Abstract
Background and aims
Current guidelines recommend antibiotic prophylaxis in all patients presenting with cirrhosis and acute variceal hemorrhage (AVH). We aimed to evaluate the characteristics and clinical impact of “early” infections (developing within 14 days) of AVH in a real-world setting.
Methods
We analyzed retrospective data from a cohort of 371 adult patients with cirrhosis and AVH all of whom had received antibiotic prophylaxis (74% men; mean age 56 years), admitted to tertiary care hospitals in Edmonton, Alberta, Canada, and Barcelona, Spain. Sensitivity analyses were presented for culture-positive (confirmed) infections.
Results
The mean MELD was 16. Fifty-two percent of patients received quinolones, 45% third-generation cephalosporins and 3% other antibiotics. Fourteen percent (51/371) developed an infection within 14 days of AVH. Seventy-five percent of infections were culture positive and occurred at a mean of six days from AVH. When all infections were considered, respiratory infections were the most common (53%) followed by urinary tract infections (17%) and bacteremia (16%). Resistance patterns differed between countries. Outpatient antibiotic prophylaxis (OR 5.4) and intubation (OR 2.6) were independent predictors of bacterial infection. Bacterial infection (OR 2.6) and the MELD (OR 1.2) were independent predictors of six-week mortality.
Conclusions
Early bacterial infections develop in 14% of cirrhotic patients with AVH despite antibiotic prophylaxis, and have a negative impact on six-week mortality. Intubation and outpatient antibiotic prophylaxis are associated with increased risk of early bacterial infections. Patients at risk should be followed closely with prompt infection workup and local antibiogram-based expansion of antibiotic therapy in case of clinical decline.
Keywords: Cirrhosis, antibiotic prophylaxis, variceal bleed, antibiotic resistance, microbiology
Introduction
Patients with cirrhosis are at an increased risk for bacterial infections due to a combination of innate and adaptive immune dysfunction, increased intestinal permeability and pathological bacterial translocation.1,2 In patients with acute variceal hemorrhage (AVH) (in the absence of antibiotic prophylaxis), approximately 20% have infection detected on the day of admission and up to 50% develop infection during their hospital stay.3 Most infections develop within the first seven days after the bleed.3–7 Bacterial infections have important clinical consequences including an increase in AVH-related mortality2,3,6 and in smaller studies, failure to control bleeding and re-bleeding.8,9 Accordingly, current clinical guidelines recommend that antibiotic prophylaxis be instituted as early as possible on presentation of AVH and continued for five to seven days in all patients.10,11 Such prophylaxis is associated with a reduction in the rates of bacterial infection (relative risk (RR) 0.35), re-bleeding (RR 0.53) and mortality (RR 0.79).12
Aggregated meta-analysis data suggest that 13%3,13 of patients develop bacterial infections in the early post-AVH period despite antibiotic prophylaxis. This number may be climbing with increasing rates of antibiotic resistance and shifts in the microbiological profile of cirrhosis-related infections.14 Given the deleterious consequences of bacterial infection in the context of AVH, a better understanding of the pathogens causing “early” infections will allow us to optimize our management of patients with AVH. Using data from two separate sites (Edmonton, Canada, and Barcelona, Spain), we evaluated the type of infections that developed within the first 14 days of starting antibiotic prophylaxis (termed “early” infections), their relative prevalence, microbiology, resistance patterns and predictors. We also evaluated the impact on six-week mortality, re-bleeding within six weeks and failure to achieve hemostasis/re-bleeding within five days. Recognizing that culture-negative infections can be more reliant on clinical variables and therefore have increased potential for misclassification, we provide a sensitivity analysis of the culture-confirmed infections (culture-positive infections) throughout the manuscript.
Patients and methods
Patients
Inclusion criteria consisted of: age ≥ 18 years, cirrhosis and AVH. Patients not given antibiotic prophylaxis at admission for AVH and those with a documented bacterial infection on the day of the bleed were excluded from the study. In order to avoid over-representation of demographic variables, each patient was included only once (the first encountered AVH episode). The choice of antibiotic for the Spanish patients was based on current guidelines.7,15 Those patients with advanced cirrhosis (two clinical signs of decompensation: ascites, jaundice, hepatic encephalopathy or malnutrition) received ceftriaxone, the rest were given norfloxacin. The Canadian cohort consisted of retrospectively collected data. There was no protocol to standardize the choice of antibiotics at either Canadian center. Health Research Ethics Board Approval was obtained at all sites.
Data collection
The data collected from hospitals in the two countries (Canada and Spain) were aggregated. Canadian data were collected from AVH-related admissions at two tertiary care centers in Edmonton, Alberta, between 1996 and 2009 (86% of patients had their bleed after the year 2000). Patients were identified using relevant International Classification of Diseases, Ninth Revision (ICD-9) (456.0, 456.20, 456.8) and ICD-10 (I98.2, I98.20, I85.0, I86.4) codes, the former applied until April 2002 when ICD-10 codes were adopted by our Health Records Department. Spanish data were extracted from a prospectively collected database of patients hospitalized at the liver intensive care unit (ICU) in the Hospital Clinic in Barcelona between 2008 and 2014.
Data collected at the time of AVH included patient demographics, co-existing medical diagnoses, etiology and severity of liver disease (as determined by the Child Pugh (CP) score and model for end-stage liver disease (MELD) score), previous medication (proton pump inhibitor (PPI), non-selective beta-blockers, outpatient antibiotic prophylaxis (OAP)), presence of concurrent hepatocellular carcinoma (HCC) and medication use at admission (including intravenous octreotide and oral or intravenous antibiotics). Data about the initial blood pressure and heart rate (Edmonton series), presence of hypovolemic shock (Barcelona series), laboratory values on the day of the AVH (sodium, creatinine, peripheral white blood cell (WBC) count, hemoglobin, platelet count, international normalized ratio (INR), bilirubin, albumin), and endoscopic therapy provided were also collected.
As most infections develop within the first few days after AVH,5,16–18 following the example of Pauwels et al.,5 infection data were collected up until 14 days post-AVH.5 In the case of culture-positive infections, all microorganisms and their antibiotic-susceptibility patterns were recorded. Data on six-week mortality, successful hemostasis with the original procedure/re-bleeding within five days and re-bleeding up to six weeks were also collected.
Definitions
Cirrhosis was identified by laboratory features of hepatic dysfunction or clinical features of portal hypertension in the presence of compatible radiologic and/or histologic findings.
Bacterial infections were defined using standard guidelines.19 Spontaneous bloodstream infection diagnosis required positive blood cultures without another associated infection or central line source. As per guideline recommendations, common skin contaminants (diptheroids, Bacillus spp, Propionibacterium spp, coagulase-negative Staphylococci, Aerococcus spp, Micrococcus spp) were counted as contaminants unless blood cultures were positive on two separate occasions or if there were clinical signs of infection. Spontaneous bacterial peritonitis/empyema (SBP/SBE) was defined by an ascites/pleural fluid polymorphonuclear cell count ≥250 cells/mm3. Pneumonia was defined as a chest radiograph with at least one of the following (new or progressive infiltrate, consolidation, cavitation) and at least one of (fever >38°, altered mental state, new onset of purulent sputum, new onset of worsening cough or dyspnea, worsening gas exchange). In the Spanish cohort, “tracheobronchitis” was understood as clinical features of respiratory infection, no radiographic infiltrates and positive bronchial aspirate or sputum culture for a respiratory specimen. Urinary tract infection (UTI) was defined by the growth of 106 colony-forming units per liter of a pure urinary tract pathogen in the presence of UTI symptoms, bacteremia or hepatic encephalopathy.19,20
Antibiotic resistance was reported for the two most common regimens used in AVH—second-generation quinolones norfloxacin/ciprofloxacin (FQ) and nonpseudomonal third-generation cephalosporin (Ceph3, primarily ceftriaxone) therapy. Cultured organisms were classified as resistant if the antibiotic class was known to be ineffective/not recommended for therapy, for example, ceftriaxone for Enterococcus or Staphylococcus aureus, or if the organisms were determined to be resistant or have intermediate susceptibility reported on standard microbiologic susceptibility testing. In vitro susceptibilities of quinolones for staphylococcus and streptococcus species defaulted to “resistant” because of pharmacokinetic concerns and/or lack of recommended breakpoints. As per routine practice at each hospital, after receipt of cultures, clinical management included antibiotic adjustments as per usual care.
Statistical analysis
Variables are presented using means and standard deviations or proportions. Comparisons between patients with and without early infections were performed with Chi-square, or t-test for unpaired samples. To assess for predictors of early infection and to assess the relationship between early infections and the outcome six-week mortality, we utilized univariate and multivariate logistic regression, adjusting for clinically and statistically significant variables. Statistical analysis was carried out using SPSS version 17.0 (SPSS Inc, Chicago, IL, USA) and R (htttp://www.r-project.org).
Results
Patient demographics
The combined dataset included 371 patients, 225 from the Canadian sites and 146 from the Spanish site. Data comparing patients from each site subdivided by the presence or absence of bacterial infection within 14 days are presented in Table 1. The comparison between the baseline demographics from each site as a whole is presented in Supplementary Table 1. Patients from Spain were more likely to be on oral PPI therapy, non-selective beta-blockers and long-term OAP prior to admission. Patients from the Canadian sites were sicker, with a mean MELD of 17.0 (standard deviation (SD) 7.2) as compared to 14.8 (SD 5.0) from the Spanish dataset, and a mean serum WBC count of 10.3 (SD 6.4) as compared to 6.9 (SD 3.5). There was no statistically significant difference in the rates of re-bleeding (Canadian site 24%, Spanish site 20.5%) or six-week mortality (Canadian site 17.3%, Spanish site 11%) between centers.
Table 3.
Documented infections in 225 patients in Edmonton, Canada, who received prophylactic antibiotics (30 infections, 21 culture positive).
| Pneumonia (N = 13) |
Urinary tract infection (N = 6) |
SBP (N = 6) |
Spontaneous bacteremia (N = 5) |
|
|---|---|---|---|---|
| Culture-positive infection | N = 7 (54%) | N = 6 (100%) | N = 3 (50%) | N = 5 (100%) |
| Gram-negative organisms | ||||
| Escherichia coli | 1 (0 QR, 0 CR) | 1 (1 QR, 1 CR) | 1 (0 QR, 0 CR) | 1 (0 QR, 0 CR) |
| Pseudomonas aeruginosa | 1 (0 QR, 1 CR) | |||
| Gram-positive organisms | ||||
| Enterococcus spp (all Vancomycin sensitive) | 5 (5 QR, 5 CR) | 1 (1 QR, 1 CR) | 2 (2QR, 2 CR) | |
| Streptococcus pneumoniae | 1 (1 QR, 1 CR) | |||
| Viridans group Streptococcus | 1 (1 QR, 0 CR) | |||
| Methicillin-susceptible Staphylococcus aureus | 4 (4 QR, 0 CR) | |||
| Methicillin-resistant Staphylococcus aureus | 1 (1 QR, 1 CR) | |||
| Mycoplasma pneumoniae | 1 (1 QR, 0 CR) | |||
| Totals | 6 QR (86%) 1CR (14%) | 6 QR (100%) 6 CR (100%) | 2 QR (66%) 2 CR (66%) | 3 QR (60%) 3 CR (60%) |
SBP: spontaneous bacterial peritonitis; CR: third-generation cephalosporin resistant; QR: quinolone resistant.
Table 4.
Documented infections in 146 patients in Barcelona, Spain, who received prophylactic antibiotics (21 infections, 17 culture positive).
| Bacterial classification | Pneumonia (N = 7) | Tracheobronchitis (N = 7) | UTI (N = 3) | Spontaneous bacteremia or line infection (N = 4) |
|---|---|---|---|---|
| Culture-positive infection |
N = 3 (43%) |
N = 7 (100%) |
N = 3 (100%) |
N = 4 (100%) |
| Gram-negative organisms | ||||
| ESBL Escherichia coli | 1 (0 QR, 1 CR)a | |||
| Pseudomonas aeruginosa | 2 (1 QR, 2 CR) | 4 (0 QR, 4 CR) | 1 (0 QR, 1 CR)b | |
| Citrobacter spp | 2 (0 QR, 2 CR) | |||
| Gram-positive organisms | ||||
| Staphylococcus ludunensis | 1 (1 QR, 0 CR) | |||
| Enterococcus (all Vancomycin sensitive) | 2 (2 QR, 2 CR) | 1 (1 QR, 1 CR) | ||
| Staphylococcus hominis | 1 (1 QR, 1 CR) | |||
| Viridans group Streptococcus | 1 (0 QR, 0 CR) | |||
| Streptococcus agalactiae | 1 (0 QR, 0 CR) | |||
| Totals | 1 QR (33%) 2 CR (66%) | 0 QR (0%) 7 CR (100%) | 2 QR (66%) 2 CR (66%) | 3 QR (75%) 3 CR (75%) |
ESBL: extended-spectrum beta-lactamase; UTI: urinary tract infection; CR: third-generation cephalosporin resistant; QR: quinolone resistant.
Mixed Gram-negative and Gram-positive infection with Methicillin-susceptible Staphylococcus aureus.
Two organisms, Pseudomonas and Stenotrophomonas maltophilia.
Table 1.
Comparison of patients with and without early bacterial infection.
| CANADIAN DATASET (n = 225) |
SPANISH DATASET (n = 146) |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Bacterial infection (n = 30) % (n) or mean ± SD | No bacterial infection (n = 195) % (n) or mean ± SD | p value | Bacterial infection (n = 21) % (n) or mean ± SD | No bacterial infection (n = 125) % (n) or mean ± SD | p value | |
| Demographics and pre-AVH meds and vitals | |||||||
| Male gender | 66.7% (20) | 73.8% (144) | 0.51 | 71.4% (15) | 76.8% (96) | 0.59 | |
| Age (years) | 54.2 ± 14.5 | 54.8 ± 11.4 | 0.80 | 59.8 ± 11.4 | 58.1 ± 13.3 | 0.59 | |
| On oral PPI therapy | 23.3% (7) | 32.8% (64) | 0.40 | 42.9% (9) | 48.8% (61) | 0.65 | |
| On non-selective beta blockers | 3.3% (1) | 11.3% (22) | 0.33 | 47.6% (10) | 59.7% (74) | 0.34 | |
| On outpatient antibiotic prophylaxis (all FQ) | 20% (6) | 3.1% (6) | 0.002 | 28.6% (6) | 16% (20) | 0.21 | |
| Systolic BP (mm Hg) | 113.8 ± 20.2 | 114.2 ± 24.0 | 0.94 | – | – | – | |
| Heart rate | 98.9 ± 17.1 | 96.6 ± 18.5 | 0.53 | – | – | – | |
| Shock | – | – | – | 57% (12) | 32% (40) | 0.046 | |
| Liver disease etiology and severity | |||||||
| Alcohol etiology | 36.7% (11) | 46.2% (90) | 0.43 | 33.3% (7) | 37.6% (47) | 0.81 | |
| HCC | 6.9% (2) | 6.7% (13) | 1.0 | 19% (4) | 12.8%(16) | 0.49 | |
| Ascites on day of bleed | 93.3% (28) | 68.7% (134) | 0.004 | 76.2% (16) | 24% (30) | 0.001 | |
| MELD | 20.3 ± 7.9 | 16.5 ± 6.9 | 0.006 | 16.4 ± 6.3 | 14.6 ± 4.7 | 0.12 | |
| CP score | 10.8 ± 2.2 | 9.2 ± 2.1 | 0.001 | 8.1 ± 2.0 | 7.6 ± 1.6 | 0.19 | |
| Labs | |||||||
| Sodium (mmol/l) | 135.6 ± 8.4 | 136.8 ± 5.6 | 0.31 | 137.7 ± 6.3 | 137.0 ± 5.3 | 0.59 | |
| Peripheral blood leukocytes ( × 109 cells/l) | 13.1 ± 8.1 | 9.9 ± 6.0 | 0.01 | 7.9 ± 3.6 | 6.8 ± 3.5 | 0.16 | |
| INR | 1.7 ± 0.5 | 1.6 ± 0.5 | 0.06 | 1.8 ± 0.7 | 1.5 ± 0.4 | 0.02 | |
| Hemoglobin (mmol/l) | 91.0 ± 24.9 | 87.3 ± 22.0 | 0.40 | 89.6 ± 21.6 | 92.1 ± 24.4 | 0.66 | |
| Creatinine (µmol/l) | 124.1 ± 73.2 | 102.1 ± 68.2 | 0.11 | 89.1 ± 32.7 | 102.3 ± 67.8 | 0.38 | |
| Bilirubin (µmol/l) | 149.2 ± 226.3 | 72.9 ± 98.7 | 0.002 | 31.2 ± 20.8 | 35.8 ± 48.3 | 0.67 | |
| Albumin (g/l) | 25.3 ± 7.4 | 26.6 ± 7.5 | 0.37 | 28.0 ± 5.8 | 28.8 ± 5.1 | 0.50 | |
| AVH treatment and outcomes | |||||||
| Failure to control bleeding or re-bleeding within five days | 16.7% (5) | 16.9% (33) | 1.0 | 28.6% (6) | 11.2% (14) | 0.04 | |
| Re-bleeding within six weeks | 33.3% (10) | 22.6% (44) | 0.25 | 38.1% (8) | 17.6% (22) | 0.04 | |
| Six-week mortality | 36.7% (11) | 14.4% (28) | 0.007 | 28.6% (6) | 8% (10) | 0.013 | |
PPI: proton pump inhibitor; FQ: fluoroquinolone; BP: blood pressure; HCC: hepatocellular carcinoma; MELD: model for end-stage liver disease; CP: Child Pugh score; AVH: acute variceal hemorrhage. Bold indicates p values statistically significant with p < 0.05.
Antibiotic prophylaxis data and the prevalence and types of “break-through” bacterial infections
There was an almost even split between the number of patients who received FQ (52%) and Ceph3 (primarily ceftriaxone) antibiotics (45%) on presentation with AVH, similar between the two cohorts (Table 2). Only 3% of the cohort received other broad-spectrum antibiotics.
Table 2.
Antibiotic prophylaxis and prevalence and types of early bacterial infections.
| Variable | Edmonton cohort (n = 225) % (n) or mean ± SD | Barcelona cohort (n = 146) % (n) or mean ± SD | Total group (n = 371) % (n) or mean ± SD |
|---|---|---|---|
| Prophylactic antibiotic therapy given at AVH presentation | |||
| -Second-generation quinolone | 50% (112) | 54% (79) | 52% (191) |
| -Third-generation cephalosporin | 45% (101) | 45% (66) | 45% (167) |
| -Other broad-spectrum antibiotic | 5% (12) | 1% (1) | 3% (13) |
| Prevalence of early bacterial infection | |||
| Bacterial infection | 13% (30/225) | 14% (21/146) | 14% (51/371) |
| Culture positive | 70% (21/30) | 80% (17/21) | 74.5% (38/51) |
| Gram-positive | 76% (16/21) | 47% (8/17) | 63% (24/38) |
| Mean timing of infection (days) | 6 ± 4.7 | 6 ± 3.8 | 6 ± 4.3 |
| Type of early bacterial infection | |||
| Respiratory infection | 43% (13/30) | 66% (14/21) | 53% (27/51) |
| -Pneumonia | 43% (13/30) | 33% (7/21) | 39% (20/51) |
| -Tracheobronchitis | – | 33% (7/21) | 14% (7/51) |
| Urinary tract infection | 20% (6/30) | 14% (3/21) | 17% (9/51) |
| Spontaneous bacteremia | 17% (5/30) | 14% (3/21) | 16% (8/51) |
| SBP | 20% (6/30) | 0% | 12% (6/51) |
| Line infection | 0% | 5% (1/21) | 2% (1/51) |
AVH: acute variceal hemorrhage; SBP: spontaneous bacterial peritonitis.
The prevalence of early bacterial infections was also similar in each cohort, overall occurring in 14% of all patients, with 75% of infections being culture positive and occurring at a mean of six days (SD 4.3) from presentation with AVH. Seventy percent of all infections occurred within the first seven days after AVH. Seventy-eight percent of all early bacterial infections and 77% of culture-positive infections were diagnosed after 48 hours of hospital, in keeping with the definition of a nosocomial infection.14,21 These rates were the same across both cohorts.
Respiratory infections predominated the series, accounting for 53% of the 51 infections seen in the combined cohort. This was followed by UTIs in 17%, spontaneous bacteremia in 16%, SBP in 12% and a single central line infection. There was no consistent pattern with regards to the timing of infection by type. Respiratory infections occurred at a mean of 5.9 days (SD 4.5), UTIs at a mean of seven days (SD 4), spontaneous bacteremia at a mean of 6.7 days (SD 4.3) and SBP at a mean of 3.5 days (SD 0.7). In a sensitivity analysis considering only the 38 culture-positive infections, respiratory infections were still the most common, occurring in 45% of patients, followed by UTIs in 24%, spontaneous bacteremia in 21%, SBP in 8% and a single line infection.
Microbiology and antibiotic susceptibility data for culture-positive infections
Information regarding antibiotic susceptibility is presented separately for each cohort.
i. Canadian data (Table 3)
Of the 21 culture-positive infections, 76% (16/21) were gram-positive organisms (most commonly Enterococcus).
FQ resistance
Of the culture-positive infections, 81% (17/21) grew organisms that were classed as FQ resistant, which were mainly gram-positive isolates. Five of these patients had been given FQ for AVH antibiotic prophylaxis, 10 patients a Ceph3 and two patients an alternate broad-spectrum antibiotic. Twenty-nine percent (6/21) of the patients with culture-positive infection had been on OAP with an FQ antibiotic.
Ceph3 resistance
Of the culture-positive infections, 57% (12/21) grew organisms that were resistant to empiric Ceph3 therapy. Five of these patients had been given FQ for AVH antibiotic prophylaxis and seven a Ceph3 antibiotic.
ii. Spanish data (Table 4)
In contrast to the predominantly gram-positive infections seen in the Canadian data, of the 17 culture-positive infections, 59% involved gram-negative organisms, 53% (9/17) were gram-negative, 6% (1/17) mixed gram-positive and gram-negative and 41% (7/17) gram-positive, with a preponderance of more resistant species (extended-spectrum beta-lactamase (ESBL) E. coli, Pseudomonas spp).
FQ resistance
Thirty-five percent (6/17) of the culture-positive infections grew organisms that were resistant to empiric FQ therapy. Fifty percent of these patients had been given FQ for AVH antibiotic prophylaxis and 50% a Ceph3. Twenty-four percent (4/17) of patients with culture-positive infections had been on OAP with an FQ antibiotic.
Ceph3 resistance
Eighty-two percent (14/17) of the culture-positive infections grew organisms that were resistant to empiric Ceph3 therapy. Fifty percent of these patients had been given FQ for AVH antibiotic prophylaxis and 50% a Ceph3 antibiotic.
AVH prophylaxis used and documented resistance to that agent
In Supplementary Table 2 we present the type and microbiology of infections divided by the type of antibiotic prophylaxis (Ceph3 versus FQ). Notably, because of indication bias, the ability to make direct comparisons between the antibiotics is limited by the differences in the groups—for example, the patients receiving Ceph3 antibiotics were sicker, with MELD 18 versus 15, p = 0.001. Of the 13 culture-positive infections diagnosed in patients on FQ, 54% were resistant to this antibiotic family. Sixty percent of the 25 culture-positive infections occurring in patients on Ceph3 were resistant to beta-lactams including cephalosporins.
The efficacy of FQ was relatively low in patients receiving OAP. Twenty-four percent (5/21) of these patients developed early infections, a finding that supports the current Baveno VI consensus guideline recommendation that patients on long-term OAP with FQ should receive non-FQ therapy when admitted with AVH.
Predictors of early bacterial infections—combined dataset of 371 patients
Using the combined dataset of 371 patients, on multivariate logistic regression analysis, the use of outpatient antibiotic prophylaxis (odds ratio (OR) 5.4 (95% confidence interval (CI): 1.4 to 21.3), p = 0.02)) and intubation at the time of the bleed (OR 2.6 (1.05 to 6.5), p = 0.04 were independent predictors of break-through bacterial infection. The presence of ascites (OR 4.3 (0.96 to 19.3), p = 0.06)) trended to significance (Table 5). On sensitivity analysis considering only the 38 culture-positive infections, OAP remained predictive of infection with a culture-positive infection (p = 0.02) with intubation trending to significance (p = 0.07).
Table 5.
Predictors of early bacterial infection in the combined dataset of 371 patients who received AVH antibiotic prophylaxis.
| Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| Characteristic | OR (95% CI) | p value | OR (95% CI) | p value |
| Male gender | 0.73 (0.38 to 1.39) | 0.34 | ||
| Age (years) | 1.0 (0.98 to 1.03) | 0.83 | ||
| Intubated for bleed | 2.4 (1.3 to 4.4) | 0.005 | 2.6 (1.1 to 6.5) | 0.04 |
| On outpatient antibiotic prophylaxis (FQ n = 36), (septra n = 2) | 3.5 (1.6 to 7.4) | 0.001 | 5.4 (1.4 to 21.3) | 0.02 |
| Oral PPI therapy | 0.71 (0.38 to 1.34) | 0.30 | ||
| Beta-blocker therapy | 0.64 (0.31 to 1.3) | 0.22 | ||
| Ascites on day of bleed | 6.0 (2.6 to 13.7) | 0.001 | 4.3 (0.96 to 19.3) | 0.06 |
| CP score | 1.27 (1.10 to 1.45) | 0.001 | ||
| MELD | 1.06 (1.02 to 1.11) | 0.003 | 1.01 (0.95 to 1.07) | 0.81 |
| Sodium (mmol/l) | 0.99 (0.94 to 1.04) | 0.64 | ||
| Peripheral blood leukocytes (×109 cells/l) | 1.06 (1.02 to 1.11) | 0.009 | 1.04 (0.97 to 1.10) | 0.28 |
| Albumin (g/l) | 0.98 (0.93 to 1.02) | 0.29 | ||
AVH: acute variceal hemorrhage; FQ: fluoroquinolone; PPI: proton pump inhibitor; CP: Child Pugh score; MELD: model for end-stage liver disease; OR: odds ratio; CI: confidence interval. Bold indicates p values statistically significant with p < 0.05.
Predictors of six-week mortality—combined dataset of 371 patients
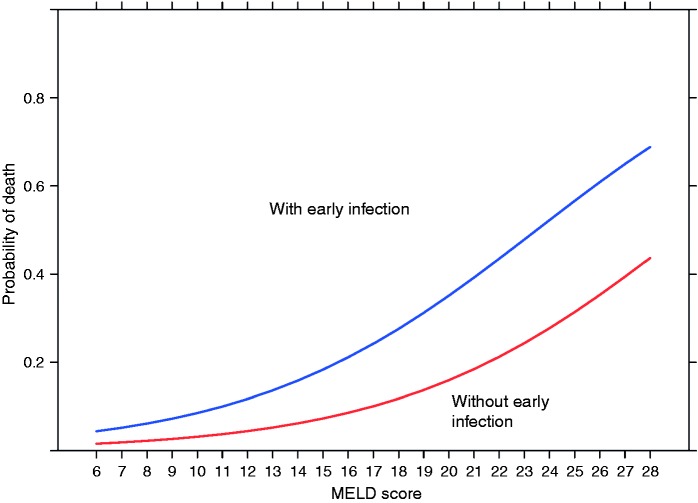
Using the combined dataset of 371 patients, on multivariate analysis, early bacterial infection (OR 2.6 (95% CI: 1.2 to 5.7)) and the MELD score (OR 1.18 (95% CI: 1.12 to 1.25)) were the two independent predictors of six-week mortality (Table 6 and Figure 1). On sensitivity analysis considering only the 38 culture-positive infections, these predictors of six-week mortality remained robust: early culture-positive bacterial infection (OR 2.7 (95% CI: 1.1 to 6.5) and the MELD score (OR 1.2 (95% CI: 1.2 to 1.3)).
Table 6.
Predictors of six-week mortality in the combined dataset of 371 patients who received antibiotic prophylaxis.
| Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| Characteristic | OR (95% CI) | p value | OR (95% CI) | p value |
| Male gender | 0.83 (0.44 to 1.56) | 0.56 | ||
| Age (years) | 0.99 (0.97 to 1.02) | 0.51 | ||
| Early bacterial infection | 3.7 (1.9 to 7.3) | 0.001 | 2.5 (1.1 to 5.6) | 0.03 |
| Ascites on day of bleed | 3.3 (1.7 to 6.5) | 0.001 | 1.5 (0.7 to 3.4) | 0.27 |
| CP score | 1.6 (1.4 to 1.9) | 0.001 | ||
| MELD | 1.20 (1.14 to 1.26) | 0.001 | 1.18 (1.12 to 1.24) | 0.001 |
| Sodium (mmol/l) | 0.99 (0.95 to 1.05) | 0.98 | ||
| Peripheral blood leukocytes (×109 cells/l) | 1.07 (1.03 to 1.12) | 0.002 | 1.01 (0.96 to 1.06) | 0.80 |
| Albumin (g/l) | 0.96 (0.92 to 1.004) | 0.07 | ||
| On outpatient antibiotic prophylaxis | 2.29 (1.04 to 5.02) | 0.04 | 1.3 (0.5 to 3.3) | 0.63 |
CP score: Child Pugh score; MELD: model for end-stage liver disease; OR: odds ratio; CI: confidence interval. Bold indicates p values statistically significant with p < 0.05.
Predictors of re-bleeding within six weeks and the combined endpoint of hemostasis/re-bleeding within five days—combined dataset of 371 patients
On multivariate analysis, after introduction of clinically significant and statistically significant variables, the MELD score (OR 1.1 (95% CI: 1.02 to 1.11)) was the only independent predictor of re-bleeding, with bacterial infection within 14 days trending to significance (OR 1.8 (95% CI: 0.9 to 3.5), p = 0.1)). Ascites and the peripheral WBC did not retain significance in a multivariate model (data not shown). Similarly, for the combined endpoint of hemostasis/re-bleeding within five days, liver dysfunction as measured by MELD or CP were the only independent predictors of re-bleeding (data not shown).
Discussion
This multicenter study of 371 patients evaluates the prevalence, predictors and significance of bacterial infections occurring within 14 days of AVH despite antibiotic prophylaxis. The main findings are three-fold. First, 14% of patients develop infection within 14 days despite antibiotic prophylaxis, with respiratory infections accounting for more than 50% of infections, and with a high proportion of culture-positive infections due to organisms resistant to the recommended FQ and Ceph3 antibiotics. Secondly, intubation and outpatient antibiotic prophylaxis are important risk factors for early infections with the presence of ascites trending to significance. And finally, in addition to the MELD score, early infections contribute independently to six-week mortality despite usual hospital management.
Figure 1.
Early bacterial infection is independent of the model for end-stage liver disease (MELD) score in predicting mortality in cirrhotic patients presenting with acute variceal hemorrhage.
At 14%, the prevalence of early infections identified in our cohorts is in keeping with data from existing meta-analyses3,13 and supports the overall efficacy of prophylactic antibiotic therapy. Respiratory infections were the most common early infection identified (diagnosed at a mean of six days post-AVH), accounting for more than half of all infections and 77% (10/13) of culture-negative infections. Pathophysiologically, occult or overt aspiration can occur with hematemesis and resuscitation and can be associated both with pneumonitis and pneumonia. As pneumonia seems to be more common when prophylactic endotracheal intubation is carried out22 avoiding unnecessary intubation may offer an opportunity to reduce risk. Even in the current series, intubation was associated with early infections. Moreover, care bundles for the prevention and control of secondary infections (ventilator-associated pneumonia, catheter-related bacteremia and UTIs) are recommended in the routine management of AVH23 and may help to reduce the rate of respiratory as well as other infections even further.
The OR of 5.4 for early infection in patients on OAP (95% of which was oral FQ) is a concerning statistic and a reminder of the dangers of antibiotic use.24 As per current guidelines, OAP is recommended for patients with low protein ascites and liver/renal dysfunction (primary prophylaxis) against SBP as well as for patients who have already had SBP (secondary prophylaxis).25
The use of OAP and intubation were the two risk factors that emerged as significant predictors of early bacterial infections with the presence of ascites trending to significance. More vigilance and closer follow-up may be required in patients with these risks. The use of OAP (almost universally FQ antibiotics in our series) has been associated with a shift toward gram-positive infections and resistant gram-negative infections because of a shift in endogenous flora. Consistent with our findings and as per current Baveno VI consensus guidelines, patients on OAP with FQ should be given a non-FQ antibiotic when they present with AVH.26 The overall rate of overall FQ non-susceptibility (81% in Edmonton, 35% in Barcelona) also supports the change in the Baveno recommendations away from using FQ as a first-line antibiotic in all patients.24,26 The FQ resistance seen in the current series was driven mainly by the preponderance of gram-positive isolates, with only one gram-negative early infection isolate found to be FQ resistant in each cohort.
Importantly, the differences in the microbiological data between the two cohorts demonstrate that the spectrum of pathogens is different from country to country (and potentially different between hospitals and regions) and therefore strategies such as adding gram-positive coverage (for example with Vancomycin) to high-risk patients on OAP upon admission may not be effective in all jurisdictions. This underscores the need to assess local data to guide clinical decision support and treatment guidelines. There is a notably higher proportion of gram-negative infections in the Spanish cohort, in spite of greater use of gram-negative active OAP, suggesting that epidemiologic factors beyond patient-specific antimicrobial use play a significant role in local bacterial etiologies. Although we were not able to account for all differences between the cohorts, statistically significant differences were seen in certain factors including their past history of variceal bleeding, hepatic encephalopathy and the number who had transjugular intrahepatic portosystemic shunt (TIPS) performed for bleeding (Supplementary Table 1). This may have contributed to the overall differences and the microbiology-related differences seen between groups. As we did not have the information available to us regarding statin use and stage of hepatocellular carcinoma, we were unable to analyze the contribution of these variables to our outcomes.
Conclusions
In conclusion therefore, these data portray a subset of patients at higher risk of early infections despite AVH antibiotic prophylaxis. Those with specific risks (intubation, previous OAP, ascites) should be followed closely for clinical deterioration, with prompt septic workup and expansion of therapy for gram-positive and resistant gram-negative isolates if there is any clinical decline. Notably, early infection significantly affects mortality risk even when correction for liver disease severity is performed. As hemorrhage control outcomes have improved, this makes early infection prophylaxis and management an important area of potential optimization in the care of these complex patients. Consistent with recent Baveno guidelines, antibiotic prophylaxis should be adapted to local sensitivities. Given the high rates of FQ resistance, these agents should be used with caution as first-line antibiotics. A prospective multicenter study with standardized data collection will help to inform potentially needed changes to antibiotic regimens in this area.
Supplementary Material
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis 2008; 28: 26–42. [DOI] [PubMed] [Google Scholar]
- 2.Jalan R, Fernández J, Wiest R, et al. Bacterial infections in cirrhosis. A position statement based on the EASL Special Conference 2013. J Hepatol 2014; 60: 1310–1324. [DOI] [PubMed] [Google Scholar]
- 3.Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila FI, et al. Antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding. Cochrane Database Syst Rev 2010, pp. CD002907–CD002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernández J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol 2012; 56(Suppl 1): S1–S12. [DOI] [PubMed] [Google Scholar]
- 5.Pauwels A, Mostefa-Kara N, Debenes B, et al. Systemic antibiotic prophylaxis after gastrointestinal hemorrhage in cirrhotic patients with a high risk of infection. Hepatology 1996; 24: 802–806. [DOI] [PubMed] [Google Scholar]
- 6.Bernard B, Grange JD, Khac EN, et al. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: A meta-analysis. Hepatology 1999; 29: 1655–1661. [DOI] [PubMed] [Google Scholar]
- 7.Fernández J, Ruiz del Arbol L, Gómez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology 2006; 131: 1049–1056. [DOI] [PubMed] [Google Scholar]
- 8.Goulis J, Armonis A, Patch D, et al. Bacterial infection is independently associated with failure to control bleeding in cirrhotic patients with gastrointestinal hemorrhage. Hepatology 1998; 27: 1207–1212. [DOI] [PubMed] [Google Scholar]
- 9.Hou MC, Lin HC, Liu TT, et al. Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: A randomized trial. Hepatology 2004; 39: 746–753. [DOI] [PubMed] [Google Scholar]
- 10.de Franchis R. Revising consensus in portal hypertension: Report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010; 53: 762–768. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 2010; 362: 823–832. [DOI] [PubMed] [Google Scholar]
- 12.Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila F, et al. Meta-analysis: Antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding—an updated Cochrane review. Aliment Pharmacol Ther 2011; 34: 509–518. [DOI] [PubMed] [Google Scholar]
- 13.Soares-Weiser K, Brezis M, Leibovici L. Antibiotics for spontaneous bacterial peritonitis in cirrhotics. Cochrane Database Syst Rev 2001, pp. CD002232–CD002232. [DOI] [PubMed] [Google Scholar]
- 14.Fernández J, Acevedo J, Castro M, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: A prospective study. Hepatology 2012; 55: 1551–1561. [DOI] [PubMed] [Google Scholar]
- 15.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 2010; 53: 397–417. [DOI] [PubMed] [Google Scholar]
- 16.Soriano G, Guarner C, Tomas A, et al. Norfloxacin prevents bacterial infection in cirrhotics with gastrointestinal hemorrhage. Gastroenterology 1992; 103: 1267–1272. [DOI] [PubMed] [Google Scholar]
- 17.Blaise M, Pateron D, Trinchet JC, et al. Systemic antibiotic therapy prevents bacterial infection in cirrhotic patients with gastrointestinal hemorrhage. Hepatology 1994; 20: 34–38. [DOI] [PubMed] [Google Scholar]
- 18.Lin YT, Lo GH, Lai KH, et al. Prophylactic antibiotics in cirrhotics with upper gastrointestinal hemorrhage: A prospective, controlled trial. Zhonghua Yi Xue Za Zhi (Taipei) 2002; 65: 365–371. [PubMed] [Google Scholar]
- 19.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36: 309–332. [DOI] [PubMed] [Google Scholar]
- 20.Tandon P, Delisle A, Topal JE, et al. High prevalence of antibiotic-resistant bacterial infections among patients with cirrhosis at a US liver center. Clin Gastroenterol Hepatol 2012; 10: 1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaulk J, Carbonneau M, Qamar H, et al. Third-generation cephalosporin-resistant spontaneous bacterial peritonitis: A single-centre experience and summary of existing studies. Can J Gastroenterol Hepatol 2014; 28: 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almashhrawi AA, Rahman R, Jersak ST, et al. Prophylactic tracheal intubation for upper GI bleeding: A meta-analysis. World J Metaanal 2015; 3: 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gines P, Fernández J, Durand F, et al. Management of critically-ill cirrhotic patients. J Hepatol 2012; 56(Suppl 1): S13–S24. [DOI] [PubMed] [Google Scholar]
- 24.Fernández J, Tandon P, Mensa J, et al. Antibiotic prophylaxis in cirrhosis: Good and bad. Hepatology 2016; 63: 2019–2031. [DOI] [PubMed] [Google Scholar]
- 25.Runyon BA. Management of adult patients with ascites due to cirrhosis: An update. Hepatology 2009; 49: 2087–2107. [DOI] [PubMed] [Google Scholar]
- 26.de Franchis R, Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63: 743–752. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.