Abstract
Background
Depression is strongly linked to increased morbidity and mortality in patients with chronic stable angina; however, its associated healthcare costs have been less well studied. Our objective was to identify the characteristics of chronic stable patients found to have depression and to determine the impact of an occurrence of depression on healthcare costs within 1 year of a diagnosis of stable angina.
Methods and Results
In this population‐based study conducted in Ontario, Canada, we identified patients diagnosed with stable angina based on angiogram between October 1, 2008, and September 30, 2013. Depression was ascertained by physician billing codes and hospital admission diagnostic codes contained within administrative databases. The primary outcome was cumulative mean 1‐year healthcare costs following index angiogram. Generalized linear models were developed with a logarithmic link and γ distribution to determine predictors of cost. Our cohort included 22 917 patients with chronic stable angina. Patients with depression had significantly higher mean 1‐year healthcare costs ($32 072±$41 963) than patients without depression ($23 021±$25 741). After adjustment for baseline comorbidities, depression was found to be a significant independent predictor of cost, with a cost ratio of 1.33 (95% confidence interval, 1.29–1.37). Higher costs in depressed patients were seen in all healthcare sectors, including acute and ambulatory care.
Conclusions
Depression is an important driver of healthcare costs in patients following a diagnosis of chronic stable angina. Further research is needed to understand whether improvements in the approach to diagnosis and treatment of depression will translate to reduced expenditures in this population.
Keywords: cost, depression, stable coronary artery disease
Subject Categories: Cost-Effectiveness, Health Services, Chronic Ischemic Heart Disease
Clinical Perspective
What Is New?
An occurrence of depression within 1 year of a diagnosis of chronic stable angina is a significant driver of mean 1‐year healthcare costs.
Among patients with chronic stable angina, depression is associated with increased costs in nearly all healthcare sectors, not only mental health services.
What Are the Clinical Implications?
Depression has important economic consequences in patients with chronic stable angina.
Higher costs in both acute and ambulatory care are seen in depressed patients.
Additional research is needed to determine whether interventions such as screening for depression will lessen healthcare utilization in this group of patients.
Introduction
Coronary artery disease (CAD) poses a major public health and economic burden globally. In Canada, the yearly estimate for direct medical costs related to cardiovascular diseases was $11.7 billion in 2008.1 CAD also incurs an indirect economic impact due to disability, lost work productivity, and premature mortality.2 Cumulatively, these figures are expected to grow with the rise in prevalence of CAD related to the aging population.3
To develop efficient strategies that will improve outcomes and reduce expenditures, it is important to understand drivers of cost among patients with CAD. Depression is a frequent comorbidity in patients with chronic diseases and is known to magnify healthcare costs.4 Among patients with CAD, depression is the most common psychiatric disorder.5 Depression is present in ≈20% of patients with CAD and is an independent risk factor for adverse outcomes, including cardiac events and mortality.6 The exact nature of this relationship is unclear but is likely related to certain behavioral and physiological characteristics of depressed patients.7 In the setting of myocardial infarction, depression has been shown to increase healthcare utilization related to higher hospital readmission rates, longer length of stay, and more frequent ambulatory care and emergency room visits.8, 9, 10, 11, 12 Similarly, this relationship has been observed in depressed patients with coronary artery bypass grafting (CABG)13 and congestive heart failure.14
In comparison, there is a paucity of data on the pattern of healthcare use and cost in patients with chronic stable angina and depression; studies to date have been small15, 16 and conducted in primary care settings.17 Chronic stable angina constitutes the most prevalent form of CAD,2 and depression also negatively affects morbidity and mortality in this large group of patients.18 A more comprehensive understanding of how healthcare costs in stable angina patients are affected by a diagnosis of depression is a necessary step toward improving care and cost containment in this population. Accordingly, to address this gap in knowledge, the aim of our study was to identify the characteristics of chronic stable patients found to have depression and evaluate the healthcare costs associated with the occurrence of depression within 1 year of a new diagnosis of chronic stable angina.
Methods
This study was approved by the institutional research ethics board at Sunnybrook Health Sciences Center, Toronto, Canada. The need for patient consent was exempted under Ontario's Personal Health Information Protection Act (the province's legislation regarding privacy of health information).
Data Sources
This retrospective cohort study was conducted using population‐based databases in Ontario, Canada, where 13.6 million residents receive universal medical coverage funded by a single third‐party payer, the Ministry of Health and Long Term Care. Data were obtained from a clinical registry that is maintained by the Cardiac Care Network of Ontario (CCN). CCN includes a network of 19 hospitals in Ontario that perform cardiac procedures, such as angiography, percutaneous coronary intervention, and CABG.19, 20 Demographics, comorbidities, cardiac stress testing, and coronary anatomy are among the details collected by the CCN Cardiac Registry on patients who have undergone cardiac procedures. The accuracy of this registry has been assessed through retrospective chart review.21, 22
Linkage of CCN Cardiac Registry data to population‐based administrative databases at the Institute for Clinical Evaluative Sciences (ICES) was performed using unique encoded identifiers to protect patient confidentiality. The administrative databases that were utilized in this study included the Ontario Health Insurance Plan (OHIP) Claims Database, the Registered Persons Database (RPDB), the Canadian Institute for Health Information Discharge Abstract Database (CIHI‐DAD), the National Ambulatory Care Reporting System (NACRS), the ICES Physician Database (IPDB), the Ontario Mental Health Reporting System (OMHRS), and the Ontario Drug Benefit (ODB) Claims Database. All physician service claims, which include diagnostic codes and dates of service, are captured by OHIP. The RPDB contains vital statistics information for all Ontario residents and was used to determine mortality. Clinical information regarding discharges from acute and chronic inpatient hospitalizations was obtained from CIHI‐DAD, and information from emergency department records was obtained from NACRS. The IPDB reports physician demographics and specialization. Data on hospitalizations for mental illness are contained within the OMHRS database. Persons aged >65 years receive full coverage for prescription medications from the ODB program, and drug claims information with reliable dispensing records is found within the ODB database.
Cohort Selection
Our cohort consisted of Ontario residents aged >20 years who underwent an index angiogram between October 1, 2008, and September 30, 2013, for the investigation of stable angina and were found to have obstructive CAD, defined as having any stenosis >70% in ≥1 epicardial vessel or any stenosis >50% in the left main coronary artery.23, 24 Patients who presented with acute coronary syndrome or who had a prior history of CAD (previous myocardial infarction, acute coronary syndrome, percutaneous coronary intervention, or CABG) were excluded. Patients were followed from the date of their index angiogram for up to 1 year. If a patient underwent >1 angiogram during the study period, only the first was considered for the purposes of eligibility and inclusion in the cohort. We excluded patients who experienced depression during the 3 years preceding the start date of accrual for the cohort.
Definition of Depression
Patients were identified as having an occurrence of depression if they were diagnosed by a medical professional or were hospitalized for depression within 1 year of their index angiogram (October 1, 2008, to September 30, 2013). The diagnostic codes from physician service claims (OHIP) that were used included reactive depression (300) and depressive or other nonpsychotic disorder not classified elsewhere (311). The occurrence of depression was also identified using the International Classification of Diseases, 10th Revision diagnostic codes obtained from in‐hospital and emergency department visits from CIHI‐DAD and NACRS, respectively (see Table S1). The validity of these codes has not been assessed by retrospective chart audits; however, they have been used in studies examining depression with administrative data.25, 26, 27 The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition codes for a single (296.20–0.26) or recurrent (296.30–0.36) episode of major depressive disorder from OMHRS were also used to identify depressed patients. Of note, any codes associated with bipolar disorder were excluded. If information regarding the same hospitalization for depression was obtained from both OMHRS and CIHI‐DAD, this was considered a single hospitalization in the statistical analyses.
Healthcare Costs
The primary outcome was the total aggregate 1‐year healthcare costs per patient following the index angiogram. Cost estimations to account for censored data were not required because the full cohort had complete cost data available from the 1‐year time period or until death. Healthcare expenditures were divided broadly into 5 sectors: acute inpatient care, chronic care, outpatient care, physician costs, and prescription medication use. The acute inpatient care sector included costs related to inpatient hospitalizations and inpatient mental health care. The chronic care sector included costs related to long‐term care, rehabilitation, home care services, and complex continuing care. In Ontario, complex continuing care programs manage medically complex inpatients who have medical and nursing care needs that cannot be met in the community or in long‐term care. Outpatient care referred to the costs of outpatient clinic visits, emergency department visits, same‐day surgery, laboratory testing, and dialysis. Last, physician costs consisted of expenditures associated with fee‐for‐service visits and physician capitation and were discrete from costs associated with the categories of inpatient, outpatient, and chronic care. Cost related to prescription medication use was available only for patients aged ≥65 years, given that only this age group has full drug coverage in Ontario.
We determined costs associated with physician visits and laboratory tests using data from the claims history of the OHIP database.28 This database also includes shadow billings from providers of organizations covered by alternative payment arrangements. We estimated the cost of hospital admission using the resource intensity weights method.29 In brief, resource intensity weights is a measure of how much resources were utilized during a patient's encounter with the healthcare system. We multiplied the resource intensity weights associated with the case‐mix group for each hospital admission in the CIHI‐DAD by the average provincial cost per weighted case for all Ontario acute and chronic care hospitals for that year.29 This method yields a mean cost per hospital admission for cases assigned to a particular case‐mix group category. We used a similar resource intensity weights method to determine the costs for emergency department visits and same day surgeries, both obtained from the NACRS database. We adjusted costs to 2013 Canadian dollars using the consumer price index.
Statistical Analyses
Differences in baseline characteristics between patients with and without depression during the first year following index angiogram were compared using the t test for continuous variables and the χ2 test for categorical variables. Mean (SD) and median (interquartile range) costs with respect to each healthcare sector were compared between these 2 groups using the t test and the Kruskal‐Wallis test, respectively. For these analyses, P<0.05 was considered the threshold for significance.
Healthcare cost data have several distinct features that are important to consider in modeling, including their highly skewed distribution due to a very small proportion of patients with large costs.30 Consequently, we used generalized linear models with a logarithmic link consistent with the previous literature.31, 32 A logarithmic link was used to ensure nonnegative values for predicted costs. The final coefficient was exponentiated to obtain a rate ratio, referred to as a cost ratio (CR), which represents the percentage change in mean cost relative to the referent group. To determine the most appropriate distribution family, we conducted a modified Park test, which recommended a γ distribution to account for the skewed distribution of our cost data. Models included a random effect for hospitals to account for clustering of patients at each of the hospitals reported within the CCN Cardiac Registry. Models were adjusted for patient‐, physician‐, and hospital‐level variables; because these models were explanatory, all clinically relevant variables were included. As a sensitivity analysis, we excluded costs associated with prescription drug use, which were available only for patients aged ≥65 years. SAS version 9.3 (SAS Institute Inc) was used for all data analyses.
Results
Cohort
During the period of interest, 272 250 coronary angiograms were performed for the investigation of stable angina. Roughly 12.7% of individuals (n=8067) were excluded from the cohort because of a diagnosis of depression within the past 3 years. Our final cohort consisted of 22 917 patients diagnosed with stable angina based on angiographic findings consistent with obstructive CAD (Figure 1). Of these patients, 1961 (8.6%) patients were diagnosed with depression within 1 year of angiogram. The mean and median time to occurrence of depression following angiogram was 145 days (SD: 108 days) and 126 days, respectively. There was no significant difference in the overall mortality rate among depressed and nondepressed patients within 1 year of diagnosis of stable angina (2.35% versus 2.02%, respectively).
Figure 1.
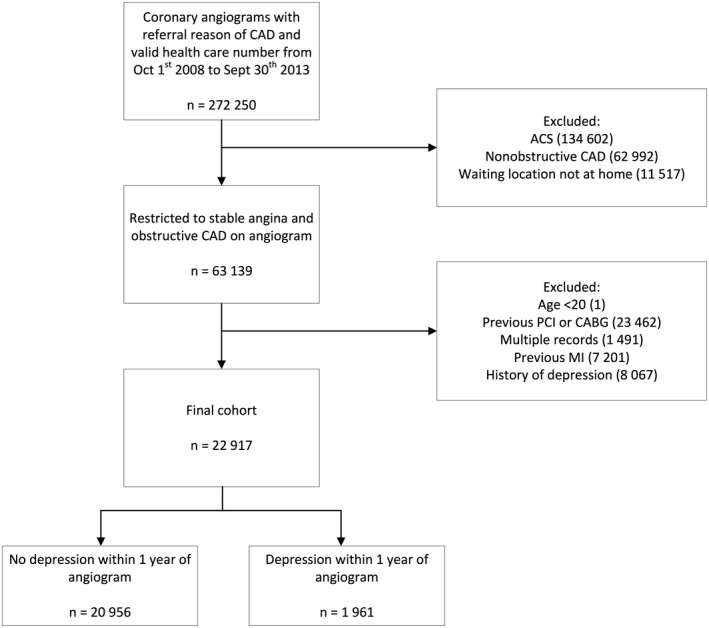
Cohort selection using the Cardiac Care Network registry. ACS indicates acute coronary syndrome; CABG, coronary artery bypass grafting; CAD, coronary artery disease; MI, myocardial infarction; and PCI, percutaneous coronary intervention.
Table 1 shows the association between baseline characteristics of the cohort and depression status within 1 year of catheterization. There were several notable differences between patients with and without depression during the first year following angiogram. A greater proportion of patients with depression were female, resided in urban areas, and had an income in the lowest quintile. Compared with nondepressed patients, depressed patients had a higher mean Carlson comorbidity index, indicating a greater number of comorbidities, and a higher rate of previous stroke. With respect to other patient‐level factors, more patients with depression within 1 year of index catheterization had a left ventricular ejection fraction >50% and underwent CABG as an initial treatment strategy. Last, fewer patients with depression underwent catheterization by a percutaneous coronary intervention physician.
Table 1.
Baseline Characteristics of the Cohort According to Diagnosis of Depression Within 1 Year of Catheterization
| Covariates | Total (N=22 917) | No Depression Within 1 Year (n=20 956) | Depression Within 1 Year (n=1961) | P Value |
|---|---|---|---|---|
| Patient‐level factors | ||||
| Demographics | ||||
| Age, y (mean±SD) | 65.6±10.2 | 65.7±10.1 | 65.0±10.7 | 0.007 |
| Female | 5280 (23.0) | 4738 (22.6) | 542 (27.6) | <0.001 |
| Rural | 3363 (14.7) | 3145 (15.0) | 218 (11.1) | <0.001 |
| Incomea | ||||
| 1 | 3919 (17.1) | 3530 (16.8) | 389 (19.8) | 0.006 |
| 2 | 4595 (20.1) | 4211 (20.1) | 384 (19.6) | |
| 3 | 4779 (20.9) | 4362 (20.8) | 417 (21.3) | |
| 4 | 4812 (21.0) | 4412 (21.1) | 400 (20.4) | |
| 5 | 4698 (20.5) | 4332 (20.7) | 366 (18.7) | |
| Medical comorbidities | ||||
| Renal dysfunction | 480 (2.1) | 440 (2.1) | 40 (2.0) | 0.86 |
| PVD | 1526 (6.7) | 1379 (6.6) | 147 (7.5) | 0.12 |
| COPD | 1194 (5.2) | 1078 (5.1) | 116 (5.9) | 0.14 |
| Previous stroke | 241 (1.1) | 211 (1.0) | 30 (1.5) | 0.03 |
| Malignancy | 587 (2.6) | 534 (2.5) | 53 (2.7) | 0.68 |
| Charlson score (mean±SD) | 0.34±0.91 | 0.33±0.91 | 0.39±0.95 | 0.008 |
| Charlson score >0 | 3913 (17.1) | 3531 (16.8) | 382 (19.5) | 0.003 |
| Cardiac risk factors | ||||
| Diabetes mellitus | 9426 (41.1) | 8593 (41.0) | 833 (42.5) | 0.21 |
| Hypertension | 19 070 (83.2) | 17 415 (83.1) | 1655 (84.4) | 0.14 |
| Hyperlipidemia | 17 157 (74.9) | 15 667 (74.8) | 1490 (76.0) | 0.23 |
| Smoking | ||||
| Former smoker | 6501 (28.4) | 5951 (28.4) | 550 (28.0) | 0.74 |
| Current smoker | 4829 (21.1) | 4394 (21.0) | 435 (22.2) | 0.21 |
| CCS angina class | ||||
| 0 | 4228 (18.4) | 3862 (18.4) | 366 (18.7) | 0.17 |
| 1 | 3768 (16.4) | 3428 (16.4) | 340 (17.3) | |
| 2 | 9382 (40.9) | 8599 (41.0) | 783 (39.9) | |
| 3 | 5238 (22.9) | 4802 (22.9) | 436 (22.2) | |
| 4 | 301 (1.3) | 265 (1.3) | 36 (1.8) | |
| Cardiac status/testing | ||||
| LV Function | ||||
| ≥50% | 12 769 (55.7) | 11 611 (55.4) | 1158 (59.1) | 0.01 |
| 35–49% | 1688 (7.4) | 1546 (7.4) | 142 (7.2) | |
| 20–34% | 534 (2.3) | 487 (2.3) | 47 (2.4) | |
| <20% | 144 (0.6) | 128 (0.6) | 16 (0.8) | |
| NA | 7782 (34.0) | 7184 (34.3) | 598 (30.5) | |
| Exercise ECG risk | ||||
| Low risk | 5821 (25.4) | 5349 (25.5) | 472 (24.1) | 0.02 |
| High risk | 7478 (32.6) | 6876 (32.8) | 602 (30.7) | |
| Uninterpretable | 1141 (5.0) | 1042 (5.0) | 99 (5.0) | |
| NA | 8477 (37.0) | 7689 (36.7) | 788 (40.2) | |
| Functional imaging risk | ||||
| Low risk | 5745 (25.1) | 5221 (24.9) | 524 (26.7) | 0.20 |
| High risk | 7850 (34.3) | 7199 (34.4) | 651 (33.2) | |
| Unknown | 9322 (40.7) | 8536 (40.7) | 786 (40.1) | |
| Native stenosisb | ||||
| LM | 2707 (11.8) | 2452 (11.7) | 255 (13.0) | 0.09 |
| Proximal LAD | 6958 (30.4) | 6371 (30.4) | 587 (29.9) | 0.67 |
| Mid/distal LAD | 11 803 (51.5) | 10 766 (51.4) | 1037 (52.9) | 0.20 |
| Circumflex | 11 123 (48.5) | 10 138 (48.4) | 985 (50.2) | 0.12 |
| RCA | 13 063 (57.0) | 11 918 (56.9) | 1145 (58.4) | 0.19 |
| Treatment within 90 d | ||||
| CABG | 6741 (29.4) | 6039 (28.8) | 702 (35.8) | <0.001 |
| MED | 6415 (28.0) | 5918 (28.2) | 497 (25.3) | |
| PCI | 9761 (42.6) | 8999 (42.9) | 762 (38.9) | |
| Hospital‐level factors | ||||
| Availability | ||||
| Cath, PCI, and CABG | 16 908 (73.8) | 15 498 (74.0) | 1410 (71.9) | 0.13 |
| Cath only | 3467 (15.1) | 3153 (15.0) | 314 (16.0) | |
| Cath and PCI only | 2542 (11.1) | 2305 (11.0) | 237 (12.1) | |
| Physician‐level factors | ||||
| Referring physician | ||||
| Cardiology | 12 849 (56.1) | 11 771 (56.2) | 1078 (55.0) | 0.46 |
| GP/FP | 5611 (24.5) | 5109 (24.4) | 502 (25.6) | |
| Other | 4457 (19.4) | 4076 (19.5) | 381 (19.4) | |
| Physician performing cath | ||||
| PCI physician | 10 965 (47.8) | 10 074 (48.1) | 891 (45.4) | 0.03 |
Data are shown as n (%) except as noted. CABG indicates coronary artery bypass grafting; Cath, catheterization; CCS, Canadian Cardiovascular Society; COPD, chronic obstructive pulmonary disease; GP/FP, general practitioner/family physician; LAD, left anterior descending; LM, left main; LV, left ventricular; MED, medical therapy; NA, not done or missing; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; RCA, right coronary artery
Income quintile: 1, lowest; 5, highest.
Significant stenosis ≥70%, except ≥50% stenosis significant for LM.
Healthcare Costs
Healthcare service sectors and their corresponding costs according to diagnosis of depression during the first year following angiogram are shown in Figure 2 and Table S2. The mean cumulative 1‐year healthcare cost was substantially greater for patients with depression ($32 072±$41 963) than for patients without depression ($23 021±$25 741, P<0.001). Depressed patients had higher mean 1‐year costs across nearly all healthcare sectors, including acute inpatient care, chronic care, outpatient care, physician costs, and medications.
Figure 2.
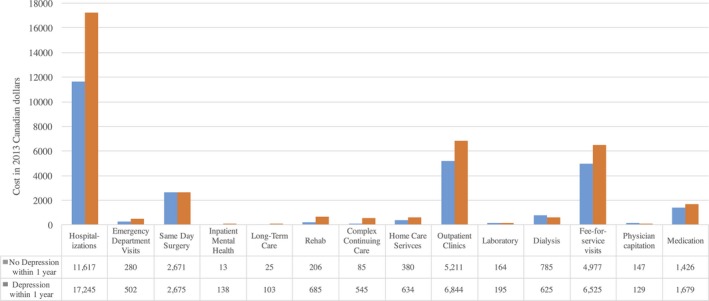
Mean 1‐year healthcare sector costs according to diagnosis of depression within 1 year of catheterization. ED indicates emergency department.
For the cohort as a whole, acute inpatient care represented the largest contributor to the aggregate 1‐year cost. In this sector, mean costs related to acute hospitalizations and inpatient mental health care were significantly greater for patients with depression compared with patients without depression. In the chronic care sector, average costs related to long‐term care, rehabilitation, complex continuing care, and home care services were all significantly higher for depressed patients. In the outpatient care sector, costs associated with outpatient clinics, emergency department visits, and laboratory services were significantly higher for patients with depression, but there was no difference in terms of average dialysis and same‐day surgery costs compared with nondepressed patients. Expenditures related to fee‐for‐service visits and medication prescriptions were significantly higher for depressed patients.
Table 2 shows the unadjusted and adjusted CRs of 1‐year cumulative healthcare costs within 1 year of index angiogram. A diagnosis of depression was a statistically significant predictor of increased healthcare expenditures, with an adjusted CR of 1.33 (95% confidence interval [CI], 1.29–1.37; P<0.001). This CR corresponds to a mean 33% increase in 1‐year healthcare costs in patients with depression compared with nondepressed patients. There was no statistically significant relationship between healthcare costs and female sex or rurality; however, greater income was associated with lower healthcare expenditures. Other significant drivers of cumulative 1‐year cost included a greater number of comorbidities denoted by the Charlson score (adjusted CR: 1.15; 95% CI, 1.14–1.17; P<0.001), as well as peripheral vascular disease (CR: 1.18; 95% CI, 1.14–1.22; P<0.001) and chronic obstructive pulmonary disease (CR: 1.08; 95% CI, 1.04–1.13; P<0.001). Previous stroke (CR: 0.87; 95% CI, 0.80–0.95; P=0.002) and malignancy (CR: 0.93; 95% CI, 0.87–0.99; P=0.023) were associated with lower cumulative 1‐year healthcare expenditures. Cardiac risk factors that were significant predictors of increased healthcare expenditures were diabetes mellitus (CR: 1.11; 95% CI, 1.09–1.13; P<0.001) and hypertension (CR: 1.07; 95% CI, 1.04–1.10; P<0.001), whereas hyperlipidemia (CR: 0.96; 95% CI 0.94–0.98; P<0.001) was associated with lower costs. More severe angina symptoms (Canadian Cardiovascular Society class 4) were associated with reduced healthcare costs, whereas worse left ventricular function and stenosis in any of the major coronary vessels were associated with higher 1‐year mean costs. One‐year costs were significantly greater for patients with CABG (CR: 2.54; 95% CI, 2.48–2.61; P<0.001) and percutaneous coronary intervention (CR: 1.29; 95% CI, 1.26–1.32; P<0.001) as the initial treatment strategy compared with medical therapy. Hospital capacity to perform catheterization, percutaneous coronary intervention, and CABG was associated with lower costs (CR: 0.89; 95% CI, 0.82–0.97; P=0.012) compared with hospitals that had the capacity to perform only catheterization. Finally, 1‐year healthcare costs were lower if the index catheterization (CR: 0.97; 95% CI, 0.95–0.99; P=0.007) was performed by an interventional cardiologist with subspecialty training in percutaneous coronary intervention. In the sensitivity analysis that excluded medication costs (Table S3), depression remained a statistically significant predictor of 1‐year cumulative healthcare costs (CR: 1.35; 95% CI, 1.31–1.39; P<0.001). Other predictors of healthcare costs also remained statistically significant following the exclusion of drug costs.
Table 2.
CRs for Mean 1‐Year Healthcare Costs
| Covariates | Unadjusted CR (95% CI) | P Value | Adjusted CR (95% CI) | P Value |
|---|---|---|---|---|
| Depression within 1 y | 1.39 (1.34–1.44) | <0.001 | 1.33 (1.29–1.37) | <0.001 |
| Patient‐level factors | ||||
| Demographics | ||||
| Female | 0.98 (0.96–1.01) | 0.21 | 1.02 (1.00–1.04) | 0.05 |
| Age index | 1.01 (1.01–1.02) | <0.001 | 1.01 (1.01–1.01) | <0.001 |
| Rural | 0.99 (0.96–1.03) | 0.7 | 1.00 (0.98–1.03) | 0.93 |
| Incomea | ||||
| 1 | Referent | Referent | ||
| 2 | 0.96 (0.93–1.00) | 0.04 | 0.95 (0.92–0.98) | <0.001 |
| 3 | 0.93 (0.89–0.96) | <0.001 | 0.94 (0.92–0.97) | <0.001 |
| 4 | 0.95 (0.92–0.99) | 0.01 | 0.96 (0.94–0.99) | 0.01 |
| 5 | 0.91 (0.87–0.94) | <0.001 | 0.93 (0.91–0.96) | <0.001 |
| Medical comorbidities | ||||
| Renal dysfunction | 2.92 (2.71–3.14) | <0.001 | 2.10 (1.96–2.26) | <0.001 |
| PVD | 1.46 (1.40–1.52) | <0.001 | 1.18 (1.14–1.22) | <0.001 |
| COPD | 1.24 (1.18–1.30) | <0.001 | 1.08 (1.04–1.13) | <0.001 |
| Previous stroke | 1.21 (1.09–1.34) | <0.001 | 0.87 (0.80–0.95) | 0.002 |
| Malignancy | 1.36 (1.27–1.45) | <0.001 | 0.93 (0.87–0.99) | 0.023 |
| Comorbidities: Charlson score | 1.23 (1.22–1.25) | <0.001 | 1.15 (1.14–1.17) | <0.001 |
| Cardiac risk factors | ||||
| Diabetes mellitus | 1.29 (1.27–1.32) | <0.001 | 1.11 (1.09–1.13) | <0.001 |
| Hypertension | 1.31 (1.27–1.35) | <0.001 | 1.07 (1.04–1.10) | <0.001 |
| Hyperlipidemia | 1.04 (1.02–1.07) | <0.001 | 0.96 (0.94–0.98) | <0.001 |
| Smoking | ||||
| Nonsmoker | Referent | Referent | ||
| Former smoker | 1.04 (1.01–1.06) | 0.008 | 1 (0.98–1.02) | 0.83 |
| Current smoker | 0.99 (0.97–1.02) | 0.71 | 1.03 (1–1.05) | 0.04 |
| CCS angina class | ||||
| 0 | Referent | Referent | ||
| 1 | 0.86 (0.83–0.89) | <0.001 | 0.91 (0.88–0.94) | <0.001 |
| 2 | 0.86 (0.83–0.88) | <0.001 | 0.91 (0.88–0.93) | <0.001 |
| 3 | 0.93 (0.90–0.96) | <0.001 | 0.93 (0.90–0.96) | <0.001 |
| 4 | 0.87 (0.79–0.96) | 0.006 | 0.86 (0.79–0.93) | <0.001 |
| Cardiac status/testing | ||||
| LV function | ||||
| ≥50% | Referent | Referent | ||
| 35–49% | 1.40 (1.09–1.19) | <0.001 | 1.01 (0.97–1.04) | 0.77 |
| 20–34% | 1.33 (1.24–1.43) | <0.001 | 1.19 (1.12–1.27) | <0.001 |
| <20% | 1.32 (1.15–1.52) | <0.001 | 1.28 (1.15–1.43) | <0.001 |
| NA | 0.98 (0.96–1.01) | 0.221 | 0.97 (0.95–0.99) | 0.01 |
| Exercise ECG risk | ||||
| Low risk | Referent | Referent | ||
| High risk | 1.13 (1.10–1.16) | <0.001 | 0.98 (0.96–1.01) | 0.24 |
| Uninterpretable | 1.15 (1.09–1.21) | <0.001 | 1.04 (0.99–1.08) | 0.12 |
| NA | 1.42 (1.28–1.46) | <0.001 | 1.17 (1.14–1.2) | <0.001 |
| Functional imaging risk | ||||
| Low risk | Referent | Referent | ||
| High risk | 1.12 (1.09–1.16) | <0.001 | 1.02 (1.00–1.05) | 0.08 |
| Unknown | 1.12 (1.09–1.15) | <0.001 | 1.04 (1.02–1.07) | 0.002 |
| Native stenosisb | ||||
| LM | 1.68 (1.63–1.74) | <0.001 | 1.14 (1.11–1.17) | <0.001 |
| Proximal LAD | 1.29 (1.26–1.32) | <0.001 | 1.13 (1.11–1.16) | <0.001 |
| Mid/distal LAD | 1.20 (1.17–1.23) | <0.001 | 1.11 (1.09–1.13) | <0.001 |
| Circumflex | 1.38 (1.36–1.41) | <0.001 | 1.13 (1.11–1.15) | <0.001 |
| RCA | 1.34 (1.31–1.37) | <0.001 | 1.13 (1.11–1.15) | <0.001 |
| Treatment within 90 d | ||||
| MED | Referent | Referent | ||
| CABG | 2.42 (2.34–2.49) | <0.001 | 2.54 (2.48–2.61) | <0.001 |
| PCI | 1.05 (1.03–1.08) | <0.001 | 1.29 (1.26–1.32) | <0.001 |
| Hospital‐level factors | ||||
| Availability | ||||
| Cath only | Referent | Referent | ||
| Cath and PCI only | 0.92 (0.85–1.00) | 0.043 | 0.93 (0.84–1.04) | 0.18 |
| Cath, PCI, and CABG | 0.89 (0.84–0.95) | <0.001 | 0.89 (0.82–0.97) | 0.012 |
| Physician‐level factors | ||||
| Referring physician | ||||
| GP/FP | Referent | Referent | ||
| Cardiology | 1.05 (1.02–1.08) | <0.001 | 1.04 (1.02–1.06) | 0.001 |
| Other | 1.16 (1.12–1.20) | <0.001 | 1.1 (1.07–1.13) | <0.001 |
| Physician performing cath | ||||
| PCI physician | 0.96 (0.94–0.98) | <0.001 | 0.97 (0.95–0.99) | 0.007 |
CABG indicates coronary artery bypass grafting; Cath, catheterization; CCS, Canadian Cardiovascular Society; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CR, cost ratio; GP/FP, general practitioner/family physician; LAD, left anterior descending; LM, left main; LV, left ventricular; MED, medical therapy; NA, not done or missing; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; RCA, right coronary artery
Income quintile: 1, lowest; 5, highest.
Significant stenosis ≥70%, except ≥50% stenosis significant for LM.
Discussion
This population‐based study demonstrated that an occurrence of depression within 1 year of receiving a diagnosis of chronic stable angina was associated with an increase in mean cumulative 1‐year healthcare costs. This increase remained significant after adjusting for other drivers of cost, such as medical comorbidity and cardiac status. Patients diagnosed with depression incurred greater expenditures in almost all healthcare domains, affecting both acute and ambulatory care including medication costs.
Previous evaluations of the implications of depression for healthcare costs among patients with cardiovascular disease have focused predominantly on populations with acute coronary syndrome, CABG, and heart failure. In contrast, a few small studies have examined resource utilization associated with depression in the setting of chronic stable angina and provided limited data on medical expenditures.15, 16, 17 The current study differs in that comprehensive healthcare cost information and clinical data on chronic stable angina patients with depression have been captured at a population level. In particular, this study estimates total and sector‐specific healthcare costs, including an illustration of overall resource utilization with respect to outpatient and inpatient services. Our findings underscore the important role of depression in the pattern of healthcare consumption in CAD patients; this study demonstrates that receiving a diagnosis of depression can intensify healthcare expenditures as soon as 1 year following diagnosis of chronic stable angina.
These results are consistent with previous literature on resource utilization in the context of depression and chronic medical illnesses, including cardiovascular diseases. Heart failure patients with depression, for instance, have been shown to have a 2‐fold greater likelihood of hospitalization and emergency department visits over a mean follow‐up of 1.6 years,14 and following myocardial infarction, depressive symptoms are associated with a 24% increase in total hospitalization days during an 18‐month follow‐up period.9 In our cohort of chronic stable angina patients, mean 1‐year expenditures among individuals diagnosed with depression within 1 year of angiogram were 33% higher compared with patients without depressive illness. These higher costs were related to acute inpatient care services, including acute hospitalizations and inpatient mental health care, as well as nonacute healthcare services such as outpatient visits, rehabilitation, home care services, and medications. This suggests that depression can have a wide‐ranging impact on healthcare utilization. Given the chronic and recurrent nature of depression, this cost difference may possibly persist or diverge even further after a 1‐year time period. Indeed, understanding the drivers of the acute phase following an occurrence of depression versus those of the chronic phase in patients with stable angina is the logical next step in future research in this area.
There are several possible explanations for the propensity toward higher healthcare costs among patients with depression and chronic stable angina. Depressed patients are recognized to have a higher rate of deleterious behaviors that may have important repercussions on the clinical trajectory of CAD. These behaviors include lifestyle habits such as decreased physical activity and tobacco use, as well as lower adherence to treatments, especially medications.7 Such impairments in self‐care may result in adverse effects over the short and long term, leading to hospital readmissions and protracted lengths of stay. From a physiological standpoint, depression may exert various biological effects on the cardiovascular system including increased sympathetic activity and platelet adhesion, resulting in a higher risk of cardiac events that require heightened medical care.7 Depressive illness may also exacerbate somatic symptoms associated with medical comorbidities, including chronic stable angina, leading patients to have more contact with their healthcare providers. In addition, patients with depression may present to their physicians for complaints specifically related to mental health.
Given the findings of this study, therapeutic strategies to attenuate costs associated with depression deserve further attention. Depression is often underrecognized by clinicians, and efforts toward improving its detection through screening may be beneficial. The integration of psychiatric interventions into cardiovascular care also warrants consideration. Examples include the participation of mental health professionals in a multidisciplinary team or involvement in cardiac rehabilitation centers. In such settings, patients may derive benefit from the support of case managers and psychiatrists who work alongside medical professionals.
Our study must be interpreted in the context of several limitations that merit discussion. First, we had medication costs only for patients aged ≥65 years, given that full coverage is available only for this age group. We would expect, however, that this would bias to the nondepressed group, given that they had older age. In addition, we lacked information about nonpharmacological approaches to depression such as psychotherapy because those are not captured in the available administrative databases. As such, we may be underestimating the incremental cost associated with depression in terms of the full range of therapeutic options. Second, we allocated patients to the depression group if they had a diagnosis at any point within 1 year. Consequently, some of the costs in this group were incurred before the formal diagnosis of depression and cannot be attributed to the diagnosis of depression itself. We chose this study design because our primary research question studied the impact of depression on 1‐year healthcare costs following an index diagnosis of coronary disease; as such, this time frame is appropriate for cost evaluation. Furthermore, our study utilizes an observational data set, and as such, there are baseline covariates that we were not able to account for; therefore, we cannot discount residual confounding. Finally, we used administrative databases and diagnostic codes to identify patients with depression, whereas previous studies have used structured psychiatric assessment tools. As a result, severity of depression was not determined, and patients with milder mood disturbances besides major depressive disorder may have been included in the cohort; however, this underscores that even potentially milder forms of mood disorders are associated with increased costs.
In summary, our results demonstrate that the occurrence of depression within 1 year of diagnosis of chronic stable angina is a significant independent predictor of mean 1‐year healthcare costs. This trend toward greater healthcare utilization among depressed patients echoes the findings of previous work in the area of depression and chronic medical diseases. The effect of depressive illness on cost permeates nearly all healthcare domains, including both ambulatory and hospital‐based care, resulting in an overall increase in medical expenditures. Depression is prevalent and a major driver of cost among patients with chronic stable angina. At this time, it is not known whether screening or treatment for depression improves cardiovascular outcomes and reduces healthcare consumption in patients with CAD. Further investigations are warranted to evaluate these aspects of care.
Sources of Funding
Wijeysundera is supported by a Distinguished Clinician Scientist award for the Heart and Stroke Foundation of Canada.
Disclosures
None.
Supporting information
Table S1. International Classification of Diseases, 10th Revision (ICD‐10‐CA) Codes for Depression
Table S2. Mean 1‐Year Healthcare Sector Costs According to Diagnosis of Depression During First Year Following Index Catheterization
Table S3. Adjusted Cost Ratios for Mean 1‐Year Healthcare Costs (With Medication Costs Excluded)
Acknowledgments
The authors acknowledge that the clinical registry data used in this publication are from participating hospitals through the Cardiac Care Network of Ontario, which serves as an advisory body to the Ministry of Health and Long‐Term Care (MOHLTC), is funded by the MOHLTC, and is dedicated to improving the quality, efficiency, access and equity in the delivery of the continuum of adult cardiac services in Ontario, Canada. Parts of this material are based on data and information compiled and provided by CIHI. However, the analyses, conclusions, opinions and statements expressed herein are those of the author, and not necessarily those of CIHI. We thank IMS Brogan Inc for use of their Drug Information Database. The following databases required acknowledgement for their use in this publication: Canadian Institute for Health Information Discharge Abstract Database, National Ambulatory Care Reporting System, Registered Persons Database, Ontario Mental Health Reporting System, and Ontario Drug Benefit database.
(J Am Heart Assoc. 2017;6:e006911 DOI: 10.1161/JAHA.117.006911.)
References
- 1. Economic Burden of Illness in Canada. 2008. Available at: http://www.phac-aspc.gc.ca/publicat/ebic-femc/2005-2008/index-eng.php. Accessed November 28, 2016.
- 2. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics‐2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 3. Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd‐Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. [DOI] [PubMed] [Google Scholar]
- 4. Bock JO, Luppa M, Brettschneider C, Riedel‐Heller S, Bickel H, Fuchs A, Gensichen J, Maier W, Mergenthal K, Schafer I, Schon G, Weyerer S, Wiese B, van den Bussche H, Scherer M, Konig HH. Impact of depression on health care utilization and costs among multimorbid patients—from the MultiCare Cohort Study. PLoS ONE. 2014;9:e91973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bankier B, Januzzi JL, Littman AB. The high prevalence of multiple psychiatric disorders in stable outpatients with coronary heart disease. Psychosom Med. 2004;66:645–650. [DOI] [PubMed] [Google Scholar]
- 6. Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure‐Smith N, Freedland KE, Jaffe AS, Leifheit‐Limson EC, Sheps DS, Vaccarino V, Wulsin L. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129:1350–1369. [DOI] [PubMed] [Google Scholar]
- 7. Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res. 2002;53:897–902. [DOI] [PubMed] [Google Scholar]
- 8. Frasure‐Smith N, Lesperance F, Gravel G, Masson A, Juneau M, Talajic M, Bourassa MG. Depression and health‐care costs during the first year following myocardial infarction. J Psychosom Res. 2000;48:471–478. [DOI] [PubMed] [Google Scholar]
- 9. Kurdyak PA, Gnam WH, Goering P, Chong A, Alter DA. The relationship between depressive symptoms, health service consumption, and prognosis after acute myocardial infarction: a prospective cohort study. BMC Health Serv Res. 2008;8:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Myers V, Gerber Y, Benyamini Y, Goldbourt U, Drory Y. Post‐myocardial infarction depression: increased hospital admissions and reduced adoption of secondary prevention measures—a longitudinal study. J Psychosom Res. 2012;72:5–10. [DOI] [PubMed] [Google Scholar]
- 11. Baumeister H, Haschke A, Munzinger M, Hutter N, Tully PJ. Inpatient and outpatient costs in patients with coronary artery disease and mental disorders: a systematic review. Biopsychosoc Med. 2015;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rodwin BA, Spruill TM, Ladapo JA. Economics of psychosocial factors in patients with cardiovascular disease. Prog Cardiovasc Dis. 2013;55:563–573. [DOI] [PubMed] [Google Scholar]
- 13. Oxlad M, Stubberfield J, Stuklis R, Edwards J, Wade TD. Psychological risk factors for cardiac‐related hospital readmission within 6 months of coronary artery bypass graft surgery. J Psychosom Res. 2006;61:775–781. [DOI] [PubMed] [Google Scholar]
- 14. Moraska AR, Chamberlain AM, Shah ND, Vickers KS, Rummans TA, Dunlay SM, Spertus JA, Weston SA, McNallan SM, Redfield MM, Roger VL. Circ Heart Fail. 2013;6:387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rutledge T, Vaccarino V, Johnson BD, Bittner V, Olson MB, Linke SE, Cornell CE, Eteiba W, Sheps DS, Francis J, Krantz DS, Bairey Merz CN, Parashar S, Handberg E, Vido DA, Shaw LJ. Depression and cardiovascular health care costs among women with suspected myocardial ischemia: prospective results from the WISE (Women's Ischemia Syndrome Evaluation) Study. J Am Coll Cardiol. 2009;53:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Versteeg H, Hoogwegt MT, Hansen TB, Pedersen SS, Zwisler AD, Thygesen LC. Depression, not anxiety, is independently associated with 5‐year hospitalizations and mortality in patients with ischemic heart disease. J Psychosom Res. 2013;75:518–525. [DOI] [PubMed] [Google Scholar]
- 17. Palacios JE, Khondoker M, Achilla E, Tylee A, Hotopf M. A single, one‐off measure of depression and anxiety predicts future symptoms, higher healthcare costs, and lower quality of life in coronary heart disease patients: analysis from a multi‐wave. Primary Care Cohort Study. PLoS ONE. 2016;11:e0158163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Szpakowski N, Bennell MC, Qiu F, Ko DT, Tu JV, Kurdyak P, Wijeysundera HC. Clinical impact of subsequent depression in patients with a new diagnosis of stable angina: a Population‐Based Study. Circ Cardiovasc Qual Outcomes. 2016;9:731–739. [DOI] [PubMed] [Google Scholar]
- 19. About Cardiac Care Network. 2016. Available at: http://www.ccn.on.ca/ccn_public/FormsAboutCCN/about.aspx. Accessed November 25, 2016.
- 20. Cardiac Care Network Annual Report 2015‐2016. 2016. Available at: http://www.ccn.on.ca/ccn_public/uploadfiles/files/CCN_Annual_Report_2015_16.pdf. Accessed November 25, 2016.
- 21. Ko DT, Guo H, Wijeysundera HC, Natarajan MK, Nagpal AD, Feindel CM, Kingsbury K, Cohen EA, Tu JV. Assessing the association of appropriateness of coronary revascularization and clinical outcomes for patients with stable coronary artery disease. J Am Coll Cardiol. 2012;60:1876–1884. [DOI] [PubMed] [Google Scholar]
- 22. Schwalm JD, Wijeysundera HC, Tu JV, Guo H, Kingsbury KJ, Natarajan MK. Influence of coronary anatomy and SYNTAX Score on the variations in revascularization strategies for patients with multivessel disease. Can J Cardiol. 2014;30:1155–1161. [DOI] [PubMed] [Google Scholar]
- 23. Bradley SM, Maddox TM, Stanislawski MA, O'Donnell CI, Grunwald GK, Tsai TT, Ho PM, Peterson ED, Rumsfeld JS. Normal coronary rates for elective angiography in the Veterans Affairs Healthcare System: insights from the VA CART program (Veterans Affairs Clinical Assessment Reporting and Tracking). J Am Coll Cardiol. 2014;63:417–426. [DOI] [PubMed] [Google Scholar]
- 24. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–e651. [DOI] [PubMed] [Google Scholar]
- 25. Hiller W, Dichtl G, Hecht H, Hundt W, Mombour W, von Zerssen D. Evaluating the new ICD‐10 categories of depressive episode and recurrent depressive disorder. J Affect Disord. 1994;31:49–60. [DOI] [PubMed] [Google Scholar]
- 26. Kessing L. A comparison of ICD‐8 and ICD‐10 diagnoses of affective disorder ‐a case register study from Denmark. Eur Psychiatry. 1998;13:342–345. [DOI] [PubMed] [Google Scholar]
- 27. Fiest KM, Jette N, Quan H, St Germaine‐Smith C, Metcalfe A, Patten SB, Beck CA. Systematic review and assessment of validated case definitions for depression in administrative data. BMC Psychiatry. 2014;14:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gurevich Y, McFarlane A, Morris K, Jokovic A, Peterson GM, Webster GK. Estimating the number of coronary artery bypass graft and percutaneous coronary intervention procedures in Canada: a comparison of cardiac registry and Canadian Institute for Health Information data sources. Can J Cardiol. 2010;26:e249–e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jacobs P, Yim R. Using Canadian administrative databases to derive economic data for health technology assessments. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2009. Available at: https://www.cadth.ca/media/pdf/H0483_Canadian_Admin_Databases_mg_e.pdf. Accessed December 5, 2016. [Google Scholar]
- 30. Blough DK, Ramsey SD. Using generalized linear models to assess medical care costs. Health Serv Outcomes Res Method. 2000;1:185–202. [Google Scholar]
- 31. Kelley AS, Ettner SL, Morrison RS, Du Q, Wenger NS, Sarkisian CA. Determinants of medical expenditures in the last 6 months of life. Ann Intern Med. 2011;154:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kang JS, Bennell MC, Qiu F, Knudtson ML, Austin PC, Ko DT, Wijeysundera HC. Relation between initial treatment strategy in stable coronary artery disease and 1‐year costs in Ontario: a population‐based cohort study. CMAJ Open. 2016;4:e409–e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. International Classification of Diseases, 10th Revision (ICD‐10‐CA) Codes for Depression
Table S2. Mean 1‐Year Healthcare Sector Costs According to Diagnosis of Depression During First Year Following Index Catheterization
Table S3. Adjusted Cost Ratios for Mean 1‐Year Healthcare Costs (With Medication Costs Excluded)


