Abstract
Study Design:
Longitudinal prospective study.
Objectives:
Whether 1-year HAL-BWSTT of chronic spinal cord injured patients can improve independent ambulated mobility further as a function of training frequency, after an initial 3-month training period.
Methods:
Eight patients with chronic SCI were enrolled. They initially received full standard physical therapy and neurorehabilitation in the acute/subacute posttrauma phase. During this trial, all patients first underwent a daily (5 per week) HAL-BWSTT for 12 weeks. Subsequently, these patients performed a 40-week HAL-BWSTT with a training session frequency of either 1 or 3 to 5 sessions per week. The patients’ functional status including HAL-associated treadmill-walking time, -distance, and -speed with additional analysis of gait pattern, and their independent (without wearing the robot suit) functional mobility improvements, were assessed using the 10-Meter-Walk Test (10MWT), Timed-Up-and-Go Test (TUG) and 6-Minute-Walk Test (6MinWT) on admission, at 6 weeks, 12 weeks, and 1 year after enrollment. The data were analyzed separately for the 2 training frequency subgroups after the initial 12-week training period, which was identical in both groups.
Results:
During the 1-year follow-up, HAL-associated walking parameters and independent functional improvements were maintained in all the patients. This result held irrespective of the training frequency.
Conclusions:
Long-term 1-year maintenance of HAL-associated treadmill walking parameters and of improved independent walking abilities after initial 12 weeks of daily HAL-BWSTT is possible and depends mainly on the patients’ ambulatory status accomplished after initial training period. Subsequent regular weekly training, but not higher frequency training, seems to be sufficient to preserve the improvements accomplished.
Keywords: spinal cord injury, neurorehabilitation, hybrid assistive limb, exoskeleton, follow up
Introduction
Spinal cord injury (SCI), with an incidence of approximately 2500 patients each year in Germany1 and 10 to 83 patients per million people worldwide,2 represents a devastating and often disabling condition for the affected individuals. It most often leads to permanent physical and functional impairments, and a higher incidence has been reported among the young and male population.3,4 Permanent impairment depends on the level of the neural lesion as well as its completeness. Patients with initial incomplete paraplegia regain ambulatory functions in approximately 75% of cases,5 and incomplete para-/tetraplegic patients regain employment in only 21% to 67%, though this differs markedly among countries.6 Nevertheless, neurological recovery is maximal within the initial 6 to 9 months and is considered to be definitive after the first year posttrauma.7 The mode, intensity, and frequency of rehabilitation seem to be important in that regard.8
Neurorehabilitative approaches are in general multifactorial and aim toward reestablishing lost or impaired sensorimotor functions and the acquisition of disease-related tasks (eg, wheelchair mobility, transfers, catheterization). However, loss of strength and coordination substantially limits the patients’ capacities for overground ambulation training,9 so they often become dependent on a wheelchair and can even be bedridden.10 This again emphasizes the importance of rehabilitation for restoring walking abilities and improving quality of life11,12 in those affected.
Standardized rehabilitation protocols have been established in recent decades. Although there is still no broad agreement about the most effective approach, locomotion training under body weight support has proven beneficial.13 It has led to greater patient mobility in numerous studies and has therefore become an established part of the treatment of acute and chronic SCI.13–15 Body weight–supported treadmill training (BWSTT) has evolved during recent years and lately been revolutionized by the introduction of driven gait orthosis (DGO). DGOs enable the patients’ legs to be trained with different degrees of paresis and spasticity and physiological gait patterns can be achieved.16 Its advantages over training with manually assisted stepping movements are a reduced workload for the therapist, longer lasting sessions and therefore more effective training,17 as well as reproducible gait patterns.18
More recently, mobile exoskeletons have been introduced into the rehabilitation of spinal cord–injured patients.19–24 Unlike other exoskeletons using autonomously generated predefined motion patterns, the Hybrid Assistive Limb robot suit (HAL, Cyberdyne Inc, Tsukuba, Japan), proposed by Yoshiyuki Sankai, a professor at Tsukuba University, is a wearable neurologically controlled robot suit, which provides gait support interactively according to the wearer’s voluntary drive.25 The system allows a direct human-machine interconnection established through electromyography electrodes on the surface of the wearer’s skin at hip and knee extensor and flexor muscle regions. This allows a voluntary machine-supported joint range of motion to be achieved in SCI patients by using voluntary, even nonfunctional, minimal bioelectric signals, recorded and amplified from the hip and knee flexors and extensors.
Since February 2013, when the HAL suit received a global safety certificate followed by an EC certificate in August 2013, the device is increasingly available throughout Europe. US Food and Drug Approval approval is currently still pending and the suit is currently available for institutional rental, in Japan only.
Several studies have been conducted to investigate the feasibility of using the neurologically controlled HAL Robot Suit for rehabilitating the ambulatory-impaired population, mainly in acute and chronic phase stroke patients, and to establish its short-term effects.26–30 In acute and chronic SCI patients, the HAL Robot Suit seems to be an effective training tool that results in improved independent (without wearing the robot suit) overground walking abilities and in beneficial effects on ambulatory mobility after a 12-week (60 sessions) training period.19,21,24 However, no long-term results of variable-frequency HAL-BWSTT training after the initial 12-week training period are currently available.
Therefore, the objective of the present study was to determine whether 1-year long-term HAL-BWSTT is capable of inducing further improvements in independent (without wearing the robot suit) ambulated mobility as a function of training frequency in chronic SCI patients, who have already completed 12 weeks of daily (60 sessions) HAL-BWSTT. The hypothesis was the following: ongoing HAL-locomotion training preserves the independent functional and ambulatory improvements achieved during the first 12-week training period.
Methods
Objective
To determine whether long-term 1-year BWSTT with the HAL Robot Suit exoskeleton is capable of inducing further improvements of independent (without wearing the robot suit) ambulated mobility as a function of training frequency in chronic SCI patients, who have initially completed 12 weeks of daily (60 sessions) HAL-locomotion training.
Patients
Eight patients (2 females) with traumatic chronic SCI were recruited for this longitudinal pilot study. The mean ± standard deviation (SD) age at the time of enrolment was 48 ± 9.4 years. All the patients had acquired SCI more than 1 year prior to enrolment in the trial, with a time period since injury of 1 to 19 years (mean 97.2 ± 88.4 months) (see Table 1). They had all received standardized SCI physical therapy and neurorehabilitation posttrauma. Inclusion criteria were traumatic SCI with chronic incomplete paraplegia (American Spinal Injury Association [ASIA] B/C/D) or complete paraplegia (ASIA A) after lesions of the conus medullaris/cauda equine with zones of partial preservation (ZPP). Irrespective of ASIA classification, the enrolled patients were required to present some motor functions (≥1 according to Janda)31 of hip and knee extensor and flexor muscle groups in order to trigger and control the HAL exoskeleton. The following exclusion criteria were defined: nontraumatic SCI, pressure sores, severe limitation of range of motion for hip and knee joints (eg, contracture, spasticity/≥4 Ashworth), severe cognitive impairment, body weight >100 kg, nonconsolidated fractures, and mild or severe heart insufficiency. Two patients had suffered from incomplete thoracic SCI (ASIA C/D) for 3 and 13 years, respectively, posttrauma. Two suffered from incomplete lumbar SCI (ASIA B/C) for 12 and 13 months, respectively, and 4 had complete SCI with ZPP in L3-S1 after lesions of the conus medullaris. The motor function and injury classification according to the ASIA were judged by an independent physician and physiotherapist when the patients were enrolled in the study and then at 6 weeks, 3 months, and 12 months after admission and during the training.
Table 1.
Subject Demographics and Clinical Characteristics.
| Case | Sex | Age | Years Since Injury | Etiology | Level | ASIA/ZPP |
|---|---|---|---|---|---|---|
| 1 | Male | 40 | 13 | # T 7/8 | T 8 | C |
| 2 | Male | 63 | 1 | # T 12 | L 1 | B/L3 |
| 3 | Male | 36 | 1.16 | # T 11/12 | T 12 | A/L3 |
| 4 | Female | 55 | 1.08 | # L 1 | L 1 | C |
| 5 | Male | 42 | 16 | # L 1 | L 1 | A/L3 |
| 6 | Male | 52 | 10 | # L 3 | L 2 | A/L3 |
| 7 | Female | 40 | 19 | # L 1 | T 11 | A/S1 |
| 8 | Male | 53 | 3 | # T 12 | T 12 | D |
Abbreviations: ASIA, American Spinal Injury Association; ZPP, zones of partial preservation; #, fracture; T, thoracic; L, lumbar; S, sacral.
The patients were not grouped according to their neurological status (eg, ASIA A/B/C/D), as this pilot study was not conducted to distinguish interindividual correlations, but rather to determine the pre-/postfunctional outcome and HAL-training applicability on a chronic SCI population.
All patients provided written informed consent prior to study admission. The trial was approved by the Ethical Board Committee of Bergmannsheil Hospital and the Ruhr-University Bochum and followed the Declaration of Helsinki strictly.
The Exoskeleton
The neurologically controlled HAL Robot Suit (Cyberdyne, Inc) is an exoskeleton with a patient size-adjustable frame and robotic actuators that attach to the patient’s lower limbs (Figure 1). The joint movements are supported by electric motors. The exoskeleton percutaneously detects minimal bioelectric signals initiated by the patient’s voluntary muscle activities (hip and knee flexors and extensors) via electromyography (EMG) electrodes and/or the floor reaction force signals caused by patient’s intended weight shifts. Through a cable connection between the exoskeleton and the patient, this system allows a voluntary robotic-supported range of motion (cybernic voluntary control mode) to occur at each motor and joint separately. Also, an individually passive, nonvoluntary range of motion (cybernic autonomous control mode) is possible if one specific muscle group signal is insufficient. The electric motor support at the hip and knee joints is gradually adjustable according to the patient’s own remnant flexor and extensor muscle function. This leads to independent individual bilateral hip and knee joint motion support synchronous with the patients’ voluntary drive, so it enables individualized and adjustable muscle group locomotion training to be established for bilateral hip and knee flexors and extensors.
Figure 1.
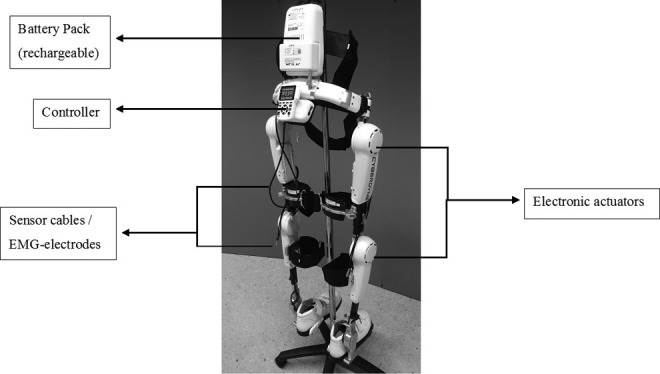
Hybrid assistive limb robot suit (Cyberdyne Inc, Japan). HAL Exoskeleton main components.
Treadmill
The treadmill system (Woodway Inc, Waukesha, WI) used in this trial features an adjustable treadmill speed of up to approximately 4.5 km/h as well as uphill and downhill adjustments of the base. A body weight support system with a patient harness was connected to a rail on the ceiling above the treadmill (see Figure 2). Depending on the patients’ ability and performance, the treadmill velocity was set individually between comfortable and maximum speed. The body weight support was approximately 50% of body weight at the beginning of the training and was reduced during the course of the intervention as far as could be tolerated without substantial knee buckling or toe dragging.
Figure 2.

Body weight–supported treadmill training with HAL Robot Suit exoskeleton. Patient performing BWSTT using HAL.
Intervention
All enrolled patients performed a BWSTT using the HAL Robot Suit for an initial period of 12 weeks with 5 training sessions a week. During the training, each individual muscle group (hip and knee flexors and extensors) was independently trained by adjusting each individual motor joint support (bilateral hip and knee motors) depending on the EMG activity and strength of the individual muscle group.
During the first 12 weeks the mean number of sessions was 51.75 ± 5.6. Prior to the subsequent HAL-training for the following 40 weeks, all patients were divided according to their preference into 2 subgroups: subgroup 1 (n = 4) with 3 to 5 training sessions per week, and subgroup 2 (n = 4) with about 1 training session a week. During the following 40 weeks the mean number of training sessions in subgroup 1 was 126.8 ± 7.9. In subgroup 2, the patients performed a mean of 32.3 ± 3.3 training sessions.
Each training session included the 10-Meter-Walk Test (10MWT) before and after the HAL-BWSTT, but all other independent functional walking ability tests were performed only at specific intervals.
The training was performed and supervised by a physical therapist and medical doctor. During the intervention neither minor nor severe adverse events occurred.
Measurements
Each patient’s neurological impairment was assessed using the ASIA Impairment Scale (AIS) modified from the Frankel classification to quantify the motor and sensory impairment resulting from the SCI.32 Initial and follow-up assessments were performed by the medical doctors of the Department of Spinal Cord Injury and the Department of Neurology.
All patients’ independent functional outcome measures (without wearing the robot suit) and HAL Robot Suit treadmill training parameters were assessed on admission into the trial, after 6 and 12 weeks of daily training, and after a total of 1 year, with variable frequency training during the concluding 40 weeks by trained physiotherapists and physicians who were involved in neither the study design nor the data analysis.
Walking time, walking distance, and walking speed were measured as HAL Robot Suit-associated treadmill training parameters.
The following standardized tools were used to assess the patients’ independent functional outcome in ambulatory mobility without wearing the robot suit: 10MWT measuring the time needed, number of steps, and the assistance required to walk a 10 meter distance33–35; Timed-Up-and-Go-test (TUG) describing the time and assistance required for standing up from the wheelchair, walking 3 meters, turning around, walking back, and sitting down again36; the 6-Minute-Walk test (6MinWT) measuring the distance traveled and assistance required while walking for 6 minutes37; and the Walking Index for SCI II (WISCI II) assessing ambulatory mobility.38 The WISCI II score is a reliable 20-item scale, measuring the walking capabilities of a patient, based on the requirements for support provided by walking aids, personal assistance, or braces.39 Furthermore, gait was analyzed throughout the entire trial to measure cadence (steps/minute) and stride length during the 10MWT.40
Statistical Analysis
All statistical analyses were carried out using IBM SPSS version 18.0 for Windows (SPSS, Inc, an IBM company, Chicago, IL). The participants’ demographic data were first analyzed using descriptive statistics. All parametric data are expressed as mean ± standard error (SE). Because the number of subjects was too small for a reliable value distribution, the Mann-Whitney U test (Wilcoxon test) was used to establish the statistical significance of differences in functional mobility between assessment on admission and after the first 6 and 12 weeks. Repeated-measure analysis of variance (rm-ANOVA) was applied to investigate differences in functional mobility between the 2 training frequency subgroups (inner-subject factors) at the 2 time points, that is, 12 weeks and 52 weeks.
Statements of Ethics
All participants gave written informed consent to participate and to have their anonymized data published. We further certify that the study was approved by the Ethical Board Committee of the Ruhr-University Bochum and was conducted according to the principles stated in the Declaration of Helsinki.
Study Funding
The study was partially supported by a governmental grant (I&K-Gender-Study, European Union, and NRW, Germany).
Results
Functional Outcome With HAL Robot Suit Support on the Treadmill
All Patients Analyzed as One Group (n = 8)
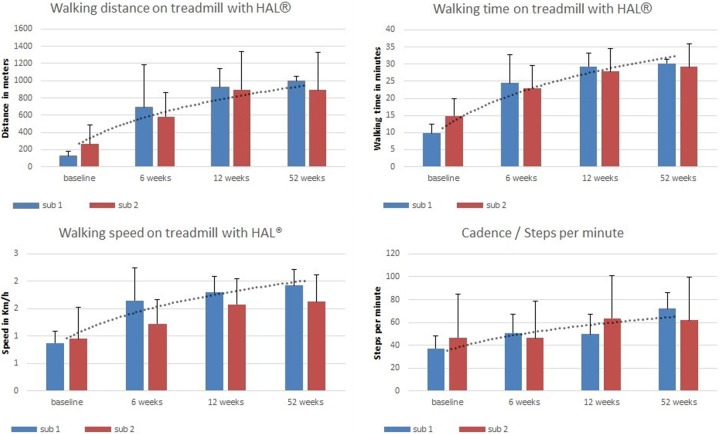
During the first 12 weeks of HAL-BWSTT all patients improved continuously in walking time, walking distance, and walking speed on the treadmill. The average walking time increased within 6 weeks from 12.4 ± 1.6 minutes to 23.8 ± 2.5 minutes and continued to increase to 32.0 ± 1.8 minutes (Z = −2.52; P = .012) after 12 weeks. The average walking distance rose from 195.9 ± 59.0 m at baseline to 638.5 ± 133.5 m at 6 weeks and to 954.1 ± 114.3 m (Z = −2.52; P = .012) at 12 weeks. Finally, the average walking speed increased from 0.91 ± 0.14 km/h (0.5-1.8 km/h) to 1.44 ± 0.18 km/h (0.8-2.3 km/h) after 6 weeks and to 1.59 ± 0.14 km/h (0.8-2.1 km/h) (Z = −2.38; P = .017) after 12 weeks.
During the following 40 weeks, patients trained either once or 3 to 5 times per week. Overall, there was no further significant improvement in walking time (29.63 ± 1.6 minutes; F[1, 6] = 1.061; P = .343), walking distance (944.5 ± 103.4 meters; F[1, 6] = 0.459; P = .523), or walking speed (1.78 ± 0.14 km/h; F[1, 6] = 1.089; P = .337) over the corresponding values at 12 weeks of training.
Long-term Outcome Related to Training Frequency/Subgroup Analysis (n = 4/4)
After a similar initial training with daily HAL-BWSTT for 12 weeks, 4 of the 8 patients continued for the rest of the year (40 weeks) with 3 to 5 training sessions per week (subgroup 1), while the other 4 continued on a lower training frequency with an average of 1 training session per week (subgroup 2).
The results for both groups reveal no significant differences between 12 weeks and 52 weeks in walking time (although in subgroup 1 the ambulated distance was slightly greater [subgroup 1: 29.4 ± 1.9 minutes vs 30 ± 0 minutes; subgroup 2: 27.82 ± 3.4 minutes vs 29.25 ± 3.4 minutes]), walking distance (subgroup 1: 921.5 ± 111.1 m vs 998 ± 27.2 m; subgroup 2: 896.25 ± 220.4 m vs 891 ± 217.5 m), or walking speed (subgroup 1: 1.8 ± 0.15 km/h vs 1.93 ± 0.14 km/h; subgroup 2: 1.85 ± 0.24 km/h vs 1.63 ± 0.25 km/h). Also, there was no significant difference between the 2 training frequency groups at 52 weeks in walking time (F[1, 6] = 0.109; P = .753), walking distance (F[1, 6] = 0.085; P = .781), or walking speed (F[1, 6] = 0.943; P = .369) on the treadmill while using the HAL robot suit.
However, mean value analysis revealed improvements in walking distance in the higher training frequency subgroup 1 (921.5 m to 998.0 m), while in the lower training frequency subgroup 2 the walking distance was nearly constant between weeks 12 and 52 (896.2 m to 891.0 m). See Figure 3.
Figure 3.
Treadmill results using HAL. Baseline, 6th-, 12th-, and 52nd-week assessments of BWSTT parameters including trendline.
Independent Functional Outcome and Walking Ability Without Wearing the HAL Robot Suit
All Patients Analyzed as One Group (n = 8)
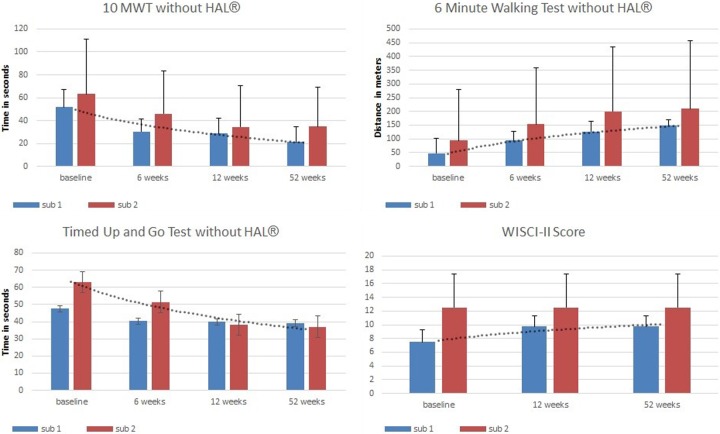
Throughout the first 12 weeks of HAL-BWSTT all patients continually improved in performing the 10MWT, TUG, and 6MinWT and in terms of the walking assistance required.
The time required to walk a 10-meter distance (10MWT) was reduced from 57.45 ± 11.92 seconds at baseline to 38.16 seconds at the 6-week assessment and 31.45 ± 9.08 seconds (Z = −2.52; P = .012) after 12 weeks. Accordingly, the number of steps needed for the 10MWT decreased significantly from 30.25 ± 2.90 (at baseline) to 21.13 ± 1.74 (at 12 weeks) (Z = −2.52, P = .012). The cadence and the stride length increased significantly from 41.85 ± 9.45 steps/min (at baseline) to 56.7 ± 9.9 steps/min (at 12 weeks) (Z = −2.10; P = .036) and from 0.71 ± 0.01 m (at baseline) to 1.00 ± 0.09 m (at 12 weeks) (Z = −2.52; P = .012), while the velocity increased significantly from 0.28 ± 0.10 m/s to 0.38 m/s at the 6-week assessment and to 0.50 ± 0.12 m/s (Z = −2.52; P = .012) after 12 weeks.
The average time needed for the TUG was reduced from 55.34 ± 11.4 seconds at baseline to 47.06 ± 13.1 seconds at the 6-week assessment and 38.94 ± 13.8 seconds after 12 weeks.
The 6MinWT results showed that all subjects improved their walking distance significantly during the 12 weeks of HAL-BWSTT from 70.25 ± 45.95 m to 163.38 ± 56.80 m (Z = −2.52; P = .012).
The WISCI II score showed that the assistance needed (personal, aids) to perform the functional testing improved during the first 12 weeks, although not significantly, from 10 ± 1.5 to 11.13 ± 1.3.
Considering all 8 patients together, irrespective of continuous training frequency during the following 40 weeks, there were no significant changes in the time required for the 10MWT (28.31 ± 8.9 seconds; F[1, 6] = 3.18; P = .125), the number of steps (20.25 ± 2.1; F[1, 6] = 1.547; P = .260), or the cadence or stride length (67.08 ± 9.4 steps/min; F[1, 6] = 4.805; P = .071; 1.06 ± 0.1 m; F[1, 6] = 2.759; P = .148). Furthermore, neither the performance of the TUG (38.06 ± 8.5 seconds) nor the walking distance in the 6MinWT (179.5 ± 58.4 m; F[1, 6] = 4.99; P = .067) differed significantly from the 12-week assessment. No patient improved or worsened in terms of assistance needed (WISCI II) to perform the 10MWT, TUG, or 6MinWT.
Overall, these independent functional measurements (without wearing the robot suit) indicate that the initial functional gain after 3 months of daily continuous HAL-BWSTT was stable and consistent over a 1-year period.
Long-Term Outcome Related to Training Frequency/Subgroup Analysis (n = 4/4)
Subgroups 1 and 2 were assessed separately after the additional 40 weeks of HAL-BWSTT. The results revealed no significant changes in either group in 10MWT (subgroup 1: 28.61 ± 6.9 seconds vs 21.22 ± 6.6 seconds; subgroup 2: 34.28 ± 18.2 seconds vs 34.61 ± 17.3 seconds; F[1, 6] = 0.255; P = .632), the number of steps (subgroup 1: 20.75 ± 1.8 vs 18.5 ± 2.3; subgroup 2: 21.5 ± 3.3 vs 22 ± 3.7; F[1, 6] = 0.288; P = .611), cadence (subgroup 1: 49.71 ± 8.8 steps/min vs 72.16 ± 6.9 steps/min; subgroup 2: 63.65 ± 18.7 steps/min vs 62 ± 18.8 steps/min; F[1, 6] = 0.009; P = .927), or stride length (subgroup 1: 0.99 ± 0.1 m vs 1.12 ± 0.1 m; subgroup 2: 1.0 ± 0.2 m vs 0.99 ± 0.2 m; F[1, 6] = 0.080, P = .787).
There was no significant difference in TUG between 12 and 52 weeks for either subgroup (subgroup 1: 39.8 ± 8.3 seconds vs 39.1 ± 12.03 seconds; subgroup 2: 38.1 ± 15.7 seconds vs 37.06 ± 13.9 seconds; F[1, 6] = 0.139; P = .722). There were no differences as a result of increased training frequency (F[1, 6] = 0.011, P = .921).
The 6MinWT revealed no significant differences consequent on 40 additional weeks of HAL-BWSTT (subgroup 1: 126.75 ± 19.25 m vs 149.5 ± 9.41 m; subgroup 2: 200 ± 117.42 m vs 209.5 ± 123.5 m) and no significant effect of training intensity (F[1, 6] = 0.302; P = .603).
Neither subgroup 1 nor subgroup 2 changed in terms of assistance required to walk without the HAL Robot Suit (WISCI II). See Figure 4.
Figure 4.
Functional mobility without HAL. Results of functional mobility assessment including trendline.
Overall, the functional results with the HAL Robot Suit support on the treadmill and the independent functional outcome and walking ability without wearing the HAL Robot Suit did not change after a 1-year follow-up (with different frequencies of HAL-BWSTT) after an initial 3 months of intensive HAL-BWSTT with 5 training sessions per week. Furthermore, in this pilot study, the functional results with HAL Robot Suit support and the independent functional outcome and walking ability without wearing the HAL Robot Suit after 1 year seem not to depend on the training frequency during the reminder of the year after the initial 3 months of intensive training.
Discussion
As a continuation of a previous pilot study, in which 8 chronic SCI patients performed 12 weeks of daily HAL-BWSTT (60 sessions),24 the objective of the present study was to determine whether long-term 1-year HAL-BWSTT can maintain the activity level already gained, or can even induce further improvements in independent ambulated mobility as a function of training frequency using the HAL Robot Suit after the initial 12 weeks of training.
The overall results indicate no significant changes in the independent functional outcome and walking ability (without wearing the HAL Robot Suit), or in the HAL Robot Suit-supported treadmill-associated functional measures. However, statistical analysis of the training frequency subgroups indicated marginal improvements after 1 year of training in the intensive frequency training group (3-5 times/week) for walking distance on the treadmill while wearing the HAL Robot Suit.
The effectiveness of locomotion training using a DGO in patients with chronic SCI has been investigated from various points of view and considered promising by several systematic reviews including a Cochrane study.13,41 In one multicenter study, Wirz et al concluded that intensive locomotor training on a treadmill with the assistance of a DGO can improve overground walking abilities and pointed out the advantages of DGOs in the training process. However, no significant changes in requirement for walking aids, orthoses, or external physical assistance (WISCI II) subsequent to the training were achieved.15 Furthermore, a Cochrane review addressed the question of which type of locomotor training would be most effective in improving walking function for people with traumatic SCI.41 The authors found there was insufficient evidence to suggest any one locomotor training strategy is superior to others in improving walking function among people with SCI. In particular, the effects of robot-assisted locomotor training remain unclear, so research in the form of large randomized controlled studies is required, particularly for robotic training.
Newly available exoskeletons promise a new developmental step in locomotor training of traumatic SCI patients. They can be distinguished according to their mode of operation and thus in their specific applications and indications. A review article published in 2015 describes the currently available exoskeletal systems and their clinical applications, including scientific and medical evidence to derive individual exoskeleton-related recommendations for clinical practice in rehabilitating patients with spinal cord injuries.21 The authors propose a classification of the systems into “posture-controlled” (eg, EksoGT, ReWALK) and “neurologically controlled” (eg, HAL) exoskeletons. They emphasize that minimal EMG signals and some residual muscular functions are mandatory for enabling SCI patients to rehabilitate with neurologically controlled exoskeletons.21 Posture-controlled devices facilitate locomotion training regardless of remaining motor function, but their primary application would be to complete SCI cases with no residual EMG signals.22,42
The HAL Robot Suit featuring its cutaneous EMG electrodes for each separate and side-independent muscle group requires the wearer’s voluntary drive to be continual and therefore represents a tailored approach to the individual patient’s neurological deficit. This interface enables the patient to be trained and to improve in daily living activities in situations without wearing the exoskeleton.24 The durability of these HAL Robot Suit training results is not yet certain. Nevertheless, regular physiotherapy is an established part in the maintenance therapy of SCI patients to ensure that the activity level reached initially is sustained.43,44
The results obtained prior to this 1-year follow-up study (baseline, 6th week, and 12th week assessments) revealed highly significant improvements in exoskeleton-independent overground walking abilities measured by the 10MWT, the 6MinWT, and the TUG, and in the partial reduction of physical assistance and walking aids in the WISCI II score.24 Muscle strength, measured using the lower extremity motor score, increased in all patients.24
The results of the 6th-week and 12th-week assessments show major changes during the first 6 weeks of HAL-BWSTT. During the following 6 weeks (12th-week assessment) the statistically assessable functional improvements are significant, but less pronounced than during the first 6 weeks (see Figure 3 and 4).
The results at the 52nd week show neither a loss nor any further significant improvements in robot suit–independent functional mobility or robot suit–supported treadmill performance over those obtained in the 12th-week assessment of the intervention, irrespective of whether the overall patient cohort or the 2 training-frequency subgroups are analyzed. This observation allows 3 inferences to be drawn regarding the results of the neurologically controlled HAL Robot Suit training. First, the independent functional activity level and ambulatory walking abilities in SCI patients are maintained after an intensive 3-month training period with the HAL Robot Suit. Second, despite variable frequency training with the HAL Robot Suit for the reminder of the year, no more changes or further improvements of robot suit–independent functions and mobility ensue. Third, the training frequency seems not to be important in the durability of the 3-month treatment results; the minor advantage of subgroup 1 (training 3-5 times/week) is not statistically significant. According to the present pilot study results from 8 chronic state traumatic SCI patients, a statistically assessable steady state in terms of functional improvements is achieved after 12 weeks of HAL-BWSTT.
No general recommendation for long-term neurorehabilitation with the HAL-exoskeleton can be given on the basis of these preliminary results owing to the absence of a control group (no HAL robot suit training after the 12th week). Otherwise, long-term conventional physiotherapy and exercise training is widely accepted and evidence-based.45 Hicks et al even provide evidence that the effects of long-term treadmill training of chronic incomplete SCI cases in improving treadmill walking abilities and indices of subjective well-being were maintained for up to 8 months following the cessation of training.46 This could suggest that long-term neurorehabilitation is at least as effective as conventional BWSTT and therefore advisable. In contrast, improved robot suit–independent functional mobility after the first 12 weeks of the intervention might enable the patient to maintain his level of rehabilitation without continuation of the training. In conclusion, the long-term perpetuation of improvements depends primarily on the ambulatory function after the first 12 weeks of daily HAL-BWSTT and only secondarily on the frequency of the continued HAL body weight–supported treadmill training.
As a downside of this pilot study, the small number of chronic state SCI patients has to be considered in relation to the results. This relatively small number of patients and the lack of a control group indicate the need for future studies to investigate the indication and significance of long-term neurorehabilitation with the HAL-Robot Suit exoskeleton.
In summary, this study provides the first long-term results of BWSTT using a neurologically controlled exoskeleton in patients suffering from chronic state traumatic SCI. The independent functional benefit of an initial intensive training period over 3 months with daily HAL-BWSTT seems evident.24 In terms of cost-benefit considerations, a long-term HAL therapy will primarily lead to an increased expenditure on the rehabilitation of SCI patients. In the long-term view these additional costs may be partially offset by decreasing follow-up costs in the treatment of chronic SCI patients by preventing pressure sores and reducing the demand of concomitant physiotherapy as the improved mobility enables the patient to maintain his level of activity independently.
BWSTT as an important part in the rehabilitation of SCI patients to enhance walking abilities, is limited by the personnel and labor requirements placed on the physical therapists. The introduction of robotic devices that provide tailored assistance and therefore support physical therapists may improve delivery of BWSTT.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Thomas A. Schildhauer is a consultant for Cyberdyne Japan. The other authors have no conflicts of interest to report.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was partially supported by a governmental grant (I&K-Gender-Study, European-Union, and NRW, Germany), as well as financial support of the New Energy and Industrial Technology Development Organization (Japan).
References
- 1. Zäch GA, et al. Demographie und Statistik der Querschnittslähmung In: Zäch GA. (Hrsg.). Querschnittslähmung—Ganzheitliche Rehabilitation. Küsnacht, Switzerland: Wüst; 1995:S16–S19. [Google Scholar]
- 2. Wyndaele M, Wyndaele JJ. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord. 2006;44:523–529. [DOI] [PubMed] [Google Scholar]
- 3. Spinal cord injuries facts and figures at a glance. J Spinal Cord Med. 2013;36:568–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ackery A, Tator C, Krassioukov A. Global perspective on spinal cord injury epidemiology. J Neurotrauma. 2004;21:1355–1370. [DOI] [PubMed] [Google Scholar]
- 5. Waters RL, Adkins R, Yakura J, Vigil D. Prediction of ambulatory performance based on motor scores derived from standards of the American Spinal Injury Association. Arch Phys Med Rehabil. 1994;75:756–760. [PubMed] [Google Scholar]
- 6. Lildal IB, Huynh TK, Biering-Sorensen F. Return to work following spinal cord injury: a review. Disabil Rehabil. 2007;29:1341–1375. [DOI] [PubMed] [Google Scholar]
- 7. Piepmeier JM, Jenkins NR. Late neurological changes following traumatic spinal cord injury. J Neurosurg. 1988;69:399–402. [DOI] [PubMed] [Google Scholar]
- 8. Brown AK, Woller SA, Moreno G, Grau JW, Hook MA. Exercise therapy and recovery after SCI: evidence that shows early intervention improves recovery of function. Spinal Cord. 2011;49:623–628. doi:10.1038/sc.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gittler MS, McKinley WO, Stiens SA, Groah SL, Kirshblum SC. Spinal cord injury medicine. 3. Rehabilitation outcomes. Arch Phys Med Rehabil. 2002;83:65–71. [DOI] [PubMed] [Google Scholar]
- 10. Paolucci S. Epidemiology and treatment of post-stroke depression. Neuropsychiatr Dis Treat. 2008;4:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cruciger O, Schildhauer TA, Meindl RC, et al. Impact of locomotion training with a neurologic controlled hybrid assistive limb (HAL) exoskeleton on neuropathic pain and health related quality of life (HRQoL) in chronic SCI: a case study. Disabil Rehabil Assist Technol. 2014;11:529–534. [DOI] [PubMed] [Google Scholar]
- 12. Lude P, Kennedy P, Elfström ML, Ballert CS. Quality of life in and after spinal cord injury rehabilitation: a longitudinal multicenter study. Top Spinal Cord Inj Rehabil. 2014;20:197–207. doi:10.1310/sci2003-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morawietz C, Moffat F. Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch Phys Med Rehab. 2013;94:2297–2308. [DOI] [PubMed] [Google Scholar]
- 14. Winchester P, McColl R, Querry R, et al. Changes in supraspinal activation patterns following robotic locomotor therapy in motor-incomplete spinal cord injury. Neurorehabil Neural Repair 2005;19:313–324. [DOI] [PubMed] [Google Scholar]
- 15. Wirz M, Zemon DH, Rupp R, et al. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Arch Phys Med Rehabil. 2005;86:672–680. [DOI] [PubMed] [Google Scholar]
- 16. Colombo G, Joerg M, Schreier R, Dietz V. Treadmill training of paraplegic patients using a robotic orthosis. J Rehabil Res Dev. 2000;37:693–700. [PubMed] [Google Scholar]
- 17. Dobkin BH, Apple D, Barbeau H, et al. Methods for a randomized trial of weight-supported treadmill training versus conventional training for walking during inpatient rehabilitation after incomplete traumatic spinal cord injury. Neurorehabil Neural Repair. 2003;17:153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwartz I, Meiner Z. The influence of locomotor treatment using robotic body-weight-supported treadmill training on rehabilitation outcome of patients suffering from neurological disorders [in Hebrew]. Harefuah. 2013;152:166–171, 181, 182. [PubMed] [Google Scholar]
- 19. Cruciger O, Tegenthoff M, Schwenkreis P, Schildhauer TA, Aach M. Locomotion training using voluntary driven exoskeleton (HAL) in acute incomplete SCI. Neurology. 2014;83:474 doi:10.1212/WNL.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 20. Sale P, Franceschini M, Waldner A, Hesse S. Use of the robot assisted gait therapy in rehabilitation of patients with stroke and spinal cord injury. Eur J Phys Rehabil Med. 2012;48:111–121. [PubMed] [Google Scholar]
- 21. Aach M, Meindl RC, Geßmann J, Schildhauer TA, Citak M, Cruciger O. Exoskeletons for rehabilitation of patients with spinal cord injuries: options and limitations [in German]. Unfallchirurg. 2015;118:130–137. doi:10.1007/s00113-014-2616-1. [DOI] [PubMed] [Google Scholar]
- 22. Kolakowsky-Hayner SA, Crew J, Moran S, Shah A. Safety and feasibility of using the Ekso™ bionic exoskeleton to aid ambulation after spinal cord injury. J Spine. 2013;S4:003 doi:10.4172/2165-7939.S4-003. [Google Scholar]
- 23. Fineberg DB, Asselin P, Harel NY, et al. Vertical ground reaction force-based analysis of powered exoskeleton-assisted walking in persons with motor-complete paraplegia. J Spinal Cord Med. 2013;36:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aach M, Cruciger O, Sczesny-Kaiser M, et al. Voluntary driven exoskeleton as a new tool for rehabilitation in chronic spinal cord Injury: a pilot study. Spine J. 2014;14:2847–2853. doi:10.1016/j.spinee.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 25. Suzuki K, Mito G, Kawamoto H, Hasegawa Y, Sankai Y. Intention-based walking support for paraplegia patients with Robot Suit HAL. Adv Robot. 2007;21:1441–1469. [Google Scholar]
- 26. Kawamoto H, Kamibayashi K, Nakata Y, et al. Pilot study of locomotion improvement using hybrid assistive limb in chronic stroke patients. BMC Neurol. 2013;13:141 doi:10.1186/1471-2377-13-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ueba T, Hamada O, Ogata T, Inoue T, Shiota E, Sankai Y. Feasibility and safety of acute phase rehabilitation after stroke using the hybrid assistive limb robot suit. Neurol Med Chir. 2013;53:287–290. [DOI] [PubMed] [Google Scholar]
- 28. Maeshima S, Osawa A, Nishio D, et al. Efficacy of a hybrid assistive limb in post-stroke hemiplegic patients: a preliminary report. BMC Neurol. 2011;11:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nilsson A, Vreede KS, Häglund V, Kawamoto H, Sankai Y, Borg J. Gait training early after stroke with a new exoskeleton—the hybrid assistive limb: a study of safety and feasibility. J Neuroeng Rehabil. 2014;11:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoshimoto T, Shimizu I, Hiroi Y, Kawaki M, Sato D, Nagasawa M. Feasibility and efficacy of high-speed gait training with a voluntary driven exoskeleton robot for gait and balance dysfunction in patients with chronic stroke: nonrandomized pilot study with concurrent control. Int J Rehabil Res. 2015;38:338–343. doi:10.1097/MRR.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 31. Janda V, ed. Muscle Function Testing. London, England: Butterworths; 1983:230–231, 241. [Google Scholar]
- 32. Maynard FM, Bracken MB, Creasey G, et al. International standards for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Spinal Cord. 1997;35:266–274. [DOI] [PubMed] [Google Scholar]
- 33. Van Hedel HJ, Wirz M, Dietz V. Standardized assessment of walking capacity after spinal cord injury: the European network approach. Neurol Res. 2008;30:61–73. [DOI] [PubMed] [Google Scholar]
- 34. Van Hedel HJ, Wirz M, Curt A. Improving walking assessment in subjects with an incomplete spinal cord injury: responsiveness. Spinal Cord. 2006;44:352–356. [DOI] [PubMed] [Google Scholar]
- 35. Van Hedel HJ, Wirz M, Dietz V. Assessing walking ability in subjects with spinal cord injury: validity and reliability of 3 walking tests. Arch Phys Med Rehabil. 2005;86:190–196. [DOI] [PubMed] [Google Scholar]
- 36. Picone EN. The timed up and go test. Am J Nurs. 2013;113:56–59. [DOI] [PubMed] [Google Scholar]
- 37. Enright PL. The six-minute walk test. Respir Care. 2003;48:783–785. [PubMed] [Google Scholar]
- 38. Myeong OK, Burns AS, Ditunno JF, Marino RJ. The assessment of walking capacity using the walking index for spinal cord injury: self-selected versus maximal levels. Arch Phys Med Rehabil. 2007;88:762–767. [DOI] [PubMed] [Google Scholar]
- 39. Scivoletto G, Tamburella F, Laurenza L, Torre M, Molinari M, Ditunno JF. Walking Index for Spinal Cord Injury version II in acute spinal cord injury: reliability and reproducibility. Spinal Cord. 2014;52:65–69. [DOI] [PubMed] [Google Scholar]
- 40. Coutts F. Gait analysis in the therapeutic environment. Man Ther. 1999;4:2–10. [DOI] [PubMed] [Google Scholar]
- 41. Mehrholz J, Kugler J, Pohl M. Locomotor training for walking after spinal cord injury. Cochrane Database Syst Rev. 2012;(11):CD006676. [DOI] [PubMed] [Google Scholar]
- 42. Esquenazi A, Talaty M, Packel A, Saulino M. The ReWalk powered exoskeleton to restore ambulatory function to individuals with thoracic-level motor-complete spinal cord injury. Am J Phys Med Rehabil. 2012;91:911–921. doi:10.1097/PHM.0b013e318269d9a3. [DOI] [PubMed] [Google Scholar]
- 43. Jacobs PL, Nash MS. Exercise recommendations for individuals with spinal cord injury. Sports Med. 2004;34:727–751. [DOI] [PubMed] [Google Scholar]
- 44. Harvey LA, Herbert RD, Glinsky J, Moseley AM, Bowden J. Effects of 6 months of regular passive movements on ankle joint mobility in people with spinal cord injury: a randomized controlled trial. Spinal Cord. 2009;47:62–66. doi:10.1038/sc.2008.71. [DOI] [PubMed] [Google Scholar]
- 45. Hicks AL, Martin KA, Ditor DS, et al. Long-term exercise training in persons with spinal cord injury: effects on strength, arm ergometry performance and psychological well-being. Spinal Cord. 2003;41:34–43. [DOI] [PubMed] [Google Scholar]
- 46. Hicks AL, Adams MM, Martin Ginis K, et al. Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: effects on functional walking ability and measures of subjective well-being. Spinal Cord. 2005;43:291–298. [DOI] [PubMed] [Google Scholar]