Abstract
Purpose of review
Recent advances in genomics and gene editing have expanded the range of model organisms to include those with interesting biological capabilities such as regeneration. Among these are the classic models of regeneration biology, the salamander. Although stimulating endogenous regeneration in humans likely is many years away, with advances in stem cell biology and biomedical engineering (e.g. bio-inspired materials), it is evident that there is great potential to enhance regenerative outcomes by approaching the problem from an engineering perspective. The question at this point is what do we need to engineer?
Recent findings
The value of regeneration models is that they show us how regeneration works, which then can guide efforts to mimic these developmental processes therapeutically. Among these models, the Accessory Limb Model (ALM) was developed in the axolotl as a gain-of-function assay for the sequential steps that are required for successful regeneration. To date, this model has identified a number of proregenerative signals, including growth factor signaling associated with nerves, and signals associated with the extracellular matrix (ECM) that induce pattern formation.
Summary
Identification of these signals through the use of models in highly regenerative vertebrates (e.g. the axolotl) offers a wide range of possible modifications for engineering bio-inspired, biomimetic materials to create a dynamic stem cell niche for regeneration and scar-free repair.
Keywords: axolotl, regeneration, regenerative engineering, hyaluronic acid, heparan sulfate, ECM
Introduction
Historically, model systems for studying developmental biology were limited to organisms with amenable genetics. These animals have short life cycles with rapid embryonic development, and as a result the field of developmental biology has been focused for decades on developmental genetics of embryos. As a consequence, we have a very good understanding of the complex gene regulatory networks that control embryogenesis, which has led to the development of an impressive toolbox of techniques for experimentally manipulating gene expression. Combined with the advances in genomics driven by ever more powerful nucleotide sequencing technologies, it now is possible to expand the range of model organisms to including those with interesting biological capabilities such as regeneration.
Compared to the decades of mechanistic studies of embryogenesis, little is known about the genetic regulation of regeneration. The challenges of working with regeneration models, which have not been amenable to classical genetic techniques, no longer are insurmountable given advances in gene editing and genomics. Thus it now is possible to embrace model systems that will allow for the discovery of the mechanisms for post-natal regenerative development. Among these is the classic model of tetrapod regeneration biology, the salamander. Over the past decades as genomics has advanced, the axolotl has emerged as the model of choice for tetrapod regeneration, and thus we focus on this model system as an assay for the discovery of signaling pathways controlling regeneration. With increasing knowledge and the development of new tools, we presume it is only a matter of time before it will be possible to control the processes of regeneration, leading to the ultimate goal of endogenous human regeneration.
An ultimate goal of regeneration research is to apply the knowledge gained from studies of animals that regenerate well to enhance the regenerative response of mammals, and thus to improve human health. Stimulating endogenous regeneration in humans likely is many years away, but with advances in stem cell biology and biomedical engineering (e.g. bio-inspired materials), it is evident that there is great potential to make important advances now through the applications of regenerative engineering [1]. The question at this point is what do we need to engineer? The value of regeneration models is that they show us how regeneration works (e.g. the spatial and temporal regulation of proregenerative signaling pathways), which then can guide efforts to mimic these developmental processes therapeutically.
From studies of regeneration models to date, there are reasons to be encouraged about the future advances and success of regenerative engineering. From the developmental biology side, the key to this optimism is the conservation of biological mechanisms between species, as well as between embryonic and regenerative development. As a consequence, lessons learned from studies of one model organism will be applicable (either broadly or specifically) to others. In addition, the explosive growth of technology for manipulating stem cell biology, as well as the ability to reprogram somatic cells (iPS cells), have made it possible to create all of the cell types needed to rebuild body parts [2]. From the engineering side, advances in biomaterials and high-resolution fabrication (e.g. 3-D printing) are making it possible to create essentially any desired structural configuration of bioactive materials. Thus the challenge today is to discover how to get the right cells into the right place to regenerate functional body parts. Therein lies the critical role of the regeneration model systems.
The reality is that regeneration is a complex and orchestrated series of events that occur in response to injury. Although all animals can regenerate to some extent, some can do this much better than others. Thus the lessons to be learned from the very good regenerators will be critical as the field of regeneration biology grows. An important mechanistic lesson learned from studies of regeneration models such as the axolotl is that endogenous regeneration requires both the cells to replace the missing structures (i.e. stem cells) and the information to instruct those cells as to where to go and what to become. This information is created by cells and multiple factors within the extracellular matrix (ECM), and ultimately becomes encoded and stabilized via synthesis of the bioactive macromolecules of the ECM. It is that bioactive and information-rich extracellular environment that we now refer to as the niche. In the case of stem cells, the importance of the niche has become well recognized and appreciated [3]. The importance of the positional information niche for endogenous regeneration is much less appreciated.
The modern day concept of the stem cell niche is comparable to the classical idea that a “permissive” environment is required for regeneration. The signals associated with this extracellular environment control the behavior of the progenitor cells for regeneration (i.e. regeneration stem cells) in terms of migration, proliferation, and state of differentiation/dedifferentiation [4,5]. Though this function of the niche is critical for recruiting regeneration-competent cells to form the blastema, there also must be information in the niche to re-create the pattern of the structures to be regenerated (positional information, PI) [5-7]. Thus the challenge of inducing regeneration lies in engineering the niche in terms of both its permissive and instructive functions. Although much effort has been focused in recent years on the permissive functions of the stem cell niche (especially in terms of the control of proliferation and differentiation), little is understood mechanistically about PI and the role of the niche in pattern formation. The unique value of regeneration models such as the axolotl is that they provide the opportunity to understand what PI is and how it is controlled during regeneration.
The axolotl regeneration model
Historically, the salamander has been the tetrapod regeneration model of choice since it can regenerate well both as a larva and as an adult. During the early years, much of the research involved newts, but over the decades the axolotl (Ambystoma mexicanum) has become the model organism for a number of reasons. Axolotls can be bred in the lab throughout the year rather than being collected seasonally from increasing depleted wild populations. The advances in genomics and techniques for transgenesis have all been developed and optimized using axolotls [8-10]. In addition, the Accessory Limb Model (ALM) has been optimized in the axolotl as a gain-of-function assay for the steps and signals that control the steps of limb regeneration (Figure 1; see below).
Figure 1. The Accessory Limb Model (ALM) as a gain-of-function assay for the signals controlling regeneration.
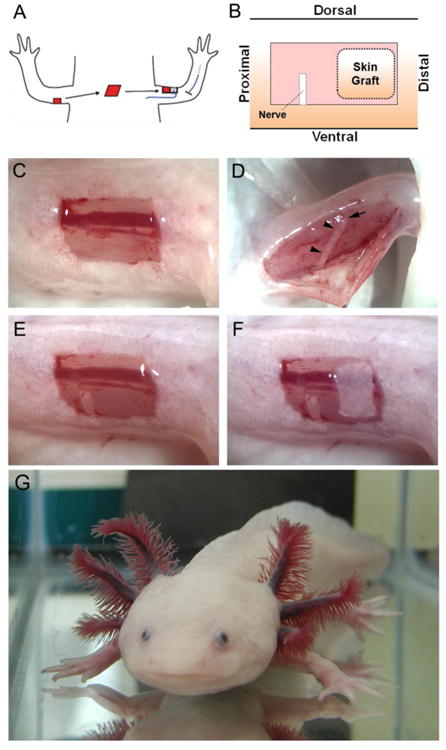
Making an accessory axolotl limb (ALM; Endo et al., 2004). A, Ectopic limbs with normal pattern can be induced to form in response to a wound to which a nerve (blue) is surgically deviated, and a piece of skin (red) from the opposite side of the limb (posterior) is grafted into the host site (anterior) as indicated by the black arrows. B, Cartoon illustrating the final arrangement of wound, deviated nerve, and skin graft that will result in formation of an ectopic limb. C–F, In vivo images of the progressive surgical steps in the ALM beginning with the full-thickness skin wound (C) that will heal without forming an ectopic blastema. If this wound is provided with signals from a nerve (D, E) it will be induced to form an ectopic blastema that does not make skeletal elements. The brachial nerve (black arrows) is isolated surgically prior to being deviated (D), and then is deviated to the anterior wound bed (E). If a piece of posterior skin is grafted in combination with the deviated nerve (F as illustrated in B), it provides additional signals that are sufficient to generate a supernumerary limb as shown in (G) (two left arms). (With permission from: Bryant SV and Gardiner DM. Regeneration 2016, 3: 103–122) [45].
As with all regeneration models, there always have been two important limitations. In almost all experiments, various body parts are amputated, and the sequence of down-stream events are described. Given the trauma of amputation, there are changes in the expression of many thousands of genes [11-15]. Since many of these also are expressed in animals that do not regenerate when wounded, it likely is the case that only a few are critical for successful regeneration. Thus there is a signal-to-noise problem that extends to all the many non regeneration-specific molecular and cellular changes that occur in response to injury. Obviously, there are regeneration-essential events, and at least one of them does not occur in animals (e.g. humans) that fail to regenerate in response to amputation. This problem is additionally complicated by the likelihood that there are many regeneration-essential events that do or could occur in non-regenerating animals, but do not because of the failure on an earlier event. This is actually good news in that we do not need to fix all the steps of regeneration, just the ones that do not work; nevertheless, there still is the challenge of finding the needle in the haystack.
The second challenge is that historically, and continuing to the present, experiments using regeneration models are designed to test for loss-of-function. Since the control samples (untreated) will regenerate, then the experimental samples will be expected to have some sort of regeneration defect. A classic example of this approach is the fact that denervated salamander limbs fail to regenerate. This result leads to the conclusion that nerves are required for regeneration, but it does shed light on the underlying mechanisms that make regeneration work. In contrast, gain-of-function experiments have the potential to identify signals and pathways that can induce regeneration when activated. Although experiments on animals that normally do not regenerate are by nature gain-of-function, if there are multiple signals/pathways involved (which is likely), it will not be possible to induce regeneration by testing single candidate molecules. The ALM was developed and optimized to address both of these experimental challenges to discovering what processes are required for endogenous regeneration. It thus provides the opportunity to approach regeneration with an engineering strategy to manipulate these processes therapeutically and thus enhance and induce regeneration in humans.
The ALM as an assay for signaling that is required for endogenous regeneration
The ALM was developed as a gain-of-function model for regeneration (Figure 1; [16,17]) based on early experiments demonstrating that ectopic limbs could be induced to form from wounds on the side of the arm of salamanders [18,19]. The induction of ectopic limbs requires three conditions; a full-thickness skin wound, a nerve, and cells with different positional information (PI). A skin wound (without damage to the underlying soft tissues) alone heals and the skin is regenerated, but no ectopic limbs are formed. If a nerve is deviated to the site of the wound, then cells are recruited to form an ectopic blastema; however, this blastema fails to form an ectopic limb and eventually in integrated back into the tissues of the host limb. Finally, if cells with PI from the side of the limb that is opposite to the wound site are grafted to a wound with a deviated nerve (e.g. posterior cells grafted into an anterior wound site), a blastema forms and continues to develop into a well-patterned ectopic limb. When analyzed in terms of patterns of gene expression and cellular behaviors, the mechanism for ectopic limb formation is the same as for limb regeneration in response to an amputation [17]. Thus the ALM is an assay for the signals that induce each of the steps of regeneration (gain-of-function) while reducing the trauma associated with amputation.
The ALM has been utilized in a number of studies to identify proregenerative signals and pathways (discussed below). From a broader, engineering perspective, one value of this model is that it demonstrates what has been intuitively obvious, i.e. that regeneration is a step-wise process. In response to amputation, the entire process of regeneration plays out and there is no way to stop in along the way. In contrast, with the ALM, there are multiple steps at which the appropriate signal(s) must be provided, otherwise the system fails to progress to the next step, resulting in regenerative failure. By this reasoning, each step is a potential barrier to successful regeneration [5,20], and the strategy to inducing regeneration in humans is to identify each step and intervene therapeutically to ensure that progression to the next step can occur. Thus the early steps must by necessity be discovered and successfully navigated before discovering what the later steps might be.
In the ALM as well as in amputated limbs, wound healing occurs by migration of epithelial cells from the cut edge of the epidermis, which is followed by formation of the blastema. This later step is dependent on signals from the nerve that interact with the newly formed wound epithelium to induce formation of a signaling center that recruits mesenchymal cells from the adjacent connective tissue (see [5,21,22]. This step that is dependent on nerve-associated signals in vivo can be regulated by the therapeutic delivery of a defined cocktail of growth factors [23-25]. In so doing, a deviated nerve is no longer required, and the downstream pathways (next steps in the regeneration cascade) leading to blastema formation can be targeted directly.
Similarly, the role of grafted cells with different PI can be substituted for by chemically defined ECM macromolecules. As demonstrated in the ALM, an ectopic blastema can be induced to form (via a deviated nerve or a growth factor cocktail), but this blastema is unable to form limb structures. When skin is grafted from the side of the limb that is opposite the host wound, PI is provided that instructs the blastema cells to make limb structures. Since the necessary PI is contained within the dermal compartment of the skin graft [16], it must be associated with either dermal fibroblasts, the ECM these cells synthesize, or both. By using the ALM to assay for pattern-inducing signaling, it has been possible to identify and characterize ECM components that provide this PI [26].
PI-associated signalling activity is encoded by modifications of proteoglycans of the ECM of axolotl limb skin [26]. At least some of this activity is associated with the patterns of sulfation of heparan sulfate (HS) that are controlled by the spatial and temporal regulation of the expression and activity of different sulfotransferases. These specific patterns of sulfation in turn function in regeneration by regulating growth factor signaling. The sulfation code can be altered over a short time course, and thus it can be stable over time, yet can be modified and reprogrammed in response to injury to enable the genesis of new PI over the course of regeneration (i.e. it is programmable). In addition, there are ECM associated activities that inhibit a regenerative response in the ALM [26], which reminds us that the lack of a regenerative response is not the same as lacking the ability to regenerate. The ability to assay for proregenerative signaling by extracellular macromolecules is exciting in that the ECM is acellular and can be engineered so as to induce desired outcomes in the context of regeneration.
Mammalian signaling molecules that induce a regeneration response in the ALM
The realization that the underlying mechanisms of embryonic and regenerative development are conserved, and used by both salamanders and humans is encouraging in that what works in an axolotl likely will work in a human, and vice versa (e.g. human recombinant growth factors induce ectopic blastema formation in the axolotl [23,25]). The lack of a regenerative response in humans may be a consequence of a failure to produce the necessary signals at the right time or place, but it is unlikely that there is a problem in the underlying pathways themselves. This hypothesis is founded on the concept of modern development biology that since the underlying mechanisms are highly conserved, the diversity and differences in outcomes are a result of differential regulation of these conserved signals in time and space [27]. In the case of regeneration, the outcome is determined by the orchestration of these signals in response to injury. The point being that since mammalian signals and conserved pathways function to lead to excellent regeneration in an axolotl, these same pathways should work as well in mammals if regulated appropriated so as to affect a regenerative outcome.
Based on this inference, the challenge is to identify when and where specific signals are required to progress to the next step along the regeneration cascade, and the ALM allows that to be done. Equally important is that the ALM can be used as an assay to determine if those signals are present in mammalian wound responses. If a signal is present then it is not a barrier to mammalian regeneration; however, if it is not present it will be necessary to provide it therapeutically in order to overcome that barrier and progress to the next steps along the regeneration cascade. The approach of using the ALM as an assay for signaling in mammalian tissues has identified proregenerative signals associated with the ECM (heparan sulfate proteoglycans in particular) of both axolotl and mouse limb skin [26]. This signaling is associated with spatial differences in the patterns of sulfation of the heparan sulfate (HS) chains that are predicted to regulate the response of cells to a number of growth factors, including different members of the FGF family of signaling molecules [28,29]. The signaling activity varies both temporally and spatially, which is consistent with observations that HS synthesis is dynamically regulated. In addition to inducing the formation of ectopic limb structures, there are ECM-associated signals that inhibit blastema formation. Finally, an artificial ECM containing porcine HS can induce formation of complex skeletal elements including joints and digit tips when grafted into ectopic axolotl blastemas [26].
The importance of HS and the closely related macromolecule hyaluronic acid (HA) is a good example of how developmental biology and regenerative engineering can inform each other and advance the goal of inducing regeneration (Table 1). The importance of HS has become increasingly appreciated as the field of glycobiology has grown in recent years, and it now is evident that it plays a critical role in regulation of a number of biological processes, in particular through its regulation of growth factor signaling [28,29]. On the bioengineering side, HA has played a central role in the development of biomaterials and hydrogels for tissue engineering because of its biocompatible properties. Thus these two macromolecules are an example of a point of convergence in terms of a role in the regulation of regeneration and the engineering of ECM/hydrogels for tissue engineering that likely will have the potential for advancing our abilities to induce or enhance regeneration in humans.
Table 1. The use of hyaluronic acid in tissue engineering and its functional role in endogenous regeneration.
| Model | Cell Type | Approach | Outcome Ref | Ref |
|---|---|---|---|---|
| Tissue Engineering | ||||
| in vivo (Rabbit) | Mesenchymal Progenitor Cells | Implantation of a HA-based three-D scaffold for cells in a full thickness osteochondral lesion | Cells adhered and proliferated on scaffold and lesions filled with this hydrogel, either seeded or unseeded with cells, achieved a faster and better healing. | [32] |
| in vivo (Rat) | Human Aortic Endothelial Cells (HAECs) | Implantation of a glycidyl methacrylate- HA (GMHA) scaffold into subcutaneous locations in rats to promote tissue repair | Implanted GMHA hydrogels showed good biocompatibility, little inflammatory response, i.e. suitable for modification with adhesive peptide sequences to use for wound healing applications. | [33] |
| in vitro, in vivo (Rat) | Human Mesenchymal Stem Cells (hMSC) | Implantation of an acrylated HA as a scaffold with BMP-2 and human mesenchymal stem cells (hMSCs) for rat calvarial defect regeneration | Viability in vitro was increased up to 55% in hydrogels with BMP-2; hydrogels with BMP-2 and MSCs had the highest expression of osteocalcin and bone formation with vascular markers for in vivo calvarial defects | [34] |
| in vitro | Ventral Mesencephalic Neural Progenitor Cells | Photoencapsulation of cells into HA hydrogels with varying numbers of photocrosslinkable methacrylate groups to investigate differentiation in 3D cultures that mimic geometry and mechanical properties of tissues | After three weeks, the majority of NPCs cultured in hydrogels with mechanical properties comparable to those of neonatal brain had differentiated into neurons, while NPCs cultured within stiffer hydrogels, with mechanical properties comparable to those of adult brain, had differentiated mostly into astrocytes. | [35] |
| in vitro, in vivo (Mouse) | Retinal Stem-Progenitor Cell (RSPC) | Development of HA and methylcellulose hydrogel for localized delivery to sub-retinal space | HAMC supported RSPC survival and proliferation in vitro and exhibiting ideal properties for delivery to the sub-retinal space for in vivo study. | [36] |
| in vivo (Rat & Rabbit) | Human Umbilical Cord Mesenchymal Stem Cells | Transplantation of hUCB-MSCs in 4% HA hydrogel composite into defect on the knee to confirm the regenerative potential in models | A composite of hUCB-MSCs with HA hydrogel to cartilage defects results in consistent cartilage regeneration and can be used for the regenerative treatment of full-thickness articular cartilage defects. | [37] |
| Regeneration | ||||
| in vivo (Newt) | Limb Blastema Cells | Investigation of changes in matrix in response to amputation and its role in blastema formation | HA is synthesized in the early-dedifferentiated blastema, is at highest level at 10 days of regeneration, and then degraded rapidly at 25 days of regeneration. | [38] |
| in vivo (Newt) | Limb Blastema Cells | Investigation of hyaluronidase activity and glycosaminoglycan synthesis in denervated (fail to regenerate) and innervated limbs | HA was the major GAG being produced, the ratio of HA to chondroitin sulfate was reduced in denervated limbs, and, hyaluronidase activity appears at the cartilage deposition stage, with or without a nerve. | [39] |
| in vivo (Newt)) | Limb Blastema Cells | Investigation of the changes in the Matrix and Glycosaminoglycan synthesis during the initiation of regeneration | HA synthesis began at onset of tissue dedifferentiation, became maximal within 1 week, and continued throughout the period of active cell proliferation. Chondroitin sulfate synthesis began later, increased steadily, and reached high levels during chondrogenesis | [40] |
| in vivo (Newt) | Muscle satellite cells | Investigation of influence of the matrix on newt myoblasts in vitro | HA, tenascin-C and fibronectin influjence cell behaviors (DNA synthesis, migration, myotube fragmentation and myoblast fusion). | [41] |
HA is HS without the complex patterns of sulfation. Both are composed of repeating disaccharide subunits of glucuronic acid and N-acetylglucosamine that form very large and abundant ECM macromolecules. The prevalence and biological properties of HA were recognized early on by tissue engineers, who were by definition looking to repair and regenerate damaged or lost tissues. The ideas of taking an engineering approach to regeneration were being actively discussed in the mid-1980s, culminating in the formalization of the concept of tissue engineering in the landmark paper by Langer and Vacanti [30]. Publications in the field based on the use of hydrogels, including HA, collagen, and fibrinogen appeared in the literature coincident with the birth of the field of tissue engineering, and HA as one of the first biomaterials as a scaffold continues to be widely used to this day [31-37]. Table 1 identifies a selected group of papers (based on a survey of approximately 250 publications over the past two decades) pertaining to the use of HA in regenerative engineering, and provides a guide to the reader who is interested in both the history and utility of HA hydrogels. The structural properties of HA make it particularly amenable for engineering into bioactive and biocompatible scaffolds. It promotes cell adhesion and proliferation, and is very good at maintaining cell viability. In vivo it is implicated as being important in a number of biological activities including the maintenance of homeostasis of a variety of tissues such as hyaline cartilage, synovial fluid, and loose connective tissues. It also is an important participant in the wound healing response to injury. Given the widespread distribution and abundance in tissues in vivo, it is no surprise that cells like HA hydrogels.
In spite of being important in a large number of biological processes, the role of HA in regeneration has not been extensively investigated in model organisms that regenerate well, such as the axolotl. There are early publications that make a strong case that HA has important functions in salamander limb regeneration (Table 1; [38-41]). Synthesis of HA is upregulated during the early stages of blastema formation, and the enzymes that degrade HA subsequently are expressed resulting a reduction of levels of HA at later stages [38,39]. Given its hydroscopic property, one hypothesized function for HA is that it creates pro-migratory space into which cells from the wound bed and margin can migrate to form the blastema [38]. Consistent with this hypothesis is the observation that the early migration of pre-blastema cells from the dermis is delayed for several days post-amputation [42,43], as if the space beneath the wound epithelium needs to become permissive to migration; e.g. synthesis of HA into that space. The role of HA in axolotl limb regeneration has not been investigated aggressively since these early reports indicating it likely is important. Given the advances in glycobiology, the availability of the ALM as an assay for proregenerative activity, and the importance of the sulfated version of HA (HS), there is an opportunity to revisit HA as another tool in the box to achieve success in the engineering of regeneration in humans.
Conclusion: Engineering the stem cell niche for regeneration
The convergence of tissue engineering and the reemergence of the classical regeneration model systems such as the axolotl, allow for the development of novel approaches for engineering the processes for successful regeneration. Among those processes, being able to control the behavior of the progenitor cells for regeneration is essential for success. These proregenerative behaviors are regulated by cell-cell and cell-ECM (the niche) interactions, and thus one important goal for regenerative medicine is to be able to engineer the stem/progenitor cell niche. Success likely will be achieved through the convergence of developmental biology and engineering, as for the example discussed above for HA and HS. The ability to make progress in this endeavor necessitates having assays for proregenerative signaling, and those assays need to be in animals that can regenerate. Ultimately the discoveries will be translated for the clinic through mammalian models such as rats, rabbits and mice; but they do not regenerate intrinsically and thus cannot be used as an assay. Although the ALM can be used to identify proregenerative signals [26,44], its major drawback is the time it takes to achieve the endpoint of differentiated limb structures (about three months). Thus one challenge is to refine the ALM in order to validate shorter, surrogate end points that could be used as higher throughput assays, in particular for the multitude of modifications of HS that are predicted to have different biological activities. The identification of more proximate functional endpoints also will lead to further discovery of the essential steps of regeneration and the mechanisms that control those steps. Finally, the utility of regeneration models such as the axolotl ALM reinforces the view that we need to have an integrated approach to the problem of engineering regeneration based on the underlying principle of embryonic and regenerative development; hence the emergence of “regenerative engineering” [1]. As discussed in this essay, an understanding of the ECM composition and associated biological functions in highly regenerative vertebrates offers a wide range of possible modifications for engineering bio-inspired, biomimetic materials to create a dynamic stem cell niche capable of regeneration and scar-free repair.
Acknowledgments
We thank Dr. Susan V. Bryant and Dr. Ken Muneoka for insights into the importance of designing gain-of-function experiments for regeneration, and Dr. Cato Laurencin for his insights into the convergence of regeneration biology and engineering leading to the emergence of regenerative engineering for inducing human regeneration.
Footnotes
Conflict of Interest: Negar Seyedhassantehrani, Takayoshi Otsuka, Shambhavi Singh, and David M. Gardiner declare that they have no conflict of interest.
Compliance with Ethical Standards: Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Laurencin CT, Khan Y. Regenerative Engineering. CRC Press; 2013. [DOI] [PubMed] [Google Scholar]
- 2.Trounson A, DeWitt ND. Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol. 2016;17:194–200. doi: 10.1038/nrm.2016.10. [DOI] [PubMed] [Google Scholar]
- 3.Vishwakarma A, Karp JM. Biology and Engineering of Stem Cell Niches. Elsevier Science; 2017. [Google Scholar]
- 4.Bryant SV, Endo T, Gardiner DM. Vertebrate limb regeneration and the origin of limb stem cells. Int J Dev Biol. 2002;46:887–96. [PubMed] [Google Scholar]
- 5•.McCusker C, Gardiner DM, Bryant SV. The Axolotl limb blastema: cellular and molecular mechanisms driving blastema formation and limb regeneration in tetrapods. Regeneration (Oxf) 2015 doi: 10.1002/reg2.32. This review provides the most current overview of the process of limb regeneration based largely on the axolotl regeneration model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.French V, Bryant PJ, Bryant SV. Pattern regulation in epimorphic fields. Science. 1976;193:969–81. doi: 10.1126/science.948762. [DOI] [PubMed] [Google Scholar]
- 7.Bryant SV, French V, Bryant PJ. Distal regeneration and symmetry. Science. 1981;212:993–1002. doi: 10.1126/science.212.4498.993. [DOI] [PubMed] [Google Scholar]
- 8.Putta S, Smith JJ, Walker JA, Rondet M, Weisrock DW, Monaghan J, et al. From biomedicine to natural history research: EST resources for ambystomatid salamanders. BMC Genomics. 2004;5:54. doi: 10.1186/1471-2164-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith JJ, Putta S, Walker JA, Kump DK, Samuels AK, Monaghan JR, et al. Sal-Site: integrating new and existing ambystomatid salamander research and informational resources. BMC Genomics. 2005;6:181. doi: 10.1186/1471-2164-6-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voss SR, Epperlein HH, Tanaka EM. Cold Spring Harb Protoc. Cold Spring Harbor Laboratory Press; 2009. Ambystoma mexicanum, the axolotl: a versatile amphibian model for regeneration, development, and evolution studies. pdb.emo128-8. [DOI] [PubMed] [Google Scholar]
- 11.Voss SR, Palumbo A, Nagarajan R, Gardiner DM, Muneoka K, Stromberg AJ, et al. Gene expression during the first 28 days of axolotl limb regeneration I: Experimental design and global analysis of gene expression. Regeneration (Oxf) 2015;2:120–36. doi: 10.1002/reg2.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu W, Pao GM, Satoh A, Cummings G, Monaghan JR, Harkins TT, et al. Activation of germline-specific genes is required for limb regeneration in the Mexican axolotl. Dev Biol. (2012) 2012;370:42–51. doi: 10.1016/j.ydbio.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monaghan JR, Walker JA, Page RB, Putta S, Beachy CK, Voss SR. Early gene expression during natural spinal cord regeneration in the salamander Ambystoma mexicanum. J Neurochem. 2007;101:27–40. doi: 10.1111/j.1471-4159.2006.04344.x. [DOI] [PubMed] [Google Scholar]
- 14.Monaghan JR, Athippozhy A, Seifert AW, Putta S, Stromberg AJ, Maden M, et al. Biol Open. Vol. 1. The Company of Biologists Ltd; 2012. Gene expression patterns specific to the regenerating limb of the Mexican axolotl; pp. 937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monaghan JR, Epp LG, Putta S, Page RB, Walker JA, Beachy CK, et al. Microarray and cDNA sequence analysis of transcription during nerve-dependent limb regeneration. BMC Biol. 2009;7:1. doi: 10.1186/1741-7007-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endo T, Bryant SV, Gardiner DM. A stepwise model system for limb regeneration. Dev Biol. 2004;270:135–45. doi: 10.1016/j.ydbio.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Satoh A, Gardiner DM, Bryant SV, Endo T. Nerve-induced ectopic limb blastemas in the Axolotl are equivalent to amputation-induced blastemas. Dev Biol. 2007;312:231–44. doi: 10.1016/j.ydbio.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 18.Maden M, Holder N. Axial characteristics of nerve induced supernumerary limbs in the axolotl. Roux's Arch Dev Biol. 1984;193:394–401. doi: 10.1007/BF00848230. [DOI] [PubMed] [Google Scholar]
- 19.Bodemer CW. The development of nerve-induced supernumerary limbs in the adult newt, Triturus viridescens. J Morphol. 1958;102:555–81. [Google Scholar]
- 20.Muller TL, Ngo-Muller V, Reginelli A, Taylor G, Anderson R, Muneoka K. Regeneration in higher vertebrates: limb buds and digit tips. Semin Cell Dev Biol. (1999) 1999;10:405–13. doi: 10.1006/scdb.1999.0327. [DOI] [PubMed] [Google Scholar]
- 21.Wallace H. Vertebrate Limb Regeneration. Chichester: John Wiley and Sons; 1981. [Google Scholar]
- 22.Tassava RA, Olsen CL. Higher vertebrates do not regenerate digits and legs because the wound epidermis is not functional. A hypothesis Differentiation. 1982;22:151–5. doi: 10.1111/j.1432-0436.1982.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 23•.Makanae A, Mitogawa K, Satoh A. Co-operative Bmp- and Fgf-signaling inputs convert skin wound healing to limb formation in urodele amphibians. Developmental Biology. 2014;396:57–66. doi: 10.1016/j.ydbio.2014.09.021. This paper demonstrates that a cocktail of human growth factors can substitute functionally for signaling from a nerve to induce blastema formation and maintain subsequent steps involved in limb regeneration. [DOI] [PubMed] [Google Scholar]
- 24.Makanae A, Mitogawa K, Satoh A. Cooperative inputs of Bmp and Fgf signaling induce tail regeneration in urodele amphibians. Dev Biol. 2016;410:45–55. doi: 10.1016/j.ydbio.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Makanae A, Hirata A, Honjo Y, Mitogawa K, Satoh A. Nerve independent limb induction in axolotls. Developmental Biology. 2013;381:213–26. doi: 10.1016/j.ydbio.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 26••.Phan AQ, Lee J, Oei M, Flath C, Hwe C, Mariano R, et al. Heparan Sulfates Mediate Positional Information by Position-Specific Growth Factor Regulation during Axolotl (Ambystoma mexicanum) Limb Regeneration. Regeneration (Oxf) 2015;2:182–201. doi: 10.1002/reg2.40. This paper demonstrates that bioactive ECM from both axolotl and mouse limb skin can stimulate formation of ectopic limb structures in the axolotl Accessory Limb Model. Characterization of this activity leads to the conclusion that the signaling properties of the ECM are encoded by spatial- and temporal-specific modifications in the patterns of sulfation of glycosaminoglycans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert SF. Developmental Biology. 10. Sunderland, MA: Sinauer Associates, Inc; 2013. [Google Scholar]
- 28.Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841–8. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 29.Sarrazin S, Lamanna WC, Esko JD. Cold Spring Harb Perspect Biol. Vol. 3. Cold Spring Harbor Lab; 2011. Heparan sulfate proteoglycans; pp. a004952–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 31.Seyedhassantehrani N, Li Y, Yao L. Dynamic behaviors of astrocytes in chemically modified fibrin and collagen hydrogels. Integr Biol (Camb) The Royal Society of Chemistry. 2016;8:624–34. doi: 10.1039/c6ib00003g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radice M, Brun P, Cortivo R, Scapinelli R, Battaliard C, Abatangelo G. Hyaluronan-based biopolymers as delivery vehicles for bone-marrow-derived mesenchymal progenitors. J Biomed Mater Res. 2000;50:101–9. doi: 10.1002/(sici)1097-4636(200005)50:2<101::aid-jbm2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 33.Baier Leach J, Bivens KA, Patrick CW, Schmidt CE. Biotechnol Bioeng. Vol. 82. Wiley Subscription Services, Inc., A Wiley Company; 2003. Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds; pp. 578–89. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Kim IS, Cho TH, Lee KB, Hwang SJ, Tae G, et al. Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials. 2007;28:1830–7. doi: 10.1016/j.biomaterials.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 35.Seidlits SK, Khaing ZZ, Petersen RR, Nickels JD, Vanscoy JE, Shear JB, et al. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials. 2010;31:3930–40. doi: 10.1016/j.biomaterials.2010.01.125. [DOI] [PubMed] [Google Scholar]
- 36.Ballios BG, Cooke MJ, van der Kooy D, Shoichet MS. A hydrogel-based stem cell delivery system to treat retinal degenerative diseases. Biomaterials. 2010;31:2555–64. doi: 10.1016/j.biomaterials.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Ha CW, Park YB, Chung JY, Park YG. Stem Cells Transl Med. Vol. 4. AlphaMed Press; 2015. Cartilage Repair Using Composites of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronic Acid Hydrogel in a Minipig Model; pp. 1044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toole BP, Gross J. The extracellular matrix of the regenerating newt limb: synthesis and removal of hyaluronate prior to differentiation. Dev Biol. 1971;25:57–77. doi: 10.1016/0012-1606(71)90019-4. [DOI] [PubMed] [Google Scholar]
- 39.Smith GN, Toole BP, Gross J. Hyaluronidase activity and glycosaminoglycan synthesis in the amputated newt limb: comparison of denervated, nonregenerating limbs with regenerates. Dev Biol. 1975;43:221–32. doi: 10.1016/0012-1606(75)90022-6. [DOI] [PubMed] [Google Scholar]
- 40.Mescher AL, Munaim SI. Anat Rec. Vol. 214. Wiley Subscription Services, Inc., A Wiley Company; 1986. Changes in the extracellular matrix and glycosaminoglycan synthesis during the initiation of regeneration in adult newt forelimbs; pp. 424–31.pp. 394–5. [DOI] [PubMed] [Google Scholar]
- 41.Calve S, Odelberg SJ, Simon HG. A transitional extracellular matrix instructs cell behavior during muscle regeneration. Dev Biol. 2010;344:259–71. doi: 10.1016/j.ydbio.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardiner DM, Muneoka K, Bryant SV. The migration of dermal cells during blastema formation in axolotls. Developmental Biology. 1986;118:488–93. doi: 10.1016/0012-1606(86)90020-5. [DOI] [PubMed] [Google Scholar]
- 43.Satoh A, Graham GM, Bryant SV, Gardiner DM. Neurotrophic regulation of epidermal dedifferentiation during wound healing and limb regeneration in the axolotl (Ambystoma mexicanum) Dev Biol. 2008;319:321–35. doi: 10.1016/j.ydbio.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 44.Satoh A, Mitogawa K, Makanae A. Regeneration inducers in limb regeneration. Dev Growth Differ. 2015;57:421–9. doi: 10.1111/dgd.12230. [DOI] [PubMed] [Google Scholar]
- 45.Bryant SV, Gardiner DM. The relationship between growth and pattern formation. Regeneration. 2016;3:103–122. doi: 10.1002/reg2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]


