vegetables are glucosinolates, which are a precursor for isothiocyanates. Isothiocyanates have been widely researched because of their anticancer properties.44, 45, 46 Isothiocyanates also have antioxidant and anti‐inflammatory effects, both of which may prevent the progression of atherosclerosis.11, 47, 48 The inflammatory properties of isothiocyanates can be supported by a study demonstrating an inverse association between cruciferous vegetables and proinflammatory cytokines.49
Organosulfur compounds found in allium vegetables have been studied in more depth. Although intake of allium vegetables was 6.2 g/d compared with 196.5 g/d in total vegetable intake, the association of allium vegetables and ASVD mortality was strong. There is evidence that organosulfur compounds found in allium vegetables may have the ability to prevent the production of reactive oxygen species, prevent vascular inflammation, inhibit platelet aggregation, and increase the bioavailability of NO.11 Recent evidence also suggests that allicin, an organosulfur compound found in garlic, is readily degraded into organic polysulfides and the subsequent interactions with thiol groups result in the generation of H2S.50 Preclinical studies have demonstrated possible cardioprotective effects of H2S, and cross talk between H2S and NO signaling may explain these cardioprotective effects.50
Leafy green vegetables are a rich source of dietary nitrate that can be reduced to NO via the entero‐salivary nitrate‐nitrite‐NO pathway.51 Although significant in the unadjusted analysis, after adjustment for covariates and especially after further adjustment of intake of nonleafy green vegetables, which included allium and cruciferous vegetables, the beneficial effect disappeared. This result differs from other studies.41 It should be noted that after excluding all deaths that occurred in the first 24 months, intake of leafy green vegetables became significant after adjustment for multiple covariates. While not consistently significant, leafy green vegetables had a consistent suggestive relationship warranting more research (HR between 0.88 and 0.91 and CI between 0.78 and 1.01). This suggests that leafy green vegetables may still be an important contributor to the benefits seen with ASVD mortality.
Study Strengths
Strengths of this prospective population‐based cohort study were that participants were representative of older adult women from the Australian population. The average vegetable intake of participants in this study was 2.6 servings per day, the same for Australian adult women 75 years and older.52 Food intake was also assessed at 3 different time points during the 15 years of follow‐up, thus reducing measurement error. Given the participants’ age at baseline and the long follow‐up period of 15 years, the ASVD death rate was also increased, giving the study further power to detect an association. Lastly, loss to follow‐up bias was likely minimized, having a complete follow‐up of death records for all participants who remained in Western Australia, which was likely to be the majority of women given their age.
Study Limitations
Our findings need to be interpreted carefully in the context of limitations applicable to prospective cohort studies. Classification by one characteristic results in many other differences in other characteristics such as food and nutrients intakes, energy intake, and physical activity, which may have a substantial effect on the outcome of interest. This problem was addressed by the use of multivariable adjustments that may not have correctly allowed for residual or unknown confounders. For example, it should be noted that the group with the highest versus the lowest intake of vegetable servings reported an ≈30% higher energy intake and higher proportions of most nutrients and food types despite similar BMI values, suggesting that they were correspondingly more active. An important consideration to be noted is that physical activity has been shown to be a strong predictor of cardiovascular health.53, 54 To address this concern, both total energy intake and self‐reported physical activity were included as covariates without affecting the outcome of interest. The self‐reported physical activity used in this study has been shown to be related to bone mineral density in the same cohort of older adult women.55 Higher intakes of nonvegetable foods such as fish and nuts, which have been shown in other studies to have cardiovascular health benefits56, 57 were observed in participants who consumed higher vegetable servings. Individual adjustments for these foods did not alter the interpretation between cruciferous, allium, and total vegetable intakes and ASVD mortality. Dietary intakes in this study were self‐reported and, therefore, measurement error and misclassification are possible. We attempted to address measurement error by averaging the intakes of cruciferous, allium and total vegetables across baseline, 5 years (2003) and 7 years (2005), and entering these values individually in multivariable‐adjusted Cox proportional hazards models for ASVD mortality. This did not substantively alter the interpretation between baseline values and ASVD‐related mortality. Lastly, given the observational nature of this study, a causal relationship between the exposure and ASVD mortality remains uncertain.
Conclusions
Intakes of cruciferous and allium vegetables were inversely associated with ASVD mortality in this cohort of women aged 70 years and older. These findings support a focus on studying the effects of increased intake of cruciferous and allium vegetables on cardiovascular disease risk. They may precipitate greater study of the role and potential mechanisms of organosulfur compounds on ASVD outcomes. If supported by other studies, efforts to increase cruciferous and allium vegetables within the diet may lead to an achievable and cost‐effective approach to reduce worldwide cardiovascular morbidity and mortality.
Author Contributions
Ms Blekkenhorst and Drs Lewis, Devine, Prince, and Hodgson designed the research; Ms Blekkenhorst and Dr Hodgson conducted the research; Drs Lewis, Zhu, Lim, Devine, and Prince provided the essential materials; Ms Blekkenhorst, and Drs Woodman and Hodgson analyzed the data; Ms Blekkenhorst and Drs Prince and Hodgson wrote the article; Ms Blekkenhorst had primary responsibility for the final content; and all authors critically revised the article for important intellectual content. All authors read and approved the final article.
Sources of Funding
The PLSAW was funded by Healthway the Western Australian Health Promotion Foundation and by project grants 254627, 303169, and 572604 from the National Health and Medical Research Council of Australia. This analysis was supported by a National Health and Medical Research Council of Australia project grant 1084922. The salaries of Drs Lewis and Hodgson were supported by National Health and Medical Research Council of Australia Fellowships. These funding agencies had no role in the design, analysis, or writing of this article.
Disclosures
None.
Acknowledgments
The authors wish to thank the staff at the Western Australian Data Linkage Branch, Hospital Morbidity Data Collection and Registry of Births, Deaths and Marriages for their work on providing the data for this study.
(J Am Heart Assoc. 2017;6:e006558 DOI: 10.1161/JAHA.117.006558.)
References
- 1. Mendis S, Puska P, Norrving B. Global Atlas on Cardiovascular Disease Prevention and Control. World Health Organization; 2011.
- 2. Gan Y, Tong X, Li L, Cao S, Yin X, Gao C, Herath C, Li W, Jin Z, Chen Y, Lu Z. Consumption of fruit and vegetable and risk of coronary heart disease: a meta‐analysis of prospective cohort studies. Int J Cardiol. 2015;183:129–137. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization . Fruit and vegetables for health: report of a joint FAO/WHO workshop. 2004. Available at: http://www.who.int/dietphysicalactivity/publications/fruit_vegetables_report.pdf. Accessed September 25, 2017.
- 4. National Health and Medical Research Council . Australian Dietary Guidelines. 2013. Available at: https://www.nhmrc.gov.au/guidelines-publications/n55. Accessed September 25, 2017.
- 5. U.S. Department of Health and Human Services and U.S. Department of Agriculture . 2015–2020 Dietary Guidelines for Americans. Available at: https://health.gov/dietaryguidelines/2015/guidelines/ 2015. Accessed September 25, 2017.
- 6. Borgi L, Muraki I, Satija A, Willett WC, Rimm EB, Forman JP. Fruit and vegetable consumption and the incidence of hypertension in three prospective cohort studies. Hypertension. 2016;67:288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu RH. Health‐promoting components of fruits and vegetables in the diet. Adv Nutr. 2013;4:384S–392S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Threapleton DE, Greenwood DC, Evans CE, Cleghorn CL, Nykjaer C, Woodhead C, Cade JE, Gale CP, Burley VJ. Dietary fibre intake and risk of cardiovascular disease: systematic review and meta‐analysis. BMJ. 2013;347:f6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta‐analyses. BMJ. 2013;346:f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Del Gobbo LC, Imamura F, Wu JH, de Oliveira Otto MC, Chiuve SE, Mozaffarian D. Circulating and dietary magnesium and risk of cardiovascular disease: a systematic review and meta‐analysis of prospective studies. Am J Clin Nutr. 2013;98:160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vazquez‐Prieto MA, Miatello RM. Organosulfur compounds and cardiovascular disease. Mol Aspects Med. 2010;31:540–545. [DOI] [PubMed] [Google Scholar]
- 12. Voutilainen S, Nurmi T, Mursu J, Rissanen TH. Carotenoids and cardiovascular health. Am J Clin Nutr. 2006;83:1265–1271. [DOI] [PubMed] [Google Scholar]
- 13. Weitzberg E, Lundberg JO. Novel aspects of dietary nitrate and human health. Annu Rev Nutr. 2013;33:129–159. [DOI] [PubMed] [Google Scholar]
- 14. Quiñones M, Miguel M, Aleixandre A. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol Res. 2013;68:125–131. [DOI] [PubMed] [Google Scholar]
- 15. Prince RL, Devine A, Dhaliwal SS, Dick IM. Effects of calcium supplementation on clinical fracture and bone structure: results of a 5‐year, double‐blind, placebo‐controlled trial in elderly women. Arch Intern Med. 2006;166:869–875. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization . Manual of the International Statistical Classification of Diseases, Injuries and Causes of Death, 9th Revision (ICD‐9). 1977.
- 17. National Coding Centre . The Australian Version of the International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM). Sydney: National Coding Centre, Faculty of Health Sciences, University of Sydney; 1996. [Google Scholar]
- 18. Britt H, Scahill S, Miller G. ICPC PLUS for community health? A feasibility study. Health Inf Manag. 1997;27:171–175. [DOI] [PubMed] [Google Scholar]
- 19. National Centre for Classification in Health . International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Australian Modification (ICD‐10‐AM). 1998. [PubMed]
- 20. Ireland P, Jolley D, Giles G, O'Dea K, Powles J, Rutishauser I, Wahlqvist ML, Williams J. Development of the Melbourne FFQ: a food frequency questionnaire for use in an Australian prospective study involving an ethnically diverse cohort. Asia Pac J Clin Nutr. 1994;3:19–31. [PubMed] [Google Scholar]
- 21. Hodge A, Patterson AJ, Brown WJ, Ireland P, Giles G. The Anti Cancer Council of Victoria FFQ: relative validity of nutrient intakes compared with weighed food records in young to middle‐aged women in a study of iron supplementation. Aust N Z J Public Health. 2000;24:576–583. [DOI] [PubMed] [Google Scholar]
- 22. Lewis J, Milligan G, Hunt A. NUTTAB 95 Nutrient Data Table for Use in Australia. 1995.
- 23. Fulgoni VL, Keast DR, Drewnowski A. Development and validation of the nutrient‐rich foods index: a tool to measure nutritional quality of foods. J Nutr. 2009;139:1549–1554. [DOI] [PubMed] [Google Scholar]
- 24. National Health and Medical Research Council . Nutrient reference values for Australia and New Zealand. 2006.
- 25. Blekkenhorst LC, Bondonno CP, Lewis JR, Devine A, Woodman RJ, Croft KD, Lim WH, Wong G, Beilin LJ, Prince RL, et al. Association of dietary nitrate with atherosclerotic vascular disease mortality: a prospective cohort study of older adult women. Am J Clin Nutr. 2017;106:207–216. [DOI] [PubMed] [Google Scholar]
- 26. Devine A, Dhaliwal SS, Dick IM, Bollerslev J, Prince RL. Physical activity and calcium consumption are important determinants of lower limb bone mass in older women. J Bone Miner Res. 2004;19:1634–1639. [DOI] [PubMed] [Google Scholar]
- 27. McArdle WD, Katch FI, Katch VL. Energy, Nutrition and Human Performance. Philadelphia, PA: Lea & Febiger; 1991. [Google Scholar]
- 28. Pollock ML, Wilmore JH, Fox SM. Health and Fitness Through Physical Activity. New York, NY: Wiley and Sons; 1978. [Google Scholar]
- 29. Australian Bureau of Statistics . Socio‐economic indexes for areas. Catalogue Number 2039.0. Canberra: ABS, 1998. Available at: https://www.ausstats.abs.gov.au/ausstats/free.nsf/0/C17E9A880591BB45CA256AE9001BCD57/$File/2039.0_1996.pdf
- 30. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lewis JR, Lim W, Dhaliwal SS, Zhu K, Lim EM, Thompson PL, Prince RL. Estimated glomerular filtration rate as an independent predictor of atherosclerotic vascular disease in older women. BMC Nephrol. 2012;13:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 33. D'Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circ J. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 34. Blekkenhorst LC, Prince RL, Hodgson JM, Lim WH, Zhu K, Devine A, Thompson PL, Lewis JR. Dietary saturated fat intake and atherosclerotic vascular disease mortality in elderly women: a prospective cohort study. Am J Clin Nutr. 2015;101:1263–1268. [DOI] [PubMed] [Google Scholar]
- 35. Joshipura KJ, Ascherio A, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Hennekens CH, Spiegelman D, Willett WC. Fruit and vegetable intake in relation to risk of ischemic stroke. J Am Med Assoc. 1999;282:1233–1239. [DOI] [PubMed] [Google Scholar]
- 36. Cornelis MC, El‐Sohemy A, Campos H. GSTT1 genotype modifies the association between cruciferous vegetable intake and the risk of myocardial infarction. Am J Clin Nutr. 2007;86:752–758. [DOI] [PubMed] [Google Scholar]
- 37. Zhang X, Shu XO, Xiang YB, Yang G, Li H, Gao J, Cai H, Gao YT, Zheng W. Cruciferous vegetable consumption is associated with a reduced risk of total and cardiovascular disease mortality. Am J Clin Nutr. 2011;94:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Buil‐Cosiales P, Toledo E, Salas‐Salvadó J, Zazpe I, Farràs M, Basterra‐Gortari FJ, Diez‐Espino J, Estruch R, Corella D, Ros E, Marti A, Gómez‐Gracia E, Ortega‐Calvo M, Arós F, Moñino M, Serra‐Majem L, Pintó X, Lamuela‐Raventós RM, Babio N, Gonzalez JI, Fitó M, Martínez‐González MA. Association between dietary fibre intake and fruit, vegetable or whole‐grain consumption and the risk of CVD: results from the PREvención con DIeta MEDiterránea (PREDIMED) trial. Br J Nutr. 2016;116:534–546. [DOI] [PubMed] [Google Scholar]
- 39. Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ. 1996;312:478–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Galeone C, Tavani A, Pelucchi C, Negri E, La Vecchia C. Allium vegetable intake and risk of acute myocardial infarction in Italy. Eur J Nutr. 2009;48:120–123. [DOI] [PubMed] [Google Scholar]
- 41. Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, Greenwood DC, Riboli E, Vatten LJ, Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all‐cause mortality: a systematic review and dose‐response meta‐analysis of prospective studies. Int J Epidemiol. 2017;46:1029–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gu X, Zhu YZ. Therapeutic applications of organosulfur compounds as novel hydrogen sulfide donors and/or mediators. Expert Rev Clin Pharmacol. 2011;4:123–133. [DOI] [PubMed] [Google Scholar]
- 43. Yu XH, Cui LB, Wu K, Zheng XL, Cayabyab FS, Chen ZW, Tang CK. Hydrogen sulfide as a potent cardiovascular protective agent. Clin Chim Acta. 2014;437:78–87. [DOI] [PubMed] [Google Scholar]
- 44. Wu QJ, Yang Y, Vogtmann E, Wang J, Han LH, Li HL, Xiang YB. Cruciferous vegetables intake and the risk of colorectal cancer: a meta‐analysis of observational studies. Ann Oncol. 2013;24:1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu X, Lv K. Cruciferous vegetables intake is inversely associated with risk of breast cancer: a meta‐analysis. Breast. 2013;22:309–313. [DOI] [PubMed] [Google Scholar]
- 46. Liu B, Mao Q, Cao M, Xie L. Cruciferous vegetables intake and risk of prostate cancer: a meta‐analysis. Int J Urol. 2012;19:134–141. [DOI] [PubMed] [Google Scholar]
- 47. Bai Y, Wang X, Zhao S, Ma C, Cui J, Zheng Y. Sulforaphane protects against cardiovascular disease via Nrf2 activation. Oxid Med Cell Longev. 2015;2015:407580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yamagishi S, Matsui T. Protective role of sulphoraphane against vascular complications in diabetes. Pharm Biol. 2016;54:2329–2339. [DOI] [PubMed] [Google Scholar]
- 49. Jiang Y, Wu SH, Shu XO, Xiang YB, Ji BT, Milne GL, Cai Q, Zhang X, Gao YT, Zheng W, Yang G. Cruciferous vegetable intake is inversely correlated with circulating levels of proinflammatory markers in women. J Acad Nutr Diet. 2014;114:700–708.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bradley JM, Organ CL, Lefer DJ. Garlic‐derived organic polysulfides and myocardial protection. J Nutr. 2016;146:403S–409S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bondonno CP, Croft KD, Hodgson JM. Dietary nitrate, nitric oxide, and cardiovascular health. Crit Rev Food Sci Nutr. 2016;56:2036–2052. [DOI] [PubMed] [Google Scholar]
- 52. Australian Bureau of Statistics . National Health Survey: first results, 2014–15. 2015. Available at: http://www.abs.gov.au/ausstats/abs@.nsf/mf/4364.0.55.001.. Accessed September 25, 2017.
- 53. Berlin JA, Colditz GA. A meta‐analysis of physical activity in the prevention of coronary heart disease. Am J Epidemiol. 1990;132:612–628. [DOI] [PubMed] [Google Scholar]
- 54. Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Siscovick DS. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–725. [DOI] [PubMed] [Google Scholar]
- 55. Nurzenski MK, Briffa NK, Price RI, Khoo BC, Devine A, Beck TJ, Prince RL. Geometric indices of bone strength are associated with physical activity and dietary calcium intake in healthy older women. J Bone Miner Res. 2007;22:416–424. [DOI] [PubMed] [Google Scholar]
- 56. He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Greenland P. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta‐analysis of cohort studies. Circulation. 2004;109:2705–2711. [DOI] [PubMed] [Google Scholar]
- 57. Luo C, Zhang Y, Ding Y, Shan Z, Chen S, Yu M, Hu FB, Liu L. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all‐cause mortality: a systematic review and meta‐analysis. Am J Clin Nutr. 2014;100:256–269. [DOI] [PubMed] [Google Scholar]


