Abstract
Background
Among infants exposed to human immunodeficiency virus (HIV) type 1, mixed breastfeeding is associated with higher postnatal HIV-1 transmission than exclusive breastfeeding, but the mechanisms of this differential risk are uncertain.
Methods
HIV-1–exposed Ugandan infants were prospectively assessed during the first year of life for feeding practices and T-cell maturation, intestinal homing (β7hi), activation, and HIV-1 coreceptor (CCR5) expression in peripheral blood. Infants receiving only breast milk and those with introduction of other foods before 6 months were categorized as exclusive and nonexclusive, respectively.
Results
Among CD4+ and CD8+ T cells, the expression of memory, activation, and CCR5 markers increased rapidly from birth to week 2, peaking at week 6, whereas cells expressing the intestinal homing marker increased steadily in the central memory (CM) and effector memory T cells over 48 weeks. At 24 weeks, when feeding practices had diverged, nonexclusively breastfed infants showed increased frequencies and absolute counts of β7hi CM CD4+ and CD8+ T cells, including the HIV-1–targeted cells with CD4+β7hi/CCR5+ coexpression, as well as increased activation.
Conclusions
The T-cell phenotype associated with susceptibility to HIV-1 infection (CCR5+, gut-homing, CM CD4+ T cells) was preferentially expressed in nonexclusively breastfed infants, a group of infants at increased risk for HIV-1 acquisition.
Keywords: HIV-1, breastfeeding, postnatal transmission, CD4+ T lymphocytes, lymphocyte activation, T cell homing, CCR5, α4β7
The World Health Organization recommends breastfeeding for mothers infected with human immunodeficiency virus (HIV) type 1 in resource-limited settings because the associated benefits of lower infant morbidity and mortality rates outweigh the risk of HIV-1 transmission, especially when antiretroviral prophylaxis is given [1, 2]. Although maternal antiretroviral therapy and infant prophylaxis dramatically reduce transmission rates during breastfeeding, breastfeeding continues to contribute to new infections owing to lack of access to care as well as incomplete efficacy of antivirals [3–5]. Therefore, understanding the mechanism of HIV-1 transmission via breastfeeding and factors that may modulate this risk is important for identifying other effective interventions.
Initially counterintuitive and post hoc observations [6], now confirmed [7–9], indicate that exclusive breastfeeding is associated with a 2–4-fold lower rate of postnatal transmission compared with mixed breastfeeding. The pathogenesis underlying the deleterious outcomes of nonexclusive breastfeeding for mother-to-child transmission remains unclear [10, 11]. The differential incidence by feeding practice of mastitis, which is associated with increased HIV-1 in breast milk and transmission, has been proposed, although mastitis may not occur more frequently in mixed breastfeeding [12]. Alternatively, breastfeeding practices may differentially modulate changes in the infant gut mucosa, resulting in altered mucosal and systemic inflammation [13–15]. Such changes might affect the development and activation of the T cells in the intestinal mucosa, cells that may serve as primary targets for HIV-1 infection.
Memory cells expressing CCR5 are prime targets for CCR5-tropic HIV-1 infant founder viruses [16–18]. In addition, gp120 can bind and signal through α4β7 integrin [19], which is found on cells that migrate to the gut [20], thereby enhancing HIV-1 infection of gut-homing cells. Indeed, blocking α4β7 integrin reduced intestinal transmission of simian immunodeficiency virus (SIV) in primate models [21]. In addition, mucosal exposure to certain microbial agents initiates and sustains inflammation and cellular activation [22], and T-cell activation can accentuate susceptibility to HIV-1 infection [23]. The intestinal mucosa supports high numbers of CD4+ T cells [24], including those coexpressing CCR5, which are present even at birth and in early infancy [25]. However, little is known about the ontogeny of gut-homing T cells during infancy, their activation status, or the effects of differential infant feeding practices on T-cell phenotype.
We considered whether the increased incidence of postnatal transmission of HIV-1 among women who practice mixed compared with exclusive breastfeeding could be related to more rapid maturation of infant memory CD4+ T cells, increased expression of the HIV-1 coreceptor CCR5, and increased activation of these cells, particularly among cells that express the intestinal homing integrin, α4β7. We found that infants fed supplemental food showed higher frequencies of gut-homing, CCR5+ memory CD4+ T cells, which are considered to have increased susceptibility for HIV-1 infection.
MATERIALS AND METHODS
Study Participants
This study was approved by the institutional review boards of the University of Colorado, Denver, and Makerere University. We prospectively enrolled 101 HIV-1–infected mothers, with written informed consent, in Kampala, Uganda, and their newborns delivered at Mulago Hospital. Eligibility criteria included maternal intention to breastfeed, maternal CD4+ T-cell count >350/µL at screening, vaginal delivery, birth weight >2500 g, no serious maternal or infant illness at enrollment, and no maternal antibiotics other than cotrimoxazole prophylaxis. Following the standard of care at the time of the study, all mothers received antiretroviral medications for prevention of perinatal transmission including single-dose nevirapine (95%) and additional short-term zidovudine alone or zidovudine with lamivudine for perinatal prevention (99%). The maternal quantitative plasma HIV-1 RNA level was assessed by means of Abbott RealTime HIV-1 RNA polymerase chain reaction (Abbott Laboratories) at Johns Hopkins University and the Roche COBAS AmpliPrep/COBAS Taqman assay version 2 (Roche Molecular Diagnostics) at Makerere University–Johns Hopkins University. All infants received single-dose nevirapine and then either zidovudine for 1–4 weeks (n = 62) or daily nevirapine for the duration of breastfeeding (n = 37). Study visits occurred at birth and at 2, 6, 12, 18, 24, and 48 weeks of life.
Definition of Feeding Groups
All mothers were counseled to practice exclusive breastfeeding at each study visit through infant age 24 weeks but made their own choice of feeding practice, which was assessed at each study visit. After study completion but before analysis, infants were categorized using study definitions for exclusive and nonexclusive breastfeeding. Exclusive breastfeeding was defined as maternal report of giving only breast milk (allowing up to 3 days of water or nonmilk liquids and drops of medications or vitamins) at all visits through week 24. Nonexclusive breastfeeding was defined as having received liquids or solids (with or without continued breast milk) at ≥2 visits between weeks 2 and 24. Infants receiving liquids and/or solids at only 1 visit at or before week 24, or with missing data, were unclassified because their exposure to non–breast milk feeding was limited or uncertain. The unclassified infants were excluded from the comparison across feeding groups but included in analyses of the full cohort.
Flow Cytometry Panels and Monoclonal Antibodies
Peripheral whole blood was collected with ethylenediaminetetraacetic acid anticoagulant and analyzed within 8 hours at the Makerere University Walter Reed Project laboratory. Numbers and percentages of CD4+CD3+and CD8+CD3+ cells from mothers (prenatal screening visit only) and infants (each visit) were determined with a FACSCalibur flow cytometer (BD Biosciences) using single-platform Multitest 4-color reagent with TruCount tubes and MultiSET software (Becton Dickinson).
CD4+ and CD8+ T-lymphocyte maturation, activation, and intestinal homing markers were characterized with 8-color panels. The CD4 panel used the following antibodies: CD4 Pacific Blue (clone S3.5; Molecular Probes); CD45RA PerCP Cy5.5 (clone HI100) and CD38 PeCy7 (clone HIT2; Biolegend); and CD3 Anemonia Majano cyan fluorescent protein (AmCyan) (clone SK7), CCR7 fluorescein isothiocyanate (FITC) (clone 3D12), CCR5 APC-Cy7 (clone 2D7), HLA-DR allophycocyanin (APC) (clone G46-6), β7 integrin phycoerythrin (PE) (clone FIB504) (BD Bioscience). The CD8 panel was identical except for CD8 Pacific Blue (clone 3B5) in place of CD4 and CD62L APC Alexa Fluor 750 (clone DREG-56) (Molecular Probes) in place of CCR5.
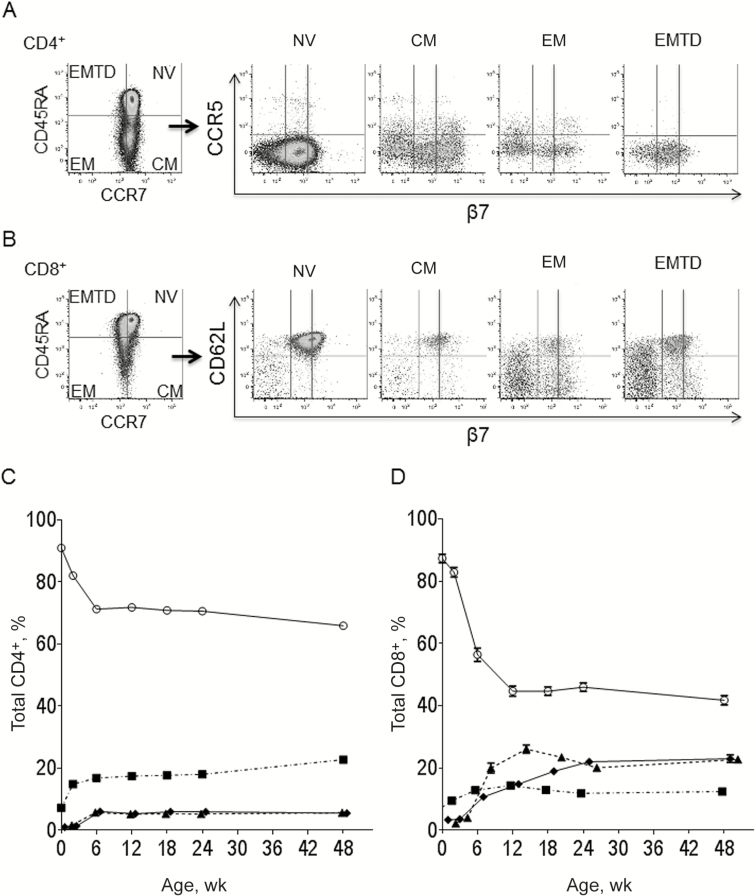
The gating strategy is shown in Figure 1. Memory subsets in the CD4+CD3+ and CD8+CD3+ T cells were classified as naive (CD45RA+/CCR7+), central memory (CM; CD45RA−/CCR7+), effector memory (EM; CD45RA−/CCR7−), and EM terminally differentiated (EMTD; CD45RA+/CCR7−) [26]. Naive and memory subsets were further analyzed for expression of HIV-1 surface coreceptor CCR5, activation (HLA-DR+/CD38+), and β7 integrin, corresponding to the heterodimer α4β7, for which high-level expression is found on cells homing to the intestinal mucosa [20]. Gating controls consisted of frequency minus 1 for CD38, HLA-DR, and CCR5. Data were acquired on a FACSCanto II sytem (BD Biosciences) with daily quality control. Flow data were analyzed with FlowJo software, version 9.6.1 (Tree Star). On average, 270 000 lymphocyte events were analyzed for each sample. For low-frequency subsets, samples with <80 events in the parent subset, resulting in wide confidence intervals for the frequency, were excluded from analysis.
Figure 1.
Gating strategy and maturation phenotype over the first year of life. Lymphocytes were identified using forward and side scatter followed by separation of CD4+CD3+ and CD8+CD3+ cells (not shown). A, B, Maturation phenotype for CD4+ (A) and CD8+ (B) T-cell subsets were identified with CCR7 and CD45RA. Naive cells (NV; CCR7+CD45RA+), central memory (CM; CCR7+CD45RA−), effector memory (EM; CCR7−CD45RA−), and EM terminally differentiated (EMTD; CCR7−CD45RA+). Naive and memory subsets were analyzed for expression of CCR5 (CD4+ T cells), CD62L (CD8+ T cells), and β7 integrin intermediate (β7int) and high (β7hi). Representative sample shown for an infant at 48 weeks of age. C, D, Mean frequency of naive and memory T-cell subsets over the first year of life: CD4+ (C; n = 89, 92, 93, 91, 90, 87, and 79 at weeks 0, 2, 6, 12, 18, 24 and 48, respectively) and CD8+ (D; n = 91, 94, 95, 93, 92, 86, and 78 at weeks 0, 2, 6, 12, 18, 24 and 48, respectively). T-cell subsets include naive (open circles), CM (squares), EM (triangles), and EMTD (diamonds); error bars represent standard errors.
Statistical Analysis
Data were analyzed using SAS 9.4 software. For comparison of demographic and clinical characteristics, Fisher exact tests, t tests, or Wilcoxon rank sum tests were used as appropriate. Paired t tests were used to compare outcomes from the same subjects between 2 time points,. Wilcoxon rank sum tests were used for comparisons by feeding practice. For the multivariate analysis, we modeled the absolute cell count of each subset using negative binomial regression and tested the difference between the feeding groups while adjusting for maternal CD4+ T-cell count, infant weight z score at week 24, and the number of gastrointestinal (GI) illnesses occurring from birth to week 24.
RESULTS
Maternal and Infant Clinical Characteristics
A total of 101 mothers and their singlet infants were enrolled. One infant was HIV-1 infected at birth and was excluded from the analysis. The majority of infants were exclusively breastfed through week 12 (99%, 95%, and 91% at weeks 2, 6, and 12, respectively). Feeding practices diverged by week 18, with a decrease in exclusively breastfed infants to 80% and 63% at weeks 18 and 24, respectively. Supplementary food was introduced for all infants after week 24. Approximately half of the infants (n = 49) met criteria for exclusive breastfeeding, and 19 met criteria for nonexclusive breastfeeding. The remaining infants (n = 32) did not meet either criteria (received non–breast milk food on a single study visit, n = 24; missing data, n = 8).
Maternal and infant clinical characteristics are summarized in Table 1. Among maternal characteristics, only CD4+ T-cell counts differed between feeding groups and were higher in the exclusively breastfeeding mothers. No women reported symptomatic mastitis throughout the first 24 weeks. Infants’ nutritional status was comparable between groups at birth and week 48, but weight, weight z score, and body mass index were lower in the nonexclusive breastfeeders at week 24. The nonexclusively breastfeeding infants had more GI illnesses.
Table 1.
Maternal and Infant Characteristics by Feeding Practicea
| Characteristic | Complete Cohort (n = 99–100) |
Exclusive Breastfeedingb (n = 47–49) |
Nonexclusive Breastfeedingb (n = 18–19) |
P Valuec |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Age, mean (range), y | 24.6 (18–40) | 24.7 (18–36) | 26.3 (18–40) | .23 |
| CD4+ T-cell count, mean (range), cells/μL | 657 (354–2095) | 710 (362–2095) | 532 (367–798) | .002 |
| HIV-1 plasma RNA, mean (range), copies/mL |
12 930 (20-408 901) |
7364 (20-73 931) |
11 198 (20-60 990) |
.34 |
| Infant characteristics | ||||
| Male sex, No. (%) | 48 (48) | 22 (45) | 9 (47) | >.99d |
| Weight, mean (range), z score | ||||
| Birth | –0.44 (−1.7 to 1.6) | −0.38 (−1.7 to 1.6) | −0.42 (−1.5 to 0.5) | .83 |
| Age 24 wk | −0.14 (−3.1 to 2.7) | 0.06 (−2.0 to 2.7) | −0.77 (−3.1 to 0.6) | .004 |
| Age 48 wk | −0.44 (−3.5 to 2.0) | −0.46 (−3.5 to 2.0) | −0.78 (−3.5 to 0.6) | .25 |
| BCG vaccine at birth, No. (%) | 86 (97) | 41 (95) | 17 (94) | >.99d |
| GI illnesses between 2 and 24 wk, mean (range), No. | 0.22 (0–2) | 0.08 (0–1) | 0.47 (0–2) | .004e |
Abbreviations: GI, gastrointestinal; HIV-1, human immunodeficiency virus type 1.
aMaternal and infant characteristics that did not differ between feeding practices also included maternal World Health Organization stage, frequency of maternal CD4+ T-cell counts >500/μL or <500 cells/μL, receipt of maternal antiretrovirals for treatment, and proportion of women and infants receiving cotrimoxazole prophylaxis. Corresponding to the differences in infant weight z score, infant weight (7.5 vs 6.9 kg; P = .009), and body mass index (18.7 vs 17.2; P = .007) were higher in the exclusive-breastfeeding than in the nonexclusive-breastfeeding group at 24 weeks but did not differ at birth or week 48. Maternal characteristics were determined at enrollment during pregnancy, unless otherwise specified. Infant were characteristics determined at birth or at the specified age.
bFeeding groups were determined by breastfeeding practice, as defined in Materials and Methods.
cDifferences between exclusive- and nonexclusive-breastfeeding groups were determined with t tests unless otherwise noted.
dDetermined with Fisher exact test.
eDetermined with Wilcoxon rank sum test.
Early Infancy Increases in Memory, CCR5-Expressing, and Activated T Cells
The frequency (Figure 1) and absolute counts (Supplemental Figure 1) of memory T-cell phenotypes were determined for the whole cohort during the first year of life. As expected, the majority of T cells were naive at birth; however, memory cells already represented 9.0% and 12.7% of the CD4+ and CD8+ T cells, respectively. Increases in the frequency of memory CD4+ and CD8+ T cells occurred rapidly by 2 weeks of life (Figure 1). CM CD4+ T cells doubled between birth and week 2 (P < .001) and plateaued by week 6 (Figure 1C). Changes thereafter were modest. Increases in the frequency of the more differentiated EM and EMTD CD4+ T cells were delayed until week 6 (week 0 vs week 6: EM, 0.7% vs 5.8%; EMTD, 1.0% vs 6.1%; both P < .001). Early maturation of CD8+ T cells was more robust than that of CD4+ T cells (Figure 1D). At age 12 weeks, 55.3% of CD8+ T cells versus 28.1% of CD4+ T cells (P < .001) had a memory phenotype. In contrast to the predominance of CM in the CD4+ T memory cell population, CD8+ memory T cells comprised similar proportions of the CM, EM, and EMTD cells. The frequency of CM and EM CD4+ T cells expressing CCR5 in peripheral blood at birth increased early, peaking at 6 weeks (Figure 2A). Expression of CCR5 was restricted primarily to CM and EM and was present on only a minor fraction of naive and EMTD CD4+ T cells.
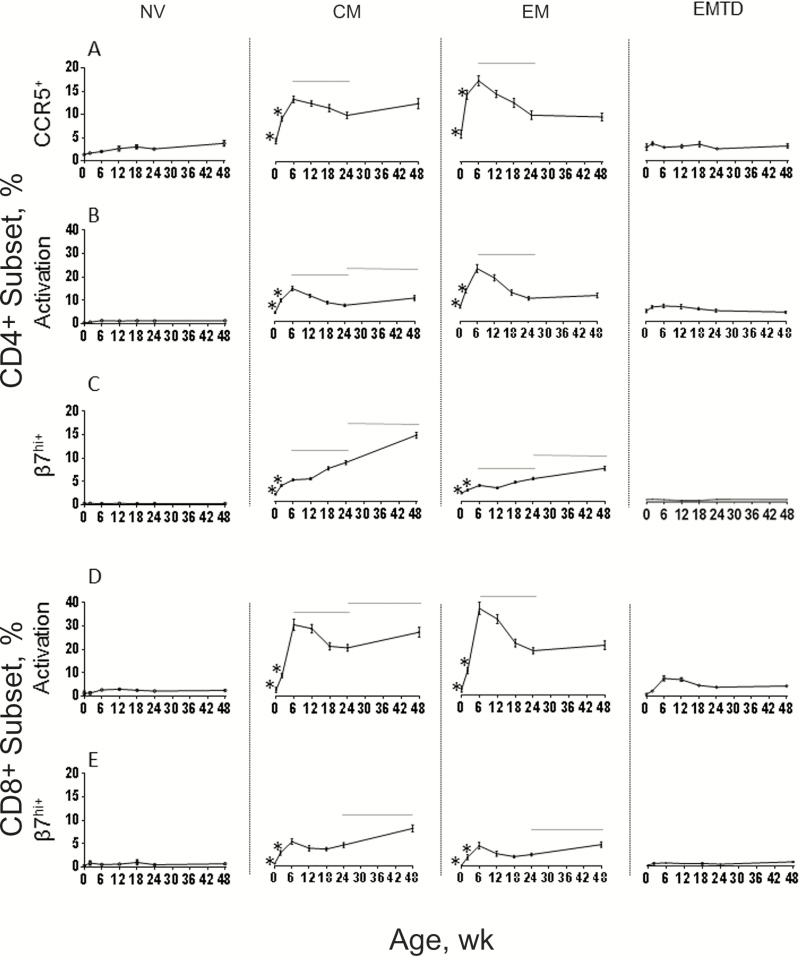
Figure 2.
Mean frequencies of CD4+ and CD8+ T cells expressing CCR5 (A), dual activation makers CD38+/HLA-DR+ (CD4+ in B and CD8+ in D), and β7hi (CD4+ in C and CD8+ in E) during the first year of life within the naive, central memory (CM), effector memory (EM), and EM terminally differentiated (EMTD) subsets. Error bars represent standard errors. *P < .05 for week 0 vs all later weeks and for week 2 vs week 6; horizontal line represents P < .05 for week 6 vs week 24 and week 24 vs week 48 (Wilcoxon rank sum test).
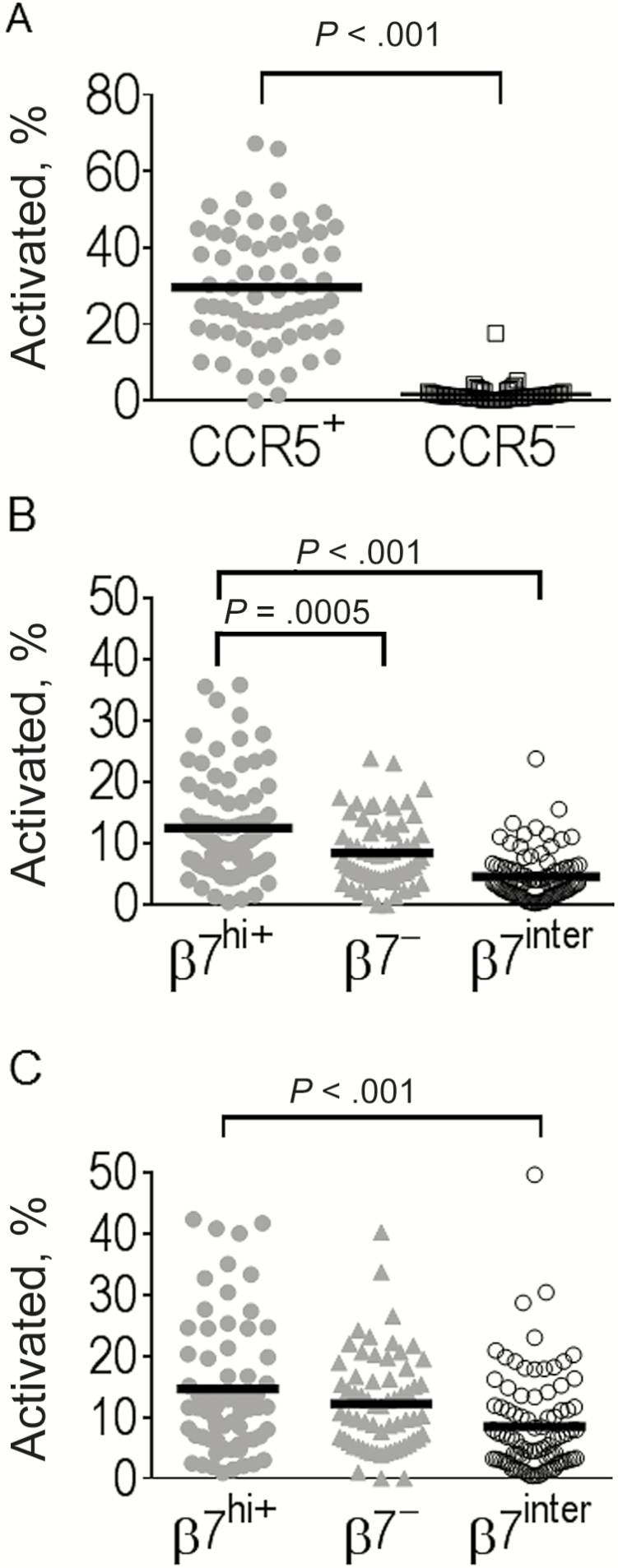
The changes in the frequency of activated cells followed a pattern similar to the changes in CCR5 expression, with an early peak at 6 weeks followed by a decline between weeks 6 to 24 (Figure 2B and 2D). Activation was more pronounced on CD8+ than on CD4+ T cells (percentage activated for total CD4+ vs CD8+ T cells at week 6, 17.7% vs 3.17%; P < .001). The mean frequency of activated memory CD8+ T cells at week 6 remarkably reached peaks of 30.0% and 36.4% of CM and EM CD8+ T cells, respectively. Among the CD4+ T cells, activation was targeted almost exclusively within the CCR5-expressing cells (Figure 3A).
Figure 3.
Activation of CD4+ T-cell subsets by CCR5 and β7 expression, showing the percentage of activated (CD38+/HLA-DR+) cells among CD4+ T cells with (gray circles) or without (open squares) CCR5 expression (A) and for CD4+T cells with high (β7hi+; gray circles), low (β7−; gray triangles), or intermediate (β7int; open circles) expression of the β7 among central memory (B) and effector memory (C) subsets. Bars represent mean values. Representative data are shown for week 6. Comparisons were performed with Wilcoxon rank sum tests.
Kinetics of the Development of Gut-Homing Marker α4β7-Integrin Expression
T cells with high-level expression of β7, a component of the heterodimer α4β7, a cellular adhesion integrin that directs circulating lymphocytes to the gut [20] ,were rare at birth in both naive and memory CD4+ and CD8+ T cells (Figure 2C and 2E). The frequencies of β7hi CD4+ and CD8+ T cells increased progressively during the first year of life in CM and EM cells but were rare in naive or EMTD subsets. The β7hi cells had a greater proportion of activated cells compared with the β7int cells throughout the first year of life (data shown for week 6 in Figure 3B and 3C).
Comparison of T-Cell Subsets by Feeding Practice
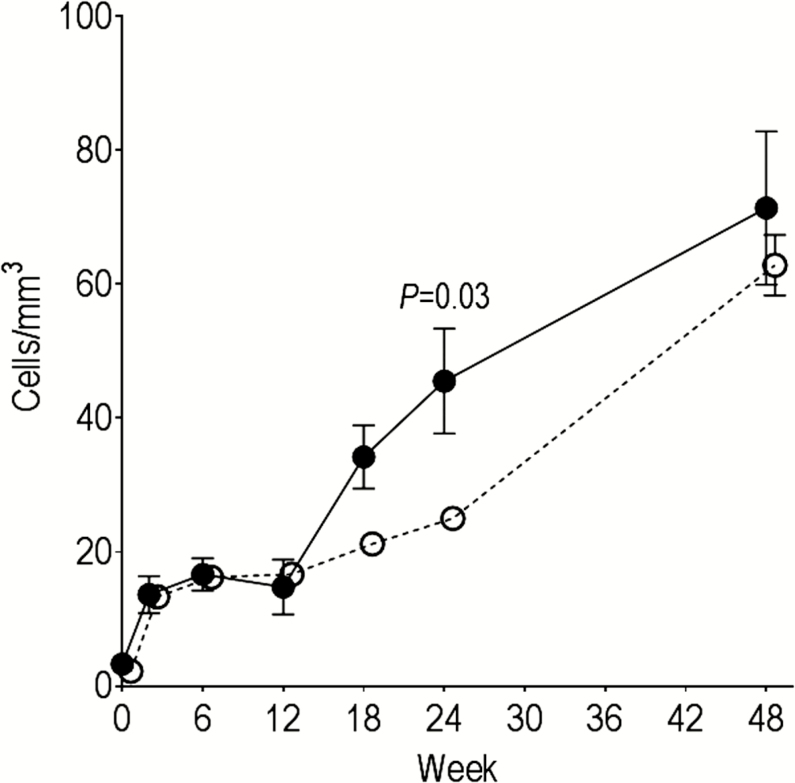
To determine the impact of feeding practices on T-cell development and activation, we compared the absolute count and frequency of T-cell subsets between exclusive- and nonexclusive-breastfeeding groups at week 24, when the feeding practices had diverged (Table 2 and Supplemental Table 1). Nonexclusively breastfeeding infants had higher frequency and absolute counts for β7hi CM CD4+ and CD8+ T cells than exclusively breastfeeding infants. Moreover, CM CD4+ T cells coexpressing both β7hi and CCR5, a phenotypic signature associated with increased susceptibility for HIV-1 infection [27, 28], were enriched in the nonexclusively breastfed group (P = .004). The difference in β7hi CM CD4+ and CD8+ T-cell subsets, including the CCR5+ subset, remained statistically significant (P < .04) when adjusted for maternal CD4+ T-cell count, infant weight z score at week 24, and the average number of GI illnesses between birth and week 24. For CD4+ T cells, the difference by feeding practice in the frequency and count of memory cells was restricted to the gut-homing subset, whereas for CD8+ T cells, both gut-homing and total memory subsets were higher in the nonexclusive breastfeeders. The number and frequency of gut-homing memory cells converged for nonexclusive and exclusive breastfeeders by week 48 when all infants were receiving supplemental food (Figure 4). In addition to the more rapid appearance of gut-homing memory cells, the nonexclusively breastfeeding infants had higher activation among the β7hi CM CD4+ T cells (P = .003), a difference that remained statistically significant in the multivariate analysis (P = .003). In the CD8+ T cells, the nonexclusively breastfeeding infants had increased activation in multiple total CD8+ memory T-cell subsets as well as the β7hi subsets (Table 3).
Table 2.
Absolute Counts of T-Cell Subsets by Breastfeeding Practice at Age 24 Weeksa
| Subset | CD4+ T Cells | CD8+ T Cells | ||||
|---|---|---|---|---|---|---|
| Cell Count, Mean (SD), Cells/μL | P Valuec | Cell Count, Mean (SD), Cells/μL | ||||
| Exclusive Breastfeeding | Nonexclusive Breastfeeding | Exclusive Breastfeeding | Nonexclusive Breastfeeding | P Valueb | ||
| Total | 2056 (728) | 2362 (1025) | .27 | 1084 (485) | 1341 (480) | .01c |
| CM | 348 (114) | 429 (198) | .19 | 111 (90) | 153 (92) | .07 |
| EM | 105 (65) | 106 (59) | .95 | 171 (108) | 292 (115) | .001c |
| EMTD | 125 (106) | 130 (121) | .85 | 228 (159) | 309 (151) | .04c |
| CM CCR5+ | 30 (17) | 51 (39) | .04c | ND | ND | … |
| EM CCR5+ | 10 (10) | 11 (12) | .97 | ND | ND | … |
| CM β7hi | 25 (11) | 46 (34) | .03c | 4 (4) | 11 (12) | .001c |
| EM β7hi | 5 (4) | 5 (5) | .88 | 4 (4) | 9 (7) | .001c |
| CM β7hi CCR5+ | 3 (3) | 10 (11) | .004c | ND | ND | … |
| EM β7hi CCR5+ | NAd | NAd | … | ND | ND | … |
Abbreviations: CM, central memory; EM, effector memory; EMTD, EM terminally differentiated; NA, not assessed; ND, not done; SD, standard deviation.
aExclusive- and nonexclusive-breastfeeding groups determined by breastfeeding practice as defined in Methods. Absolute counts of designated CD4+ or CD8+ T-cell subsets at week 24 were determined with flow cytometry.
bComparison of feeding groups by means of Wilcoxon rank sum test.
cSignificant at P < .05.
dThere were too few infants (n = 5) with sufficient cells in this low-frequency subset to assess differences.
Figure 4.
Change in β7hi central memory (CM) CD4+ T cells during the first year of life by feeding practice. The mean absolute count of β7hi CM CD4+ T cells for exclusively (open circles) and nonexclusively (closed circles) breastfeeding infants was determined through week 48. Error bars represent standard errors. Comparisons at 24 weeks were performed with Wilcoxon rank sum tests.
Table 3.
Absolute Counts of Activated Cells by Breastfeeding Practice at Age 24 Weeksa
| T-Cell Subset | CD4+ T cells | CD8+ T cells | P Valueb | |||
|---|---|---|---|---|---|---|
| Activated Cell Count, Mean (SD), Cells/μL | P Valueb | Activated Cell Count, Mean (SD), Cells/μL | ||||
| Exclusive Breastfeeding | Nonexclusive Breastfeeding | Exclusive Breastfeeding | Nonexclusive Breastfeeding | |||
| CM | 10.76 (8) | 20.14 (21) | .03c | 20.28 (26) | 50.13 (55) | <.001c |
| EM | 4.51 (4) | 6.84 (7) | .36 | 30.05 (33) | 71.38 (38) | <.001c |
| EMTD | 1.81 (2) | 2.81 (5) | .54 | 7.12 (8) | 18.00 (12) | <.001c |
| CM CCR5+ | 3.82 (3) | 7.61 (6) | .03c | ND | ND | … |
| EM CCR5+ | 1.26 (1) | 2.30 (2) | .09 | ND | ND | … |
| CM β7hi | 1.58 (1) | 3.70 (3) | .003c | 2.46 (3) | 8.29 (11) | .03c |
| EM β7hi | 0.37 (.28) | 0.58 (.47) | .06 | 2.08 (3) | 4.59 (4) | .02c |
| CM β7hi CCR5+ | 0.71 (1) | 1.94 (2) | .001c | ND | ND | … |
| EM β7hi CCR5+ | NAd | NAd | … | ND | ND | … |
Abbreviations: CM, central memory; EM, effector memory; EMTD, EM terminally differentiated; NA, not assessed; ND, not done; SD, standard deviation.
aExclusive and nonexclusive feeding groups were determined by breastfeeding practice as defined in Materials and Methods. The absolute count of activated cells in designated CD4+ or CD8+ T-cell subsets at week 24 were determined with flow cytometry.
bComparison of feeding groups by means of Wilcoxon rank sum test.
cSignificant at P < .05.
dThere were too few infants (n = 5) with sufficient cells in this low-frequency subset to assess differences.
DISCUSSION
We investigated a potential immunologic basis underlying the increased risk of HIV-1 transmission to infants who receive breast milk mixed with other foods, compared with those who receive only breast milk. Previous work has evaluated subclinical mastitis and breast milk HIV-1 RNA as predisposing factors for this increased incidence [12, 29, 30]. However, ours is the first study to prospectively characterize the maturation, activation, and homing signature of CD4+ T cells, a principle target HIV-1 infection, by feeding practices in a high-risk population. Infants receiving non–human milk food showed higher CD4+ T cells of the CM phenotype expressing the gut-homing marker α4β7 and CCR5, 2 phenotypic markers associated with increased HIV-1 susceptibility. In addition, CD8+ T cells with a memory phenotype were more prevalent, as were activated cells.
The vast majority of infant founder viruses are CCR5 tropic [16–18], and α4β7 integrin increases CD4+ T-cell susceptibility to HIV-1 infection [19, 27, 28, 31]. Thus, the increase in CCR5-expressing memory CD4+ T cells homing to the gut in the nonexclusively breastfeeding infants may provide enhanced targets for HIV-1 infection in the mucosa. A higher frequency of memory CD4+ and CD8+ T cells in cord blood is associated with an increased risk of early HIV-1 transmission (at age ≤1 mo) [32], indicating that differences in circulating T-cell phenotype can indicate differential risk of perinatal HIV-1 acquisition. The level of CCR5 expression is implicated in perinatal SIV transmission [23], as well as several modes of HIV-1 transmission [23]. Administering α4β7 monoclonal antibody provides significant protection against intravaginal SIV challenge of rhesus macaques, indicating a role for α4β7 in facilitating infection [21]. In our study, receiving non–human milk food was associated with an increase in CD4+ T cells with a phenotype that facilitates primary infection, which may contribute to higher rates of HIV-1 acquisition.
Our data suggest that early exposure to non–breast milk food leads to earlier and preferential T-cell maturation and activation in cells homing to the intestinal compartment. The potential mechanisms by which breastfeeding practice may modify T-cell phenotype are many but uncertain. GI illnesses could potentially alter mucosal T-cell populations, and, as in other cohorts, the nonexclusively breastfeeding infants did have a higher frequency of GI illnesses [33, 34], but the differences in T-cell subsets remained significant when adjusted for GI illness. Differences in the intestinal microbiome in HIV-1–infected adults are associated with immune activation [22], and breastfeeding practices clearly modify the intestinal microbiome [35, 36], including among HIV-1–exposed infants [37]. Such differences may affect mucosal inflammation, epithelial cell integrity, gut permeability, and the nutrients available to infants [38, 39]. Indeed, we have identified differences in both the microbial diversity of these exclusively and nonexclusively breastfeeding infants (E. N. Janoff, unpublished data), results that, like those in T-cell phenotype, are time dependent, diverge at 18–24 weeks when the feeding practice differed most between groups, but are not differentiated at 48 weeks, when virtually all infants are no longer breastfeeding.
T-cell dynamics began very soon after birth in both feeding groups, when some memory T cells were already present in the circulation [40]. Intestinal T cells bearing CCR5 are present in newborn gut mucosa from the earliest days [25]. The increase in memory T cells was rapid during the first 2–6 weeks after birth but then remained relatively stable during the first year. T-cell activation also increased rapidly during this period. Our data delineate the kinetics of the elevated CCR5 expression previously found on memory CD4+ T cells in HIV-1–exposed infants [41]. The early increases and subsequent declines in activation and CCR5 expression suggest environmental pressure early in life. In a similar HIV-1–unexposed breastfed cohort of infants in the United States, rates of activation were lower (<10% of CD4+ and CD8+ memory T cells; authors’ unpublished data) than in the Ugandan infants. The African children did receive BCG vaccine at birth, which may alter immune ontogeny [42]. Cytomegalovirus infection might be another cause of T-cell activation during early infancy, in that 20% of HIV-1–exposed uninfected infants acquire cytomegalovirus infection by 1 month of age [43, 44]. Early T-cell maturation and altered responses to vaccination are observed in HIV-1–exposed but uninfected infants compared with unexposed infants [45–48]. We did not include HIV-1–unexposed infants, so we are unable to compare them directly, but our study provides a more detailed picture of T-cell maturation in HIV-1–exposed infants than prior studies.
In particular, our results provide a unique and more detailed ontogeny of gut-homing T cells in the newborn than has been previously available. Almost all newborn naive cells expressed intermediate levels of β7, as described elsewhere [20]. Unlike CCR5 findings, the increase in the proportion of cells with high-level β7 was more gradual and constant though the first year of life, perhaps indicating ongoing, progressive maturation of T cells in the gut mucosa. Expression of β7hi was restricted to CM and EM T cells but negligible on EMTD cells. Because the EMTD subset is farther on the differentiation pathway, one might expect a similar fraction to have matured in the gut. Perhaps on homing to the gut, these cells are retained and are less likely to circulate.
The comparisons in this study have limitations. First, the groups were not randomized, because this would have been unethical with the knowledge that exclusive breastfeeding is protective against HIV-1 transmission to the infant. Therefore, it is possible that feeding practice may be a marker of other factors not directly assessed in this study. Nevertheless, the differences in CD4+memory subsets remained significant after adjustment for differences in maternal CD4+ T cell counts, infant weight, and rates of GI illnesses. Despite our initial study projections, the majority of women practiced exclusive breastfeeding for 6 months—a testimony to the counseling provided but resulting in unbalanced numbers in the groups and a relatively small group receiving non–human milk feeding before 24 weeks. Owing to the delayed introduction of supplementary food, feeding patterns did not diverge until 18–24 weeks of age. Despite this imbalance, time-dependent and significant differences emerged. Few women chose mixed feeding for a prolonged time, and most weaned soon after introducing other foods. Therefore, we are unable to know whether the alterations we observed are specific to exposure to other food versus the absence of breast milk. Finally, for ethical and practical reasons, we sampled the peripheral blood rather than primary intestinal tissues over time. However, the frequency of circulating β7hi CD4+ cells correlates with CD4+ cells in the gut [49].
In summary, in the context of clinical and epidemiologic evidence that exclusive breastfeeding is associated with a lower rate of postnatal transmission of HIV-1, we found significant differences in maturation and activation among the gut-homing T cells in infants exposed to exclusive versus nonexclusive breastfeeding. The prominence of a gut-homing phenotype in CD4+ T memory cells coexpressing CCR5, identified in the nonexclusive-breastfeeding group, may enhance susceptibility of the mixed fed infant to HIV-1 infection. Because the effects of breastfeeding practice probably derived from conditions in the gut, ongoing studies are directed to determine whether local inflammation or permeability, microbiome diversity and constituents, and nutritional factors in the infants contribute to the described differences in T cells. The current study of putative gut-homing cells in the circulation is an indirect but accessible approach to linking events in the intestine with the T cells in blood. A case-control study embedded in a larger program of an intervention to prevent breast milk transmission could be used to determine whether increased numbers of these susceptible HIV-1 target cells (activated memory phenotype α4β7hi and coexpressing CCR5) are a risk factor for transmission. Even in the absence of further knowledge, our data provide additional support for the recommendation for exclusive breastfeeding for the first 6 months.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank Paul A. Harding for technical support, the dedicated staff at the Johns Hopkins–Makerere University Program, the staff of the Mulago Hospital maternal and infant clinics for their support, and the participants for their commitment to advancing research in prevention and care of HIV-1–infected women and their children.
Financial support. This work was supported by the National Institute of Health (grants R21AI083615, R01AI097265, and R01 HD059527), the National Center for Advancing Translational Sciences/National Institute of Health (Colorado Clinical and Translational Science awardUL1 TR001082), the Elizabeth Glaser Pediatric AIDS Foundation, the University of Colorado Department of Medicine Medical Student Research Track program, and the Mucosal and Vaccine Research Program Colorado.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army, the Department of Defense, or the National Institutes of Health.
Footnotes
Presented in part: Conference on Retroviral Infections and Opportunistic Infections, Boston, Massachusetts, 3–6 March 2014. Abstract 561.
References
- 1. World Health Organization, United Nations Children’s Fund. Guideline: updates on HIV and infant feeding: the duration of breastfeeding, and support from health services to improve feeding practices among mothers living with HIV. Geneva: World Health Organization, 2016. http://www.who.int/maternal_child_adolescent/documents/hiv-infant-feeding-2016/en/. Accessed 30 January 2017. [PubMed] [Google Scholar]
- 2. Nagot N, Kankasa C, Tumwine JK, et al. ; ANRS 12174 Trial Group Extended pre-exposure prophylaxis with lopinavir-ritonavir versus lamivudine to prevent HIV-1 transmission through breastfeeding up to 50 weeks in infants in Africa (ANRS 12174): a randomised controlled trial. Lancet 2016; 387:566–73. [DOI] [PubMed] [Google Scholar]
- 3. Luzuriaga K, Mofenson LM. Challenges in the elimination of pediatric HIV-1 Infection. N Engl J Med 2016; 374:761–70. [DOI] [PubMed] [Google Scholar]
- 4. Aizire J, Fowler MG, Coovadia HM. Operational issues and barriers to implementation of prevention of mother-to-child transmission of HIV (PMTCT) interventions in Sub-Saharan Africa. Curr HIV Res 2013; 11:144–59. [DOI] [PubMed] [Google Scholar]
- 5. Fouda GG, Cunningham CK, Permar SR. Infant HIV-1 vaccines: supplementing strategies to reduce maternal-child transmission. JAMA 2015; 313:1513–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia HM. Influence of infant-feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South Africa: a prospective cohort study. South African vitamin A study group. Lancet 1999; 354:471–6. [DOI] [PubMed] [Google Scholar]
- 7. Iliff PJ, Piwoz EG, Tavengwa NV, et al. ; ZVITAMBO Study Group Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS 2005; 19:699–708. [DOI] [PubMed] [Google Scholar]
- 8. Coovadia HM, Rollins NC, Bland RM, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet 2007; 369:1107–16. [DOI] [PubMed] [Google Scholar]
- 9. Kuhn L, Sinkala M, Kankasa C, et al. High uptake of exclusive breastfeeding and reduced early post-natal HIV transmission. PLoS One 2007; 2:e1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuhn L. Milk mysteries: Why are women who exclusively breast-feed less likely to transmit HIV during breast-feeding? Clin Infect Dis 2010; 50:770–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rollins N, Coovadia HM. Breastfeeding and HIV transmission in the developing world: past, present, future. Curr Opin HIV AIDS 2013; 8:467–73. [DOI] [PubMed] [Google Scholar]
- 12. Lunney KM, Iliff P, Mutasa K, et al. Associations between breast milk viral load, mastitis, exclusive breast-feeding, and postnatal transmission of HIV. Clin Infect Dis 2010; 50:762–9. [DOI] [PubMed] [Google Scholar]
- 13. Hanson LA. Session 1: feeding and infant development breast-feeding and immune function. Proc Nutr Soc 2007; 66:384–96. [DOI] [PubMed] [Google Scholar]
- 14. Walker WA, Iyengar RS. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr Res 2015; 77:220–8. [DOI] [PubMed] [Google Scholar]
- 15. Turfkruyer M, Verhasselt V. Breast milk and its impact on maturation of the neonatal immune system. Curr Opin Infect Dis 2015; 28:199–206. [DOI] [PubMed] [Google Scholar]
- 16. Kishko M, Somasundaran M, Brewster F, Sullivan JL, Clapham PR, Luzuriaga K. Genotypic and functional properties of early infant HIV-1 envelopes. Retrovirology 2011; 8:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rainwater SM, Wu X, Nduati R, et al. Cloning and characterization of functional subtype A HIV-1 envelope variants transmitted through breastfeeding. Curr HIV Res 2007; 5:189–97. [DOI] [PubMed] [Google Scholar]
- 18. Church JD, Huang W, Mwatha A, et al. Analysis of HIV tropism in Ugandan infants. Curr HIV Res 2010; 8:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arthos J, Cicala C, Martinelli E, et al. HIV-1 envelope protein binds to and signals through integrin α4β7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol 2008; 9:301–9. [DOI] [PubMed] [Google Scholar]
- 20. Johansson-Lindbom B, Agace WW. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol Rev 2007; 215:226–42. [DOI] [PubMed] [Google Scholar]
- 21. Byrareddy SN, Kallam B, Arthos J, et al. Targeting α4β7 integrin reduces mucosal transmission of simian immunodeficiency virus and protects gut-associated lymphoid tissue from infection. Nat Med 2014; 20:1397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dillon SM, Lee EJ, Kotter CV, et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol 2014; 7:983–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McKinnon LR, Kaul R. Quality and quantity: mucosal CD4+ T cells and HIV susceptibility. Curr Opin HIV AIDS 2012; 7:195–202. [DOI] [PubMed] [Google Scholar]
- 24. Gustafson CE, Higbee D, Yeckes AR, et al. Limited expression of APRIL and its receptors prior to intestinal IgA plasma cell development during human infancy. Mucosal Immunol 2014; 7:467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bunders MJ, van der Loos CM, Klarenbeek PL, et al. Memory CD4+CCR5+ T cells are abundantly present in the gut of newborn infants to facilitate mother-to-child transmission of HIV-1. Blood 2012; 120:4383–90. [DOI] [PubMed] [Google Scholar]
- 26. Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A 2008; 73:975–83. [DOI] [PubMed] [Google Scholar]
- 27. Cicala C, Martinelli E, McNally JP, et al. The integrin α4β7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc Natl Acad Sci U S A 2009; 106:20877–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ding J, Tasker C, Lespinasse P, et al. Integrin α4β7 expression increases HIV susceptibility in activated cervical CD4+ T cells by an HIV attachment-independent mechanism. J Acquir Immune Defic Syndr 2015; 69:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Semrau K, Kuhn L, Brooks DR, et al. Exclusive breastfeeding, maternal HIV disease, and the risk of clinical breast pathology in HIV-infected, breastfeeding women. Am J Obstet Gynecol 2011; 205:344 e1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rossenkhan R, Novitsky V, Sebunya TK, et al. Infant feeding practices were not associated with breast milk HIV-1 RNA levels in a randomized clinical trial in Botswana. AIDS Behav 2012; 16:1260–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kader M, Wang X, Piatak M, et al. α4+β7hiCD4+ memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol 2009; 2:439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gasper MA, Kunwar P, Itaya G, et al. Natural killer cell and T-cell subset distributions and activation influence susceptibility to perinatal HIV-1 infection. AIDS 2014; 28:1115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Victora CG, Smith PG, Vaughan JP, et al. Evidence for protection by breast-feeding against infant deaths from infectious diseases in Brazil. Lancet 1987; 2:319–22. [DOI] [PubMed] [Google Scholar]
- 34. Fawzy A, Arpadi S, Kankasa C, et al. Early weaning increases diarrhea morbidity and mortality among uninfected children born to HIV-infected mothers in Zambia. J Infect Dis 2011; 203:1222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Madan JC, Hoen AG, Lundgren SN, et al. Association of cesarean delivery and formula supplementation with the intestinal microbiome of 6-week-old infants. JAMA Pediatr 2016; 170:212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O’Sullivan A, Farver M, Smilowitz JT. The influence of early infant-feeding practices on the intestinal microbiome and body composition in infants. Nutr Metab Insights 2015; 8(suppl 1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. González R, Maldonado A, Martín V, et al. Breast milk and gut microbiota in African mothers and infants from an area of high HIV prevalence. PLoS One 2013; 8:e80299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hinde K, Lewis ZT. Mother’s littlest helpers. Science 2015; 348:1427–8. [DOI] [PubMed] [Google Scholar]
- 39. Le Huërou-Luron I, Blat S, Boudry G. Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev 2010; 23:23–36. [DOI] [PubMed] [Google Scholar]
- 40. Zhang X, Mozeleski B, Lemoine S, et al. CD4 T cells with effector memory phenotype and function develop in the sterile environment of the fetus. Science Transl Med 2014; 6:238ra72. [DOI] [PubMed] [Google Scholar]
- 41. Tuttle DL, Coberley CR, Xie X, Kou ZC, Sleasman JW, Goodenow MM. Effects of human immunodeficiency virus type 1 infection on CCR5 and CXCR4 coreceptor expression on CD4 T lymphocyte subsets in infants and adolescents. AIDS Res Hum Retroviruses 2004; 20:305–13. [DOI] [PubMed] [Google Scholar]
- 42. Hanekom WA. The immune response to BCG vaccination of newborns. Ann N Y Acad Sci 2005; 1062:69–78. [DOI] [PubMed] [Google Scholar]
- 43. Slyker JA, Lohman-Payne BL, John-Stewart GC, et al. Acute cytomegalovirus infection in Kenyan HIV-infected infants. AIDS 2009; 23:2173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Slyker JA, Rowland-Jones SL, Dong T, et al. Acute cytomegalovirus infection is associated with increased frequencies of activated and apoptosis-vulnerable T cells in HIV-1-infected infants. J Virol 2012; 86:11373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pfeifer C, Bunders MJ. Maternal HIV infection alters the immune balance in the mother and fetus; implications for pregnancy outcome and infant health. Curr Opin HIV AIDS 2016; 11:138–45. [DOI] [PubMed] [Google Scholar]
- 46. Bunders MJ, van Hamme JL, Jansen MH, Boer K, Kootstra NA, Kuijpers TW. Fetal exposure to HIV-1 alters chemokine receptor expression by CD4+T cells and increases susceptibility to HIV-1. Sci Rep 2014; 4:6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kidzeru EB, Hesseling AC, Passmore JA, et al. In-utero exposure to maternal HIV infection alters T-cell immune responses to vaccination in HIV-uninfected infants. AIDS 2014; 28:1421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ono E, Nunes dos Santos AM, de Menezes Succi RC, et al. Imbalance of naive and memory T lymphocytes with sustained high cellular activation during the first year of life from uninfected children born to HIV-1-infected mothers on HAART. Braz J Med Biol Res 2008; 41:700–8. [DOI] [PubMed] [Google Scholar]
- 49. Wang X, Xu H, Gill AF, et al. Monitoring α4β7 integrin expression on circulating CD4+ T cells as a surrogate marker for tracking intestinal CD4+ T-cell loss in SIV infection. Mucosal Immunol 2009; 2:518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.