Abstract
N,N-dimethylformamide (DMF) has been widely used as an organic solvent in industries. DMF is a potential medication. However, the antitumorigenic role of DMF in breast cancer remains unclear. Here, we examined dose-dependent effects of DMF on proliferation and apoptosis in breast cancer MCF-7 and nontumorous MCF-12A cells. We found that DMF had a growth inhibitory effect in MCF-12A cells in a dose-dependent manner. By contrast, however, DMF had dual effects on cell proliferation and apoptosis in MCF-7 cells. DMF at a high dose (100 mM) significantly inhibited MCF-7 cell growth while at a low dose (1 mM) significantly stimulated MCF-7 cell growth (both P < .05). The inhibitory effect of DMF on cell proliferation was accompanied by the decrease of cyclin D1 and cyclin E1 protein expression, leading to the cell cycle arrest at the G0/G1 phase. Furthermore, a high-dose DMF significantly increased the number of early apoptotic cells by increasing cleaved caspase-9 and proapoptotic protein Bax expression and decreased the ratio of Bcl-xL/Bax (P < .01). Thus, our data demonstrated for the first time that DMF has dual effects on breast cancer cell behaviors depending upon its dose. Caution must be warranted in determining its effective dose for targeting breast cancer.
Keywords: antitumor, apoptosis, cell cycle, dose dependent, DMF, proliferation
Introduction
N,N-dimethylformamide (DMF) has multiple effects on living cell behavior in mammals. It is colorless at room temperature and has been widely used as an organic solvent in industries and presents in the production of synthetic leather, fibers, films, and surface coatings.1,2 DMF can easily be absorbed through oral, dermal, or inhalation exposure.3 Because of its wide application, long-term or short-term occupational exposure to DMF may result in the liver and digestive disorder in workers.4,5 DMF reduces the cholesterol level and increases the concentration of bile acid, indicating that there is a relationship between DMF and gut microbiotas.6 Previous studies have also shown that DMF can promote apoptosis and inhibit tumor growth, such as head and neck cancer,7 prostate cancer,8 and ovarian cancer.9 On the other hand, DMF enhances hepatocarcinogenicity in animal model.3
Breast cancer is the first common female malignancy in 2012 worldwide and the leading cause of cancer death in women.10 According to recent statistics, it is expected to account for 30% of all new cancer cases and 14% of cancer deaths of female breast cancer in the United States.11 Interestingly, the incidence rate of hormone receptor–positive breast cancers increased, whereas the rate of hormone receptor–negative breast cancers decreased, from 2005 to 2014.12 In China, the incidence rate of breast cancer is about 15% of all new cancer diagnoses in 2015.13 Although many advances in early screening, optimal chemotherapy, and a better understanding of breast cancer molecular interaction, recurrent patients with early-stage breast cancer account for over 30%.14 There is an emerging urge to weaken chemoresistance and improve sensitivity after drugs uptake accompanied by epigenetic modification and regulatory dysfunction at the level of protein interaction in cancer cells.15 It is pivotal to match susceptible tumors with multichemotherapeutic drugs to improve clinical effects.16
Based on its wide application and a wide range of toxic effects, DMF has been paid attention to look at the possible association between DMF exposure and cancer development or treatment. In this study, we explored the biological effects of DMF in breast cancer MCF-7 and nontumorous MCF-12A cells.
Materials and Methods
Cell Culture
A nontumorous mammary epithelial cell line MCF-12A and human breast adenocarcinoma cell line MCF-7 were obtained from American Type Culture Collection (Manassas, Virginia). MCF-12A cells were cultured in DMEM/F12 (Cat# 11330032; Gibco, Carlsbad, California), supplemented with 5% horse serum (Cat# 16050122; Gibco, Grand Island, California), 20 ng/mL recombinant human epidermal growth factor (Cat# AF100-15; PeproTech, Rocky Hill, New Jersey), 10 ng/mL cholera toxin (C8052-2MG; Sigma, St Louis, Missouri), 500 ng/mL hydrocortisone (Cat# H0888; Sigma), and 0.01 mg/mL bovine insulin (Cat# I5500; Sigma). MCF-7 cells were cultured in RPMI-1640 medium (Corning Inc, Manassas, Virginia) supplemented with 10% fetal bovine serum (Cat# 26140079; Invitrogen, Carlsbad, California) at 37°C in 5% CO2.
N,N-dimethylformamide Treatment and Trypan Blue Dye Exclusion Assay
Both MCF-7 and MCF-12A cells were treated with DMF (D4551-250ML; Sigma) at the dose of 0, 0.1, 1, 10, 31.25, 62.5, 100, 125, 250, and 500 mM for 24, 48, and 72 hours, respectively. Cells were then incubated with trypan blue dye (Cat# KGY015; Keygen, Nanjing, Jiangsu, China) for 15 minutes and subsequently washed 3 times with phosphate-buffered saline (PBS). The dead and live cells were observed after trypan blue staining and were determined under an inverted microscope.
Protein Extraction and Western Blot
After treatment, MCF-7 cells were washed with PBS and lysed on ice with radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime, Nantong, Jiangsu, China), supplemented with 1% phenylmethylsulfonyl fluoride (Beyotime). After sonication, the lysates were centrifuged at 14 000 revolutions per minute (rpm) for 25 minutes, and the supernatant was then collected. Protein concentration was measured by the Bicinchoninic Acid (BCA) Protein Assay kit (Cat# 23227; Thermo Scientific, Rockford, Illinois). Equal protein samples (28 µg) were subjected to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis under denaturing/reducing conditions. Proteins from the gels were transferred electrophoretically to polyvinylidene difluoride membranes (IVPH 00010; Millipore, Bedford, Massachusetts) with the semi-film transfer apparatus (BioRad, Hercules, California) for 1 A, 20 minutes. The membranes were blocked with 5% fat-free milk for 1 hour at room temperature. The membranes were then incubated overnight with primary antibody at 4°C. After wash, the membranes were incubated with the horseradish peroxidase–conjugated secondary antibody (anti-mouse, anti-rabbit, or anti-goat, chosen with respect to the source of primary antibody in 1:5000 dilution) for 1 hour at room temperature. Protein bands of interest were visualized and analyzed with Tanon-4500 gel imaging system (Tanon Science and Technology, Shanghai, China). Primary antibodies and their sources and dilutions used in this study are as follows: rabbit anti-cyclin D1 (1:2000 dilution, Cat# CY5404) and rabbit anti-cyclin E1 (1:2000 dilution, Cat# CY1028) from Abways Technology Inc (Shanghai, China); rabbit anti-Bax (1:5000 dilution, Cat# 2772), rabbit anti-Bcl-xL (1:5000 dilution, Cat# 2764), rabbit anti-caspase-9 (1:2000 dilution, Cat# 9508), rabbit anti-caspase-8 (1:2000 dilution, Cat# 4790), and mouse anti-β-actin (1:5000 dilution, Cat# 4970) from Cell Signaling Technology (Danvers, Massachusetts). After densitometric analysis, the ratio of Bcl-xL to Bax in different treatment groups was calculated. Data were represented as the mean ± standard deviation (SD).
Cell Proliferation Assay
Both MCF-7 and MCF-12A cells were cultured in 96-well plates at 4000 cells/100 µL/well. After incubation for 24 hours, the culture medium was discarded and cells were exposed to DMF at a dose of 0, 1, and 100 mM for 24 hours. WST-1 Kit (Cat# 11644807001; Roche Diagnostics, Indianapolis, Indiana) was used to determine cell proliferation as described previously.17 Briefly, a 10 µL of the WST-1 solution was added per well and incubated for 2 hours at 37°C. Absorbance was read at 450 nm with BioTek Epoch Microplate Reader (Winooski, Vermont). Experiments were performed in triplicate.
Cell Cycle and Flow Cytometry
MCF-7 cells were seeded in 6-well plates at 70% to 80% confluence. After culturing for 24 hours, cells were treated with DMF at a different concentration (0, 1, and 100 mM) and incubated for 72 hours. After detaching by trypsin, the cells were fixed with 70% alcohol overnight at 4°C. After washing cells twice with cold 1× PBS at 1000 rpm for 6 minutes, the cell pellet was resuspended with 500 µL PI/RNase staining buffer (Cat #5508825; BD Pharmingen, San Diego, California). After incubation for 15 minutes, the cells were detected by a flow cytometry (Gallios; Beckman Coulter, Inc, Brea, California).18
Apoptosis Assay
MCF-7 cells were seeded in a 6-well plate overnight at a density of 3 × 105/well, and the next day they were treated with 0, 1, and 100 mM DMF for 72 hours. Apoptosis assay was performed using the Cell Apoptosis Kit (BD Pharmingen), as per the manufacturer’s protocol. Briefly, the floating cells in culture media and washing buffer were collected together with detached cells after trypsinization. After washing with cold 1× PBS twice, the cells were resuspended in 1× binding buffer. After adding 5 µL of fluorescein isothiocyanate (FITC)-conjugated annexin-V and 5 µL propidium iodide (PI) into cells in 100 µL 1× binding buffer and incubated for 15 minutes at room temperature, the cells were further diluted in 400 µL 1× binding buffer and detected by a flow cytometry.
Statistical Analyses
For multiple groups’ comparison, a 1-way analysis of variance with post hoc test was used. A Student t test was applied to compare 2 groups (treatment vs control). Data from 3 independent experiments were presented as mean (standard deviation). A P value <.05 was considered statistically significant. All analyses and graphs were made with SPSS Statistics 21.0 software (SPSS, Chicago, Illinois) and GraphPad Prism software v 6.0 (GraphPad Software, Inc, La Jolla, California).
Results
N,N-dimethylformamide Differentially Affects the Cell Proliferation Between Cancerous and Noncancerous Cells
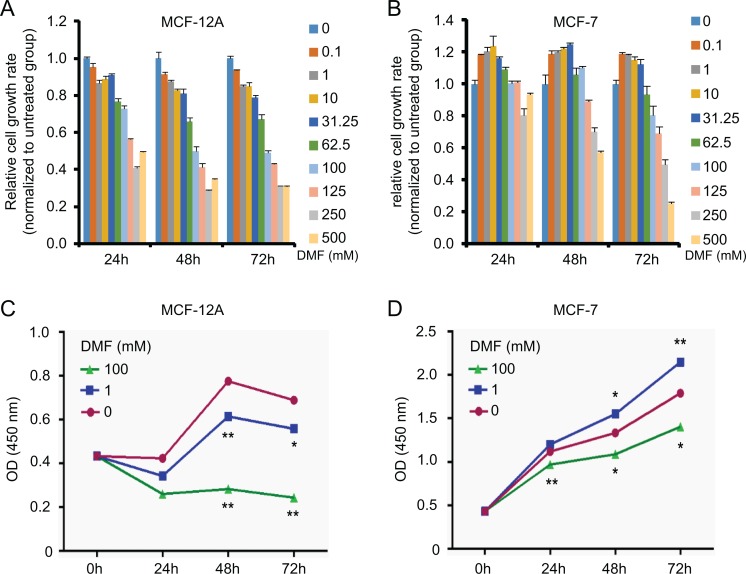
To investigate the effect of DMF on the growth of breast epithelial cells, we used a normal mammary epithelial cell line (MCF-12A) and an adenocarcinoma mammary epithelial cell line (MCF-7) treated with increasing doses of DMF (from 0.1 mM to 500 mM) for 24, 48, and 72 hours, respectively. Trypan blue dye exclusion assay showed that DMF inhibited normal MCF-12A cell growth in a dose-dependent manner (Figure 1A). Interestingly, DMF promoted cell growth at low doses (from 0.1 to 31.25 mM) and suppressed cell growth at high doses (100 to 500 mM) in cancerous MCF-7 cells (Figure 1B). WST-1 assay confirmed the results obtained by trypan blue dye exclusion assay. By comparing cells without DMF (0 mM), cell growth of MCF-12A was significantly inhibited after DMF treatment at the doses of 1 mM and 100 mM for 48 and 72 hours (Figure 1C). In MCF-7 cells, however, dual effects were observed. N,N-dimethylformamide at 1 mM significantly increased cell proliferation at 48 and 72 hours posttreatment, while DMF at 100 mM significantly decreased cell proliferation at 24, 48, and 72 hours posttreatment, compared to the untreated controls (Figure 1D).
Figure 1.
Effect of DMF on cell proliferation in nontumorous and tumorous mammary epithelial cells. Effect of DMF on MCF-12A (A) and MCF-7 (B) cell growth determined by trypan blue dye exclusion assay. Cells were treated with DMF at different doses (0-500 mM) for 24, 48, and 72 hours. Effect of DMF on MCF-12A (C) and MCF-7 (D) cell proliferation detected by the WST-1 assay. Cells were treated with DMF at different doses (0, 1, and 100 mM) for 24, 48, and 72 hours. The experiment was repeated at least 3 times. Data represent the mean ±SD. *P < .05; **P < .01. DMF indicates N,N-dimethylformamide; SD, standard deviation.
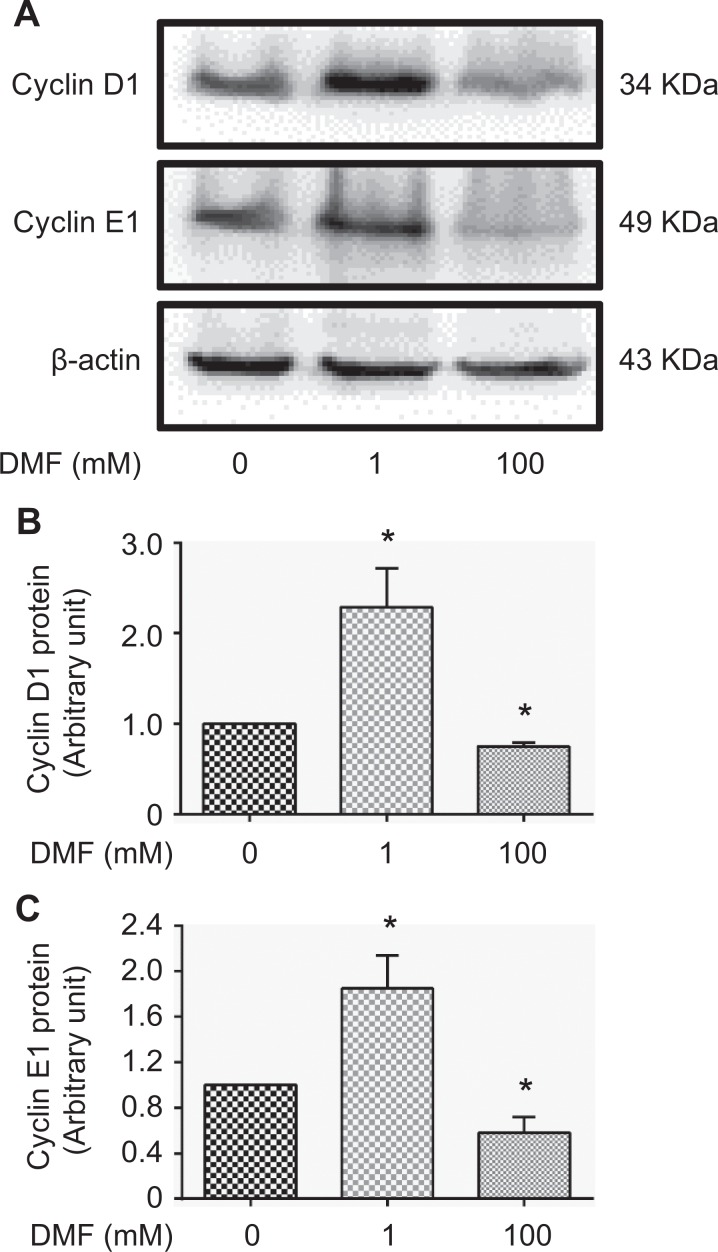
Furthermore, we found that the protein expression of cyclin D1 and cyclin E1, 2 cell proliferation markers, was significantly increased in MCF-7 cells treated with a low dose of DMF (1 mM) but was significantly decreased in cells treated with a high dose of DMF (100 mM; Figure 2A–C; P < .05).
Figure 2.
Effect of DMF on the expression of cyclin D1 and cyclin E1 in MCF-7 cells. A, The expression of cyclin D1 and cyclin E1 protein detected by Western blot analysis in MCF-7 cells treated with DMF at different doses (0, 1, and 100 mM) for 72 hours. Representative images are shown. B, Densitometric analysis of Western blots for cyclin D1 normalized to β-actin. C, Densitometric analysis of Western blots for cyclin E1 normalized to β-actin. Data represent the mean ±SD. n = 3; *P < .05. DMF indicates N,N-dimethylformamide; SD, standard deviation.
N,N-dimethylformamide Has Dual Effects on Breast Cancer Cell Cycle
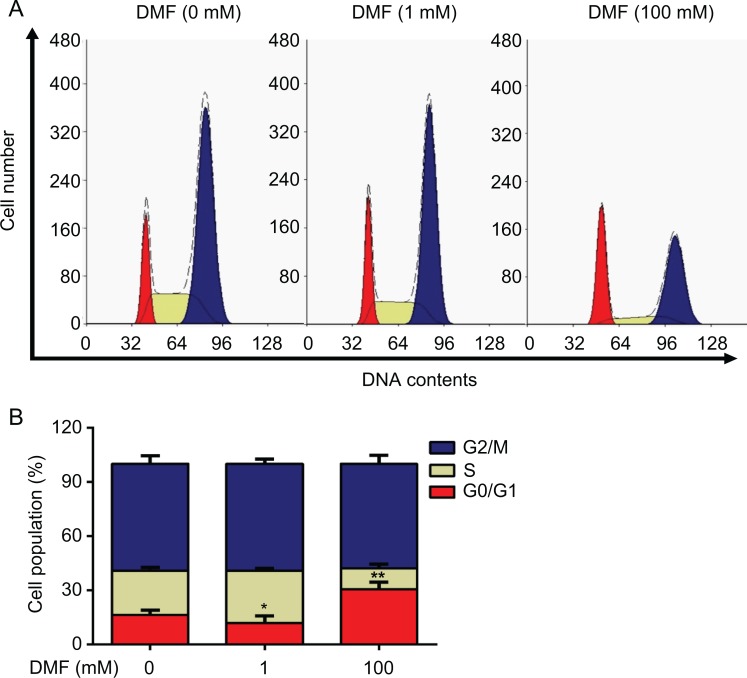
To evaluate the effect of DMF treatment on cell cycle, flow cytometry was applied in MCF-7 cells treated with low dose (1 mM) or high dose (100 mM) of DMF for 72 hours (Figure 3A). We found that cell number was significantly decreased at the G0/G1 phase after a low dose (1 mM) of DMF treatment and was significantly increased after a high dose (100 mM) of DMF treatment compared to the untreated cells (0 mM; Figure 3B). These data indicated that DMF has a dual role in breast cancer cells.
Figure 3.
Effect of DMF on cell cycle in MCF-7 cells. Cells were treated with different doses (0, 1, and 100 mM) of DMF for 72 hours. A, Cell cycle was determined by flow cytometry. B, The histogram shows the percentage of the cell population in different phases of cell cycle. The experiment was repeated at least 3 times. Data represent the mean ±SD. *P < .05; **P < .01. DMF indicates N,N-dimethylformamide; SD, standard deviation.
Dose-Dependent Effect of DMF on Apoptosis in Breast Cancer Cells
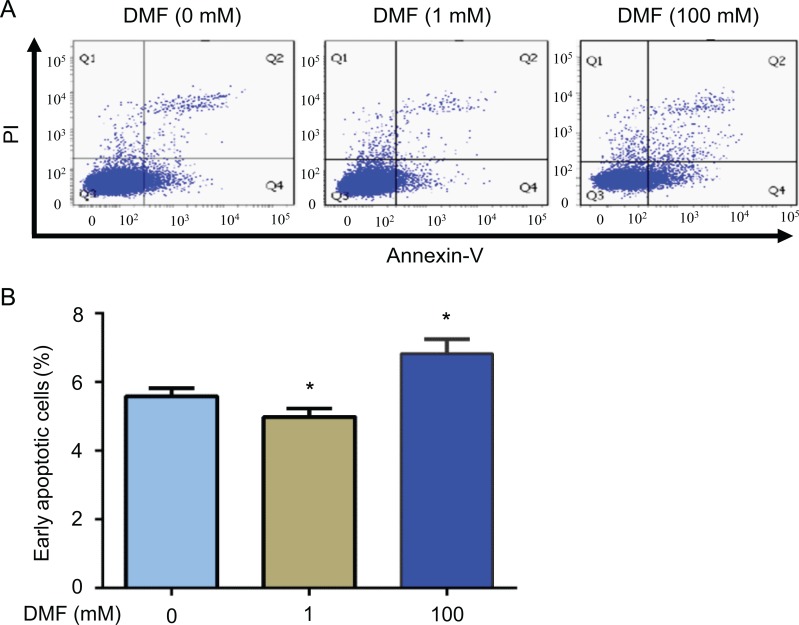
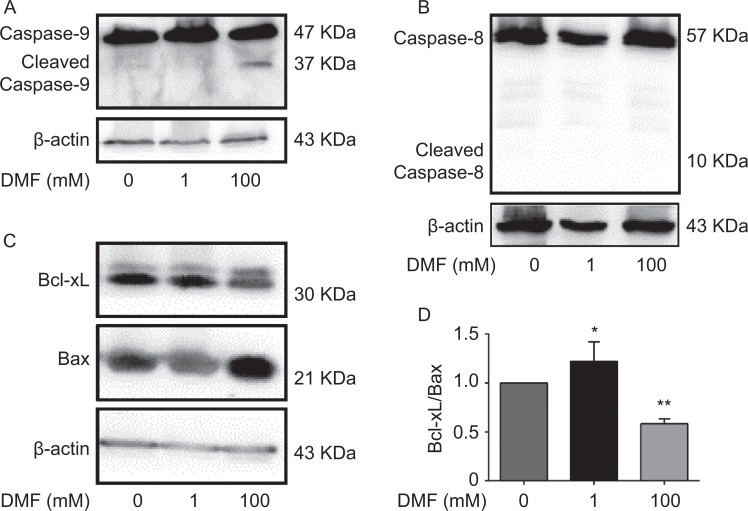
Next, we examined whether DMF influences cell apoptosis in breast cancer cells by flow cytometric analysis. After MCF-7 cells were treated with different doses of DMF (0, 1, or 100 mM) for 72 hours, we found that DMF at a high dose (100 mM) induced cell apoptosis. However, DMF at a low dose (1 mM) inhibited cell apoptosis (Figure 4A). Low-dose DMF significantly decreased early apoptotic cells, whereas high-dose DMF significantly increased early apoptotic cells (P < .05; Figure 4B). Apoptosis was further confirmed by Western blot analysis in MCF-7 cells. We found that 100 mM DMF increased a band of cleaved caspase-9 (Figure 5A) rather than caspase-8 (Figure 5B). We also found that 100 mM DMF decreased the expression of an antiapoptotic protein Bcl-xL and increased a proapoptotic protein Bax (Figure 5C). The ratio of Bcl-xL/Bax was significantly decreased after the high dose of DMF treatment but increased after the low dose of DMF treatment (Figure 5D).
Figure 4.
Effect of DMF on apoptosis in MCF-7 cells. Cells were treated with different doses (0, 1, and 100 mM) of DMF for 72 hours. A, Flow cytometric analysis of cell apoptosis. B, The histogram shows the percentage of early apoptotic cells in different treatment groups. The experiment was repeated at least 3 times. Data represent the mean ±SD. n = 3, *P < .05; **P < .01. DMF indicates N,N-dimethylformamide; SD, standard deviation.
Figure 5.
N,N-dimethylformamide-induced apoptosis via caspase 9 pathway in MCF-7 cells. Cells were treated with control (0 mM), low-dose DMF (1 mM), or high-dose DMF (100 mM) for 72 hours. Representative Western blots for caspase-9 (A) and caspase-8 (B) along with β-actin as their loading control, n = 2. C, Representative Western blots for antiapoptotic protein Bcl-xL and proapoptotic protein Bax. D, Densitometric analysis of ratio between Bcl-xL and Bax protein in different treatment groups is shown. Data represent the mean ±SD. n = 3; *P < .05; **P < .01. DMF indicates N,N-dimethylformamide; SD, standard deviation.
Discussion
The present study demonstrated that DMF treatment elicits a differential response to the breast cancer cell fate in vitro, depending upon its dose. The low dose (1 mM) of DMF promoted cell proliferation and inhibited apoptosis, while the high dose (100 mM) resulted in inhibition of cell proliferation and induction of apoptosis via caspase 9 activation. For the normal mammary epithelial cells, however, both doses of DMF were cytotoxic.
Many studies show the toxicity of DMF in both human and experimental animals.19–21 N,N-dimethylformamide can be absorbed into the body via skin absorption or inhalation. The hepatotoxic effect of DMF has been documented.22,23 Cells exposure to DMF (0.25-0.5 M) suppressed the extracellular acidification rate below baseline in mouse hepatoma cells (Hepa 1C1C7).24 Some protective compounds (eg, graphene oxide and cupric oxide) against DMF treatment can reduce the tissue damage, but an affordable protection is unguaranteed.25 Therefore, it is necessary to determine more effective and low-toxic drugs for therapeutic purposes. We showed here that the different doses of DMF modulate cancer cell behavior differently and therefore warrant caution in its use. However, the mechanism for this differential behavior of the low and high dose of DMF is still unclear and needs further investigation.
We elucidated that DMF at the dose of 100 mM attenuated the expression of antiapoptotic protein Bcl-xL through the intrinsic caspase-9 pathway. Furthermore, we found that the extrinsic caspase-8 signal was not involved in the initiation of the apoptotic program. Early discoveries also indicated that DMF can induce several malignancies to form well-differentiated phenotypes through the increase of tonofilaments and desmosomes in colon carcinoma26 and rhabdomyosarcoma.27
The dose of DMF is a key factor in the influence of cancer cell behavior. We found that a high dose (100 mM) of DMF suppressed breast cancer cell proliferation and arrested the cell cycle at the G0/G1 phase, which was similar to the data obtained by the other group that 1% DMF promotes an increase in doubling time in MCF-7 cells and cell arrest at the G0/G1 phase.28 However, the reason why low and high doses of DMF behave differently in regulating breast cancer cell proliferation and apoptosis remains unclear. Our results may explain in part by shortening the G0/G1 phase via the upregulation of cyclin D1 and cyclin E1 protein and via the caspase-9 apoptotic signaling pathway.
Interestingly, DMF has shown to inhibit alloreactive T-cell proliferation by reducing the secretion of immune regulatory factors such as TNF-α, IL-12, and IFN-γ and inducing the expression of anti-inflammatory factor heme oxygenase 1.29 We have shown here that DMF at different doses modulates cyclin D1 and cyclin E1 expression in a different way. We can also decipher that the high levels of cyclin protein promote cell viability, and hence, these 2 cyclins can be used to judge the effects of DMF treatment.
In conclusion, we have elucidated for the first time that DMF can have contrasting effects on cancer cell viability depending upon its dose such that a high dose exhibits antitumorigenic effects while a low dose can indeed promote the growth of cancer cells. Therefore, caution is warranted in determining its effective dose for targeting breast cancer.
Footnotes
Authors’ Note: Jihong Zhang and Daibing Zhou contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from National Natural Science Foundation of China (81272880), Shanghai Municipal Commission of Health and Family Planning (201640287), Natural Science Foundation of Shanghai (17ZR1404100), and Science and Technology Commission of Shanghai Municipality (124119b1300) to G. Xu and a start-up fund of research from Jinshan Hospital (2014-9) to J. Zhang.
References
- 1. Kim TH, Kim YW, Shin SM, Kim CW, Yu IJ, Kim SG. Synergistic hepatotoxicity of N,N-dimethylformamide with carbon tetrachloride in association with endoplasmic reticulum stress. Chem Biol Interact. 2010;184(3):492–501. [DOI] [PubMed] [Google Scholar]
- 2. Kim TH, Kim SG. Clinical outcomes of occupational exposure to n, n-dimethylformamide: perspectives from experimental toxicology. Saf Health Work. 2011;2(2):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ohbayashi H, Umeda Y, Senoh H, et al. Enhanced hepatocarcinogenicity by combined inhalation and oral exposures to N,N-dimethylformamide in male rats. J Toxicol Sci. 2009;34(1):53–63. [DOI] [PubMed] [Google Scholar]
- 4. Scailteur V, Lauwerys RR. Dimethylformamide (DMF) hepatotoxicity. Toxicology. 1987;43(3):231–238. [DOI] [PubMed] [Google Scholar]
- 5. Wang C, Huang C, Wei Y, Zhu Q, Tian W, Zhang Q. Short-term exposure to dimethylformamide and the impact on digestive system disease: an outdoor study for volatile organic compound. Environ Pollut. 2014;190:133–138. [DOI] [PubMed] [Google Scholar]
- 6. Zhang M, Zheng M, Wu Z, et al. Alteration of gut microbial community after N, N-dimethylformamide exposure. J Toxicol Sci. 2017;42(2):241–250. [DOI] [PubMed] [Google Scholar]
- 7. van Dongen GA, Braakhuis BJ, Leyva A, et al. Anti-tumor and differentiation-inducing activity of N,N-dimethylformamide (DMF) in head-and-neck cancer xenografts. Int J Cancer. 1989;43(2):285–292. [DOI] [PubMed] [Google Scholar]
- 8. Chen JL, Fayerweather WE, Pell S. Cancer incidence of workers exposed to dimethylformamide and/or acrylonitrile. J Occup Med. 1988;30(10):813–818. [DOI] [PubMed] [Google Scholar]
- 9. Grunt TW, Somay C, Ellinger A, Pavelka M, Dittrich E, Dittrich C. The differential effects of N,N-dimethylformamide and transforming growth factor-beta 1 on a human ovarian cancer cell line (HOC-7). J Cell Physiol. 1992;151(1):13–22. [DOI] [PubMed] [Google Scholar]
- 10. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 11. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 12. DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67(6):439–448. [DOI] [PubMed] [Google Scholar]
- 13. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. [DOI] [PubMed] [Google Scholar]
- 14. Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chavez JD, Schweppe DK, Eng JK, et al. Quantitative interactome analysis reveals a chemoresistant edgotype. Nat Commun. 2015;6:7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13(10):714–726. [DOI] [PubMed] [Google Scholar]
- 17. Sun W, Gui L, Zuo X, et al. Human epithelial-type ovarian tumour marker beta-2-microglobulin is regulated by the TGF-beta signaling pathway. J Transl Med. 2016;14(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou D, Zhang L, Sun W, et al. Cytidine monophosphate kinase is inhibited by the TGF-beta signalling pathway through the upregulation of miR-130b-3p in human epithelial ovarian cancer. Cell Signal. 2017;35:197–207. [DOI] [PubMed] [Google Scholar]
- 19. Hamada M, Abe M, Tokumoto Y, et al. Occupational liver injury due to N,N-dimethylformamide in the synthetics industry. Intern Med. 2009;48(18):1647–1650. [DOI] [PubMed] [Google Scholar]
- 20. Buylaert W, Calle P, De Paepe P, et al. Hepatotoxicity of N,N-dimethylformamide (DMF) in acute poisoning with the veterinary euthanasia drug T-61. Hum Exp Toxicol. 1996;15(8):607–611. [DOI] [PubMed] [Google Scholar]
- 21. Koh SB, Cha BS, Park JK, Chang SH, Chang SJ. The metabolism and liver toxicity of N,N-dimethylformamide in the isolated perfused rat liver. Yonsei Med J. 2002;43(4):491–499. [DOI] [PubMed] [Google Scholar]
- 22. Lynch DW, Placke ME, Persing RL, Ryan MJ. Thirteen-week inhalation toxicity of N,N-dimethylformamide in F344/N rats and B6C3F1 mice. Toxicol Sci. 2003;72(2):347–358. [DOI] [PubMed] [Google Scholar]
- 23. Roure MB, Lambert AM, Cour C, Bonnet P, Saillenfait AM. Hepatotoxicity of N,N-dimethylformamide in rats following intraperitoneal or inhalation routes of administration. J Appl Toxicol. 1996;16(3):265–267. [DOI] [PubMed] [Google Scholar]
- 24. Twiner MJ, Hirst M, Valenciano A, Zacharewski TR, Dixon SJ. N, N-Dimethylformamide modulates acid extrusion from murine hepatoma cells. Toxicol Appl Pharmacol. 1998;153(2):143–151. [DOI] [PubMed] [Google Scholar]
- 25. Zhang S, Gao W, Li J, Zhou X, Qu Y. Interfacial effects of the CuO/GO composite to mediate the side reactions of N,N-dimethylformamide fragments. ACS Appl Mater Interfaces. 2014;6(24):22174–22182. [DOI] [PubMed] [Google Scholar]
- 26. Dexter DL, Barbosa JA, Calabresi P. N,N-dimethylformamide-induced alteration of cell culture characteristics and loss of tumorigenicity in cultured human colon carcinoma cells. Cancer Res. 1979;39(3):1020–1025. [PubMed] [Google Scholar]
- 27. Dexter DL. N,N-Dimethylformamide-induced morphological differentiation and reduction of tumorigenicity in cultured mouse rhabdomyosarcoma cells. Cancer Res. 1977;37(9):3136–3140. [PubMed] [Google Scholar]
- 28. Guilbaud NF, Gas N, Dupont MA, Valette A. Effects of differentiation-inducing agents on maturation of human MCF-7 breast cancer cells. J Cell Physiol. 1990;145(1):162–172. [DOI] [PubMed] [Google Scholar]
- 29. Lehmann JC, Listopad JJ, Rentzsch CU, et al. Dimethylfumarate induces immunosuppression via glutathione depletion and subsequent induction of heme oxygenase 1. J Invest Dermatol. 2007;127(4):835–845. [DOI] [PubMed] [Google Scholar]