ABSTRACT
The Abelson tyrosine kinase (Abl) lies at the heart of one of the small set of ubiquitous, conserved signal transduction pathways that do much of the work of development and physiology. Abl signaling is essential to epithelial integrity, motility of autonomous cells such as blood cells, and axon growth and guidance in the nervous system. However, though Abl was one of the first of these conserved signaling machines to be identified, it has been among the last to have its essential architecture elucidated. Here we will first discuss some of the challenges that long delayed the dissection of this pathway, and what they tell us about the special problems of investigating dynamic processes like motility. We will then describe our recent experiments that revealed the functional organization of the Abl pathway in Drosophila neurons. Finally, in the second part of the review we will introduce a different kind of complexity in the role of Abl in motility: the discovery of a previously unappreciated function in protein secretion and trafficking. We will provide evidence that the secretory function of Abl also contributes to its role in axon growth and guidance, and finally end with a discussion of the challenges that Abl pleiotropy provide for the investigator, but the opportunities that it provides for coordinating biological regulation.
KEYWORDS: Abl, Abi1, axon guidance, disabled, enabled, Trio
Introduction
Axonal growth cones encounter an ensemble of guidance cues in the extracellular milieu that direct expansion of the leading edge plasma membrane. Constellations of axon guidance receptors housed in the plasma membrane respond to those cues by regulating the dynamics of the actin cytoskeleton through a variety of cytoplasmic signaling networks.1,2 Of these, one central regulator of the neuronal cytoskeleton in growing axons is the non-receptor, cytoplasmic tyrosine kinase, Abl.3-7 Indeed, almost all of the common, phylogenetically conserved families of guidance receptors - Notch, DCC, Robo, Plexin, Eph and others - signal through Abl kinase.8-16 Furthermore, Abl links diverse extracellular stimuli to other signaling pathways that control survival, invasion and adhesion, in processes including early development, immune function, leukemia, solid tumors and Alzheimer disease.17-23 The mechanism of Abl signaling, therefore, marks a fundamental process underpinning the cellular and molecular mechanisms of cell motility, growth cone guidance and epithelial integrity, from neurodevelopment to neurodegenerative disease.
Many studies of axon patterning have applied powerful genetic tools to try to dissect the molecular mechanism underlying Abl-dependent cytoskeletal remodeling. Numerous genetic experiments identified a cloud of potential components of Abl signaling that act in concert during axon patterning. They include Disabled (Dab), an adaptor protein; Trio, a GEF for Rac and Rho GTPases; Enabled, an actin regulator and Abi1, a component of the WAVE/SCAR complex that activates Arp2/3.10,11,15,22,24-26 However, to establish the mechanism by which the Abl signaling network achieves its diverse effects, it was imperative to figure out precisely what are the molecular relationships between individual members of the pathway.
Undeniably, the mechanistic basis of this genetic pathway is intricate and complex. But beyond this, the challenges furnished by the Abl network are instructive for thinking about how we might best investigate other dynamic signaling pathways. In part, the challenges of investigating Abl arise from pleiotropy. Since Abl and its signaling partners are ubiquitous, acting in parallel in several cell types to perform any single morphogenetic function, the phenotype assayed in wholly mutant animals often reflects a conglomerate effect of unrelated individual functions in different cells. Therefore, seemingly similar experiments could produce quite divergent results just because the reagents acted differentially in space and time within the animal. It was therefore essential to control gene function, acutely, in selected cells, and also to monitor phenotypic consequences in individual cells.15 Second, the effects of Abl on motility are time-dependent, with many opportunities for compensation or phenotypic bypass. This makes immediate readouts of pathway activity essential for drawing reliable conclusions.27 Finally, the dynamic processes in the cytoskeleton are typically not linear pathways; instead they are cyclical. Manipulations that simply lock a piece of the machine into an ON or OFF state generally are not an effective way to study the mechanism of the cycle phenotypically since either condition just breaks the cycle.28-30 Instead, more nuanced treatments that modulate the cycle without interrupting it tend to give more consistent and interpretable results.9,15,31,32 Thus, for example, the biological role of Rac GTPase in Notch-dependent axon guidance choices was difficult to infer from the effects of strong genetic manipulations due to the similar overt phenotypes of gain- vs. loss-of-function. Use of mild genetic manipulations, such as introducing heterozygous mutations into sensitized genetic backgrounds and relying on statistical effects on large sample sizes, was far more effective for obtaining interpretable results from this cyclical, dynamic cell biologic process.32
Recently, using a combination of direct FRET measurements of the biochemical outputs of pathway activity and analysis of protein localization, in addition to genetic epistasis, we explored the architecture of the Abl pathway in Drosophila neurons.27 This 3-pronged approach allowed us to establish that Abl defines a bifurcating pathway that achieves a balance between the 2 major classes of actin structures in the cell, linear actin filaments vs. branched actin networks27 (Fig. 1). The adaptor protein Dab, in addition to having a role in Abl protein localization, stimulates Abl kinase activity in neurons. Downstream of this, Abl simultaneously suppresses the actin-regulatory factor Enabled to limit extension of actin filaments, and activates the GEF Trio to stimulate Rac signaling. Finally, active Rac engages the WAVE complex to promote formation of branched actin networks.
Figure 1.

Functional architecture of the Abl signaling network. Abl signaling bifurcates to balance different actin filaments during axon guidance. Solid arrows indicate positive interaction, T-bar indicates negative relationship, and dashed line indicates physical association. Schematic modified from ref.27
Abl signaling bifurcates to balance actin dynamics at the leading edge
Dab acts upstream of Abl while Trio functions between Abl and Rac
Disabled, a tyrosine phosphorylated adaptor protein, has been thought to serve as a possible point of linkage of cell surface receptors to cytoplasmic signaling elements.24,33 For some time, the role of Dab in Abl signaling was controversial, although several studies have now convincingly established the central role of Dab in the Abl network.27,34,35 Dab interacts synergistically with Abl and trio mutations and antagonistically to ena, demonstrating that Dab is a positive component of the Abl pathway, and Dab protein co-immunoprecipitates with Abl. In addition, it was found that Dab regulates Abl localization in the retinal neuroepithelium and embryonic epithelia of Drosophila, suggesting it may be upstream of Abl.35 This has now been confirmed by FRET measurements of Abl kinase activity that showed Dab stimulates the kinase activity of Abl, presumably by physical binding.27
It has long been known that Abl interacts genetically with Trio/Rac signaling in neurons.36-38 Genetic experiments showed that axonal phenotypes of trio mutants mimic those of Abl mutations and heterozygous mutations of Trio enhance Abl phenotypes and vice versa.28,38,39 Whether these proteins work in parallel or serially, and if serially, in which order, has been unclear, however. We therefore designed a pair of complementary fluorescence resonance energy transfer (FRET) experiments to resolve this issue. We measured the activity of the 2 key signaling outputs of Abl in neurons, Rac GTPase and Abl kinase itself, and we found that altering Abl activity changed the level of Rac signaling, acutely as well as chronically, but altering Rac had no effect on Abl kinase. This demonstrated that Abl is upstream of Trio/Rac. We further verified this conclusion by demonstrating that UAS-Trio suppresses the phenotype of an Abl mutant, but not vice versa, confirming that Abl acts linearly upstream of Trio in axon growth and guidance.27
Mechanisms by which Abl regulates Trio to stimulate Rac GTPase
If Abl regulates Trio, how does it do so? At a molecular level, we ruled out the hypothesis that Abl acts by directly stimulating the Trio GEF1 domain, by showing that the activity of the isolated Trio GEF1 is not altered in an Abl mutant. Therefore, to identify the region of Trio protein that mediates the effect of Abl, we generated and tested a panel of Trio derivatives, both in the presence and absence of endogenous Abl activity. The key observations were as follows (Fig. 2). As mentioned above, activity of wild type Trio is severely diminished in the absence of Abl kinase. In contrast, while deletion of the Trio N-terminal domain (NTD) had no effect on Trio activity in the presence of Abl kinase, when Abl was inhibited, TrioΔNTD activity was not suppressed. This suggested that the NTD plays a repressive role in the absence of Abl. Furthermore, the spectrin repeats of Trio were essential for Trio activity if the NTD was present, but entirely dispensable if the NTD was deleted (regardless of presence or absence of Abl). We inferred, therefore, that in the wild type protein the role of the NTD is to inhibit Rac GEF activity of Trio in the absence of Abl, and that the role of the spectrin repeats is to work together with Abl to counteract this repressive interaction.
Figure 2.
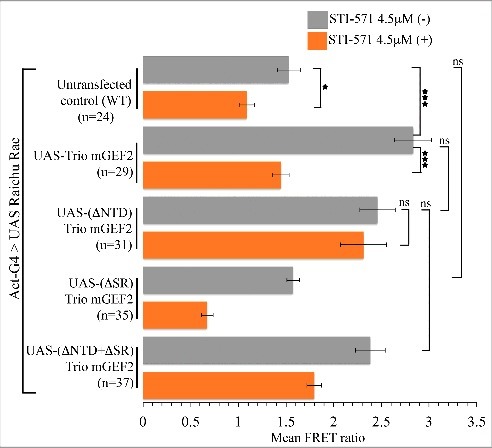
Stimulation of Rac by Trio derivatives and its regulation by Abl kinase. Mean FRET ratio measured in Drosophila S2 cells co-transfected with Raichu-Rac FRET reporter and Trio derivatives with and without the Abl kinase inhibitor, STI-571. Error bars indicate s.e.m. *P < 0.05 and ***P < 0.001. Data reproduced from Ref.27
What is the mechanism by which Abl kinase relieves the auto-inhibited state of Trio?One simple hypothesis is that Abl binds and/or phosphorylates Trio protein directly. Indeed, in mammalian cells it is known that Abl and Src phosphorylate tyrosine residues in the C-terminal portion of Trio to stimulate its Rho GEF activity.40 The Trio domain that is phosphorylated in that model, however, is not conserved in the fly protein, ruling it out as the mechanism of Rac GEF activation in our experiments. Previous studies showed in vitro binding of purified Drosophila Abl and Trio fragments from cells, and overexpression of Abl was capable of increasing Trio phosphorylation.11 Those studies, however, were unable to demonstrate that the native level of Trio phosphorylation depends on Abl. Moreover, we were unable to immunoprecipitate complexes of full-length Abl with Trio from embryo or cultured cell lysates, though this could be due to technical issues if complexes are labile, or form only transiently in response to local external cues. Nonetheless, details of our Rac FRET results suggest an alternative possibility, that an additional protein may be required to mediate the interaction of Abl and Trio. One such putative factor seems to exists in mammalian systems, where DISC1 binds to the amino-terminal half of the Trio spectrin repeats, preventing them from binding to the Trio GEF1 domain and thus allowing recruitment and activation of Rac1 by Trio.41 Finally, we cannot entirely rule out that a non-kinase, scaffolding function of Abl might contribute to relieving NTD repression of Trio.42 It is difficult to test this in vivo in Drosophila since overexpression of Abl bypasses the normal regulation and induces neomorphic, non-physiologic activation of other Rac GEFs (RK and EG, unpublished observations). However, if such a non-kinase mechanism of regulation exists it must be in addition to an Abl kinase-dependent mechanism since pharmacological inhibition of Abl kinase activity acutely suppresses the activation of Rac signaling by Trio in our experiments.
Abl signaling regulates structure and function of the secretory apparatus through actin remodeling
Axonal navigation requires integration of cytoskeletal remodeling, protein secretion and membrane trafficking to achieve directed expansion of the growth cone plasma membrane, with membrane trafficking and protein secretion tuned to the needs of the extending axon.43-46 Consequently, the secretory pathway must be responsive to signaling from the plasma membrane, and likely enough, the secretory apparatus in return signals back to the plasma membrane. Although we know how many signaling molecules regulate these processes independently, the mechanisms that coordinate them remain mysterious.47 In this section, we will first discuss how Abl/Ena signaling regulates the organization of the Golgi complex, and second, provide evidence that changes in Golgi architecture are essential to meet the qualitative and quantitative demands of axonal growth cones during axon extension.
Abl/Ena signaling controls Golgi architecture through actin remodeling in Drosophila neurons
Abl and its pathway partners associate with multiple guidance receptors to regulate context-dependent dynamic behavior in axon guidance. Though remodeling of cortical actin is obviously central, our data suggests that Abl has an additional role regulating the secretory apparatus through its effects on actin remodeling. While investigating the subcellular localization of Abl pathway proteins, we discovered that Ena selectively marks the cis-Golgi compartment.48 Moreover, increased expression of ena, or loss of function of Abl or Dab, caused Golgi fragmentation due to an increase in fissions of Golgi cisternae and a decrease in fusions, and also caused subcellular relocalization of Golgi in neurons, with cisternae collecting at the most basal part of the cell soma, near the axon exit point. Finally, we found that the regulation of Golgi architecture by Abl and Ena was mediated through the effects of these proteins on actin structure (as opposed, for example, to a direct effect on Golgi-resident proteins). These data are consistent with several other studies that reinforce the importance of actin-dependent regulation of Golgi organization.49-52 Key regulators of actin structure have been localized to this organelle, and manipulation of those proteins regulates Golgi structure and function. These include Abi and SCAR/WAVE complex,50 the cdc42/N-WASP/Arp2/3 pathway,51 FMNL1,53 LIM kinase1 and ADF/cofilin.54,55
Abl/Ena acts in parallel to Dar3 signaling to regulate axon patterning
Our data on the requirement for the Abl network in Golgi architecture raises an important question whether Abl-mediated axon patterning might also be executed through control of protein secretion and membrane trafficking via regulation of Golgi structure. Certainly precedent from the literature suggests a key role of protein secretion in dendritic morphogenesis.56-58
To test whether mutants that inhibit protein secretion affect axon pathfinding, we first focused on embryonic motor axons where altering Abl levels leads to characteristic axon patterning defects.7,9,15,35,38 Intersegmental Nerve b (ISNb) comprises axons from 9 CNS motor neurons that exit the CNS in association with the intersegmental nerve (ISN) and establish synaptic connections with ventrolateral muscles.59 In Abl gain-of-function or Ena zygotic mutant embryos, the ISNb axons fail to defasciculate from the ISN and do not invade the muscle field but instead continue growing in association with the ISN (termed a “bypass” phenotype) (Fig. 3E). In embryos with loss-of-function mutations of Abl or dab, ISNb defasciculates from ISN but selectively fails to innervate its most distal target, muscle 12, and instead does not extend beyond the cleft between muscles 6 and 13 (termed a “stall” phenotype;7,9,35) (Fig. 3E). To manipulate the secretory machinery we took advantage of partial loss-of-function mutations in dar3, the Drosophila ortholog of the Sar1 small GTPase that regulates the formation of COPII-coated cargos for anterograde trafficking from endoplasmic reticulum (ER) to Golgi complex. This mutant therefore halts secretory cargo immediately before entry into the cis-Golgi. Drosophila dar3 mutations disrupt dendritic arborization of class IV larval sensory neurons due to defects in dendritic Golgi localization58 and Sar1 activity is limiting for growth of mouse hippocampal axons in culture.56
Figure 3.
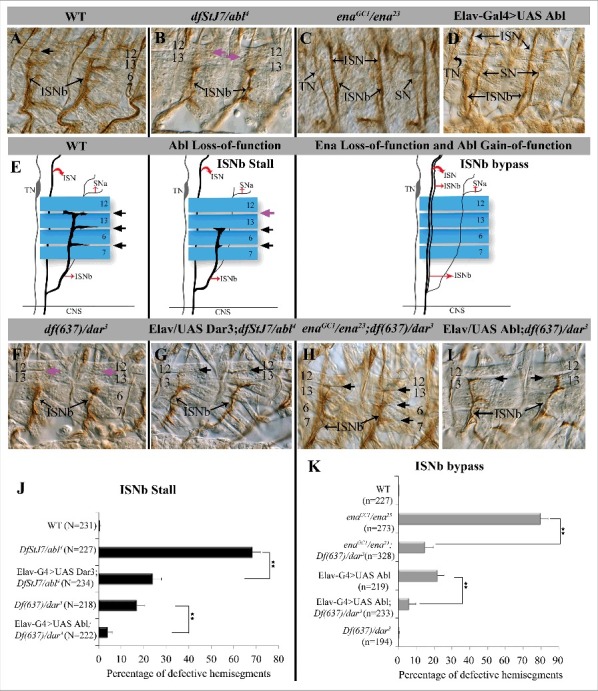
Genetic interactions between dar3 and Abl signaling pathway in embryonic motor axon defasciculation and target recognition. (A-D, F-I) Fillet preparations of anti-FasII (mAb 1D4)-stained late stage 16 embryos to visualize peripheral motor axonal projections. Dorsal is up and anterior is left in all panels. Black arrows indicate ISNb innervation upon the 4 muscles, purple arrows highlight missing ISNb innervation between muscles 13/12 in Abl and dar3 mutants. (A) WT. (B) Abl mutant (Df stJ7/abl4). (C) ena mutant (enaGC1/ena23). (D) neuronal overexpression of Abl. E. Schematic illustration of different peripheral motor axonal branches relative to the 4 ventro-lateral muscles (blue) at a single focal plane as they would appear in filleted embryos. (F) dar3 mutant (df(637)/dar3). (G) neuronal expression of dar3 in Abl mutant, Elav-G4/UAS-dar3; df stJ7/abl4 mutants. (H) ena; dar3 double mutant, enaGC1/ena23; df(637)/dar3. (I) neuronal expression of Abl in dar3 mutant, Elav-G4/UAS-abl; df(637)/dar3. (J) Quantification of ISNb stall phenotypes observed in panels A, B, F, G, I. Statistical significance calculated by ANOVA. (**P < 0.001). Number of abdominal hemisegments (n) scored for each genotype is reported. Error bars represent SEM. (K) Quantification of ISNb bypass phenotypes observed in panels A, C, D, H, I.
The ISNb phenotype of dar3 mutants (Fig. 3F) was quite similar to that of Abl pathway mutants7,35,36 (Fig. 3B). In dar3 hypomorphic mutants, ISNb axons defasciculate from ISN but failed to innervate the distal muscle 12, and instead stall at the cleft between muscles 6 and 13 (16.9% ± 3.5 of hemisegments defective (n = 218 hemisegments), vs. 0.6% ± 0.3 in WT (n = 231 hemisegments) and 68.3% ± 3.6 in Abl−/− (n = 227 hemisegments) (Fig. 3J). Overexpression of dar3 had no obvious effect on ISNb patterning (data not shown).
We next investigated the epistatic relationship between dar3 and Abl. Neuronal overexpression of Abl leads to the ISNb bypass phenotype in 21.8% ± 3.1 of hemisegments (n = 219). This was substantially suppressed by the dar3 mutation (6.8% ± 2.1 bypass in UAS Abl; dar3−/−; n = 233) (Fig. 3K). Similarly, the ISNb bypass phenotype of ena mutants alone (79.7% ± 4.3 defective hemisegments, n = 273) was significantly suppressed in combination with dar3 mutants (14.8 ± 4.9 defective hemisegments in ena−; dar3−, n = 328). Moreover, the ISNb stall phenotype of Abl mutant embryos (68.3% ± 3.6 of hemisegments defective; n = 227) was significantly suppressed by overexpression of dar3 (24% ± 3.9 defective hemisegments in abl−/−; UAS-dar3; n = 234) (Fig. 3J). Conversely Abl overexpression suppressed the ISNb stall phenotype in dar3 mutant embryos (to 4% ± 1.9; n = 222).
We also used clonal analysis with the MARCM system60 to examine the function of dar3 and Abl in projection of photoreceptor (PR) axons to the medulla neuropile of the adult brain (Fig. 4). In the medulla, axons of 67.6% of dar3 mutant R7 PR cells (GMR-Flp; n = 42) and 4.3% of Abl mutant R7 cells (ey3.5-Flp; n = 78) fail to reach their M6 target layer, suggesting that dar3 GTPase and Abl both function cell autonomously to promote R7 PR axon targeting. Abl overexpression in dar3 MARCM clones significantly suppressed defective R7 targeting (to 45.8% defective; Fig. 4F). We also observed a characteristic PR axon overshooting phenotype at low expressivity in MARCM clones of both dar3 (8.1%, N = 37) and Abl (3.5%, N = 57) using GMR-Flp and ey3.5-Flp, respectively (Fig. 4F). Thus, as in embryonic motor neurons, inhibiting secretion in PRs produces phenotypes bearing similarity to those from Abl mutations.
Figure 4.
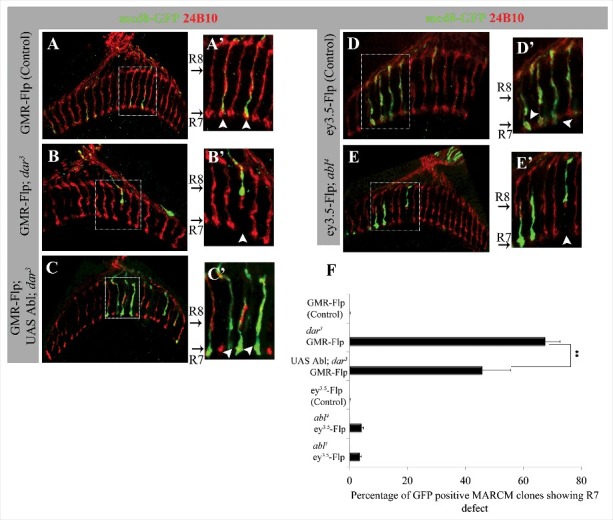
dar3 and Abl are required autonomously for R7 photoreceptor synaptic targeting. (A-E) Projected confocal sections of adult brain stained for anti-chaoptin (24B10) to visualize PR axonal projections and anti-GFP to label both wild type and mutant MARCM clones in a heterozygous genetic background. Enlarged panels (A’-E’) from the boxed region of corresponding genotypes show GFP positive MARCM clones in R7 and R8 synaptic layers (white arrow head). (A) GMR-Flp; Act-G4 UAS mcd8GFP; FRT82B iso/FRT82B tub-Gal80 (Control). (B) GMR-Flp; Act-G4 UAS mcd8GFP; FRT82B dar3/FRT82B tub-Gal80 (dar3 mutant MARCM). (C) GMR-Flp; Act-G4 UAS mcd8-GFP/UAS Abl; FRT82B dar3/FRT 82B tub-Gal80 (Abl overexpression in dar3 MARCM clones). (D) ey3.5-Flp; Act-G4 UAS mcd8-GFP; FRT2A iso/FRT2A tub-Gal80 (Control). (E) ey3.5-Flp; Act-G4 UAS mcd8-GFP; FRT2A abl4/FRT2A tub-Gal80 (abl4 mutant MARCM). F. Quantification of R7 synaptic targeting defects MARCM experiments. Statistical significance by ANOVA ** represents P < 0.001. Error bars represent SEM.
Mutations of dar3 (Sar1 GTPase) that impair the secretory pathway produce axonal phenotypes that mimic the characteristic guidance defects observed in Abl pathway mutants. Moreover, dar3 interacts genetically with Abl in control of embryonic ISNb motor axons and adult photoreceptor axons in a manner consistent with the secretory machinery acting genetically downstream of Abl signaling in the regulation of axon guidance and synaptic targeting in Drosophila. In embryonic ISNb guidance (where the phenotypes were sufficiently penetrant to allow the experiment), increased or decreased dar3 levels suppressed the Abl loss- and gain-of-function phenotypes, respectively. These observations are consistent with the axonal phenotypes of Abl being mediated in part, through its effects on the secretory machinery. We note that the axonal phenotypes of dar3 mutants were also partly suppressed by overexpression of Abl, but as we could only use partial-loss-of-function conditions of dar3 (complete loss of dar3 activity appeared to be cell-inviable; RK and EG unpublished observations), it is not informative for addressing the question of epistasis: we hypothesize that hyperactivation of Abl signaling partly compensated for reduced dar3 by stimulating residual dar3-dependent secretory capacity in the mutants. Therefore, the pattern of epistasis is consistent with secretory function lying downstream of Abl in axon patterning, though additional experiments will be necessary to test this conclusively.
How does protein secretion modulate Abl-dependent axon patterning?
Two main classes of models could account for how altered regulation of the secretory apparatus in Abl pathway mutants disrupts axon growth and guidance. It may be that protein secretion and membrane trafficking are impaired non-specifically, such that there are not enough bulk raw materials available to support axon growth. Alternatively, it could be that one or a small number of specific components, such as particular guidance cues and receptors, become limiting or mistargeted.61-63 At least for embryonic motor axons, we do not favor the former, non-specific hypothesis. It is quite striking that mutation of different genes in the Abl pathway cause stalling of ISNb at the same highly characteristic choice point.7,32,35 The percent of segments affected depends on the mutated gene, the allele strength, and presence or absence of maternal product, but the terminal phenotypes are essentially the same across many different genetic conditions. The simplest explanation is that some specific, required component is absent or mistargeted due to the defect in secretion. We cannot distinguish whether this is because one of a cohort of underproduced proteins becomes quantitatively limiting at the choice point, or because of a selective failure to properly sort a particular protein at the Golgi compartment in the mutant.64 There is precedent for actin-dependent sorting of specific cargoes at the Golgi complex, such as the acid hydroxylase receptor CI-MPR,65 and the lysosomal enzyme cathepsin D.53,55 Additional experiments will be required to distinguish among these models, and in particular to determine whether one or more specific guidance molecules are selectively absent or mistargeted in Abl pathway mutants.
In cultured mammalian hippocampal neurons, the Sar1 GTPase that regulates construction of ER exit sites is necessary, and indeed limiting, for differentiation and growth of axons.56 Moreover, the Drosophila ortholog of Sar1 GTPase is critical for accumulation of dendritic Golgi outposts and thus for dendrite development in class IV larval sensory neurons58 and as we show here, also for motor neuron outgrowth and PR axon targeting. Also in Drosophila, loss of rich, a guanine nucleotide exchange factor (GEF) for Golgi-localized Rab6 GTPase leads to defective projection of R7 PR axons57 similar to what we observed in dar3 mutants. Our data show that these processes are under the control of the Abl signaling pathway, and that mutations in dar3 interact genetically with manipulations that alter Abl signaling. This provides a direct link between the regulatory machinery that senses guidance information and the secretory machinery that helps execute those patterning choices.
On the face of it, the finding that the Abl/Ena pathway affects axon guidance in part through protein secretion raises a legitimate question of whether we can be certain that the action of this pathway at the cortex of growth cones and migrating cells is also essential for motility. Several lines of evidence suggest that it is. First, Ena/VASP orthologs have been found to localize at the tips of filopodia in growing axons, correlating with invasion of the leading edge by microtubules,66 and at the leading edge of migrating keratocytes,67 correlating quantitatively with the speed and persistence of migration. Moreover, forced localization of Ena to the cell cortex produces a gain-of-function phenotype akin to that from overexpression of Ena, identifying a major site of Ena action at the plasma membrane.68 Finally, manipulations that promote actin assembly, such as overexpression of the actin nucleation factor diaphanous, can functionally take the place of Abl/Ena signaling in growth of Drosophila longitudinal pioneer axons15 even though they do not affect Golgi organization in our assay (RK and EG, unpublished observations). This argues that the effects of the Abl pathway on axon patterning arise from a convergence of its influences at the growth cone cortex with those on the secretory apparatus.
Summary
The Abl signaling network is essential for many aspects of morphology and motility during development, but a clear picture of its function and mechanism has long been elusive. Solving these problems required special attention to the complexities inherent in analyzing dynamic processes and pleiotropic molecular machinery. Similarly, the existence of many parallel downstream targets of the Abl network introduces extra complexity to discriminating the mechanisms by which Abl executes it biologic effects. It is this very complexity, however, and more specifically the ability of Abl to coordinate disparate aspects of cell biology and physiology, that likely accounts for why so many signaling pathways have evolved to converge on the Abl network to implement their effects.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We wish to thank all of the members of our laboratory who contributed to developing the ideas presented in this review, particularly JK Song, Madhuri Shivalkar and Lucy Kotlyanskaya.
Funding
This work was supported by the Basic Neuroscience Program of the Intramural Research Program of NINDS, NIH (Z01-NS003013, to EG). RK was supported, in part by a DBT Ramalingaswami re-entry fellowship and Accelerator program for Discovery in Brain disorders using Stem cells (ADBS) from the Department of Biotechnology (DBT), Government of India.
References
- [1].Dickson BJ. Molecular mechanisms of axon guidance. Science 2002; 298:1959-64; PMID:12471249; https://doi.org/ 10.1126/science.1072165 [DOI] [PubMed] [Google Scholar]
- [2].Song H, Poo M. The cell biology of neuronal navigation. Nat Cell Biol 2001; 3(3):E81-8; PMID:11231595; https://doi.org/ 10.1038/35060164 [DOI] [PubMed] [Google Scholar]
- [3].Goodman CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell/Neuron 1993; 72:77-98 [DOI] [PubMed] [Google Scholar]
- [4].Hoffmann FM. Drosophila abl and genetic redundancy in signal transduction. TIGS 1991; 7:351-55; https://doi.org/ 10.1016/0168-9525(91)90254-F [DOI] [PubMed] [Google Scholar]
- [5].Lanier LM, Gertler FB. From Abl to actin: Abl tyrosine kinase and associated protein in growth cone motility. Curr Opin Neurobiol 2000; 10:80-7; PMID:10679439; https://doi.org/ 10.1016/S0959-4388(99)00058-6 [DOI] [PubMed] [Google Scholar]
- [6].Qiu Z, Cang Y, Goff SP. Abl family tyrosine kinases are essential for basement membrane integrity and cortical lamination in the cerebellum. J Neurosci 2010; 30(43):14430-9; PMID:20980600; https://doi.org/ 10.1523/JNEUROSCI.2861-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wills Z, Bateman J, Korey CA, Comer A, Van Vactor D. The tyrosine kinase Abl and its substrate enabled collaborate with the receptor phosphatase Dlar to control motor axon guidance. Neuron 1999; 22(2):301-12; PMID:10069336; https://doi.org/ 10.1016/S0896-6273(00)81091-0 [DOI] [PubMed] [Google Scholar]
- [8].Bashaw GJ, Kidd T, Murray D, Pawson T, Goodman CS. Repulsive axon guidance: Abelson and enabled play opposing roles downstream of the roundabout receptor. Cell 2000; 101(7):703-15; PMID:10892742; https://doi.org/ 10.1016/S0092-8674(00)80883-1 [DOI] [PubMed] [Google Scholar]
- [9].Crowner D, Le Gall M, Gates MA, Giniger E. Notch steers Drosophila ISNb motor axons by regulating the Abl signaling pathway. Curr Biol 2003; 13(11):967-72; PMID:12781136; https://doi.org/ 10.1016/S0960-9822(03)00325-7 [DOI] [PubMed] [Google Scholar]
- [10].Deinhardt K, Kim T, Spellman DS, Mains RE, Eipper BA, Neubert TA, Chao MV, Hempstead BL. Neuronal growth cone retraction relies on proneurotrophin receptor signaling through Rac. Sci Signal 2011; 4(202):ra82; PMID:22155786; https://doi.org/ 10.1126/scisignal.2002060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Forsthoefel DJ, Liebl EC, Kolodziej PA, Seeger MA. The Abelson tyrosine kinase, the Trio GEF and enabled interact with the Netrin receptor Frazzled in Drosophila. Development 2005; 132(8):1983-94; PMID:15790972; https://doi.org/ 10.1242/dev.01736 [DOI] [PubMed] [Google Scholar]
- [12].Garbe DS, O'Donnell M, Bashaw GJ. Cytoplasmic domain requirements for Frazzled-mediated attractive axon turning at the Drosophila midline. Development 2007; 134(24):4325-34; PMID:18003737; https://doi.org/ 10.1242/dev.012872 [DOI] [PubMed] [Google Scholar]
- [13].Gupton SL, Riquelme D, Hughes-Alford SK, Tadros J, Rudina SS, Hynes RO, Lauffenburger D, Gertler FB. Mena binds alpha5 integrin directly and modulates alpha5beta1 function. J Cell Biol 2012; 198(4):657-76; PMID:22908313; https://doi.org/ 10.1083/jcb.201202079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hsouna A, Kim YS, VanBerkum MF. Abelson tyrosine kinase is required to transduce midline repulsive cues. J Neurobiol 2003; 57(1):15-30; PMID:12973825; https://doi.org/ 10.1002/neu.10232 [DOI] [PubMed] [Google Scholar]
- [15].Kuzina I, Song JK, Giniger E. How Notch establishes longitudinal axon connections between successive segments of the Drosophila CNS. Development 2011; 138(9):1839-49; PMID:21447553; https://doi.org/ 10.1242/dev.062471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yu HH, Zisch AH, Dodelet VC, Pasquale EB. Multiple signaling interactions of Abl and Arg kinases with the EphB2 receptor. Oncogene 2001; 20(30):3995-4006; PMID:11494128; https://doi.org/ 10.1038/sj.onc.1204524 [DOI] [PubMed] [Google Scholar]
- [17].Bradley WD, Koleske AJ. Regulation of cell migration and morphogenesis by Abl-family kinases: emerging mechanisms and physiological contexts. J Cell Sci 2009; 122(Pt 19):3441-54; PMID:19759284; https://doi.org/ 10.1242/jcs.039859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cancino GI, Toledo EM, Leal NR, Hernandez DE, Yevenes LF, Inestrosa NC, Alvarez AR. STI571 prevents apoptosis, tau phosphorylation and behavioural impairments induced by Alzheimer's beta-amyloid deposits. Brain 2008; 131(Pt 9):2425-42; PMID:18559370; https://doi.org/ 10.1093/brain/awn125 [DOI] [PubMed] [Google Scholar]
- [19].Colicelli J. ABL tyrosine kinases: evolution of function, regulation, and specificity. Sci Signal 2010; 3(139):re6; PMID:20841568; https://doi.org/ 10.1126/scisignal.3139re6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gonzalez-Zuniga M, Contreras PS, Estrada LD, Chamorro D, Villagra A, Zanlungo S, Seto E, Alvarez AR. c-Abl stabilizes HDAC2 levels by tyrosine phosphorylation repressing neuronal gene expression in Alzheimer's disease. Mol Cell 2014; 56(1):163-73; PMID:25219501; https://doi.org/ 10.1016/j.molcel.2014.08.013 [DOI] [PubMed] [Google Scholar]
- [21].Gruber F, Knapek S, Fujita M, Matsuo K, Bracker L, Shinzato N, Siwanowicz I, Tanimura T, Tanimoto H. Suppression of conditioned odor approach by feeding is independent of taste and nutritional value in Drosophila. Curr Biol 2013; 23(6):507-14; PMID:23477724; https://doi.org/ 10.1016/j.cub.2013.02.010 [DOI] [PubMed] [Google Scholar]
- [22].Lin TY, Huang CH, Kao HH, Liou GG, Yeh SR, Cheng CM, Chen MH, Pan RL, Juang JL. Abi plays an opposing role to Abl in Drosophila axonogenesis and synaptogenesis. Development 2009; 136(18):3099-107; PMID:19675132; https://doi.org/ 10.1242/dev.033324 [DOI] [PubMed] [Google Scholar]
- [23].Pendergast AM. The Abl family kinases: mechanisms of regulation and signaling. Adv Cancer Res 2002; 85:51-100; PMID:12374288 [DOI] [PubMed] [Google Scholar]
- [24].Gertler FB, Hill KK, Clark MJ, Hoffmann FM. Dosage-sensitive modifiers of Drosophila abl tyrosine kinase function: prospero, a regulator of axonal outgrowth, and disabled, a novel tyrosine kinase substrate. Genes Dev 1993; 7:441-53; PMID:7680635; https://doi.org/ 10.1101/gad.7.3.441 [DOI] [PubMed] [Google Scholar]
- [25].Gertler FB, Doctor JS, Hoffmann FM. Genetic suppression of mutations in the Drosophila abl proto-oncogene homolog. Science 1990; 248(4957):857-60; PMID:2188361; https://doi.org/ 10.1126/science.2188361 [DOI] [PubMed] [Google Scholar]
- [26].Gautreau A, Ho HY, Li J, Steen H, Gygi SP, Kirschner MW. Purification and architecture of the ubiquitous Wave complex. Proc Natl Acad Sci U S A 2004; 101(13):4379-83; PMID:15070726; https://doi.org/ 10.1073/pnas.0400628101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kannan R, Song JK, Karpova T, Clarke A, Shivalkar M, Wang B, Kotlyanskaya L, Kuzina I, Gu Q, Giniger E. The Abl pathway bifurcates to balance Enabled and Rac signaling in axon patterning in Drosophila. Development 2017; 144(3):487-98; PMID:28087633; https://doi.org/ 10.1242/dev.143776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Luo L. Trio quartet in D. (melanogaster). Neuron 2000; 26(1):1-2; PMID:10798384; https://doi.org/ 10.1016/S0896-6273(00)81129-0 [DOI] [PubMed] [Google Scholar]
- [29].Luo L, Liao J, Jan LY, Jan YN. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev 1994; 8:1787-802; PMID:7958857; https://doi.org/ 10.1101/gad.8.15.1787 [DOI] [PubMed] [Google Scholar]
- [30].Ng J, Nardine T, Harms M, Tzu J, Goldstein A, Sun Y, Dietzl G, Dickson BJ, Luo L. Rac GTPases control axon growth, guidance and branching. Nature 2002; 416(6879):442-7; PMID:11919635; https://doi.org/ 10.1038/416442a [DOI] [PubMed] [Google Scholar]
- [31].Giniger E. A role for Abl in Notch signaling. Neuron 1998; 20(4):667-81; PMID:9581760; https://doi.org/ 10.1016/S0896-6273(00)81007-7 [DOI] [PubMed] [Google Scholar]
- [32].Song JK, Giniger E. Noncanonical Notch function in motor axon guidance is mediated by Rac GTPase and the GEF1 domain of Trio. Dev Dyn 2011; 240(2):324-32; PMID:21246649; https://doi.org/ 10.1002/dvdy.22525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Howell BW, Gertler FB, Cooper JA. Mouse disabled (mDab1): a Src binding protein implicated in neuronal development. Embo J 1997; 16(1):121-32; PMID:9009273; https://doi.org/ 10.1093/emboj/16.1.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liebl EC, Rowe RG, Forsthoefel DJ, Stammler AL, Schmidt ER, Turski M, Seeger MA. Interactions between the secreted protein Amalgam, its transmembrane receptor Neurotactin and the Abelson tyrosine kinase affect axon pathfinding. Development 2003; 130(14):3217-26; PMID:12783792; https://doi.org/ 10.1242/dev.00545 [DOI] [PubMed] [Google Scholar]
- [35].Song JK, Kannan R, Merdes G, Singh J, Mlodzik M, Giniger E. Disabled is a bona fide component of the Abl signaling network. Development 2010; 137(21):3719-27; PMID:20940230; https://doi.org/ 10.1242/dev.050948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Awasaki T, Saito M, Sone M, Suzuki E, Sakai R, Ito K, Hama C. The Drosophila trio plays an essential role in patterning of axons by regulating their directional extension. Neuron 2000; 26(1):119-31; PMID:10798397; https://doi.org/ 10.1016/S0896-6273(00)81143-5 [DOI] [PubMed] [Google Scholar]
- [37].Newsome TP, Schmidt S, Dietzl G, Keleman K, Asling B, Debant A, Dickson BJ. Trio combines with dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell 2000; 101(3):283-94; PMID:10847683; https://doi.org/ 10.1016/S0092-8674(00)80838-7 [DOI] [PubMed] [Google Scholar]
- [38].Liebl EC, Forsthoefel DJ, Franco LS, Sample SH, Hess JE, Cowger JA, Chandler MP, Shupert AM, Seeger MA. Dosage-sensitive, reciprocal genetic interactions between the Abl tyrosine kinase and the putative GEF trio reveal trio's role in axon pathfinding. Neuron 2000; 26(1):107-18; PMID:10798396; https://doi.org/ 10.1016/S0896-6273(00)81142-3 [DOI] [PubMed] [Google Scholar]
- [39].Bateman J, Shu H, Van Vactor D. The guanine nucleotide exchange factor trio mediates axonal development in the Drosophila embryo. Neuron 2000; 26(1):93-106; PMID:10798395; https://doi.org/ 10.1016/S0896-6273(00)81141-1 [DOI] [PubMed] [Google Scholar]
- [40].Sonoshita M, Itatani Y, Kakizaki F, Sakimura K, Terashima T, Katsuyama Y, Sakai Y, Taketo MM. Promotion of colorectal cancer invasion and metastasis through activation of NOTCH-DAB1-ABL-RHOGEF protein TRIO. Cancer Discov 2015; 5(2):198-211; PMID:25432929; https://doi.org/ 10.1158/2159-8290.CD-14-0595 [DOI] [PubMed] [Google Scholar]
- [41].Chen SY, Huang PH, Cheng HJ. Disrupted-in-Schizophrenia 1-mediated axon guidance involves TRIO-RAC-PAK small GTPase pathway signaling. Proc Natl Acad Sci U S A 2011; 108(14):5861-6; PMID:21422296; https://doi.org/ 10.1073/pnas.1018128108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rogers EM, Spracklen AJ, Bilancia CG, Sumigray KD, Allred SC, Nowotarski SH, Schaefer KN, Ritchie BJ, Peifer M. Abelson kinase acts as a robust, multifunctional scaffold in regulating embryonic morphogenesis. Mol Biol Cell 2016; 27(16):2613-31; PMID:27385341; https://doi.org/ 10.1091/mbc.E16-05-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Giniger E. How do Rho family GTPases direct axon growth and guidance? A proposal relating signaling pathways to growth cone mechanics. Differentiation 2002; 70(8):385-96; PMID:12366376; https://doi.org/ 10.1046/j.1432-0436.2002.700801.x [DOI] [PubMed] [Google Scholar]
- [44].Horton AC, Ehlers MD. Secretory trafficking in neuronal dendrites. Nat Cell Biol 2004; 6(7):585-91; PMID:15232591; https://doi.org/ 10.1038/ncb0704-585 [DOI] [PubMed] [Google Scholar]
- [45].Pfenninger KH. Plasma membrane expansion: a neuron's Herculean task. Nat Rev Neurosci 2009; 10(4):251-61; PMID:19259102; https://doi.org/ 10.1038/nrn2593 [DOI] [PubMed] [Google Scholar]
- [46].Dent EW, Gupton SL, Gertler FB. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol 2011; 3(3); PMID:21106647; https://doi.org/ 10.1101/cshperspect.a001800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Vitriol EA, Zheng JQ. Growth cone travel in space and time: the cellular ensemble of cytoskeleton, adhesion, and membrane. Neuron 2012; 73(6):1068-81; PMID:22445336; https://doi.org/ 10.1016/j.neuron.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kannan R, Kuzina I, Wincovitch S, Nowotarski SH, Giniger E. The Abl/enabled signaling pathway regulates Golgi architecture in Drosophila photoreceptor neurons. Mol Biol Cell 2014; 25(19):2993-3005; PMID:25103244; https://doi.org/ 10.1091/mbc.E14-02-0729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Almeida CG, Yamada A, Tenza D, Louvard D, Raposo G, Coudrier E. Myosin 1b promotes the formation of post-Golgi carriers by regulating actin assembly and membrane remodelling at the trans-Golgi network. Nat Cell Biol 2011; 13(7):779-89; PMID:21666684; https://doi.org/ 10.1038/ncb2262 [DOI] [PubMed] [Google Scholar]
- [50].Kondylis V, van Nispen tot Pannerden HE, Herpers B, Friggi-Grelin F, Rabouille C. The golgi comprises a paired stack that is separated at G2 by modulation of the actin cytoskeleton through Abi and Scar/WAVE. Dev Cell 2007; 12(6):901-15; PMID:17543863; https://doi.org/ 10.1016/j.devcel.2007.03.008 [DOI] [PubMed] [Google Scholar]
- [51].Matas OB, Martinez-Menarguez JA, Egea G. Association of Cdc42/N-WASP/Arp2/3 signaling pathway with Golgi membranes. Traffic 2004; 5(11):838-46; PMID:15479449; https://doi.org/ 10.1111/j.1600-0854.2004.00225.x [DOI] [PubMed] [Google Scholar]
- [52].von Blume J, Duran JM, Forlanelli E, Alleaume AM, Egorov M, Polishchuk R, Molina H, Malhotra V. Actin remodeling by ADF/cofilin is required for cargo sorting at the trans-Golgi network. J Cell Biol 2009; 187(7):1055-69; PMID:20026655; https://doi.org/ 10.1083/jcb.200908040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Colon-Franco JM, Gomez TS, Billadeau DD. Dynamic remodeling of the actin cytoskeleton by FMNL1gamma is required for structural maintenance of the Golgi complex. J Cell Sci 2011; 124(Pt 18):3118-26; PMID:21868368; https://doi.org/ 10.1242/jcs.083725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Salvarezza SB, Deborde S, Schreiner R, Campagne F, Kessels MM, Qualmann B, Caceres A, Kreitzer G, Rodriguez-Boulan E. LIM kinase 1 and cofilin regulate actin filament population required for dynamin-dependent apical carrier fission from the trans-Golgi network. Mol Biol Cell 2009; 20(1):438-51; PMID:18987335; https://doi.org/ 10.1091/mbc.E08-08-0891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].von Blume J, Alleaume AM, Cantero-Recasens G, Curwin A, Carreras-Sureda A, Zimmermann T, van Galen J, Wakana Y, Valverde MA, Malhotra V. ADF/cofilin regulates secretory cargo sorting at the TGN via the Ca2+ ATPase SPCA1. Dev Cell 2011; 20(5):652-62; PMID:21571222; https://doi.org/ 10.1016/j.devcel.2011.03.014 [DOI] [PubMed] [Google Scholar]
- [56].Aridor M, Fish KN. Selective targeting of ER exit sites supports axon development. Traffic 2009; 10(11):1669-84; PMID:19761544; https://doi.org/ 10.1111/j.1600-0854.2009.00974.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tong C, Ohyama T, Tien AC, Rajan A, Haueter CM, Bellen HJ. Rich regulates target specificity of photoreceptor cells and N-cadherin trafficking in the Drosophila visual system via Rab6. Neuron 2011; 71(3):447-59; PMID:21835342; https://doi.org/ 10.1016/j.neuron.2011.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell 2007; 130(4):717-29; PMID:17719548; https://doi.org/ 10.1016/j.cell.2007.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Landgraf M, Bossing T, Technau GM, Bate M. The origin, location, and projections of the embryonic abdominal motorneurons of Drosophila. J Neurosci 1997; 17(24):9642-55; PMID:9391019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 1999; 22(3):451-61; PMID:10197526; https://doi.org/ 10.1016/S0896-6273(00)80701-1 [DOI] [PubMed] [Google Scholar]
- [61].Bashaw GJ, Klein R. Signaling from axon guidance receptors. Cold Spring Harb Perspect Biol 2010; 2(5):a001941; PMID:20452961; https://doi.org/ 10.1101/cshperspect.a001941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Williamson WR, Yang T, Terman JR, Hiesinger PR. Guidance receptor degradation is required for neuronal connectivity in the Drosophila nervous system. PLoS Biol 2010; 8(12):e1000553; PMID:21151882; https://doi.org/ 10.1371/journal.pbio.1000553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Winckler B, Mellman I. Trafficking guidance receptors. Cold Spring Harb Perspect Biol 2010; 2(7):a001826; PMID:20504966; https://doi.org/ 10.1101/cshperspect.a001826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kamikura DM, Cooper JA. Clathrin interaction and subcellular localization of Ce-DAB-1, an adaptor for protein secretion in Caenorhabditis elegans. Traffic 2006; 7(3):324-36; PMID:16497226; https://doi.org/ 10.1111/j.1600-0854.2006.00386.x [DOI] [PubMed] [Google Scholar]
- [65].Gomez TS, Billadeau DD. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell 2009; 17(5):699-711; PMID:19922874; https://doi.org/ 10.1016/j.devcel.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Dent EW, Kwiatkowski AV, Mebane LM, Philippar U, Barzik M, Rubinson DA, Gupton S, Van Veen JE, Furman C, Zhang J, Alberts AS, Mori S, Gertler FB. Filopodia are required for cortical neurite initiation. Nat Cell Biol 2007; 9(12):1347-59; PMID:18026093; https://doi.org/ 10.1038/ncb1654 [DOI] [PubMed] [Google Scholar]
- [67].Lacayo CI, Pincus Z, VanDuijn MM, Wilson CA, Fletcher DA, Gertler FB, Mogilner A, Theriot JA. Emergence of large-scale cell morphology and movement from local actin filament growth dynamics. PLoS Biol 2007; 5(9):e233; PMID:17760506; https://doi.org/ 10.1371/journal.pbio.0050233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bear JE, Loureiro JJ, Libova I, Fassler R, Wehland J, Gertler FB. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell 2000; 101:717-28; PMID:10892743; https://doi.org/ 10.1016/S0092-8674(00)80884-3 [DOI] [PubMed] [Google Scholar]


