Abstract
Background
The safety of testosterone supplements in men remains unclear. In the present study, we tested the hypothesis that in young and old male spontaneously hypertensive rats (SHR), long‐term testosterone supplements increase blood pressure and that the mechanism is mediated in part by activation of the renin‐angiotensin system.
Methods and Results
In untreated males, serum testosterone exhibited a sustained decrease after 5 months of age, reaching a nadir by 18 to 22 months of age. The reductions in serum testosterone were accompanied by an increase in body weight until very old age (18 months). Testosterone supplements were given for 6 weeks to young (12 weeks‐YMSHR) and old (21–22 months‐OMSHR) male SHR that increased serum testosterone by 2‐fold in young males and by 4‐fold in old males. Testosterone supplements decreased body weight, fat mass, lean mass, and plasma leptin, and increased plasma estradiol in YMSHR but had no effect in OMSHR. Mean arterial pressure (MAP) was significantly higher in OMSHR than in YMSHR and testosterone supplements decreased MAP in OMSHR, but significantly increased MAP in YMSHR. Enalapril, the angiotensin‐converting enzyme inhibitor, reduced MAP in both control and testosterone‐supplemented YMSHR, but had a greater effect on MAP in testosterone‐treated rats, suggesting the mechanism responsible for the increase in MAP in YMSHR is mediated at least in part by activation of the renin‐angiotensin system.
Conclusions
Taken together with previous studies, these data suggest that testosterone supplements may have differential effects on men depending on age, cardiovascular and metabolic status, and dose and whether given long‐term or short‐term.
Keywords: aging, hypertension, leptin, testosterone
Subject Categories: Hypertension
Clinical Perspective
What Is New?
The effect of testosterone supplements on blood pressure in hypertensive animals is age‐dependent.
While in young adult male spontaneously hypertensive rats testosterone supplements have a positive effect to decrease fat mass and body weight, they increase blood pressure.
In contrast, in old male spontaneously hypertensive rats, testosterone has no effect on the lean mass, fat mass, or body weight, but decreases blood pressure.
What Are the Clinical Implications?
This study highlights the fact that in a specific population such as hypertensive middle‐aged men, testosterone supplements could have adverse cardiovascular consequences and caution should be taken in prescribing them.
In contrast, in men of advancing age, testosterone supplements may provide maintenance of body weight and mass and actually reduce cardiovascular risk factors such as hypertension.
Introduction
Androgen supplementation as a therapeutic option for hypogonadal men is very common. In addition, increasing numbers of aging men are taking testosterone supplements to improve libido, protect against bone loss (osteoporosis), and to improve feelings of well‐being.1 In obese patients with hypogonadism, androgen supplementation also improves body weight, lipid profile, and insulin resistance.2
However, the safety of androgen supplementation is still not clear since it could lead to hypertension, a major risk factor for myocardial infarction and stroke.3, 4, 5, 6 The role of endogenous androgens and cardiovascular disease is also not clear since young men have higher blood pressure and greater risk of developing myocardial infarction than matched‐age women.7 Recently, 2 clinical studies showed that testosterone supplementation increased the mortality rate attributable to cardiovascular disease.6, 8 Men who used anabolic steroids, a synthetic derivative of testosterone, had a higher risk of hypertension, ventricular remodeling, and sudden cardiac death.9 In contrast, men who had ST‐segment depression during a stress test were improved with testosterone supplements due to the short‐term vasodilator action of androgen supplements.10 Therefore, whether testosterone supplements have beneficial or deleterious cardiovascular effects still remains controversial and may depend on the preparation and the length of treatment.
In recent years we have evaluated the effect of androgens in male animal models. We reported that long‐term androgen supplementation in obese male Zucker rats caused an increase in blood pressure, but improved other cardiovascular risk factors, such as metabolic syndrome and inflammation.11 In contrast, in young male spontaneously hypertensive rats (SHR), we found that endogenous androgens play a role in the regulation of blood pressure since the hypertension was attenuated when the rats were castrated.12 The mechanisms responsible for the elevated blood pressure in young intact male SHR include activation of the renin‐angiotensin system (RAS) and activation of the sympathetic nervous system. We also showed that renal denervation reduces blood pressure in young adult male SHR.13 We and others reported that intracerebroventricular blockade of the melanocortin 4 receptor in young and old male SHR reduces their blood pressure, but has no effect on either young or old females,14, 15 showing a sex difference in this pressor mechanism that may be related to androgens since the old males are not hypogonadal. Finally, we showed that tempol, the superoxide dismutase mimetic, reduced blood pressure in young male SHR, but not young females when begun after puberty,16 also showing a sex difference. As mentioned, these sex differences may be related to the levels of androgens since castration of male SHR attenuates the blood pressure to levels found in females.12 These data suggest that testosterone, whether endogenous or exogenous, plays a role in the regulation of blood pressure in males, but the mechanisms need to be further evaluated.
In the present study, we tested the hypothesis that in old and young male SHR, long‐term testosterone supplements increase blood pressure and that the mechanism is mediated in part by activation of the RAS.
Material and Methods
All of the protocols complied with the Guidelines for the Care and Use of Laboratory Animals by the National Institutes of Health and were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Young male SHR were obtained at 10 weeks of age from the vendor (Harlan, Indianapolis, IN), and maintained in 12 hour:12 hour light:dark cycle with ad libitum access to diet (Envigo Tecklad 22/5 Rodent Diet, cat.# 8640, Madison, WI) and tap water. Rats were aged to 4 months (young) or 18 to 22 months (old). They were randomly divided into 4 groups (n=6/group): control young or old males that were implanted with silastic placebo pellets; young or old males that were implanted with silastic testosterone pellets, as described below (old male SHR with placebo—OMSHR+P or testosterone—OMSHR+T, n=6; and young male SHR with placebo—YMSHR+P or testosterone—YMSHR+T; respectively). Throughout the testosterone treatment period, food and water intake were measured weekly.
Testosterone Supplement and Plasma Testosterone Levels
Silastic implants (10 mm, ID: 0.062 in; OD: 0.125 in; Dow Chemical Co, Midland, MI) were packed with testosterone propionate (8 mg/pellet; Sigma, St Louis, MO) and sealed with silastic glue. Pellets were soaked overnight in 70% ethanol before implantation. Rats were implanted with testosterone pellets at ages either 16 weeks (young males) or 18 to 22 months (old males). Pellets were changed once at 4 weeks, and rats were studied 2 weeks later.
Measurement of Metabolic Parameters
Testosterone levels were measured in untreated rats at various ages (3, 5, 7, 8, 9, 12, 14, 18, and 22 months) and in rats, aged 22 weeks or 18 to 22 months receiving testosterone pellets or controls, in serum drawn from the jugular vein with rats under isoflurane anesthetic, and using a commercially available radioimmunoassay kit (Coat‐A‐Count testosterone kit; Diagnostic Products Corporation, Los Angeles, CA), as we have described previously.11 Plasma estradiol was measured at the end of the experiment by Ultrasensitive Estradiol Kit (Diagnostic Systems Laboratories, Inc, Webster, TX), according to manufacturer's recommendations, as we previously described.17 Plasma leptin was measured by commercially available kits (leptin: Linco Research, St Charles, MO), as described by the manufacturer.15
Body Composition by ECHO MRI
In young and old male SHR (n=6/group), body composition including total body fat mass, lean mass, and total body water, were measured by ECHO‐MRI (41‐900 model Body Composition Analyzer, ECHO MRI, Houston, TX), according to manufacturer's directions. Data are presented in grams and total body adipose fat was factored for body weight in all animals and expressed as a percentage.
Measurement of Blood Pressure
After 4 weeks of testosterone supplementation in young or old males, radiotelemeters (Model: HD‐S10; DSI, Minneapolis, MN) were implanted, as we have described previously.18 After a 2‐week recovery period, baseline mean arterial pressure (MAP) was measured for 5 days and recorded.
Angiotensin‐Converting Enzyme Inhibitor Treatment
To determine whether the pressor effect of testosterone supplements was mediated through the RAS, young male SHR (n=6), implanted with testosterone supplements or placebo pellets, were treated with enalapril (angiotensin‐converting enzyme inhibitor, 250 mg/L drinking water) and MAP measured for 7 days.
Statistical Analyses
All results are expressed as mean±SEM. Multiple groups were analyzed by 2‐way ANOVA followed by Student‐Newman‐Keul's comparisons. Time series blood pressure data were analyzed by repeated‐measures 2‐way ANOVA followed by Student‐Newman‐Keul's comparisons. Differences were considered statistically significant at P<0.05. Statistical analyses were performed with SigmaStat software package version 3.1 (Systat Software, San Jose, CA).
Results
Plasma Testosterone Levels and Body Weight
Figure 1A shows that endogenous serum testosterone levels began to fall by 7 months of age in male SHR, but were maintained fairly constant through 14 months of age. By 18 months of age, testosterone levels were reduced by 8‐fold compared with YMSHR at 3 and 5 months. This reduction in testosterone from 7 to 14 months is accompanied by an increase in body weight (Figure 1B). By 14 months of age, male SHR reached a plateau in body weight (Figure 1B). However, by 22 months of age, the body weight was decreased to levels not different from 5‐month‐old males.
Figure 1.
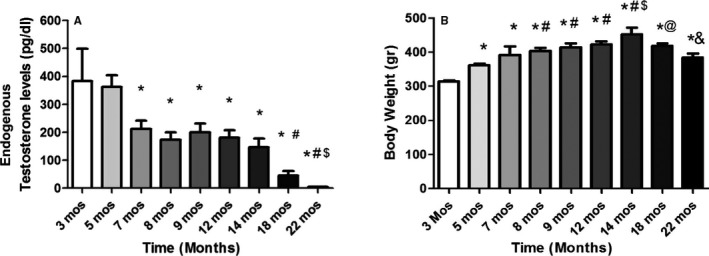
Serum testosterone and body weight in aging rats. A, Endogenous serum testosterone levels decrease with age in male SHR, (n=6/group). *P<0.05 compared with rats aged 3 and 5 mo; # P<0.05, compared with rats, aged 7 to 14 mo; $ P<0.05, compared with rats aged 18 mo. B, Body weight increases with aging until 18 mo of age or later (n=6/group). *P<0.05, compared with rats, aged 3 mo; # P<0.05 compared with rats aged 5 mo; $ P<0.05, compared with rats, aged 7–12 mo; @ P<0.05 vs 14 mo; & P<0.05 vs 18 mo. SHR indicates spontaneously hypertensive rats.
Testosterone levels were reduced by almost 90% in old male controls compared with young male controls. Testosterone supplements increased serum testosterone by 2‐fold in YMSHR‐T compared with YMSHR‐P, and by 4‐fold in OMSHR‐T compared with OMSHR‐P (Figure 2A). Testosterone supplements caused a reduction in body weight in YMSHR‐T compared with YMSHR‐P but had no effect on body weight in OMSHR‐T compared with OMSHR‐P (Figure 2B).
Figure 2.
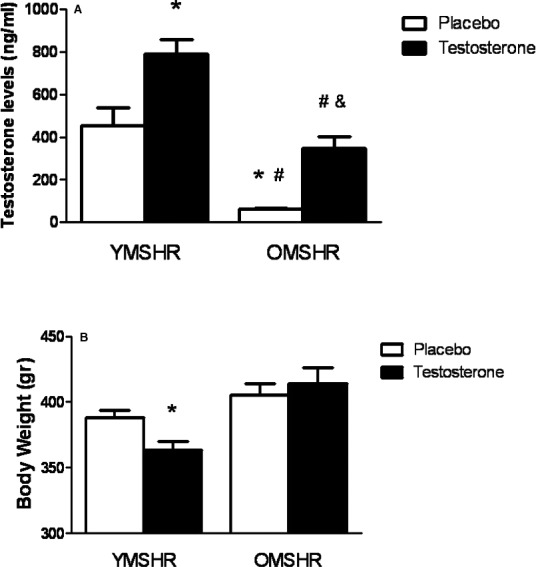
Serum testosterone and body weight in young and old male rats treated with testosterone supplements. A, Testosterone supplements increase serum testosterone by 2‐fold in young male SHR (YMSHR‐T), but by 4‐fold in old male SHR (OMSHR‐T). *P<0.05 vs YMSHRP; # P<0.05 vs YMSHR‐T; & P<0.05 vs OMSHR‐P. B, Testosterone supplements decreased body weight in YMSHR‐T but not OMSHR‐T. *P<0.05 vs placebo treated rats. OMSHR‐P indicates old male SHR with placebo; OMSHR‐T, old male SHR with testosterone; SHR, spontaneously hypertensive rats; YMSHR‐P, young male SHR with placebo; YMSHR‐T, young male SHR with testosterone.
Fat and Lean Mass With Testosterone Supplements
Fat and lean mass were measured by ECHO MRI. As shown in Figure 3, OMSHR had more than 2‐fold higher fat mass than in YMSHR. Testosterone supplements had no effect on either fat mass or lean mass in OMSHR‐T (Figure 3C and 3D), but reduced fat mass and lean mass in YMSHR‐T (Figure 3A and 3B).
Figure 3.
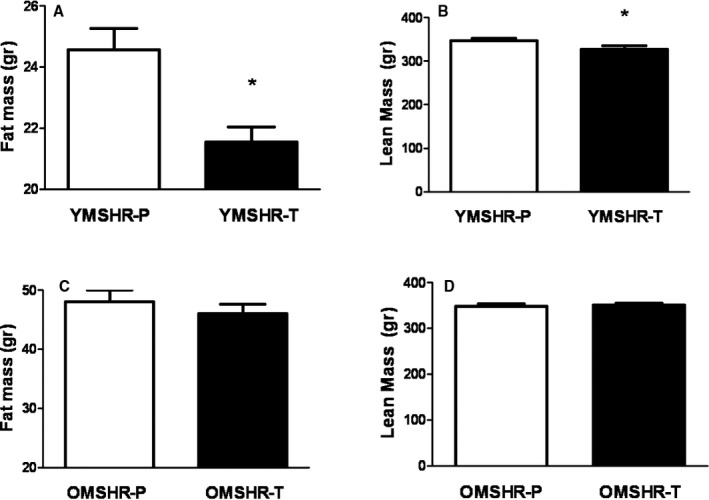
Age‐related differences in fat mass and lean mass in young and old males given testosterone supplements. Testosterone supplements reduced fat mass (A) and lean mass (B) in young male spontaneously hypertensive rats (SHR)—testosterone (YMSHR‐T) compared with controls young male SHR—placebo (YMSHR‐P), but had no effect on either lean mass (C) or fat mass (D) in old male SHR—testosterone (OMSHR‐T). *P<0.05 vs YMSHR‐P.
Estradiol and Leptin
Because testosterone is converted to estradiol by aromatase, we measured the level of estradiol in these animals. In YMSHR‐T, estradiol levels were significantly higher than in OMSHR (Figure 4A). Testosterone supplements increased plasma estradiol by 3‐fold in YMSHR but had no effect on estradiol on OMSHR.
Figure 4.
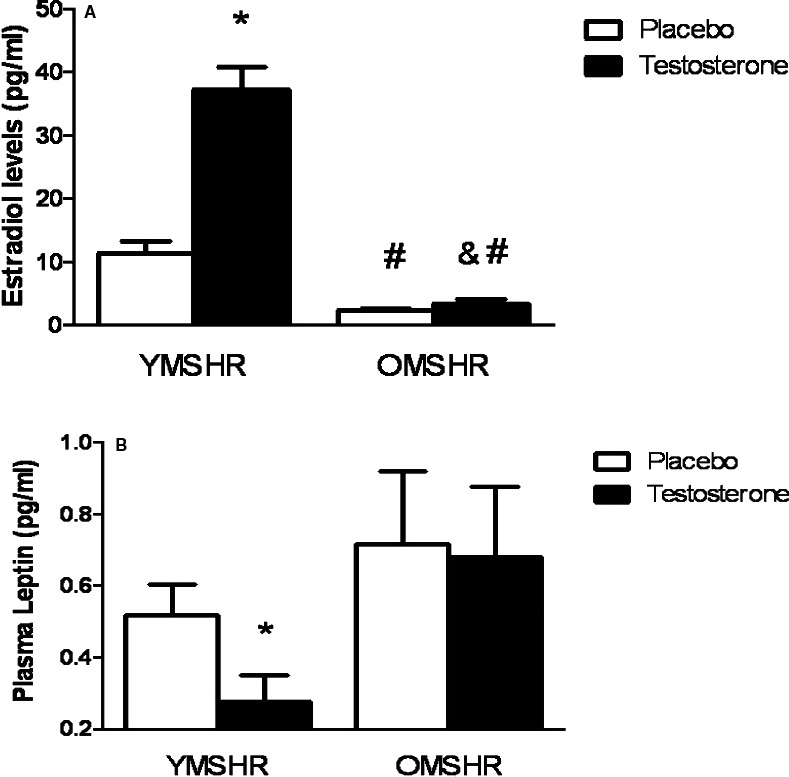
Plasma estradiol and leptin levels in young and old male SHR with or without testosterone supplements. A, Testosterone supplements increased plasma estradiol in young male SHR (YMSHR) but had no effect in old male SHR (OMSHR). *P<0.05 vs YMSHR‐P (placebo); # P<0.05 vs YMSHR‐T (testosterone). B, Testosterone supplements reduced plasma leptin in YMSHR but had no effect on OMSHR. *P<0.05 vs YMSHR‐P. SHR indicates spontaneously hypertensive rats.
As shown in Figure 4B, plasma leptin levels tended to be higher in OMSHR‐P than YMSHR‐P, but the differences were not significant. Along with the reduction in fat mass and body weight, testosterone supplements reduced plasma leptin in YMSHR but failed to affect plasma leptin in OMSHR‐T.
Mean Arterial Pressure
Mean arterial pressure was significantly higher in OMSHR than YMSHR (OMSHR: 166±7 mm Hg; YMSHR: 148±0.5 mm Hg, n=6, P<0.05, Figure 5A). In YMSHR‐T, testosterone supplements significantly increased MAP compared with YMSHR‐P (Figure 5B). However, in OMSHR‐T, testosterone supplements significantly attenuated MAP (Figure 5C).
Figure 5.
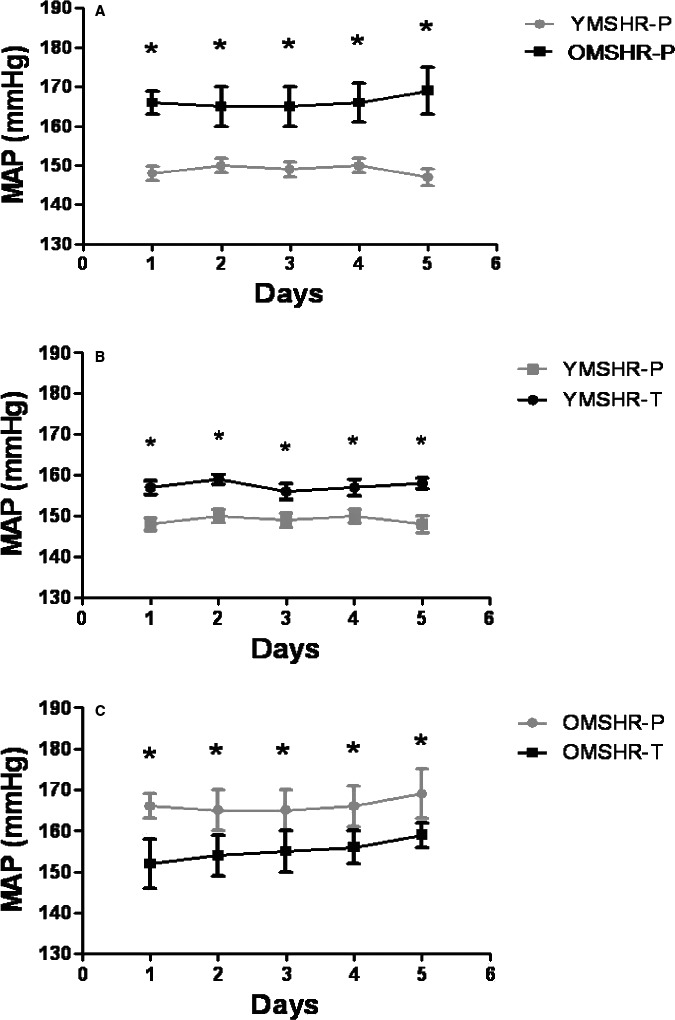
Mean arterial pressure (MAP) in young and old male SHR with and without testosterone supplements. MAP was higher in old male SHR—placebo (OMSHR‐P) than young male SHR—placebo (YMSHR‐P, A). Testosterone supplements increased MAP in young male SHR—testosterone (YMSHR‐T) compared with YMSHR‐P (B), but reduced MAP in old male SHR—testosterone (OMSHR‐T) compared with OMSHR‐P (C). A, *P<0.05 for OMSHR‐P vs YMSHR‐P; B, *P<0.05, OMSHR‐P vs OMSHR‐T; C, *P<0.05, YMSHR‐T vs YMSHR‐P. SHR indicates spontaneously hypertensive rats.
Depressor Response to Enalapril
In order to determine the mechanism by which testosterone supplements increased blood pressure in YMSHR‐T, rats were treated with enalapril, the angiotensin I‐converting enzyme inhibitor. As shown in Figure 6, enalapril reduced MAP in both YMSHR‐P and YMSHR‐T, but had a greater effect on MAP in YMSHR‐T, suggesting that androgens stimulated the RAS to increase the blood pressure in YMSHR.
Figure 6.
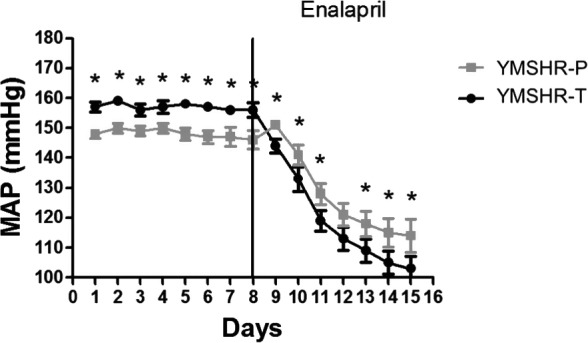
Enalapril reduced mean arterial pressure (MAP) in both young male SHR—placebo (YMSHR‐P) and young male SHR—testosterone (YMSHR‐T), but had a greater effect in YMSHR‐T. *P<0.05, vs YMSHR‐T. SHR indicates spontaneously hypertensive rats.
Discussion
The main findings of this study are the following: (1) Aging is accompanied by a decrease in endogenous testosterone and an increase in body weight in male SHR; (2) Testosterone supplementation reduced body weight and fat mass but increased blood pressure in young male SHR; (3) In contrast, testosterone supplementation causes a reduction of blood pressure with no effect on either body weight or fat mass in OMSHR; (4) The RAS likely contributes to the testosterone‐mediated increase in blood pressure in young male SHR, but the mechanism by which androgens in old males attenuates hypertension remains to be determined.
Aging in men is accompanied by a decrease in endogenous testosterone, and is associated with an increase in body weight, visceral fat, dyslipidemia, hypertension, and other cardiovascular risk factors.8 As a part of the treatment for this condition, the use of testosterone supplements has been on the rise around the world, with several reports indicating that this treatment improved lifestyle, libido, sexual performance, and muscle strength.19 However, recent evidence also showed that testosterone supplements in aging men are associated with increased mortality as a consequence of cardiovascular events.8 These data open questions regarding the safe use of testosterone replacement therapy, particularly in aging men.
In our study, male rats exhibited an increase in body weight until 14 months of age (middle age) followed by a reduction in body weight with advanced aging. Testosterone levels were highest at 3 to 5 months of age, and then decreased and plateaued from 7 to 14 months of age. By 18 months of age, testosterone levels were significantly reduced by 90% compared with the levels in young males. Testosterone supplements in young male SHR reduced body weight, fat mass, and plasma leptin levels. In aging male SHR, while body weight was higher, testosterone supplements had no effect on body weight, fat mass, or plasma leptin levels. Most studies in hypogonadal men show that testosterone supplements increase lean mass and decrease fat mass.20, 21 Our young males were not hypogonadal when they were treated with testosterone, and this may explain why we did not see an increase in lean mass but did see a reduction in fat mass.
Importantly, our study showed that testosterone supplements increased the blood pressure in young male SHR. This finding is not surprising since we showed previously that castration reduces blood pressure in male SHR.12 Moreover, castration at 8 months of age, when androgen levels are still above hypogonadal range, prevented renal injury by 18 months of age, with a reduction in blood pressure of ≈20 mm Hg.22 Castration of male Dahl salt‐sensitive rats also attenuates the pressor response to high salt diet,23 although castration alone did not affect the baseline blood pressure. In other studies, testosterone supplements in male obese Zucker rats caused improvement in metabolic syndrome parameters, such as reduced insulin, leptin, cholesterol, and inflammation, but caused an increase in blood pressure.11 In contrast to the young male SHR in our current study and these other studies, the obese Zucker rats are morbidly obese and hypogonadal and have low serum levels of testosterone (60% to 70% lower than lean Zucker littermates).11 Thus, the responses to testosterone supplements may be different in hypogonadal males than in testosterone‐sufficient males. In any case, our data suggest that in young men, especially men who are already hypertensive, blood pressure should be monitored closely during testosterone supplementation in order to prevent further cardiovascular disease events. Also, more data are needed to evaluate the effect of testosterone supplementation in aging men.
While the present studies evaluated the long‐term effects of androgen supplements, Perusquía et al showed that short‐term intravenous infusion of testosterone or testosterone derivatives caused a reduction in blood pressure in young (18–21 weeks) male SHR or WKY.24 The response was nongenomic and mediated through L‐type voltage‐operated Ca2+channels (L‐VOCC). These investigators also reported that castration increased systolic blood pressure over 10 weeks in Wistar and WKY rats, as measured by tail plethysmography.24 In addition, these investigators previously reported that short‐term testosterone supplements caused reductions in blood pressure that were neuronal nitric oxide synthase‐dependent.25 We have made similar observations with telemetry blood pressure when testosterone or dihydrotestosterone pellets are implanted in male or female rats; there is a transient reduction in blood pressure that returns back to baseline levels within 24 hours (Maranon RO, MD, PhD and Reckelhoff JF, PhD, 2016, unpublished data). Thus, in addition to the age of the individual, whether androgens are given long term or short term makes a difference in the blood pressure response.
In order to determine the mechanism by which the blood pressure was elevated with long‐term androgen supplements in young male SHR, we evaluated the depressor response to angiotensin‐converting enzyme inhibitor. We observed that enalapril caused a greater reduction in MAP in young males who received testosterone supplements, even decreasing blood pressure to a level lower than in untreated males, suggesting that testosterone increases blood pressure through a RAS‐mediated process. We showed in previous studies in SHR that enalapril could reduce the blood pressure in young adult intact males and females, or gonadectomized males or females.26 Thus, further studies are necessary for young and middle‐aged men to determine whether androgen supplements upregulate the RAS specifically, which could also increase the risk for cardiovascular disease.
Why testosterone supplements failed to increase but rather decreased the blood pressure in older male SHR is not clear. The mechanism is independent of improvement of metabolic parameters since the androgen supplements did not reduce fat mass, body weight, or plasma leptin levels in the old males. In addition to being different than in young male SHR, the data are also different than we previously found in obese Zucker rats.11 As noted above, testosterone supplements in obese Zucker rats did improve their metabolic parameters and renal inflammation but also increased their blood pressure significantly.
In the present study, since the old males showed a reduction in blood pressure with testosterone, it is not likely that the renal vasoconstrictor RAS was activated, and thus we did not evaluate the response to enalapril. It is also not likely that the mechanism responsible was mediated via testosterone‐mediated inhibition of the sympathetic nervous system since we have shown that blockade of the MC4R, which causes activation of the sympathetic nervous system, is important in blood pressure in both old and young male SHR.15 It is also not very likely that a reduction in oxidative stress with testosterone supplements impacted the blood pressure in old males, since we have shown that in order for a reduction in oxidative stress to reduce blood pressure, there must be nitric oxide present.27 With the long‐term hypertension and the age of these animals, it is likely that they have endothelial dysfunction and reduced nitric oxide, and thus even if testosterone reduced the oxidative stress, there would be little nitric oxide to reduce blood pressure.
It is possible that testosterone activated other systems in the kidney that produced a depressor effect, such as 20‐HETE in the tubules. We and others have shown that androgens increase 20‐HETE via activation of ω‐hydroxylases such as cytochrome P450 4A2.28, 29 If the increase in 20‐HETE occurs in the tubules rather than the vasculature, the result may be sodium excretion and a reduction in blood pressure. Future studies will be necessary to determine the mechanisms responsible for the attenuation of hypertension in old male SHR with testosterone supplements. In addition, whether these data with androgen supplements are applicable to aging men has also not been extensively studied to determine whether this is an anomaly in the aging male rat or whether it can be generalized to aging men as well.
In summary, testosterone supplements in young, nonhypogonadal male SHR cause an increase in blood pressure along with reductions in body weight, fat mass, and leptin levels. The elevated blood pressure is likely mediated by activation of the RAS since angiotensin‐converting enzyme reduces their blood pressure more than in placebo control males. In contrast, long‐term testosterone supplements in aging male SHR have no effect on body weight, fat mass, or leptin levels and decreases their blood pressure. Future studies are necessary to determine the mechanisms responsible for the attenuation of hypertension in aging male SHR, and whether these data can be generalized to aging men.
Sources of Funding
The authors acknowledge the support of the American Heart Association Scientific Development Awards #0830239N (Cardozo) and #12SDG8980032 (Romero), and NIH grants P20GM‐121334 (Cardozo) and R21 DK‐113500 (Romero) and American Heart Association Southeast Affiliate Postdoctoral Fellowship Award 14POST18640015 (Maranon).
Disclosures
None
Acknowledgments
The authors would like to thank Jane Reckelhoff for her critical reading of the article and Norma Ojeda for her statistical support.
(J Am Heart Assoc. 2017;6:e007074 DOI: 10.1161/JAHA.117.007074.)
References
- 1. Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens‐Shields AJ, Cauley JA, Gill TM, Barrett‐Connor E, Swerdloff RS, Wang C, Ensrud KE, Lewis CE, Farrar JT, Cella D, Rosen RC, Pahor M, Crandall JP, Molitch ME, Cifelli D, Dougar D, Fluharty L, Resnick SM, Storer TW, Anton S, Basaria S, Diem SJ, Hou X, Mohler ER III, Parsons JK, Wenger NK, Zeldow B, Landis JR, Ellenberg SS; Testosterone Trials Investigators . Effects of testosterone treatment in older men. N Engl J Med. 2016;374:611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Corona G, Giagulli E, Maseroli VA, Vignozzi L, Aversa A, Zitzmann M, Saad F, Mannucci E, Maggi M. Testosterone supplementation and body composition: results from a meta‐analysis of observational studies. J Endocrinol Invest. 2016;39:967–981. [DOI] [PubMed] [Google Scholar]
- 3. Basaria S, Harman SM, Travison TG, Hodis H, Tsitouras P, Budoff M, Pencina KM, Vita J, Dzekov C, Mazer NA, Coviello AD, Knapp PE, Hally K, Pinjic E, Yan M, Storer TW, Bhasin S. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low‐normal testosterone levels: a randomized clinical trial. JAMA. 2015;314:570–581. [DOI] [PubMed] [Google Scholar]
- 4. Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, Fraumeni JF Jr, Hoover RN. Infarction following testosterone therapy prescription in men. PLoS ONE. 2014;9:e85805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta‐analysis of placebo‐controlled randomized trials. BMC Med. 2013;11:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vigen R, O'Donnell CI, Barón AE, Grunwald GK, Maddox TM, Bradley SM, Barqawi A, Woning G, Wierman ME, Plomondon ME, Rumsfeld JS, Ho PM. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. [DOI] [PubMed] [Google Scholar]
- 7. Towfighi A, Zheng L, Ovbiagele B. Sex‐specific trends in midlife coronary heart disease risk and prevalence. Arch Intern Med. 2009;169:1762–1766. [DOI] [PubMed] [Google Scholar]
- 8. Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede‐Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frati P, Busardò FP, Cipolloni L, Dominicis ED, Fineschi V. Anabolic androgenic steroid (AAS) related deaths: autoptic, histopathological and toxicological findings. Curr Neuropharmacol. 2015;13:146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malkin CJ, Pugh PJ, Morris PD, Kerry KE, Jones RD, Jones TH, Channer KS. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004;90:871–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis DD, Ruiz AL, Yanes LL, Iliescu R, Yuan K, Moulana M, Racusen LC, Reckelhoff JF. Testosterone supplementation in male obese Zucker rats reduces body weight and improves insulin sensitivity but increases blood pressure. Hypertension. 2012;59:726–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reckelhoff JF, Granger JP. Role of androgens in mediating hypertension and renal injury. Clin Exp Pharmacol Physiol. 1999;26:127–131. [DOI] [PubMed] [Google Scholar]
- 13. Iliescu R, Yanes LL, Bell W, Dwyer T, Baltatu OC, Reckelhoff JF. Role of the renal nerves in blood pressure in male and female SHR. Am J Physiol Regul Integr Comp Physiol. 2006;290:341–344. [DOI] [PubMed] [Google Scholar]
- 14. da Silva AA, do Carmo JM, Kanyicska B, Dubinion J, Brandon E, Hall JE. Endogenous melanocortin system activity contributes to the elevated arterial pressure in spontaneously hypertensive rats. Hypertension. 2008; 51:884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maranon RO, Lima R, Mathbout M, do Carmo JM, Hall JE, Roman RJ, Reckelhoff JF. Postmenopausal hypertension: role of the sympathetic nervous system in an animal model. Am J Physiol Regul Integr Comp Physiol. 2014;306:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fortepiani LA, Reckelhoff JF. Role of oxidative stress in the sex differences in blood pressure in spontaneously hypertensive rats. J Hypertens. 2005;23:801–805. [DOI] [PubMed] [Google Scholar]
- 17. Sartori‐Valinotti JC, Venegas‐Ponta MR, LaMarca BB, Romero DG, Yanes LL, Racusend LC, Jonesa AV, Ryan MJ, Reckelhoff JF. Rosiglitazone reduces blood pressure in female Dahl salt‐sensitive rats. Steroids. 2010;75:794–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maranon RO, Reckelhoff JF. Mechanisms responsible for postmenopausal hypertension in a rat model: roles of the renal sympathetic nervous system and the renin‐angiotensin system. Physiol Rep. 2016; 4:pii: e12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang C, Swerdloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G, Matsumoto AM, Weber T, Berman N; Testosterone Gel Study Group . Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2839–2853. [DOI] [PubMed] [Google Scholar]
- 20. Allan CA, Strauss BJG, McLachlan RI. Body composition, metabolic syndrome and testosterone in ageing men. Int J Impot Res. 2007;19:448–457. [DOI] [PubMed] [Google Scholar]
- 21. Singh R, Artaza JN, Taylor WE, Gonzalez‐Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor‐mediated pathway. Endocrinology. 2003;144:5081–5508. [DOI] [PubMed] [Google Scholar]
- 22. Fortepiani LA, Yanes L, Zhang H, Racusen LC, Reckelhoff JF. Role of androgens in mediating renal injury in aging SHR. Hypertension. 2003;42:952–955. [DOI] [PubMed] [Google Scholar]
- 23. Yanes LL, Sartori‐Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone‐dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt‐sensitive rats. Am J Physiol Renal Physiol. 2009;296:771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perusquía M, Herrera N, Ferrer M, Stallone JN. Antihypertensive effects of androgens in conscious, spontaneously hypertensive rats. J Steroid Biochem Mol Biol. 2017;167:106–114. [DOI] [PubMed] [Google Scholar]
- 25. Perusquía M, Greenway CD, Perkins LM, Stallone JN. Systemic hypotensive effects of testosterone are androgen structure‐specific and neuronal nitric oxide synthase‐dependent. Am J Physiol Regul Integr Comp Physiol. 2015;309:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin‐angiotensin system. Hypertension. 2000;35:480–483. [DOI] [PubMed] [Google Scholar]
- 27. Yanes L, Romero D, Iliescu R, Cucchiarelli VE, Fortepiani LA, Santacruz F, Bell W, Zhang H, Reckelhoff JF. Systemic arterial pressure response to two weeks of Tempol therapy in SHR: involvement of NO, the RAS, and oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288:903–908. [DOI] [PubMed] [Google Scholar]
- 28. Dalmasso C, Maranon R, Patil C, Moulana M, Romero DG, Reckelhoff JF. 20‐HETE and CYP4A2 ω‐hydroxylase contribute to the elevated blood pressure in hyperandrogenemic female rats. Am J Physiol Renal Physiol. 2016;311:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakagawa K, Marji JS, Schwartzman ML, Waterman MR, Capdevila JH. Androgen‐mediated induction of the kidney arachidonate hydroxylases is associated with the development of hypertension. Am J Physiol Regul Integr Comp Physiol. 2003;284:1055–1062. [DOI] [PubMed] [Google Scholar]


