Abstract
Background:
The oral glucose tolerance test (OGTT) is the current established method performed worldwide to diagnose gestational diabetes mellitus (GDM). The purpose of this study was to assess the utility of the use of long- and short-term markers of glycemic status.
Methods:
The study group was composed of 80 pregnant women, 40 with GDM and 40 with normal glucose tolerance. GDM was diagnosed with the American Diabetes Association criteria. Glycemic markers were measured in the OGTT blood samples of women at 24–28 weeks of gestation.
Results:
HbA1c was significantly higher in the GDM group when compared with the controls, whereas 1,5-anhydroglucitol (1,5-AG) levels were significantly lower. There was not a significant difference between the groups for glycated albumin. Whereas HbA1c levels were correlated with fasting and 1 h glucose and negatively correlated with mean corpuscular volume, 1,5-AG was only negatively correlated with the first hour glucose. No difference was found for the diagnostic performances of HbA1c and 1,5-AG (receiver operating characteristic of the area under the concentration curve values were 0.756 and 0.722, respectively).
Conclusion:
HbA1c and 1,5-AG alone does not have sufficient diagnostic accuracy to diagnose GDM. 1,5-AG values were correlated with post-load glucose values in pregnant women so will improve the GDM management and be useful to predict complications.
Keywords: 1,5-anhydroglucitol; gestational diabetes mellitus; glycated albumin; HbA1c
Introduction
Parallel to the global rise in the prevalence of diabetes, there has also been an increase in gestational diabetes mellitus (GDM) in pregnant women.1,2 The rate of hyperglycemia in pregnant women between 20 and 49 years old is estimated to be 16.9%.1 GDM causes various complications in the mother and fetus, and the incidence of these complications can be lowered with treatment. Timely diagnosis remains important in this respect. There is a lack of international consensus regarding the screening and diagnosis of GDM. Some health organizations recommend screening all pregnant women for GDM, whereas others recommend screening only women with risk factors.2–4 The internationally recommended timeframe for GDM screening is between the 24th and 28th weeks of gestation.
Although the oral glucose tolerance test (OGTT) is accepted as the gold standard in GDM diagnosis, the optimal glucose load amount and cut-off values continue to be a topic of debate.2–5 The results obtained by the International Association of Diabetes and Pregnancy Study Group (IADPSG) in the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study using a 75 g OGTT have been adapted for clinical use and their recommendations are recognized by health organizations such as the American Diabetes Association (ADA) and the World Health Organization (WHO).5 The National Institutes of Health (NIH) and American College of Obstetricians and Gynecologists (ACOG) do not recognize these cut-off values and continue to advise using diagnostic criteria based on the 100 g OGTT.3
OGTT is a time-consuming test and may induce or aggravate nausea, vomiting, and headache especially in pregnant women and, thus, some participants fail to complete the test.6 The IADPSG predicts that simpler and more cost-effective strategies for the diagnosis of GDM will replace OGTT in the future, and that fasting glucose level, HbA1c, and more short-term glycemic status markers will be used especially in the evaluation of low-risk patients. HbA1c provides information about the previous 2–3 months of glycemic status and glycated albumin (GA) reflects a 2- to 3-week period.7,8 1,5-Anhydroglucitol (1,5-AG) provides insight into patients’ short-term (approximately 3–7 days) glycemic status.7,9–11
Serum 1,5-AG levels rapidly change inversely related to glucose variations. Increased blood glucose levels inhibit reabsorption from kidney tubules by competing with 1,5-AG.10,11 This is a stable compound and the detection method is cheaper and more reliable than other glycemic status indicators.7 Studies have reported that measurement of 1,5-AG is valuable in assessing short-term and postprandial glycemic excursions.10 The use of 1,5-AG levels in pregnancy is controversial. Whereas some studies have shown that it is a good indicator of glycemic control for diabetic pregnant women, there are reports stating that it has limited benefit because of changes in renal glucose thresholds in pregnancy.12–14 It was demonstrated that 1,5-AG is associated with glycemic exposure related neonatal complications of pregnancy.9 The aim of this study is to investigate the potential utility of HbA1c and the short-term glycemic status indicators GA and 1,5-AG in patients with GDM.
Materials and methods
The study was designed as a cross-sectional case–control study. Of 250 Turkish pregnant women between 24 and 28 weeks of gestation who attended the Gynecology and Obstetrics outpatient clinic of the Dokuz Eylul University Hospital between February and June 2014, 40 women with normal glucose tolerance and 40 with GDM were included in the study. All patients underwent a 75 g OGTT after overnight fasting and GDM was diagnosed using one or more of the criteria (fasting glucose ⩾ 5.1 mmol/l, 1 h glucose ⩾ 10 mmol/l, 2 h glucose ⩾ 8.5 mmol/l) as recommended by ADA.2
The study was approved by the Dokuz Eylul University Non-Interventional Studies Ethics Committee. Signed informed consent was obtained from all study participants. Women previously diagnosed with diabetes or having any hepatic or renal disease that would influence 1,5-AG levels were excluded from the study. With the power of 80%, the sample size was calculated as 80, having 40 patients in each group, to detect a difference of 3.7 µg/ml between diabetes mellitus (DM) and control groups for 1,5-AG, with a standard deviation of 6.0 and an alpha error of 0.05.15
Glucose was analyzed by hexokinase assay. HbA1c was measured using HPLC (Tosoh Bioscience Inc., CA, USA; standardized according to DCCT/NGSP) at the time of sampling. Samples (sodium fluoride plasma and serum) were centrifuged immediately and stored at −80°C for analysis.
1,5-AG concentrations were measured with the GlycoMark kit [New York, USA; intra-assay coefficient of variation (CV) 1.84%, inter-assay CV 1.78%] in sodium fluoride plasma at 0, 1, and 2 h of OGTT. GA levels were measured by an enzymatic method (GSP-glycated serum protein, Diazyme Laboratories, CA, USA; intra-assay CV 0.79%, inter-assay CV 2.16%) in serum samples. Measurements were performed with AU-5800 and Dxl (Beckman Coulter Inc., Miami, FL, USA) analyzers. Serum albumin-adjusted GA (adjusted GA) was calculated after GSP and serum albumin measurement using a formula reported in previous publications.16 The estimated glomerular filtration rate (eGFR) was calculated for each patient using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula with creatinine levels assessed from serum samples.17 Automated peripheral blood counts were performed with LH750 hematology analyzer (Beckman Coulter Inc., Miami, FL, USA).
Statistical analyses
SPSS for Windows version 22.0 software was used for statistical analyses. Parameters were evaluated for normal distribution with the Kolmogorov–Smirnov test. As the data distribution was normal, data are expressed as mean ± standard deviation (SD). Differences between the two groups were assessed by Student’s t-test. Pearson’s test was used for correlation analysis. Receiving operator characteristic (ROC) curves were plotted to determine the diagnostic performance of glycemic markers and p values of <0.05 were considered statistically significant.
Results
The diagnostic criteria for GDM based on a 75 g OGTT carried out between 24 and 28 weeks of gestation. From the 250 pregnant women screened for inclusion in the current study, 40 (16.6%) were diagnosed with GDM. The patients’ basic characteristics and biochemical data are presented in Table 1. Compared with pregnant patients with normal glucose tolerance, patients in the GDM group had significantly lower plasma 1,5-AG levels and significantly higher HbA1c levels. No significant difference was detected in GA levels between the two groups.
Table 1.
Demographic and clinical characteristics of study participants.
| Characteristics | GDM group (n = 40) | Control group (n = 40) | p value |
|---|---|---|---|
| Maternal age (years) | 32.5 ± 5.27 | 29.7 ± 5.71 | 0.025* |
| Fasting glucose (mmol/l) | 4.79 ± 0.52 | 4.18 ± 0.34 | <0.001* |
| 1 h glucose (mmol/l) | 10.5 ± 1.3 | 7.02 ± 1.46 | <0.001* |
| 2 h glucose (mmol/l) | 8.2 ± 1.6 | 6.2 ± 0.98 | <0.001* |
| AST (U/l) | 17.6 ± 4.33 | 18.3 ± 4.74 | 0.478 |
| ALT (U/l) | 13.9 ± 5.59 | 13.8 ± 6.18 | 0.986 |
| Albumin (g/l) | 34.5 ± 1.7 | 34.6 ± 1.6 | 0.809 |
| Hemoglobin (g/l) | 118 ± 8.4 | 114 ± 8.2 | 0.055 |
| MCV (fl) | 89 ± 6.33 | 89 ± 4.13 | 0.917 |
| HbA1c (%) | 5.28 ± 0.34 | 4.95 ± 0.34 | <0.001* |
| Fasting 1,5-AG (µg/ml) | 9.1 ± 4.77 | 13.2 ± 5.39 | 0.001* |
| Adjusted GA (%) | 12.4 ± 1.95 | 12.1 ± 1.63 | 0.495 |
| GSP (µmol/l) | 180 ± 30.4 | 176 ± 21.5 | 0.470 |
Data are expressed as mean ± standard deviation (SD), *p < 0.05.
1,5-AG, 1,5-anhydroglucitol; ALT, alanine transaminase; AST, aspartate transaminase; GA, glycated albumin; GSP, glycated serum protein; MCV, mean corpuscular volume.
We found no correlations between 1,5-AG and HbA1c or GA (p = 0.42 and 0.47, respectively) or between HbA1c and GA (p = 0.84). 1,5-AG levels were negatively correlated with glucose level at 1 h, the point of the OGTT at which glucose reached its maximum concentration (r = −0.357, p = 0.001), though no statistically significant association was found between plasma 1,5-AG and fasting or 2-h glucose.
HbA1c was positively correlated with fasting and 1 h glucose (r = 0.448 and 0.449, respectively; p < 0.001) and negatively correlated with mean corpuscular volume (MCV; r = −0.423, p < 0.001). No statistically significant relationship between 2 h glucose and HbA1c was found. There were no significant associations between GA and glucose values during the OGTT.
ROC area under the curve (AUC) values [95% confidence intervals (95% CI)] for 1,5-AG in diagnosing GDM were 0.722 (0.609–0.834) at fasting, 0.725 (0.613–0.838) at 1 h, and 0.723 (0.610–0.836) at 2 h of OGTT. Comparison of ROC-AUC values for 1,5-AG presented same diagnostic accuracy at fasting and post-load (p > 0.05). ROC curves were created to test the value of alternate glycemic markers to diagnose GDM. ROC-AUC values (95% CI) for plasma HbA1c, fasting 1,5-AG, and GA in diagnosing GDM were 0.756 (0.651–0.861), 0.722 (0.609–0.834), and 0.550 (0.421–0.678), respectively (Figure 1). Comparison of ROC-AUC values revealed no significant difference between the discriminatory power of 1,5-AG and HbA1c (p = 0.679).
Figure 1.
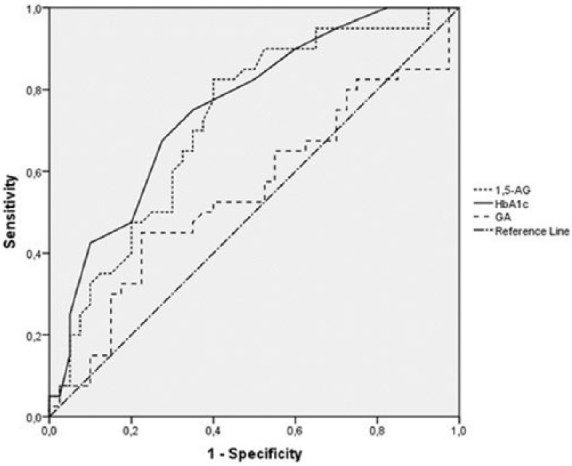
Receiver operating characteristic (ROC) curve analysis of glycemic markers for diagnosing gestational diabetes mellitus (GDM). Area under the curve (AUC) values were 0.756 for HbA1c, 0.722 for 1,5-anhydroglucitol (1,5-AG), and 0.550 for glycated albumin (GA).
Discussion
The laboratory diagnosis of GDM is being updated because of the increased incidence of this disorder and increased evidence of complications for both the mother and the baby. In this study, we investigated the diagnostic value of three alternate glycemic markers for GDM and found that healthy pregnant women and those with GDM could be differentiated with 1,5-AG and HbA1c, but not with GA.
GA still lacks an established reference interval. The differences in reference values could be explained by dissimilar demographics, population age, and/or ethnicity and seasonal variation.16 Studies are being performed for standardization and validation of the method.16 There are few reports on the relationship between GA and the occurrence of GDM, and all of them are Asian studies. Some publications indicate that GA can be used as a glycemic control indicator in GDM patients.18,19 Hiramatsu et al. reported lower GA values in pregnant women with proteinuria and in obese women.20 Body weight has a direct influence on GA levels during pregnancy.8 Since GA is expressed as the ratio to total albumin, conditions affecting turnover of serum albumin, such as thyroid disorders, hepatic and renal diseases could affect albumin and, hence, GA levels.16 In a recent study, it was reported that GA was not suitable as a screening tool for GDM, which is consistent with the findings in the present study.8 The authors reported a ROC-AUC value similar to ours to identify GDM in patients 24–28 weeks’ gestation (0.542 versus 0.550). One study showed that the incidence of macrosomia in GDM women with GA levels ⩾12% was increased at 36–38 weeks of gestation.18 Sugawara et al. reported a positive correlation between the number of complications seen in their infants and their GA levels at late pregnancy.21 The association can be explained by the relation of GA with short-term and postprandial hyperglycemia. It may be concluded that GA may be useful in monitoring GDM rather than diagnosis.
In a study of diabetic pregnant women, Dworacka et al. showed that 1,5-AG levels were primarily determined by hyperglycemic peaks.14 A correlation between 1,5-AG and maximum glucose concentration has also been found in pregnant women with type 1 DM.13 We found that 1,5-AG was negatively correlated with 1 h glucose values, which were the maximum glucose concentrations reached during the OGTT. Recently, several groups reported that elevated 1 h glucose during OGTT provides a better tool to identify subjects with beta-cell dysfunction compared with HbA1c and these subjects are more prone to developing type 2 DM.22,23 In addition, it has been reported that the combination of fasting and 1 h glucose gives higher predictability for large-for-gestational-age newborns of mothers with GDM.24
Tam et al. conducted a study with pregnant women having risk factors for GDM and reported that there was no relation between fasting glucose and 1,5-AG, and that 1,5-AG was unable to discriminate GDM in the ROC curve.25 Kilpatrick et al. demonstrated that glucosuria caused variation in 1,5-AG levels of pregnant women considered normoglycemic according to 2 h OGTT results.12 After the GDM diagnostic criteria update, glucose level at 1 h (the time point at which post-load glucose concentration reaches its maximum level) alone is sufficient for diagnosis, which may increase the utility of 1,5-AG in pregnancy. Likewise, in our study, 23 of 40 GDM patients exceeded the 1 h glucose cut-off value; 12 of those 23 patients were diagnosed with GDM based on exceeding only the 1 h cut-off.
Two groups found significantly higher levels of HbA1c in their GDM groups.26,27 Rajput et al. reported that HbA1c could not replace OGTT for the diagnosis of GDM, but may be beneficial when used in combination.26 Sevket et al. claimed that HbA1c may not decrease the need for OGTT for GDM diagnosis.27
In a recent study, Odsæter et al. studied HbA1c as a screening test for GDM using the modified IADPSG criteria.28 They found that around 30% of pregnant women could potentially have avoided an OGTT by using HbA1c with a sensitivity of 87.5% at pregnancy weeks 18–22 and 97% at weeks 32–36. Currently, GDM diagnosis is made during the late second trimester and controlling of blood glucose levels can reduce maternal and neonatal complications. In our study, we found that HbA1c could discriminate GDM cases from controls at weeks 24–28. Specifically, the ROC-AUC values of HbA1c were higher than those of the other markers we evaluated.
It has been reported that HbA1c shows changes during pregnancy due to iron deficiency, especially increasing in late pregnancy independent of glucose.8 In our study, HbA1c levels were correlated with fasting and 1 h glucose, as well as MCV levels. A recent study showed that low or high mean corpuscular hemoglobin or MCV levels are associated with increased risk of erroneous HbA1c based identification of glycemia status.29 Therefore, regardless of the capacity of HbA1c to discriminate GDM patients from controls, hematologic changes that arise during pregnancy limit the use of this marker in pregnant women.
The HAPO study showed that pregnant women with fasting glucose under 4.4 mmol/l were at lower risk of developing GDM complications and the recommended criterion of diagnosis of GDM is 5.1 mmol/l or more.5 Zhu et al, conducted a study to evaluate the usefulness of fasting plasma glucose to screen for GDM at 24–28 weeks of gestation.30 They concluded that this stepwise approach reduces the need for OGTT about half the formal diagnostic criteria and reduce the cost for GDM diagnosis.
In the present study 40 women diagnosed with GDM, of these 13.2% was based on fasting and 1 h glucose. The correlation between 1,5-AG and 1-h OGTT glucose level suggests that the combined use of 1,5-AG and fasting glucose could reduce the need for the OGTT in diagnosing GDM. The ability to perform both analyses with a single blood sample enhances patient comfort and allows the side effects of OGTTs to be avoided.
According to our results, HbA1c and 1,5-AG alone have poor diagnostic value. Their correlation with post-load glucose may prove beneficial because of the association of postprandial hyperglycemia with GDM complications. There are reports showing that controlling blood glucose in the nonfasting state, especially in the postprandial period can reduce the risk of diabetic complications.10,11 Given the presence of dysglycemic individuals who are only postprandial hyperglycemia, serum 1,5-AG may be a useful marker of diabetes.7
Recent evidence shows that plasma concentrations of 1,5-AG show decreases at the highest levels of blood glucose and reflect glucose excursions.10 These observations support the usefulness of 1,5-AG as a marker of short-term glycemic variability for the assessment and preventing of GDM complications.11
The strength of our study is the inclusion of 1 h glucose values. In some previous studies using different diagnostic criteria 1 h glucose values were not available.28 There are some limitations of this study. The number of pregnant women included was small. Nevertheless, the observed rate of GDM in our study population was comparable with previous prevalence studies.1 Data concerning the patients’ body mass index (BMI) and weight gained during pregnancy were not collected, although these are risk factors of GDM. Furthermore, daily consumption of dairy products is known to affect 1,5-AG levels; however, the study did not assess dairy consumption or urine glucose levels. The final limitation was not questioning the patients about their use of iron supplements routinely given during pregnancy.
Conclusion
We conclude that 1,5-AG alone does not have sufficient diagnostic accuracy to diagnose GDM. 1,5-AG values were an indicator of 1-h post-load glucose values in pregnant women so will improve the glycemic control and be useful to predict and prevent GDM complications.
Footnotes
Funding: This study was supported by the Dokuz Eylul University Scientific Research Projects Coordination Unit. Project number 2013.KB.SAG.084.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Baris Saglam, Faculty of Medicine, Department of Biochemistry, Dokuz Eylul University, Izmir, Turkey.
Sezer Uysal, Faculty of Medicine, Department of Biochemistry, Dokuz Eylul University, Izmir, 35340, Turkey.
Sadik Sozdinler, Faculty of Medicine, Department of Obstetrics and Gynecology, Dokuz Eylul University, Izmir, Turkey.
Omer Erbil Dogan, Faculty of Medicine, Department of Obstetrics and Gynecology, Dokuz Eylul University, Izmir, Turkey.
Banu Onvural, Faculty of Medicine, Department of Biochemistry, Dokuz Eylul University, Izmir, Turkey.
References
- 1. Guariguata L, Linnenkamp U, Beagley J, et al. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract 2013; 103: 176–185. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2015; 38: S8–S16. [DOI] [PubMed] [Google Scholar]
- 3. Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 137: gestational diabetes mellitus. Obstet Gynecol 2013; 122: 406–416. [DOI] [PubMed] [Google Scholar]
- 4. Bilous R. Diagnosis of gestational diabetes, defining the net, refining the catch. Diabetologia 2015; 58: 1965–1968. [DOI] [PubMed] [Google Scholar]
- 5. Metzger BE, Gabbe SG, Persson B, et al. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fuller KP, Borgida AF. Gestational diabetes mellitus screening using the one-step versus two-step method in a high-risk practice. Clin Diabetes 2014; 32: 148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Yuan Y, Zhang Y, et al. Serum 1,5-anhydroglucitol level as a screening tool for diabetes mellitus in a community-based population at high risk of diabetes. Acta Diabetol 2017; 54: 425–431. [DOI] [PubMed] [Google Scholar]
- 8. Zhu J, Chen Y, Li C, et al. The diagnostic value of glycated albumin in gestational diabetes mellitus. J Endocrinol Invest. Epub ahead of print 6 June 2017. DOI: 10.1007/s40618-016-0605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee JE. Alternative biomarkers for assessing glycemic control in diabetes: fructosamine, glycated albumin, and 1,5-anhydroglucitol. Ann Pediatr Endocrinol Metab 2015; 20: 74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pramodkumar TA, Jayashri R, Gokulakrishnan K, et al. Relationship of glycemic control markers - 1,5 anhydroglucitol, fructosamine, and glycated hemoglobin among Asian Indians with different degrees of glucose intolerance. Indian J Endocrinol Metab 2016: 690–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ma C, Sheng J, Liu Z, et al. Excretion rates of 1,5-anhydro-D-glucitol, uric acid and microalbuminuria as glycemic control indexes in patients with type 2 diabetes. Sci Rep 2017; 7: 44291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kilpatrick ES, Keevilt BG, Richmond KL, et al. Plasma 1,5-anhydroglucitol concentrations are influenced by variations in the renal threshold for glucose. Diabet Med 1999; 16: 496–499. [DOI] [PubMed] [Google Scholar]
- 13. Nowak N, Skupien J, Cyganek K, et al. 1,5-Anhydroglucitol as a marker of maternal glycaemic control and predictor of neonatal birthweight in pregnancies complicated by type 1 diabetes mellitus. Diabetologia 2013; 56: 709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dworacka M, Wender-Ozegowska E, Winiarska H, et al. Plasma anhydro-D-glucitol (1,5-AG) as an indicator of hyperglycaemic excursions in pregnant women with diabetes. Diabet Med 2006; 23: 171–175. [DOI] [PubMed] [Google Scholar]
- 15. Ouchi M, Oba K, Aoyama J, et al. Serum uric acid in relation to serum 1,5-anhydroglucitol levels in patients with and without type 2diabetes mellitus. Clin Biochem 2013; 46: 1436–1441. [DOI] [PubMed] [Google Scholar]
- 16. Rodriguez-Capote K, Tovell K, Holmes D, et al. Analytical evaluation of the Diazyme glycated serum protein assay on the siemens ADVIA 1800: comparison of results against HbA1c for diagnosis and management of diabetes. J Diabetes Sci Technol 2015; 9: 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li HP, Wang FH, Tao MF, et al. Association between glycemic control and birthweight with glycated albumin in Chinese women with gestational diabetes mellitus. J Diabetes Investig 2016; 7: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang Y, Hu Y, Ma YU, et al. Glycated albumin is an optimal biomarker for gestational diabetes mellitus. Exp Ther Med 2015; 10: 2145–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hiramatsu Y, Shimizu I, Omori Y, et al. Determination of reference intervals of glycated albumin and hemoglobin A1c in healthy pregnant Japanese women and analysis of their time courses and influencing factors during pregnancy. Endocr J 2012; 59: 145–151. [DOI] [PubMed] [Google Scholar]
- 21. Sugawara D, Sato H, Ichihashi K, et al. Glycated albumin level during late pregnancy as a predictive factor for neonatal outcomes of women with diabetes. J Matern Fetal Neonatal Med. Epub ahead of print 9 June 2017. DOI: 10.1080/14767058.2017.1333103. [DOI] [PubMed] [Google Scholar]
- 22. Priya MM, Amutha A, Pramodkumar TA, et al. β-cell function and insulin sensitivity in normal glucose-tolerant subjects stratified by 1-hour plasma glucose values. Diabetes Technol Ther 2016; 18: 29–33. [DOI] [PubMed] [Google Scholar]
- 23. Jagannathan R, Sevick MA, Li H, et al. Elevated 1-hour plasma glucose levels are associated with dysglycemia, impaired beta-cell function, and insulin sensitivity: a pilot study from a real world health care setting. Endocrine 2016; 52: 172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brankica K, Valentina VN, Slagjana SK, et al. Maternal 75-g OGTT glucose levels as predictive factors for large-for-gestational age newborns in women with gestational diabetes mellitus. Arch Endocrinol Metab 2016; 60: 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tam WH, Rogers MS, Lau TK, et al. The predictive value of serum 1,5-anhydro-D-glucitol in pregnancies at increased risk of gestational diabetes mellitus and gestational impaired glucose tolerance. BJOG 2001; 108: 754–756. [DOI] [PubMed] [Google Scholar]
- 26. Rajput R, Yadav Yogesh, Rajput M, et al. Utility of HbA1c for diagnosis of gestational diabetes mellitus. Diabetes Res Clin Pract 2012; 98: 104–107. [DOI] [PubMed] [Google Scholar]
- 27. Sevket O, Sevket A, Ozel A, et al. The use of HbA1c as an aid in the diagnosis of gestational diabetes mellitus. J Obstet Gynaecol 2014; 34: 690–692. [DOI] [PubMed] [Google Scholar]
- 28. Odsæter IH, Åsberg A, Vanky E, et al. Hemoglobin A1c as screening for gestational diabetes mellitus in Nordic Caucasian women. Diabetol Metab Syndr 2016; 8: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rodriguez-Segade S, Garcia JR, García-López JM, et al. Impact of mean cell hemoglobin on HbA1c-defined glycemia status. Clin Chem 2016; 62: 1570–1578. [DOI] [PubMed] [Google Scholar]
- 30. Zhu WW, Fan L, Yang HX, et al. Fasting plasma glucose at 24–28 weeks to screen for gestational diabetes mellitus: new evidence from China. Diabetes Care 2013; 36: 2038–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]


