Abstract
Background
In cardiac resynchronization therapy (CRT), quadripolar (QUAD) left ventricular (LV) leads are less prone to postoperative complications than non‐QUAD leads. Some studies have suggested better clinical outcomes.
Methods and Results
Clinical events were assessed in 847 patients after CRT‐pacing or CRT‐defibrillation using either QUAD (n=287) or non‐QUAD (n=560), programmed to single‐site site LV pacing. Over a follow‐up period of 3.2 years (median [interquartile range, 1.90–5.0]), QUAD was associated with a lower total mortality (adjusted hazard ratio [aHR]: 0.32, 95% confidence interval [CI], 0.20–0.52), cardiac mortality (aHR: 0.36, 95% CI, 0.20–0.65), and heart failure (HF) hospitalization (aHR: 0.62, 95% CI, 0.39–0.99), after adjustment for age, sex, New York Heart Association class, HF etiology, device type (CRT‐pacing or CRT‐defibrillation), comorbidities, atrial rhythm, medication, left ventricular ejection fraction, and creatinine. Death from pump failure was lower with QUAD (aHR: 0.33; 95% CI, 0.18–0.62), but no group differences emerged with respect to sudden cardiac death. There were no differences in implant‐related complications. Re‐interventions for LV displacement or phrenic nerve stimulation, which were lower with QUAD, predicted total mortality (aHR: 1.68, 95% CI, 1.11–2.54), cardiac mortality (aHR: 2.61, 95% CI, 1.66–4.11) and HF hospitalization (aHR: 2.09, 95% CI, 1.22–3.58).
Conclusions
CRT using QUAD, programmed to biventricular pacing with single‐site LV pacing, is associated with a lower total mortality, cardiac mortality, and HF hospitalization. These trends were observed for both CRT‐defibrillation and CRT‐pacing, after adjustment for HF cause and other confounders. Re‐intervention for LV lead displacement or phrenic nerve stimulation was associated with worse outcomes.
Keywords: arrhythmia, bipolar lead, cardiac resynchronization therapy, heart failure, quadripolar lead, sudden cardiac death
Clinical Perspective
What Is New?
In this nonrandomized, retrospective, observational study of patients undergoing cardiac resynchronization therapy (CRT) with quadripolar (QUAD) and non‐QUAD left ventricular leads, programmed to biventricular, single‐site left ventricular pacing, QUAD was associated with a lower total mortality, cardiac mortality, and heart failure hospitalization.
These benefits were observed after both CRT‐defibrillation and CRT‐pacing, after adjustment for heart failure etiology.
Re‐interventions for left ventricular displacement or phrenic nerve stimulation, which were lower with QUAD, were associated with worse outcomes.
What Are the Clinical Implications?
The markedly better outcomes after CRT observed with QUAD supports their preferential use over non‐QUAD in clinical practice.
The relative benefits of CRT‐defibrillation over CRT‐pacing requires further evaluation in the QUAD era.
Introduction
Cardiac resynchronization therapy (CRT), with CRT‐defibrillation (CRT‐D) or without (CRT‐pacing [CRT‐P]) defibrillation, is a standard treatment for selected patients with heart failure (HF) with severe left ventricular (LV) dysfunction and a wide QRS complex.1 Since the first transvenous CRT implantations were undertaken in the 1990s,2, 3 improvements in delivery catheter and LV lead design, as well as implantation techniques using venoplasty and snaring, have helped to improve implantation success. Prominent among the challenges still encountered at implantation and thereafter is achieving acceptable LV pacing thresholds without phrenic nerve stimulation (PNS).4 Deactivation of the LV lead mainly occurs as a result of LV lead displacement which, in studies using unipolar and bipolar leads, occurs more frequently than with atrial or right ventricular leads.5, 6, 7, 8, 9
Since their launch in 2010, quadripolar LV leads (QUAD) have been considered by implanters as a “game‐changer,” even before robust clinical evidence emerged in their favor. Observational studies and a randomized, controlled trial10 have since shown that QUAD is associated with higher implant success rates and lower rates of re‐interventions for LV lead displacement or PNS.11, 12
Some observational studies have suggested that CRT‐D using QUAD programmed to single‐site LV pacing also improves survival.12, 13, 14 These findings, however, are not consistent,15 and there is uncertainty as to whether they also apply to CRT‐P. Moreover, the possible influence of HF etiology and the effects of QUAD on HF hospitalization and mode of death remain largely unexplored.
Methods
This is a nonrandomized, retrospective, observational study comparing clinical outcomes of patients undergoing CRT‐D and CRT‐P device implantation using unipolar, bipolar, and quadripolar leads in a single center (Queen Elizabeth Hospital, Birmingham, United Kingdom) from February 2010 to January 2017. The study was approved by the Clinical Audit Department at the Queen Elizabeth Hospital, which does not require informed consent for audit of clinical care delivery. The study conforms to the Declaration of Helsinki.
Implantation
Device implantation was undertaken using standard techniques with patients under local anesthesia and intravenous sedation. Access was gained via subclavian, axillary, and cephalic veins. The LV pacing site was chosen by the implanter on the basis of lead stability, absence of PNS, and adequate pacing parameters. An implant was considered a failure in the event of failure to deploy all desired leads and device at the index procedure. The first QUAD was implanted in February 2010. The following QUAD leads were used: Quartet 1458Q (St. Jude Medical, Sylmar, CA), Attain Performa (Medtronic Inc, Minneapolis, MN), and Acuity X4 (Boston Scientific, Marlborough, MA). The choice of vector was made at implantation and was made on the basis of presence or absence of PNS.
Follow‐Up
Patients were followed up in dedicated device therapy clinics. Before 2013, patients underwent systematic echocardiographic optimization. To this end, patients in sinus rhythm underwent transmitral Doppler‐directed optimization of atrioventricular delay using an iterative technique before discharge and at every scheduled visit thereafter. In patients with sinus rhythm, atrial pacing was set at 60 beats/min, and the pacing mode was set to DDDR with an interventricular delay of 0 to 4 ms, according to the manufacturer. In patients with permanent atrial fibrillation, right ventricular and LV leads were implanted and a CRT generator was used, plugging the atrial port and programming the generator to a ventricular triggered mode. In patients with uncontrolled atrial fibrillation despite medical therapy with suboptimal biventricular pacing capture (<98%), atrioventricular junction ablation was undertaken, according to the individual clinician's decision. After 2013, echocardiographic optimization was only undertaken in symptomatic nonresponders.
End Points
The primary end point was total mortality, which included cardiac transplantation. Secondary end points included cardiac mortality and unplanned HF hospitalization. The first event was included in the analysis. With respect to mode of death, sudden cardiac death was defined as a “natural, unexpected death due to cardiac causes, heralded by an abrupt loss of consciousness within 1 hour of the onset of acute symptoms,”16 whereas death from pump failure was defined as “death after a period of clinical deterioration in signs and symptoms of heart failure despite medical treatment”17 or cardiac transplantation. Mortality data were collected through medical records every 3 months by investigators who were blinded to all other patient data. Mortality and event data were collected by separate investigators who were blinded to all other data, except patient identifiers.
Statistical Analysis
In preliminary analyses, no differences in outcomes emerged between unipolar and bipolar LV leads (data not shown). On this basis, the latter were classified as “non‐QUAD” in statistical analyses. Normality was tested using the Shapiro–Wilk test. Continuous variables are expressed as mean (±SD) and compared using the Student t test. Categorical variables were compared using the χ2 tests. Kaplan–Meier curves and the log‐rank tests were used to assess observed cumulative survival and to test for differences in survival, respectively. Cox proportional hazard models were used to compare hazard rates of subgroups. Variables reaching a P<0.10 on univariable analyses were entered in multivariable models, and further backward elimination was applied for the final multivariable models. Confounders included in final models were the following: quadripolar lead, sex (male), age at implantation, New York Heart Association (NYHA) class, creatinine, QRS duration, and medication of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers. Proportionality hypotheses were verified by visual examination of log (survival) graphs to ensure parallel slopes, and by examining Schoenfeld residuals. Statistical analyses were undertaken using Stata 14 (StataCorp, Houston, TX). A 2‐sided P≤0.05 was considered statistically significant.
Results
Baseline Characteristics
Over the study period of 6.9 years, 847 patients underwent CRT (CRT‐D: 436 [51.5%]; CRT‐P: 411 [48.5%]), using QUAD (287 [33.9%]), unipolar (63 [7.43%]), or bipolar (497 [58.7%]) leads. Implantations using unipolar and bipolar leads were classified as non‐QUAD. As shown in Table 1, the groups were well matched for age, sex, cause of cardiomyopathy, comorbidities, proportion of upgrades from pacemaker, atrial rhythm (sinus rhythm or atrial fibrillation), QRS morphology, QRS duration, and left ventricular ejection fraction. Compared with the non‐QUAD group, QUAD were more likely to be in NYHA class I and II (P<0.001) and to undergo CRT‐D (62.0% versus 46.1%, P<0.001). In addition, QUAD had a lower uptake of loop diuretics (P=0.003) and a higher uptake of β‐blockers (P=0.004).
Table 1.
Characteristics of the Study Group
| QUAD | Non‐QUAD | P Valuea | |
|---|---|---|---|
| N | 287 | 560 | |
| Sex (male), n (%) | 209 (72.8) | 398 (71.1) | 0.592 |
| Age, y | 72.5±12.2 | 73.2±11.3 | 0.517 |
| NYHA class, n (%) | |||
| I | 40 (14.3) | 32 (5.8) | <0.001 |
| II | 87 (31.1) | 66 (11.9) | |
| III | 148 (52.9) | 419 (75.5) | |
| IV | 5 (1.79) | 38 (6.9) | |
| Cause of cardiomyopathy, n (%) | |||
| Ischemic | 151 (52.6) | 279 (49.8) | 0.442 |
| Nonischemic | 136 (47.4) | 281 (50.2) | |
| Device type, n (%) | |||
| CRT‐D | 178 (62.0) | 258 (46.1) | <0.001 |
| CRT‐P | 109 (38.0) | 302 (53.9) | |
| Comorbidities, n (%) | |||
| Diabetes mellitus | 80 (28.1) | 127 (22.9) | 0.099 |
| Hypertension | 87 (30.5) | 165 (29.7) | 0.811 |
| CABG | 48 (16.7) | 102 (18.2) | 0.591 |
| Upgrade from pacemaker | 56 (19.5) | 119 (21.6) | 0.554 |
| ECG variables | |||
| Sinus rhythm, n (%) | 195 (67.9) | 356 (63.6) | 0.206 |
| Atrial fibrillation, n (%)b | 92 (32.1) | 204 (36.4) | |
| QRS morphology (LBBB), n (%) | 228 (79.4) | 438 (78.2) | 0.680 |
| QRS duration, msc | 152.6±24.0 | 152.4±25.0 | 0.896 |
| Medication, n (%) | |||
| Loop diuretics | 274 (95.5) | 553 (98.8) | 0.003 |
| ACEIs/ARA | 257 (89.6) | 489 (87.3) | 0.344 |
| β‐Blockers | 227 (79.1) | 391 (69.2) | 0.004 |
| MRA | 124 (43.2) | 244 (43.6) | 0.919 |
| LVEF, % | 24.9±9.1 | 25.8±11.2 | 0.249 |
| Creatinine, μmol/Ld | 106 (89–129) | 104 (87–132) | 0.727 |
Variables are expressed as mean±SD, unless indicated otherwise. ACEI indicates angiotensin‐converting enzyme inhibitors; ARA, angiotensin receptor blockers; CABG, coronary artery bypass grafting; CRT‐D, cardiac resynchronization therapy‐defibrillation; CRT‐P, cardiac resynchronization therapy‐pacing; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; QUAD, quadripolar left ventricular lead.
Refers to differences between the groups from ANOVA for continuous variables and from χ2 tests for categorical variables.
Includes permanent, persistent, and paroxysmal atrial fibrillation.
Excludes upgrades to pacemaker.
Log‐transformed for statistical analyses.
Total Mortality
Over a follow‐up period of 3.2 years (median [interquartile range,1.90 to 5.0; 1.8 years [interquartile range, 1.0–2.6] for QUAD; 4.7 years [interquartile range, 3.4–5.7] for non‐QUAD), QUAD was associated with a lower total mortality in Kaplan–Meier survival analyses (log rank P<0.001, Figure 1). The annualized total mortality rate was 3.6% (n=19) for QUAD and 10.9% (n=218) for non‐QUAD. Event rates are shown in Table 2. Univariable Cox proportional hazards analyses are shown in Table 3. In multivariable analyses (Table 4), QUAD was associated with a lower mortality (adjusted hazard ratio [aHR]: 0.32, 95% confidence interval [CI], 0.20–0.52), after adjustment for age, sex, NYHA class, and creatinine. Other confounders failed to reach significance in multivariable models. In order to exclude a possible time‐related bias, we explored whether date of implant emerged as a predictor of total mortality. In multivariable Cox proportional hazards analysis, date of implant did not predict total mortality (HR: 1.46, 95% CI, 0.95–2.26).
Figure 1.
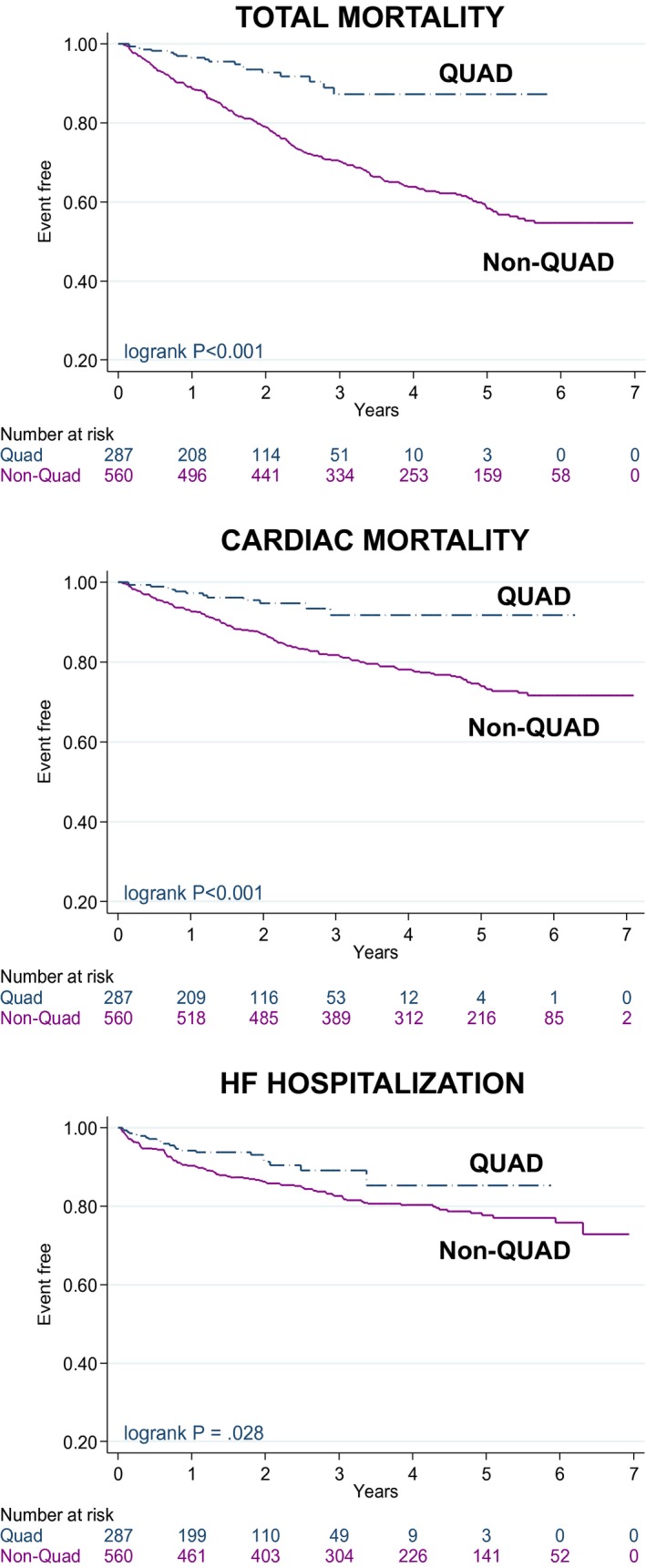
Clinical outcomes according to lead type. Kaplan–Meier survival curves for clinical outcomes according to device and lead type. HF indicates heart failure; QUAD, quadripolar lead.
Table 2.
Event Rates According to Lead Type
| QUAD (n=287) | Non‐QUAD (n=560) | |||
|---|---|---|---|---|
| n | %a | n | %a | |
| Total mortality | 19 | 3.6 | 218 | 10.9 |
| Cardiac mortality | 13 | 2.4 | 136 | 6.0 |
| HF hospitalization | 22 | 4.4 | 104 | 5.6 |
| Death from pump failure | 11 | 2.1 | 122 | 5.4 |
| SCD | 2 | 0.4 | 13 | 0.6 |
HF indicates heart failure; QUAD, quadripolar left ventricular lead; SCD, sudden cardiac death.
Data are expressed in terms of annualized event rates.
Table 3.
Univariable Cox Proportional Hazards Analyses of Baseline Variables in Relation to Clinical Outcomes
| Total Mortality | Cardiac Mortality | HF Hospitalization | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | ||||
| Lead type (QUAD) | 0.31 | 0.19 | 0.49 | <0.001 | 0.35 | 0.20 | 0.63 | <0.001 | 0.60 | 0.37 | 0.95 | 0.030 |
| Sex (male) | 1.76 | 1.28 | 2.42 | 0.001 | 1.99 | 1.30 | 3.04 | 0.001 | 1.23 | 0.82 | 1.84 | 0.309 |
| Age | 1.04 | 1.02 | 1.05 | <0.001 | 1.02 | 1.00 | 1.03 | 0.029 | 1.03 | 1.01 | 1.04 | 0.004 |
| NYHA class | ||||||||||||
| III | 1.50 | 1.04 | 2.18 | 0.031 | 1.41 | 0.90 | 2.21 | 0.138 | 1.25 | 0.80 | 1.94 | 0.329 |
| IV | 3.74 | 2.25 | 6.21 | <0.001 | 2.40 | 1.25 | 4.61 | 0.009 | 1.24 | 0.51 | 3.03 | 0.634 |
| Cause (ischemic) | 1.23 | 0.95 | 1.59 | 0.112 | 1.21 | 0.88 | 1.67 | 0.249 | 1.31 | 0.92 | 1.87 | 0.130 |
| Device type (CRT‐D) | 0.81 | 0.63 | 1.04 | 0.100 | 0.96 | 0.70 | 1.33 | 0.810 | 0.98 | 0.69 | 1.39 | 0.922 |
| Comorbidities | ||||||||||||
| Diabetes mellitus | 1.33 | 1.00 | 1.77 | 0.050 | 1.57 | 1.11 | 2.22 | 0.010 | 1.48 | 1.02 | 2.16 | 0.042 |
| Hypertension | 1.10 | 0.83 | 1.45 | 0.509 | 0.91 | 0.63 | 1.31 | 0.620 | 0.96 | 0.65 | 1.41 | 0.820 |
| CABG | 1.16 | 0.85 | 1.60 | 0.352 | 1.05 | 0.70 | 1.59 | 0.801 | 1.04 | 0.66 | 1.63 | 0.872 |
| ECG variables | ||||||||||||
| Atrial fibrillation | 1.36 | 1.05 | 1.76 | 0.019 | 1.26 | 0.91 | 1.74 | 0.171 | 0.94 | 0.65 | 1.36 | 0.741 |
| QRS morphology (LBBB) | 0.81 | 0.60 | 1.09 | 0.161 | 0.71 | 0.50 | 1.02 | 0.064 | 0.57 | 0.39 | 0.84 | 0.004 |
| QRS duration, ms | 1.00 | 0.99 | 1.00 | 0.115 | 0.99 | 0.99 | 1.00 | 0.041 | 0.99 | 0.98 | 0.99 | <0.001 |
| Medication | ||||||||||||
| Loop diuretics | 1.38 | 0.44 | 4.32 | 0.578 | 2.73 | 0.38 | 19.51 | 0.317 | ||||
| ACEIs/ARA | 0.54 | 0.39 | 0.76 | <0.001 | 0.52 | 0.35 | 0.79 | 0.002 | 1.28 | 0.69 | 2.39 | 0.428 |
| β‐Blockers | 0.87 | 0.66 | 1.14 | 0.309 | 0.81 | 0.57 | 1.13 | 0.215 | 0.91 | 0.62 | 1.33 | 0.612 |
| MRA | 0.90 | 0.69 | 1.16 | 0.410 | 1.01 | 0.73 | 1.40 | 0.949 | 1.25 | 0.88 | 1.77 | 0.210 |
| LVEF, % | 1.00 | 0.98 | 1.01 | 0.462 | 0.99 | 0.97 | 1.01 | 0.211 | 1.01 | 0.99 | 1.02 | 0.404 |
| Creatinine, log μmol/L | 2.20 | 1.72 | 2.82 | <0.001 | 1.92 | 1.38 | 2.67 | <0.001 | 1.93 | 1.34 | 2.76 | <0.001 |
ACEIs indicates angiotensin‐converting enzyme inhibitors; ARA, angiotensin receptor blockers; CABG, coronary artery bypass grafting; CRT‐D, cardiac resynchronization therapy‐defibrillation; HF, heart failure; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; QUAD, quadripolar left ventricular lead.
Results are expressed as hazard ratios and 95% confidence intervals (CI) from Cox proportional hazards analyses.
Table 4.
Multivariable Analyses of Baseline Variables in Relation to Clinical Outcomes
| Total Mortality | Cardiac Mortality | HF Hospitalization | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | ||||
| Lead type (QUAD) | 0.32 | 0.20 | 0.52 | <0.001 | 0.36 | 0.20 | 0.65 | 0.001 | 0.62 | 0.39 | 0.99 | 0.047 |
| Sex, male | 1.65 | 1.18 | 2.31 | 0.003 | 1.74 | 1.13 | 2.70 | 0.013 | ··· | ··· | ··· | ··· |
| Age, y | 1.03 | 1.02 | 1.05 | <0.001 | 1.02 | 1.00 | 1.03 | 0.043 | 1.03 | 1.01 | 1.05 | 0.001 |
| NYHA class (IV) | 1.89 | 1.25 | 2.86 | 0.003 | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· |
| QRS duration, ms | ··· | ··· | ··· | ··· | 0.99 | 0.99 | 1.00 | 0.019 | 0.99 | 0.98 | 0.99 | <0.001 |
| ACEIs/ARAs | ··· | ··· | ··· | ··· | 0.64 | 0.42 | 0.99 | 0.044 | ··· | ··· | ··· | ··· |
| Creatinine, log μmol/L | 1.68 | 1.25 | 2.25 | 0.001 | 1.50 | 1.04 | 2.16 | 0.030 | 1.91 | 1.30 | 2.80 | 0.001 |
Only variables with P<0.10 on univariable analyses were included in multivariable models. ACEI, angiotensin‐converting enzyme inhibitors; ARA, angiotensin receptor blockers; HF, heart failure; NYHA, New York Heart Association; QUAD, quadripolar left ventricular lead.
Results are expressed as hazard ratios and 95% confidence intervals (CI) from Cox proportional hazards analyses.
Cardiac Mortality
The annualized cardiac mortality rate was 2.40% (n=13) for QUAD and 6.0% (n=136) for non‐QUAD (Table 2). In Kaplan–Meier survival analyses, QUAD was associated with a lower cardiac mortality (log rank P<0.001, Figure 1). In multivariable analyses (Table 4), QUAD was associated with a lower cardiac mortality (aHR: 0.36, 95% CI, 0.20–0.65), after adjustment for known confounders.
HF Hospitalization
The annualized HF hospitalization rate was 4.40% for QUAD (n=22) and 5.6% (n=104) for non‐QUAD (Table 2). In Kaplan–Meier survival analyses, QUAD was associated with a lower risk of HF hospitalization (log rank P=0.028, Figure 1). In multivariable analyses (Table 4), QUAD was associated with a lower risk of HF hospitalization (aHR: 0.62, 95% CI, 0.39–0.99), after adjustment for potential confounders.
QUAD and Device Type
In univariable (Table 3) and mutivariable (Table 4) analyses, CRT‐D did not emerge as a predictor of any end point. Separate analyses according to device type were also undertaken. As shown in Figure 2, QUAD was superior to non‐QUAD with respect to all end points in Kaplan–Meier survival analyses. In univariable analyses including CRT‐D patients only, QUAD was superior to non‐QUAD with respect to total mortality (HR: 0.44, 95% CI, 0.25–0.76) and cardiac mortality (HR: 0.43, 95% CI, 0.22–0.85). A lower, albeit nonsignificant reduction with QUAD was observed in HF hospitalization (HR: 0.54, 95% CI, 0.29–1.01). In univariable analyses including CRT‐P patients only, QUAD was superior to non‐QUAD with respect to total mortality (HR: 0.16, 95% CI, 0.06–0.45) and cardiac mortality (HR: 0.23, 95% CI, 0.07–0.73), but no differences emerged in HF hospitalization (HR: 0.67, 95% CI, 0.32–1.37).
Figure 2.
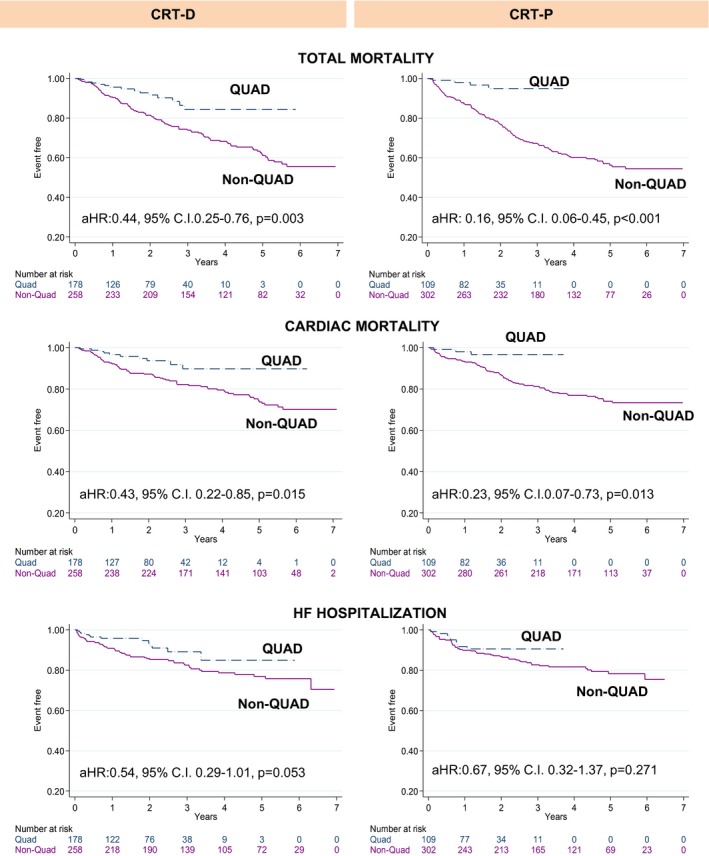
Clinical outcomes according to device and lead type. Kaplan–Meier survival curves for clinical outcomes according to device and lead type. aHR indicates adjusted hazard ratio; C.I., confidence interval; CRT‐D, cardiac resynchronization therapy‐defibrillation; CRT‐P, cardiac resynchronization therapy‐pacing; HF, heart failure; QUAD, quadripolar lead.
Mode of Death
Over the follow‐up period, there were 11/287 (3.83%) deaths because of pump failure with QUAD and 122/558 (21.8%) with non‐QUAD. There were 2/287 (0.70%) sudden cardiac deaths with QUAD and 13/560 (2.32%) with non‐QUAD. Noncardiac deaths accounted for 3/287 (1.04%) deaths with QUAD and 42/560 (7.5%) deaths with non‐QUAD. The cause and mode of death was unknown in 3 (1.04%) patients with QUAD and in 40 (7.14%) patients with non‐QUAD. Excluding these patients, QUAD was associated with a lower mortality from pump failure (log rank P<0.001; aHR: 0.33; 95% CI, 0.18–0.62), but no differences emerged with respect to sudden cardiac death (aHR: 0.58; 95% CI, 0.13–2.68, Figure 3).
Figure 3.
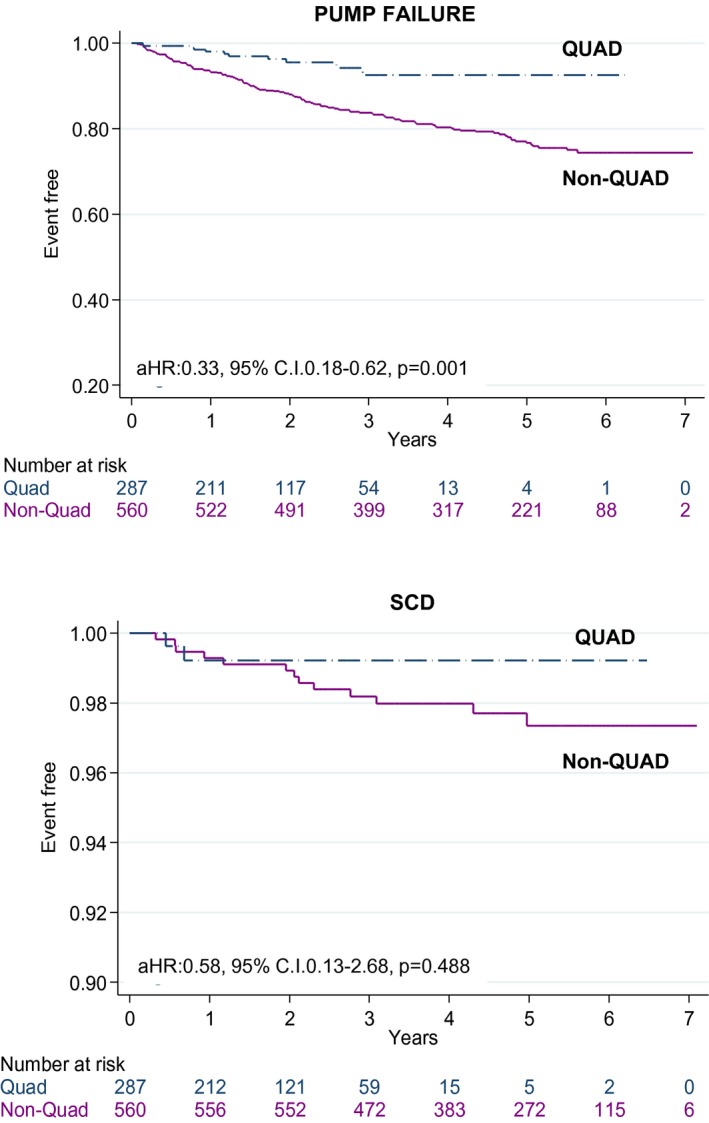
Mode of death according to lead type. Kaplan–Meier survival curves for death from pump failure or sudden cardiac death (SCD) according to lead type. aHR indicates adjusted hazard ratio; C.I., confidence interval; QUAD, quadripolar lead.
Implantation
There were 856 first attempts at CRT device implantation, 833 (97.3%) of which were successful at the first attempt and 847 (98.9%) after ≥1 attempts. Re‐interventions for LV displacement or PNS were lower with QUAD than with non‐QUAD (Table 5, Figure 4). In univariable analyses, re‐interventions for LV displacement or PNS predicted total mortality (aHR: 1.68, 95% CI, 1.11–2.54), cardiac mortality (aHR: 2.61, 95% CI, 1.66–4.11), and HF hospitalization (aHR: 2.09, 95% CI, 1.22–3.58).
Table 5.
Implant‐Related Complications and Re‐Interventions
| All | QUAD | Non‐QUAD | P Valuea | |
|---|---|---|---|---|
| 847 | 287 | 560 | ||
| Implant‐related complications, n (%) | ||||
| Hematoma treated conservatively | 23 (2.72) | 10 (3.48) | 14 (2.50) | 0.390 |
| Hematoma requiring evacuation | 4 (0.47) | 0 | 4 (0.71) | |
| Pneumothorax treated conservatively | 5 (0.59) | 2 (0.70) | 3 (0.54) | |
| Pneumothorax requiring drainage | 1 (0.12) | 0 | 1 (0.18) | |
| Perforation by RV lead | 2 (0.24) | 1 (0.35) | 1 (0.18) | |
| Coronary sinus dissectionb | 5 (0.59) | 4 (1.39) | 3 (0.54) | |
| Subclavian artery aneurysm | 1 (0.12) | 1 (0.35) | 0 | |
| Arrhythmia requiring cardioversion | 1 (0.12) | 1 (0.35) | 0 | |
| Anemia postprocedure | 1 (0.12) | 0 | 1 (0.18) | |
| Pulmonary edema | 1 (0.12) | 0 | 1 (0.18) | |
| Total, n (%) | 44 (5.19) | 19 (6.62) | 28 (5.00) | |
| Extractions for infection | ||||
| Within 1 y | 8 (1.43) | 3 (1.05) | 5 (0.89) | 0.297 |
| After 1 y | 3 (0.53) | 0 | 3 (0.54) | |
| Total, n (%) | 11 (1.96) | 3 (1.05) | 8 (1.43) | |
| LV lead re‐interventions | ||||
| LV lead displacement | 34 (4.01) | 6 (2.09) | 28 (5.0) | 0.007 |
| Phrenic nerve stimulation | 19 (2.24) | 3 (1.05) | 16 (2.86) | |
| Total | 53 (6.26) | 9 (3.14) | 44 (7.86) | |
RV indicates right ventricular.
Refers to χ2 tests of quadripolar (QUAD) compared with non‐QUAD left ventricular (LV) leads.
No coronary sinus dissections required re‐interventions.
Figure 4.
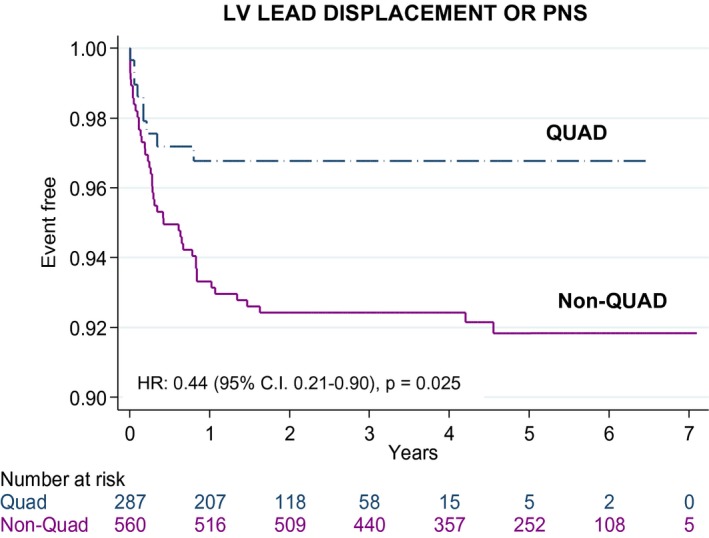
Reinterventions for left ventricular lead displacement or phrenic nerve stimulation. Kaplan–Meier survival curves of re‐interventions for left ventricular (LV) lead displacement or phrenic nerve stimulation (PNS) after device implantation using quadripolar (QUAD) or non‐QUAD leads. aHR indicates adjusted hazard ratio.
Other Complications
As shown in Table 5, implant‐related complications were similar for QUAD and non‐QUAD (odds ratio: 1.30, 95% CI, 0.71–2.36). A total of 8 extractions for system infection were undertaken within 1 year of implantation (QUAD: 3 (1.05%); non‐QUAD: 5 (0.90%; P=0.828) and 3 after 1 year (QUAD: 0); non‐QUAD: 3 (0.54%; P=0.214). No device‐related infection or subsequent extraction led to death.
Lead Design
Three LV lead families from 3 manufacturers were used, namely, Quartet (n=189, St. Jude Medical, Sylmar, CA), Attain Performa (n=87, Medtronic Inc, Minneapolis, MN), and Acuity X4 (n=11, Boston Scientific, Marlborough, MA). Compared with non‐QUAD leads, Quartet leads (aHR: 0.36, 95% CI, 0.21–0.6; sample size: 560 non‐QUAD and 189 Quartet leads) as well as the Attain Performa leads (aHR: 0.11, 95% CI, 0.03–0.45; sample size: 560 non‐QUAD and 87 Attain Performa) were associated with lower total mortality. Comparison of Quartet (n=189) with Attain Performa (n=87) revealed no difference in total mortality (Quartet HR: 3.06, 95% CI, 0.70–13.38). Boston Scientific Acuity X4 leads were excluded from these analysis because of the small numbers involved (n=11).
Discussion
In this study, we have compared clinical outcomes after CRT using QUAD and non‐QUAD, programmed to biventricular, single‐site LV pacing. Several findings have emerged. First, QUAD was associated with a 68% lower total mortality. Second, QUAD was associated with a marked reduction in cardiac mortality (by 64%) and in HF hospitalization (by 38%). Third, QUAD was associated with a lower mortality from pump failure, while no differences emerged in sudden cardiac death. Fourth, HF cause did not impact on the superior outcomes of QUAD over non‐QUAD. Fifth, QUAD was superior to non‐QUAD after both CRT‐D and CRT‐P. Sixth, no group differences emerged in implant complications, but QUAD was associated with fewer re‐interventions for LV lead displacement or PNS. Seventh, re‐interventions for LV displacement or PNS predicted total mortality, cardiac mortality, and HF hospitalization.
Mortality
A recent retrospective study comparing QUAD with bipolar leads showed no difference in survival at 12 months (mean follow‐up 256 days for QUAD).15 In contrast, a US‐wide study based on data from device implant records and telemonitoring showed that CRT‐D using QUAD was associated with a better survival than CRT‐D using bipolar leads.13 Observational data from 3 centers in the United Kingdom showed similar findings.12
Our annualized total mortality rate for non‐QUAD (10.9%) is comparable to that found in randomized, controlled trials using non‐QUAD, which amounted to 9.7% in CARE‐HF (Cardiac Resynchronization Heart Failure)18 and 15% in COMPANION (Comparison of Medical Therapy, Pacing and Defibrillation in heart failure)19, 20 after CRT‐P. In the CRT‐D arm of COMPANION, the annualized total mortality rate was 12%.20 In contrast, the annualized total mortality rate in the present study was 3.6% with QUAD.
HF Hospitalization
This is the first study to explore HF hospitalization after CRT using QUAD. Survival free from cardiovascular hospitalization at 1 and 2 years with QUAD was 94% and 91%, respectively. Among the few studies to address the long‐term effects of CRT on HF hospitalizations in the non‐QUAD LV lead era, van Bommel et al found survival free from cardiovascular hospitalization at 1 and 2 years was 80% and 70%, respectively.21
The reasons for reduced HF hospitalizations with QUAD are not entirely clear. Several cofounders, however, could potentially explain our findings. In the telemonitoring study of Turakhia et al, potential confounders for a benefit of QUAD was limited to age, sex, remote monitoring enrollment, and socioeconomic status.13 Behar et al did not adjust for NYHA class, QRS duration, left ventricular ejection fraction, HF medication, or comorbidities.12 In the present study, which comprises a longer follow‐up period, the survival advantage of QUAD versus bipolar LV leads was observed after adjustment for age, sex, device type (CRT‐P or CRT‐D), NYHA class, QRS duration, QRS morphology, left ventricular ejection fraction, HF cause, medication, or history of hypertension, coronary artery bypass grafting, or diabetes mellitus.
Lower rates of LV lead re‐interventions may also be relevant. In this respect, we observed that no LV lead revision led to death and that LV lead re‐interventions were lower for QUAD than for non‐QUAD. On the other hand, LV lead re‐interventions were associated with an increased risk of total mortality, cardiac mortality, and HF hospitalization. These findings suggest that LV lead deactivation and the associated re‐intervention contributed to a higher risk of HF hospitalization. This, however, does not explain the lower risk of total mortality observed with QUAD. It would appear that the survival benefit of QUAD relates to the lead itself.
CRT‐D and CRT‐P
Previous studies on QUAD12, 13 have exclusively focused on CRT‐D. We have shown better outcomes for QUAD after both CRT‐D and CRT‐P. In fact, the magnitude of the survival benefit of QUAD over non‐QUAD after CRT‐P (by 84%) was higher than after CRT‐D (by 56%). While we should be careful with overinterpreting the results of a retrospective study, such marked differences raise the possibility that the benefit of QUAD is proportionally higher after CRT‐P than after CRT‐D. If that is the case, we should reconsider the findings of COMPANION, which was underpowered to compare CRT‐D and CRT‐P. At the low event rates observed in the present study, proof of superiority of CRT‐D over CRT‐P may require much higher numbers of patients than those included in COMPANION. Meta‐analyses of CRT‐D versus CRT‐P may need to be revisited in the QUAD era.
LV Lead Re‐Interventions
In the MORE‐CRT (More Options Available With a Quadripolar LV Lead Provide In‐Clinic Solutions to CRT Challenges) trial, 1074 patients undergoing CRT‐D were randomized in 1:2 ratio to bipolar leads or QUAD. Freedom from the composite end point of intraoperative and postoperative LV lead–related events at 6 months was greater with QUAD than with bipolar leads (83.0% versus 74.4%, P=0.0002), but this was because of differences in the intraoperative rather than postoperative events.10 In the present study, which involved a longer follow‐up period, QUAD was associated with a lower incidence of PNS and LV lead displacement. This might be expected in view of the fact that vector optimization almost invariably eliminates PNS in patients who initially have PNS with QUAD.12
HF Cause
In the QUAD era, Forleo et al22 showed that the cause of cardiomyopathy did not influence the LV reverse remodeling response to CRT‐D using QUAD. Similarly, Behar et al found no interaction between HF cause and the survival benefit of CRT‐D using QUAD, compared with bipolar leads.12 We have also found that HF cause has no bearing on survival benefit of QUAD over non‐QUAD. Importantly, however, the event rate with QUAD is much lower than with non‐QUAD. It is possible that with QUAD, higher numbers of patients are needed to show a HF cause‐specific difference in outcomes after CRT.
Single‐Site and Multipoint Pacing
In this study, multipoint pacing was not activated in any patient, suggesting that the survival advantage of QUAD is simply because of lead design or availability of multiple pacing vector configurations. We should also consider that electrical stimulation over a dipole in a QUAD could depolarize the myocardium at the anodal pole if this has a comparatively low threshold.23 We cannot determine whether anodal capture (and effectively multipoint pacing) could account for some of the observed effects of QUAD. These questions could not be addressed in the present study.
Limitations
This study has the typical limitations of a single‐center, nonrandomized, retrospective study and therefore we cannot discount the possible influence of unobserved variables. While we adjusted for potential confounders, only randomization could fully correct for their biological effects. In particular, we should stress that, despite covariate adjustment, the better outcomes observed with QUAD might be because, at least in part, of different patient characteristics towards the end of the recruitment period, rather than primarily or uniquely to the utilization of QUAD. The greater proportion of patients in NYHA class I and II and in sinus rhythm as well as higher uptake of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers and β‐blockers may still contribute to better outcomes with QUAD. Moreover, we cannot exclude the possibility that the time interval from actual LV lead displacement (deactivation) to re‐intervention may have adversely influenced outcomes. As we did not use telemonitoring, we cannot quantify the duration of LV lead deactivation before re‐intervention. A further possibility is that allowing programming over a wider range of vectors, QUAD could have converted nonresponders to responders.24 Unfortunately, the present study does not address vector locations or how vector configurations changed during follow‐up. It is hoped that ongoing prospective studies25 may shed further light on this issue. We did not collect data as on Q‐LV as an aid for targeting LV lead positions, but it is possible that this approach could influence outcomes.
Conclusions
In this study of real‐world clinical practice, we have shown that CRT using QUAD, programmed to biventricular, single‐site LV pacing, was associated with a dramatic reduction in total mortality, cardiac mortality, and HF hospitalization, compared with non‐QUAD. These findings emerged after both CRT‐D and CRT‐P, after adjustment for HF etiology and other potential confounders. The remarkably low event rate observed with QUAD in this study has implications for clinical practice and the design of future CRT trials.
Disclosures
Leyva is a consultant and has received research support from Medtronic Inc, St Jude Medical, Boston Scientific, and LivaNova. Marshall is a consultant for Spectranetics. The other authors report no conflicts of interest.
(J Am Heart Assoc. 2017;6:e007026 DOI: 10.1161/JAHA.117.007026.)
References
- 1. Leyva F, Nisam S, Auricchio A. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol. 2014;64:1047–1058. [DOI] [PubMed] [Google Scholar]
- 2. Daubert JC, Ritter P, Le Breton H, Gras D, Leclercq C, Lazarus A, Mugica J, Mabo P, Cazeau S. Permanent left ventricular pacing with transvenous leads inserted into the coronary veins. Pacing Clin Electrophysiol. 1998;21:239–245. [DOI] [PubMed] [Google Scholar]
- 3. Auricchio A, Klein H, Tockman B, Sack S, Stellbrink C, Neuzner J, Kramer A, Ding J, Pochet T, Maarse A, Spinelli J. Transvenous biventricular pacing for heart failure: can the obstacles be overcome? Am J Cardiol. 1999;83:136D–142D. [DOI] [PubMed] [Google Scholar]
- 4. Sperzel J, Danschel W, Gutleben KJ, Kranig W, Mortensen P, Connelly D, Trappe HJ, Seidl K, Duray G, Pieske B, Stockinger J, Boriani G, Jung W, Schilling R, Saberi L, Hallier B, Simon M, Rinaldi CA. First prospective, multi‐centre clinical experience with a novel left ventricular quadripolar lead. Europace. 2012;14:365–372. [DOI] [PubMed] [Google Scholar]
- 5. Alonso C, Leclercq C, d'Allonnes FR, Pavin D, Victor F, Mabo P, Daubert J‐C. Six year experience of transvenous left ventricular lead implantation for permanent biventricular pacing in patients with advanced heart failure: Technical aspects. Heart. 2001;86:405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. León AR, Abraham WT, Curtis AB, Daubert JP, Fisher WG, Gurley J, Hayes DL, Lieberman R, Petersen‐Stejskal S, Wheelan K. Safety of transvenous cardiac resynchronization system implantation in patients with chronic heart failure: combined results of over 2,000 patients from a multicenter study program. J Am Coll Cardiol. 2005;46:2348–2356. [DOI] [PubMed] [Google Scholar]
- 7. Gras D, Böcker D, Lunati M, Wellens HJJ, Calvert M, Freemantle N, Gervais R, Kappenberger L, Tavazzi L, Erdmann E, Cleland JGF, Daubert J‐C. Implantation of cardiac resynchronization therapy systems in the care‐hf trial: Procedural success rate and safety. Europace. 2007;9:516–522. [DOI] [PubMed] [Google Scholar]
- 8. Cheng A, Wang Y, Curtis JP, Varosy PD. Acute lead dislodgements and in‐hospital mortality in patients enrolled in the national cardiovascular data registry implantable cardioverter defibrillator registry. J Am Coll Cardiol. 2010;56:1651–1656. [DOI] [PubMed] [Google Scholar]
- 9. van Rees JB, de Bie MK, Thijssen J, Borleffs CJW, Schalij MJ, van Erven L. Implantation‐related complications of implantable cardioverter‐defibrillators and cardiac resynchronization therapy devices: a systematic review of randomized clinical trials. J Am Coll Cardiol. 2011;58:995–1000. [DOI] [PubMed] [Google Scholar]
- 10. Boriani G, Connors S, Kalarus Z, Lemke B, Mullens W, Osca Asensi J, Raatikainen P, Gazzola C, Farazi TG, Leclercq C. Cardiac resynchronization therapy with a quadripolar electrode lead decreases complications at 6 months. J Am Coll Cardiol. 2016;2:212–220. [DOI] [PubMed] [Google Scholar]
- 11. Forleo GB, Mantica M, Di Biase L, Panattoni G, Della Rocca DG, Papavasileiou LP, Santamaria M, Santangeli P, Avella A, Sergi D, Santini L, Tondo C, Natale A, Romeo F. Clinical and procedural outcome of patients implanted with a quadripolar left ventricular lead: early results of a prospective multicenter study. Heart Rhythm. 2012;9:1822–1828. [DOI] [PubMed] [Google Scholar]
- 12. Behar JM, Bostock J, Zhu Li AP, Chin HM, Jubb S, Lent E, Gamble J, Foley PW, Betts TR, Rinaldi CA, Herring N. Cardiac resynchronization therapy delivered via a multipolar left ventricular lead is associated with reduced mortality and elimination of phrenic nerve stimulation: long‐term follow‐up from a multicenter registry. J Cardiovasc Electrophysiol. 2015;26:540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turakhia MP, Cao M, Fischer A, Nabutovsky Y, Sloman LS, Dalal N, Gold MR. Reduced mortality associated with quadripolar compared to bipolar left ventricular leads in cardiac resynchronization therapy. JACC Clin Electrophysiol. 2016;2:426–433. [DOI] [PubMed] [Google Scholar]
- 14. Corbisiero R, Kazemian P, Bharmi R, Shah R, Muller D. Less with more: hospitalization cost and event rates with quadripolar versus bipolar CRT‐D system. Pacing Clin Electrophysiol. 2016;39:1038–1045. [DOI] [PubMed] [Google Scholar]
- 15. Rijal S, Wolfe J, Rattan R, Durrani A, Althouse AD, Marroquin OC, Jain S, Mulukutla S, Saba S. Lead related complications in quadripolar versus bipolar left ventricular leads. Indian Pacing Electrophysiol J. 2017;17:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Myerburg RJ, Castellanos A. Cardiac arrest and sudden cardiac death In: Braunwald E, ed. Heart Disease: A Textbook of Cardiovascular Medicine. New York: WB Saunders Publishing Co; 1997:742–779. [Google Scholar]
- 17. Rockman HA, Juneau C, Chatterjee K, Rouleau JL. Long‐term predictors of sudden and low output death in chronic congestive heart failure secondary to coronary artery disease. Am J Cardiol. 1989;64:1344–1348. [DOI] [PubMed] [Google Scholar]
- 18. Cleland J, Daubert J, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 19. Bristow M, Saxon L, Boehmer J, Krueger S, Kass D, De Marco T, Carson P, DiCarlo L, DeMets D, White B. Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. [DOI] [PubMed] [Google Scholar]
- 20. Colquitt J, Mendes D, Clegg A, Harris P, Cooper K, Picot J, Bryant J. Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure: systematic review and economic evaluation. Health Technol Assess. 2014;18:1–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Bommel RJ, Borleffs CJ, Ypenburg C, Marsan NA, Delgado V, Bertini M, van der Wall EE, Schalij MJ, Bax JJ. Morbidity and mortality in heart failure patients treated with cardiac resynchronization therapy: influence of pre‐implantation characteristics on long‐term outcome. Eur Heart J. 2010;31:2783–2790. [DOI] [PubMed] [Google Scholar]
- 22. Forleo GB, Di Biase L, Della Rocca DG, Panattoni G, Mantica M, Santamaria M, Pappalardo A, Panigada S, Santini L, Natale A, Romeo F. Impact of previous myocardial infarction on outcomes of CRT patients implanted with a quadripolar left ventricular lead. Results from a multicentric prospective study. Int J Cardiol. 2012;160:145–146. [DOI] [PubMed] [Google Scholar]
- 23. Trolese L, Biermann J, Hartmann M, Schluermann F, Faber TS, Bode C, Asbach S. Haemodynamic vector personalization of a quadripolar left ventricular lead used for cardiac resynchronization therapy: use of surface electrocardiogram and interventricular time delays. Europace. 2014;16:1476–1481. [DOI] [PubMed] [Google Scholar]
- 24. Umar F, Taylor RJ, Stegemann B, Marshall H, Flannigan S, Lencioni M, De Bono J, Griffith M, Leyva F. Haemodynamic effects of cardiac resynchronization therapy using single‐vein, three‐pole, multipoint left ventricular pacing in patients with ischaemic cardiomyopathy and a left ventricular free wall scar: the maestro study. Europace. 2016;18:1227–1234. [DOI] [PubMed] [Google Scholar]
- 25. Leclercq C. More response on cardiac resynchronization therapy with multipoint pacing (MORE‐CRT MPP). 2015. Available at: https://www.clinicaltrials.gov. Accessed August 28, 2017.


