Abstract
Background
Although 24‐hour blood pressure (BP) variability (BPV) is predictive of cardiovascular outcomes independent of absolute BP levels, it is not regularly assessed in clinical practice. One possible limitation to routine BPV assessment is the lack of standardized methods for accurately estimating 24‐hour BPV. We conducted a systematic review to assess the predictive power of reported BPV indexes to address appropriate quantification of 24‐hour BPV, including the average real variability (ARV) index.
Methods and Results
Studies chosen for review were those that presented data for 24‐hour BPV in adults from meta‐analysis, longitudinal or cross‐sectional design, and examined BPV in terms of the following issues: (1) methods used to calculate and evaluate ARV; (2) assessment of 24‐hour BPV determined using noninvasive ambulatory BP monitoring; (3) multivariate analysis adjusted for covariates, including some measure of BP; (4) association of 24‐hour BPV with subclinical organ damage; and (5) the predictive value of 24‐hour BPV on target organ damage and rate of cardiovascular events. Of the 19 assessed studies, 17 reported significant associations between high ARV and the presence and progression of subclinical organ damage, as well as the incidence of hard end points, such as cardiovascular events. In all these cases, ARV remained a significant independent predictor (P<0.05) after adjustment for BP and other clinical factors. In addition, increased ARV in systolic BP was associated with risk of all cardiovascular events (hazard ratio, 1.18; 95% confidence interval, 1.09–1.27). Only 2 cross‐sectional studies did not find that high ARV was a significant risk factor.
Conclusions
Current evidence suggests that ARV index adds significant prognostic information to 24‐hour ambulatory BP monitoring and is a useful approach for studying the clinical value of BPV.
Keywords: blood pressure variability, cardiovascular events, target organ damage
Subject Categories: High Blood Pressure, Hypertension, Blood Pressure, Prognosis, Risk Factors
Clinical Perspective
What Is New?
Until now, there has not been a systematic review or meta‐analysis comparing the prognostic value of the different indexes used to measure 24‐hour blood pressure (BP) variability.
Average real variability was a better estimator of 24‐hour BP variability than other measures of dispersion. Average real variability remained as an independent predictor after adjustment for BP and other clinical factors for the presence and progression of subclinical organ damage. Incidences of cardiovascular events in the general population and hypertensive individuals were considered.
What Are the Clinical Implications?
The average real variability index adds significant prognostic information to 24‐hour ambulatory BP monitoring, and is a useful approach for studying the clinical value of BP variability. However, further investigation is required to define potential diagnostic thresholds of abnormal BP variability before this parameter becomes a regular tool for clinical use.
Introduction
Blood pressure (BP) is a dynamic physiological parameter, influenced by behavioral, emotional, and environmental factors, as well as intrinsic cardiovascular control. Daily variation in BP is the sum of responses to extrinsic pressor stimuli, spontaneous and regulatory fluctuations attributable to influences of the central nervous system,1, 2 mechanical forces generated by respiration,3 and effects of humoral and local vasomotor phenomena.4, 5 Regardless of the causes, arterial and cardiopulmonary reflexes act as modulators of short‐term changes in BP. Reduced efficacy of those reflexes can result in significant increases in BP oscillations.6, 7 Within‐subject BP variability (BPV) also increases with elevated average BP and age.8, 9 The clinical relevance of BPV during a 24‐hour period was initially examined through intra‐arterial beat‐to‐beat measurements.9, 10, 11 Early studies showed that increased BPV in hypertensive individuals is directly related to severity of target organ damage.10, 11, 12 Later studies, using noninvasive, intermittent, reading‐to‐reading, 24‐hour ambulatory BP monitoring (ABPM), provided evidence that an initial increase in BPV is an independent predictor of cardiovascular events/complications.13, 14, 15, 16 However, despite evidence of the clinical relevance of BPV, its pathological role remains a hypothesis, and it is not regularly used in clinical practice. One possible limitation to routine BPV assessment is the lack of standardized methods for accurately estimating 24‐hour BPV.17, 18
The clinical significance and prognostic implications of BPV depend on the measurement method6, 19 and sampling frequency.17, 18, 19, 20 Most studies have evaluated the predictive value of BPV based on the SD of 24‐hour average ABPM recordings, but this index is an approximate indicator of BP dynamics and does not capture many characteristics of BPV.21, 22, 23 Because the SD is correlated with mean BP, it can be inadequate to use both measurements in a multivariate prognostic model.24 One solution is to use the coefficient of variation (CV),25 which is the SD divided by the corresponding mean; or to calculate the variation independent of mean,26 which is similar to the CV, but with the mean raised to a power, x, that removes the correlation. Another important limitation of the 24‐hour SD is that its magnitude is significantly affected by the nocturnal BP decrease in individuals with large daily fluctuations.27, 28 This “dipping” phenomenon can reduce the prognostic power of 24‐hour SD.21 Calculation of a “weighted” 24‐hour SD (wSD; computed as the average of day and night SDs, weighted for their respective durations) can minimize the effect of nocturnal dipping without discarding information about BPV.28, 29
Hypertensive individuals are generally characterized by steep, rapid, short‐duration BPV that might have clinical implications.30, 31 However, the SD does not reflect the steepness or rapidity. Therefore, the time rate of BP variation (TRBPV) was proposed as an alternative index by Zakopoulos et al.32 The TRBPV measures the steepness and speed of changes in BP, calculated as the mean of the absolute ratios of the difference between successive BP readings and the time between them.
To improve the predictive power of 24‐hour BPV, Mena et al proposed the average real variability (ARV) index.33 This method focuses on changes occurring over short time intervals and, thus, corrects some of the limitations of SD, which only reflects the dispersion of BP measurements around the mean. The ARV index calculates the average of absolute changes between consecutive BP readings:
where N denotes the number of valid BP measurements, and k is the order of measurements. Mena et al33 concluded that the ARV index has a greater predictive value than the SD and is more useful for determining therapeutic measures aimed at controlling BPV. However, the relatively high event rate observed in that study indicated that its results could not be completely extrapolated to the general population of hypertensive subjects, and that further study of the prognostic significance of the ARV index was required.21
This review systematically examines recent studies of the ARV index and its prognostic value, specifically its association with subclinical target organ damage and the incidence of cardiovascular events in the general population and hypertensive individuals. We critically discuss the methods that should be considered for standardized estimation of 24‐hour BPV and other fundamental issues that need to be addressed before this parameter becomes a regular tool for clinical use.
Methods
Only original publications in English language were considered. The quality of the relevant articles was assessed using the guidelines recommended by Hayden et al.34
One author (V.G.F.) performed a literature search of potentially relevant articles in the PubMed/MEDLINE database using the keywords “ARV,” “average,” “real,” “blood pressure,” and “variability” and in Google Scholar service based on publications that cited Mena et al33 (up to July 2016). Two authors (V.G.F., J.D.M.) reviewed the abstracts and titles to identify relevant articles, and disagreements were resolved by discussion and debate with a third author (L.J.M.). The full texts of selected articles were obtained, and data were extracted, verified, and checked independently by 2 authors (L.J.M., G.E.M.). Discrepancies were discussed to reach a consensus.
The studies chosen for review were those that presented data for 24‐hour BPV in adults from meta‐analysis, longitudinal or cross‐sectional design, and examined BPV in terms of the following issues: (1) method used to calculate and evaluate ARV; (2) assessment of 24‐hour BPV, determined using noninvasive ABPM; (3) multivariate analysis adjusted for covariates, including some measure of BP; (4) association of 24‐hour BPV with subclinical organ damage; and (5) the predictive value of 24‐hour BPV on target organ damage and rate of cardiovascular events.
Data extracted from the reviewed studies included the type of population, study design, sample size, sex distribution, mean age, BPV indexes, confounding variables included in multivariate analyses, and minimum number of valid BP readings used to quantify BPV. For studies with several multivariate analyses, we focused on fully adjusted models that included BP as a predictor variable to avoid selection bias. Only significant BPV predictors (P<0.05) were selected to address independent risk contribution of BP variation over and above the influence of elevated mean BP levels. When the minimum number of 24‐hour BP readings was not specified, approximate values were estimated on the basis of sampling interval and quality criteria defined by authors or assumed to be 80% of the maximum possible BP measurements.
Data from long‐term prognostic fatal and nonfatal cardiovascular events were pooled using a random‐effects meta‐analysis. Only studies that provided hazard ratios were included in this analysis. Heterogeneity was assessed using the χ2 test and I2 statistic.35 The Egger test36 was used to assess the extent of publication bias. P<0.10 was considered as heterogeneity and asymmetry between studies.
Results
The initial search identified 176 articles citing the article by Mena et al (Figure 1).33 On the basis of our criteria, 157 were excluded, leaving 19 articles in our systematic review. All studies received ethical approval, and each participant gave informed consent. Ten of these37, 38, 39, 40, 41, 42, 43, 44, 45, 46 investigated the prognostic value of 24‐hour BPV (Table 1). Nine studies47, 48, 49, 50, 51, 52, 53, 54, 55 examined its relation to target organ damage (cardiac, cerebral, arterial, renal, endothelial, and vascular).
Figure 1.
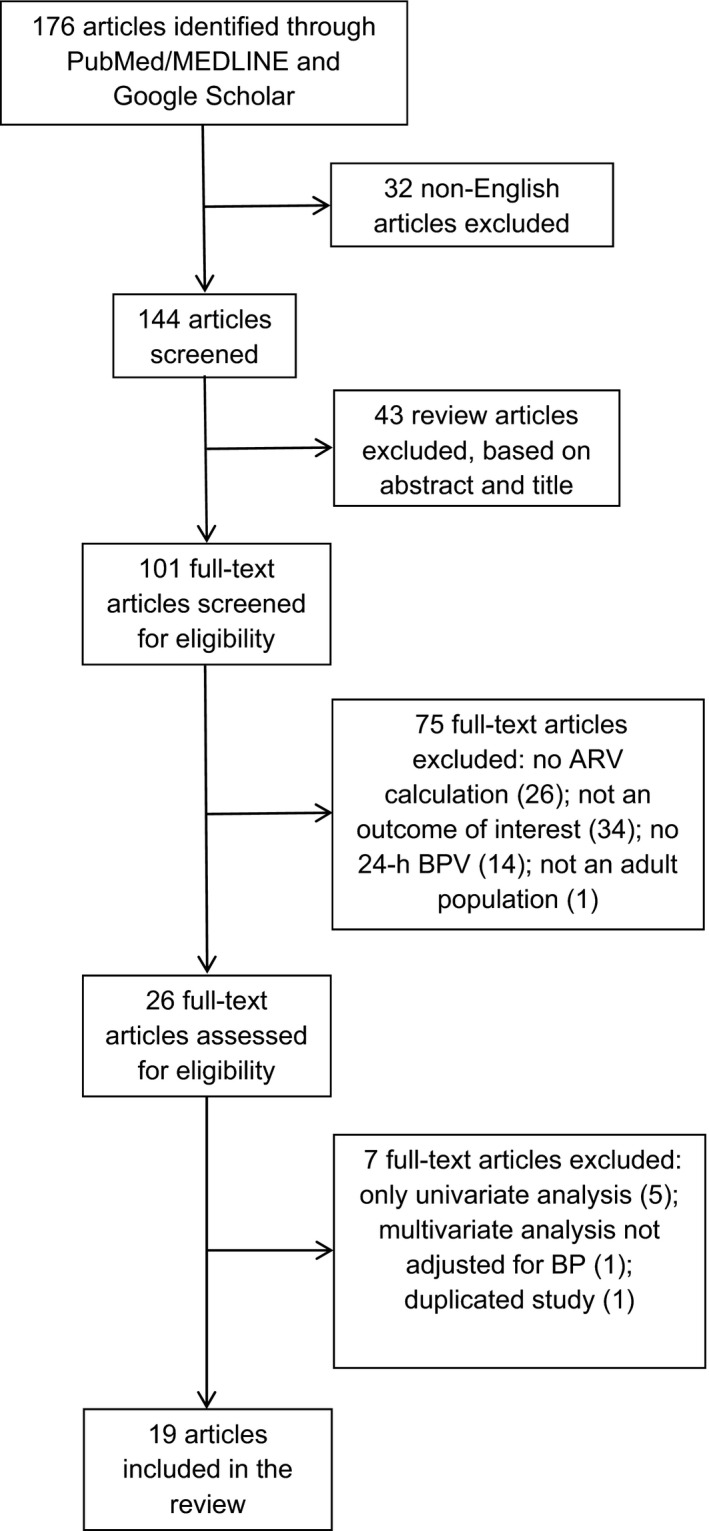
Flow chart showing the process for selection of articles. ARV indicates average real variability; BP, blood pressure; and BPV, BP variability.
Table 1.
Studies That Assessed the Prognostic Value of ARV by Multivariate Analysis
| Study | Population | Design | N, (Women, %) | Age, Mean±SD, y | Estimated Minimum of BP Readings (↑/↓) | Outcome Measure | Significant BVP Index | Confounding Variables Included in Multivariate Analyses |
|---|---|---|---|---|---|---|---|---|
| Hansen et al37 | General | Meta‐analysis | 8938 (46.8) | 53.0±15.8 | 15 (↓) | Cardiovascular events | ARV, wSD | Age, sex, 24‐h HR, BMI, smoking, drinking, TC, history of CVD, DM, TAD, corresponding 24‐h BP level |
| Mena et al38 | General | Meta‐analysis | 5343 (45.6) | 54.0±16.1 | 48 (↑) | Cardiovascular events | ARV | Age, sex, BMI, smoking, drinking, TC, history of CVD, TAD, corresponding 24‐h BP level |
| Hsu et al39 | Normotensive and untreated hypertensive patients | Longitudinal | 1257 (46.8) | 53.1±13.1 | 42 (↓) | Cardiovascular events | ARV | Age, sex, smoking, TC, HDL‐C, IFG, office SBP |
| Pierdomenico et al40 | Untreated and treated hypertensive patients | Longitudinal | 1280 (51.0) | 58.0±10.0 | 59 (↑) | Cardiovascular events | ARV | Age, DM, LVH, daytime SBP level |
| Yamaguchi et al41 | General | Longitudinal | 210 (55.2) | 70.9±0.9 | 27 (↓) | CSVD | ARV, wSD | Age, sex, hypertension, hyperlipidemia, DM, IGT, or IFG, 24‐h SBP level |
| Filomena et al42 | Asymptomatic hypertensive patients | Cross‐sectional | 487 (46.8) | 64.0±3.0 | 47 (↑) | CSVD | ARV | Age, sex, DM, waist circumference, TAD, office DBP, corresponding SBP level |
| Leoncini et al43 | Untreated hypertensive patients | Cross‐sectional | 169 (33.1) | 47.1±9.5 | 64 (↑) | Multiple OD (GFR, IMT, LVH) | ARV, SD, wSD | Age, sex, BMI, smoking, triglycerides, LDL‐C, glucose, SBP load |
| Madden et al46 | General | Cross‐sectional | 1134 (53.5) | 60.2±5.5 | 27 (↓) | Multiple OD (LVH, MAU) | Not significant | Age, sex, smoking, BMI, DM, TAD, 24‐h SBP level |
| Ryu et al44 | Hypertensive patients with CKD | Cross‐sectional | 1173 (37.0) | 56.6±11.9 | 28 (↓) | LVH | ARV | Age, sex, smoking, DM, TAD, exercise, GFR, proteinuria, 24‐h SBP level |
| Mulè et al45 | Untreated hypertensive patients | Cross‐sectional | 315 (58.4) | 45.2±12.4 | 70 (↑) | MAU | ARV | Age, sex, BMI or waist circumference, uric acid, DM or glucose, 24‐h SBP level |
↑, indicates number of BP readings above the minimum sampling rate; ↓, number of BP readings below the minimum sampling rate; ARV, average real variability; BMI, body mass index; BP, blood pressure; BPV, BP variability; CKD, chronic kidney disease; CSVD, cerebral small‐vessel disease; CVD, cardiovascular disease; DBP diastolic BP; DM, diabetes mellitus; GFR, glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; HR, heart rate; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; IMT, intima‐media thickness; LDL‐C, low‐density lipoprotein cholesterol; LVH, left ventricular hypertrophy; MAU, microalbuminuria; OD, organ damage; SBP, systolic BP; TAD, treatment with antihypertensive drug; TC, total cholesterol; and wSD, weighted SD.
The design of the 19 studies included 2 meta‐analyses,37, 38 3 prospective studies,39, 40, 41 and 14 cross‐sectional studies.42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55 Sample sizes ranged from 3652, 54 to 893837 individuals, and the study populations included European, Asian, and North American cohorts, as well as compounded international databases. Eight studies focused on hypertensive populations,40, 42, 43, 44, 45, 48, 49, 55 and 3 of those included patients with diabetes mellitus48 or chronic kidney disease.44, 55 The remaining studies involved healthy subjects,52, 53 populations with mixed hypertensive status,39, 50, 51, 54 or subjects drawn from the general population.37, 38, 41, 46, 47
Analytical approaches used by the 19 studies included multivariate analyses using Cox,37, 38, 39, 40 logistic,41, 42, 43, 44, 45, 46 or linear47, 48, 49, 50, 51, 52, 53, 54, 55 regression models, which incorporated ARV as an independent variable. Six studies used ARV as a unique index for estimating BPV.38, 39, 44, 53, 54, 55 In addition to ARV, 10 studies included measures of dispersion, such as SD,37, 40, 41, 42, 43, 45, 46, 47, 48, 49 CV,41, 42, 46, 47 and wSD.37, 41, 42, 43, 45, 46, 47 Three studies used alternative indexes, such as variation independent of mean50 or TRBPV,51, 52 to assess BPV. The estimated minimum number of BP measurements required for accurate estimates of ARV ranged from 1537 to 77.49 The mean of the minimum sampling rate was of 42.9±16.7 BP readings. P<0.05 was considered statistically significant in all cases.
Prognostic Significance of ARV
Four studies examined the long‐term prognostic significance of 24‐hour BPV, estimated using ARV, for cardiovascular outcome37, 38, 39, 40 (Table 2). Two of the studies used large samples from the International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcome and combined results across cohorts in fully adjusted models.37, 38 The results showed that ARV, SD, and wSD predicted cardiovascular mortality over a median of 11.3 years of follow‐up.37 ARV and wSD predicted all fatal plus nonfatal cardiovascular outcomes. ARV predicted fatal outcomes plus nonfatal stroke. The authors concluded that ARV was a better predictor than SD and wSD for most outcomes and, thus, might be a more accurate measure of BPV than SD.37 A later International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcome study that used a minimum of 48 BP readings similarly found that ARV was a significant and independent predictor of cardiovascular and cardiac deaths and all cerebrovascular events.38
Table 2.
Multivariable‐Adjusted Standardized HRs Associated With Higher Systolic ARV as a Continuous Variable
| Study | Cardiovascular Outcome | No. of Events | Median Follow‐Up, y | Systolic ARV HR (95% CI) |
|---|---|---|---|---|
| Hansen et al37 | Fatal and nonfatal | 1049 | 11.3 | 1.07 (1.00–1.48)a |
| Mena et al38 | Fatal | 335 | 10.2 | 1.17 (1.04–1.31)b |
| Hsu et al39 | Fatal | 90 | 20.0 | 1.20 (1.00–1.45)a |
| Pierdomenico et al40 | Fatal and nonfatal | 104 | 4.8 | 1.27 (1.06–1.51)a |
Standardized HRs (95% CIs) were associated with a 1‐SD increase in ARV for cardiovascular events by fully adjusted Cox proportional hazards regression models. ARV indicates average real variability; CI, confidence interval; and HR, hazard ratio.
P<0.05, HR significance.
P<0.01, HR significance.
Hsu et al investigated the prognostic value of a high BPV in normotensive and hypertensive subjects over a median follow‐up of 20 years.39 The results showed that ARV predicted cardiovascular mortality independent of conventional risk factors plus office BP. ARV was not a significant predictor when 24‐hour BP was included in the multivariable models. However, in subgroup analysis, ARV significantly predicted cardiovascular deaths in subjects with hypertension, independently of 24‐hour mean BP and in addition to well‐known risk factors. The authors concluded that BPV might be more useful than BP in long‐term risk stratification for untreated hypertensive subjects. Pierdomenico et al also evaluated the independent prognostic significance of SD and ARV as indexes of BPV in initially untreated and treated hypertensive patients.40 Multivariate analysis adjusted for other covariates, including BP, showed that ARV as either a categorical or a continuous variable was an independent predictor of cardiovascular events, whereas SD was not. The authors concluded that ARV could be a more appropriate index of BPV than SD and a more useful predictor of outcomes.
Two studies evaluated the prognostic capacity of different BPV parameters (SD, CV, wSD, and ARV) as risk factors for cerebral small‐vessel disease (CSVD), an important contributor to stroke and cognitive decline in elderly patients.56 Yamaguchi et al conducted a 4‐year, community‐based, longitudinal study to determine whether CSVD progression was independently related to BPV.41 Multivariable logistic regression analysis using BPV as a continuous variable showed that ARV and wSD were independent predictors of CSVD progression after adjustment for confounding factors and mean BP. The study also found that lacunar infarction was significantly associated with cognitive impairment and that only ARV differed significantly between subjects with and without cognitive decline. Similarly, Filomena et al conducted a cohort study on asymptomatic hypertensive patients to evaluate the potential usefulness of BPV as a predictor of CSVD, compared with BP and other clinical factors.42 Only BPV, measured as ARV, was independently related to the presence of CSVD. Furthermore, ARV was the only metric significantly associated with lacunar brain infarcts and periventricular and deep white matter hyperintensities.
Four cross‐sectional studies examined the predictive relationship of BPV to the development of organ damage. Leoncini et al assessed the independent influence of BPV on multiple target organ damage, including left ventricular hypertrophy, carotid atheromatosis, and renal abnormalities.43 High BPV, measured as SD, wSD, and ARV, was associated with the simultaneous presence of 2 or more signs of subclinical organ damage, regardless of several confounding variables, including BP. Other multicenter studies of patients with hypertensive chronic kidney disease showed that ARV was an independent predictor of left ventricular hypertrophy, after adjustment for 24‐hour mean BP and other factors.44 In a study of BPV as a predictor of renal complications, multiple logistic regression analysis revealed that the presence of microalbuminuria was independently associated with ARV, after adjustment for average BP value and other potential confounders.45 When ARV was replaced by SD or wSD in the same model, BPV was not independently associated with microalbuminuria. When the independent correlates of urinary albumin excretion rate were explored using stepwise linear multiple regression analysis, only ARV was significantly related to the outcome variable. In contrast, Madden et al found no significant correlations of BPV (SD, CV, wSD, and ARV) with left ventricular hypertrophy in both unadjusted and fully adjusted models. The association between BPV and microalbuminuria did not persist for all indexes when mean BP was added to the model.46 However, a subanalysis that included only subjects taking antihypertensive treatment showed a significant association between ARV and microalbuminuria, after adjustment for all confounders, including mean BP. Table 3 shows odds ratios of studies that determined that BPV assessed with ARV was an independent predictor of organ damage.
Table 3.
ORs of Higher Systolic ARV for Progression of Target OD
| Study | Outcome Measure | Systolic ARV OR (95% CI) |
|---|---|---|
| Yamaguchi et al41 | CSVD | 2.05 (1.04–4.03)a |
| Filomena et al42 | CSVD | 1.16 (1.02–1.33)a |
| Leoncini et al43 | Multiple OD | 1.14 (1.00–1.29)a |
| Ryu et al44 | LVH | 1.05 (1.02–1.09)b |
| Mulè et al45 | MAU | 5.34 (1.23–23.10)a |
ORs (95% CIs) per 1–mm Hg increase in ARV by a fully adjusted multivariate logistic regression model. ARV indicates average real variability; CI, confidence interval; CSVD, cerebral small‐vessel disease; LVH, left ventricular hypertrophy; MAU, microalbuminuria; OD, organ damage; and OR, odds ratio.
P<0.05, OR significance.
P<0.01, OR significance.
Meta‐Analysis
Figure 2 presents random‐effects meta‐analysis of hazard ratios for increases in systolic ARV and all cardiovascular events. Four studies that examined incidence of hard end points show a significant association (hazard ratio, 1.18; 95% confidence interval, 1.09–1.27; I2=0.0%; P=0.64). The Egger test indicated no evidence of publication bias (P=0.95).
Figure 2.
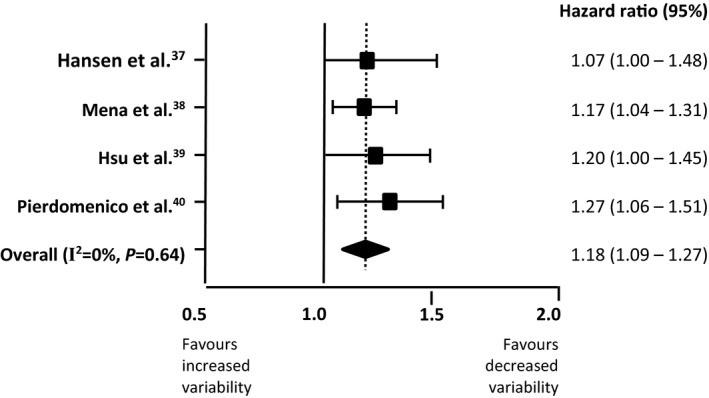
Random‐effects meta‐analysis of hazard ratios for increases of systolic average real variability and all cardiovascular events.
Association of ARV With Target Organ Damage
Nine studies evaluated the correlations between BPV and target organ damage (Table 4). Two studies examined carotid artery alteration, using carotid intima‐media thickness as a sign of early atherosclerosis. Xiong et al found that ARV generally had a stronger relationship than SD or CV to intima‐media thickness.47 Comparison on the BPV parameters in a mixed model with backward selection regression showed that only ARV maintained a significant relationship to intima‐media thickness, which remained significant after adjustment for baseline characteristics and mean BP. Wu et al concluded that ARV had greater prognostic significance than SD for evaluating the carotid intima‐media thickness in hypertensive patients with diabetes mellitus.48 Another study found that ARV had the strongest relationship to arterial stiffness, followed by the wSD and SD, after adjustment for age, sex, and 24‐hour BP.49 This result was confirmed through stepwise multiple linear regression analysis, showing the relationship between ARV and arterial stiffness was independent from, and in addition to, office BP and average 24‐hour BP. In contrast, Wei et al found no significant association between BPV and 3 indexes of organ damage, except for an increase in aortic pulse wave velocity with increasing variation independent of mean and increasing difference between maximum and minimum BP.50 However, the same study used the beat‐to‐beat recordings method and found that the left ventricular mass index was related to ARV, variation independent of mean, and difference between maximum and minimum, independently of BP and other covariables.
Table 4.
Studies That Assessed the Association of ARV With Target OD by Multivariate Analysis
| Study | Population | Design | N, (Women, %) | Age, Mean±SD, y | Estimated Minimum of BP Readings (↑/↓) | Outcome Measure | Significant BVP Index | Confounding Variables Included in Multivariate Analyses |
|---|---|---|---|---|---|---|---|---|
| Wei et al50 | Normotensive and untreated hypertensive patients | Cross‐sectional | 256 (50.4) | 51.1±10.3 | 45 (↑) | Multiple OD (LVH, MAU, PWV) | VIM, MMD | Age, sex, BMI, 24‐h HR, smoking, drinking, TC, glucose, 24‐h SBP level |
| Xiong et al47 | General | Cross‐sectional | 60 (46.7) | 58.7±12.1 | 38 (↓) | IMT | ARV, SD, CV | Age, sex, smoking, hypertension, corresponding PP and BP level |
| Wu et al48 | Treated hypertensive patients with and without DM | Cross‐sectional | 148 (40.7) | 57.7±13.0 | 38 (↓) | IMT | ARV | Age, sex, smoking, heart disease, 24‐h and daytime DBP level |
| Schillaci et al49 | Untreated hypertensive patients | Cross‐sectional | 911 (52.0) | 49.0±11.0 | 77 (↑) | PWV | ARV | Age, HR, GFR, IFG, office SBP, 24‐h SBP level |
| Diaz et al51 | Normotensive or prehypertensive patients | Cross‐sectional | 38 (86.8) | 52.6±1.5 | 32 (↓) | GFR | ARV, TRBPV | Age, BMI, 24‐h DBP level |
| Ozkayar et al55 | Hypertensive renal transplant recipients | Cross‐sectional | 73 (28.8) | 42.3±12.4 | 62 (↑) | Endothelial dysfunction | ARV | Age, sex, corresponding BP level |
| Diaz et al52 | Nondiabetic, nonsmoking, and free of renal disease and CVD | Cross‐sectional | 36 (75.0) | 52.0±1.0 | 32 (↓) | Endothelial dysfunction | ARV, TRBPV | Age, sex, BMI, corresponding 24‐h BP level |
| Diaz et al53 | Nondiabetic, nonsmoking, and free of CVD | Cross‐sectional | 72 (83.3) | 51.7±0.7 | 32 (↓) | Smooth muscle function | ARV | Age, sex, BMI, corresponding 24‐h BP level |
| Veerabhadrappa et al54 | Normotensive or prehypertensive patients | Cross‐sectional | 36 (83.3) | 52.0±7.0 | 32 (↓) | Circulating inflammatory markers | ARV | Age, sex, BMI, 24‐h SBP level |
↑, indicates number of BP readings above the minimum sampling rate; ↓, number of BP readings below the minimum sampling rate; ARV, average real variability; BMI, body mass index; BP, blood pressure; BPV, BP variability; CV, coefficient of variation; CVD, cardiovascular disease; DBP diastolic BP; DM, diabetes mellitus; GFR, glomerular filtration rate; HR, heart rate; IFG, impaired fasting glucose; IMT, intima‐media thickness; LVH, left ventricular hypertrophy; MAU, microalbuminuria; MMD, difference between maximum and minimum; OD, organ damage; PP, pulse pressure; PWV, pulse wave velocity; SBP, systolic BP; TC, total cholesterol; TRBPV, time rate of BP variation; VIM, variation independent of mean.
For renal damage, Diaz et al identified ARV and TRBPV as independent determinants of renal function, positively correlated with glomerular filtration rate in a multivariate regression model that included age, body mass index, and mean 24‐hour BP.51 Later work by the same authors provided evidence that BPV was higher in subjects with impaired endothelial function and was associated with the vascular smooth muscle response to nitric oxide.52 High ARV was also significantly associated with a greater vasodilatory response to nitroglycerin‐mediated vasodilation53 and elevated levels of high‐sensitivity C‐reactive protein,54 independent of mean BP. High ARV was the strongest risk factor for endothelial dysfunction in a study that evaluated the relationship between various ambulatory BP parameters and flow‐mediated dilatation in kidney transplant recipients.55
Discussion
We conducted a systematic review to assess the predictive power of various BPV indexes on target organ damage and cardiovascular outcomes. Most studies found that ARV was a better estimator of 24‐hour BPV than other measures of dispersion, including SD, CV, and wSD. Of the 19 assessed studies, 17 reported significant associations between high ARV and the presence and progression of subclinical organ damage,41, 42, 43, 44, 45, 47, 48, 49, 51, 52, 53, 54, 55 as well as the incidence of hard end points, such as cardiovascular events.37, 38, 39, 40 For all these cases, ARV remained an independent predictor after adjustment for BP and other clinical factors. Moreover, in 11 of the 13 studies that included several BPV indexes, ARV was the best37, 41, 43, 47, 51, 52 or unique40, 42, 45, 48, 49 significant predictor. In addition, our meta‐analysis showed that increased ARV in systolic BP was associated with risk of all cardiovascular events. Only 2 cross‐sectional studies did not find that high ARV was a significant risk factor. A study that computed ARV on the basis of a low minimum number of BP measurements (<20 daytime and 7 nighttime BP readings) found no association with preclinical organ damage.46 Another study found no association between organ damage and 24‐hour BPV, but did find a significant association with beat‐to‐beat recordings.50
Despite extensive evidence that 24‐hour BPV, particularly ARV, provides superior predictive value than conventional BP indexes (SD, CV, and wSD), the regular use of 24‐hour BPV in clinical practice remains problematic. Some studies have found that the contribution of ARV to stratification risk, although statistically significant, is low with for average BP levels. For example, the International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcome reports indicated that the incremental risk explained by adding ARV to models that included 24‐hour ambulatory BP and other covariables was only 0.1%.37, 38 However, these results might have been influenced by different methods of 24‐hour ABPM used in the different cohort studies whose data were pooled in the analysis.6, 57
On the other hand, heterogeneity in the metrics and protocols used in different studies is an overall problem in evaluating the predictive value of BPV.17, 58 Therefore, several methodological problems need to be addressed before BPV is routinely used in clinical practice. An important constraint is that the discontinuous nature of noninvasive ABPM devices might limit its accuracy in assessing rapid changes in BP.19, 59 Identification of an optimal measurement interval is key to the reliable estimation of the beat‐by‐beat oscillations that occur in seconds. Di Rienzo et al20 demonstrated that quantification of SD using a time interval >15 minutes increases the potential error in estimating the true variance. However, short sampling intervals are rarely used in clinical settings, for practical reasons and because of the potential discomfort of patients.21 The influence of the measurement interval gives the use of ARV an advantage over alternative approaches, such as TRBPV, which is potentially more sensitive to the sampling rate.22
Another methodological problem is that the minimum number of valid BP measurements needed to provide a reproducible and reliable assessment of BPV might not have been reached in all studies that use ARV. Mena et al concluded that 48 BP readings during a 24‐hour period is sufficient to compute ARV without meaningful loss of prognostic information.39 In fact, ARV might be less sensitive to missing readings and low sampling frequency than other BPV indexes. Eguchi et al60 tested the reproducibility of ARV, SD, and wSD in hypertensive patients, before and after antihypertensive treatment. ARV exhibited the greatest reproducibility during both the observation and treatment periods. In agreement with those results, ARV was a significant predictor in 10 of the 11 studies with a minimum number of BP readings below the average lowest sampling rate.37, 39, 41, 44, 47, 48, 51, 52, 53, 54
Although ARV has been shown to be a better predictor of risk than SD for most adverse outcomes,37, 40, 41, 42, 48, 49 studies to date have not estimated ARV using real‐time monitoring, because no existing ABPM devices incorporate automatic quantification of ARV or other novel BPV indexes. The procedures for collection, computation, and storage of electronic BP recordings need to automatically provide accurate calculations of 24‐hour BPV.
In conclusion, the current evidence suggests that the ARV index adds significant prognostic information to 24‐hour ABPM, and is a useful approach for studying the clinical value of BPV. Moreover, technical features of ARV could resolve some methodological problems: ARV (1) is simple to compute, (2) is relatively insensitive to low sampling frequency, (3) is not strongly influenced by cyclic components, (4) is sensitive to the sequential order of BP readings, and (5) provides insight into BP changes. However, even if ARV is accepted as the optimal index for reliable and reproducible evaluation of 24‐hour BPV, methodological standardization is only an initial step for incorporating it in clinical practice. Further investigation is required to define potential diagnostic thresholds of abnormal BPV, to determine if reduction below those thresholds is associated with a decrease in target organ damage and/or risk of cardiovascular disease, to identify treatments that reduce BPV, and to clarify whether those therapeutic interventions provide additional prognostic benefit, independent of a reduction in mean BP.
Sources of Funding
This work was supported by grants 1R01AG036469‐01A1 and R03AG054186 from the National Institute on Aging and Fogarty International Center, and grants DSA/103.5/15/11115 and PFCE/2458/16 from Secretaria de Educación Pública, México.
Disclosures
None.
Acknowledgments
We acknowledge the South Texas Diabetes and Obesity Institute for support in preparation of this report.
(J Am Heart Assoc. 2017;6:e006895 DOI: 10.1161/JAHA.117.006895.)
References
- 1. Narkiewicz K, Winnicki M, Schroeder K, Phillips BG, Kato M, Cwalina E, Somers VK. Relationship between muscle sympathetic nerve activity and diurnal blood pressure profile. Hypertension. 2002;39:168–172. [DOI] [PubMed] [Google Scholar]
- 2. Nishi EE, Bergamaschi CT, Campos RR. The crosstalk between the kidney and the central nervous system: the role of renal nerves in blood pressure regulation. Exp Physiol. 2015;100:479–484. [DOI] [PubMed] [Google Scholar]
- 3. Elghozi JL, Laude D, Girard A. Effects of respiration on blood pressure and heart rate variability in humans. Clin Exp Pharmacol Physiol. 1991;18:735–742. [DOI] [PubMed] [Google Scholar]
- 4. Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension. 1999;34:724–728. [DOI] [PubMed] [Google Scholar]
- 5. Parati G, Saul JP, Di Rienzo M, Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation: a critical appraisal. Hypertension. 1995;25:1276–1286. [DOI] [PubMed] [Google Scholar]
- 6. Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood‐pressure variability. Nat Rev Cardiol. 2013;10:143–155. [DOI] [PubMed] [Google Scholar]
- 7. Mancia G, Parati G, Pomidossi G, Casadei R, Di Rienzo M, Zanchetti A. Arterial baroreflexes and blood pressure and heart rate variabilities in humans. Hypertension. 1986;8:147–153. [DOI] [PubMed] [Google Scholar]
- 8. Mancia G, Ferrari A, Gregorini L, Parati G, Pomidossi G, Bertinieri G, Grassi G, di Rienzo M, Pedotti A, Zanchetti A. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ Res. 1983;53:96–104. [DOI] [PubMed] [Google Scholar]
- 9. Mancia G, Ferrari A, Gregorini L, Parati G, Pomidossi G, Bertinieri G, Zanchetti A. Blood pressure variability in man: its relation to high blood pressure, age and baroreflex sensitivity. Clin Sci. 1980;59:401s–404s. [DOI] [PubMed] [Google Scholar]
- 10. Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24‐hour blood pressure mean and variability to severity of target‐organ damage in hypertension. J Hypertens. 1987;5:93–98. [DOI] [PubMed] [Google Scholar]
- 11. Frattola A, Parati G, Cuspidi C, Albini F, Mancia G. Prognostic value of 24‐hour blood pressure variability. J Hypertens. 1993;11:1133–1137. [DOI] [PubMed] [Google Scholar]
- 12. Palatini P, Penzo M, Racioppa A, Zugno E, Guzzardi G, Anaclerio M, Pessina AC. Clinical relevance of nighttime blood pressure and of daytime blood pressure variability. Arch Intern Med. 1992;152:1855–1860. [PubMed] [Google Scholar]
- 13. Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, Ota M, Nagai K, Araki T, Satoh H, Ito S, Hisamichi S, Imai Y. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension. 2000;36:901–906. [DOI] [PubMed] [Google Scholar]
- 14. Sander D, Kukla C, Klingelhöfer J, Winbeck K, Conrad B. Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: a 3‐year follow‐up study. Circulation. 2000;102:1536–1541. [DOI] [PubMed] [Google Scholar]
- 15. Mancia G, Parati G, Hennig M, Flatau B, Omboni S, Glavina F, Costad B, Scherze R, Bondf G, Zanchetti A. Relation between blood pressure variability and carotid artery damage in hypertension: baseline data from the European Lacidipine Study on Atherosclerosis (ELSA). J Hypertens. 2001;19:1981–1989. [DOI] [PubMed] [Google Scholar]
- 16. Pringle E, Phillips C, Thijs L, Davidson C, Staessen JA, de Leeuw PW, Jaaskivi M, Nachev C, Parati G, O'Brien ET, Tuomilehto J, Webster J, Bulpitt CJ, Fagard RH. Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens. 2003;21:2251–2257. [DOI] [PubMed] [Google Scholar]
- 17. Parati G, Ochoa JE, Lombardi C, Bilo G. Blood pressure variability: assessment, predictive value, and potential as a therapeutic target. Curr Hypertens Rep. 2015;17:1–18. [DOI] [PubMed] [Google Scholar]
- 18. Dolan E, O'Brien E. Is it daily, monthly, or yearly blood pressure variability that enhances cardiovascular risk? Curr Cardiol Rep. 2015;17:1–5. [DOI] [PubMed] [Google Scholar]
- 19. Parati G, Bilo G, Valentini M. Blood pressure variability: methodological aspects, pathophysiological and clinical implications In: Mancia G, Grassi G, Kjeldsen SE, eds. Manual of Hypertension of the European Society of Hypertension. London, England: Informa UK Ltd; 2008:61–71. [Google Scholar]
- 20. Di Rienzo M, Grassi G, Pedotti A, Mancia G. Continuous vs intermittent blood pressure measurements in estimating 24‐hour average blood pressure. Hypertension. 1983;5:264–269. [DOI] [PubMed] [Google Scholar]
- 21. Parati G, Rizzoni D. Assessing the prognostic relevance of blood pressure variability: discrepant information from different indices. J Hypertens. 2005;23:483–486. [DOI] [PubMed] [Google Scholar]
- 22. Bilo G, Parati G. Rate of blood pressure changes assessed by 24 h ambulatory blood pressure monitoring: another meaningful index of blood pressure variability? J Hypertens. 2011;29:1054–1058. [DOI] [PubMed] [Google Scholar]
- 23. Stergiou GS, Kollias A, Ntineri A. Assessment of drug effects on blood pressure variability: which method and which index? J Hypertens. 2014;32:1197–1200. [DOI] [PubMed] [Google Scholar]
- 24. Dolan E, O'Brien E. Blood pressure variability clarity for clinical practice. Hypertension. 2010;56:179–181. [DOI] [PubMed] [Google Scholar]
- 25. Clement DL, Mussche MM, Vanhoutte G, Pannier R. Is blood pressure variability related to activity of the sympathetic system? Clin Sci. 1979;57:217s–219s. [DOI] [PubMed] [Google Scholar]
- 26. Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. [DOI] [PubMed] [Google Scholar]
- 27. Imai Y, Aihara A, Ohkubo T, Nagai K, Tsuji I, Minami N, Satoh H, Hisamichi S. Factors that affect blood pressure variability: a community‐based study in Ohasama, Japan. Am J Hypertens. 1997;10:1281–1289. [DOI] [PubMed] [Google Scholar]
- 28. Parati G, Bilo G, Villani A, Maronati A, Vettorello M, Kawecka‐Jaszcz K, Mancia G. A new method for assessing 24 h blood pressure variability correcting for the contribution of nocturnal blood pressure fall. J Hypertens. 2004;22:S167. [DOI] [PubMed] [Google Scholar]
- 29. Bilo G, Giglio A, Styczkiewicz K, Caldara G, Maronati A, Kawecka‐Jaszcz K, Mancia G, Parati G. A new method for assessing 24 h blood pressure variability after excluding the contribution of nocturnal blood pressure fall. J Hypertens. 2007;25:2058–2066. [DOI] [PubMed] [Google Scholar]
- 30. Mancia G, Parati G, Castiglioni P, Tordi R, Tortorici E, Glavina F, Di Rienzo M. Daily life blood pressure changes are steeper in hypertensive than in normotensive subjects. Hypertension. 2003;42:277–282. [DOI] [PubMed] [Google Scholar]
- 31. van Gestel AJ, Camen G, Clarenbach CF, Sievi N, Rossi VA, Kohler M. Quantifying the speed of fluctuations in systolic blood pressure. Hypertens Res. 2013;36:1039–1044. [DOI] [PubMed] [Google Scholar]
- 32. Zakopoulos NA, Tsivgoulis G, Barlas G, Papamichael C, Spengos K, Manios E, Ikonomidis I, Kotsis V, Spiliopoulou I, Vemmos K, Mavrikakis M, Moulopoulos SD. Time rate of blood pressure variation is associated with increased common carotid artery intima‐media thickness. Hypertension. 2005;45:505–512. [DOI] [PubMed] [Google Scholar]
- 33. Mena L, Pintos S, Queipo NV, Aizpurua JA, Maestre G, Sulbaran T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens. 2005;23:505–511. [DOI] [PubMed] [Google Scholar]
- 34. Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144:427–437. [DOI] [PubMed] [Google Scholar]
- 35. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 36. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Björklund‐Bodegård K, Richart T, Ohkubo T, Jeppesen J, Torp‐Pedersen C, Dolan E, Kuznetsova T, Stolarz‐Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka‐Jaszcz K, Imai Y, Wang J, Ibsen H, O'Brien E, Staessen JA. Prognostic value of reading‐to‐reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55:1049–1057. [DOI] [PubMed] [Google Scholar]
- 38. Mena LJ, Maestre GE, Hansen TW, Thijs L, Liu Y, Boggia J, Li Y, Kikuya M, Björklund‐Bodegård K, Ohkubo T, Jeppesen J, Torp‐Pedersen C, Dolan E, Kuznetsova T, Stolarz‐Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka‐Jaszcz K, Filipovsky J, lmai Y, Wang J, O'Brien E, Staessen JA. How many measurements are needed to estimate blood pressure variability without loss of prognostic information? Am J Hypertens. 2016;27:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hsu PF, Cheng HM, Wu CH, Sung SH, Chuang SY, Lakatta EG, Yin FCP, Chou P, Chen CH. High short‐term blood pressure variability predicts long‐term cardiovascular mortality in untreated hypertensives but not in normotensives. Am J Hypertens. 2016;29:806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pierdomenico SD, Di Nicola M, Esposito AL, Di Mascio R, Ballone E, Lapenna D, Cuccurullo F. Prognostic value of different indices of blood pressure variability in hypertensive patients. Am J Hypertens. 2009;22:842–847. [DOI] [PubMed] [Google Scholar]
- 41. Yamaguchi Y, Wada M, Sato H, Nagasawa H, Koyama S, Takahashi Y, Kawanami T, Kato T. Impact of ambulatory blood pressure variability on cerebral small vessel disease progression and cognitive decline in community‐based elderly Japanese. Am J Hypertens. 2014;27:1257–1267. [DOI] [PubMed] [Google Scholar]
- 42. Filomena J, Riba‐Llena I, Vinyoles E, Tovar JL, Mundet X, Castañé X, Vilar A, López‐Rueda A, Jiménez‐Baladó J, Cartanyà A, Montaner J, Delgado P. Short‐term blood pressure variability relates to the presence of subclinical brain small vessel disease in primary hypertension. Hypertension. 2015;66:634–640. [DOI] [PubMed] [Google Scholar]
- 43. Leoncini G, Viazzi F, Storace G, Deferrari G, Pontremoli R. Blood pressure variability and multiple organ damage in primary hypertension. J Hum Hypertens. 2013;27:663–670. [DOI] [PubMed] [Google Scholar]
- 44. Ryu J, Cha RH, Kim DK, Lee JH, Yoon S, Ryu DR, Oh JE, Kim S, Han SY, Lee EY, Kim YS. The clinical association of the blood pressure variability with the target organ damage in hypertensive patients with chronic kidney disease. J Korean Med Sci. 2014;29:957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mulè G, Calcaterra I, Costanzo M, Morreale M, D'Ignoto F, Castiglia A, Geraci G, Rabbiolo G, Vaccaro F, Cottone S. Average real variability of 24‐h systolic blood pressure is associated with microalbuminuria in patients with primary hypertension. J Hum Hypertens. 2015;30:164–170. [DOI] [PubMed] [Google Scholar]
- 46. Madden JM, O'Flynn AM, Dolan E, Fitzgerald AP, Kearney PM. Short‐term blood pressure variability over 24 h and target organ damage in middle‐aged men and women. J Hum Hypertens. 2015;29:719–725. [DOI] [PubMed] [Google Scholar]
- 47. Xiong H, Wu D, Tian X, Lin WH, Li C, Zhang H, Cai Y, Zhang YT. The relationship between the 24 h blood pressure variability and carotid intima‐media thickness: a compared study. Comput Math Methods Med. 2014;2014:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu D, Li C, Chen Y, Xiong H, Tian X, Wu W, Huang W, Zhang YT, Zhang H. Influence of blood pressure variability on early carotid atherosclerosis in hypertension with and without diabetes. Medicine. 2016;95:e3864–e3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schillaci G, Bilo G, Pucci G, Laurent S, Macquin‐Mavier I, Boutouyrie P, Battista F, Settimi L, Desamericq G, Dolbeau G, Faini A, Salvi P, Mannarino E, Parati G. Relationship between short‐term blood pressure variability and large‐artery stiffness in human hypertension findings from 2 large databases. Hypertension. 2012;60:369–377. [DOI] [PubMed] [Google Scholar]
- 50. Wei FF, Li Y, Zhang L, Xu TY, Ding FH, Wang JG, Staessen JA. Beat‐to‐beat, reading‐to‐reading, and day‐to‐day blood pressure variability in relation to organ damage in untreated Chinese. Hypertension. 2014;63:790–796. [DOI] [PubMed] [Google Scholar]
- 51. Diaz KM, Feairheller DL, Sturgeon KM, Veerabhadrappa P, Williamson ST, Crabbe DL, Brown MD. Increased nitric oxide and attenuated diastolic blood pressure variability in African Americans with mildly impaired renal function. Int J Hypertens. 2010;2010:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Diaz KM, Veerabhadrappa P, Kashem MA, Feairheller DL, Sturgeon KM, Williamson ST, Crabbe DL, Brown MD. Relationship of visit‐to‐visit and ambulatory blood pressure variability to vascular function in African Americans. Hypertens Res. 2012;35:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Diaz KM, Veerabhadrappa P, Kashem MA, Thakkar SR, Feairheller DL, Sturgeon KM. Visit‐to‐visit and 24‐h blood pressure variability: association with endothelial and smooth muscle function in African Americans. J Hum Hypertens. 2013;27:671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Veerabhadrappa P, Diaz KM, Feairheller DL, Sturgeon KM, Williamson S, Crabbe DL, Kashem A, Ahrensfield D, Brown MD. Enhanced blood pressure variability in a high cardiovascular risk group of African Americans: FIT4Life Study. J Am Soc Hypertens. 2010;4:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ozkayar N, Altun B, Yildirim T, Yilmaz R, Dede F, Arik G, Turkmen E, Hayran M, Aki FT, Arici M, Erdem Y. Blood pressure measurements, blood pressure variability and endothelial function in renal transplant recipients. Clin Exp Hypertens. 2014;36:392–397. [DOI] [PubMed] [Google Scholar]
- 56. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. [DOI] [PubMed] [Google Scholar]
- 57. Stergiou GS, Ntineri A, Kollias A, Ohkubo T, Imai Y, Parati G. Blood pressure variability assessed by home measurements: a systematic review. Hypertens Res. 2014;37:565–572. [DOI] [PubMed] [Google Scholar]
- 58. Taylor KS, Heneghan CJ, Stevens RJ, Adams EC, Nunan D, Ward A. Heterogeneity of prognostic studies of 24‐hour blood pressure variability: systematic review and meta‐analysis. PLoS ONE. 2015;10:e0126375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Parati G, Valentini M. Prognostic relevance of blood pressure variability. Hypertension. 2006;47:137–138. [DOI] [PubMed] [Google Scholar]
- 60. Eguchi K, Hoshide S, Hoshide Y, Ishikawa S, Shimada K, Kario K. Reproducibility of ambulatory blood pressure in treated and untreated hypertensive patients. J Hypertens. 2010;28:918–924. [DOI] [PubMed] [Google Scholar]


