ABSTRACT
Myoglianin, the Drosophila homolog of the secreted vertebrate proteins Myostatin and GDF-11, is an important regulator of neuronal modeling, and synapse function and morphology. While Myoglianin suppression during development elicits positive effects on the neuromuscular system, genetic manipulations of myoglianin expression levels have a varied effect on the outcome of performance tests in aging flies. Specifically, Myoglianin preserves jumping ability, has no effect on negative geotaxis, and negatively regulates flight performance in aging flies. In addition, Myoglianin exhibits a tissue-specific effect on longevity, with myoglianin upregulation in glial cells increasing the median lifespan. These findings indicate complex role for this TGF-β-like protein in governing neuromuscular signaling and consequent behavioral outputs and lifespan in adult flies.
KEYWORDS: Drosophila, myoglianin, lifespan, performance
Introduction
In vertebrates, growth differentiation factor 11 (GDF-11) and Myostatin (MST, also known as GDF-8) are closely related ligands of the Transforming growth factor-β (TGF-β) superfamily of proteins. While MST functions as a potent negative regulator of skeletal muscle growth,1,2 GDF-11 is a well-documented suppressor of neurogenesis and neuronal number.3-5 Both proteins are detected in the blood serum and act primarily by binding to activin type II receptors and eliciting response via intracellular transducers and transcriptional modulators SMAD2 and SMAD3.6-9 Myoglianin (MYO) is the Drosophila homolog of Myostatin and GDF-11, with strong expression detected in muscle and glial cells.10,11
Recently, we identified MYO as a regulator of muscle size and body weight in Drosophila larvae (Augustin et al.12). In addition, genetic attenuation of myoglianin (myo) in muscles or glial cells strengthens the synaptic transmission and upregulates the density of critical pre- and post-synaptic markers at the fly NMJ (neuromuscular junction) via modulation of Smad2/Smox and GSK-3/shaggy signaling (Fig. 1). The same manipulation improves an electrical synapse output in adult flies, pointing toward a broader mechanism for synapse regulation.12 These findings led us to believe that Myoglianin in flies could combine some of the main roles of MST and GDF-11 in vertebrates. Downregulation of myo throughout development in either muscle or glial compartment leads to improved larval motility (faster crawling speed),12 demonstrating a way to enhance performance parameters in flies and, possibly, other species. Interestingly, injections of human MST into developing larvae reversed the positive affect of Myoglianin suppression on synaptic function, suggesting the possibility of MST having a similar role in the mammalian nervous system.
Figure 1.
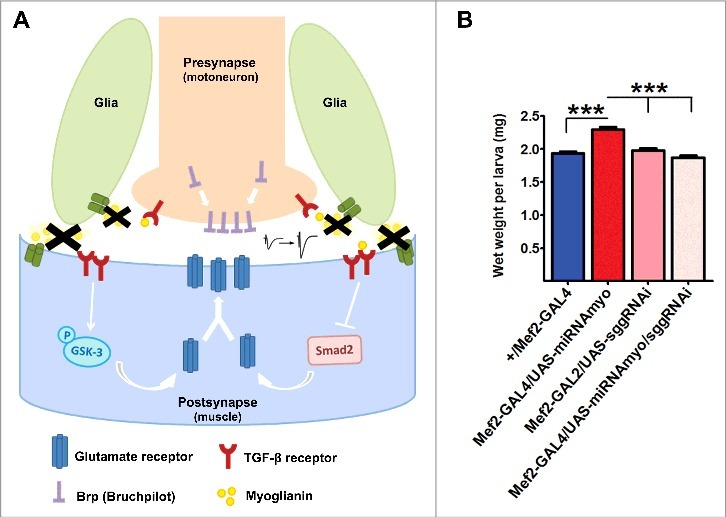
Reduced Myoglianin levels promote membrane targeting of the presynaptic active zone assembly regulator (Brp) and postsynaptic glutamate receptors (type A) via inhibition of Smad2/ Smox and stimulation of GSK-3/shaggy signaling, resulting in enhanced electrophysiological response.
Both MST and GDF-11 have been implicated in the control of age-related processes. For example, inhibition of Myostatin increases skeletal muscle mass and strength and improves exercise-induced performance outcome in aging mice.13,14 Myostatin levels decline with age in healthy men,15 and MST inhibition is being investigated as a potential treatment of sarcopenia and other muscle-wasting disorders.16 Conflicting results exist regarding the role of GDF-11 in aging. While early findings indicated a positive effect of GDF-11 on brain17 and skeletal muscle18 aging, the subsequent reports identified GDF-11 as a likely pro-aging factor.19-21
We therefore wanted to assess the impact of Myoglianin on age-dependent muscle function in adult flies. Myoglianin transcript is detected at low to moderate levels in various fly adult tissues.22 However, as the muscular system in adult flies appear to be extremely limited in terms of its ability to grow and regenerate,23 the role of MYO in aging flies might be significantly different from its role in rapidly growing tissues during development.
Results and discussion
In flies with MYO levels reduced in muscles (using the UAS-miRNAmyo construct driven by the Mef2-GAL4 driver), we observed improved flight ability throughout adult life compared with controls, with a small decrease in body weight measured in these flies with age (Fig. 2A). These results parallel the improved motility of 3rd instar larvae with genetically silenced myo.12 Equally, the opposite effect on the flight ability was seen in myo-overexpressing flies (Mef2-GAL4/UAS-myoglianin, Fig. 2B).
Figure 2.
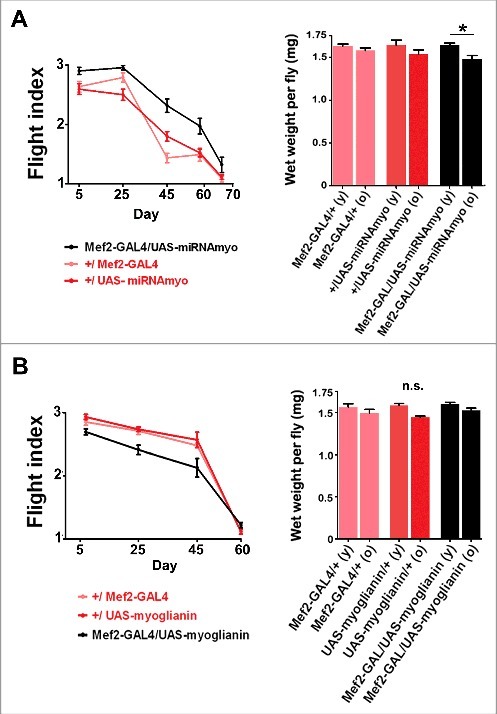
MYO negatively regulates flight performance in adult flies. (A) Left: Flight performance index measured in Mef2-GAL4/UAS-miRNAmyo (black) and control (red) flies throughout the adult life (n = 31–33, ANOVA genotype/age interaction P = 0.0055). Right: Total wet weight of individual flies measured in young (day 5) and old (day 50) animals (n = 10–12). (B) Left: Flight performance index measured in Mef2-GAL4/UAS-myoglianin (black) and control (red) flies (n = 26–29). The genotype/age interaction was significant (P=0.0056). Right: Total wet weight of individual flies measured in young (day 5) and old (day 50) animals (n = 10–12).
In disagreement with a recent paper reporting impaired climbing in the flies with RNAi-reduced myo expression in muscles,24 we saw no effect on the climbing ability upon muscle-specific myo silencing (Fig. 3A). Interestingly, increased myo expression prevented age-related decline in the jumping ability, resulting in ‘youthful’ jump test outcomes even in late adulthood (day 48) (Fig. 3B).
Figure 3.
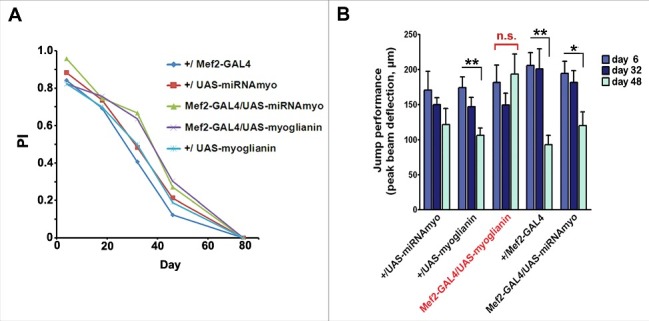
MYO affects jumping ability, but not climbing performance. (A) The climbing ability (‘negative geotaxis’) of the adult flies of denoted genotypes. No significant difference between the genotypes was observed. (B) Jump muscle performance is sustained by expression of myoglianin by Mef2 (n = 6–19 per genotype/age). In the full data set, including Mef2-GAL4/UAS-myoglianin, ANOVA genotype interaction with age P=0.042; but without the 48 day-old data, or the Mef2-GAL4/UAS-myoglianin data the genotype-age interaction is not significant (P = 0.57 and P = 0.49, respectively).
Demontis et al.24 reported lifespan extension in myo-overexpressing flies and reduced longevity in myo-silenced animals. In our hands, variable myo expression levels in the muscle did not significantly affect lifespan (Fig. 4A). It is possible that these phenotypic differences stem from different muscle drivers and RNAi constructs used to manipulate myoglianin levels in adult muscles. For example, while the MHC-GAL4 driver (used by Demontis et al., 201424) drives strongly in thoracic muscles, the Mef2-GAL4 drives “far more extensively, in most somatic musculature”.25 Intriguingly, myo overexpression in glial cells, previously shown to have a negative impact on the weight, motility and synaptic parameters in Drosophila larvae,12 resulted in significantly increased median lifespan, with glial silencing of myo having the opposite effect (Fig. 4B). These findings further our understanding of the lifespan-modulating role of the glial compartment in Drosophila.26,27
Figure 4.
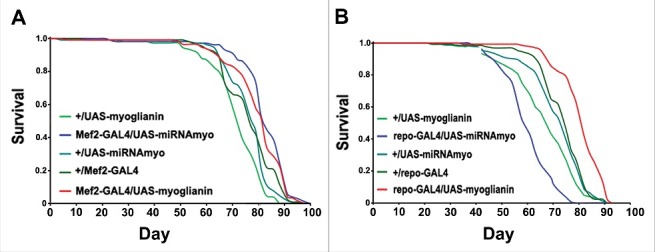
Myoglianin in glia, but not in muscle, affects lifespan. (A) Lifespans in flies expressing various myo transgenes in the muscle. Median survival times for female flies (in days) were 82 (Mef2-GAL4/UAS-miRNAmyo), 82 (Mef2-GAL4/UAS-myoglianin), 71 (+/UAS-myoglianin), 78 (+/UAS-miRNAmyo) and 76 (+/Mef2-GAL4); (B) The survival curves from flies expressing myo constructs driven by the glial repo-GAL4 driver. Median survival times for female flies were 59 (repo-GAL4/UAS-miRNAmyo), 80 d (repo-GAL4/UAS-myoglianin), 67 (+/UAS-myoglianin), 73 (+/UAS-miRNAmyo) and 73 d (+/repo-GAL4).
Our results imply a complex role for Myoglianin in modulating neuromuscular system function in adult flies, with different muscles/tissues requiring different levels of MYO for achieving optimal functional output. Furthermore, the combinatorial effect of these inputs is likely to play an important role in determining the overall health- and life-span in aging flies. Overall, in addition to its role as an important regulator of muscle size, body weight, motility, and synaptic composition and function in larvae,12 Myoglianin appears to have a highly regulated and context-dependent impact on adult tissues and whole organism in D. melanogaster.
Materials and methods
Fly stocks and husbandry
Tissue- and cell type-specific expression was achieved with the GAL4-UAS system.28 To standardize genetic background, parental GAL4 and UAS strains used to generate experimental and control genotypes were backcrossed to laboratory control strain wDah for at least 6 generations. All stocks were maintained and all experiments were conducted at 25ºC on a 12h:12h light:dark cycle at constant humidity using standard sugar/yeast/agar (SYA) medium29 (the food contained 5% sucrose (w/v), 1.5% agar (w/v), 0.3% propionic acid (v/v), 0.3% nipagen (w/v) and either 1% (0.1 × yeast), 5% (0.5 × yeast), 10% autolysed brewer's yeast (w/v) and was prepared as described previously.30 UAS-miRNAmyo line11 was a gift from T. Awasaki and from the T. Lee laboratory at Janelia Farm; UAS-myoglianin (2nd chromosome) was a kind gift from M. O'Connor, University of Minnesota; Mef2-GAL4 (#27390) and repo-GAL4 (#7415) were obtained from the Bloomington Stock Center. wDah was the “wild-type” strain used in all experiments. The white Dahomey (wDah) stock was derived by incorporation of the w1118 mutation into the outbred Dahomey background by backcrossing.
Flies were mated for 48 h before separating females from males. Only female flies were used in the experiments.
Flight and climbing tests
Flight testing was done using a described previously protocol.31 Individual flies were released from the bottom of a perspex (‘Sparrow’) box 40 cm high with a light source at the top, and scored for flight as follows: top (2 points), middle (1 point) or bottom (no points). The mean point achieved by 3–6 flies was determined; the procedure was repeated 5–10 and mean calculated for each genotype/time point.
For the climbing (negative geotaxis) test, adult female flies were housed at 15 flies/vial and 3 populations were tested for each genotype/experiment. The flies were assayed at 5 time points (days 4, 18, 32, 46 and 70) for climbing activity in a modified 25 ml ‘stripette’ tube. The flies were gently tapped to the base of the climbing tube and their climbing progress was recorded after 45 s. Each population of flies were assessed 3 times per assay and the average values were used to calculate the performance index as described previously.32
Lifespan experiments
Flies in lifespan experiments were performed as described previously.33 Animals were reared at standard larval density and eclosing adults were collected over a 12 h period. Females were mated for 48 h before being separated from the males. Flies were maintained in vials on standard SYA medium at a density of 10 flies per vial and transferred to new vials every 2–3 d and scored for deaths.
Jump muscle performance
Jump muscle performance was determined using a miniature ergometer.34,35 Briefly, flies were anaesthetized with CO2 and mounted on a tungsten pin and allowed to recover for over 20 min. They were mounted in the apparatus and the jump elicited by electrical stimulation between the eyes. The jump response was determined optically from the movement of the platform below the fly. For each fly the biggest response to 4–10 stimuli was determined. The best response of each fly was used in the calculation of mean and SEM and in statistical evaluations. The experimenter was blind to the exact genotypes during testing.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism 5 software (GraphPad Software Inc., USA). A 2 way ANOVA test was used to perform (age x genotype) interaction calculations. For other comparisons between 2 or more groups, a one-way ANOVA followed by a Tukey-Kramer post hoc test was used. In all instances, P < 0.05 is considered to be statistically significant (*P < 0.05; **P < 0.01; ***P < 0.001). Log-rank tests were performed for survival. Values are reported as the mean ± SEM.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgements
We would like to thank Michael O'Connor (University of Minnesota, USA) and Takeshi Awasaki (Janelia Farm, USA, and Kyorin University, Japan) for myoglianin lines, and the Bloomington Drosophila Stock Center for additional reagents.
Funding
This work was funded by a Wellcome Trust Strategic Award to L.P., and by the Max Planck Society.
References
- [1].McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83-90. doi: 10.1038/387083a0. PMID:9139826 [DOI] [PubMed] [Google Scholar]
- [2].Patel K, Amthor H. The function of Myostatin and strategies of Myostatin blockade-new hope for therapies aimed at promoting growth of skeletal muscle. Neuromuscul Disord. 2005;15:117-26. doi: 10.1016/j.nmd.2004.10.018. PMID:15694133 [DOI] [PubMed] [Google Scholar]
- [3].Kawauchi S, Kim J, Santos R, Wu HH, Lander AD, Calof AL. Foxg1 promotes olfactory neurogenesis by antagonizing Gdf11. Development. 2009;136:1453-64. doi: 10.1242/dev.034967. PMID:19297409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kim J, Wu HH, Lander AD, Lyons KM, Matzuk MM, Calof AL. GDF11 controls the timing of progenitor cell competence in developing retina. Science. 2005;308:1927-30. doi: 10.1126/science.1110175. PMID:15976303 [DOI] [PubMed] [Google Scholar]
- [5].Wu HH, Ivkovic S, Murray RC, Jaramillo S, Lyons KM, Johnson JE, Calof AL. Autoregulation of neurogenesis by GDF11. Neuron. 2003;37:197-207. doi: 10.1016/S0896-6273(02)01172-8. PMID:12546816 [DOI] [PubMed] [Google Scholar]
- [6].Oh SP, Yeo CY, Lee Y, Schrewe H, Whitman M, Li E. Activin type IIA and IIB receptors mediate Gdf11 signaling in axial vertebral patterning. Genes Dev. 2002;16:2749-54. doi: 10.1101/gad.1021802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L. Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol. 2003;23:7230-42. doi: 10.1128/MCB.23.20.7230-7242.2003. PMID:14517293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Andersson O, Reissmann E, Ibanez CF. Growth differentiation factor 11 signals through the transforming growth factor-beta receptor ALK5 to regionalize the anterior-posterior axis. EMBO Rep. 2006;7:831-7. PMID:16845371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Budi EH, Xu J, Derynck R. Regulation of TGF-beta Receptors. Methods Mol Biology. 2016;1344:1-33. doi: 10.1007/978-1-4939-2966-5_1. PMID:26520115 [DOI] [PubMed] [Google Scholar]
- [10].Lo PC, Frasch M. Sequence and expression of myoglianin, a novel Drosophila gene of the TGF-beta superfamily. Mechanisms of development. 1999;86:171-5. doi: 10.1016/S0925-4773(99)00108-2. PMID:10446278 [DOI] [PubMed] [Google Scholar]
- [11].Awasaki T, Huang Y, O'Connor MB, Lee T. Glia instruct developmental neuronal remodeling through TGF-beta signaling. Nature neuroscience. 2011;14:821-3. doi: 10.1038/nn.2833. PMID:21685919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Augustin H, McGourty K, Steinert JR, Cocheme HM, Adcott J, Cabecinha M, Vincent A, Halff EF, Kittler JT, Boucrot E, et al.. Myostatin-like proteins regulate synaptic function and neuronal morphology. Development. 2017. doi: 10.1242/dev.152975. PMID:28533206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].LeBrasseur NK, Schelhorn TM, Bernardo BL, Cosgrove PG, Loria PM, Brown TA. Myostatin inhibition enhances the effects of exercise on performance and metabolic outcomes in aged mice. J Gerontol A Biol Sci Med Sci. 2009;64:940-8. doi: 10.1093/gerona/glp068. PMID:19483181 [DOI] [PubMed] [Google Scholar]
- [14].Whittemore LA, Song K, Li X, Aghajanian J, Davies M, Girgenrath S, Hill JJ, Jalenak M, Kelley P, Knight A, et al.. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun. 2003;300:965-71. doi: 10.1016/S0006-291X(02)02953-4. PMID:12559968 [DOI] [PubMed] [Google Scholar]
- [15].Schafer MJ, Atkinson EJ, Vanderboom PM, Kotajarvi B, White TA, Moore MM, Bruce CJ, Greason KL, Suri RM, Khosla S, et al.. Quantification of GDF11 and Myostatin in Human Aging and Cardiovascular Disease. Cell Metab. 2016;23:1207-15. doi: 10.1016/j.cmet.2016.05.023. PMID:27304512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ali S, Garcia JM. Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options – a mini-review. Gerontology. 2014;60:294-305. doi: 10.1159/000356760. PMID:24731978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630-4. doi: 10.1126/science.1251141. PMID:24797482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, et al.. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649-52. doi: 10.1126/science.1251152. PMID:24797481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, Mallozzi C, Jacobi C, Jennings LL, Clay I, et al.. GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration. Cell Metab. 2015;22:164-74. doi: 10.1016/j.cmet.2015.05.010. PMID:26001423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hammers DW, Merscham-Banda M, Hsiao JY, Engst S, Hartman JJ, Sweeney HL. Supraphysiological levels of GDF11 induce striated muscle atrophy. EMBO Mol Med. 2017;9:531-44. doi: 10.15252/emmm.201607231. PMID:28270449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rodgers BD, Eldridge JA. Reduced Circulating GDF11 Is Unlikely Responsible for Age-Dependent Changes in Mouse Heart, Muscle, and Brain. Endocrinology. 2015;156:3885-8. doi: 10.1210/en.2015-1628. PMID:26372181 [DOI] [PubMed] [Google Scholar]
- [22].mod EC, Roy S, Ernst J, Kharchenko PV, Kheradpour P, Negre N, Eaton ML, Landolin JM, Bristow CA, Ma L, et al.. Identification of functional elements and regulatory circuits by Drosophila modENCODE. Science. 2010;330:1787-97. doi: 10.1126/science.1198374. PMID:21177974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Augustin H, Partridge L. Invertebrate models of age-related muscle degeneration. Biochim Biophys Acta. 2009;1790:1084-94. doi: 10.1016/j.bbagen.2009.06.011. PMID:19563864 [DOI] [PubMed] [Google Scholar]
- [24].Demontis F, Patel VK, Swindell WR, Perrimon N. Intertissue control of the nucleolus via a myokine-dependent longevity pathway. Cell Rep. 2014;7:1481-94. doi: 10.1016/j.celrep.2014.05.001. PMID:24882005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Viswanathan MC, Blice-Baum AC, Schmidt W, Foster DB, Cammarato A. Pseudo-acetylation of K326 and K328 of actin disrupts Drosophila melanogaster indirect flight muscle structure and performance. Front Physiol. 2015;6:116. doi: 10.3389/fphys.2015.00116. PMID:25972811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kounatidis I, Chtarbanova S, Cao Y, Hayne M, Jayanth D, Ganetzky B, Ligoxygakis P. NF-kappaB Immunity in the Brain Determines Fly Lifespan in Healthy Aging and Age-Related Neurodegeneration. Cell Rep. 2017;19:836-48. doi: 10.1016/j.celrep.2017.04.007. PMID:28445733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sanchez D, Lopez-Arias B, Torroja L, Canal I, Wang X, Bastiani MJ, Ganfornina MD. Loss of glial lazarillo, a homolog of apolipoprotein D, reduces lifespan and stress resistance in Drosophila. Current biology: CB. 2006;16:680-6. doi: 10.1016/j.cub.2006.03.024. PMID:16581513 [DOI] [PubMed] [Google Scholar]
- [28].Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993;118:401-15. PMID:8223268 [DOI] [PubMed] [Google Scholar]
- [29].Bass TM, Grandison RC, Wong R, Martinez P, Partridge L, Piper MD. Optimization of dietary restriction protocols in Drosophila. J Gerontol A Biol Sci Med Sci. 2007;62:1071-81. doi: 10.1093/gerona/62.10.1071. PMID:17921418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science. 2004;305:361. doi: 10.1126/science.1098219. PMID:15192154 [DOI] [PubMed] [Google Scholar]
- [31].Drummond DR, Hennessey ES, Sparrow JC. Characterisation of missense mutations in the Act88F gene of Drosophila melanogaster. Molecular & general genetics: MGG 1991;226:70-80. doi: 10.1007/BF00273589 [DOI] [PubMed] [Google Scholar]
- [32].Sofola O, Kerr F, Rogers I, Killick R, Augustin H, Gandy C, Allen MJ, Hardy J, Lovestone S, Partridge L. Inhibition of GSK-3 ameliorates Abeta pathology in an adult-onset Drosophila model of Alzheimer's disease. PLoS Genet. 2010;6:e1001087. doi: 10.1371/journal.pgen.1001087. PMID:20824130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Piper MD, Partridge L. Protocols to Study Aging in Drosophila. Methods Mol Biol. 2016;1478:291-302. doi: 10.1007/978-1-4939-6371-3_18. PMID:27730590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Elliott CJ, Brunger HL, Stark M, Sparrow JC. Direct measurement of the performance of the Drosophila jump muscle in whole flies. Fly. 2007;1:68-74. doi: 10.4161/fly.3979. PMID:18820447 [DOI] [PubMed] [Google Scholar]
- [35].Elliott CJ, Sparrow JC. In vivo measurement of muscle output in intact Drosophila. Methods. 2012;56:78-86. doi: 10.1016/j.ymeth.2011.10.005. PMID:22037247 [DOI] [PubMed] [Google Scholar]


