Abstract
Background
Clostridium difficile toxin A is responsible for colonic damage observed in infected patients. Drugs able to restore Clostridium difficile toxin A-induced toxicity have the potential to improve the recovery of infected patients. Cannabidiol is a non-psychotropic component of Cannabis sativa, which has been demonstrated to protect enterocytes against chemical and/or inflammatory damage and to restore intestinal mucosa integrity.
Objective
The purpose of this study was to evaluate (a) the anti-apoptotic effect and (b) the mechanisms by which cannabidiol protects mucosal integrity in Caco-2 cells exposed to Clostridium difficile toxin A.
Methods
Caco-2 cells were exposed to Clostridium difficile toxin A (30 ng/ml), with or without cannabidiol (10−7–10−9 M), in the presence of the specific antagonist AM251 (10−7 M). Cytotoxicity assay, transepithelial electrical resistence measurements, immunofluorescence analysis and immunoblot analysis were performed in the different experimental conditions.
Results
Clostridium difficile toxin A significantly decreased Caco-2 cells’ viability and reduced transepithelial electrical resistence values and RhoA guanosine triphosphate (GTP), bax, zonula occludens-1 and occludin protein expression, respectively. All these effects were significantly and concentration-dependently inhibited by cannabidiol, whose effects were completely abolished in the presence of the cannabinoid receptor type 1 (CB1) antagonist, AM251.
Conclusions
Cannabidiol improved Clostridium difficile toxin A-induced damage in Caco-2 cells, by inhibiting the apoptotic process and restoring the intestinal barrier integrity, through the involvement of the CB1 receptor.
Keywords: Clostridium difficile, cannabinoids, cannabidiol, clostridium difficile toxin A, intestinal permeability
Introduction
Clostridium difficile infection (CDI) is responsible for the pseudomembranous colitis, a serious pathological condition of the large intestine, characterised by massive inflammation and bleeding.1 It is known that Clostridium difficile produces two enterotoxins, named Clostridium difficile toxin A and B (TcdA and TcdB, respectively) that, in turn, are responsible for the extensive colonic mucosal damage, causing severe diarrhoea, colitis, shock and death in most severe cases.2,3 TcdA is the major cause of Clostridium difficile enterotoxicity. TcdA is a glucosyltransferase, that once internalised into the host cell via receptor-mediated endocytosis, inactivates small GTPases.4 Among these proteins, RhoA, a small GTPase member of the Rho subfamily that is a critical regulator of actin cytoskeleton and tight junction assembly, is the primary target of TcdA.5 TcdA-induced inactivation of RhoA results in the transition from guanosine triphosphate (GTP)-bound form (active) to guanosine diphosphate (GDP)-bound form (inactive), leading to an alteration of cellular structure and tight junction integrity, and consequently to increased epithelial barrier permeability; this process is also sustained by the acute inflammation of colonic mucosa and contributes to the leaky gut and massive ions’ secretion.6 Due to its role in the mucosal homeostasis and functions, the targeting of RhoA may represent an innovative pharmacological strategy for the treatment of CDI.
In the last decade, cannabinoids extracted from the marijuana plant (Cannabis sativa) and synthetic cannabinoids have shown numerous beneficial effects on gastrointestinal (GI) functions.7 Non-psychotropic phytocannabinoid cannabidiol (CBD) is one of the most interesting compounds, since it exerts a wide range of beneficial pharmacological actions on GI functions, ranging from antioxidant to antinflammatory activities.8,9 Unlike psychoactive cannabinoids such as tetrahydrocannabidiol (THC), CBD has little binding affinity to cannabinoid receptors (either CB1 and CB2); whereas, by acting on peroxisome proliferator-activated receptor gamma (PPARγ)10 and 5-hydroxytryptamine (5HT)-1 A receptors,11 it displays antinflammatory and antioxidant effects.12 Unlike other phytocannabinoids, CBD has been shown to act as a non-competitive negative allosteric modulator of CB1 receptors.13 Notably, CBD is able to restore in vitro intestinal permeability increased by ethylenediaminetetraacetic acid (EDTA) or pro-inflammatory stimuli.14,15 So far, no evidence has been produced about the putative protective role exerted by CBD in CDI. To further this aim, the present study was addressed at evaluating the in vitro effects of CBD on TcdA-induced apoptosis in Caco-2 cells and at investigating the effects of CBD and its mechanism of action.
Materials and methods
Materials
The experiments were performed in human Caucasian colon adenocarcinoma (Caco-2) cells that have been shown to be a good model to address TcdA toxicity in vitro.16 Caco-2 cells were purchased from European Collection of Cell Cultures (ECACC, Public Health England, Porton Down, Salisbury, UK). Cell medium, chemicals and reagents used for cell culture, and TcdA were obtained from Sigma-Aldrich (St. Louis, Missouri, USA). Instruments, reagents, and materials used for Western blot analysis were obtained from Bio-Rad Laboratories (Milan, Italy). CBD and AM251 (CB1 receptor antagonist) were purchased from Tocris Cookson, Inc. (Ballwin, Missouri, USA). The antibodies rabbit anti-zonula occludens-1 (ZO-1), rabbit anti-occludin, rabbit anti-bax and rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies were procured from Cell Signalling Technology (Danvers, Massachusetts, USA). Mouse anti-ZO-1 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, California, USA). Mouse monoclonal antibody anti-active RhoA by New East Bioscience (Pennsylvania, USA) has been used. Fluorescein isothiocyanate-conjugated anti-rabbit antibody and Texas red conjugated anti-mouse antibody were purchased from Abcam (Cambridge, UK) and horseradish peroxidase (HRP) was obtained from Dako (Milan, Italy).
Cell culture and experimental conditions
Caco-2 cells were grown at 37℃ with 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) in addition with 10% foetal bovine serum (FBS), 1% penicillin–streptomycin, 2 mM L-glutamate, and 1% non-essential amino acids. Caco-2 cells were plated at a density of 1 × 106 cells/well in six-well plates and incubated for 24 h. Every 24–48 h the medium was replaced with fresh medium to confluence. After reaching confluence, the cells were washed three times with phosphate-buffered saline (PBS), detached with trypsin/EDTA, plated in six-well plates some containing and on polyethylene-terephtalate (PET) filter inserts (Falcon Becton-Dickinson, 0.4 mm pore diameter, area 4.21 cm2, pore density 2 ± 0.2 106/cm2) to measure the transepithelial electrical resistance (TEER), and allowed to adhere for an appropriate time. Caco-2 cells were randomly divided into the following groups: vehicle, 30 ng/ml TcdA, 30 ng/ml TcdA plus CBD at 10−9, 10−8 and 10−7 M CBD and 30 ng/ml TcdA plus 10−7 M CBD plus 10−7 M CB1 receptor antagonist AM251. The concentrations of CBD and AM251 were selected on the basis of previous reports14,15 and our preliminary experiments (Supplementary Material, Figure 1, data not shown); in brief, cells were treated with different concentrations of CBD and/or AM251 for 24 h and then incubated at 37℃ in the presence of TcdA for 24 h.
TEER
Caco-2 (TEER) was measured using the EVOM volt-ohm meter (World Precision Instruments Germany, Berlin, Germany) according to the method described by Wells and colleagues.17
In brief, cells were used for experimentation between 14–21 days and each epithelial cell layer with a TEER value greater than 1000 Ω × cm2, was considered to have tight adhesion. At this point, cell monolayers were treated according to experimental protocol described above and TEER measurements were performed at different time points (2, 3, 5, 7, 12, 18 and 24 h, respectively). TEER values were measured at a current of 20 mA, corrected for background, resistance value without cells, and normalised by multiplying the determined resistance by effective membrane growth area, 4.71 cm2.
Cytotoxicity assay
The 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide (MTT) assay was used to determine Caco-2 cell proliferation and survival.18 At least (5 × 104 cells/well) were plated in 96-well plates and allowed to adhere for 3 h. Then DMEM was replaced with fresh medium and then cells were treated according to the different experimental protocols (see above). After 24 h, 25 μl MTT (5 mg/ml MTT in DMEM) was added to the cells and the mixture was incubated for further 3 h at 37℃. Subsequently, the cells were lysed and the dark blue crystals were solubilised using a 100 μl solution containing 50% N,N-dimethylformamide and 20% (w/v) sodium dodecyl sulphate (SDS) (pH 4.5). The optical density (OD) of each well was determined using a microplate spectrophotometer equipped with a 620 nm filter (PerkinElmer, Inc.; Waltham, Massachusetts, USA).
Western blot analysis
Twenty-four hours after treatment, the cells (1 × 106/well) were washed with ice-cold PBS, were harvested into Separate Eppendorf tubes for different treatment groups and collected by centrifugation at 180 g for 10 min at 4℃. The cell pellet, obtained after centrifugation, was re-suspended in 100 µl ice-cold hypotonic lysis buffer (10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM phenylmethylsulphonylfluoride, 1.5 µg/ml soybean trypsin inhibitor, 7 µg/ml pepstatin A, 5 µg/ml leupeptin, 0.1 mM benzamidine and 0.5 mM dithiothreitol (DTT)) and incubated on ice for an additional 15 min.
The suspension was rapidly passed through a syringe needle five to six times to lyse the cells and then centrifuged for 15 min at 13,000 × g to obtain the cytoplasmic fraction. The cytoplasmic fraction proteins were used to determine the protein concentration with Bradford assay and mixed with non-reducing gel loading buffer (50 mM Tris (hydroxymethyl) aminomethane (Tris),10% SDS, 10% glycerol, 2 mg bromophenol/ml) at a 1:1 ratio. The solutions were then boiled for 3 min, centrifugated at 10,000 g for 10 min and 50 µg of each homogenate was used for electrophoresis using 12% discontinuous polyacrylamide mini gels. Proteins were then transferred to nitrocellulose membranes that were saturated by incubation with 10% non-fat dry milk in 1X PBS overnight at 4℃ and then incubated with rabbit anti-ZO-1 (1:1000), rabbit anti-occludin (1:1000), mouse anti-active RhoA (1:1000), rabbit anti-bax (1:1000) and rabbit anti-GAPDH (1:1000) antibodies. After being extensively washed in TBS 1X with 0.1% Tween 20, membranes were then incubated for 2 h at room temperature with the specific secondary antibodies conjugated to HRP anti-mouse (1:2000) or anti-rabbit (1:3000). Immune complexes were identified by enhanced chemiluminescence detection reagents (Amersham Biosciences, Milan, Italy) and the blots were analysed by scanning densitometry (GS-700 Imaging 143 Densitometer; Bio-Rad, Segrate, Italy). Results are expressed as OD; (arbitrary units; mm2) and normalised against the expression of the housekeeping protein GAPDH.
Immunofluorescence
For these experiments, Caco-2 cells were cultured onto coverslips until confluence, and then treated according to the different above-described protocols. Cells were then fixed for 30 min in 4% formaldehyde, washed with ice-cold PBS and permeabilised with 0.3% Triton-X100 in PBS for one hour. Subsequently, 2% bovine serum albumin (BSA) was used to block the nonspecific binding sites. The cells were then incubated overnight with mouse anti-ZO-1 (1:100), or rabbit anti-occludin antibody (1:100), following PBS washing and further incubated in the dark for half an hour with the appropriate secondary antibody (fluorescein isothiocyanate (FITC)-conjugated anti-rabbit or Texas red conjugated anti-mouse). After final PBS washing, the cells were analysed using a microscope (Nikon Eclipse 80i), and images were captured with a high-resolution digital camera (Nikon Digital Sight DS-U1). Texas Red was excited at a wavelength of 568 nm and collected through a long pass filter (590LP). FITC was excited with a wavelength of 488 nm and collected with a narrow band filter (515–540BP). Texas Red and FITC were assigned to the red and green channels respectively of the generated RGB channel image.
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM) of four or five experiments and each experiment was performed in triplicate. Statistical analysis was performed using parametric one-way analysis of variance (ANOVA) and Bonferroni’s post-hoc test was used for multiple comparisons. Values of p < 0.05 were considered significant.
Results
CBD affects TcdA-induced damage of epithelial barrier integrity and restores the expression of ZO-1 and occludin
TEER measurements were performed to evaluate the effect of CBD (10−7, 10−8 and 10−9 M) alone, or in the presence of CB1 antagonist AM251, on epithelial barrier integrity of Caco-2 cells layers exposed to TcdA (30 ng/ml) for 24 h.
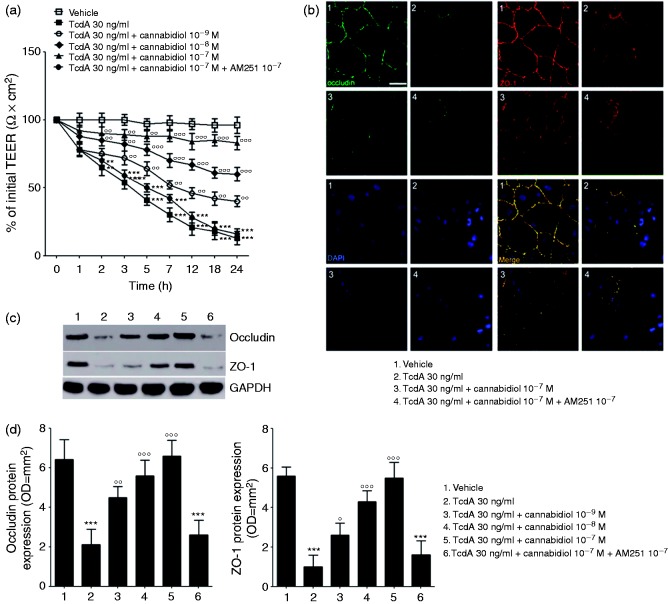
As shown in Figure 1(a), TcdA exposure induced a significant and time-dependent reduction of TEER (by −35, −46, −57, −69, −78, −81 and −86%, at 2, 3, 5, 7, 12, 18 and 24 h, respectively; p < 0.01 at 2 h and p < 0.001 at all other time-points). Starting from 2 h after toxin challenge, the effect of TcdA on electrical resistance was significantly and concentration-dependently counteracted by CBD treatment (Figure 1(a)); TEER values at 2, 3, 5, 7, 12, 18 and 24 h were indeed significantly increased by 15, 33, 56, 73, 119, 133 and 225% in CBD 10−9 M-treated cells (p = ns at 2 h and p < 0.01 at all other time-points), while CBD 10−8 M and CBD 10−7 M treatments yield to a significant increase of TEER values by 31, 52, 90, 133, 219, 239, 361%, and 38, 65, 114, 193, 300, 372 and 538%, respectively (p < 0.01 at 2 and 3 h and p < 0.001 at all other time points for CBD 10−8 M; p < 0.01 at 2, 3 and 5 h and p < 0.001 at all other time-points for CBD 10−7 M).
Figure 1.
Effect of cannabidiol (CBD) on transepithelial electrical resistance (TEER) and barrier integrity of Clostridium difficile toxin A (TcdA)-exposed Caco-2 cells. (a) 24 h Time course TEER changes following treatment (n = 4); (b) immunofluorescent staining showing the effects of TcdA on zonula occludens-1 (ZO-1) and occludin co-expression at 24 h. Nuclei were stained by DAPI (scalebar = 25 µm); (c) immunoreactive bands corresponding to ZO-1 and occludin expression at 24 h following the TcdA challenge; (d) relative densitometric analysis of immunoreactive bands (arbitrary units normalised against the expression of the housekeeping glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein; n = 5). Results are expressed as mean ± standard error of the mean (SEM) of experiments performed in triplicate. ***p < 0.001 and **p < 0.01 vs vehicle group; °°°p < 0.001, °°p < 0.01 and °p < 0.05 vs TcdA group. DAPI (4',6-diamidino-2-phenylindole).
Interestingly, the effect of CBD on the TcdA-induced TEER reduction was completely abolished in the presence of the CB1 antagonist, AM251 (p < 0.01, Figure 1(a)).
Immunofluorescence analysis, showed that 10−7 M CBD, markedly reversed the TcdA-induced decrease of both occludin and ZO-1 co-expression in cultured cells, thus restoring the epithelial barrier architecture (Figure 1(b)). This finding was confirmed by quantitative analysis showing that TcdA-reduced expression of occludin and ZO-1 (0.3 ± 0.1 and 0.2 ± 0.1 vs 1.0 ± 0.1 fold-change in the vehicle group, respectively; all p < 0.001) was significantly and concentration dependently restored by CBD at the doses of 10−9 M (occludin: 2.1 ± 0.2 vs 1.0 ± 0.3 fold-change in TcdA-treated cells, p < 0.01; ZO-1: 2.6 ± 0.5 vs 1.0 ± 0.5 fold-change in TcdA-treated cells, p < 0.05), 10−8 M (occludin: 2.6 ± 0.3 vs 1.0 ± 0.3 fold-change in TcdA-treated cells, p < 0.001; ZO-1: 4.4 ± 0.4 vs 1.0 ± 0.5 fold-change in TcdA-treated cells, p < 0.001) and 10−7 M (occludin: 3.1 ± 0.3 vs 1.0 ± 0.3 fold-change in TcdA-treated cells, p < 0.001; ZO-1: 5.5 ± 0.5 vs 1.0 ± 0.5 fold-change in TcdA-treated cells, p < 0.001) (Figure 1(c) and (d)). Once again, AM251 significantly inhibited the CBD-mediated rescue of ZO-1 and occludin proteins (all p < 0.001) (Figure 1(b)–(d)).
CBD inhibits TcdA-induced apoptosis and cells’ toxicity
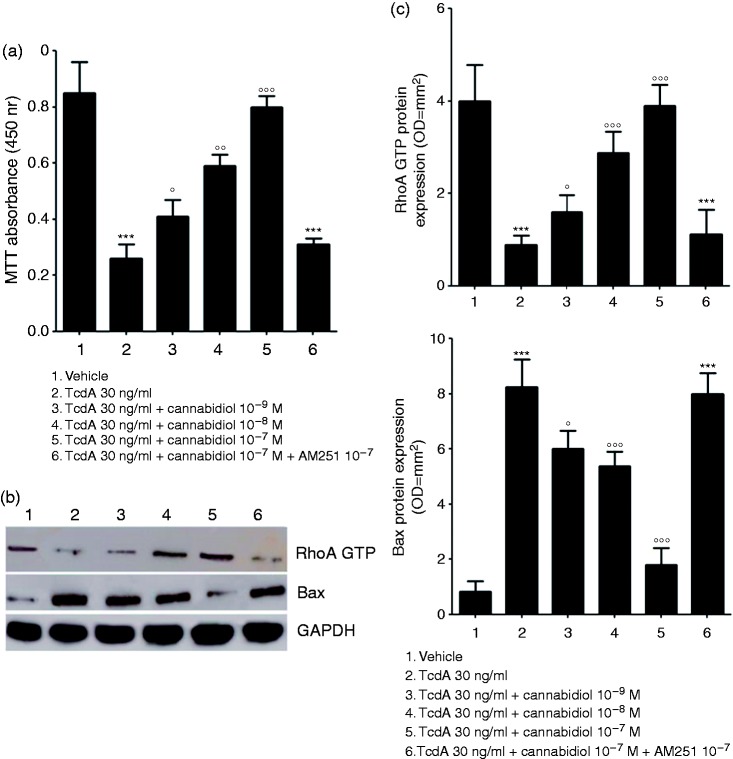
As shown in Figure 2(a), a significant decrease in Caco-2 cell viability was observed at 24 h following the TcdA challenge (−70% as compared to vehicle group assumed as 100% viable cells, p < 0.001). Under the same experimental conditions, CBD caused a significant and concentration-dependent inhibition of TcdA-induced cytotoxicity, resulting in an increased cells’ viability (by 61, 133 and 328% at 10−9, 10−8 and 10−7 M, respectively, vs TcdA group (p < 0.05, p < 0.01 and p < 0.001, respectively).
Figure 2.
Effect of cannabidiol (CBD) on Clostridium difficile toxin A (TcdA)-induced cells toxicity and apoptosis. (a) 3-[4,5-Dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide (MTT) cell viability absorbance at 24 h (n = 5); (b) immunoreactive bands corresponding to RhoA GTP and Bax expression at 24 h following the TcdA challenge; (c) relative densitometric analysis of immunoreactive bands (arbitrary units normalised against the expression of the housekeeping glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein; n = 5). Results are expressed as mean ± standard error of the mean (SEM) of experiments performed in triplicate. ***p < 0.001 and vs vehicle group; °°°p < 0.001, °°p < 0.01 and °p < 0.05 vs TcdA group. GTP: guanosine triphosphate.
Exposure to TcdA significantly reduced the expression of RhoA GTP (0.2 ± 0.1 vs 1.0 ± 0.3 fold-change in the vehicle group, p < 0.001) and increased the expression of the pro-apoptotic Bax protein (10.5 ± 1.2 vs 1.0 ± 0.5 fold-change in the vehicle group, p < 0.001) (Figure 2(b) and (c)); these effects were significantly restored by CBD, that at 10−9, 10−8 and 10−7 M increased the expression of RhoA GTP (1.8 ± 0.4, 3.3 ± 0.5 and 4.5 ± 0.5 vs 1.0 ± 0.3 fold-change in TcdA-treated cells; p < 0.05, p < 0.001 and p < 0.001, respectively) and decreased the expression of Bax (0.7 ± 0.1, 0.6 ± 0.1 and 0.2 ± 0.1 vs 1.0 ± 0.1 fold-change in TcdA-treated cells; p < 0.05, p < 0.001 and p < 0.001) (Figure 2(b) and (c)). As shown for the TcdA-impaired barrier function the protective effects of CBD on cells toxicity were completely abolished in the presence of AM251 (all p < 0.001) (Figure 2(a)–(c)).
Discussion
CDI is one of the main causes of nosocomial diarrhoea and it is responsible for pseudomembranous colitis. An annual incidence of ∼450,000 cases in the USA has been estimated, turning CDI into a very important sanitary emergency;19 since it is associated with significant morbidity, 5% infection-related mortality and an overall mortality of 13–20 %.3,20 There is an urgent need for new drugs able to improve CDI outcome, maximising the recovery of patients.
Due to its ability to inhibit Rho GTP activation,4,21 TcdA has been postulated as the main enterotoxin involved in gut mucosal disruption,5,22 leaky gut and loss of cell-to-cell integrity, leading to massive apoptosis.23,24 The inhibition of TcdA effects might thus represent the key for a targeted therapy of CDI.
In this perspective, cannabinoids might display a wide range of protective effects on the GI epithelial barrier, due to their antinflammatory, anticancer and antioxidant properties.25,26 Among the almost 113 active phytocannabinoids isolated from Cannabis sativa plant, CBD is one of the most interesting compounds considered for medical use, as different clinical reports showed its almost complete lack of side effects in humans.27 Remarkably, CBD is a non-psychotropic cannabinoid (unlike Δ9-THC) and does not interfere with psychomotor learning and psychological functions.28
In this study we have demonstrated, for the first time, that CBD is able to preserve mucosal integrity and to reduce cellular permeability in in vitro cultured Caco-2 cells, counteracting the effects of TcdA. CBD, indeed, caused a concentration-dependent increase of transepithelial resistance, significantly preventing the enterotoxin-evoked damage. Moreover, CBD caused a marked inhibition of cell death in TcdA-exposed cells, due to a concentration-dependent up-regulation of both occludin and ZO-1 protein, two of the main cell-to-cell tight junction proteins.29 Furthermore, CBD caused a significant RhoA GTP rescue that raised in parallel with the inhibition of pro-apoptotic Bax protein expression; these combined effects likely account for the restoration of the TcdA-induced intestinal barrier dysfunction and apoptosis.
CBD effects were, at least partially, mediated by critical involvement of the CB-1 receptor, since they were almost completely abolished in the presence of the specific CB-1 receptor antagonist AM251.
Although different receptors have been proposed to mediate CBD activity,11,30 it has been postulated that CBD may represent a non-competitive negative allosteric modulator of CB1 receptors.13 Consequently, the presence of a specific CB1 antagonist markedly impairs CBD activity, as previously demonstrated by different studies.14,15 Accordingly, CBD was able to contain cellular damage in the in vitro model of mucosal disruption, as it occurs in our experimental conditions. Our results indicated that CBD is able to increase RhoA GTP expression, via the selective involvement of CB-1 receptors. However, CBD exhibits both antioxidant and antinflammatory properties, labelled as generic neuroprotective functions,30 mediated by a number of different pathways and cellular effectors, that have been only partially recognised so far. These so-called ‘entourage’ effects are not to be excluded a priori when considering the potential therapeutic effects of this compound in CDI.
One can speculate that this entourage activity might synergistically cooperate with CB-1 dependent negative allosteric modulation, further enhancing the protective effects on gut epithelial cells; preventing the cytotoxic effects of reactive oxygen species products and pro-inflammatory cytokines,31,32 released in the mucosa following TcdA stimulus.
In recent decades, CBD has been proposed as an effective therapeutic option in a variety of GI pathologies, ranging from inflammatory bowel disease8 to colon cancer,33 inflammatory hypermotility in mice34 and intestinal sepsis.35 The results of our preliminary report indicate that CBD might be an intriguing candidate in CDI treatment, as well.
Although to be confirmed in vivo, the multifaceted activities exerted by CBD might prevent the cytotoxic damage in CDI and from a translational standpoint, given its lack of any significant toxic effect in humans, may ideally represent an effective adjuvant treatment in this high-mortality and morbidity rate condition.
Conclusion
Clostridium difficile infection is the leading cause of hospital-acquired diarrhoea and pseudomembranous colitis. Clostridium difficile-Toxin A significantly affects enterocytes permeability leading to apoptosis and colonic mucosal damage. In the present study, we showed that Cannabidiol, a non-psychotropic component of Cannabis sativa significantly inhibit the apoptosis rate in TcdA-exposed cells and restores barrier function by a significant RhoA GTP rescue. We also provide evidence that the effects of Cannabidiol are mediated by CB-1 receptor. Given the absence of any significant toxic effect in humans, cannabidiol may ideally represent an effective adjuvant treatment for Clostridium difficile-associated colitis.
Knowledge on this subject:
Clostridium difficile infection is the leading cause of hospital-acquired diarrhoea and pseudomembranous colitis
Clostridium difficile-Toxin A is responsible for extensive colonic mucosal damage and altered barrier function
Cannabidiol is a non-psychotropic component of Cannabis sativa with potent anti-inflammatory activities on the gastrointestional tract
What are the significant and/or new findings of this study?
Clostridium difficile-Toxin A significantly affects enterocytes permeability and apoptosis
Cannabidiol caused a marked inhibition of apoptosis in TcdA-exposed cells and restores barrier function by a significant RhoA GTP rescue
The protective effects of Cannabidiol are mediated by CB-1 receptor
Given the absence of any significant toxic effect in humans, cannabidiol may ideally represent an effective adjuvant treatment for Clostridium difficile-associated colitis
Supplementary Material
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.McFarland LV, Surawicz CM, Rubin M, et al. Recurrent Clostridium difficile disease: Epidemiology and clinical characteristics. Infect Control Hosp Epidemiol 1999; 20: 43–50. [DOI] [PubMed] [Google Scholar]
- 2.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: New developments in epidemiology and pathogenesis. Nat Rev Microbiol 2009; 7: 526–536. [DOI] [PubMed] [Google Scholar]
- 3.Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med 2015; 372: 1539–1548. [DOI] [PubMed] [Google Scholar]
- 4.Voth DE, Ballard JD, Studi D, et al. Clostridium difficile toxins: Mechanism of action and role in disease. Clin Microbiol Rev 2005; 18: 247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun X, Savidge T, Feng H. The enterotoxicity of Clostridium difficile toxins. Toxins 2010; 2: 1848–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly CP, Lamont JT. Clostridium difficile infection. Annu Rev Med 1998; 49: 375–390. [DOI] [PubMed] [Google Scholar]
- 7.Borrelli F, Aviello G, Romano B, et al. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med 2009; 87: 1111–1121. [DOI] [PubMed] [Google Scholar]
- 8.De Filippis D, Esposito G, Cirillo C, et al. Cannabidiol reduces intestinal inflammation through the control of neuroimmune axis. PloS One 2011; 6: e28159–e28159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izzo A, Sharkey K. Cannabinoids and the gut: New developments and emerging concepts. Pharmacol Ther 2010; 126: 21–38. [DOI] [PubMed] [Google Scholar]
- 10.O’Sullivan SE, Kendall D. Cannabinoid activation of peroxisome proliferator-activated receptors: Potential for modulation of inflammatory disease. Immunobiology 2010; 215: 611–616. [DOI] [PubMed] [Google Scholar]
- 11.Mishima K, Hayakawa K, Abe K, et al. Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor-dependent mechanism. Stroke 2005; 36: 1077–1082. [DOI] [PubMed] [Google Scholar]
- 12.Hampson AJ, Grimaldi M, Axelrod J, et al. Cannabidiol and (-)D9-tetrahydrocannabinol are neuroprotective antioxidants. PNAS 1998; 95: 8268–8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laprairie RB, Bagher AM, Kelly ME, et al. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Brit J Pharmacol 2015; 172: 4790–4805. [DOI] [PMC free article] [PubMed]
- 14.Alhamoruni A, Lee AC, Wright KL, et al. Pharmacological effects of cannabinoids on the Caco-2 Cell culture model of intestinal permeability. J Pharmacol Exp Ther 2010; 335: 92–102. [DOI] [PubMed] [Google Scholar]
- 15.Alhamoruni A, Wright KL, Larvin M, et al. Cannabinoids mediate opposing effects on inflammation-induced intestinal permeability. Brit J Pharmacol 2012; 165: 2598–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esposito G, Nobile N, Gigli S, et al. Rifaximin improves clostridium difficile toxin A-induced toxicity in Caco-2 cells by the PXR-dependent TLR4/MyD88/NF-κB pathway. Front Pharmacol 2016; 7: 1–8. [DOI] [PMC free article] [PubMed]
- 17.Wells CL, Westerlo E, Jechorek RP, et al. Cytochalasin-induced actin disruption of polarized enterocytes can augment internalization of bacteria. Infect Immun 1998; 66: 2410–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65: 55–63. [DOI] [PubMed] [Google Scholar]
- 19.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372: 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lofgren ET, Cole SR, Weber DJ, et al. Hospital-acquired Clostridium difficile infections: Estimating all-cause mortality and length of stay. Epidemiology 2014; 25: 570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Just I, Wilm M, Selzer J, et al. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the rho proteins. J Biol Chem 1995; 270: 13932–13936. [DOI] [PubMed] [Google Scholar]
- 22.Nusrat A, Madara JL, Parkos CA. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun 2001; 69: 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerhard R, Nottrott S, Schoentaube J, et al. Glucosylation of Rho GTPases by Clostridium difficile toxin A triggers apoptosis in intestinal epithelial cells. J Med Microbiol 2008; 57: 765–770. [DOI] [PubMed] [Google Scholar]
- 24.Brito GAC, Fujji J, Carneiro-filho BA, et al. Mechanism of Clostridium difficile toxin A – induced apoptosis in T84 cells. J Infect Dis 2002; 186: 1438–1447. [DOI] [PubMed] [Google Scholar]
- 25.Esposito G, Ligresti A, Izzo A, et al. The endocannabinoid system protects rat glioma cells against HIV-1 Tat protein-induced cytotoxicity. Mechanism and regulation. J Biol Chem 2002; 277: 50348–50354. [DOI] [PubMed] [Google Scholar]
- 26.Scuderi C, Filippis D De, Iuvone T, et al. Cannabidiol in medicine: A review of its therapeutic potential in CNS disorders. Phytother Res 2009; 602: 597–602. [DOI] [PubMed]
- 27.Mechoulam R, Hanus L. Cannabidiol: An overview of some chemical and pharmacological aspects. Part I: Chemical aspects. Chem Phys Lipids 2002; 121: 35–43. [DOI] [PubMed] [Google Scholar]
- 28.Bergamaschi MM, Queiroz RHC, Zuardi AW, et al. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf 2011; 6: 237–249. [DOI] [PubMed] [Google Scholar]
- 29.Hartsock A, Nelson WJ. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 2008; 1778: 660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esposito G, Scuderi C, Valenza M, et al. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PloS One 2011; 6: e28668–e28668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frädrich C, Beer L-A, Gerhard R. Reactive oxygen species as additional determinants for cytotoxicity of Clostridium difficile Toxins A and B. Toxins 2016; 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JM, Kim JS, Jung HC, et al. Differential expression and polarized secretion of CXC and CC chemokines by human intestinal epithelial cancer cell lines in Response to Clostridium difficile Toxin A. Microbiol Immunol 2002; 46: 333–342. [DOI] [PubMed] [Google Scholar]
- 33.Aviello G, Romano B, Borrelli F, et al. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J Mol Med 2012; 90: 925–934. [DOI] [PubMed] [Google Scholar]
- 34.Capasso R, Borrelli F, Aviello G, et al. Cannabidiol, extracted from Cannabis sativa, selectively inhibits inflammatory hypermotility in mice. Brit J Pharmacol 2008; 154: 1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Filippis D, Iuvone T, D’Amico A, et al. Effect of cannabidiol on sepsis-induced motility disturbances in mice: Involvement of CB receptors and fatty acid amide hydrolase. Neurogastroenterol Motil 2008; 20: 919–927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.