Abstract
Background
Hospitalizations for acute myocardial infarctions (AMIs) are associated with changes in statin adherence. It is unclear to what extent adherence changes, which patients are likely to change, and how post‐discharge follow‐up is associated with statin adherence change.
Methods and Results
This retrospective study used Medicare data for all fee‐for‐service beneficiaries 66 years and older with an AMI hospitalization in 2008–2010 and statin use before their index AMI. Multivariable multinomial logistic regression models (odds ratio [OR] and 99% confidence interval [CI]) were applied to assess associations between both patient characteristics and follow‐up with a primary care provider and/or cardiologist with the outcome of statin adherence change (increase or decrease) from the 6‐month pre‐ to 6‐month post‐AMI periods. Of 113 296 patients, 64.0% had no change in adherence, while 19.7% had increased and 16.3% had decreased adherence after AMI hospitalization. Black and Hispanic patients were more likely to have either increased or decreased adherence than white patients. Patients who required coronary artery bypass graft surgery (OR, 1.34; 99% CI, 1.21–1.49) or percutaneous transluminal coronary angioplasty/stent procedure (OR, 1.25; 99% CI, 1.17–1.32) during their index hospitalization were more likely to have increased adherence. Follow‐up with a primary care provider was only mildly associated with increased adherence (OR, 1.08; 99% CI, 1.00–1.16), while follow‐up with a cardiologist (OR, 1.15; 99% CI, 1.05–1.25) or both provider types (OR, 1.21; 99% CI, 1.12–1.30) had stronger associations with increased adherence.
Conclusions
Post‐AMI changes in statin adherence varied by patient characteristics, and improved adherence was associated with post‐discharge follow‐up care, particularly with a cardiologist or both a primary care provider and a cardiologist.
Keywords: behavior change, medication adherence, myocardial infarction, secondary prevention
Subject Categories: Myocardial Infarction, Secondary Prevention, Aging, Epidemiology
Clinical Perspective
What Is New?
Follow‐up with a clinician within 30 days of an acute myocardial infarction (AMI) hospital discharge was associated with increased statin adherence among patients already taking these medications pre‐AMI.
Follow‐up with a cardiologist or both a cardiologist and a primary care provider was more strongly associated with increased adherence than follow‐up with only a primary care provider.
Patients who required invasive procedures during AMI hospitalization were more likely to have increased statin adherence after AMI.
The association of race/ethnicity with statin adherence change was complex, with many black and Hispanic patients being more likely to have decreases in adherence compared with white patients.
What Are the Clinical Implications?
Early follow‐up with clinicians, especially cardiologists, should be encouraged for patients after AMI to address barriers to and the importance of medication adherence.
Primary care providers should be aware of all post‐AMI care that patients are receiving. For patients who are unable to follow up with a cardiologist, primary care providers should place a strong emphasis on medication adherence and consider innovative strategies to address barriers to adherence.
Patient sociodemographic characteristics, comorbidity burden, and severity of the AMI hospitalization should all be considered when counseling patients on medication adherence.
Introduction
Acute myocardial infarction (AMI) is a common cardiovascular event with significant public health implications. Pharmacotherapy after an AMI, including the use of statins, is a vital part of the secondary prevention treatment strategy, and numerous studies have shown that patients cannot experience the full benefit realized in clinical trials if they are not adherent to these prescribed medications.1, 2, 3, 4 Many patients older than 65 years are already taking statins before an AMI, and it is unclear whether patients’ adherence to statins may increase, decrease, or not change following an AMI.
Interaction with healthcare providers may also affect a patient's response to a negative health event. However, discontinuity during transitions in care––including both lack of follow‐up and breakdown of communication among multiple providers––may also negatively impact adherence.5, 6, 7 It is unclear how post‐AMI follow‐up with either a primary care provider (PCP) and/or a cardiologist may affect changes in adherence among patients who are already using statins.8
In order to effectively develop clinical and policy interventions to improve adherence to secondary prevention therapies, it is important to understand how medication adherence may change in response to an AMI, which patients are more likely to have increased or decreased adherence, and how follow‐up with healthcare providers may affect this change. The evidence available from prior studies is insufficient to address these questions.8, 9, 10, 11 Therefore, the aims of this study were (1) to determine to what extent older patients’ adherence to statins may change after an AMI, (2) to identify patient characteristics associated with change in statin adherence after an AMI, and (3) to assess whether post‐discharge follow‐up with PCPs and/or cardiologists is associated with change in statin adherence after an AMI.
Methods
Study Design, Data Sources, and Participants
This is a retrospective cohort study using the US Centers for Medicare & Medicaid Services (CMS) Medicare Chronic Conditions Data Warehouse (CCW) enrollment summary, medical services, and prescription Part D event files from 2007 to 2011. We assembled a cohort of all Medicare fee‐for‐service eligible beneficiaries who had an AMI from 2008 to 2010 (Figure 1). The eligibility criteria were (1) index AMI hospitalization between January 1, 2008, and December 31, 2010; (2) age 66 years and older at the time of index hospitalization; (3) survived hospitalization and discharged to home/self‐care; (4) continuous enrollment in Medicare Parts A, B, and D for at least 12 months pre‐hospital admission and 6 months post‐hospital discharge or until death, whichever occurred first; (5) filled at least one statin prescription in the period between 360 and 14 days before index hospitalization; and (6) did not have end‐stage renal disease in the baseline period. Index AMI hospitalization was identified using an International Classification of Diseases, Ninth Revision (ICD‐9), code of 410.x1 in either the primary or secondary discharge diagnosis field in the Medicare inpatient claims.12, 13 If a patient had multiple AMIs in the base year, only the first was considered the index hospitalization. See Figure 2 for patient selection and attrition based on eligibility criteria.
Figure 1.
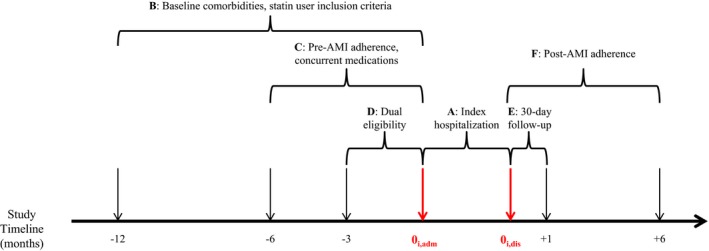
Study timeline. A, Index hospitalization (duration is length of stay). B, Twelve‐month period used to identify baseline comorbidities. This period was used to identify prevalent users of statins for study inclusion. C, Six‐month period used to identify concurrent users of angiotensin‐converting (ACE) enzyme inhibitors/angiotensin receptor blockers (ARB) and β‐blockers. Also used to measure pre–acute myocardial infarction (AMI) statin adherence. If a patient's first prescription claim occurred during this period, adherence was measured from the date of that first fill until the first day of hospital admission for index AMI (0i,adm). D, Three‐month period used to identify patients with dual Medicare and Medicaid eligibility. If a patient had dual eligibility during any of these 3 months, they were considered dual eligible for the entire study. E, Thirty‐day period after index hospitalization discharge used to measure whether patient followed up with a primary care provider and/or cardiologist. F, Follow‐up period for all patients used to measure post‐AMI statin adherence. This period lasted 6 months after hospital discharge except for those individuals who died within 6 months of hospital discharge (n=12 281, 10.8%). Date of death was the end of follow‐up for these patients. 0i,dis indicates discharge date for index hospitalization (which was the beginning of the follow‐up period for all patients).
Figure 2.
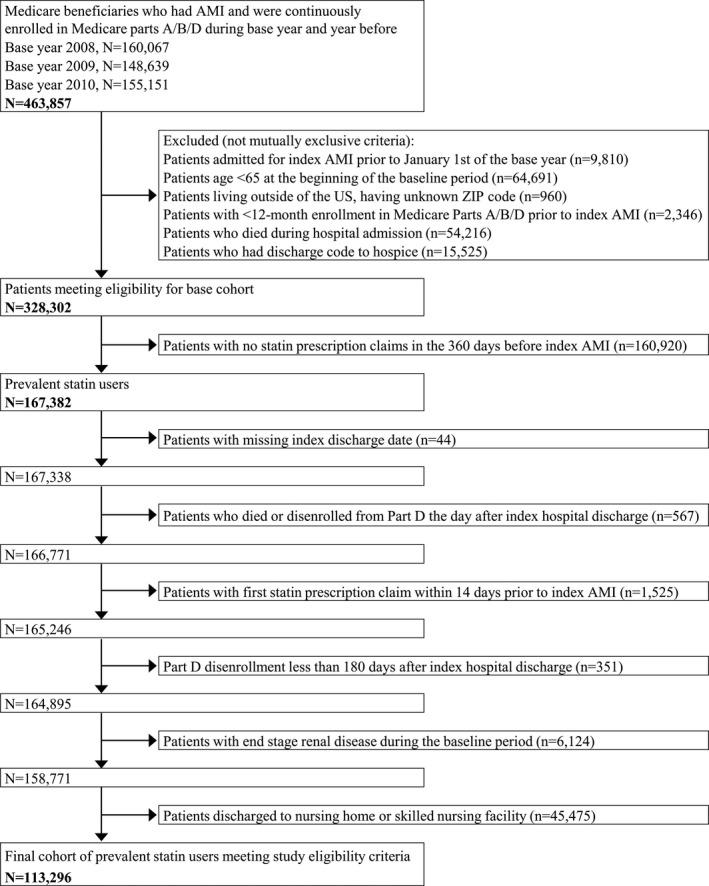
Patient attrition and eligibility criteria. AMI indicates acute myocardial infarction.
The study was approved by the institutional review board of the University of North Carolina at Chapel Hill. Because this was a secondary analysis of deidentified administrative claims data, the need for informed consent was waived.
Statin Adherence and Adherence Change
Prescription claims for statins were identified in the prescription Part D files. Adherence to statins was measured using the proportion of days covered (PDC) (0–100%). Pre‐AMI adherence was measured in the 180 days before AMI hospital admission; patients were left‐censored if their first prescription claim was identified during this time period. Post‐AMI adherence was measured for 180 days after discharge, but patients were right‐censored if they died within 6 months after hospital discharge. The adherence measure was adjusted for hospital stays and oversupply from previous statin prescription fills. Patients were also categorized into severely nonadherent (PDC <40%), moderately nonadherent (PDC 40–79.9%), and adherent (PDC ≥80%); previous research has shown a dose‐response relationship with these statin adherence categories and health outcomes.3
The outcome for this study was change in statin adherence, a categorical variable defined with either 3 levels or 5 levels (Figure 3). For the 3‐level adherence change outcome, change was defined as a “decrease” if the post‐AMI adherence was lower categorically than pre‐AMI adherence, an “increase” if the post‐AMI adherence was higher categorically, and “no change” if there was no movement from one adherence category to another. For the 5‐level adherence change outcome, change was defined as a “major decrease” if pre‐AMI adherence was ≥80% and post‐AMI adherence was <40%, a “moderate decrease” for all other adherence decreases, “no change,” a “major increase” if pre‐AMI adherence was <40% and post‐AMI adherence was ≥80%, and a “moderate increase” for all other adherence increases. A continuous adherence change variable was calculated as post‐AMI PDC minus pre‐AMI PDC, with values ranging from −100% to +100%.
Figure 3.
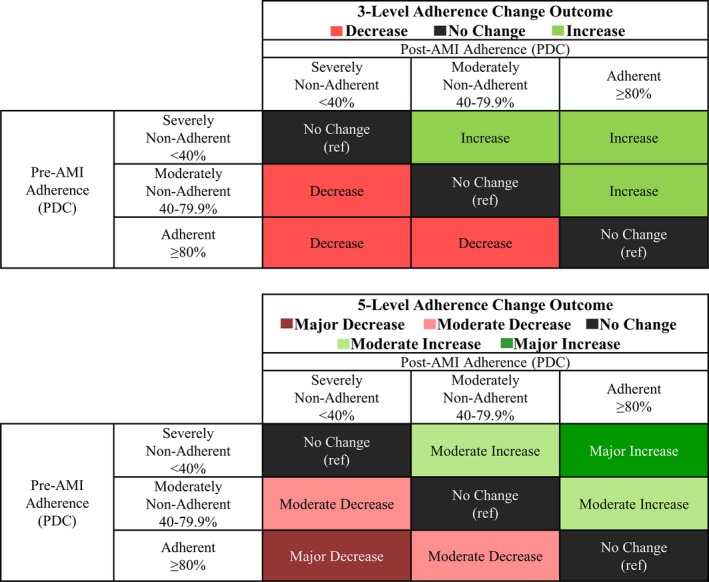
Definition of 3‐level and 5‐level categories of adherence change. AMI indicates acute myocardial infarction; PDC, proportion of days covered; ref, reference.
Patient Characteristics
Sociodemographic data were ascertained from the enrollment summary files. Characteristics measured included age at the time of index hospital admission, sex, race/ethnicity, dual eligibility in Medicare and Medicaid, and median household income of the US Census block groups for individuals aged 65 years and older. Patients were identified as dually eligible for Medicaid in the 3 months before AMI hospital admission.
Comorbidities and cardiovascular procedures were measured in the 12 months preceding the index AMI admission. These variables included previous AMI, dementia/Alzheimer's disease, depression, ischemic heart disease, unstable angina, lipid abnormalities, history of rhabdomyolysis/myopathy, coronary artery bypass surgery (CABG), stent/percutaneous transluminal coronary angioplasty (PTCA), and the Charlson Comorbidity Index (CCI). Additionally, a modified CCI was calculated that excluded AMI and dementia, as both variables were included as separate variables in regression models. Variables related to the index admission were measured including type of AMI (subendocardial or transmural infarction), procedures (CABG, PTCA/stent, cardiac catheterization, angiocardiography, infusion of platelet inhibitors, and infusion of thrombolytics), complications (cardiogenic shock, cardiac dysrhythmias, hypotension, acute renal failure, and heart failure), length of index hospitalization, admission to an intensive care unit or a coronary care unit, and a cardiologist consultation during the admission. These patient characteristics were measured using standardized algorithms from the Chronic Conditions Data Warehouse14 and health services research literature.15, 16 Statin adherence in the pre‐AMI period was included as a predictor of adherence change. Prescription claims for a β‐blocker or angiotensin‐converting enzyme (ACE) inhibitor/angiotensin receptor blocker (ARB) in the 6 months before the index admission were also identified.
Follow‐Up With a Provider
Follow‐up with a cardiologist or a PCP within 30 days after hospital discharge was measured using outpatient and physician office visit claims. Provider type was measured using CMS Medicare Specialty Codes. PCPs included nonspecialist physicians, physician assistants, and nurse practitioners as defined in clinical practice guidelines available during the study period.17
Statistical Analysis
Distributions of patient sociodemographic and clinical characteristics by categories of statin adherence change were described. Absolute standardized differences were used to assess unadjusted differences between groups stratified by change in statin adherence.18 An absolute standardized difference of ≥0.10 with “no change” as the reference group was used as a cutoff to determine whether there were significant differences in patient characteristics between groups.19
Multivariable multinomial logistic regression models were developed for the 3‐level and 5‐level categories of adherence change with “no change” as the reference outcome group. Multivariable regression models were utilized to identify predictors of adherence change after an AMI in the domains of sociodemographic characteristics, baseline clinical characteristics, clinical events during index hospitalization, and post‐discharge follow‐up. Adjusted odds ratios (ORs) with 99% confidence intervals (CIs) were reported for all multinomial logistic regression models.
We assessed the robustness and consistency of our model estimates in 11 sensitivity analyses. First, using our full sample, 5 additional sets of analyses were conducted: (1) to alleviate the influence of small changes in adherence, a categorical change in adherence also required an absolute change of 10% in PDC to officially be defined as an adherence change,8 (2) the period for post‐AMI follow‐up with clinicians was extended to 42 days,20 (3) change in the simvastatin‐equivalent average daily dose from the pre‐ to post‐AMI period was added to a separate model,21, 22 (4) categorical variables indicating a high‐, moderate‐, or low‐intensity statin for the last fill before index AMI and first fill after index AMI were added to a separate model,21 and (5) liver disease (a potential contraindication for statins) was added to a separate model. Second, we altered the cohort eligibility criteria in 3 separate models: (6) patients must have had their first statin prescription fill at least 6 months before index AMI, (7) patients must have survived 6 months post‐AMI discharge, and (8) patients with another AMI within 6 months after discharge from their index event were excluded. In addition, (9) adherence and change in adherence were measured in the 12‐month baseline and 12‐month post‐discharge periods. Given the conditional nature of the outcome (eg, a patient with PDC <40% before their AMI could have “no change” in adherence or an “increase” but would not be able to “decrease”), (10) a single sensitivity analysis on the 3‐level outcome using PROC NLMIXED in SAS defined the likelihood function as conditional on the pre‐AMI adherence category. Finally, (11) a linear regression model was developed for the continuous adherence change outcome (post‐AMI PDC minus pre‐AMI PDC).
All analyses were conducted with SAS 9.4 (SAS Institute Inc).
Results
A total of 113 296 prevalent statin users met our study eligibility criteria. The distributions of sociodemographic and clinical characteristics stratified by statin adherence change categories are displayed in Table 1. Most patients were aged 66 to 85 years (84.5%), female (54.3%), and white (84.3%), and 5228 (4.6%) had an AMI in the 12 months before their index AMI. Overall 17 186 patients (15.2%) did not follow‐up with either a cardiologist or PCP within 30 days of hospital discharge. See Tables S1 through S3 for a full list of the distribution of population characteristics stratified by adherence change, pre‐AMI adherence, and post‐AMI adherence.
Table 1.
Patient Characteristics Stratified by Change in Statin Adherence After AMI
| Patient Characteristics | Total | Adherence Change | ||
|---|---|---|---|---|
| Decrease | No Change | Increase | ||
| N=113 296 | n=18 502 | n=72 510 | n=22 284 | |
| No. (%) | % | % | % | |
| Age, y | ||||
| Mean±SD | 77.3±7.4 | 77.7±7.4 | 77.4±7.4 | 76.5±7.1a |
| 66 to 75 | 51 010 (45.0) | 42.6 | 44.3 | 49.3a |
| 76 to 85 | 44 751 (39.5) | 40.2 | 39.7 | 38.2 |
| 86+ | 17 535 (15.5) | 17.2 | 16.0 | 12.5 |
| Women | 61 555 (54.3) | 55.1 | 53.7 | 55.7 |
| Race/ethnicity | ||||
| White | 95 481 (84.3) | 83.8 | 85.5 | 80.5a |
| Black | 9700 (8.6) | 9.0 | 7.7 | 10.9a |
| Hispanic | 3413 (3.0) | 3.2 | 2.6 | 4.0 |
| Asian | 2504 (2.2) | 1.9 | 2.2 | 2.5 |
| Other | 2198 (1.9) | 2.1 | 1.9 | 2.1 |
| Dual eligibilityb | 28 462 (25.1) | 23.6 | 24.9 | 27.1 |
| Baseline CCI | ||||
| Mean±SD | 2.5±2.3 | 2.8±2.4a | 2.5±2.3 | 2.4±2.3 |
| 0 | 22 608 (20.0) | 17.4 | 19.8 | 22.6 |
| 1 or 2 | 43 540 (38.4) | 36.5 | 38.7 | 39.3 |
| 3 to 5 | 34 947 (30.8) | 33.1 | 31.0 | 28.5 |
| 6 to 8 | 9868 (8.7) | 10.4 | 8.6 | 7.6 |
| ≥9 | 2333 (2.1) | 2.6 | 2.0 | 1.9 |
| Baseline comorbidities | ||||
| Prior AMIc | 5228 (4.6) | 5.4 | 4.6 | 4.0 |
| Dementia/Alzheimer's diseased | 11 574 (10.2) | 12.1 | 9.9 | 9.7 |
| Depression | 17 464 (15.4) | 17.3 | 15.0 | 15.3 |
| CABG | 1136 (1.0) | 1.1 | 1.0 | 1.0 |
| PTCA/stent | 7441 (6.6) | 7.2 | 6.6 | 5.8 |
| IHD | 68 689 (60.6) | 64.1 | 61.4 | 55.4a |
| Rhabdomyolysis/myopathy | 7553 (6.7) | 7.6 | 6.3 | 7.0 |
| Index admission | ||||
| CABG | 6988 (6.2) | 5.3 | 6.0 | 7.6 |
| PTCA/stent | 41 596 (36.7) | 30.3a | 36.4 | 43.1a |
| Cardiac catheterization | 64 954 (57.3) | 51.4a | 56.8 | 63.9a |
| Cardiac dysrhythmias | 35 365 (31.2) | 31.7 | 31.6 | 29.4 |
| Acute renal failure | 17 876 (15.8) | 17.7 | 15.5 | 14.9 |
| Length of stay, d | ||||
| Mean±SD | 5.3±5.0 | 5.7±5.8 | 5.2±4.8 | 5.3±4.9 |
| 1 to 3 | 49 896 (44.0) | 40.6 | 44.7 | 44.9 |
| 4 to 6 | 35 187 (31.1) | 31.6 | 31.1 | 30.4 |
| 7 to 11 | 19 525 (17.2) | 18.9 | 17.0 | 16.7 |
| 12+ | 8688 (7.7) | 8.8 | 7.3 | 8.1 |
| 30‐d follow‐up | ||||
| None | 17 186 (15.2) | 15.8 | 15.1 | 14.9 |
| PCP onlye | 33 332 (29.4) | 31.0 | 29.4 | 28.0 |
| Cardiologist only | 20 292 (17.9) | 16.2 | 18.1 | 18.8 |
| Both | 42 486 (37.5) | 37.0 | 37.4 | 38.3 |
See Table S1 for a full list of patient characteristics. AMI indicates acute myocardial infarction; CABG, coronary artery bypass surgery; IHD, ischemic heart disease; PCP, primary care provider; PTCA, percutaneous transluminal coronary angioplasty; SD, standard deviation.
Absolute standardized difference ≥0.10 with no change in statin adherence as the reference group.
Enrolled in Medicare and Medicaid.
Charlson Comorbidity Index (CCI) definition.
Medicare Chronic Condition Data Warehouse definition.
Primary care physician, physician assistant, or nurse practitioner.
Of the 756 059 prescription fills used to measure adherence, 80.4% and 15.1% had a 30‐ and 90‐day supply, respectively. After index AMI hospitalization, 22 284 (19.7%) patients had an increase in statin adherence, 18 502 (16.3%) had a decrease, and 72 510 (64.0%) had no categorical change in adherence (Figure 4). Patient characteristics associated with different categories of adherence change are presented in Table 1.
Figure 4.
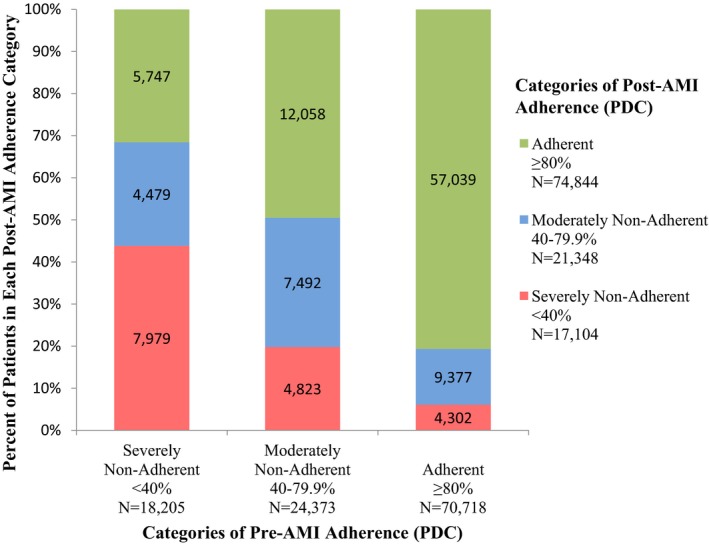
Distribution of post‐AMI adherence stratified by pre‐AMI adherence. AMI indicates acute myocardial infarction; PDC, proportion of days covered.
Model 1 Results: 3‐Level Multinomial Regression
The associations of patient sociodemographic and clinical characteristics with statin adherence change from the 3‐level multinomial logistic regression model (model 1) are presented in Table 2. Compared with white patients, black patients were more likely to have decreased adherence (model 1: OR, 1.21; 99% CI, 1.12–1.31) or increased adherence (OR, 1.14; 99% CI, 1.05–1.23) than to have no change in adherence. Hispanic patients had similar patterns of adherence change compared with white patients. Patients dually enrolled in both Medicare and Medicaid were less likely to have decreased adherence (OR, 0.80; 99% CI, 0.75–0.84) and more likely to have increased adherence (OR, 1.18; 99% CI, 1.11–1.25) than to have no change in adherence. Patients with greater chronic disease burden (ie, higher CCI scores) were more likely to have decreases in adherence and less likely to have increases compared with no change in adherence. The association of statin adherence change with patients receiving invasive procedures during the index hospital, such as CABG or PTCA/stent, had the inverse relationship; these patients were less likely to have decreases in adherence and more likely to have increases in adherence. Finally, as length of stay increased, decreased statin adherence was typically more likely.
Table 2.
Association Between Patient Characteristics and 3‐Level Statin Adherence Change After an AMI
| Patient Characteristics | Model 1: 3‐Level Adherence Changea Odds Ratio (99% CI) (“No Change” as Reference) | |
|---|---|---|
| Decrease | Increase | |
| Age, y | ||
| 66 to 75 | 1. | 1. |
| 76 to 85 | 0.99 (0.94–1.04) | 0.97 (0.92–1.02) |
| 86+ | 0.99 (0.92–1.06) | 0.88 (0.81–0.95) |
| Women | 1.03 (0.98–1.08) | 1.06 (1.01–1.12) |
| Race/ethnicity | ||
| White | 1. | 1. |
| Black | 1.21 (1.12–1.31) | 1.14 (1.05–1.23) |
| Hispanic | 1.30 (1.15–1.48) | 1.29 (1.13–1.48) |
| Asian | 0.97 (0.83–1.14) | 1.16 (0.99–1.36) |
| Other | 1.22 (1.05–1.42) | 1.15 (0.97–1.36) |
| Dual eligibilityb | 0.80 (0.75–0.84) | 1.18 (1.11–1.25) |
| Adjusted baseline CCIc | ||
| 0 | 1. | 1. |
| 1 or 2 | 1.03 (0.97–1.10) | 0.88 (0.83–0.94) |
| 3 to 5 | 1.08 (1.01–1.15) | 0.84 (0.79–0.91) |
| 6 to 8 | 1.14 (1.04–1.26) | 0.81 (0.73–0.91) |
| 9 or more | 1.39 (1.19–1.62) | 0.77 (0.64–0.93) |
| Baseline comorbidities | ||
| Prior AMId | 1.04 (0.94–1.15) | 0.92 (0.81–1.04) |
| Dementia/Alzheimer's diseasee | 1.13 (1.06–1.21) | 1.05 (0.97–1.14) |
| Depression | 1.11 (1.05–1.18) | 1.02 (0.95–1.09) |
| CABG | 1.13 (0.92–1.39) | 1.28 (1.01–1.62) |
| PTCA/stent | 1.12 (1.02–1.23) | 0.85 (0.76–0.95) |
| Ischemic heart disease | 1.09 (1.04–1.15) | 0.84 (0.79–0.89) |
| Rhabdomyolysis/myopathy | 1.15 (1.05–1.24) | 1.01 (0.92–1.11) |
| Index admission | ||
| CABG | 0.78 (0.71–0.87) | 1.34 (1.21–1.49) |
| PTCA/stent | 0.82 (0.78–0.87) | 1.25 (1.17–1.32) |
| Cardiac catheterization | 1.03 (0.95–1.12) | 1.09 (1.00–1.19) |
| Cardiac dysrhythmias | 1.00 (0.95–1.05) | 0.93 (0.88–0.98) |
| Acute renal failure | 1.05 (0.99–1.12) | 1.00 (0.93–1.07) |
| Length of stay, d | ||
| 1 to 3 | 1. | 1. |
| 4 to 6 | 1.05 (1.00–1.11) | 1.00 (0.94–1.06) |
| 7 to 11 | 1.14 (1.07–1.22) | 1.02 (0.95–1.10) |
| 12+ | 1.27 (1.16–1.39) | 1.08 (0.97–1.20) |
Models were adjusted for all variables in the table as well as pre–acute myocardial infarction (AMI) adherence, area‐level household income, other baseline conditions, concurrent use of other AMI secondary prevention medications, other events during the index hospitalization, and 30‐day follow‐up care with a provider. See Table S4 for the full list of results for model 1. CABG indicates coronary artery bypass surgery; CI, confidence interval; IHD, ischemic heart disease; OR, odds ratio; PDC, proportion of days covered; PTCA, percutaneous transluminal coronary angioplasty.
Three‐level multinomial logistic regression comparing decrease in adherence to no change and comparing increase in adherence to no change.
Enrolled in Medicare and Medicaid.
Charlson Comorbidity Index (CCI) score does not include counts for AMI and dementia.
CCI definition.
Medicare Chronic Condition Data Warehouse definition.
The association of follow‐up with clinicians and the 3‐level adherence change outcome can be seen in Figure 5. Patients who followed up with only a PCP compared with no follow‐up within 30 days after the index discharge date had an 8% increase in the likelihood of an increase in adherence (OR, 1.08; 99% CI, 1.00–1.16) compared with no change in adherence. Follow‐up with a cardiologist (OR, 1.15; 99% CI, 1.05–1.25) or both types of providers (OR, 1.21; 99% CI, 1.12–1.30) had a stronger association with an increase in adherence. The association of clinician follow‐up with a decrease in adherence was not significant except for patients who followed up with a cardiologist; these patients were less likely to have a decrease in adherence than no change in adherence (OR, 0.91; 99% CI, 0.84–0.98) compared with patients with no follow‐up. See Table S4 for the full list of results for model 1.
Figure 5.
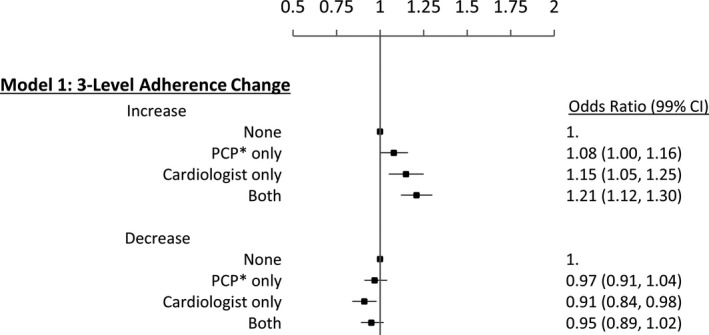
Association between follow‐up care within 30 days of index hospital discharge and 3‐level statin adherence change after an acute myocardial infarction (AMI). Models were adjusted for sociodemographic characteristics, pre‐AMI adherence, baseline conditions and Charlson Comorbidity Index, concurrent use of other AMI secondary prevention medications, and events during the index hospitalization. See Table S4 for a full list of these variables and estimates. The 3‐level multinomial logistic regression compared a decrease in adherence with no change and compared an increase in adherence with no change. *Primary care physician, physician assistant, or nurse practitioner. CI indicates confidence interval; PCP, primary care provider.
Model 2 Results: 5‐Level Multinomial Regression
Results using the 5‐level adherence change outcome were consistent with results using the 3‐level outcome but provided additional insight into patient characteristics that were associated with a major increase or a major decrease in statin adherence. Results from the 5‐level multinomial logistic regression model (model 2) are presented in Table 3. Patients enrolled in both Medicare and Medicaid were 13% more likely to have a moderate increase in adherence (model 2: OR, 1.13; 99% CI, 1.06–1.20) and, to a greater extent, they were 46% more likely to have a major increase in adherence (OR, 1.46; 99% CI, 1.31–1.63). Patients who had an invasive procedure such as CABG during their index hospital admission were 37% less likely to have a major decrease (model 2: OR, 0.73; 99% CI, 0.59–0.89) and 25% less likely to have a moderate decrease in adherence (OR, 0.80; 99% CI, 0.71–0.91); they were also 28% more likely to have a moderate increase (OR, 1.28; 99% CI, 1.15–1.43) and 77% more likely to have a major increase in statin adherence (OR, 1.77; 99% CI, 1.46–2.14). Patients with a PTCA/stent procedure during index hospitalization had similar patterns of adherence change.
Table 3.
Association Between Patient Characteristics and 5‐Level Statin Adherence Change After an AMI
| Patient Characteristics | Model 2: 5‐Level Adherence Changea Odds Ratio (99% CI) (“No Change” as Reference) | |||
|---|---|---|---|---|
| Major Decrease | Moderate Decrease | Moderate Increase | Major Increase | |
| Age, y | ||||
| 66 to 75 | 1. | 1. | 1. | 1. |
| 76 to 85 | 0.95 (0.87–1.05) | 1.00 (0.95–1.06) | 0.96 (0.91–1.02) | 1.01 (0.92–1.11) |
| 86+ | 0.99 (0.88–1.12) | 0.98 (0.91–1.06) | 0.86 (0.79–0.93) | 0.94 (0.81–1.08) |
| Women | 1.06 (0.97–1.15) | 1.02 (0.97–1.07) | 1.07 (1.01–1.12) | 1.04 (0.95–1.14) |
| Race/ethnicity | ||||
| White | 1. | 1. | 1. | 1. |
| Black | 1.25 (1.07–1.45) | 1.21 (1.11–1.32) | 1.15 (1.06–1.25) | 1.16 (1.01–1.34) |
| Hispanic | 1.29 (1.00–1.64) | 1.32 (1.15–1.52) | 1.32 (1.15–1.51) | 1.25 (1.00–1.58) |
| Asian | 0.87 (0.64–1.19) | 1.01 (0.84–1.20) | 1.18 (1.00–1.39) | 1.09 (0.82–1.47) |
| Other | 1.19 (0.88–1.59) | 1.24 (1.04–1.46) | 1.14 (0.96–1.36) | 1.27 (0.94–1.71) |
| Dual eligibilityb | 0.81 (0.73–0.90) | 0.79 (0.74–0.84) | 1.13 (1.06–1.20) | 1.46 (1.31–1.63) |
| Adjusted baseline CCIc | ||||
| 0 | 1. | 1. | 1. | 1. |
| 1 or 2 | 1.01 (0.90–1.14) | 1.04 (0.97–1.11) | 0.89 (0.83–0.95) | 0.82 (0.73–0.92) |
| 3 to 5 | 1.07 (0.94–1.22) | 1.08 (1.00–1.17) | 0.87 (0.81–0.94) | 0.69 (0.61–0.79) |
| 6 to 8 | 1.15 (0.96–1.37) | 1.14 (1.02–1.27) | 0.82 (0.73–0.92) | 0.74 (0.61–0.91) |
| 9 or more | 1.37 (1.02–1.83) | 1.40 (1.18–1.66) | 0.81 (0.66–0.99) | 0.58 (0.41–0.83) |
| Baseline comorbidities | ||||
| Prior AMId | 1.14 (0.95–1.38) | 1.01 (0.90–1.13) | 0.92 (0.81–1.05) | 0.90 (0.72–1.14) |
| Dementia/Alzheimer's diseasee | 1.30 (1.15–1.47) | 1.08 (1.00–1.17) | 1.06 (0.97–1.15) | 0.97 (0.83–1.14) |
| Depression | 1.14 (1.02–1.28) | 1.10 (1.03–1.18) | 1.01 (0.94–1.09) | 1.03 (0.91–1.16) |
| CABG | 1.24 (0.84–1.83) | 1.10 (0.87–1.38) | 1.26 (0.99–1.61) | 1.35 (0.85–2.17) |
| PTCA/stent | 1.21 (1.02–1.45) | 1.09 (0.99–1.21) | 0.86 (0.77–0.96) | 0.79 (0.64–0.97) |
| Ischemic heart disease | 1.07 (0.97–1.18) | 1.10 (1.04–1.17) | 0.89 (0.84–0.94) | 0.61 (0.56–0.68) |
| Rhabdomyolysis/myopathy | 1.15 (0.98–1.35) | 1.15 (1.04–1.26) | 1.03 (0.94–1.14) | 0.91 (0.77–1.08) |
| Index admission | ||||
| CABG | 0.73 (0.59–0.89) | 0.80 (0.71–0.91) | 1.28 (1.15–1.43) | 1.77 (1.46–2.14) |
| PTCA/stent | 0.80 (0.72–0.89) | 0.83 (0.78–0.88) | 1.19 (1.12–1.27) | 1.60 (1.43–1.78) |
| Cardiac catheterization | 0.99 (0.84–1.17) | 1.04 (0.95–1.15) | 1.07 (0.97–1.17) | 1.20 (1.02–1.41) |
| Cardiac dysrhythmias | 1.01 (0.92–1.10) | 0.99 (0.94–1.05) | 0.93 (0.88–0.98) | 0.90 (0.82–0.99) |
| Acute renal failure | 1.05 (0.94–1.17) | 1.05 (0.98–1.13) | 1.00 (0.93–1.07) | 0.99 (0.87–1.13) |
| Length of stay, d | ||||
| 1 to 3 | 1. | 1. | 1. | 1. |
| 4 to 6 | 1.11 (1.01–1.23) | 1.04 (0.98–1.10) | 0.99 (0.93–1.05) | 1.04 (0.94–1.16) |
| 7 to 11 | 1.36 (1.21–1.53) | 1.08 (1.01–1.17) | 1.00 (0.93–1.08) | 1.10 (0.96–1.25) |
| 12+ | 1.67 (1.42–1.96) | 1.16 (1.05–1.28) | 1.07 (0.96–1.19) | 1.11 (0.91–1.34) |
Models were adjusted for all variables in table as well as pre–acute myocardial infarction (AMI) adherence, area‐level household income, other baseline conditions, concurrent use of other AMI secondary prevention medications, other events during the index hospitalization, and 30‐day follow‐up care with a provider. See Table S5 for the full list of results for model 2. CABG, coronary artery bypass surgery; CI, confidence interval; IHD, ischemic heart disease; OR, odds ratio; PDC, proportion of days covered; PTCA, percutaneous transluminal coronary angioplasty.
Five‐level multinomial logistic regression model comparing all four outcomes in the table with the reference group of no change in adherence.
Enrolled in Medicare and Medicaid.
Charlson Comorbidity Index (CCI) score does not include counts for AMI and dementia.
CCI definition.
Medicare Chronic Condition Data Warehouse definition.
Figure 6 shows that follow‐up with a PCP within 30 days after index hospital discharge trended towards an association with a moderate increase in adherence (OR, 1.07; 99% CI, 0.99–1.15) and a major increase in adherence (OR, 1.10; 99% CI, 0.96–1.27). Seeing a cardiologist was associated with a 13% increase in the likelihood of a moderate increase in adherence (OR, 1.13; 99% CI, 1.03–1.23) and a 27% increase in the likelihood of a major increase in adherence (OR, 1.27; 99% CI, 1.09–1.48). Follow‐up with both a cardiologist and a PCP (OR, 1.16; 99% CI, 1.08–1.26) was associated with a moderate adherence increase compared with no follow‐up; follow‐up with both of these providers also had a greater impact on the odds of a major increase in adherence (OR, 1.48; 99% CI, 1.29–1.69). See Table S5 for the full list of results for model 2.
Figure 6.

Association between follow‐up care within 30 days of index hospital discharge and 5‐level statin adherence change after an acute myocardial infarction (AMI). Models were adjusted for sociodemographic characteristics, pre‐AMI adherence, baseline conditions and Charlson Comorbidity Index, concurrent use of other AMI secondary prevention medications, and events during the index hospitalization. See Table S5 for a full list of these variables and estimates. The 5‐level multinomial logistic regression model compared all four adherence change outcomes with the reference group of no change in adherence. *Primary care physician, physician assistant, or nurse practitioner. CI indicates confidence interval; PCP, primary care provider.
Sensitivity Analyses
Our sensitivity analyses found mostly similar and consistent results that did not change the interpretation of our findings from model 1 (Tables S6 through S10). When patient eligibility was restricted to those who survived at least 6 months post‐hospital discharge, a CCI score of 9+ compared with 0 was no longer statistically associated with increased adherence (Table S7 in the “post‐AMI 6‐month survival” model; OR, 0.82 [99% CI, 0.65–1.04]). The sensitivity analysis with adherence measured over 12 months in the pre‐ and post‐AMI periods showed similar and consistent results, although fewer patients had increased and more patients had decreased statin adherence (Figure S1). When investigating 1‐year adherence changes, black patients were still likely to have reduced adherence (Table S8; OR, 1.17; 99% CI, 1.09–1.26) but were no longer as likely to have increased adherence (OR, 1.05; 99% CI, 0.96–1.15). In the sensitivity analysis using a likelihood function conditional on pre‐AMI adherence, black patients (OR, 0.94; 99% CI, 0.86–1.02) and Hispanic patients (OR, 1.09; 99% CI, 0.94–1.25) were no longer more likely than white patients to have increased adherence (Table S9).
Discussion
In a cohort of 113 296 Medicare beneficiaries who were prevalent statin users before their index AMI hospitalization, 64% had no change in adherence, 20% had increased adherence, and 16% had decreased adherence after hospitalization. Interestingly, both black and Hispanic patients responded more sensitively to an AMI hospitalization compared with white patients, with adherence changes likely in both directions. Patients who had a greater burden of chronic comorbidities were more likely to have decreased adherence and less likely to have increased adherence. On the other hand, patients who required invasive interventions during the index hospitalization were more likely to have increases in adherence after discharge. Notably, patients who followed up with a PCP were only mildly more likely to have increased adherence, but patients who saw a cardiologist or both a cardiologist and a PCP appeared to have markedly greater odds of having increased statin adherence.
Change in adherence to statins after an AMI has been reported in a few prior studies.8, 9, 10, 11 In a study that used a 5% random sample of Medicare patients, Kronish et al8 found that 33% of patients with pre‐AMI PDC ≥80% became nonadherent after an AMI, while 38% of patients with PDC <80% became adherent after AMI. They also found that patients with a greater baseline comorbidity burden were less likely to change from nonadherent to adherent and were more likely to change from adherent to nonadherent after an AMI. Another study with 855 patients with type 2 diabetes mellitus found that 26% of patients became more adherent and 20% of patients became less adherent after incident cardiovascular events.11 However, we found strong relationships with adherence change after AMI associated with race/ethnicity and CABG during hospitalization that were not found in these earlier studies. Our larger sample size––based on a 100% sample of Medicare fee‐for‐service beneficiaries––also allowed us to investigate more risk factors and categorize race/ethnicity more granularly than white patients compared with all other groups.
In this study, we found that patients dually enrolled in Medicare and Medicaid were less likely to have decreased adherence and more likely to have a moderate or major increase in statin adherence. Prior research also supports the finding that lower out‐of‐pocket costs are associated with better adherence to preventive medications after an AMI.23, 24 Therefore, patients with Medicare who are eligible but not enrolled in Medicaid or the low‐income subsidy program may benefit if providers and their clinical support staff inform patients about these programs.
Similar to previous findings,8 patients with greater comorbidity burden at baseline were more likely to have decreased adherence and less likely to have increased adherence after AMI hospital discharge. It is also possible that clinicians stopped preventative medications in patients with a short life expectancy. When analyses were restricted to patients who survived 6 months after hospital discharge, the magnitude of the association between CCI and decreased adherence was slightly weaker; however, this finding did not significantly impact our interpretation of the results. Clinicians should continue to emphasize the importance of preventive medications in patients with depression, a history of ischemic heart disease, or multiple comorbidities. Given that greater comorbidity burden is associated with decreased adherence, coordinated care with outpatient clinicians to manage these other conditions has the potential to improve adherence to preventive medications after an AMI, in addition to improving patient health directly associated with these other conditions. Further research is needed to completely understand the association between comorbidity burden and statin adherence change after an AMI.
Patients who required invasive procedures such as a CABG or PTCA/stent during their hospitalization were more likely to have increased adherence after an AMI. The finding that these more severe hospitalizations may have acted as a “wake‐up call” to patients about the need for statin adherence also aligns with the Sentinel Event Effect.25, 26 Studies have shown that perceived necessity for statins is associated with better adherence27 and that illness severity, such as the need for stents or CABG, is predictive of positive behavior change after a cardiac event.28 While clinical severity often influences which patients receive the most attention, our results suggest that patients with “minor” AMIs may benefit as much if not more from counseling if providers can utilize this event as a teachable moment.
Our study also showed that complex patterns of statin adherence change after an AMI were associated with race and ethnicity. These complex patterns may suggest heterogeneity in statin adherence change within these subgroups. Black and Hispanic patients were more likely to have adherence changes in both directions compared with white patients. The associations between race/ethnicity and interactions with the healthcare system are multifaceted, with biology, culture, perceived racism, social support, socioeconomic status, and structural racism among other factors playing a role.29, 30 Future hypothesis‐driven studies are warranted to further understand the complex associations of statin adherence change within and between these race/ethnicity groups.
Finally, an important finding from our study was that follow‐up with a clinician within 30 days of hospital discharge was associated with increased adherence. Follow‐up with a clinician may not be a direct predictor of increased statin adherence (ie, the same patients who decided to adhere to their statins also decided it was important to follow up with a clinician); however, current literature does support the view that early follow‐up with providers after a hospitalization as an intervention can improve medication adherence20, 31, 32 and clinical outcomes.33, 34, 35 In addition, we found that a cardiologist visit compared with no follow‐up care was associated with a major increase in adherence (pre‐AMI PDC <40% to post‐AMI PDC ≥80%), while this association was not significant among patients who followed up with a PCP only. This finding is intriguing, but more research is needed to verify and fully understand these results. More importantly, however, follow‐up care had an even stronger association with increased statin adherence among patients who followed up with both a cardiologist and a PCP. This supports the importance of collaborative team care and continuity of care after hospital discharge.36, 37 While our results may suggest post‐AMI follow‐up that includes a cardiologist is the preferred intervention, it is unrealistic that all patients who have an AMI will be able to see a cardiologist. Previous research found that a post‐AMI intervention including pharmacist collaborative care with primary care physicians and/or cardiologists resulted in improved 1‐year adherence for most secondary prevention medications.38 If future research supports the finding that increases in adherence are suboptimal among patients who follow‐up with a PCP only, then pharmacist involvement in collaborative care transitions to synergistically optimize medication utilization could be a potential intervention worth considering, especially for patients not able to follow‐up with a cardiologist.
Study Limitations
This study has several limitations. Because administrative claims are used for billing purposes and not designed for research, there is the possibility of variable misclassification. Some predictive factors (eg, smoking status) were not available in the data. These may be important variables for clinicians to consider and may also confound relationships between other predictive factors and statin adherence change. We followed existing standards using secondary claims data for health services research to mitigate these limitations. Second, several factors can result in measurement error when administrative claims data are used to measure adherence. Prescription fill data may overestimate adherence as there is no information on whether patients used the medication, and our data did not have information available on the type of pharmacy (ie, physical versus mail‐order pharmacies) or whether automatic refill programs were used; however, prescription fill records have been shown to have good validity and correlation with other adherence measures––self‐report, pill counts, and electronic devices39, 40, 41––as well as clinical outcomes.3 It is also possible that adherence was underestimated if prescriptions were paid for outside of the Medicare Part D plan. However, research has shown that this does not often happen for Medicare beneficiaries, and even when it does, these claims are often adjudicated into Part D records42, 43, 44 Medicare patients are also less likely to use medication samples than those in the privately insured population.45 Third, we did not have a control group in this study, but previous research using two control groups––patients without a hospitalization and patients hospitalized for pneumonia––found that AMI hospitalizations were associated with changes in statin adherence.8
Study Strengths
This study possesses several strengths. It is the first to employ a nationally representative sample of all Medicare fee‐for‐service beneficiaries who had an AMI in the period 2008–2010, enabling us to investigate more risk factors with improved generalizability. We also investigated the impact of post‐AMI follow‐up with a clinician, which was noted as an important factor in previous research on this topic.8 Further, our several definitions of adherence change allowed assessment of varying magnitudes of adherence change and the inclusion of all study patients in a single statistical model.
Conclusions
An AMI hospitalization may lead to changes in statin adherence among patients already taking these medications. The direction of statin adherence change after an AMI varied by patient sociodemographic and clinical characteristics. Improved adherence was also associated with post‐discharge follow‐up care, particularly among patients who saw a cardiologist or both a PCP and a cardiologist. Innovative strategies that address these complex changes in medication adherence after an AMI and emphasize continuity of care are needed to improve post‐AMI secondary prevention.
Sources of Funding
This study is supported in part by the National Institutes of Health's National Institute on Aging grant 1R01AG046267‐01A1 (principal investigator: Fang) and 1R21AG043668‐01A1 (principal investigator: Fang). The findings and conclusions in this article are those of the authors, who are responsible for its contents, and do not necessarily represent the views of the funding agency. Dr Hickson received support from the American Foundation for Pharmaceutical Education as the 2015 Phi Lambda Sigma First Year Graduate School Fellow.
Disclosures
Hickson, Annis, Killeya‐Jones, and Fang have no conflicts of interests to report. Robinson worked on grants from Amarin, Amgen, Astra‐Zeneca, Eli Lilly, Esai, GlaxoSmithKline, Merck, Pfizer, Regeneron/Sanofi, and Takeda. Robinson was a consultant for Amgen, Pfizer, and Regeneron/Sanofi (significant), and Akcea/Ionis, Eli Lilly, Esperion, and Merck (modest). Korhonen worked on grants from the Social Insurance Institution of Finland. Cole is a paid (part‐time) employee of IBM Watson Health (significant).
Supporting information
Table S1. Patient Characteristics Stratified by Change in Statin Adherence After AMI
Table S2. Patient Characteristics Stratified by Pre‐AMI Statin Adherence
Table S3. Patient Characteristics Stratified by Post‐AMI Statin Adherence
Table S4. Full Model for 3‐Level Multinomial Regression Model Predicting Statin Adherence Change After an AMI
Table S5. Full Model for 5‐Level Multinomial Regression Model Predicting Statin Adherence Change After an AMI
Table S6. Crude Estimates, Fully Adjusted Model, and Sensitivity Analyses Involving Changing Variable Definitions or Adding New Variables to the Model for the 3‐Level Multinomial Regression Model Predicting Statin Adherence Change After an AMI
Table S7. Fully Adjusted Model and Sensitivity Analyses With Restricted Cohort Eligibility for the 3‐Level Multinomial Regression Model Predicting Statin Adherence Change After an AMI
Table S8. Three‐Level Multinomial Regression Models Predicting Change From 1‐Year Pre‐AMI Statin Adherence to 1‐Year Post‐AMI Statin Adherence (N=112 780)
Table S9. Three‐Level Multinomial Regression Model Predicting Statin Adherence Change After an AMI Using a Likelihood Function Conditional on the Pre‐AMI Adherence Category†
Table S10. Linear Regression Model Predicting Statin Adherence Change After an AMI
Figure S1. Sensitivity analysis showing distribution of 1‐year post‐AMI adherence stratified by 1‐year pre‐AMI adherence.
(J Am Heart Assoc. 2017;6:e007106 DOI: 10.1161/JAHA.117.007106.)
This article was handled independently by Daniel Edmundowicz, MD, as a guest editor.
References
- 1. Choudhry NK, Glynn RJ, Avorn J, Lee JL, Brennan TA, Reisman L, Toscano M, Levin R, Matlin OS, Antman EM, Shrank WH. Untangling the relationship between medication adherence and post‐myocardial infarction outcomes: medication adherence and clinical outcomes. Am Heart J. 2014;167:51–58.e5. [DOI] [PubMed] [Google Scholar]
- 2. Kuepper‐Nybelen J, Hellmich M, Abbas S, Ihle P, Griebenow R, Schubert I. Association of long‐term adherence to evidence‐based combination drug therapy after acute myocardial infarction with all‐cause mortality. A prospective cohort study based on claims data. Eur J Clin Pharmacol. 2012;68:1451–1460. [DOI] [PubMed] [Google Scholar]
- 3. Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence‐based pharmacotherapy and long‐term mortality after acute myocardial infarction. JAMA. 2007;297:177–186. [DOI] [PubMed] [Google Scholar]
- 4. Zhang Y, Kaplan CM, Baik SH, Chang CC, Lave JR. Medication adherence and readmission after myocardial infarction in the Medicare population. Am J Manag Care. 2014;20:e498–e505. [PMC free article] [PubMed] [Google Scholar]
- 5. Schnipper JL, Kirwin JL, Cotugno MC, Wahlstrom SA, Brown BA, Tarvin E, Kachalia A, Horng M, Roy CL, McKean SC, Bates DW. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565–571. [DOI] [PubMed] [Google Scholar]
- 6. Garcia‐Caballos M, Ramos‐Diaz F, Jimenez‐Moleon JJ, Bueno‐Cavanillas A. Drug‐related problems in older people after hospital discharge and interventions to reduce them. Age Ageing. 2010;39:430–438. [DOI] [PubMed] [Google Scholar]
- 7. Kripalani S, Henderson LE, Jacobson TA, Vaccarino V. Medication use among inner‐city patients after hospital discharge: patient‐reported barriers and solutions. Mayo Clin Proc. 2008;83:529–535. [DOI] [PubMed] [Google Scholar]
- 8. Kronish IM, Ross JS, Zhao H, Muntner P. The impact of hospitalization for acute myocardial infarction on adherence to statins among older adults. Circ Cardiovasc Qual Outcomes. 2016;9:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beloin‐Jubinville B, Joly‐Mischlich T, Rouleau ED, Noiseux P, Blais L, Forget A, Beauchesne MF. Does hospitalization influence patients’ medication adherence and community pharmacists’ interventions? Ann Pharmacother. 2013;47:1143–1152. [DOI] [PubMed] [Google Scholar]
- 10. Cohen MJ, Shaykevich S, Cawthon C, Kripalani S, Paasche‐Orlow MK, Schnipper JL. Predictors of medication adherence postdischarge: the impact of patient age, insurance status, and prior adherence. J Hosp Med. 2012;7:470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Vries FM, Denig P, Vegter S, Bos HJ, Postma MJ, Hak E. Does a cardiovascular event change adherence to statin treatment in patients with type 2 diabetes? A matched cohort design. Curr Med Res Opin. 2015;31:595–602. [DOI] [PubMed] [Google Scholar]
- 12. Choma NN, Griffin MR, Huang RL, Mitchel EF Jr, Kaltenbach LA, Gideon P, Stratton SM, Roumie CL. An algorithm to identify incident myocardial infarction using Medicaid data. Pharmacoepidemiol Drug Saf. 2009;18:1064–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims‐based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. [DOI] [PubMed] [Google Scholar]
- 14. Buccaneer, Computer Systems and Services, Inc . Chronic condition data warehouse user manual. 2009;1.5.
- 15. Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–1079; discussion 1081‐90. [DOI] [PubMed] [Google Scholar]
- 16. Andrade SE, Graham DJ, Staffa JA, Schech SD, Shatin D, La Grenade L, Goodman MJ, Platt R, Gurwitz JH, Chan KA. Health plan administrative databases can efficiently identify serious myopathy and rhabdomyolysis. J Clin Epidemiol. 2005;58:171–174. [DOI] [PubMed] [Google Scholar]
- 17. Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC Jr, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK. ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation. 2004;110:e82–e292. [PubMed] [Google Scholar]
- 18. Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS®. SAS Institute Inc. 2012. Proceedings of the SAS® Global Forum 2012 Conference. Statistics and Data Analysis, Paper 335–2012. Cary, NC: SAS Institute Inc; http://support.sas.com/resources/papers/proceedings12/335-2012.pdf. Accessed June 21, 2016. [Google Scholar]
- 19. Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. [DOI] [PubMed] [Google Scholar]
- 20. Faridi KF, Peterson ED, McCoy LA, Thomas L, Enriquez J, Wang TY. Timing of first postdischarge follow‐up and medication adherence after acute myocardial infarction. JAMA Cardiol. 2016;1:147–155. [DOI] [PubMed] [Google Scholar]
- 21. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 22. Scranton RE, Young M, Lawler E, Solomon D, Gagnon D, Gaziano JM. Statin use and fracture risk: study of a US veterans population. Arch Intern Med. 2005;165:2007–2012. [DOI] [PubMed] [Google Scholar]
- 23. Choudhry NK, Avorn J, Glynn RJ, Antman EM, Schneeweiss S, Toscano M, Reisman L, Fernandes J, Spettell C, Lee JL, Levin R, Brennan T, Shrank WH. Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365:2088–2097. [DOI] [PubMed] [Google Scholar]
- 24. Stuart B, Davidoff A, Erten M, Gottlieb SS, Dai M, Shaffer T, Zuckerman IH, Simoni‐Wastila L, Bryant‐Comstock L, Shenolikar R. How Medicare Part D benefit phases affect adherence with evidence‐based medications following acute myocardial infarction. Health Serv Res. 2013;48:1960–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boudreaux ED, Bock B, O'Hea E. When an event sparks behavior change: an introduction to the sentinel event method of dynamic model building and its application to emergency medicine. Acad Emerg Med. 2012;19:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18:156–170. [DOI] [PubMed] [Google Scholar]
- 27. Allen LaPointe NM, Ou FS, Calvert SB, Melloni C, Stafford JA, Harding T, Peterson ED, Alexander KP. Association between patient beliefs and medication adherence following hospitalization for acute coronary syndrome. Am Heart J. 2011;161:855–863. [DOI] [PubMed] [Google Scholar]
- 28. O'Hea E, Abar B, Bock B, Chapman G, Boudreaux ED. Understanding smoking after acute illness: an application of the sentinel event method. Psychol Health. 2015;30:879–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cuffee YL, Hargraves JL, Rosal M, Briesacher BA, Schoenthaler A, Person S, Hullett S, Allison J. Reported racial discrimination, trust in physicians, and medication adherence among inner‐city African Americans with hypertension. Am J Public Health. 2013;103:e55–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smedley BD, Stith AY, Nelson AR. Institute of Medicine, Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal treatment: confronting racial and ethnic disparities in health care. 2003. [PubMed]
- 31. Mathews R, Peterson ED, Honeycutt E, Chin CT, Effron MB, Zettler M, Fonarow GC, Henry TD, Wang TY. Early medication nonadherence after acute myocardial infarction: insights into actionable opportunities from the TReatment with ADP receptor iNhibitorS: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE‐ACS) Study. Circ Cardiovasc Qual Outcomes. 2015;8:347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shah ND, Dunlay SM, Ting HH, Montori VM, Thomas RJ, Wagie AE, Roger VL. Long‐term medication adherence after myocardial infarction: experience of a community. Am J Med. 2009;122:961.e7–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428. [DOI] [PubMed] [Google Scholar]
- 34. Misky GJ, Wald HL, Coleman EA. Post‐hospitalization transitions: examining the effects of timing of primary care provider follow‐up. J Hosp Med. 2010;5:392–397. [DOI] [PubMed] [Google Scholar]
- 35. Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828. [DOI] [PubMed] [Google Scholar]
- 36. Brookhart MA, Patrick AR, Schneeweiss S, Avorn J, Dormuth C, Shrank W, van Wijk BL, Cadarette SM, Canning CF, Solomon DH. Physician follow‐up and provider continuity are associated with long‐term medication adherence: a study of the dynamics of statin use. Arch Intern Med. 2007;167:847–852. [DOI] [PubMed] [Google Scholar]
- 37. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314–323. [DOI] [PubMed] [Google Scholar]
- 38. Ho PM, Lambert‐Kerzner A, Carey EP, Fahdi IE, Bryson CL, Melnyk SD, Bosworth HB, Radcliff T, Davis R, Mun H, Weaver J, Barnett C, Baron A, Del Giacco EJ. Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge: a randomized clinical trial. JAMA Intern Med. 2014;174:186–193. [DOI] [PubMed] [Google Scholar]
- 39. Hansen RA, Kim MM, Song L, Tu W, Wu J, Murray MD. Comparison of methods to assess medication adherence and classify nonadherence. Ann Pharmacother. 2009;43:413–422. [DOI] [PubMed] [Google Scholar]
- 40. Lau HS, de Boer A, Beuning KS, Porsius A. Validation of pharmacy records in drug exposure assessment. J Clin Epidemiol. 1997;50:619–625. [DOI] [PubMed] [Google Scholar]
- 41. Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. [DOI] [PubMed] [Google Scholar]
- 42. Stuart B, Loh FE. Medicare Part D enrollees’ use of out‐of‐plan discounted generic drugs. J Am Geriatr Soc. 2012;60:387–388. [DOI] [PubMed] [Google Scholar]
- 43. Roberto PN, Stuart B. Out‐of‐plan medication in Medicare Part D. Am J Manag Care. 2014;20:743–748. [PubMed] [Google Scholar]
- 44. Zhou L, Stearns SC, Thudium EM, Alburikan KA, Rodgers JE. Assessing Medicare Part D claim completeness using medication self‐reports: the role of veteran status and Generic Drug Discount Programs. Med Care. 2015;53:463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brown JD, Doshi PA, Talbert JC. Utilization of free medication samples in the United States in a nationally representative sample: 2009–2013. Res Social Adm Pharm. 2016;13:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient Characteristics Stratified by Change in Statin Adherence After AMI
Table S2. Patient Characteristics Stratified by Pre‐AMI Statin Adherence
Table S3. Patient Characteristics Stratified by Post‐AMI Statin Adherence
Table S4. Full Model for 3‐Level Multinomial Regression Model Predicting Statin Adherence Change After an AMI
Table S5. Full Model for 5‐Level Multinomial Regression Model Predicting Statin Adherence Change After an AMI
Table S6. Crude Estimates, Fully Adjusted Model, and Sensitivity Analyses Involving Changing Variable Definitions or Adding New Variables to the Model for the 3‐Level Multinomial Regression Model Predicting Statin Adherence Change After an AMI
Table S7. Fully Adjusted Model and Sensitivity Analyses With Restricted Cohort Eligibility for the 3‐Level Multinomial Regression Model Predicting Statin Adherence Change After an AMI
Table S8. Three‐Level Multinomial Regression Models Predicting Change From 1‐Year Pre‐AMI Statin Adherence to 1‐Year Post‐AMI Statin Adherence (N=112 780)
Table S9. Three‐Level Multinomial Regression Model Predicting Statin Adherence Change After an AMI Using a Likelihood Function Conditional on the Pre‐AMI Adherence Category†
Table S10. Linear Regression Model Predicting Statin Adherence Change After an AMI
Figure S1. Sensitivity analysis showing distribution of 1‐year post‐AMI adherence stratified by 1‐year pre‐AMI adherence.


