Abstract
Synthetic cannabinoids refer to a wide variety of chemicals engineered to bind cannabinoid receptors (CB1 and CB2) and mimic the effects of delta-9-tetrahydrocanabinol. The potential for severe toxicity and limited in vivo data make synthetic cannabinoid intake an important public health and safety concern. Neurologic toxidromes associated with their use include mental status changes, panic attacks, memory distortions, acute psychosis (e.g. paranoia, delusional thoughts), disorganized behavior, and suicidal and homicidal thoughts. Systemic complications include vomiting, sinus tachycardia, myocardial infarction, and acute kidney injury. Seizures are common; however, status epilepticus is not widely reported. In this case report, we describe a patient who developed acute psychosis and new-onset refractory status epilepticus necessitating emergent neurological life-support and prolonged admission to an intensive care unit following abuse of synthetic cannabinoids. We include a brief review of the literature to prepare the treating clinician for the broad clinical spectrum of this increasingly common intoxication.
Keywords: Spice, synthetic cannabinoids, psychosis, seizure, status epilepticus, new-onset refractory status epilepticus, drug abuse
Introduction
Synthetic cannabinoids (SCs) refer to a wide variety of chemicals engineered to bind cannabinoid receptors (CB1 and CB2) and mimic the effects of delta-9-tetrahydrocanabinol (THC)—the major active constituent in marijuana.1 SCs were initially developed in the 1940s as a research tool to explore the endocannabinoid system and its use as a potential therapeutic target.2 SCs activate CB1 receptors, which are G-protein-coupled receptors predominantly located at pre-synaptic terminals. CB1 receptor activation decreases cellular cyclic adenosine monophosphate (cAMP) levels and elicits cannabimimetic responses.1–3 SC agonists also interact with voltage-gated ion channels and inhibit potassium, sodium, and N-and P/Q-type-calcium channels by reducing membrane potentials.3
SCs, synthesized in clandestine labs, were first marketed as a legal cannabis alternative in Europe and North America in the late 1990s and early 2000s.3 Widespread use of the Internet has facilitated the dissemination of these substances. To date, many SCs are Schedule I drugs under the US Controlled Substance Act,4,5 though more recently structurally diverse cannabimimetic compounds have emerged, which are not covered under current regulations. SCs popularity are attributed to intense psychoactive effects, lack of detectability in routine urine drug tests, and, until recently, legal status in most jurisdictions.6–8
Evidence of serious adverse effects and limited in vivo data make SCs intake an important public health and safety concern. Neurologic toxidromes associated with their use include mental status changes, panic attacks, memory distortions, acute psychosis (e.g. paranoia, delusional thoughts), disorganized behavior, and suicidal and homicidal thoughts.7–9 Systemic complications include vomiting and tachycardia, myocardial infarction, and acute kidney injury.7,8,10–14 Reports of acute ischemic stroke have also been described.10,15 Severe acute psychosis and seizures are not uncommon.16–26 The diagnostic dilemma associated with SCs and their use is a current lack of conventional serum and toxicology screens. Diagnosis at this time is made solely based on historical and clinical grounds.
In this case report, we describe a patient who developed acute psychosis and new-onset refractory status epilepticus (NORSE) necessitating emergent neurological life-support and prolonged admission to an intensive care unit following abuse of SCs. Although convulsions associated with the recreational use of SCs have been reported,16,18,24–28 there are no previously written reports of protracted refractory status epilepticus or NORSE in the setting of SC use.18,23–26
Case report
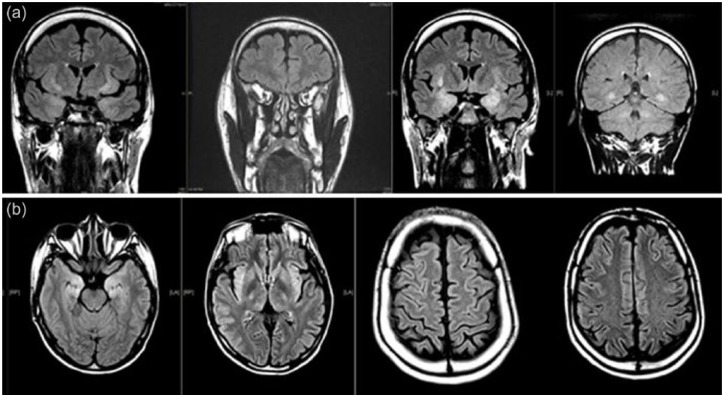
A 40-year-old man presented to our institution with NORSE. His prior medical history was only remarkable for uncomplicated febrile seizure during early childhood without recurring seizures. He was initially reported to have taken no medications prior to presentation. Upon admission, his examination was notable for stupor, left gaze deviation, lip smacking, and unresponsiveness, concerning for ongoing status epilepticus. The patient was intubated for airway protection. He was treated with intravenous lorazepam, valproic acid, levetiracetam, and lacosamide. Electroencephalography (EEG) demonstrated non-convulsive right temporal seizures and generalized period discharges (GPDs) admixed with a background of diffuse slowing. Lumbar puncture was essentially unremarkable revealing normal opening pressure, 5 red blood cells/µL, 6 white blood cells/µL, protein of 35 mg/dL, and glucose 71 mg/dL. An extensive workup for viral encephalitis, prion disease, and paraneoplastic meningoencephalitis were all unremarkable. Metabolic panels, including liver function tests, and both a comprehensive serum and urine toxicology screen were all negative. Computed tomography (CT) of chest, abdomen, and pelvis failed to demonstrate evidence of occult malignancy or any other focal abnormalities. An magnetic resonance imaging (MRI) of the brain with and without gadolinium obtained 48 h after presentation revealed bilateral hyperintensities within the limbic structures, predominantly in the insula and hippocampi (Figure 1, arrow), thought to be related to epileptiform activity. No initial history could be elicited regarding any prior recreational drug use as family was not available for further clarification.
Figure 1.
(a) MRI brain: coronal T2-flair sequence demonstrating hyperintensities within the peri-insular regions bilaterally and medial temporal lobes bilaterally. (b) MRI brain: axial T2-flair (left to right Rostral to Caudal) demonstrating T2-flair hyperintensities within bilateral medial temporal lobes, peri-insular region, as well as diffuse peri-sulcal enhancement.
Leading differential diagnoses for NORSE at that time included paraneoplastic or autoimmune encephalitis. The patient continued to be treated with an aggressive anti-epileptic regimen including lacosamide, valproic acid, levetiracetam, and clobazam. He was also treated with high-dose intravenous steroids (methylprednisolone 250 mg every 6 h) and two doses of gamma immunoglobulin (IVIg, 2 g/kg total). Following extubation, the patient’s course was further complicated by severe psychosis and aggression toward healthcare personnel. He repeatedly exhibited homicidal and violent behavior toward staff, requiring intervention with repeated doses of anti-psychotic, sedatives, and hypnotics as well as physical restraints. Following a 3-week hospitalization in the intensive care unit, the patient was ultimately discharged. The patient returned for outpatient follow-up, 1 month following discharge, where he and his family attested to the recent experimentation with SCs, noting a significant increase in the use of “Spice” mere days preceding hospitalization. The patient had no permanent neurological or systemic sequelae following his initial hospitalization, although dedicated neuropsychiatric testing was not performed to identify minor cognitive sequelae.
Discussion
The recreational use of psychoactive drugs has been increasing over the years. This trend is likely to continue, given the elusive confirmatory tests of these substances, wide consumer availability, ambiguous legal jurisdiction, and ease of access. The use of SCs poses a serious health problem, with the emergence of numerous reports of unique manifestations associated with SCs and risk of severe acute brain injury. Data from the American Association of Poison Control Centers suggest that the majority of SC exposures do not result in major adverse events; however, they may result in adverse events more serious than marijuana. There is a current emergence of numerous case reports and series detailing the life-threatening effects of SCs.12,13,16–20,29 The variability and adverse outcomes associated with the use of SCs is likely a result of multiple different chemical compounds, dose dependency, and analogue-receptor targets involved.10–12,14,15,27,28
Marijuana and its active ingredient THC possess anticonvulsant properties, as demonstrated in animal models and humans.30 Historically, cannabis has been used as a remedy for seizure,31 though the exact anti-epileptic mechanism is still under investigation. One potential mechanism includes the decrease of both glutamate and γ-aminobutyric acid (GABA) synaptic transmission in the brain. The decrease in excitatory neurotransmitter release may have an effect on increasing the seizure threshold.1,32 In addition, numerous studies have also emerged on the impact of cannabinoids on dopamine release and behavioral output. One particular study demonstrated in vivo characteristics of a biphasic motor profile coupled with increases in dopamine and decreases in glutamate release with the use of SCs.1,32
Despite how prevalent the recreational use of marijuana is in both contemporary and historical societies, convulsions associated with its use are rarely described, and literature thus far is limited to occasional case reports of accidental ingestions in children.20–24
Nonetheless, the mechanisms responsible for SC-induced convulsions are currently unknown. It therefore remains unclear as to whether certain synthetics or combinations thereof are responsible for the various observed side effects.
Our patient had no pertinent medical history except for a remote uncomplicated febrile seizure during early childhood. Generally, risk of epilepsy recurrence is slightly higher in children who experienced prior febrile seizure compared to those without.33–35 NORSE is a new entity in the medical literature, implying a term for different etiologies, with often a devastating clinical outcome.36,37 This term was first coined in 2005 to describe a group of previously healthy adults who presented with NORSE, which was associated with high morbidity and mortality.36–42 There is mounting evidence of an array of pathogenic antibodies against neuronal surface antigens, such as voltage-gated potassium channels, glutamate receptors, and N-methyl-d-aspartate receptor (NMDA) receptors that have been identified in cases of NORSE.43–46 As such, one could wonder whether there is an intrinsic association between the specific chemoreceptor targets of SCs and NORSE in the setting of its use. However, this remains speculative without proven link.
Conclusion
In our case report, we add to the literature by describing the first case of NORSE in association with SC (Spice) use. We highlight the importance of this class of substance as an emerging public health problem, as new analogues are synthesized and marketed on a daily basis. Clinicians, whether in primary care or acute emergency settings, should be aware of the wide array of both acute and chronic health consequences observed with SCs. In fact, the relative high risk for serious systemic adverse effects and potential risk of developing an acute devastating neurologic injury with SCs allow for a clear distinction from its natural occurring cousin THC. The constellation of clinical effects may range from psychosis, behavioral abnormalities, and memory disturbances to more serious consequences, such as stroke, acute kidney injury, and status epilepticus. Given the evolving nature of these substances, physicians should be aware of their elusive diagnostic nature. Having a high index of suspicion to explore further the history and send specific tests for these substances is imperative for the recognition of this toxicological syndrome. Obtaining additional history about recreational use of these substances is often helpful in making a clinical diagnosis until more available laboratory testing becomes more readily available.
There are no specific treatment or antidotes for SC and treatment remains supportive, with management of secondary complications. While most written case reports describe self-limited symptoms, there are additional numerous written reports on more serious consequences.10–13,16–20,29
We recognize the limitations of our case report. Primarily, the association of SC use with NORSE does not prove causation, although the association and timing by itself is curious and cannot be overlooked. This patient’s co-existing remote history of febrile seizure is, by itself, considered an independent risk factor for recurrent seizures in the general population, although there are no prior reports of remote childhood febrile seizure and NORSE. Finally, it is difficult to completely exclude any other substances that may have been undisclosed by the patient, although the severity of the presentation and the current clinical history appear to implicate SCs. These questions alone prompt the authors to indicate a need for further research on this topic.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Disclosures: The authors agree to the conditions outlined in the Authorship and Contributorship section of the Information for authors. No statistical analysis was performed.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
References
- 1. Polissidis A, Chouliara O, Galanopoulos A, et al. Cannabinoids negatively modulate striatal glutamate and dopamine release and behavioural output of acute D-amphetamine. Behav Brain Res 2014; 270: 261–269. [DOI] [PubMed] [Google Scholar]
- 2. Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol 2006; 147(Suppl. 1): S163–S171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pertwee RG. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr Med Chem 2010; 17: 1360–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. US Drug Enforcement Administration. Schedules of controlled substances: temporary placement of four synthetic cannabinoids into schedule I. Final order. Fed Regist 2014; 79: 7577–7582. [PubMed] [Google Scholar]
- 5. US Drug Enforcement Administration. Establishment of drug codes for 26 substances. Final rule. Fed Regist 2013; 78: 664–666. [PubMed] [Google Scholar]
- 6. Vandrey R, Dunn KE, Fry JA, et al. A survey study to characterize use of spice products (synthetic cannabinoids). Drug Alcohol Depend 2012; 120: 238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gunderson EW, Haughey HM, Ait-Daoud N, et al. “Spice” and “K2” herbal highs: a case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict 2012; 21: 320–326. [DOI] [PubMed] [Google Scholar]
- 8. Winstock AR, Barratt MJ. Synthetic cannabis: a comparison of patterns of use and effect profile with natural cannabis in a large global sample. Drug Alcohol Depend 2013; 131: 106–111. [DOI] [PubMed] [Google Scholar]
- 9. McGrath J, Welham J, Scott J, et al. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry 2010; 67(5): 440–447. [DOI] [PubMed] [Google Scholar]
- 10. Hermanns-Clausen M, Kneisel S, Szabo B, et al. Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction 2013; 108: 534–544. [DOI] [PubMed] [Google Scholar]
- 11. Glue P, Al-Shaqsi S, Hancock D, et al. Hospitalisation associated with use of the synthetic cannabinoid K2. N Z Med J 2013; 126: 18–23. [PubMed] [Google Scholar]
- 12. Thomas S, Bliss S, Malik M. Suicidal ideation and self-harm following K2 use. J Okla State Med Assoc 2012; 105: 430–433. [PubMed] [Google Scholar]
- 13. Bhanushali GK, Jain G, Fatima H, et al. AKI associated with synthetic cannabinoids: a case series. Clin J Am Soc Nephrol 2013; 8: 523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mir A, Obafemi A, Young A, et al. Myocardial infarction associated with use of the synthetic cannabinoid K2. Pediatrics 2011; 128: e1622–e1627. [DOI] [PubMed] [Google Scholar]
- 15. Freeman MJ, Rose DZ, Myers MA, et al. Ischemic stroke after use of the synthetic marijuana “spice.” Neurology 2013; 81: 2090–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McQuade D, Hudson S, Dargan PI, et al. First European case of convulsions related to analytically confirmed use of the synthetic cannabinoid receptor agonist AM-2201. Eur J Clin Pharmacol 2013; 69: 373–376. [DOI] [PubMed] [Google Scholar]
- 17. Ustundag MF, OzhanIbis E, Yucel A, et al. Synthetic cannabis-induced mania. Case Rep Psychiatry 2015; 2015: 310930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schep LJ, Slaughter RJ, Hudson S, et al. Delayed seizure-like activity following analytically confirmed use of previously unreported synthetic cannabinoid analogues. Hum Exp Toxicol 2015; 34(5): 557–560. [DOI] [PubMed] [Google Scholar]
- 19. Van Amsterdam J, Brunt T, Van den Brink W. The adverse health effects of synthetic cannabinoids with emphasis on psychosis-like effects. J Psychopharmacol 2015; 29(3): 254–263. [DOI] [PubMed] [Google Scholar]
- 20. Ng SK, Brust JC, Hauser WA, et al. Illicit drug use and the risk of new-onset seizures. Am J Epidemiol 1990; 132: 47–57. [DOI] [PubMed] [Google Scholar]
- 21. Gordon E, Devinsky O. Alcohol and marijuana: effects on epilepsy and use by patients with epilepsy. Epilepsia 2001; 42: 1266–1272. [DOI] [PubMed] [Google Scholar]
- 22. Keeler MH, Reifler CB. Grand mal convulsions subsequent to marijuana use. Case report. Dis Nerv Syst 1967; 28: 474–475. [PubMed] [Google Scholar]
- 23. Bonkowsky JL, Sarco D, Pomeroy SL. Ataxia and shaking in a 2-year-old girl: acute marijuana intoxication presenting as seizure. Pediatr Emerg Care 2005; 21(8): 527–528. [DOI] [PubMed] [Google Scholar]
- 24. Harris CR, Brown A. Synthetic cannabinoid intoxication: a case series and review. J Emerg Med 2013; 44(2): 360–366. [DOI] [PubMed] [Google Scholar]
- 25. Schneir AB, Baumbacher T. Convulsions associated with the use of a synthetic cannabinoid product. J Med Toxicol 2012; 8: 62–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Louh IK, Freeman WD. A “spicy” encephalopathy: synthetic cannabinoids as cause of encephalopathy and seizure. Crit Care 2014; 2018(5): 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoyte CO, Jacob J, Monte AA, et al. A characterization of synthetic cannabinoid exposures reported to the National poison data system in 2010. Ann Emerg Med 2012; 60: 435–438. [DOI] [PubMed] [Google Scholar]
- 28. McQuade D, Hudson S, Dargan PI, et al. First European case of convulsions related to analytically confirmed use of the synthetic cannabinoid receptor agonist AM-2201. Eur J Clin Pharmacol 2013; 69: 373–376. [DOI] [PubMed] [Google Scholar]
- 29. Nacca N, Vatti D, Sullivan R, et al. The synthetic cannabinoid withdrawal syndrome. J Addict Med 2013; 7: 296–298. [DOI] [PubMed] [Google Scholar]
- 30. Mortati K, Dworetzky B, Devinsky O. Marijuana: an effective antiepileptic treatment in partial epilepsy? A case report and review of the literature. Rev Neurol Dis 2007; 4(2): 103–106. [PubMed] [Google Scholar]
- 31. Grinspoon L, Bakalar J. Marihuana: the forbidden medicine. New Haven, CT: Yale University Press, 1997, pp. 66–82. [Google Scholar]
- 32. Lutz B. On-demand activation of the endocannabinoid system in the control of neuronal excitability and epileptiform seizures. Biochem Pharmacol 2004; 68(9): 1691–1698. [DOI] [PubMed] [Google Scholar]
- 33. Annegers JF, Hauser WA, Shirts SB, et al. Factors prognostic of unprovoked seizures after febrile convulsions. N Engl J Med 1987; 316: 493–498. [DOI] [PubMed] [Google Scholar]
- 34. Vestergaard M, Pedersen CB, Sidenius P, et al. The long-term risk of epilepsy after febrile seizures in susceptible subgroups. Am J Epidemiol 2007; 165: 911–918. [DOI] [PubMed] [Google Scholar]
- 35. Neligan A, Bell GS, Giavasi C, et al. Long-term risk of developing epilepsy after febrile seizures: a prospective cohort study. Neurology 2012; 78: 1166–1170. [DOI] [PubMed] [Google Scholar]
- 36. Khawaja AM, DeWolfe JL, Miller DW, et al. New-onset refractory status epilepticus (NORSE)—the potential role for immunotherapy. Epilepsy Behav 2015; 47: 17–23. [DOI] [PubMed] [Google Scholar]
- 37. Waheed S, Sabeen A, UllahKhan N. New onset refractory status epilepticus as an unusual presentation of a suspected organophosphate poisoning. Case Rep Emerg Med 2014; 2014: 676358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilder-Smith EP, Lim EC, Teoh HL, et al. The NORSE (new-onset refractory status epilepticus) syndrome: defining a disease entity. Ann Acad Med Singapore 2005; 34(7): 417–420. [PubMed] [Google Scholar]
- 39. Costello DJ, Kilbride RD, Cole AJ. Cryptogenic new onset refractory status epilepticus (NORSE) in adults—infectious or not? J Neurol Sci 15; 277(1–2): 26–31. [DOI] [PubMed] [Google Scholar]
- 40. Rathakrishnan R, Wilder-Smith EP. New onset refractory status epilepticus (NORSE). J Neurol Sci 2009; 284(1–2): 220. [DOI] [PubMed] [Google Scholar]
- 41. Nair PP, Wadwekar V, Murgai A, et al. Refractory status epilepticus complicated by drug-induced involuntary movements. BMJ Case Rep 2014; 2014: bcr-2013-202691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wilder-Smith EP, Lim EC, Teoh HL, et al. The NORSE (new-onset refractory status epilepticus) syndrome: defining a disease entity. Ann Acad Med Singapore 2005; 34(7): 417–420. [PubMed] [Google Scholar]
- 43. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008; 7(12): 1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ataller L, Kleopa KA, Wu GF, et al. Autoimmune limbic encephalitis in 39 patients: immunophenotypes and outcomes. J Neurol Neurosurg Psychiatry 2007; 78(4): 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Graus F, Saiz A, Lai M, et al. Neuronal surface antigen antibodies in limbic encephalitis: clinical-immunologic associations. Neurology 2008; 71(12): 930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lousa M, Sanchez-Alonso S, Rodriguez-Diaz R, et al. Status epilepticus with neuron-reactive serum antibodies: response to plasma exchange. Neurology 2000; 54(11): 2163–2165. [DOI] [PubMed] [Google Scholar]