Highlights
-
•
There is a demand from countries for a hepatitis B vaccine product licensed for Controlled Temperature Chain (CTC).
-
•
CTC is considered to be a cost efficient approach to improve coverage and equity.
-
•
CTC was viewed as potential relief for facilities without continuous cold chain.
-
•
Vaccination of babies born at home would be greatly facilitated by the CTC approach.
-
•
This study highlights that countries still require comprehensive orientation to CTC.
Keywords: Hepatitis B, Vaccination, Birth dose, Thermostability, Controlled Temperature Chain (CTC)
Abstract
Chronic hepatitis B infection can be prevented by hepatitis B vaccine birth dose (hepB-BD) given within 24 h after birth, followed by two hepatitis B vaccinations within the first year of life. Yet nearly half of World Health Organization (WHO) Member States do not provide a hepB-BD. Barriers are primarily attributed to vaccine storage and transportation, as well as high rates of home births. Delivering the vaccine outside the cold chain could potentially increase coverage. To do this, WHO recommends vaccines be licensed for use in a “controlled temperature chain” (CTC), which requires a given product to tolerate temperature excursions up to at least 40 °C for a minimum of three days. To date, no hepB vaccine is labelled for CTC.
To inform dialogue with manufacturers, WHO conducted a survey among countries in the African and Western Pacific Regions (AFR and WPR) to assess demand for a hepatitis B product licensed for use in a CTC. Twenty-five (44%) countries responded, with 8 of 11 (73%) from the WPR and 17 of 46 (37%) from the AFR.
Of these responding countries, 5 in AFR and all 8 in WPR have introduced universal hepB-BD. Seventy-two percent indicated that CTC would facilitate the provision of hepB-BD. While no overall difference in responses was detected between countries either providing or not providing hepB-BD, countries that already introduced hepB-BD but had low hepB-BD coverage were particularly interested in CTC. Irrespective of hepB-BD policy, responding countries suggested that a CTC-licenced product would be beneficial, though the price of such a vaccine would influence procurement decisions.
This survey was beneficial to inform the CTC agenda. However, countries' lack of experience with HepB-BD as well as with CTC and the fact that countries were commenting on a product that is not yet on the market should be acknowledged.
1. Introduction
Chronic hepatitis B virus (HBV) infection is an important cause of morbidity and mortality worldwide. Up to 90% of infants who acquire HBV infection at birth become chronically infected [1]. Evidence from clinical trials suggests that chronic HBV infection can be prevented by hepatitis B vaccine (hepB) administration within 24 h after birth, followed by at least two additional hepB doses given within the first year of life [2]. Accordingly, the World Health Organization (WHO) recommends that “the birth dose should be followed by 2 or 3 additional doses to complete the primary series,” including in countries where HBV is of low endemicity [2]. One of the targets of WHO’s Global Health Sector Strategy on Viral Hepatitis is to reach 90% coverage of hepatitis B birth dose (hepB-BD) vaccination by 2030 [3]. However, currently, 49% of all Member States do not provide hepB-BD, most of which are located in the WHO African region (AFR) [4].
Administration of the hepB-BD cannot be scheduled like other vaccinations in the Expanded Programme on Immunization (EPI) due to the uncertain timing of newborn delivery and the need to administer the vaccine within 24 h after birth. Barriers and facilitators to the provision of birth dose are linked to vaccine storage and transportation in an appropriate cold chain; births outside health facilities are associated with lower coverage of hepB-BD [5], [6]. In Africa almost half (47%) of births take place outside of a health facility; while in Southern Asia and Southeast Asia these numbers are slightly higher, at 55% and 60% respectively [7].
A systematic literature review identified a number of effective practices to facilitate timely hepB-BD administration, which included ensuring that the vaccine is available in the delivery room or postnatal ward and reaching infants born outside health facilities [5]. Both of these practices could be facilitated by vaccine transport and storage outside the traditional +2 °C to +8 °C cold chain.
Some countries have used hepatitis B vaccines outside the cold chain (OCC) for limited periods of time, relying on Vaccine Vial Monitors (VVMs) for accumulated temperature exposure and on occasion, an additional temperature threshold indicator to capture sudden spikes in temperature. The WHO Regional Office for the Western Pacific (WPRO) published operational field guidelines in 2006 advocating that HepB-BD may be stored and used OCC when the regular cold chain cannot be maintained [8]. However, although field studies have suggested that potency of certain hepatitis B vaccine products remains unaffected when the vaccine is used OCC [9], [10], [11], [12], this is considered ‘off-label’, unless the product is licensed and labelled for use in this manner by the relevant national regulatory body. More recently, WHO has been advocating for ‘on-label’ use through compliance with the “controlled temperature chain” (CTC), a specifically defined approach to vaccine delivery allowing vaccines to be kept at temperatures beyond the traditional cold chain of +2 °C to +8 °C for a limited period of time, under monitored and controlled conditions. For a vaccine to be licensed for use in a CTC, it must be able to tolerate ambient temperatures of at least +40 °C for a minimum of three days just prior to administration and should be accompanied by a VVM on each vial [13]. OCC does not have a clear definition or monitoring regulations, and is currently not approved by the WHO prequalification program nor by most national regulatory authorities. The WHO Immunization Practices Advisory Committee (IPAC) strongly recommends countries to only use vaccines outside of the traditional cold chain if they have been licensed and prequalified for use in a CTC [14]. This recommendation was also adopted for hepatitis B vaccines by the Strategic Advisory Group of Experts (SAGE) in October 2016 [15] and included in the HepB vaccines: WHO position paper [2].
The first vaccine to be licensed and prequalified for CTC use was the Meningitis A vaccine, MenAfriVac® in December 2012. The possibility of removing MenAfriVac® from the cold chain for a limited number of days was highly appreciated by staff and their supervisors in the 6 African countries where CTC was used. Without ice packs, the vaccine carriers were lighter than usual and vaccinators could stay in the field longer, spared from travelling back to the health facility in the evenings to refrigerate unused vaccines and recondition ice packs [16]. Two economic evaluations further found that the CTC approach reduced logistics costs [17], [18].
No hepatitis B vaccine has been licensed for use in a CTC to date. However, the possibility to deliver vaccines this way has a great potential to increase vaccine coverage, considering the successful use of hepatitis B OCC in increasing timely birth dose coverage [9], [10], [11], [12], [19]. For this reason, the CTC working group under IPAC identified hepB-BD as a priority vaccine for CTC licensure.
To inform dialogue with manufacturers on CTC vaccine demand and knowledge, WHO conducted a survey among countries in the African and Western Pacific regions, aiming to obtain more information about the necessary characteristics of this product and potential demand.
2. Methods
WHO works across 6 official geographic regions, each represented by regional offices which were contacted about this survey. WPRO and WHO African Region proceeded with the survey. The European region declined after reporting they were not in need of additional interventions such as CTC to improve already sufficiently high birth dose coverage. The South-East Asia and the Eastern Mediterranean regions, though interested in the subject matter, ultimately did not participate in the survey, due to competing priorities and lack of immediate relevance. The Region for the Americas did not participate as they consider their countries to not have significant cold chain challenges at present. This survey was conducted only shortly after the World Health Assembly (WHA) resolution to eliminate perinatal hepB transmission, which may increase regional and country interest in the future.
The survey, consisting of 13 questions along with background information on CTC, was sent by email to 57 WHO country offices in the two participating Regions (46 in AFR and 11 in WPR) between May and August 2016. The country offices in turn shared the survey with immunization personnel in ministries of health (MoH) and local UNICEF offices. The survey queried current or anticipated challenges in hepB-BD provision, the potential advantages that CTC could bring and which price countries would be willing to pay for a CTC-licensed hepatitis B vaccine. Responses were analysed using Microsoft Excel 2010.
2.1. Response rate
Twenty-five of 57 (44%) countries responded; eight out of 11 (73%) from the WPR and 17 out of 46 (37%) from the AFR. One survey out of the 17 from AFR was only partially completed, but all available answers were incorporated into the analysis. It was noted that when multiple institutions within a single country provided responses, there was no variance, thereby allowing just one survey per country to be retained (see Table 1).
Table 1.
Response rate.
| Total number of countries contacted | Responding countries | Universal HepB BD introduced | |
|---|---|---|---|
| AFR | 46 | 17 (37%) | 5 |
| WPR | 11 | 8 (73%) | 8 |
| Total | 57 | 25 | 13 |
3. Results
Of the 25 responding countries, eight WPR countries and five AFR countries had introduced universal hepB-BD, whereby official policy states that the vaccine should be given to all newborns within 24 h after birth. In AFR, one country indicated that hepB-BD was only provided in private health facilities and another stated that hepB-BD was only provided to children born to mothers known to be HBsAg-positive. These two countries were therefore not counted as having introduced universal hepB-BD. It should be noted that, irrespective of survey participation, at the time of the survey a total of only six countries in AFR had begun implementing universal hepB-BD, five of which responded to this survey.
According to the WHO/UNICEF Estimates of National Immunization Coverage, hepB-BD coverage in the responding countries from the WPR ranged from 32 to 99%, with a mean of 74% (19). In four of the five responding countries in the AFR that have introduced hepB-BD, coverage ranged from 43 to 100%. One country only introduced hepB-BD recently, for which reason coverage data were not available.
3.1. Challenges for hepatitis B birth dose provision
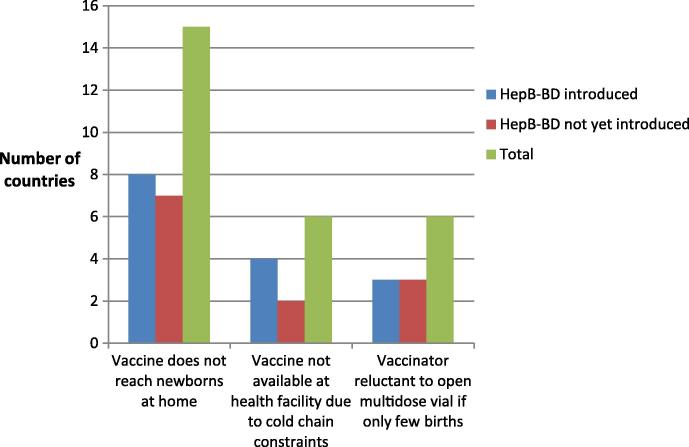
As illustrated in Fig. 1, the most commonly faced or anticipated challenge by countries regarding the provision of hepB-BD was “The vaccine does not reach newborns at home”, with 15 of the 24 countries responding to this question (63%) choosing this response. This was followed by “The vaccine is not available in all health facilities due to cold chain constraints” and “Health workers are reluctant to open multidose vials if only few births take place in a facility”, which each were selected by six (25%) countries. Two AFR countries stated that they did not face any issues in hepB-BD provision. There was no difference in responses between those countries, which implement universal hepB-BD and those that do not.
Fig. 1.
Perceived challenges to Hepatitis B birth dose provision.
It is most common that newborns receive hepB-BD in health facilities either by immunization staff (17/25, 68%)), by obstetrics/newborn staff (15/25, 60%)) or in an EPI clinic (11/25, 44%). Provision by a skilled community health worker or midwife at home was indicated by three countries in WPR and one country in AFR. Three countries expressed the need for good training or the option of providing this vaccine in oral form, instead of injectable.
3.2. Role of CTC in the hepatitis B birth dose provision
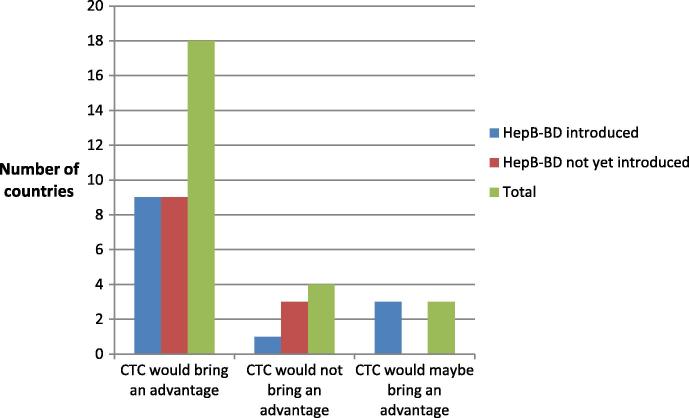
Eighteen out of the 25 responding countries (72%) confirmed that a Hepatitis B vaccine licensed for CTC would facilitate the provision of birth doses (seven from WPR and 11 from AFR), while three, all of which provide hepB-BD, responded “maybe”. The status of hepB-BD introduction was not listed to be a significant factor (see Fig. 2).
Fig. 2.
Number of countries considering that CTC would bring an advantage to HepB BD provision.
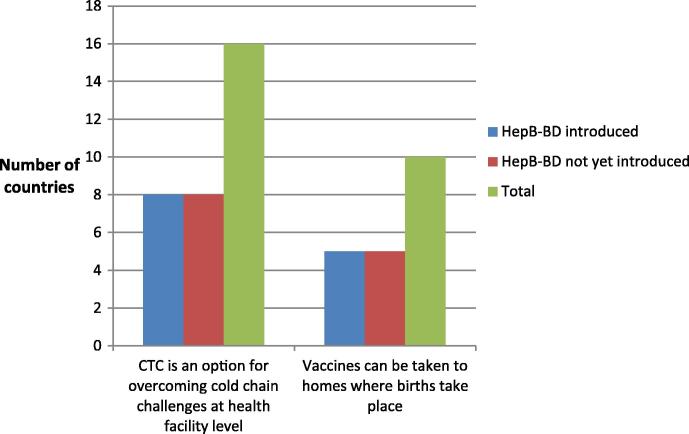
The main relief that countries suggested CTC would bring was in supplying vaccines to facilities without adequate cold chain equipment (16 countries (64%), 6 in WPR and 10 in AFR) and bringing vaccines to home deliveries (10 countries (40%), 4 in WPR and 6 in AFR) with a number of countries choosing both responses. Responses were evenly distributed between countries having introduced hepB-BD and those not yet having introduced hepB-BD (see Fig. 3). This indicates that, though only six countries acknowledged continuous cold chain at facilities as a challenge, compared to 15 countries claiming it is difficult to reach babies born at home, more countries view CTC as an option for overcoming cold chain constraints (16 countries) at the health facility level rather than as an option for facilitating vaccine administration during home deliveries (10 countries). Only five of the 16 countries, which consider that CTC can help overcome cold chain challenges at facility level, had indicated continuous cold chain as a barrier to HepB-BD provision.
Fig. 3.
Challenges that could be overcome by CTC.
A country that claimed no difficulty with the cold chain, nevertheless stated that CTC would be useful, given the challenge of constant cold chain monitoring. The four countries, which indicated that CTC would not bring any advantages reported that they did not have any cold chain issues and virtually no home births. One also indicated that HepB-BD was not presently a priority.
3.3. Price and procurement
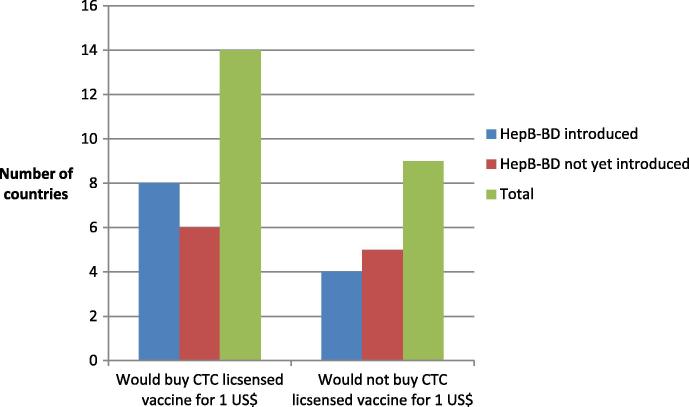
Of the 25 countries that participated in this survey, 14 (56%) (five from WPR and nine from AFR) would procure a HepB vaccine licensed for CTC for 28 days at 40 °C at the price of 1 US$ per dose and nine stated they would not (six from AFR, three from WPR). Two countries did not respond to this question. Of those 14 countries not opposed to 1 US$ per CTC dose, 11 reported intended use for all births (3 in WPR, 8 in AFR); one country in WPR indicated use only for home birth and two countries in AFR opted only for facilities without continuous cold chain. Slightly more countries that currently implement hepB BD would buy the CTC-licensed vaccine for 1 US$, than those that have not yet introduced hepB BD (see Fig. 4).
Fig. 4.
Number of countries, which would buy the vaccine for 1 US$.
The main reason for opposition to 1 US$ per dose was that it was too high; many countries were outspoken about vaccine prices being challenging for their governments to cover, including those that receive support from Gavi, the Vaccine Alliance. Four of the nine countries, which had said that they would not buy the vaccine at this proposed price indicated that they were already struggling to pay for their current vaccines (1 in WPR; 3 in AFR). The remaining countries reiterated that they did not need a CTC-licensed vaccine due to a lack of cold chain issues. Only one country stated that price was not an issue, though they also did not consider CTC of interest, given their sufficient command on their cold chain.
Countries stating that they would buy such a vaccine had a percentage of home births between 12% and 85%, with an average of 46% according to 2016 WUENIC data [20]; whereas most of those stating they would not buy this vaccine, had a percentage of home births between 0.2 and 9% [20]. Only one country with a high percentage of home births (72%) nevertheless suggested not being able to afford the vaccine. One country indicated they would not purchase a CTC vaccine due to the required training.
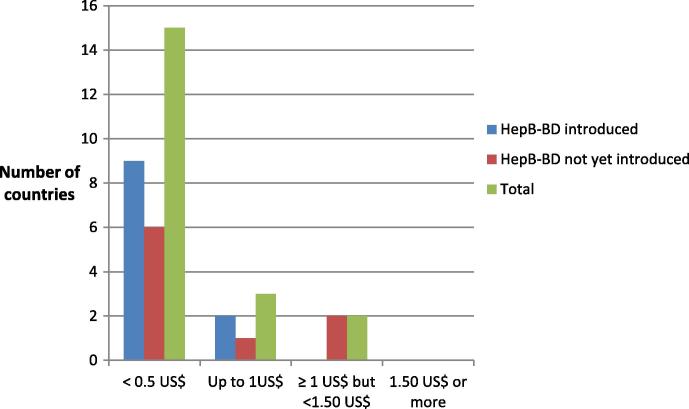
When asked about a more favourable price for a CTC-licensed vaccine, 15 countries (60%) indicated “below 0.50 US$”, (11 in AFR and five in WPR), three indicated “up to 1.00 US$” (one in AFR and two in WPR), and two (both in AFR) indicated ≥1.00 US$, but <1.50 US$ (see Fig. 5). Five countries did not respond to this question, but their comments together with the above information suggested that more countries would be interested in this vaccine if it were available for less than 0.50 US$.
Fig. 5.
Price countries are willing to pay for a Hepatitis B vaccine licensed for use in a CTC.
Besides pricing, current hepB-BD coverage also seems to influence interest in a CTC-licensed vaccine. Countries that have already introduced hepB-BD and attained high coverage (96–100%) reported that they would not buy a vaccine licensed for use in a CTC, while those with low coverage (32–65%) would. One country stated that they were very interested in using hepB-BD in a CTC as their hepB-BD coverage had been stagnating, and HBV prevalence was still high. Two countries with high coverage of 98 and 99% indicated that they would buy such a vaccine, with one of these countries reporting CTC-licensed HepB-BD as a facilitator to reach disaster affected areas.
Only one country pointed out potential advantages that CTC could bring to efforts to reduce high HBV endemicity.
3.4. Understanding of CTC by responding countries
One objective of this survey had been to determine the most desirable duration for a CTC excursion with hepB-BD. Responses across countries were quite varied and did not allow for a useful conclusion. Furthermore, it became apparent that many respondents had not fully understood the concept of CTC, with this being particularly the case in AFR.
4. Discussion
The main driver in conducting this survey was the need to gauge potential demand among countries for a CTC-licensed hepatitis B vaccine, which appears to be unknown and unstudied to date. While the results offer valuable insights, they must be interpreted carefully, taking into account three main limitations: (1) only two out of six WHO regions participated in this survey, limiting the generalizability of findings, and the AFR response rate was relatively low; (2) the vaccine under discussion had yet to be introduced by the majority of countries surveyed; and (3) the CTC approach under discussion, which is new and complex, may not have been fully understood by respondents, especially in countries which have not introduced birth dose or had no prior OCC experience.
The low response rate could be explained by unfamiliarity with CTC as a subject, thereby possibly rendering the survey a low priority. Likewise, countries that have yet to introduce hepB-BD may consider themselves insufficiently knowledgeable to answer questions related to hepB-BD. This is corroborated by the fact that five of the six AFR countries implementing hepB-BD responded to this survey.
Overall, countries surveyed would be interested in a hepatitis B vaccine licensed for CTC to facilitate reaching home deliveries and facilities without continuous cold chain, though only if this vaccine were available for less than 1US$. Currently, hepB-BD is not supported financially by Gavi, which might send the message to countries that this vaccine is less important. Gavi’s next Vaccine Investment Strategy cycle, during which decisions are made about the vaccine introductions to be supported, will be considering hepB-BD, as well as the new delivery mechanisms and policy changes required for its introduction.
When considering vaccine pricing, total system costs should be taken into account, along with other factors, such as facilitation of vaccine administration and potential savings linked to the prevention of chronic HBV infection. Only one country mentioned the cost of treating chronic infection and consequent interest in a Hepatitis B vaccine that can be used in a CTC.
Countries with a high percentage of home births appeared to clearly see CTC as a potential facilitator for the provision of hepB-BD. It was not apparent whether these same countries would ensure the vaccine is brought to home deliveries. However, it may be premature to discuss this option when the majority of these countries have not yet introduced hepB-BD.
The survey highlights the need for comprehensive orientation and training for countries on the subject matter, including raising awareness that CTC is an opportunity to reduce implementation costs and increase program efficiencies, thereby allowing for higher coverage and equity, rather than just a potential solution to cold chain constraints. This applies even more to AFR countries, where the CTC practice is relatively new in contrast to WPR’s long-standing promotion and experience in using hepatitis B vaccine out of the cold chain [8], [9], [10], [21], [22].
This survey was conducted shortly after the 2016 WHA resolution which endorsed a plan and targets of the Global Health Sector Strategy on Viral Hepatitis, 2016–2021, setting targets of 50% hepB-BD coverage by 2020 and 90% by 2030 [3]. This resolution is likely to have an impact on demand for a CTC-licensed hepatitis B vaccine, as commitment to hepatitis B vaccination is growing in different regions. This is particularly demonstrated by the South-East Asia Region’s Immunization Technical Advisory Group’s (SEAR-ITAG) request that member states conduct a systematic review of hepatitis B vaccination coverage to identify gaps and causes of under immunization- especially with regard to birth dose and to implement strategies to bring coverage to target levels. These recommendations of the Seventh SEAR-ITAG Meeting (held in 2016) were made after this survey was conducted.
HepB-BD was identified as a priority vaccine by the CTC Working Group under IPAC, as it is expected that CTC will bring advantages to outreach activities targeting babies born at home as well as provision of the Hep B vaccine in small rural facilities lacking adequate cold chain capacity. Currently, there exist seven WHO prequalified Hepatitis B vaccines, none of which are licensed for use in a CTC to date. However, a few manufacturers are currently working towards licensing hepatitis B vaccine products for CTC use. Communicating country interest for a CTC-compatible HepB-BD to manufacturers will be impactful. Moreover, increasing awareness at country level about the potential benefits associated with the CTC approach will be key to boosting interest and eventual uptake of this valuable vaccine delivery strategy, and in turn improving coverage and equity.
Acknowledgment
This survey was conducted as part of a grant from the Bill and Melinda Gates Foundation
Declarations
a. The authors do not declare any conflict of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the World Health Organization.
b. Ethical approval: This study did not involve any clinical trials on humans.
References
- 1.McMahon B., Alward W.L., Hall D.B., Heyward W.L., Bender T.R., Francis D.P., Maynard J.E. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis. 1985;151:599–603. doi: 10.1093/infdis/151.4.599. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Hepatitis B vaccines WHO position paper. 2017; http://apps.who.int/iris/bitstream/10665/255841/1/WER9227.pdf?ua=1.
- 3.World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016-2021; 2016 http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf?ua=1.
- 4.World Health Organization. WHO/IVB Database as at 14 April 2017 and ECDC published data; 2017 http://vaccine-schedule.ecdc.europa.eu/Pages/Scheduler.aspx.
- 5.World Health Organization. Practices to improve coverage of the hepatitis B birth dose vaccine; 2013 http://apps.who.int/iris/bitstream/10665/78616/1/WHO_IVB_12.11_eng.pdf.
- 6.Levin C.E., Nelson C.M., Widjaya A., Moniaga V., Anwar C. The costs of home delivery of a birth dose of hepatitis B vaccine in a prefilled syringe in Indonesia. Bull World Health Org. 2005;83:456–461. [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond-Smith N., Sudhinaraset M. Drivers of facility deliveries in Africa and Asia: regional analysis using the demographic and health surveys. Reprod Health. 2015 doi: 10.1186/1742-4755-12-6. 12.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization Western Pacific Region. Preventing Mother-to-Child Transmission of Hepatitis B. Operational Field Guidelines for Delivery of the Birth Dose of Hepatitis B Vaccine; 2006 http://www.wpro.who.int/entity/hepatitis/resource/hepb_operationalfieldguidelines.pdf.
- 9.Kolwaite A., Yeuatvongsa A., Ramirez-Gonzalez A., Wannemuehler K., Vongxay V., Vilayyone V., Hennessey K., Patel M. Hepatitis B vaccine stored outside the cold chain setting: a pilot study in rural Lao PDR. Vaccines. 2016;34:3324–3330. doi: 10.1016/j.vaccine.2016.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L., Li J., Chen H., Li F., Armstrong G., Nelson C., Ze W., Shapiro C. Hepatitis B vaccination of newborn infants in rural China: evaluation of a village-based, out-of-cold chain delivery strategy. Bull World Health Org. 2007;85:688–694. doi: 10.2471/BLT.06.037002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huong VM, Hipgrave D, Hills S, Nelson C, Hien DS, Cuong NV. Out-of-Cold-Chain delivery of the Hepatitis B birth dose in four districts of Vietnam. PATH; 2006 http://www.path.org/publications/files/TS_hepb_coldchain_vietnam.pdf.
- 12.Breakwell L., Anga J., Dadari I., Sadr-Azodi-Sadr N., Ogaoga D., Patel M. Evaluation of storing hepatitis B vaccine outside the cold chain in the Solomon Islands: Identifying opportunities and barriers to implementation. Vaccine. 2017;35:2770–2774. doi: 10.1016/j.vaccine.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Controlled Temperature Chain (CTC) http://www.who.int/immunization/programmes_systems/supply_chain/ctc/en/.
- 14.Immunization Practices Advisory Committee (IPAC). Immunization Practices (IPAC) Statement Out of Cold Chain (OCC) and Controlled Temperature Chain (CTC) use of vaccines; 2016 http://www.who.int/immunization/programmes_systems/policies_strategies/IPAC_statement_OCC_CTC_October_2016.pdf?ua=1.
- 15.World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization October 2016. Weekly epidemiological record 2016 http://apps.who.int/iris/bitstream/10665/251810/1/WER9148.pdf?ua=1.
- 16.Zipursky S., Djingarey M.H., Lodjo J.C., Olodo L., Tiendrebeogo S., Ronveaux O. Benefits of using vaccines out of the cold chain: delivering meningitis A vaccine in a controlled temperature chain during the mass immunization campaign in Benin. Vaccine. 2014;32(13):1431–1435. doi: 10.1016/j.vaccine.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lydon P., Zipursky S., Tevi-Benissan C., Djingarey M.H., Gbedonou P., Youssouf B.O., Zaffran M. Economic benefits of keeping vaccines at ambient temperature during mass vaccination: the case of meningitis A vaccine in Chad. Bull World Health Org. 2014;92:86–92. doi: 10.2471/BLT.13.123471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mvundura M, Lydon P, Gueye A, Diaw IK, Landoh DE, Toi B, Kahn AL, Kristensen D An economic evaluation of the controlled temperature chain approach for vaccine logistics: evidence from a study conducted during a meningitis A vaccine campaign in Togo. Pan African Med J 2017; Supp 3: 27. [DOI] [PMC free article] [PubMed]
- 19.Sutanto A., Suarnawa I.M., Nelson C.M., Stewart T., Soewarso I. Home delivery of heat-stable vaccines in Indonesia: outreach immunization with a prefilled, single-use injection device. Bull World Health Org. 1999;77:119–126. [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization and UNICEF. WHO/UNICEF Estimates of National Immunization Coverage (WUENIC); 2016 http://www.who.int/immunization/monitoring_surveillance/data/en/.
- 21.Hipgrave D., Maynard J., Biggs B.A. Improving birth dose coverage of hepatitis B vaccine. Bull World Health Org. 2006;84:65–71. doi: 10.2471/blt.04.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hipgrave D., Tran T.N., Huong V.M., Dat D.T., Nga N.T., Long H.T., Van N.T., Maynard J., Biggs B.A. Immunogenicity of a locally produced hepatitis b vaccine with the birth dose stored outside the cold chain in rural Vietnam. Am J Tropical Med Hygiene. 2006;74:255–260. [PubMed] [Google Scholar]