Abstract
Background
Vitamin D and calcium are essential nutrients for bone health. In addition, vitamin D suppresses inflammatory cytokines and increases bone resorption. Therefore, improvements in bone health by calcium and vitamin D supplementation have the potential to not only improve calcium metabolism but also suppress inflammation associated with exercise training. The purpose of this study was to determine whether ongoing vitamin D supplementation and low-fat milk intake by female high-school endurance runners would improve bone metabolism by suppressing inflammatory cytokines and the parathyroid hormone (PTH).
Methods
Twenty female high-school runners were assigned to a vitamin D supplement and low-fat milk intake group (MKD) or a control group (CON). Participants in the MKD group consumed a vitamin D supplement (1,000 IU/day) and low-fat milk (Ca 315 mg/day) for 6 months. Bone mineral density measurements, blood samples, and questionnaires (regarding menses and diet) were carried out. The UMIN Clinical Trials Registry number is UMIN000027854.
Results
The 25-hydroxyvitamin D (25(OH)D) concentration in MKD was sustained and PTH concentration was decreased regardless of the state of menses. The correlation coefficients of 25(OH)D or PTH concentrations and bone metabolism markers were analyzed by partial correlation coefficient via adjusting the model for frequency of menses. CTX and 25(OH)D concentration were significantly and inversely correlated at baseline (r = -0.61, P < 0.01), 3 months (r = -0.54, P = 0.02), and 6 months (r = -0.53, P = 0.02). CTX and PTH were significantly and positively correlated at 3 months (r = 0.63, P < 0.01) and 6 months (r = 0.52, P = 0.02). The bone alkaline phosphatase (BAP)/CTX ratio and 25(OH)D concentration were significantly and positively correlated at 3 months (r = 0.59, P = 0.01) and 6 months (r = 0.56, P = 0.01). The BAP/CTX ratio and PTH were significantly and inversely correlated at 3 months (r = -0.59, P = 0.01) and 6 months (r = -0.58, P < 0.01).
Conclusions
This study suggested that vitamin D and low-fat milk supplementation improves bone metabolism by sustaining the 25(OH)D concentration and decreasing the PTH concentration in female high-school endurance runners regardless of the state of menses.
Keywords: Adolescent runners, Bone metabolism, Parathyroid hormone, 25-hydroxyvitamin D, Inflammatory cytokines
Introduction
Physical activity is associated with an increase in bone mineral accumulation during childhood and with a decrease in the bone loss rate after peak bone mass is reached [1]. However, some athletes, such as endurance runners, have been observed to have low bone mineral density (BMD) and subsequent stress fractures [2-4]. These result from the excess bone resorption caused by an increase in exercise volume and the abnormal repetitive load on the bones [5]. In addition, low BMD in female athletes has been reported caused by amenorrhea for low energy availability [2-4].
The dietary strategy for prevention of low BMD and stress fractures in athletes is calcium and vitamin D supplementation. In a 2-year cohort study of female endurance runners, a high intake of dairy products (including calcium, vitamin D, and protein) was observed to increase the BMD and decrease the incidence of stress fractures [6]. In addition, calcium and vitamin D supplementation during military training was observed to be associated with increased ionized calcium levels, maintenance of parathyroid hormone (PTH) concentration, a decreased bone resorption rate, and improved BMD and bone structure [7]. Thus, the benefits of calcium and vitamin D supplementation have been reported in many previous studies [6-8]. Vitamin D is thought to aid in calcium absorption from the small intestine, thus increasing the serum calcium concentration and decreasing the PTH concentration, thus decreasing bone resorption [9, 10].
On the other hand, inflammatory cytokines were reported to accelerate bone resorption and suppress bone formation [11]. In a previous study, tumor necrosis factor-alpha (TNF-α), an inflammatory cytokine, was increased after high-intensity exercise, and the percent change of TNF-α showed positive correlations with the percent change of bone resorption markers [12]. On the contrary, vitamin D has been shown to suppress inflammatory cytokines such as interleukin (IL)-6, IL-8, and TNF-α via vitamin D receptors [13]. Furthermore, the 25-hydroxyvitamin D (25(OH)D) and TNF-α concentrations in runners reportedly have a reverse correlation [14]. Therefore, improvement in bone health by calcium and vitamin D supplementation has the potential to not only improve calcium metabolism but also to suppress inflammation.
The purpose of this study was to determine whether the intake of vitamin D supplements and low-fat milk for 6 months in female high-school endurance runners would improve bone metabolism through suppression of PTH and inflammatory cytokines.
Materials and Methods
Subjects
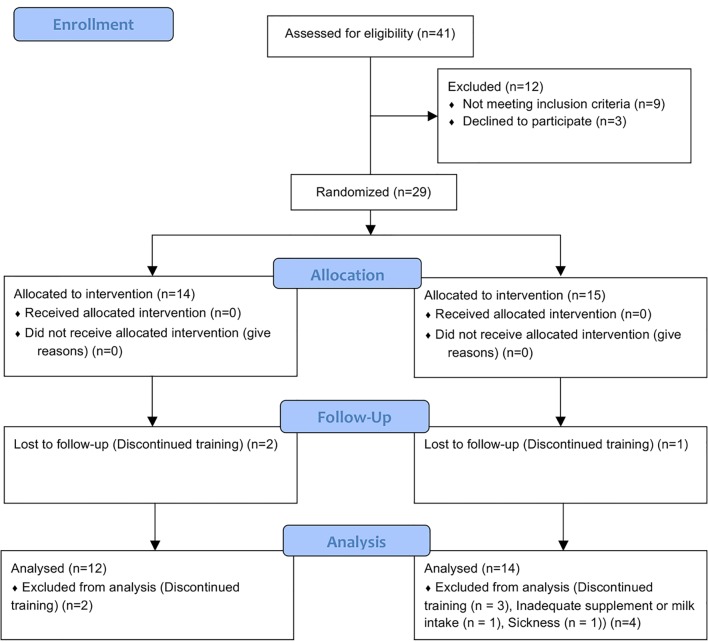
This study recruited 29 high-school female endurance runners (age 16.4 ± 0.6 years). The trial period was 6 months from September 2016 to April 2017. During the study, the subjects adhered to the following guidelines: 1) maintain a normal lifestyle, 2) take vitamin D supplements and low-fat milk as specified every day for those in the study group, 3) refrain from the use of supplements, health foods, medicines (including general medicines), or quasi-drugs that may affect this research, and 4) report to the research director when consulting medical institutions or using medicine. During the trial period, nine people were excluded (reasons included sickness, inadequate supplement or milk intake, and discontinued training), and finally 20 subjects were analyzed (Fig. 1).
Figure 1.
Study flow diagram.
Study design
This was a 6-month randomized controlled trial. Subjects were assigned to 1) the vitamin D supplement + low-fat milk (MKD) group (n = 10, age 16.3 ± 0.5 years) or 2) the control (CON) group (n = 10, age 16.3 ± 0.6 years) that received no intervention. Before and after the 6-month trial period, we measured the participants’ BMD, fat mass, and fat-free mass (FFM) using dual-energy X-ray absorptiometry (DXA). In addition, at baseline, after 3 months, and after 6 months, we performed blood sampling and administered questionnaires (assessment of menses and running distance). The study protocol was approved by the Ethics Committee for Human Experiments at Ritsumeikan University and was conducted in accordance with the Declaration of Helsinki. All subjects and legal guardians of subjects provided informed consent for participation in this study.
Nutrient supplementation
The study group participants consumed 1,000 IU of vitamin D (Nature Made, Super D, Otsuka Pharmaceutical Co., Ltd, Japan; 0.96 kcal energy, 0 - 0.1 g protein, 0 - 0.1 g fat, 0.231 g carbohydrates, 1,000 IU vitamin D, and 0 - 2 mg sodium/pill) and 200 mL of low-fat milk (SAVAS MILK PROTEIN, Meiji Co., Ltd; 107 kcal energy, 10.0 g protein, 3.2 g fat, 9.6 g carbohydrates, 0.22 g table salt equivalent, 315 mg calcium, and 0.65 mg vitamin B6). Vitamin D supplementation was taken 1 pill a day after dinner. The low-fat milk (200 mL) was consumed at the club on the practice day prior to exercise (up to 1 h before), and on days off, the milk was consumed during free time.
Assessment of menses and diet
The status of menses (age of menarche, frequency of menses (times/year), and menstrual cycle) was assessed using a questionnaire. In a 3-day diet record using a food weighing method, daily energy and nutrient intake were assessed at baseline.
Blood analysis
Blood samples were collected from a vein in the cubital fossa at rest in a fasting state (8:00 - 10:00 am). Blood samples were centrifuged at 3,000 rpm at 4 °C for 10 min, and separated serum samples were frozen and stored at -20 °C until analysis. The levels of 25(OH)D (25-OH Vitamin D Xpress ELISA Kit), TNF-α (Quantikine® ELISA, R&D systems), and type I collagen cross-linked C-telopeptides (CTX; FRELISA®β CrossLaps®-N, Fujirebio Inc.) were measured using an enzyme-linked immunoassay (ELISA). The intra- and inter-assay CV values were 4.9-9.4% and 7.4-8.8% for 25(OH)D, 3.1-8.7% and 7.2-10.4% for TNF-α, and 4.6-6.1% and 2.1-5.3% for CTX, respectively. PTH and bone alkaline phosphatase (BAP) levels were measured using an electrochemiluminescence immunoassay (ECLIA) (Medic, Inc., Japan). Intra- and inter-assay CV values were within 15%.
Body composition and BMD
The body mass, fat mass, percent body fat, appendicular FFM, and bone mass were evaluated by DXA (Lunar Prodigy; GE Healthcare, Tokyo, Japan). During DXA measurements, subjects remained in a supine position. To evaluate the whole body scans, we used enCORE version 15 software (GE Medical Systems Lunar, Tokyo, Japan), which automated measurements of FFM and fat-mass (arms, legs, torso, gynoid (gluteal area), and total body), BMD (total bone less head (TBLH), arms, spine, pelvis, and legs), and percent body fat. For screening of athletes younger than 20 years of age who are at risk for low BMD, measurement of TBLH BMD is recommended [15].
Statistical analysis
Data are shown as the mean ± standard deviation (SD) unless otherwise noted. The concentration of bone formation markers (BAP) divided by the concentration of resorption markers (CTX) was used to calculate the bone formation to resorption ratios (BAP/CTX). The calculated ratios were used to assess the change of bone turnover balance [16]. Comparison of baseline values between groups was evaluated using unpaired t-tests. Differences in time (baseline, 3 months, and 6 months) and group (MKD and CON) were evaluated with repeated two-way ANOVAs for subject characteristics, BMD, and biomarkers. The Bonferroni method for post hoc analysis was performed when time × group interactions were observed. To determine the effects of supplementation on 25(OH)D concentrations, PTH, TNF-α, and bone metabolism markers, we first calculated the changes from baseline by subtracting the baseline values from the 3-month and 6-month trial values. Then we applied an analysis of covariance. To identify whether the significant differences were influenced by the baseline values of 25(OH)D and PTH concentration, TNF-α, and bone metabolism markers, we adjusted for the baseline values of each biomarker. We also controlled for running distance and frequency of menses, because these were observed to be significantly different between the groups (P = 0.03). Correlations between indexes were evaluated using the Pearson correlation coefficient. The correlation coefficients between 25(OH)D or PTH concentrations and bone metabolism markers were analyzed by partial correlation coefficients with the model adjusted for the frequency of menses. The statistical significance level was set at 5%, and a level below 10% was defined as a tendency. Statistical analysis was performed using SPSS Statistics Version 24.0 for Microsoft Windows (IBM Corp., Tokyo, Japan). The UMIN Clinical Trials Registry number is UMIN000027854.
Results
Subject characteristics
Subject characteristics are shown in Table 1. There were no significant differences in any of subject characteristics between groups at baseline. None of the confounding factors were significant for time × group interactions. Running distance (P = 0.03) and frequency of menses (P = 0.04) were significantly different between the groups (Table 1). Age of menarche was not significantly different between the groups (P = 0.69) (CON: 13.2 ± 1.0 years, two subjects were delayed menarche; MKD: 13.5 ± 1.4 years; two subjects in CON group who reported menarche delay were excluded from the calculation). In addition, energy and nutrient intakes at baseline were not significantly different between the groups (energy: MKD 2,103 ± 366 kcal/day, CON 2,043 ± 340 kcal/day, P = 0.71, calcium: MKD 445 ± 265 mg/day, CON 495 ± 106 mg/day, P = 0.59, vitamin D: MKD 10.5 ± 7.5 µg/day, CON 16.5 ± 18.8 µg/day, P = 0.37).
Table 1. Subject Characteristics.
| Group | Baseline | 3 months | 6 months | P |
|||
|---|---|---|---|---|---|---|---|
| Time | Group | Time × group | |||||
| Height (cm) | MKD | 158.9 ± 4.2 | 159.0 ± 4.4 | 159.3 ± 4.3 | < 0.01 | n.s | n.s |
| CON | 158.3 ± 5.0 | 158.5 ± 5.0 | 158.9 ± 5.0 | ||||
| Weight (kg) | MKD | 47.3 ± 3.3 | 48.5 ± 2.8 | 49.3 ± 2.8 | < 0.01 | n.s | n.s |
| CON | 45.3 ± 3.2 | 46.8 ± 3.2 | 47.6 ± 3.6 | ||||
| BMI (kg/m2) | MKD | 18.7 ± 1.5 | 19.2 ± 1.4 | 19.5 ± 1.3 | < 0.01 | n.s | n.s |
| CON | 18.1 ± 1.1 | 18.7 ± 1.5 | 18.9 ± 1.7 | ||||
| Fat mass (%) | MKD | 19.6 ± 5.3 | - | 21.8 ± 5.2 | < 0.01 | n.s | n.s |
| CON | 17.9 ± 4.8 | - | 21.0 ± 5.6 | ||||
| Appendicular FFM (kg) | MKD | 16.1 ± 1.3 | - | 16.6 ± 1.4 | < 0.01 | n.s | n.s |
| CON | 15.7 ± 1.3 | - | 16.1 ± 1.2 | ||||
| Running distance (km/month) | MKD | 187.8 ± 88.3 | 215.5 ± 117.7 | 179.4 ± 85.2 | n.s | 0.03 | n.s |
| CON | 124.5 ± 70.7 | 103.9 ± 73.9 | 106.7 ± 61.0 | ||||
| Frequency of menses (time/year) | MKD | 11.1 ± 2.2 | 11.8 ± 3.3 | 12.5 ± 2.9 | n.s | 0.04 | n.s |
| CON | 6.8 ± 5.3 | 8.2 ± 5.7 | 7.7 ± 5.1 | ||||
Values are represented as mean ± SD. Differences in time (baseline, 3 months, and 6 months) and group (MKD and CON) were evaluated with repeated two-way ANOVAs for subject characteristics. Because fat mass and fat-free mass (FFM) were measured in before and after the 6-month trial period only, the values in 3 months were not measured (-). MKD: vitamin D supplement + low-fat milk group; CON: control group.
BMD
Changes in the subjects’ BMD are shown in Table 2. There were no significant differences in any of BMDs between groups at baseline. The change in BMD for all sites tested was not significant for time × group interactions.
Table 2. Changes in BMD.
| Group | Baseline | 6 months | P |
|||
|---|---|---|---|---|---|---|
| Time | Group | Time × group | ||||
| Arms BMD (g/cm2) | MKD | 0.838 ± 0.063 | 0.858 ± 0.051 | < 0.01 | n.s | n.s |
| CON | 0.818 ± 0.061 | 0.828 ± 0.051 | ||||
| Legs BMD (g/cm2) | MKD | 1.217 ± 0.064 | 1.230 ± 0.061 | < 0.01 | n.s | n.s |
| CON | 1.199 ± 0.077 | 1.212 ± 0.072 | ||||
| Pelvic BMD (g/cm2) | MKD | 1.068 ± 0.082 | 1.086 ± 0.076 | < 0.01 | n.s | n.s |
| CON | 1.051 ± 0.079 | 1.077 ± 0.075 | ||||
| Spine BMD (g/cm2) | MKD | 0.967 ± 0.093 | 0.999 ± 0.098 | < 0.01 | n.s | n.s |
| CON | 0.981 ± 0.073 | 1.014 ± 0.069 | ||||
| TBLH BMD (g/cm2) | MKD | 0.992 ± 0.054 | 1.002 ± 0.050 | < 0.01 | n.s | n.s |
| CON | 0.978 ± 0.059 | 0.991 ± 0.050 | ||||
Values are represented as mean ± SD. Differences in time (baseline, 3 months, and 6 months) and group (MKD and CON) were evaluated with repeated two-way ANOVAs for BMD. TBLH: total bone less head; MKD: vitamin D supplement + low-fat milk group; CON: control group.
Biomarkers
The changes in concentration of 25(OH)D, PTH, TNF-α, and bone metabolism markers are shown in Table 3. There were no significant differences in baseline values of 25(OH)D, PTH, or bone metabolism markers between groups. TNF-α concentration at baseline was significantly higher in the MKD group compared with that in the CON group (P = 0.02). A significant effect of time (P < 0.01) and time × group interaction (P < 0.01) was observed in 25(OH)D concentration. In a post hoc analysis, 25(OH)D concentration in the CON group significantly decreased from baseline to 3 months (P < 0.01) and from baseline to 6 months (P < 0.01). In contrast, the 25(OH)D concentration in the MKD group did not change significantly during the trial period. Time × group interaction (P = 0.04) exhibited a significant effect on TNF-α concentration. TNF-α concentration at baseline was significantly higher in the MKD group (1.08 ± 0.28 pg/mL) compared with that in the CON group (0.71 ± 0.38 pg/mL) (P = 0.02). Moreover, TNF-α concentration in the MKD group decreased significantly from baseline to 6 months (P = 0.02). PTH concentration was significant effect of time (P < 0.01), and tend effect of time × group interaction (P = 0.06). However, the concentration of the bone metabolism markers did not change significantly during the trial period.
Table 3. Changes in 25(OH)D, PTH, TNF-α, and Bone Metabolism Marker Concentrations.
| Group | Baseline | 3 months | 6 months | P |
|||
|---|---|---|---|---|---|---|---|
| Time | Group | Time × group | |||||
| 25(OH)D (ng/mL) | MKD | 31.8 ± 6.5 | 31.8 ± 5.0 | 30.2 ± 3.8 | < 0.01 | n.s | < 0.01 |
| CON | 34.7 ± 8.2 | 28.0 ± 7.1†† | 27.9 ± 5.3†† | ||||
| PTH (pg/mL) | MKD | 33.9 ± 10.0 | 29.2 ± 7.3 | 36.9 ± 11.2 | < 0.01 | n.s | 0.06 |
| CON | 30.0 ± 8.5 | 32.0 ± 6.6 | 40.5 ± 8.7 | ||||
| TNF-α (pg/mL) | MKD | 1.08 ± 0.28* | 0.87 ± 0.26 | 0.83 ± 0.30† | n.s | n.s | 0.04 |
| CON | 0.71 ± 0.38 | 0.85 ± 0.29 | 0.71 ± 0.34 | ||||
| CTX (ng/mL) | MKD | 0.66 ± 0.19 | 0.54 ± 0.18 | 0.55 ± 0.18 | 0.04 | n.s | n.s |
| CON | 0.85 ± 0.23 | 0.70 ± 0.25 | 0.76 ± 0.48 | ||||
| BAP (µg/L) | MKD | 15.7 ± 5.5 | 15.3 ± 4.1 | 16.1 ± 3.3 | n.s | n.s | n.s |
| CON | 17.5 ± 9.5 | 17.7 ± 11.1 | 18.2 ± 10.8 | ||||
| BAP/CTX | MKD | 24.8 ± 10.4 | 31.9 ± 17.6 | 33.5 ± 16.4 | < 0.01 | n.s | n.s |
| CON | 20.2 ± 7.3 | 24.7 ± 7.4 | 24.8 ± 6.8 | ||||
Values are represented as mean ± SD. Differences in time (baseline, 3 months, and 6 months) and group (MKD and CON) were evaluated with repeated two-way ANOVAs for biomarkers. *P < 0.05 vs. CON, †P < 0.05, ††P < 0.01 vs. Baseline. BAP/CTX: bone formation to resorption ratio; MKD: vitamin D supplement + low-fat milk group; CON: control group.
Association between vitamin D supplement and low-fat milk intake and biomarkers
The association between biomarkers and vitamin D supplementation and low-fat milk intake is shown in Table 4. The change in concentration of each biomarker in both groups was compared after each biomarker was adjusted for baseline values, frequency of menses, and running distance. The decrease in 25(OH)D concentration from baseline to 3 months in the MKD group was significantly attenuated compared to that in the CON group even after adjusting for baseline values and frequency of menses (model 1, 2, 3: P < 0.01). Although the decrease in 25(OH)D concentration from baseline to 6 months in the MKD group was significantly attenuated compared to that in the CON group, even after adjusting for baseline values and frequency of menses (model 1: P < 0.01; model 2: P = 0.02), the change was not significant at 6 months after adjusting for baseline values, frequency of menses, and running distance (model 3, P = 0.11). PTH concentration in the MKD group decreased significantly from baseline to 3 months compared with that in the CON group even after adjusting for baseline values, frequency of menses, and running distance (model 1: P = 0.03; model 2: P = 0.01; model 3: P = 0.04). However, the change in PTH concentration from baseline to 6 months was not significantly different between the groups (model 1: P = 0.10; model 2: P = 0.18; model 3: P = 0.77). TNF-α, CTX, and BAP concentrations were not significantly different between the groups during the trial period.
Table 4. Effect of Vitamin D Supplementation and Low-Fat Milk Intake on Each Biomarker.
| Baseline to 3 months |
Baseline to 6 months |
|||||
|---|---|---|---|---|---|---|
| CON (n = 10) | MKD (n = 10) | P | CON (n = 10) | MKD (n = 10) | P | |
| 25(OH)D (ng/mL) | ||||||
| Model 1 | -6.33 ± 0.76 | -0.32 ± 0.76 | < 0.01 | -6.12 ± 0.78 | -2.21 ± 0.78 | < 0.01 |
| Model 2 | -6.17 ± 0.83 | -0.48 ± 0.83 | < 0.01 | -5.87 ± 0.85 | -2.45 ± 0.85 | 0.02 |
| Model 3 | -6.19 ± 0.96 | -0.46 ± 0.96 | < 0.01 | -5.46 ± 0.95 | -2.87 ± 0.95 | 0.11 |
| PTH (pg/mL) | ||||||
| Model 1 | 1.17 ± 1.50 | -3.87 ± 1.50 | 0.03 | 9.91 ± 2.53 | 3.59 ± 2.53 | 0.10 |
| Model 2 | 2.01 ± 1.53 | -4.71 ± 1.53 | 0.01 | 9.67 ± 2.77 | 3.83 ± 2.77 | 0.18 |
| Model 3 | 1.63 ± 1.65 | -4.33 ± 1.65 | 0.04 | 7.32 ± 2.43 | 6.18± 2.43 | 0.77 |
| TNF-α (pg/mL) | ||||||
| Model 1 | 0.03 ± 0.08 | -0.11 ± 0.08 | 0.25 | -0.07 ± 0.08 | -0.19 ± 0.08 | 0.36 |
| Model 2 | 0.04 ± 0.10 | -0.12 ± 0.10 | 0.35 | -0.10 ± 0.10 | -0.16 ± 0.10 | 0.71 |
| Model 3 | -0.03 ± 0.08 | -0.05 ± 0.08 | 0.94 | -0.10 ± 0.11 | -0.16 ± 0.11 | 0.77 |
| CTX (ng/mL) | ||||||
| Model 1 | -0.13 ± 0.04 | -0.13 ± 0.04 | 0.95 | -0.10 ± 0.09 | -0.09 ± 0.09 | 0.92 |
| Model 2 | -0.15 ± 0.04 | -0.12 ± 0.04 | 0.60 | -0.14 ± 0.09 | -0.05 ± 0.09 | 0.50 |
| Model 3 | -0.14 ± 0.04 | -0.13 ± 0.04 | 0.86 | -0.15 ± 0.09 | -0.04 ± 0.09 | 0.44 |
| BAP (µg/L) | ||||||
| Model 1 | 0.31 ± 1.58 | -0.53 ± 1.58 | 0.71 | 0.85 ± 1.39 | 0.33 ± 1.39 | 0.80 |
| Model 2 | -0.18 ± 1.72 | -0.04 ± 1.72 | 0.96 | 0.90 ± 1.54 | 0.29 ± 1.54 | 0.80 |
| Model 3 | 0.25 ± 2.01 | -0.47 ± 1.96 | 0.82 | 1.05 ± 1.77 | 0.13 ± 1.77 | 0.75 |
Values are represented as mean ± SE. The change in concentration of each biomarker in both groups was compared after each biomarker was adjusted for Baseline values, frequency of menses, and running distance, respectively. Model 1: baseline value. Model 2: baseline value, frequency of menses (baseline value). Model 3: baseline value, frequency of menses (baseline value), running distance (baseline value). MKD: vitamin D supplement + low-fat milk group; CON: control group.
Correlation between 25(OH)D or PTH concentration and bone metabolism markers
The correlation between 25(OH)D or PTH concentration and bone metabolism markers was examined. Because CTX or BAP concentration and the frequency of menses were significantly correlated, the correlation coefficients of 25(OH)D or PTH concentrations and bone metabolism markers were analyzed by partial correlation coefficient via adjusting the model for frequency of menses. The correlation between 25(OH)D or PTH concentrations and bone metabolism markers with adjustment for frequency of menses is shown in Table 5. CTX and 25(OH)D concentration were significantly and inversely correlated at baseline, 3 months, and 6 months. CTX and PTH were significantly and positively correlated at 3 and 6 months. However, BAP was not correlated with 25(OH)D and PTH concentrations. The BAP/CTX ratio and 25(OH)D concentration were significantly and positively correlated at 3 and 6 months. The BAP/CTX ratio and PTH were significantly and inversely correlated at 3 and 6 months. In addition, 25(OH)D and PTH concentrations were significantly and inversely correlated at baseline (r = -0.50, P = 0.03) and 24 weeks (r = -0.64, P < 0.01). PTH and 25(OH)D concentrations at 3 months were inversely correlated (r = -0.44, P = 0.051).
Table 5. The Correlation Between 25(OH)D or PTH Concentrations and Bone Metabolism Markers With Adjustment for Frequency of Menses.
| CTX (ng/mL) |
BAP/CTX |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline |
3 months |
6 months |
Baseline |
3 months |
6 months |
|||||||
| r | P | r | P | r | P | r | P | r | P | r | P | |
| 25(OH)D (ng/mL) | ||||||||||||
| Baseline | -0.61 | < 0.01 | - | - | - | - | 0.31 | 0.19 | - | - | - | - |
| 3 months | - | - | -0.54 | 0.02 | - | - | - | - | 0.59 | 0.01 | - | - |
| 6 months | - | - | - | - | -0.53 | 0.02 | - | - | - | - | 0.56 | 0.01 |
| PTH (pg/mL) | ||||||||||||
| Baseline | 0.20 | 0.42 | - | - | - | - | -0.16 | 0.50 | - | - | - | - |
| 3 months | - | - | 0.63 | < 0.01 | - | - | - | - | -0.59 | 0.01 | - | - |
| 6 months | - | - | - | - | 0.52 | 0.02 | - | - | - | - | -0.58 | < 0.01 |
Pooled analysis by partial correlation coefficient. Values are r and P values.
Discussion
The purpose of this randomized controlled trial was to determine whether vitamin D supplementation and low-fat milk intake for 6 months by female high-school endurance runners resulted in improved bone metabolism by suppressing the concentration of PTH and inflammatory cytokines. The main finding of our study was that the 25(OH)D concentration was sustained and PTH concentration was decreased by vitamin D and low-fat milk supplementation regardless of the state of menses. In comparison to bone metabolism markers with 25(OH)D or PTH concentration, we found that high 25(OH)D and low PTH concentration decreased CTX and increased the BAP/CTX ratio (increased bone formation ratio) regardless of the state of menses. In addition, 25(OH)D and PTH concentrations were significantly and inversely correlated. Accordingly, vitamin D and low-fat milk supplementation improved bone metabolism while sustaining 25(OH)D concentration and decreasing PTH concentration regardless of the state of menses.
In 25(OH)D concentration, after adjusting for the frequency of menses and running distance, no significant change was observed at 6 months. Thus, the 25(OH)D concentration may have been influenced by the running distance. The decrease in 25(OH)D concentration has previously been reported during basic military training in male and female soldiers [17-19]. Although the mechanism is still unclear, an increased running distance may potentially influence increased PTH concentration. PTH concentration has been reported to be transient-increase dependent on exercise intensity and duration [20, 21]. In addition, teriparatide (recombinant human PTH), a therapeutic osteoporosis drug, has been reported to decrease 25(OH)D concentration [22]. 25(OH)D concentration decreased during teriparatide therapy, suggesting the conversion of 25(OH)D to 1,25(OH)2D (active vitamin D) metabolite. From these previous studies, an increased running distance can potentially decrease the 25(OH)D concentration by accelerating the conversion of 25(OH)D to 1,25(OH)2D by increasing the PTH concentration during exercise. However, because this study could not determine the underlying relationships, future study is needed.
Vitamin D and low-fat milk supplementation significantly decreased the PTH concentration from baseline to 3 months even after adjusting for the confounders. However, PTH concentration did not change significantly from baseline to 6 months, suggesting that the changes were influenced by the changes in 25(OH)D concentration. Although the change in 25(OH)D concentrations in the CON group did not differ between baseline and 3 months (approximately -6.3 ng/mL) and between baseline and 6 months (approximately -6.1 ng/mL), in the MKD group, the concentration decreased between baseline and 6 months (approximately -2 ng/mL) compared with that between baseline and 3 months (approximately -0.3 ng/mL). The 25(OH)D concentration likely decreases more during these seasons (winter season) owing to fewer sunshine hours [17]. The 3 months measurement was performed in December or January, and the 6 months measurement was performed in March or April. Previous study of Japanese indoor and outdoor female athletes reported that the 25(OH)D concentration was lowest in March and highest in September, and December was significantly lower compared with September [23]. The change in 25(OH)D concentration of this study was akin to previous study. In addition, a correlation was observed between 25(OH)D and PTH concentrations. Therefore, the seasonal variations in 25(OH)D concentration suggested that was influenced to the change in PTH concentration.
In this study, regardless of the frequency of menses, vitamin D and low-fat milk supplementation was associated with a sustained 25(OH)D concentration and a decreased PTH concentration, and a significant correlation was observed between bone metabolism markers and 25(OH)D or PTH concentration. In a previous study, when the effects of calcium and vitamin D supplementation were studied in female navy recruits, the stress fracture incidence rate in female recruits with amenorrhea decreased from 91% to 83% [8]. Thus, although the state of menses negatively affects bone metabolism, calcium and vitamin D supplementation can potentially attenuate the negative effect of menstrual irregularity.
On the contrary, vitamin D and low-fat milk supplementation did not suppress TNF-α, and TNF-α was not associated with bone metabolism. This may be because the TNF-α concentration of the subjects was low at baseline. The TNF-α concentration (1.1 ± 0.3 pg/mL) of the present study subjects was low levels and was not comparable to that of patients with type 2 diabetes (13 ± 8.6 pg/mL), in whom the inflammatory suppress effects of vitamin D and calcium supplementation have been observed [24]. Furthermore, the decrease in the cortical bone cross-sectional area during soldiers’ army training involves IL-6 polymorphism [25]. Moreover, it has been reported that vitamin D sufficiency is associated with an increase in anti-inflammatory (IL-10 and IL-13) cytokines after intense exercise [26]. Therefore, investigation of the effects of vitamin D and low-fat milk intake on other inflammatory and anti-inflammatory cytokines and the association of other cytokines and bone metabolism markers is needed.
Limitation
The present study has several limitations. This study included a relatively small sample size. Furthermore, because the state of menses was assessed by a questionnaire only, we did not evaluate sex hormones (e.g., estrogen and progesterone) or reproductive maturation factors (such as Tanner breast stage). Therefore, a larger sample size and detailed analysis of the reproductive function is needed when investigating the effect of calcium and vitamin D supplementation on bone metabolism and inflammation in the future.
Conclusions
In conclusion, this study suggested that vitamin D and low-fat milk supplementation improves bone metabolism by sustaining 25(OH)D concentration and decreasing PTH concentration, regardless of the state of menses in female high-school endurance runners.
Acknowledgments
The authors wish to acknowledge the volunteers who participated in this study.
Conflict of Interest
The authors declare no conflict of interest.
Grant Support
The Japan Dairy Association (J-milk) and the Yamaha Motor Foundation for Sports provided grants to support this study.
References
- 1.Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR, American College of Sports M. American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36(11):1985–1996. doi: 10.1249/01.MSS.0000142662.21767.58. [DOI] [PubMed] [Google Scholar]
- 2.Barrack MT, Rauh MJ, Nichols JF. Prevalence of and traits associated with low BMD among female adolescent runners. Med Sci Sports Exerc. 2008;40(12):2015–2021. doi: 10.1249/MSS.0b013e3181822ea0. [DOI] [PubMed] [Google Scholar]
- 3.Bilanin JE, Blanchard MS, Russek-Cohen E. Lower vertebral bone density in male long distance runners. Med Sci Sports Exerc. 1989;21(1):66–70. doi: 10.1249/00005768-198902000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Tenforde AS, Fredericson M, Sayres LC, Cutti P, Sainani KL. Identifying sex-specific risk factors for low bone mineral density in adolescent runners. Am J Sports Med. 2015;43(6):1494–1504. doi: 10.1177/0363546515572142. [DOI] [PubMed] [Google Scholar]
- 5.Matcuk GR Jr, Mahanty SR, Skalski MR, Patel DB, White EA, Gottsegen CJ. Stress fractures: pathophysiology, clinical presentation, imaging features, and treatment options. Emerg Radiol. 2016;23(4):365–375. doi: 10.1007/s10140-016-1390-5. [DOI] [PubMed] [Google Scholar]
- 6.Nieves JW, Melsop K, Curtis M, Kelsey JL, Bachrach LK, Greendale G, Sowers MF. et al. Nutritional factors that influence change in bone density and stress fracture risk among young female cross-country runners. PM R. 2010;2(8):740–750. doi: 10.1016/j.pmrj.2010.04.020. quiz 794. [DOI] [PubMed] [Google Scholar]
- 7.Gaffney-Stomberg E, Lutz LJ, Rood JC, Cable SJ, Pasiakos SM, Young AJ, McClung JP. Calcium and vitamin D supplementation maintains parathyroid hormone and improves bone density during initial military training: a randomized, double-blind, placebo controlled trial. Bone. 2014;68:46–56. doi: 10.1016/j.bone.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin d supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23(5):741–749. doi: 10.1359/jbmr.080102. [DOI] [PubMed] [Google Scholar]
- 9.Fleet JC. The role of vitamin D in the endocrinology controlling calcium homeostasis. Mol Cell Endocrinol. 2017;453:36–45. doi: 10.1016/j.mce.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi N, Udagawa N, Suda T. Vitamin D endocrine system and osteoclasts. Bonekey Rep. 2014;3:495. doi: 10.1038/bonekey.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takayanagi H. New developments in osteoimmunology. Nat Rev Rheumatol. 2012;8(11):684–689. doi: 10.1038/nrrheum.2012.167. [DOI] [PubMed] [Google Scholar]
- 12.Mezil YA, Allison D, Kish K, Ditor D, Ward WE, Tsiani E, Klentrou P. Response of Bone Turnover Markers and Cytokines to High-Intensity Low-Impact Exercise. Med Sci Sports Exerc. 2015;47(7):1495–1502. doi: 10.1249/MSS.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 13.Muller K, Bendtzen K. 1,25-Dihydroxyvitamin D3 as a natural regulator of human immune functions. J Investig Dermatol Symp Proc. 1996;1(1):68–71. [PubMed] [Google Scholar]
- 14.Willis KS, Smith DT, Broughton KS, Larson-Meyer DE. Vitamin D status and biomarkers of inflammation in runners. Open Access J Sports Med. 2012;3:35–42. doi: 10.2147/OAJSM.S31022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek S, Lorenc RS. et al. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11(1):43–58. doi: 10.1016/j.jocd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Jiang J, Boyle LJ, Mikus CR, Oberlin DJ, Fletcher JA, Thyfault JP, Hinton PS. The effects of improved metabolic risk factors on bone turnover markers after 12 weeks of simvastatin treatment with or without exercise. Metabolism. 2014;63(11):1398–1408. doi: 10.1016/j.metabol.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Davey T, Lanham-New SA, Shaw AM, Hale B, Cobley R, Berry JL, Roch M. et al. Low serum 25-hydroxyvitamin D is associated with increased risk of stress fracture during Royal Marine recruit training. Osteoporos Int. 2016;27(1):171–179. doi: 10.1007/s00198-015-3228-5. [DOI] [PubMed] [Google Scholar]
- 18.Lutz LJ, Karl JP, Rood JC, Cable SJ, Williams KW, Young AJ, McClung JP. Vitamin D status, dietary intake, and bone turnover in female Soldiers during military training: a longitudinal study. J Int Soc Sports Nutr. 2012;9(1):38. doi: 10.1186/1550-2783-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans RK, Antczak AJ, Lester M, Yanovich R, Israeli E, Moran DS. Effects of a 4-month recruit training program on markers of bone metabolism. . Med Sci Sports Exerc. 2008;40(11 Suppl):S660–670. doi: 10.1249/MSS.0b013e318189422b. [DOI] [PubMed] [Google Scholar]
- 20.Scott JP, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD. The role of exercise intensity in the bone metabolic response to an acute bout of weight-bearing exercise. J Appl Physiol (1985) 2011;110(2):423–432. doi: 10.1152/japplphysiol.00764.2010. [DOI] [PubMed] [Google Scholar]
- 21.Scott JP, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD. Treadmill running reduces parathyroid hormone concentrations during recovery compared with a nonexercising control group. J Clin Endocrinol Metab. 2014;99(5):1774–1782. doi: 10.1210/jc.2013-3027. [DOI] [PubMed] [Google Scholar]
- 22.Cosman F, Dawson-Hughes B, Wan X, Krege JH. Changes in vitamin D metabolites during teriparatide treatment. Bone. 2012;50(6):1368–1371. doi: 10.1016/j.bone.2012.02.635. [DOI] [PubMed] [Google Scholar]
- 23.Maruyama-Nagao A, Sakuraba K, Suzuki Y. Seasonal variations in vitamin D status in indoor and outdoor female athletes. Biomed Rep. 2016;5(1):113–117. doi: 10.3892/br.2016.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabesh M, Azadbakht L, Faghihimani E, Tabesh M, Esmaillzadeh A. Calcium-vitamin D cosupplementation influences circulating inflammatory biomarkers and adipocytokines in vitamin D-insufficient diabetics: a randomized controlled clinical trial. J Clin Endocrinol Metab. 2014;99(12):E2485–2493. doi: 10.1210/jc.2014-1977. [DOI] [PubMed] [Google Scholar]
- 25.Dhamrait SS, James L, Brull DJ, Myerson S, Hawe E, Pennell DJ, World M. et al. Cortical bone resorption during exercise is interleukin-6 genotype-dependent. Eur J Appl Physiol. 2003;89(1):21–25. doi: 10.1007/s00421-002-0750-x. [DOI] [PubMed] [Google Scholar]
- 26.Barker T, Martins TB, Hill HR, Kjeldsberg CR, Dixon BM, Schneider ED, Henriksen VT. et al. Vitamin D sufficiency associates with an increase in anti-inflammatory cytokines after intense exercise in humans. Cytokine. 2014;65(2):134–137. doi: 10.1016/j.cyto.2013.12.004. [DOI] [PubMed] [Google Scholar]