Abstract
Background
More than 60% of patients decline participation in cardiac rehabilitation after a myocardial infarction. Options to improve physical activity (PA) and other risk factors in these high‐risk individuals are limited. We conducted a phase 2 randomized controlled trial to determine feasibility, safety, acceptability, and estimates of effect of tai chi on PA, fitness, weight, and quality of life.
Methods and Results
Patients with coronary heart disease declining cardiac rehabilitation enrollment were randomized to a “LITE” (2 sessions/week for 12 weeks) or to a “PLUS” (3 sessions/week for 12 weeks, then maintenance classes for 12 additional weeks) condition. PA (accelerometry), weight, and quality of life (Health Survey Short Form) were measured at baseline and 3, 6, and 9 months after baseline; aerobic fitness (stress test) was measured at 3 months. Twenty‐nine participants (13 PLUS and 16 LITE) were enrolled. Retention at 9 months was 90% (LITE) and 88% (PLUS). No serious tai chi–related adverse events occurred. Significant mean between group differences in favor of the PLUS group were observed at 3 and 6 months for moderate‐to‐vigorous PA (100.33 min/week [95% confidence interval, 15.70–184.95 min/week] and 111.62 min/week; [95% confidence interval, 26.17–197.07 min/week], respectively, with a trend toward significance at 9 months), percentage change in weight, and quality of life. No changes in aerobic fitness were observed within and between groups.
Conclusions
In this community sample of patients with coronary heart disease declining enrollment in cardiac rehabilitation, a 6‐month tai chi program was safe and improved PA, weight, and quality of life compared with a 3‐month intervention. Tai chi could be an effective option to improve PA in this high‐risk population.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02165254.
Keywords: cardiac rehabilitation, coronary heart disease, physical exercise, risk factor, secondary prevention
Subject Categories: Cardiovascular Disease, Secondary Prevention, Exercise, Risk Factors, Lifestyle
Clinical Perspective
What Is New?
Compared with a shorter intervention, a 6‐month tai chi exercise intervention was safe, feasible, and enjoyable and increased moderate‐to‐vigorous physical activity among deconditioned patients with coronary heart disease who had declined enrollment in cardiac rehabilitation.
Other benefits included weight loss and improvements in quality of life.
This is the first study showing that tai chi may improve exercise behaviors in this high‐risk population.
What Are the Clinical Implications?
Tai chi is a promising and safe exercise alternative for patients with coronary heart disease who are unable or unwilling to attend traditional cardiac rehabilitation.
If proved effective in larger studies, tai chi could be offered as an alternative exercise option within existing cardiac rehabilitation programs or within the context of community‐based rehabilitation programs.
Introduction
Approximately 1 of 4 survivors of an acute coronary event will experience another cardiovascular event or die within the following 5 years.1, 2 This unfavorable prognosis is, however, avoidable. The aftermath of an acute coronary event can represent, in fact, a critical window for behavioral change.3 For example, smoking cessation rates of 60% are reported in long‐term smokers in the period immediately after a diagnosis of myocardial infarction.4 Physical activity (PA) is another critical target for post–coronary event behavioral change. Substantial epidemiological evidence indicates that PA delays the development of atherosclerosis and is associated with reduced risk of future coronary events.5, 6 Each 1–metabolic equivalent increase in fitness is associated with a 19% decrease in the risk of cardiovascular mortality.7, 8 Cardiac rehabilitation (CR) programs leverage this critical window for behavioral change by offering exercise training and education on coronary risk factor modification and reduce both all‐cause and cardiovascular mortality.9, 10, 11, 12 Given these substantial benefits, current guidelines recommend referral to CR after an acute coronary event, percutaneous coronary interventions, and coronary bypass surgery.13
The large majority of this high‐risk population, however, misses the opportunity for healthy behavioral changes offered by the participation in CR, because <40% of eligible patients join these programs in the United States.14 Older men, women of all ages, and ethnic minorities are particularly unlikely to enroll.15 Reasons for nonenrollment include dislike of exercise, perception of exercise as tiring or painful, low exercise capacity, travel distance to the CR center, high copays, and psychosocial factors, such as depression and low social support.16, 17, 18, 19 In addition, CR programs have high attrition rates for reasons ranging from the limited number of available exercise options to the lack of social interactions during programs.18, 20 These discouraging data have prompted calls for the “design of evidence‐based, alternative approaches to traditional CR that help provide affordable access to clinically effective secondary prevention interventions (p. 2954).”14
Tai chi, a traditional Chinese martial art based on gentle body movements accompanied by relaxation and breathing exercises,21 could be a promising exercise option for patients not attending CR. First, the exercise intensity during tai chi can be adjusted by varying session duration, body position during practice, and training style to meet the needs of elderly and deconditioned individuals.22 Second, because tai chi is safe even in high‐risk patients,23 it can be offered in community centers,24, 25 thus overcoming transportation barriers associated with CR. Third, tai chi gentle training may be particularly attractive for women, who are less likely than men to attend CR and typically dislike the type of exercise offered at CRs.19 Fourth, tai chi energy expenditure can reach 4.6 metabolic equivalents (moderate‐intensity aerobic activity)26, 27 and has been shown to improve self‐reported PA28, 29, 30 and other coronary risk factors.31 Studies have also suggested that tai chi may improve cardiac fitness.28 Last, the emphasis placed on breathing and relaxation exercises during tai chi may improve psychological distress.31 Recent research has shown that the integration of stress reduction within a comprehensive CR program results in additional clinical benefits compared with CR alone.32
To our knowledge, no study has to date evaluated whether tai chi could be offered as an alternative exercise option for patients who declined enrollment in CR. In preparation for a future large efficacy trial, we conducted a phase 2 study to determine which dose of tai chi is most feasible, acceptable, and safe (primary outcome) and to obtain preliminary estimates of effect of each dose on PA and aerobic fitness (secondary outcomes). Other secondary outcomes included quality of life (QOL) and body weight.
Methods
Population
Methods for this study have been described in detail elsewhere.33 Briefly, participants were recruited via flyers placed in cardiology practices, community centers, and other public venues; on‐line resources; and advertisements on local radio programs and community newspapers. Because tai chi was offered in a class format, we recruited 3 consecutive cohorts of participants. Inclusion criteria were as follows: (1) history of coronary heart disease; (2) ability to understand and speak English; (3) aged ≥21 years; and (4) being physically inactive (7 days PA Recall Questionnaire)34 and defined as not meeting current recommendations for PA35 (ie, 30 minutes of moderate‐intensity aerobic PA 5 d/week or 20 minutes of vigorous‐intensity activity 3 d/week). Exclusion criteria included the following: (1) inability or unwillingness to give informed consent; (2) unstable angina; (3) blood pressure >200/110 mm Hg or orthostatic systolic blood pressure decrease >20 mm Hg; (4) uncontrolled atrial or ventricular arrhythmias; (5) third‐degree AV block; (6) pericarditis or myocarditis; (7) recent thrombophlebitis; (8) abnormal stress test result without study cardiologist's clearance; (9) medical conditions likely to limit life span to <1 year; (10) New York Heart Association functional class IV; (11) severe cognitive impairment (Blessed Orientation‐Memory‐Concentration test scores ≥1036); (12) orthopedic problems prohibiting tai chi practice; (13) ongoing tai chi or other mind‐body training; (14) current enrollment in CR; (15) severe depression (Hospital Anxiety and Depression Scale depression subscale scores, >14); and (16) conditions likely to affect study compliance (ie, moving out of study area and substance use).
Once interest and preliminary eligibility were confirmed, participants were invited for a screening visit. After providing in‐person informed consent, participants completed baseline assessments and entered a run‐in period of 1 week during which they wore accelerometers for the assessment of baseline level of PA and underwent a symptom‐limited exercise stress test for evaluation of cardiac risk37 and baseline aerobic fitness. A cardiologist (W‐C.W.) gave the final clearance for the participant's enrollment in the study. Participants received a $40 stipend at each study visit. The Rhode Island Hospital (Providence) Institutional Review Board approved the study protocol (docket 599729).
The randomization schedule (1:1 ratio) was generated in “R” (http://cran.us.r-project.org)38 on the basis of a permuted block randomization procedure using small random‐sized blocks. The allocation table was uploaded to an Access database; once all assessments were completed, the research assistant (J.K.) assigned each participant to either condition by clicking the “randomize” button.
Tai Chi Interventions
Classes were held in a room located in the CR building. For each cohort, we offered 3 1‐hour tai chi classes/week during weeks 1 to 12. Patients in the LITE (2 sessions/week for 12 weeks) group were invited to come twice a week, and patients in the PLUS (3 sessions/week for 12 weeks, then maintenance classes for 12 additional weeks) group were invited to attend 3 times a week. Participants in the PLUS group continued with 2 classes weekly during week 13 to 16, followed by 1 maintenance class every other week (weeks 17–24). Both groups were instructed to practice tai chi at home at least 3 times per week using a DVD (30‐minute duration) containing the same sequence of tai chi exercises taught in class. Regardless of dose assignment, the content of the intervention was identical in the 2 groups.
A panel of experts in CR, cardiovascular prevention, tai chi, and exercise physiology reviewed the protocol used in previous studies conducted by our group in patients with heart failure and chronic obstructive lung disease.29, 39 On the basis of the panel's recommendations, a tai chi research expert (P.M.W.) worked with the tai chi instructors (3 certified instructors with 10+ years' experience) to modify existing protocols by increasing aerobic intensity over time (ie, by gradually adjusting the pace of practices [slightly faster over time] and depth of stances [slightly lower over time]).33
Intervention fidelity was assessed according to the Treatment Fidelity Workgroup guidelines.40 Tai chi sessions were video recorded, and the study auditor (P.M.W.) reviewed 10% of videos for protocol consistency using a checklist developed for the study.
Blinding
Participants were informed they would be randomized to 1 of 2 doses of tai chi and, thus, were not blinded to their dose allocation. Study personnel, other than the research assistant responsible for participant randomization and monitoring of adverse events, were blinded.
Measures
Assessments were performed at baseline and at 3, 6, and 9 months after enrollment. Data were collected using Access software for direct data entry from study participant into electronic surveys. Study data were collected between January 2015 and December 2017 and analyzed in March‐April 2017.
Primary Outcomes
Indicators of feasibility were retention rates, tai chi session attendance (recorded by the research assistant at each session), and individual tai chi home practice (self‐reported using a log to be kept daily and collected in class each week).
Acceptability was evaluated at the end of the intervention using a self‐reported rating scale ranging from 0 (did not like) to 4 (liked very much).29
For safety, the PA Readiness questionnaire was used to determine before each session whether the participant had experienced any chest pain, shortness of breath, dizziness, or muscle injury since the previous class.41 At each study visit, we collected information about unplanned clinic visits and hospital readmissions. Adverse events were validated with information from medical records whenever possible.
Secondary Outcomes
PA was measured using accelerometers. Accelerometers were not worn during tai chi classes. Measures includes number of valid days, wear time, min/week of moderate‐to‐vigorous intensity PA (MVPA), min/week of MVPA recorded in bouts of at least 10 minutes, and min/week of light activity. Data were processed using ActiLife software, with minimum wear time set at 10 h/d and nonwear time set at 60 minutes. Given the increased burden associated with participants wearing the accelerometer for a second week in case of mechanical failure of the accelerometer, the 7‐day PA Recall Questionnaire was used as a backup.34 Although absolute amounts of PA differ between these 2 measures, there is a good correlation between PA Recall Questionnaire and accelerometry collected data.42
For aerobic fitness, oxygen consumption in metabolic equivalents was calculated in all patients from stress test parameters, according to the protocol used in the test. Formulas for the Bruce Protocol and Modified Bruce Protocol were based on the American College of Sports Medicine's metabolic equations,43, 44 which are functions based on sex and time on the treadmill. Because of logistical problems, we were able to obtain direct measures of oxygen uptake (VO2) at peak exercise (mL/kg per minute) using breath‐by‐breath expired gas analysis only in a subset of participants (n=21). There were no significant differences in baseline demographics between those with and without a direct measure of VO2 (all P>0.05).
Body weight was measured on a calibrated balance beam scale. QOL was assessed using a short version (8 items) of the Health Survey Short Form, a self‐reported measure widely used for routine monitoring and assessment of care outcomes in adult patients.45
Covariates included sociodemographic characteristics (age, sex, race/ethnicity, education, and income) measured using validated forms; medical history (coronary risk factors, coronary revascularization procedures, ejection fraction, New York Heart Association class, comorbidities, and medications), abstracted from medical records; body mass index (calculated as weight [kg]/height [meters squared]); depression (using the Hospital Anxiety and Depression Scale,46 a self‐administered questionnaire with 2 subscales [score range, 0–21] measuring anxiety and depression, with higher scores indicating greater psychological morbidity); and patients' expectations about the intervention.47 We also gathered information about reasons for not attending CR using a questionnaire developed for this study, as in the study by Dunlay et al.48
Statistical Analyses
All analyses were conducted according to the intention‐to‐treat principle (with the exception of the attendance outcome, for which both intention‐to‐treat and per‐protocol analyses were conducted). t Tests, nonparametric tests, and χ2 tests, as applicable, were used to compare between‐group baseline differences as well as retention rates, attendance rates, and individual home tai chi practice.
Unadjusted average min/week of MVPA over time were calculated separately by group and between‐group differences in unadjusted mean and median MVPA at each time point and were tested using t tests and Mann‐Whitney tests, respectively. To estimate treatment effects, we used a longitudinal mixed‐effects regression model to simultaneously regress absolute MVPA (min/week) at each follow‐up on treatment group, time, group×time, and baseline MVPA. Models included a subject‐specific intercept to account for repeated measures within individual over time. A statistically significant interaction indicates that between‐group differences vary across time points.49 Contrasts were used to test between‐group differences in MVPA at each follow‐up time, controlling for baseline. Baseline variables that differed between groups and were predictive of PA were included as covariates in the multivariate model.50 Because of the large variances in mean MVPA over time, we attempted to transform towards normality. Because there were no substantial differences in the resulting model, we present the untransformed version in the Results, for ease of interpretation. Similar mixed‐effects models were used to estimate mean between‐dose differences for light activity and other secondary outcomes. All study analyses were performed using SAS, version 9.3, statistical software. Before study inception, we established to deem either dose feasible if at least 80% of participants were retained at the end of each intervention, if they attended at least 70% of the planned classes, and if they completed at least 70% of the prescribed home tai chi exercises. The intervention was considered acceptable if 80% of participants rated it a 4 (liked very much).
Results
Participants were on average 67.9 (SD, 10.3) years old, 27% were female, and 93% were white, with almost two thirds reporting at least some college education (Table 1). Overall, this was a high‐risk population: half of the study participants were diabetic, two thirds had high cholesterol levels or hypertension, more than half were obese, and one third were smoking at enrollment. More than 60% had a history of myocardial infarction, and 80% had had a percutaneous coronary intervention. Although baseline characteristics were overall balanced by randomization, LITE participants were slightly older, had a higher body mass index, were less physically active, and were more likely to have heart failure. The most common reasons for not having attended CR included dislike of intensive exercise and the perception that CR was dangerous (indicated by more than half of study participants), followed by insurance, scheduling, and transportation issues.
Table 1.
Baseline Characteristics of the Study Sample
| Characteristics | Overall (N=29) | PLUS Group (n=13) | LITE Group (n=16) |
|---|---|---|---|
| Age, mean (SD), y | 67.9 (10.3) | 65.2 (10.5) | 70.1 (9.9) |
| Female sex | 7 (26.9) | 3 (25.0) | 4 (28.6) |
| Race | |||
| American Indian or Alaskan Native | 1 (3.4) | 1 (7.7) | 0 |
| Asian or Pacific Islander | 0 | 0 | 0 |
| Black | 1 (3.4) | 1 (7.7) | 0 |
| Hispanic/Latino | 0 | 0 | 0 |
| White (not Hispanic) | 27 (93.1) | 11 (84.6) | 16 (100) |
| Education | |||
| High school or less | 8 (27.6) | 5 (38.5) | 3 (18.8) |
| College or some college | 15 (51.7) | 7 (53.8) | 8 (50.0) |
| Postgraduate | 6 (20.7) | 1 (7.7) | 5 (31.3) |
| Current smoker | 8 (27.6) | 3 (23.1) | 5 (31.2) |
| Diabetes mellitus | 14 (48.3) | 6 (46.2) | 8 (50.0) |
| High cholesterol | 22 (75.9) | 8 (61.5) | 14 (87.5) |
| BMI, mean (SD), kg/m2 | 30.4 (5.8) | 28.3 (3.8) | 32.2 (6.8) |
| Normal (<25 kg/m2) | 4 (20.0) | 3 (33.3) | 1 (9.1) |
| Overweight (25–<30 kg/m2) | 7 (35.0) | 2 (22.2) | 5 (45.5) |
| Obese (≥30 kg/m2) | 9 (45.0) | 4 (44.4) | 5 (45.5) |
| Hypertension | 23 (79.3) | 9 (69.2) | 14 (87.5) |
| Angina | 23 (79.3) | 11 (84.6) | 12 (75.0) |
| Myocardial infarction | 17 (58.6) | 9 (69.2) | 8 (50.0) |
| Heart failure | 7 (24.1) | 2 (15.4) | 5 (31.3) |
| CABG | 9 (31.0) | 7 (53.8) | 2 (12.5) |
| PCI | 24 (82.8) | 8 (61.5) | 16 (100) |
| Implantable cardioverter defibrillator | 3 (10.3) | 1 (7.7) | 2 (12.5) |
| Cerebrovascular disease | 3 (10.3) | 2 (15.4) | 1 (6.3) |
| Peripheral artery disease | 2 (6.9) | 1 (7.7) | 1 (6.3) |
| Cancer | 4 (13.8) | 1 (7.7) | 3 (18.8) |
| COPD | 2 (6.9) | 0 | 2 (12.5) |
| Depression, mean (SD) HADS scores | 4.5 (3.5) | 4.6 (2.3) | 4.5 (4.3) |
| Medications | |||
| Antiplatelets | 27 (93.1) | 12 (92.3) | 15 (93.8) |
| Anticoagulants | 3 (10.3) | 1 (7.7) | 2 (12.5) |
| β blockers | 26 (89.7) | 12 (92.3) | 14 (87.5) |
| ACE inhibitors | 11 (37.9) | 6 (46.2) | 5 (31.3) |
| Statins | 24 (82.8) | 11 (84.6) | 13 (81.3) |
| Intervention expectation scores, mean (SD) | 12.6 (2.2) | 12.1 (2.3) | 12.9 (2.2) |
| Reasons for not attending CRa | |||
| Does not like high‐intensity exercise | 18 (62.1) | 7 (53.9) | 11 (68.8) |
| CR too dangerous | 17 (58.6) | 6 (46.2) | 11 (68.7) |
| CR schedule not convenient | 12 (41.3) | 5 (38.5) | 7 (43.8) |
| No insurance coverage | 8 (27.5) | 4 (30.8) | 4 (25.0) |
| No transportation | 7 (24.1) | 2 (15.4) | 5 (31.3) |
| Professional/personal commitments | 6 (20.7) | 1 (7.7) | 5 (31.2) |
Values are n (%) unless otherwise indicated. ACE indicates angiotensin‐converting enzyme; BMI, body mass index; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; CR, cardiac rehabilitation; HADS, Hospital Anxiety and Depression Scale; LITE, 2 sessions/week for 12 weeks; PCI, percutaneous coronary intervention; and PLUS, 3 sessions/week for 12 weeks, then maintenance classes for 12 additional weeks.
Those responding “agree” to “strongly agree.”
Of the 214 subjects initially contacted, 108 were ineligible, and 46 were not interested in participating. The most common reasons for refusal were schedule conflicts and lack of interest. Of the 33 randomized participants, 4 left the study immediately after randomization (Figure 1) and 29 started the intervention (13 in the PLUS group and 16 in the LITE group).
Figure 1.
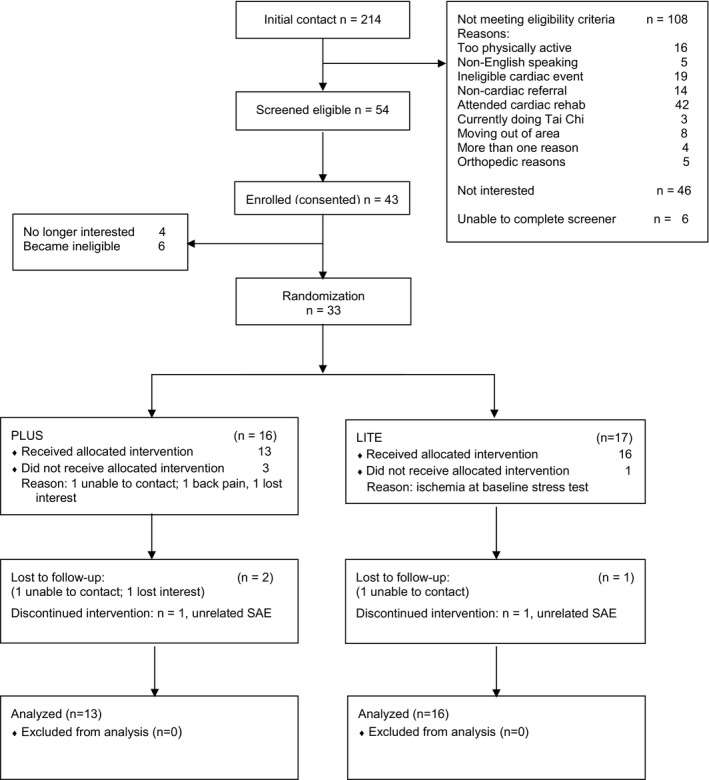
Consort diagram. LITE indicates 2 sessions/week for 12 weeks; PLUS, 3 sessions/week for 12 weeks, then maintenance classes for 12 additional weeks; and SAE, serious adverse event.
Primary Outcomes
Retention rates were 90% (94% in the LITE group and 88% in the PLUS group) at the 9‐month follow‐up visit, with no significant differences by condition. Three participants were unavailable for follow‐up, and 2 discontinued the intervention because of serious adverse events but remained in the study. Among the intention‐to‐treat sample, 39% of PLUS participants and 69% of LITE participants attended at least 70% of planned classes (Table 2). Participants' ratings showed high satisfaction, with 95% indicating that tai chi was highly enjoyable (score, ≥3 on a range of 0–4) and 100% reporting they would recommend it to a friend. Average acceptability scores were similar between conditions (Table 2). Three serious adverse events were observed (1 in the LITE group and 2 in the PLUS group); none was related to the tai chi intervention (Table 3). No cardiac adverse events occurred during the study. The most common adverse events were musculoskeletal, and none was deemed related to tai chi practice.
Table 2.
Class Attendance and Acceptability Scores
| Variable | Overall | LITE Group | PLUS Group | P Value |
|---|---|---|---|---|
| ITT analysis | ||||
| Attendance (classes attended/planned), % | 66.0 | 69.4 | 53.2 | 0.19 |
| Months 1–3, % | 69.4 | 54.0 | 0.21 | |
| Months 4–6, % | NA | 50.0 | NA | |
| Classes attended, mean (SD) | 16.6 (7.2) | 25.1 (16.4) | 0.07 | |
| Per‐protocol analysis | ||||
| Attendance (classes attended/planned), % | 66.5 | 73.3 | 61.2 | 0.35 |
| Months 1–3, % | 73.3 | 62.5 | 0.36 | |
| Months 4–6, % | NA | 59.9 | NA | |
| Classes attended, mean (SD) | 17.5 (6.3) | 29.2 (14.9) | 0.01 | |
| Acceptability score, mean (SD) | 3.6 (0.6) | 3.6 (0.7) | 3.7 (0.5) | 0.61 |
ITT indicates intention to treat; LITE, 2 sessions/week for 12 weeks; NA, not applicable; and PLUS, 3 sessions/week for 12 weeks, then maintenance classes for 12 additional weeks.
Table 3.
Adverse Events
| Event | LITE Group | PLUS Group | Relatedness |
|---|---|---|---|
| Vaginal bleedinga | 1 | 0 | Definitely unrelated |
| Abdominal discomforta | 0 | 1 | Definitely unrelated |
| Muscular chest paina | 0 | 1 | Definitely unrelated |
| Sore throat | 1 | 0 | Definitely unrelated |
| Bruise on left thumb | 0 | 1 | Unlikely related |
| Bruise on left forearm | 0 | 1 | Definitely unrelated |
| Muscle cramps | 0 | 1 | Possibly related |
| Thoracic pain | 1 | 1 | Definitely unrelated |
| Back pain | 1 | 0 | Definitely unrelated |
| Cellulitis, right foot | 1 | 0 | Definitely unrelated |
| Fall | 1 | 1 | Definitely unrelated |
| Bronchitis | 0 | 1 | Definitely unrelated |
| Total | 6 | 8 |
LITE indicates 2 sessions/week for 12 weeks; and PLUS, 3 sessions/week for 12 weeks, then maintenance classes for 12 additional weeks.
Serious adverse events (participants were admitted to the hospital, but no serious condition was diagnosed as the cause of the patient's complaint).
Secondary Outcomes
Complete accelerometer data were available for all participants. Unadjusted means of MVPA (min/week) at each time point are presented in Table 4 and Figure 2. We found significant between‐group differences in mean MVPA at baseline, 3 months, and 6 months (P=0.04, P=0.04, and P=0.02, respectively) in favor of the PLUS group. Because PA outcomes are subject to large variances (particularly in small samples), we also examined between‐group differences in median MVPA over time. Results suggested significant differences in unadjusted median MVPA at 9 months (P=0.03).
Table 4.
Unadjusted MVPA Levels (Accelerometry)
| MVPA, min/week | Overall | LITE Group | PLUS Group |
|---|---|---|---|
| Baseline | 94.4 (96.9) | 61.8 (64.8) | 134.6 (116.0) |
| 3 mo | 105.8 (122.7) | 67.1 (67.4) | 170.3 (167.0) |
| 6 mo | 117.6 (154.2) | 64.8 (63.7) | 181.0 (205.7) |
| 9 mo | 87.6 (103.5) | 44.4 (42.2) | 145.1 (133.4) |
Values are mean (SD). LITE indicates 2 sessions/week for 12 weeks; MVPA, moderate‐to‐vigorous physical activity; and PLUS, 3 sessions/week for 12 weeks, then maintenance classes for 12 additional weeks.
Figure 2.
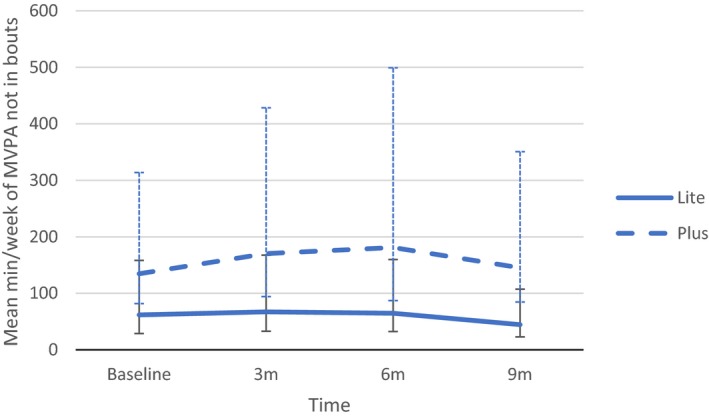
Unadjusted moderate‐to‐vigorous physical activity (MVPA), by study condition. LITE indicates 2 sessions/week for 12 weeks; and PLUS, 3 sessions/week for 12 weeks, then maintenance classes for 12 additional weeks.
Regression models adjusting for baseline MVPA and including subject‐specific intercepts indicated significant treatment effects on mean MVPA at 3 and 6 months favoring the PLUS groups. Specifically, between‐group differences in MVPA were 100.33 min/week at 3 months (95% confidence interval [CI], 15.70–184.95 min/week) and 111.62 min/week at 6 months (95% CI, 26.17–197.07 min/week). Effects at 9 months trended towards significance (P=0.08) in favor of the PLUS group. In addition, MVPA did not significantly decrease between 6 and 9 months in the aggregated sample (P>0.05). We also tested a model that controlled for accelerometer wear time and variables not balanced by randomization, but model fit was not improved and these variables were removed from the final model.
Unadjusted average maximum VO2 (VO2 max) at baseline and 3 months is presented in Table 5 (calculated and measured). As shown by similarly low baseline VO2 max values, both groups were severely deconditioned, confirming this was a particularly frail population. There were no significant between‐group differences in calculated VO2 max at 3 months, controlling for protocol type and baseline differences between groups (β=0.22; 95% CI, −1.91 to 2.35). For the subset of participants in whom we were able to obtain direct measures of VO2 max, we also found no significant between‐group difference in mean VO2 max at 3 months, controlling for baseline (β=−0.30; 95% CI, −2.92 to 2.31).
Table 5.
Unadjusted Calculated and Measured VO2 Max
| VO2 max, mL/kg per minute | Overall | LITE Group | PLUS Group |
|---|---|---|---|
| Calculated | |||
| Baseline | 15.0 (7.5) | 13.7 (8.0) | 16.6 (6.8) |
| 3 mo | 15.0 (6.4) | 13.8 (6.8) | 16.6 (5.8) |
| Measured | |||
| Baseline | 11.8 (3.0) | 10.8 (3.2) | 13.0 (2.2) |
| 3 mo | 12.2 (3.6) | 11.4 (3.8) | 13.4 (3.2) |
Values are mean (SD). n=28 for the calculated group, and n=21 for the measured group. LITE indicates 2 sessions/week for 12 weeks; PLUS, 3 sessions/week for 12 weeks, then maintenance classes for 12 additional weeks; and VO2 max, maximum oxygen uptake.
Unadjusted findings for weight and QOL are shown in Table 6. Longitudinal models suggest significant effects in favor of the PLUS arm for percentage change in body weight51 at 3 months, controlling for baseline (β=3.31; 95% CI, 0.05–7.09). Specifically, mean percentage weight loss was higher in the PLUS group (2.86 kg; SD, 3.35 kg) than in the LITE group (−0.45 kg; SD, 4.99 kg). Weight loss was maintained from 3 to 9 months.
Table 6.
Unadjusted Weight, Sleep Quality, and Quality‐of‐Life Scores
| Variable | Baseline | ||
|---|---|---|---|
| Entire Sample | LITE Group | PLUS Group | |
| Weight, kg | |||
| Baseline | 88.4 (24.3) | 97.9 (29.1) | 77.4 (9.8) |
| 3 mo | 90.5 (23.5) | 98.0 (26.8) | 79.3 (11.1) |
| 6 mo | 89.7 (24.0) | 98.4 (27.9) | 78.5 (11.0) |
| 9 mo | 90.3 (25.0) | 98.7 (28.9) | 78.7 (11.6) |
| Sleep quality scores | |||
| Baseline | 7.7 (4.3) | 7.9 (4.8) | 7.5 (3.6) |
| 3 mo | 6.7 (3.2) | 6.8 (3.1) | 6.7 (3.5) |
| 6 mo | 6.9 (3.6) | 6.3 (3.4) | 7.3 (3.8) |
| 9 mo | 6.8 (4.6) | 6.0 (4.5) | 8.0 (4.7) |
| Quality‐of‐life scores | |||
| Baseline | 2.7 (1.0) | 2.9 (1.0) | 2.5 (1.1) |
| 3 mo | 2.7 (0.9) | 3.1 (0.9) | 2.2 (0.8) |
| 6 mo | 2.9 (0.9) | 3.1 (0.5) | 2.6 (1.1) |
| 9 mo | 3.0 (0.7) | 3.1 (0.5) | 2.7 (0.9) |
Values are mean (SD). LITE indicates 2 sessions/week for 12 weeks; and PLUS, 3 sessions/week for 12 weeks, then maintenance classes for 12 additional weeks.
Models for QOL (where lower scores indicate better QOL) show a significant difference in favor of the PLUS condition at 3 months (β=−0.69; 95% CI, −1.34 to −0.04). Effects at 6 and 9 months were in the same direction, albeit not significant (P=0.16 and P=0.27, respectively).
Post Hoc Analyses
Because this population was particularly deconditioned, we thought it would be important to assess the effect of the intervention on light activity. Models showed a significant effect on light activity at 3 and 6 months favoring the PLUS group (mean between‐group differences in light activity were 397.88 [95% CI, 78.50–717.27] min/week and 376.75 [95% CI, 53.34–700.15] min/week), respectively. We observed a trend towards significance at 9 months (P=0.09) in favor of the PLUS arm.
Discussion
In this community‐based sample of patients who declined enrollment in CR, we found that a longer (6‐month) tai chi exercise intervention improved objectively assessed PA, weight, and QOL compared with a shorter (3‐month) intervention. Overall, both interventions were safe, acceptable, and feasible, with the exception of class attendance, which was slightly lower in the PLUS group.
Notably, once patients enrolled in the tai chi program, 90% were retained until the end of the study; those who dropped out did so for serious adverse events and not because they disliked the program. This high retention rate compares favorably with dropout rates reaching up to 60% in CR.20 With regard to safety, and consistent with a recent systematic review,23 our findings indicate that tai chi is safe among deconditioned patients with coronary heart disease provided that participants are screened following the same criteria adopted for CR.
We found that participation in a longer tai chi program improved both moderate and light PA, effects that trended toward significance at 9 months in the PLUS group. Such a trend is important because it suggests a more enduring behavioral change that persisted even when support from the instructor and group was no longer present. This finding is particularly remarkable considering that our population was clearly resistant to behavioral change, as shown by the high proportion of current smokers and obese individuals in our study population.
Both fear of exercise and the perception of CR as dangerous were the most commonly reported reasons for declining participation in CR. Tai chi can clearly overcome these barriers because it is a different form of exercise. During training, participants are constantly reminded they do not need to strive or struggle to achieve predetermined goals in terms of heart rate or exercise intensity. Instead, they are invited to focus their attention on the breath and/or on the movements of the body. As a result, participants do not see tai chi exercise as threatening, and this may result in improvements in exercise self‐efficacy. In fact, an analysis of data from a randomized controlled trial of tai chi for patients with heart failure has shown that increases in exercise self‐efficacy mediated changes in functional status.52
To our knowledge, this is the first study that examined the effect of tai chi on objectively assessed PA among patients not attending CR. One study conducted in outpatients with class I to III systolic heart failure found that participation in a 12‐week tai chi training program improved self‐reported PA (CHAMPS (Community Healthy Activities Model Program for Seniors/CHAMPS activities questionnaire)) compared with controls.29 Another study reported similar results with a slightly longer intervention (16 weeks).30 A second element of novelty is the use of tai chi as a possible alternative to CR‐based exercise programs. To date, studies have examined the role of tai chi as an adjunct to CR exercise training,53, 54 whereas, to our knowledge, no study has investigated the possible role of tai chi as an alternative exercise option for patients not attending traditional CR.
Increasing class frequency (2 versus 3 classes) did not affect aerobic fitness after 3 months of tai chi training compared with baseline. Findings from a recent meta‐analysis suggest that tai chi produces modest improvements in VO2 max28; however, the duration of the tai chi intervention in the studies included in this meta‐analysis ranged from 1 to 4.7 years.55, 56, 57 It is possible that the duration of tai chi training may positively affect aerobic fitness; in fact, it has been suggested that a longer duration of tai chi training is needed to affect fitness.58 For logistical reasons, we were unable to perform a third stress test at intervention completion among PLUS participants and, thus, we do not know if aerobic fitness would have increased after 6 months of training in this group. Furthermore, 1 of the aforementioned studies55 assessed exercise intensity during steady‐state tai chi performance and found average heart rates exceeding 70% of the maximal heart rate. Although we did not measure participants' heart rate during exercise, another explanation for our results is that the intensity of tai chi intervention may not have been sufficient to improve peak VO2. Finally, aerobic fitness did not improve despite improvements in overall PA. This finding is not surprising, given that several factors, including, among others, heritability,59 age and adiposity,60 and smoking,61 influence aerobic fitness in addition to PA.
Our work has several important strengths. First, this was the first study to examine the effect of tai chi on objectively measured PA. Second, we were able to enroll a community‐based sample of severely deconditioned, high‐risk patients who had declined enrollment in CR. Third, retention rates at the end of the study were superior to those commonly reported in CRs. Last, we included rigorous assessments of intervention fidelity.
Our study has also some limitations. First, as expected, given the type of population selected, recruitment was a challenging aspect of this study. Because these patients had already declined a recommended treatment (CR), it is not surprising that they were hard to enroll in a research study of a new approach. Despite their history of coronary heart disease, one third of our patients were still smoking and 80% were overweight or obese, indicating that we were targeting a high‐risk group with high resistance to behavioral change. Competition with CR in our catchment area was another problem, because an intensive enrollment campaign was launched while our study was ongoing. Second, the sample size was small, which, despite randomization, resulted in groups being unbalanced for certain baseline characteristics. However, baseline differences in activity and other characteristics were accounted for in analyses. Third, another limitation is the underrepresentation of women and minorities. Fourth, our exercise protocol may not have been intense enough to increase aerobic fitness. Once participants feel more confident, improvements in aerobic fitness could be achieved (eg, by increasing the pace of exercises, modifying body position during practice [ie, squatting or raising hands over head], or simply providing patients with a heart rate monitor to self‐monitor pace during practice). Finally, this study measured only aerobic fitness, whereas other important determinants of overall physical fitness (eg, balance, agility, and muscular strength) were not assessed.
Conclusion
In sum, tai chi is a promising and safe exercise alternative for patients with coronary heart disease who are unable or unwilling to attend traditional CR, in particular for older people, women, and deconditioned individuals. If our findings are confirmed in larger studies, tai chi could be offered either as an alternative exercise training option within existing CR programs or within the context of community‐based rehabilitation programs.
Sources of Funding
This project was funded by National Center for Complementary and Integrative Health (NCCIH) grant R34 AT007569 to Salmoirago‐Blotcher. Wayne is supported by NCCIH grant K24 AT009282. The funding agency had no involvement in the study design; in the collection, analysis, and interpretation of data; in the writing of this report; and in the decision to submit this article for publication.
Disclosures
Salmoirago‐Blotcher, Dunsiger, Bock, Breault, Wu, and Yeh have no conflict of interest to declare. Wayne is the founder and sole owner of the Tree of Life Tai Chi Center. Wayne's interests were reviewed and managed by the Brigham and Women's Hospital and Partner's HealthCare in accordance with their conflict of interest policies.
Acknowledgments
We thank the study tai chi instructors Joe Anzalone, Jamee Culbertson, and Marie Favorito.
(J Am Heart Assoc. 2017;6:e006603 DOI: 10.1161/JAHA.117.006603.)
References
- 1. Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D'Agostino R, Liau CS, Mas JL, Röther J, Smith SC Jr, Salette G, Contant CF, Massaro JM, Steg PG; REACH Registry Investigators . Comparative determinants of 4‐year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–1357. [DOI] [PubMed] [Google Scholar]
- 2. Walker S, Asaria M, Manca A, Palmer S, Gale CP, Shah AD, Abrams KR, Crowther M, Timmis A, Hemingway H, Sculpher M. Long‐term healthcare use and costs in patients with stable coronary artery disease: a population‐based cohort using linked health records (CALIBER). Eur Heart J Qual Care Clin Outcomes. 2016;2:125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baldacchino D. Myocardial infarction: a turning point in meaning in life over time. Br J Nurs. 2011;20:107–114. [DOI] [PubMed] [Google Scholar]
- 4. Dawood N, Vaccarino V, Reid KJ, Spertus JA, Hamid N, Parashar S. Predictors of smoking cessation after a myocardial infarction: the role of institutional smoking cessation programs in improving success. Arch Intern Med. 2008;168:1961–1967. [DOI] [PubMed] [Google Scholar]
- 5. Hu G, Tuomilehto J, Borodulin K, Jousilahti P. The joint associations of occupational, commuting, and leisure‐time physical activity, and the Framingham risk score on the 10‐year risk of coronary heart disease. Eur Heart J. 2007;28:492–498. [DOI] [PubMed] [Google Scholar]
- 6. Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease. Circulation. 2003;107:3109–3116. [DOI] [PubMed] [Google Scholar]
- 7. Lee DC, Sui X, Artero EG, Lee IM, Church TS, McAuley PA, Stanford FC, Kohl HW III, Blair SN. Long‐term effects of changes in cardiorespiratory fitness and body mass index on all‐cause and cardiovascular disease mortality in men: the Aerobics Center Longitudinal Study. Circulation. 2011;124:2483–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wessel TR, Arant CB, Olson MB, Johnson BD, Reis SE, Sharaf BL, Shaw LJ, Handberg E, Sopko G, Kelsey SF, Pepine CJ, Merz NB. Relationship of physical fitness vs body mass index with coronary artery disease and cardiovascular events in women. JAMA. 2004;292:1179–1187. [DOI] [PubMed] [Google Scholar]
- 9. Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long‐term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation. 2010;121:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heran BS, Chen JM, Ebrahim S, Moxham T, Oldridge N, Rees K, Thompson DR, Taylor RS. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;(7):CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Connor GT, Buring JE, Yusuf S, Goldhaber SZ, Olmstead EM, Paffenbarger RS Jr, Hennekens CH. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation. 1989;80:234–244. [DOI] [PubMed] [Google Scholar]
- 12. Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation. 2011;123:2344–2352. [DOI] [PubMed] [Google Scholar]
- 13. Kushner FG, Hand M, Smith SC Jr, King SB III, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE Jr, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO. 2009 Focused updates: ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:2271–2306. [DOI] [PubMed] [Google Scholar]
- 14. Balady GJ, Ades PA, Bittner VA, Franklin BA, Gordon NF, Thomas RJ, Tomaselli GF, Yancy CW. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation. 2011;124:2951‐2960. [DOI] [PubMed] [Google Scholar]
- 15. Ades PA, Waldmann ML, Polk DM, Coflesky JT. Referral patterns and exercise response in the rehabilitation of female coronary patients aged greater than or equal to 62 years. Am J Cardiol. 1992;69:1422–1425. [DOI] [PubMed] [Google Scholar]
- 16. Ades PA, Waldmann ML, McCann WJ, Weaver SO. Predictors of cardiac rehabilitation participation in older coronary patients. Arch Intern Med. 1992;152:1033–1035. [PubMed] [Google Scholar]
- 17. Jackson L, Leclerc J, Erskine Y, Linden W. Getting the most out of cardiac rehabilitation: a review of referral and adherence predictors. Heart. 2005;91:10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grace SL, Gravely‐Witte S, Kayaniyil S, Brual J, Suskin N, Stewart DE. A multisite examination of sex differences in cardiac rehabilitation barriers by participation status. J Womens Health (Larchmt). 2009;18:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benz Scott LA, Ben‐Or K, Allen JK. Why are women missing from outpatient cardiac rehabilitation programs? A review of multilevel factors affecting referral, enrollment, and completion. J Womens Health (Larchmt). 2002;11:773–791. [DOI] [PubMed] [Google Scholar]
- 20. Scotto CJ, Waechter D, Rosneck J. Factors affecting program completion in phase II cardiac rehabilitation. Can J Cardiovasc Nurs. 2011;21:15–20. [PubMed] [Google Scholar]
- 21. Wayne PM, Kaptchuk TJ. Challenges inherent to t'ai chi research, part I: t'ai chi as a complex multicomponent intervention. J Altern Complement Med. 2008;14:95–102. [DOI] [PubMed] [Google Scholar]
- 22. Fuzhong L, Harmer P, McAuley E, Duncan TE, Duncan SC, Chaumeton N, Fisher KJ. An evaluation of the effects of Tai Chi exercise on physical function among older persons: a randomized controlled trial. Ann Behav Med. 2001;23:139–146. [DOI] [PubMed] [Google Scholar]
- 23. Wayne PM, Berkowitz DL, Litrownik DE, Buring JE, Yeh GY. What do we really know about the safety of Tai Chi? A systematic review of adverse event reports in randomized trials. Arch Phys Med Rehabil. 2014;95:2470–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones AY, Dean E, Scudds RJ. Effectiveness of a community‐based Tai Chi program and implications for public health initiatives. Arch Phys Med Rehabil. 2005;86:619–625. [DOI] [PubMed] [Google Scholar]
- 25. Taylor‐Piliae RE, Haskell WL, Froelicher ES. Hemodynamic responses to a community‐based Tai Chi exercise intervention in ethnic Chinese adults with cardiovascular disease risk factors. Eur J Cardiovasc Nurs. 2006;5:165–174. [DOI] [PubMed] [Google Scholar]
- 26. Lan C, Chen SY, Lai JS. The exercise intensity of Tai Chi Chuan. Med Sport Sci. 2008;52:12–19. [DOI] [PubMed] [Google Scholar]
- 27. Zhuo D, Shephard RJ, Plyley MJ, Davis GM. Cardiorespiratory and metabolic responses during Tai Chi Chuan exercise. Can J Appl Sport Sci. 1984;9:7–10. [PubMed] [Google Scholar]
- 28. Zheng G, Li S, Huang M, Liu F, Tao J, Chen L. The effect of Tai Chi training on cardiorespiratory fitness in healthy adults: a systematic review and meta‐analysis. PLoS One. 2015;10:e0117360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yeh GY, McCarthy EP, Wayne PM, Stevenson LW, Wood MJ, Forman D, Davis RB, Phillips RS. Tai Chi exercise in patients with chronic heart failure: a randomized clinical trial. Arch Intern Med. 2011;171:750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barrow DE, Bedford A, Ives G, O'Toole L, Channer KS. An evaluation of the effects of Tai Chi Chuan and Chi Kung training in patients with symptomatic heart failure: a randomised controlled pilot study. Postgrad Med J. 2007;83:717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang XQ, Pi YL, Chen PJ, Liu Y, Wang R, Li X, Chen BL, Zhu Y, Yang YJ, Niu ZB. Traditional Chinese exercise for cardiovascular diseases: systematic review and meta‐analysis of randomized controlled trials. J Am Heart Assoc. 2016;5:e002562 DOI: 10.1161/JAHA.115.002562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blumenthal JA, Sherwood A, Smith PJ, Watkins L, Mabe S, Kraus WE, Ingle K, Miller P, Hinderliter A. Enhancing cardiac rehabilitation with stress management training: a randomized, clinical efficacy trial. Circulation. 2016;133:1341–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salmoirago‐Blotcher E, Wayne P, Bock BC, Dunsiger S, Wu WC, Stabile L, Yeh G. Design and methods of the Gentle Cardiac Rehabilitation Study: a behavioral study of Tai Chi exercise for patients not attending cardiac rehabilitation. Contemp Clin Trials. 2015;43:243–251. DOI: 10.1016/j.cct.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Blair SN, Haskell WL, Ho P, Paffenbarger RS Jr, Vranizan KM, Farquhar JW, Wood PD. Assessment of habitual physical activity by a seven‐day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122:794–804. [DOI] [PubMed] [Google Scholar]
- 35. Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. [DOI] [PubMed] [Google Scholar]
- 36. Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation‐Memory‐Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140:734–739. [DOI] [PubMed] [Google Scholar]
- 37. American College of Sports Medicine . Guidelines for Exercise Testing and Prescription. 6th ed Baltimore, MD: Lippincott Williams & Wilkins; 2000:22–32. [Google Scholar]
- 38. Everitt B, Hothorn T. A Handbook of Statistical Analyses Using R. 2nd ed Boca Raton, FL: CRC Press; 2010. [Google Scholar]
- 39. Yeh GY, Roberts DH, Wayne PM, Davis RB, Quilty MT, Phillips RS. Tai Chi exercise for patients with chronic obstructive pulmonary disease: a pilot study. Respir Care. 2010;55:1475–1482. [PMC free article] [PubMed] [Google Scholar]
- 40. Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, Ogedegbe G, Orwig D, Ernst D, Czajkowski S. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23:443–451. [DOI] [PubMed] [Google Scholar]
- 41. Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR‐Q). Can J Sport Sci. 1992;17:338–345. [PubMed] [Google Scholar]
- 42. Sloane R, Snyder DC, Demark‐Wahnefried W, Lobach D, Kraus WE. Comparing the 7‐day physical activity recall with a triaxial accelerometer for measuring time in exercise. Med Sci Sports Exerc. 2009;41:1334–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. di Prampero PE, Salvadego D, Fusi S, Grassi B. A simple method for assessing the energy cost of running during incremental tests. J Appl Physiol. 2009;107:1068–1075. [DOI] [PubMed] [Google Scholar]
- 44. Glass S, Gregory B. ACSM's Metabolic Calculations Handbook. Baltimore, MD: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 45. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36), I: conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 46. Zigmond A, Snaith R. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- 47. Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31:73–86. DOI: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 48. Dunlay SM, Witt BJ, Allison TG, Hayes SN, Weston SA, Koepsell E, Roger VL. Barriers to participation in cardiac rehabilitation. Am Heart J. 2009;158:852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chuang‐Stein C, Tong DM. The impact and implication of regression to the mean on the design and analysis of medical investigations. Stat Methods Med Res. 1997;6:115–128. [DOI] [PubMed] [Google Scholar]
- 50. Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med. 2002;21:2917–2930. [DOI] [PubMed] [Google Scholar]
- 51. Sharma AM, Karmali S, Birch DW. Reporting weight loss: is simple better? Obesity (Silver Spring). 2010;18:219. [DOI] [PubMed] [Google Scholar]
- 52. Yeh GY, Mu L, Davis RB, Wayne PM. Correlates of exercise self‐efficacy in a randomized trial of mind‐body exercise in patients with chronic heart failure. J Cardiopulm Rehabil Prev. 2016;36:186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Caminiti G, Volterrani M, Marazzi G, Cerrito A, Massaro R, Arisi A, Franchini A, Sposato B, Rosano G. Tai Chi enhances the effects of endurance training in the rehabilitation of elderly patients with chronic heart failure. Rehabil Res Pract. 2011;2011:761958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Taylor‐Piliae RE, Silva E, Sheremeta SP. Tai Chi as an adjunct physical activity for adults aged 45 years and older enrolled in phase III cardiac rehabilitation. Eur J Cardiovasc Nurs. 2012;11:34–43. DOI: 10.1016/j.ejcnurse.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lai JS, Wong MK, Lan C, Chong CK, Lien IN. Cardiorespiratory responses of Tai Chi Chuan practitioners and sedentary subjects during cycle ergometry. J Formos Med Assoc. 1993;92:894–899. [PubMed] [Google Scholar]
- 56. Lan C, Chou SW, Chen SY, Lai JS, Wong MK. The aerobic capacity and ventilatory efficiency during exercise in Qigong and Tai Chi Chuan practitioners. Am J Chin Med. 2004;32:141–150. [DOI] [PubMed] [Google Scholar]
- 57. Lan C, Lai JS, Chen SY, Wong MK. 12‐Month Tai Chi training in the elderly: its effect on health fitness. Med Sci Sports Exerc. 1998;30:345–351. [DOI] [PubMed] [Google Scholar]
- 58. Taylor‐Piliae RE. The effectiveness of Tai Chi exercise in improving aerobic capacity: an updated meta‐analysis. Med Sport Sci. 2008;52:40–53. [DOI] [PubMed] [Google Scholar]
- 59. Schutte NM, Nederend I, Hudziak JJ, Bartels M, de Geus EJ. Twin‐sibling study and meta‐analysis on the heritability of maximal oxygen consumption. Physiol Genomics. 2016;48:210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Serrano‐Sanchez JA, Delgado‐Guerra S, Olmedillas H, Guadalupe‐Grau A, Arteaga‐Ortiz R, Sanchis‐Moysi J, Dorado C, Calbet JA. Adiposity and age explain most of the association between physical activity and fitness in physically active men. PLoS One. 2010;5:e13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Borba AT, Jost RT, Gass R, Nedel FB, Cardoso DM, Pohl HH, Reckziegel MB, Corbellini VA, Paiva DN. The influence of active and passive smoking on the cardiorespiratory fitness of adults. Multidiscip Respir Med. 2014;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]


