Abstract
Background: The 21-gene recurrence score (RS) predicts risk of locoregional recurrence (LRR) in node-negative, estrogen receptor (ER)–positive breast cancer. We evaluated the association between RS and LRR in node-positive, ER-positive patients treated with adjuvant chemotherapy plus tamoxifen in National Surgical Adjuvant Breast and Bowel Project B-28.
Methods: B-28 compared doxorubicin/cyclophosphamide (AC X 4) with AC X 4 followed by paclitaxel X 4. Tamoxifen was given to patients age 50 years or older and those younger than age 50 years with ER-positive and/or progesterone receptor–positive tumors. Lumpectomy patients received breast radiotherapy. Mastectomy patients received no radiotherapy. The present study includes 1065 ER-positive, tamoxifen-treated patients with RS assessment. Cumulative incidence functions and subdistribution hazard regression models were used for LRR to account for competing risks including distant recurrence, second primary cancers, and death from other causes. Median follow-up was 11.2 years. All statistical tests were one-sided.
Results: There were 80 LRRs (7.5%) as first events (68% local/32% regional). RS was low: 36.2%; intermediate: 34.2%; and high: 29.6%. RS was a statistically significant predictor of LRR in univariate analyses (10-year cumulative incidence of LRR = 3.3%, 7.2%, and 12.2% for low, intermediate, and high RS, respectively, P < .001). In multivariable regression analysis, RS remained an independent predictor of LRR (hazard ratio [HR] = 2.59, 95% confidence interval [CI] = 1.28 to 5.26, for a 50-point difference, P = .008) along with pathologic nodal status (HR = 1.91, 95% CI = 1.20 to 3.03, for four or more vs one to three positive nodes, P = .006) and tumor size (HR = 1.28, 95% CI = 1.05 to 1.55, for a 1 cm difference, P = .02).
Conclusions: RS statistically significantly predicts risk of LRR in node-positive, ER-positive breast cancer patients after adjuvant chemotherapy plus tamoxifen. These findings can help in the selection of appropriate candidates for comprehensive radiotherapy.
Locoregional recurrence (LRR) is a statistically significant predictor of distant recurrence (1,2). All types of LRR (in-breast recurrence, chest wall recurrence, and regional-nodal recurrence) have been found to increase risk for subsequent distant recurrence, although the magnitude of risk varies depending on the type of LRR (1,2).
Despite considerable progress in identifying genomic profiles predicting risk of distant recurrence, LRR risk assessment is still primarily based on traditional anatomic-pathologic factors (ie, tumor size, grade, pathologic nodal status, and lymphovascular invasion). More recently, several investigations have demonstrated that genomic classifiers that predict risk for distant recurrence also predict risk for LRR (3–6).
We have previously demonstrated that the 21-gene recurrence score (RS) assay predicts risk of LRR in node-negative, estrogen receptor (ER)–positive breast cancer patients treated with adjuvant endocrine therapy and adjuvant chemo-endocrine therapy (3). These findings led us to hypothesize that a similar association may exist between RS and risk of LRR in node-positive breast cancer patients treated with adjuvant chemo-endocrine therapy.
The primary objective of this study was to evaluate the association between RS assay and risk of LRR in node-positive, ER-positive patients treated with adjuvant chemo-endocrine therapy in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-28 trial. We further wanted to explore whether RS can be combined with traditional clinico-pathologic factors in order to derive an improved algorithm for prediction of LRR risk and as a result possibly identify subgroups of ER-positive, node-positive patients who may or may not need postmastectomy chest wall and regional nodal radiotherapy (RT) or the addition of regional nodal RT to breast RT postlumpectomy.
Methods
The Parent NSABP B-28 Trial
The NSABP B-28 trial evaluated whether the sequential addition of four cycles of paclitaxel (P; 225 mg/m2) to four cycles of doxorubicin/cyclophosphamide (AC) would improve disease-free (DFS) and overall survival (OS) compared with four cycles of AC alone in patients with resected operable, node-positive breast cancer. Between Augustu of 1995 and May of 1998, 3060 patients were randomly assigned (AC: n = 1529 patients and ACP: n = 1531 patients). Patients age 50 years or older and those younger than age 50 years with ER- or progesterone receptor (PR)–positive tumors also received tamoxifen for five years starting with the first dose of AC. Postlumpectomy breast RT was mandated, but regional-nodal RT was prohibited. Postmastectomy chest wall or regional nodal RT were prohibited.
The study was approved by the Essex Institutional Review Board (IRB) (NJ), the Aultman Hospital IRB (OH), and the University of Pittsburgh IRB (PA). These trials were approved by local human investigations committees or IRBs in accordance with assurances filed with and approved by the Department of Health and Human Services. Written informed consent was required.
Aims, Eligibility, and End Points
The aims of the present study (Correlative 21-Gene Recurrence Score Study: ClinicalTrials.gov: NSABP B-28: https://clinicaltrials.gov/show/NCT01420185) (7) were to evaluate the association between RS and risk of LRR and to examine the independent prognostic contribution of RS beyond traditional clinico-pathologic factors such as age, tumor size, grade, number of positive nodes, and adjuvant chemotherapy assignment. Eligible patients had to be ER positive by tissue microarray, tamoxifen treated, and with successful 21-gene RS assay assessment. The primary prespecified end point was LRR as first event, defined as time from study entry to first LRR considering competing risks such as distant recurrence, second primary cancer, and death from other causes.
RNA Assessment Methodology
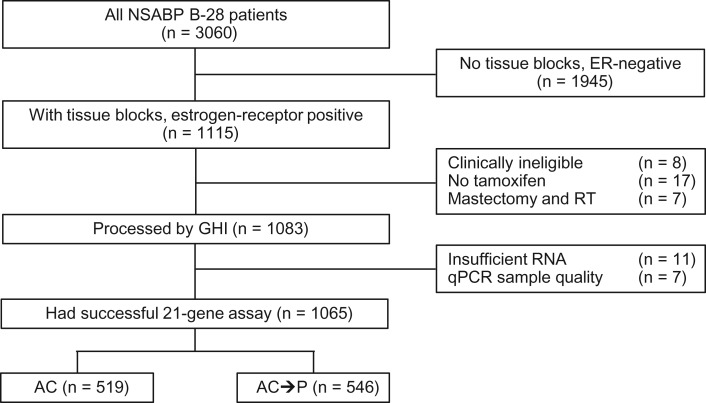
Available tumor specimens from B-28 that met the above criteria were centrally evaluated for histologic grade using the modified Bloom-Richardson score using five micron tissue sections stained by hematoxylin and eosin (H&E) (8). All specimens were then analyzed for the OncotypeDX recurrence score as previously described (9–11). Three five micron-thick sections were cut by the NSABP Division of Pathology laboratory. Tumor-rich area in the tumor block was marked by the Genomic Health, Inc. (GHI), pathologist using H&E-stained sections as references and manually microdissected with clean blades. RNA was extracted according to standard operating procedure for the OncotypeDX assay. RNA was then assessed for quantity (using the Ribogreen assay) and residual genomic DNA (using a DNA-specific polymerase chain reaction [PCR] assay). RNA was subjected to reverse transcription (with a universal RNA [Stratgene] as a positive control and water as a negative control for each set of RT reactions), followed by quantitative PCR (qPCR) analysis. The average reference gene expression served as a quality metric for each sample, and the limit of detection and quantitation cutoffs and other quality metrics, as defined for the 21-gene assay, was applied as appropriate for the 21 genes in RS. In the end, the OncotypeDX assay was successfully performed in 1065 patients with follow-up as described in the CONSORT diagram (Figure 1).
Figure 1.
CONSORT diagram. AC = doxorubicin/cyclophosphamide; ER = estrogen receptor; GHI = Genomic Health, Inc.; NSABP = National Surgical Adjuvant Breast and Bowel Project; qPCR = quantitative polymerase chain reaction; P = paclitaxel; RT = radiotherapy.
Statistical Methodology
Although RS is continuous, it is reported as integers after round-off. Patients were grouped into low (<18), intermediate (18–30), and high (≥31) RS. We tested whether RS risk groups are associated with differential risk of LRR by considering other events such as distant recurrence, second primary cancers, and death prior to any cancer as competing events (12). The cumulative incidence functions of LRR were estimated and compared via a one-sided K-sample test developed by Gray (12). The subdistribution proportional hazards regression models were used to describe the association between RS and risk of LRR with or without adjusting for clinical factors such as treatment, age, number of positive nodes, type of surgery (lumpectomy vs mastectomy), pathologic tumor size, and tumor grade (13). Residuals that are analogous to the Schoenfeld residuals in Cox models were used to check the proportionality assumption. Tests on the effect of individual covariates followed chi-square distributions and were one-sided. P values of less than .05 were considered statistically significant.
Results
LRR Rates in the Parent B-28 Trial
With median follow-up of 11.2 years, the overall 10-year cumulative incidence of LRR in B-28 was 9.3% in B-28 (95% CI = 8.3% to 10.4%), 10.0% in the AC arm (95% CI = 8.5% to 11.6%), and 8.6% in the ACP arm (95% CI = 7.3% to 10.1%, P = .24). The 10-year cumulative incidence of LRR was 8.0% for one to three positive nodes (95% CI = 6.9% to 9.2%), 12.4% for four or more positive nodes (95% CI = 10.4% to 14.7%), 6.5% for ER positive (95% CI = 5.5 to 7.7%), and 14.8% for ER-negative (95% CI = 12.6% to 17.0%).
Patient Population for the Current Study
Of the 3060 patients in the B-28 trial, 1945 were excluded because of either ER-negative tumors or no available tumor block. An additional 32 were excluded for various reasons (eight were clinically ineligible, 17 did not receive tamoxifen, and seven received postmastectomy RT). Of the remaining 1083 tumors processed by Genomic Health, 11 had insufficient RNA and seven had poor qPCR sample quality, leaving 1065 patients that constitute the core group for the present study.
Comparison of Included vs Excluded ER-Positive Patients
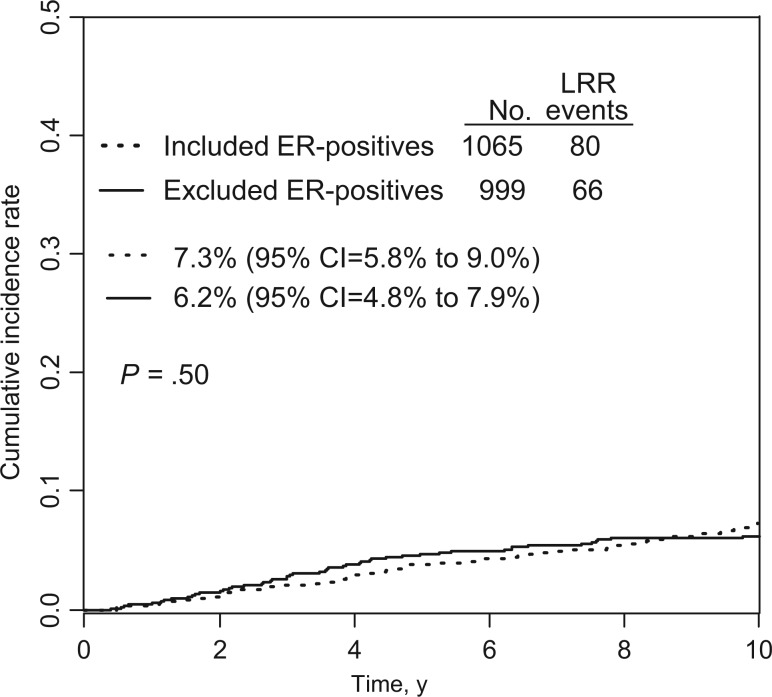
Among 1995 patients who were not included in this study, 999 were clinically eligible and had ER-positive tumors according to assessment from participating institutions. When the 1065 B-28 ER-positive patients included in the present study were compared with the 999 ER-positive patients who were excluded, there were no statistically significant differences in the distribution of age, number of positive nodes, or treatment group (AC or ACP) (Table 1). Compared with excluded patients, those who were included were statistically significantly more likely to have undergone mastectomy (P = .007), have larger tumors (P < .001), and have higher-grade tumors (P = .004) (Table 1). When the 10-year cumulative incidence of LRR in the 1065 patients included in the study was compared with that in the 999 ER-positive patients who were excluded, there were no statistically significant differences (7.3%, 95% CI = 5.8% to 9.0%, vs 6.2%, 95% CI = 4.8% to 7.9%, P = .50) (Figure 2).
Table 1.
Comparison of distribution of age, number of positive nodes, treatment, tumor size, grade, and surgery type between ER-positive patients included in the present study and those who were excluded
| Included ER+ patients
(n = 1065) |
Not included ER+ patients
(n = 999) |
||||
|---|---|---|---|---|---|
| Variable | No. (%) | No. (%) | P* | ||
| Age, y | .53 | ||||
| <50 | 511 (48.0) | 466 (46.7) | |||
| ≥50 | 553 (52.0) | 533 (53.3) | |||
| Tumor size, cm | <.001 | ||||
| ≤2.0 | 481 (45.4) | 566 (57.4) | |||
| 2.1-4.0 | 465 (43.9) | 319 (32.3) | |||
| ≥4.1 | 114 (10.7) | 102 (10.3) | |||
| Positive lymph nodes | .17 | ||||
| 1–3 | 722 (67.8) | 708 (71.2) | |||
| 4-9 | 300 (28.2) | 244 (24.5) | |||
| ≥10 | 43 (4.0) | 43 (4.3) | |||
| Tumor grade | .004 | ||||
| Well | 120 (11.3) | 152 (15.2) | |||
| Moderate | 499 (46.9) | 456 (45.7) | |||
| Poor | 405 (38.0) | 318 (31.8) | |||
| Unknown | 41 (3.8) | 73 (7.3) | |||
| Treatment | .16 | ||||
| AC | 519 (48.7) | 518 (51.8) | |||
| AC-P | 546 (51.3) | 481 (48.2) | |||
| Surgery type | .007 | ||||
| Lumpectomy | 461 (43.3) | 492 (49.3) | |||
| Mastectomy | 604 (56.7) | 507 (50.7) | |||
All statistical tests were chi-square distributed. All P values were one-sided. AC = doxorubicin/cyclophosphamide; ER = estrogen receptor; P = paclitaxel.
Figure 2.
Comparison of the 10-year cumulative incidence of locoregional recurrence between 1065 estrogen receptor (ER)–positive, B-28 patients who were included in the present study and 999 ER-positive, B-28 patients who were excluded. Gray’s k-sample test was used, and all P values were one-sided (10-year cumulative incidence of LRR = 7.3%, 95% confidence interval [CI] = 5.8% to 9.0%, vs 6.2%, 95% CI = 4.8% to 7.9%, P = .50 on graph). ER = estrogen receptor; LRR = locoregional recurrence.
Distribution of the Recurrence Score in the Study Population
Among the 1065 patients included in the present study, 386 (36.2%) had low RS (0–<18), 364 (34.2%) had intermediate RS (18–30), and 315 (29.6%) had high (≥31) RS. RS distribution was not statistically significantly different according to treatment, surgery type, or number of positive nodes. However, there were statistically significant differences in the distribution of RS according to age and tumor size, with older patients and those with small tumors being more likely to have low RS (data not shown).
Univariate Analysis of LRR According to RS Categories
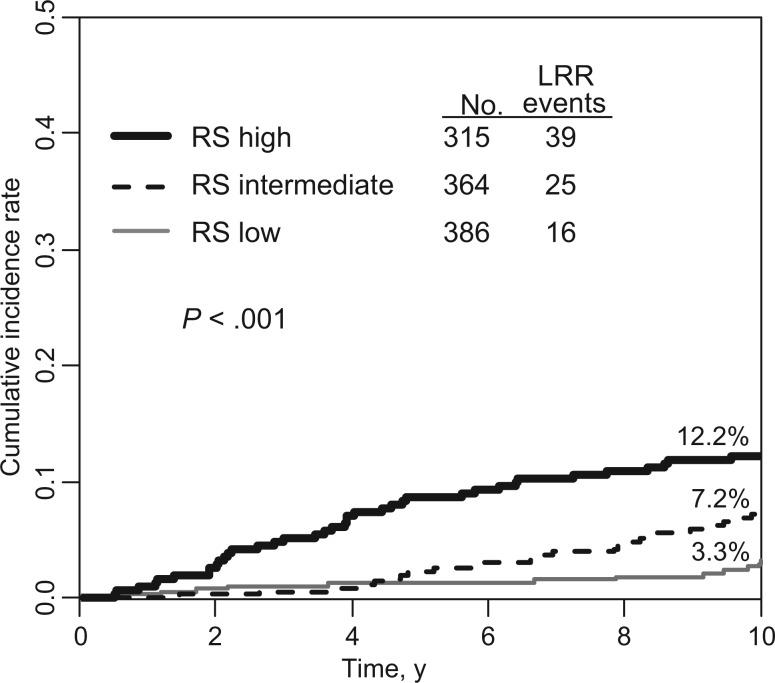
There were 80 LRRs (7.5%) as first events (68% local/32% regional). In univariate analyses, RS was a statistically significant predictor of LRR (Figure 3). The 10-year cumulative incidence of LRR was 3.3% (95% CI = 1.8% to 5.4%), 7.2% (95% CI = 4.8% to 10.2%), and 12.2% (95% CI = 8.8% to 16.1%) for low, intermediate, and high RS, respectively (P < .001). Similar associations between RS and risk of LRR were observed in patient subsets according to treatment assignment (AC or ACP), surgery type, age, tumor size, and number of positive nodes (Table 2).
Figure 3.
Cumulative incidence of locoregional recurrence by recurrence score status. Gray’s k-sample test was used, and all P values were one-sided (10-year cumulative incidence of LRR for low, intermediate, and high recurrence score, respectively = 3.3%, 95% confidence interval [CI] = 1.8% to 5.4%; 7.2%, 95% CI = 4.8% to 10.2%; and 12.2%, 95% CI = 8.8%, 16.1% on graph). LRR = locoregional recurrence; RS = recurrence score.
Table 2.
Cumulative incidence of locoregional recurrence by recurrence score risk groups in various subsets
| Clinical Factor Subsets | No. events /No. Pts | 10-year CIF (%) in
Recurrence
Score |
|||
|---|---|---|---|---|---|
| Low | Intermediate | High | P* | ||
| Treatment | |||||
| AC | 42/519 | 3.4 | 8.3 | 13.2 | .004 |
| AC+P | 38/546 | 3.1 | 6.2 | 11.3 | .04 |
| Surgery | |||||
| Lump.+ RT | 34/461 | 3.0 | 8.7 | 11.0 | .02 |
| Mastectomy | 46/604 | 3.5 | 5.9 | 12.9 | .004 |
| Age, years | |||||
| <50 | 41/511 | 3.4 | 7.4 | 11.1 | .09 |
| ≥50 | 39/553 | 3.2 | 7.0 | 13.8 | .001 |
| Tumor size, cm | |||||
| ≤2.0 | 20/351 | 2.6 | 6.1 | 7.1 | .54 |
| ≥2.1 | 60/714 | 3.9 | 8.1 | 15.3 | <.001 |
| No. of positive nodes | |||||
| 1–3 | 40/722 | 3.2 | 5.1 | 7.9 | .12 |
| ≥4 | 40/343 | 3.5 | 11.6 | 20.3 | .001 |
P values were calculated using a one-sided Gray’s test. AC = doxorubicin/cyclophosphamide; CIF = cumulative incidence function; P = paclitaxel; RT = radiation therapy.
Multivariable Analysis of LRR Adjusted for Clinico-Pathologic Variables
RS was an independent predictor of LRR in multivariable analysis (Table 3). Multivariable Cox subdistribution hazard regression model adjusting for treatment, age, number of positive nodes, type of surgery, tumor size, and tumor grade demonstrated a hazard ratio (HR) associated with a 50-unit increment in RS of 2.59 (95% CI = 1.28 to 5.26, P = .008). Additional independent predictors on multivariable analysis included number of positive nodes (HR = 1.91, 95% CI = 1.20 to 3.03 for ≥4 vs 1–3, P = .006) and tumor size (HR = 1.28, 95% CI = 1.05 to 1.55 for a 1 cm difference, P = .02).
Table 3.
Multivariable regression analysis for locoregional recurrence: NSABP B-28
| Variables | Subdistribution HR (95% CI) | P* |
|---|---|---|
| Recurrence score† | 2.59 (1.28 to 5.26) | .008 |
| AC + P vs AC | 0.83 (0.53 to 1.31) | .43 |
| Age ≥ 50 vs < 50 | 0.91 (0.56 to 1.48) | .7 |
| ≥4 positive nodes vs 1–3 | 1.91 (1.20 to 3.03) | .006 |
| Mastectomy vs lumpectomy | 0.81 (0.51 to 1.28) | .36 |
| Tumor size, cm‡ | 1.28 (1.05 to 1.55) | .02 |
| Intermediate vs low grade | 0.92 (0.38 to 2.23) | .85 |
| High vs low grade | 1.20 (0.48 to 2.99) | .7 |
All statistical tests were Wald-type tests and chi-square distributed. All P values were one-sided. AC = doxorubicin/cyclophosphamide; CI = confidence interval; HR = hazard ratio; P = paclitaxel.
Rescaled with a range from 0 to 2.
An upper threshold was imposed at 5 cm.
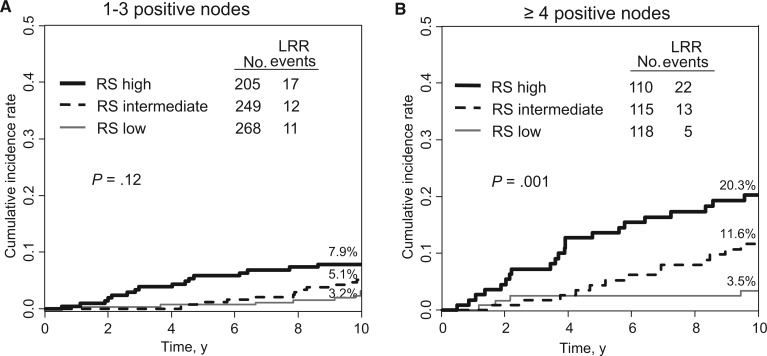
When the effect of RS on LRR was examined according to number of positive nodes, there was a statistically nonsignificant trend between RS and risk of LRR in patients with one to three positive nodes. The 10-year cumulative incidence of LRR was 3.2% (95% CI = 1.5% to 5.9%), 5.1% (95% CI = 2.8% to 8.4%), and 7.9% (95% CI = 4.7% to 12.1%) for low, intermediate, and high RS, respectively (P = .12) (Figure 4A). However, in patients with four or more positive nodes, RS was statistically significantly associated with risk of LRR, with 10-year cumulative incidence of LRR of 3.5% (95% CI = 1.1% to 8.0%), 11.6% (95% CI = 6.5% to 18.4%), and 20.3% (95% CI = 13.2% to 28.3%) for low, intermediate, and high RS, respectively (P = .001) (Figure 4B). Test of interaction between the prognostic impact of RS according to number of positive nodes was not statistically significant (P = .33)
Figure 4.
Cumulative incidence of locoregional recurrence by recurrence score (RS) and nodal status. A) Patients with one to three positive nodes (n = 722). Ten-year cumulative incidence of locoregional recurrence (LRR) was 3.2% (95% CI = 1.5% to 5.9%), 5.1% (95% CI = 2.8% to 8.4%), and 7.9% (95% CI = 4.7% to 12.1%) for low, intermediate, and high RS, respectively (P = .12). B) Patients with four or more positive nodes (n = 343). Ten-year cumulative incidence of LRR was 3.5% (95% CI = 1.1% to 8.0%), 11.6% (95% CI = 6.5% to 18.4%), and 20.3% (95% CI = 13.2% to 28.3%) for low, intermediate, and high RS, respectively (P = .001). Gray’s k-sample test was used, and all P values were one-sided. LRR = locoregional recurrence; RS = recurrence score.
Association Between RS and Risk of LRR According to Number of Positive Nodes and Surgery Type
We further examined the effect of RS according to nodal status and type of surgery received. For patients treated with mastectomy, there was no statistically significant association between RS and risk of LRR in patients with one to three positive nodes. The 10-year cumulative incidence of LRR was 2.4% (95% CI = 0.7% to 6.4%), 4.1% (95% CI = 1.5% to 8.7%), and 6.0% (95% CI = 2.6% to 11.4%) for low, intermediate, and high RS, respectively (P = .64) (Supplementary Figure 1A, available online). However, for patients with four or more positive nodes treated with mastectomy, RS was statistically significantly associated with risk of LRR with 10-year cumulative incidence of LRR of 5.5% (95% CI = 1.7% to 12.4%), 9.6% (95% CI = 3.8% to 18.5%), and 23.5% (95% CI = 14.6% to 33.5%) for low, intermediate, and high RS, respectively (P = .006) (Supplementary Figure 1B, available online). A test of interaction between the prognostic impact of RS according to number of positive nodes was not statistically significant (P = .49).
For patients treated with lumpectomy-plus-breast RT there was a statistically nonsignificant trend between RS and risk of LRR in patients with one to three positive nodes. The 10-year cumulative incidence of LRR was 3.9% (95% CI = 1.5% to 8.3%), 6.2% (95% CI = 2.7% to 11.8%), and 10.5% (95% CI = 5.1% to 18.0%) for low, intermediate, and high RS, respectively (P = .13) (Supplementary Figure 1C, available online). However, for patients with four or more positive nodes treated with lumpectomy-plus-breast RT, RS was statistically significantly associated with risk of LRR with 10-year cumulative incidence of LRR of 0.0%, 14.3% (95% CI = 6.2% to 25.7%), and 12.8% (95% CI = 3.8% to 27.3%) for low, intermediate, and high RS, respectively (P = .04) (Supplementary Figure 1D, available online). The regression model with the interaction term between RS and number of positive nodes did not converge.
Finally, we examined the effect of RS separately on the rates of local and regional recurrence according to type of surgery and number of positive nodes (Supplementary Figure 2, available online). For patients receiving mastectomy with one to three positive nodes, rates of local and regional recurrence were low (local recurrence: 1.6%, 95% CI = 0.3% to 5.3%; 1.7%, 95% CI = 0.3% to 5.4%; and 2.6%, 95% CI = 0.7% to 6.8%, for low, intermediate, and high RS, respectively; regional recurrence: 0.8%, 95% CI = 0.1% to 4.1%; 2.4%, 95% CI = 0.6% to 6.3%; and 3.4%, 95% CI = 1.1% to 8.0%, for low, intermediate, and high RS, respectively) (Supplementary Figure 2A, available online). For mastectomy patients with four or more positive nodes, rates of local recurrence increased with RS but remained moderate. Rates of regional recurrence were very low for low- and intermediate-RS patients but rose considerably for high-RS patients (local recurrence: 5.5%, 95% CI = 1.7% to 12.4%; 6.4%, 95% CI = 2.0% to 14.3%; and 9.1%, 95% CI = 4.0% to 16.9%, for low, intermediate, and high RS, respectively; regional recurrence: 0.0%; 3.2%, 95% CI = 0.6% to 10.1%; and 14.4%, 95% CI = 7.6% to 23.3%, for low, intermediate, and high RS, respectively) (Supplementary Figure 2B, available online). For lumpectomy patients with one to three positive nodes who also received breast RT, rates of local recurrence were generally low and rates of regional recurrence were extremely low (local recurrence: 3.9%, 95% CI = 1.5% to 8.3%; 6.2%, 95% CI = 2.7% to 11.8%; and 6.9%, 95% CI = 2.8% to 13.6%, for low, intermediate, and high RS, respectively; regional recurrence: 0.0%; 0.0%; and 3.5%, 95% CI = 0.9% to 9.1%, for low, intermediate, and high RS, respectively) (Supplementary Figure 2C, available online). For lumpectomy patients with four or more positive nodes, rates of local recurrence increased with RS but remained moderate, although rates of regional recurrence were very low for all RS subgroups (local recurrence: 0%; 12.2%, 95% CI = 4.9% to 23.2%; and 12.8%, 95% CI = 3.8% to 27.3%, for low, intermediate, and high RS, respectively; regional recurrence: 0.0%; 2.0%, 95% CI = 0.2% to 9.6%; and 0.0% for low, intermediate, and high RS, respectively) (Supplementary Figure 2D, available online). All regional recurrences occurred in the axilla (42%) and the supraclavicular area (58%).
Discussion
Our results of a statistically significant and independent association between RS and LRR in node-positive, ER-positive breast cancer patients treated with adjuvant chemo-endocrine therapy are concordant with those reported previously in patients with ER-positive, node-negative breast cancer treated with adjuvant endocrine therapy or with adjuvant chemo-endocrine therapy (14). In fact, rates of LRR according to RS in ER-positive/node-positive patients treated with adjuvant chemo-endocrine therapy in our study (3.3%, 7.2%, and 12.2% for low, intermediate, and high RS, respectively) were very similar to those observed in the ER-positive/node-negative patients treated with endocrine therapy alone in NSABP B-14/B-20 (4.3%, 7.2%, and 15.8% for low, intermediate, and high RS, respectively). Our findings are also concordant with those by Solin, who studied the effect of RS on LRR in 388 lumpectomy patients with zero to three positive nodes treated with adjuvant chemo-endocrine therapy and breast RT in ECOG E2197 (15). With 9.7 years’ median follow-up, the overall 10-year rates of LRR were 6.6% (6.3% for hormone receptor–positive, human epidermal growth factor receptor 2 [HER2]–negative tumors). For hormone receptor–positive tumors, the 10-year rates of LRR were 3.8%, 5.1%, and 12.0% for low, intermediate, and high 21-gene RS, respectively (P = .12), which is similar to our findings in lumpectomy patients with one to three positive nodes (10-year cumulative incidence of LRR: 3.9%, 6.2%, and 10.4% for low, intermediate, and high RS, respectively, P = .12).
One important locoregional therapy question in early-stage breast cancer relates to the use of postmastectomy RT (or regional-nodal RT after lumpectomy) in patients with a low number of positive axillary nodes. Despite an OS improvement with the addition of postmastectomy RT in patients with any number of positive nodes (16–18), this approach has not been uniformly accepted for those with one to three positive nodes. One of the main reasons for the continuing debate is that rates of LRR in the control arm of the postmastectomy RT trials and overview analyses (ie, the arm without RT) are considerably higher than those reported in more recent adjuvant trials such as B-28. For example, in the recently reported overview analysis of 22 randomized clinical trials of postmastectomy RT, the 10-year rate of LRR among 1133 women with one to three positive nodes treated with mastectomy and systemic therapy without RT was 21% (19). This is in stark contrast to the 10-year rate of 7.2% among mastectomy patients with one to three positive nodes in B-28. This rate was even lower in patients with ER-positive disease. Even within RS categories, our reported rates of LRR in ER-positive patients with one to three positive nodes ranged from 2.4% to 4.1% to 6.0% for low, intermediate, and high RS, respectively, although the differences were not statistically significant. In fact, our reported 10-year rate of LRR without RT in mastectomy patients with one to three positive nodes and low RS (2.4%) is similar to that reported in the overview analysis for 700 node-negative patients treated without RT (1.6%), a group for which no mortality reduction was shown with the addition of RT (17).
Although postmastectomy RT is generally recommended for all patients with four or more positive nodes because of their overall high rate of LRR, our findings—if confirmed by others—could potentially challenge the conventional wisdom that all patients with ER-positive tumors and four or more positive nodes are at high risk for LRR and that postmastectomy RT should be uniformly applied to all such patients. The 10-year rate of LRR without RT for patients with four or more positive nodes in B-28 was 13.6% overall and 12.2% for the ER-positive subset, considerably lower than the 32.1% reported in the overview analysis. More importantly, our finding that RS can identify a subgroup of ER-positive patients with four or more positive nodes who have a low 10-year rate of LRR (5.5%) suggests that genomic profiling could potentially identify a favorable subset of patients with four or more positive nodes for whom the role of postmastectomy RT could be revisited.
More recently, benefit from adding regional-nodal RT to breast RT was also demonstrated by Whelan in the NCIC (NCIC was National Cancer Institute of Canada, Clinical Trials Group [NCIC-CTG]; now Canadian Cancer Trials Group [CCTG]). MA.20 trial.19 That trial randomly assigned lumpectomy-treated patients primarily with one to three positive nodes (although 10% were high-risk node-negative and 5% had more than three positive nodes) to either breast RT or breast-plus-regional-nodal RT. Results showed a statistically significant improvement in LRR-free survival, disease-free survival, and distant DFS in those assigned to regional-nodal RT. However, there was no statistically significant improvement in OS with the addition of regional-nodal RT. In a prespecified subgroup analysis, the DFS treatment effects were greater for patients with ER-negative tumors (HR = 0.56) or PR-negative tumors (HR = 0.57) than for those with hormone receptor–positive tumors (ER-positive HR = 0.88; PR-positive HR = 0.91; Pinteraction value for ER = .04 and for PR = .03). These results will probably lead to an expansion of the use of regional-nodal RT in patients with one to three positive nodes who undergo breast-conserving surgery-plus-breast RT, making it increasingly important to identify subsets for which the addition of regional-nodal RT could be omitted. Our data suggest that lumpectomy patients with ER-positive, node-positive breast cancer who receive breast RT and adjuvant chemo-endocrine therapy have very low rates of regional nodal recurrence. This observation may be the result of a more favorable effect of breast RT in patients with ER-positive breast cancer and is supported by the MA.20 data and indirectly by other reports (6,20,21). However, it is important to note that our results showing low rates of regional nodal recurrence in patients with four or more positive nodes and high RS treated with lumpectomy-plus-breast RT represent unstable estimates that may be due to small cohort size (n = 33).
There are limitations to our study. This was a retrospective assessment of the RS as part of a prospective clinical trial. Although assignment to RT vs no RT was not by random assignment, the protocol specified that postmastectomy chest wall RT and regional-nodal RT were prohibited and postlumpectomy breast RT was mandated. Our results are exploratory, but if confirmed in other data sets they could have potential clinical implications for tailoring the locoregional RT approach for patients with ER-positive breast cancer and positive axillary nodes. By integrating RS with standard clinico-pathologic characteristics, such as number of positive nodes, a more tailored approach could be developed for the use of postmastectomy and regional nodal RT that will maximize efficacy while minimizing unnecessary toxicity.
Funding
This work is supported by: grants from the Susan G. Komen for the Cure, Public Health Service grants U10CA-180868, U10-CA-180822, and UG1-189867 from the National Cancer Institute, Department of Health and Human Services, and grants from Bristol-Myers Squibb Pharmaceutical Research Institute.
Notes
Trial registration: The original parent NSABP B-28 study predates ClinicalTrials.gov and PDQ registration requirements; this study, The Correlative 21-Gene Recurrence Score Study, is registered as follows: ClinicalTrials.gov: NSABP B-28: https://clinicaltrials.gov/show/NCT01420185.
The study sponsor(s) played no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors thank Barbara C. Good, PhD, for editorial assistance with this manuscript.
Potential conflicts of interest: Dr. Mamounas: honoraria: Genentech/Roche; consulting/advisory role: Genomic Health, Celgene, Pfizer, GSK, Novartis; Speakers' Bureau: Genomic Health, Genentech/Roche; travel/accommodations/expenses: Genomic Health, Genentech/Roche, Celgene. Dr. Baehner: employment and stock with Genomic Health. Dr. Tang: consulting/advisory role: Incyte. Dr. Butler: employment with Genomic Health and Avalanche Biotechnologies; stock with Genomic Health and Avalanche Biotechnologies; consulting/advisory role: Avalanche Biotechnologies. Dr. Jamshidian: employment, stock, and travel/accommodations/expenses with Genomic Health and Roche/Genentech. Dr. Cherbavaz: employment and travel/accommodations/expenses: Genomic Health, Inc. Dr. Sing: employment/leadership, stock: GHI. Dr. Shak: employment/leadership role and stock with Genomic Health; patents/royalties/other intellectual property filed with OncotypeDx, with institution. Dr. Lembersky: Speakers' Bureau: Genentech. Dr. Costantino: honoraria: Celgene. All other authors declare no other potential conflicts of interest.
Supplementary Material
References
- 1. Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;2413:2028–2037. [DOI] [PubMed] [Google Scholar]
- 2. Taghian A, Jeong JH, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: Results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004;2221:4247–4254. [DOI] [PubMed] [Google Scholar]
- 3. Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: Results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;2810:1677–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nuyten DS, Kreike B, Hart AA, et al. Predicting a local recurrence after breast-conserving therapy by gene expression profiling. Breast Cancer Res. 2006;85:R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;2618:2373–2378. [DOI] [PubMed] [Google Scholar]
- 6. Voduc KD, Cheang MC, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;2810:1684–1691. [DOI] [PubMed] [Google Scholar]
- 7.Correlative 21-Gene Recurrence Score Study. ClinicalTrials.gov: NSABP B-28. https://clinicaltrials.gov/show/NCT01420185. Accessed May 20, 16.
- 8. Elston EW, Ellis IO.. Method for grading breast cancer. J Clin Pathol. 1993;462:189–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;35127:2817–2826. [DOI] [PubMed] [Google Scholar]
- 10. Cronin M, Ghosh K, Sistare F, et al. Universal RNA reference materials for gene expression. Clin Chem. 2004;508:1464–1471. [DOI] [PubMed] [Google Scholar]
- 11. Cronin M, Sangli C, Liu ML, et al. Analytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer. Clin Chem. 2007;536:1084–1091. [DOI] [PubMed] [Google Scholar]
- 12. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;163:1141–1154. [Google Scholar]
- 13. Fine JP, Gray RJ.. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94446:496–509. [Google Scholar]
- 14. Solin LJ, Gray R, Goldstein LJ, et al. Prognostic value of biologic subtype and the 21-Gene Recurrence Score relative to local recurrence after breast conservation treatment with radiation for early stage breast carcinoma: Results from the Eastern Cooperative Oncology Group E2197 study. Breast Cancer Res Treat. 2012;1342:683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Early Breast Cancer Trialists' Collaborative Group. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: An overview of the randomised trials. Lancet. 2000;3559217:1757–1770. [PubMed] [Google Scholar]
- 16. Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;33714:949–955. [DOI] [PubMed] [Google Scholar]
- 17. Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;33714:956–962. [DOI] [PubMed] [Google Scholar]
- 18. Early Breast Cancer Trialists' Collaborative Group (EBCTCG), McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet .2014;3839935:2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Whelan TJ, Olivotto IA, Levine MN.. Regional nodal irradiation in early-stage breast cancer. New Engl J Med. 2015;3734:1878–1879. [DOI] [PubMed] [Google Scholar]
- 20. Kyndi M, Sørensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: The Danish Breast Cancer Cooperative Group. J Clin Oncol .2008;269:1419–1426. [DOI] [PubMed] [Google Scholar]
- 21. Fitzal F, Filipits M, Rudas M, et al. The genomic expression test EndoPredict is a prognostic tool for identifying risk of local recurrence in postmenopausal endocrine receptor-positive, her2neu-negative breast cancer patients randomised within the prospective ABCSG 8 trial. Br J Cancer. 2015;1128:1405–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.