Abstract
Background
Brief interventions (BIs) delivered in primary care have been shown to be effective in reducing risky drinking, but implementation is limited. Facilitated access to a digital application offers a novel alternative to face-to-face intervention, but its relative effectiveness is unknown.
Methods
Primary care-based, non-inferiority, randomised controlled trial comparing general practitioner (GP) facilitated access to an interactive alcohol reduction website (FA) with face-to-face BI for risky drinking. Patients screening positive on the short Alcohol Use Disorders Identification Test (AUDIT-C) were invited to participate in the trial. Assessment at baseline, 3 months and 12 months was carried out using AUDIT and EQ-5D-5L questionnaires.
Findings
58 participating GPs approached 9080 patients of whom 4529 (49.9%) logged on, 3841 (84.8%) undertook screening, 822 (21.4%) screened positive and 763 (19·9%) were recruited. 347 (45.5%) were allocated to FA and 416 (54.5%) to BI. At 3 months, subjects in FA group with an AUDIT score of ≥8 reduced from 95 (27.5%) to 85 (26.8%) while those in BI group increased from 123 (20.6%) to 141 (37%). Differences between groups were principally due to responses to AUDIT question 10. Analysis of primary outcome indicated non-inferiority of FA compared with BI, and prespecified subgroup analysis indicated benefits for older patients and those with higher levels of computer literacy and lower baseline severity. Additional analyses undertaken to take account of bias in response to AUDIT question 10 failed to support non-inferiority within the prespecified 10% boundary.
Interpretation
Prespecified protocol-driven analyses of the trial indicate that FA is non-inferior to BI; however, identified bias in the outcome measure and further supportive analyses question the robustness of this finding. It is therefore not possible to draw firm conclusions from this trial, and further research is needed to determine whether the findings can be replicated using more robust outcome measures.
Trial registration number
NCT01638338; Results.
Keywords: Mental health, Health informatics, Public health
Strengths and limitations of this study.
The trial evaluated a potentially important development for primary care, namely the use by general practitioners of facilitated access to a digital application as an alternative to traditional face-to-face consultation, in this case for patients with risky drinking.
All components of the trial were delivered online with the exception of the face-to-face intervention, thus, reducing the cost of the trial, allowing real-time tracking of the findings, ensuring consistency of conduct and avoiding errors of transcription.
Follow-up rates exceeded 90% at 3 months and 80% at 12 months.
Levels of hazardous and harmful drinking in trial participants were lower than anticipated.
Probable bias in the brief intervention group indicates that caution should be exercised in interpreting the main findings.
Introduction
Alcohol is the third leading cause of diseases and premature death globally1 and accounts for 3.8% of deaths and 4.6% of disability-adjusted life years.2 Brief interventions (BIs) delivered in primary healthcare settings have been demonstrated repeatedly to be effective in reducing hazardous and harmful drinking.3 However, barriers prevent their widespread implementation, including insufficient training, lack of resources and constraints in time.4 Digital applications including websites and apps that are based on behaviour change techniques may be helpful in overcoming these barriers,5 6 and clinicians may actively encourage patients to use approved applications through a process known as facilitated access. Initially adopted primarily for the management of patients with mental health problems including depression and anxiety, facilitated access has been extended to digital applications for addictive behaviours including smoking cessation and alcohol screening, and to health promotion and the management of some long-term conditions.7 8
Facilitated access offers a novel alternative to face-to-face BI for risky drinking, but it is not known whether it is as effective. A review of trials of computer-based interventions offered to college drinkers found them to be more effective than no treatment and as effective as alternative treatment approaches.9 A systematic review of electronic interventions for risky drinkers concluded that there were significant reductions in weekly alcohol consumption between intervention and control conditions between 3 months and less than 12-month follow-up, indicating this may be an effective intervention.10
A review of digital and computer-based alcohol intervention programmes promoted in primary care settings identified 15 small-scale trials of which 9 were associated with a reduction in alcohol use at follow-up.11 The indications from these studies about the likely effectiveness and cost-effectiveness of internet applications in primary care were generally positive, but firm conclusions could not be drawn because of limitations of sample size and study design. An adequately powered and appropriately designed trial was, therefore, required to provide more definitive evidence on the use of facilitated access as an alternative to face-to-face BI for the reduction of hazardous and harmful drinking and to indicate the potential for this approach to be adopted more generally in the management of health conditions by general practitioners (GPs). The research question addressed by the trial was ‘Is facilitated access to an interactive alcohol reduction website as effective in reducing hazardous and harmful drinking as face-to-face BI?’
Methods
Study design
Primary care-based, non-inferiority, randomised controlled trial of BI for risky drinkers comparing GP facilitated access to an interactive alcohol reduction website (facilitated access) with standard face-to-face BI. With the exception of face-to-face intervention, all components of the trial were delivered online to patients following receipt of a brochure describing the website and providing a unique trial log-on number. Access to the website was via the healthy lifestyle portal of the official website of the Region of Friuli-Venezia Giulia (www.downyourdrink.org.uk/). GPs were recruited via the official register of the Friuli-Venezia Giulia region of Northern Italy. All participating GPs attended a 1-day training event including an overview of the trial and interactive sessions on the delivery of face-to-face BI using the principles of brief motivational interviewing. They were encouraged to familiarise themselves with the trial website and to use the menu-driven online GP personalisation facility to create their own tailored patient messages at up to four key points of the programme (see figure 1). They were also given brief guidance about how to actively encourage patients to access the website.
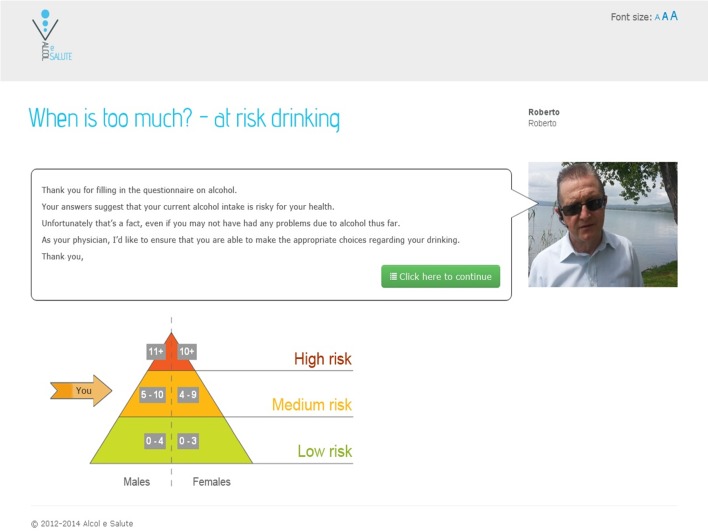
Figure 1.
Screenshot showing tailored feedback on Alcohol Use Disorders Identification Test with general practitioner personalisation (translated from original Italian).
The protocol was approved by the Isontina Independent Local Health Unit Ethics Committee on 14 June 2012.12
Patients
All patients aged ≥18 years who attended the participating practices during the study period were eligible for the trial, but those known by the GPs to suffer from severe psychiatric disorder, alcohol dependence, serious visual impairment or terminal illness were excluded, as were those judged to have inadequate command of the Italian language.
Randomisation and masking
Randomisation was at the individual level and was automated, concealed and undertaken online using software which generated randomisation with an allocation ratio 1:1. There was no stratification or blinding.
Procedures
For the purposes of screening, GPs spoke briefly to eligible patients, gave them a trial brochure with a unique login code and actively encouraged them to access the trial website. Those who logged on were asked to complete the three-question short Alcohol Use Disorders Identification Test (AUDIT-C)13 and to provide consent for the result of the test to be sent to their practice. For the purposes of the trial, cut points of 4 for women and 5 for men were used to identify probable hazardous or harmful drinkers. Patients screening below the cut points received an online message advising that their responses indicated that their stated drinking patterns fell within the guidelines for sensible drinking. Those scoring at or above the cut points received a personalised online message from their GP advising that their stated drinking patterns indicated that they were likely to be at risk from their drinking and encouraging them to take part in the study. They were then invited to review the online patient information leaflet and to complete the consent module. Following consent, patients were invited to complete online questionnaires including a questionnaire seeking information on age, gender, level of education and occupation, the 10-question AUDIT validated Italian version14 15 and the EQ-5D-5 L quality-of-life questionnaire, validated Italian version.16 Completion of baseline questionnaires was followed automatically by concealed online randomisation to either facilitated access to the alcohol reduction website or to face-to-face BI. The alcohol reduction website was adapted from the Down Your Drink Website (http://www.downyourdrink.org.uk), details of which have been reported elsewhere.17 Country-specific information for Italy such as the recommended guidelines for alcohol intake, definitions of standard drinks and alcohol-related laws were included in the website. The website was further adapted to include a menu-driven facility which enabled the participating GPs to create personalised automated tailored online messages for their patients. These were available at four key points in the programme, and included options to customise written text, add photographs and insert audio/video recorded messages.18 An example of a screenshot of tailored feedback with GP personalisation is shown in figure 1.
Patients allocated to facilitated access were directed to the opening page of the alcohol reduction website containing a personalised online message from their GP with tailored feedback about their responses to the AUDIT questionnaire. Further online messages emphasised the importance of adopting healthy drinking choices. They provided encouragement to spend at least 15 min engaging with the alcohol reduction website, including making entries in the personal Thinking Drinking Record (TDR) about their assessment of costs and benefits of their current levels of drinking. An automated email was sent 1 week later encouraging further log on. Patients were also asked online to review their alcohol consumption and were invited to discuss their website experience when they next saw their GP.
Patients allocated to face-to-face BI were invited to check a box online which automatically generated an email to their GP requesting an appointment within the next 7–10 days. GPs were instructed to offer a BI lasting 5–15 min based on the brief motivational interview.19 Non-attenders were offered up to three additional appointments.
Follow-up assessment
Follow-up took place 3 and 12 months after randomisation and a series of approaches were adopted to optimise response rates. In the first instance, each patient in the trial received an automated email requesting them to login to the website to complete their assessment questionnaires. Failure to do so resulted in further automated emails at 1-week and 2-week intervals. Persistent failure was notified to the patient’s GP, who was asked to ensure that they were contacted by letter, phone or in person in order to complete their assessment. Where necessary, assessment was completed over the phone.
Outcomes
The prespecified primary outcome measure was the proportion of hazardous or harmful drinkers as defined by a score of ≥8 points on the AUDIT questionnaire at 3-month follow-up.20 The secondary outcome measure was the EQ-5D quality of life questionnaire, validated Italian version for use in economic evaluations. Advice to seek additional medical advice was given online to all patients scoring >20 on the AUDIT. Regular checks of the quality of the data were carried out under the supervision of the research team. Data files generated by the patients’ interactions with the alcohol reduction website were stored securely on servers in accordance with EU regulations. The only identifiers were the unique login number. The files generated by the practices linking the unique login numbers to the patient identifiers were stored securely along with other clinical data in the practice and were accessible only to practice staff.
Statistical analysis
Facilitated access was deemed not inferior to face-to-face treatment at a one-sided α of 2.5% if the difference between the proportions of hazardous or harmful drinkers in the facilitated access group and the face-to-face BI group was below a specified absolute margin of non-inferiority of 10%. Assuming a reduction of 30% in the proportion of hazardous or harmful drinkers in the face-to-face BI group and allowing for an overall attrition of 10% of patients in the trial, it was calculated that 500 patients would be required in each group to give the trial 90% power (1-β) to reject the null hypothesis that facilitated access is inferior to face-to-face intervention. Analyses were described in a statistical analysis plan completed before database lock. To assess the non-inferiority of facilitated access compared with face-to-face BI, the proportions of hazardous or harmful drinkers in each group were computed and compared using generalised non-linear mixed models accounting for general practices as random effects in order to address possible therapist effects and other practice level clustering. Additional, prespecified, supportive analyses designed to provide further information about the trial outcomes were conducted as follows: Supportive 1=random intercept term for practices and baseline values for hazardous or harmful drinkers; Supportive 2=included a random residual term in replacement for the generalised random intercept term and baseline values for hazardous/harmful drinkers; Supportive 3=AUDIT score as a continuous outcome, including the baseline AUDIT score as a patient level explanatory variable, with generalised random intercept terms for GP practices.
Post hoc analyses were designed to address the unexpected finding that less than 30% of the participants were classified at baseline as hazardous or harmful drinkers by a score of ≥8 points on AUDIT, and the unexpected rise at follow-up in the proportions of patients in the face-to-face BI group scoring ≥8 points on AUDIT. Analysis was therefore carried out for the 3 months principal outcome measure on the basis of subjects who were and were not, classified as hazardous or harmful drinkers at baseline, and additionally by removing the final question of the AUDIT which may have introduced bias favouring the experimental condition. All calculations were performed on the basis of intention to treat. An independent trial steering committee oversaw the general conduct of the trial and undertook data monitoring.
Health economic analysis was undertaken to evaluate the cost-effectiveness of facilitated access to a website for hazardous drinkers compared with face-to-face BI, and the findings are reported in a separate paper.21
Results
The trial was conducted in two phases—a pilot phase involving 11 GPs who recruited 89 subjects between 14 January 2013 and 31 May 2013,1and the main trial phase involving 58 GPs who recruited 674 subjects between 20 January 2014 and 31 August 2014. The trial design was identical in both phases. Brochures were distributed to a total of 9080 patients across the 58 practices, and resulted in 4529 (49.9%) patients logging on to the healthy lifestyle website. Of these, 3841 (84.5%) undertook screening with the AUDIT-C and 822 (21.4%) screened positive. Of the screen positives, 763 (92.8%) were recruited to the trial, following consent, completion of baseline measures and randomisation. The minimum number of subjects recruited per practice was 1 and the maximum 89. The median number of subjects recruited per practice was 10 and the IQR was 3–19.
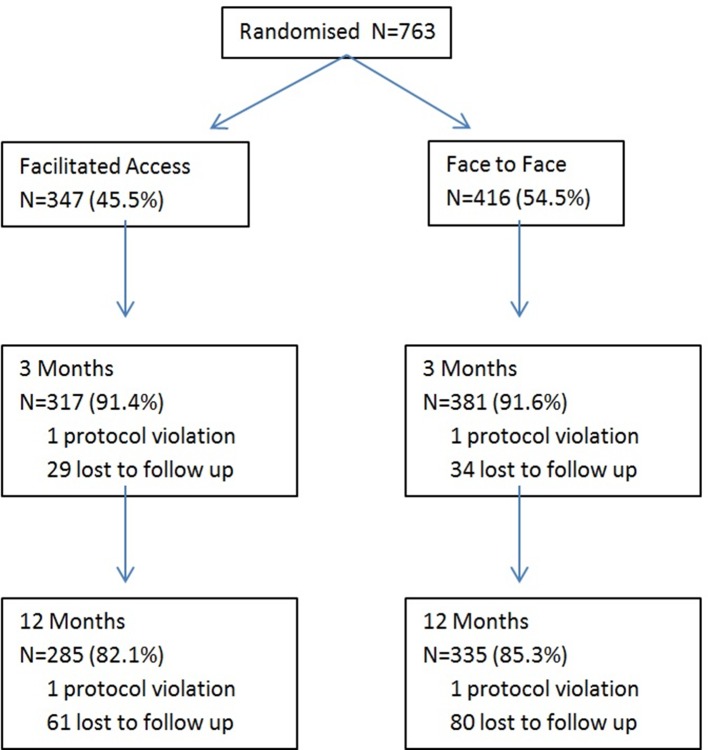
Figure 2 describes the progress of the 763 subjects through the trial. Three hundred and forty-seven (45.5%) subjects were allocated to facilitated access to the alcohol reduction website and 416 (54.5%) to face-to-face BI. A total of 698 (91.5%) subjects completed the 3-month follow-up assessment and 620 (81.2%) the 12-month follow-up assessment. One subject was excluded due to inadvertent randomisation to both the intervention and control groups.
Figure 2.
Subject progress through the trial.
Baseline characteristics
Table 1 describes the baseline characteristics of the subjects in each group. The median age of the subjects was 49 years (IQR 35–61) and 469 (61.9%) were men. The median score on the AUDIT was 5.5 (IQR 4–9). Two hundred and eighteen (28.6%) of the participants were classified at baseline as hazardous or harmful drinkers by a score of ≥8 points on the AUDIT.
Table 1.
Baseline characteristics
| Item | Facilitated access n=346 | Face-to-face n=415 |
| Male (%) | 214 (62.0) | 255 (61.9) |
| Marital status (%) | ||
| Single | 95 (27.9) | 116 (28.4) |
| Married | 208 (61.0) | 247 (60.4) |
| Separated | 28 (8.2) | 36 (8.8) |
| Widowed | 10 (2.9) | 10 (2.4) |
| Ethnicity (%) | ||
| Bengali | 1 (0.3) | 1 (0.25) |
| Indian | 1 (0.3) | 2 (0.5) |
| Italian | 328 (98.2) | 391 (97.8) |
| North African | 0 (0) | 1 (0.25) |
| Mixed race | 1 (0.3) | 1 (0.25) |
| Black African | 3 (0.9) | 4 (1.0) |
| Familiarity with IT (%) | ||
| Not familiar | 58 (16.9) | 62 (15.2) |
| Fairly familiar | 84 (24.5) | 93 (22.8) |
| Familiar | 91 (26.5) | 119 (29.2) |
| Very familiar | 110 (32.1) | 134 (32.8) |
| Qualifications (%) | ||
| None | 2 (0.6) | 2 (0.5) |
| Elementary/junior school | 112 (32.9) | 126 (30.9) |
| High school | 174 (51.2) | 184 (45.1) |
| University | 45 (13.2) | 78 (19.1) |
| Higher degree | 7 (2.1) | 18 (4.4) |
| Age, median (IQR) | 49 (37–59) | 50 (35–61) |
| No of children, median (IQR) | 1 (0–2) | 1 (0–2) |
| AUDIT-10, median (IQR) | 5 (4–8) | 6 (4–9) |
| Hazardous/harmful drinker (AUDIT ≥8) (%) | 95 (27.5) | 123 (29.6) |
AUDIT: Alcohol Use Disorders Identification Test;
Engagement with face-to-face BI and facilitated access
Of the 416 patients allocated to face-to-face BI, 325 (78.1%) were offered an appointment and 304 (73.1%) received a BI from their GP. Of the BIs, 171 (56.3%) were recorded as lasting less than 5 min, 87 (28.6) from 5 to 10 min and 46 (15.1%) more than 10 min.
Table 2 describes engagement with the alcohol reduction website by the 342 patients in the facilitated access group as assessed in terms by numbers of logins, numbers of pages downloaded and the numbers of occasions on which an entry was made to the TDR section of the website.
Table 2.
Engagement with alcohol reduction website by patients in facilitated access group (n=346)
| Engagement variable | Mean (SD) | IQR |
| User logins/patient | 1.2 (0.85) | 1–1 |
| User page views/patient | 33.5 (75.17) | 1–41 |
| TDR* total submissions/patient | 18.5 (22.54) | 3–27 |
| TDR* total records/patient | 14.8 (16.53) | 3–22 |
| TDR* total pages/patient | 6.9 (6.88) | 2–10 |
*TDR, Thinker Drinker Record entries made by patients on website pages.
AUDIT scores
At baseline, 95 (27.5%) of the patients allocated to facilitated access were classified as hazardous or harmful drinkers by a score of ≥8 points on the AUDIT, compared with 123 (20.6%) of the patients allocated to face-to-face BI.
The numbers (%) of risky drinkers at the three assessment points of the trial are shown in table 3.
Table 3.
Number of risky drinkers at baseline, 3 and 12 months by randomised condition
| Time period, n in follow-up | Face-to-face, n (%) | Facilitated, n (%) |
| Baseline, n=761 | 123 (29.6) | 95 (27.5) |
| 3 months, n=698 | 141 (37.1) | 85 (26.8) |
| 12 months, n=620 | 88 (26.3) | 71 (24.9) |
In the patients assessed at 3 months, the number in this category in the facilitated access group reduced to 85 (26.8%) while in the face-to-face BI group it rose unexpectedly to 141 (37%), dropping at 12 months to 88 (26.3%). The difference at 3 months was largely accounted for by responses to AUDIT question 10: “Has a relative or friend, doctor or other health worker been concerned about your drinking or suggested that you cut down?”
Prespecified analyses
Table 4 describes the results for the prespecified analysis of the main outcome at 3 months and the additional supportive analyses.
Table 4.
Primary analysis and supportive analyses
| Analysis | Estimate | Lower 95% CI | Upper 95% CI | p-Value |
| Primary—proportion of hazardous or harmful drinkers (OR) | 0.63 | 0.45 | 0.89 | 0.008 |
| Supportive analysis 1* (OR) | 0.62 | 0.43 | 0.90 | 0.012 |
| Supportive analysis 2† (OR) | 0.61 | 0.42 | 0.88 | 0.009 |
| Supportive analysis 3‡ (OR) | −0.17 | −0.58 | 0.25 | 0.43 |
*Proportion of hazardous or harmful drinkers; including baseline values for risky drinkers and random intercept term for practice.
†Including a random residual term in replacement for the generalised random intercept term for practice and baseline values for risky drinkers.
‡Alcohol Use Disorders Identification Test-10 score as a continuous outcome, including the baseline score as a patient level explanatory variable, with generalised random intercept terms for general practitioner practices.
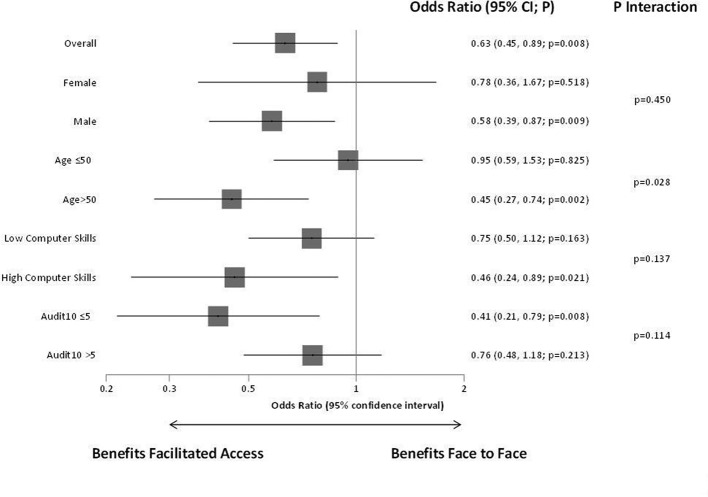
Analysis of the primary outcome, difference in the odds of hazardous and harmful drinkers, shows statistically significant benefit for facilitated access compared with face-to-face BI. This is replicated in the additional prespecified analyses and in all cases non-inferiority for facilitated access was demonstrated. Figure 3 describes the effects and interactions for the prespecified subgroups. There was a significant interaction for age, and some indication of an interaction effect for computer literacy and baseline severity.
Figure 3.
Primary outcome—prespecified subgroup analyses.
Table 5 describes the results of the analyses at 12 months on the proportion of hazardous or harmful drinkers per group, and the difference in mean AUDIT scores. The 12 months OR for hazardous or harmful drinking demonstrated non-inferiority of facilitated access compared with face-to-face BI, but non-inferiority was not demonstrated for the mean AUDIT scores at this time point.
Table 5.
Twelve-month results—difference in hazardous/harmful drinkers and mean AUDIT-10
| Analysis | Estimate | Lower 95% CI | Upper 95% CI | p Value |
| Hazardous/harmful drinkers (OR) | 0.943 | 1.432 | 0.621 | 0.784 |
| Mean AUDIT-10 | −0.3126 | −0.8159 | 0.1906 | 0.2229 |
AUDIT-10, Alcohol Use Disorders Identification Test-10.
Post hoc analyses
Table 6 shows the findings of post hoc analysis on the subsets of participants who were and were not hazardous/harmful drinkers at baseline. The analysis did not support non-inferiority of facilitated access at 3-month follow-up for those with hazardous/harmful drinking at baseline.
Table 6.
Hazardous/harmful drinking at 3 months by hazardous/harmful drinking at baseline
| Analysis | Estimate | Lower 95% CI | Upper 95% CI | p Value |
| Not hazardous/harmful drinkers at baseline n=545 (OR) |
0.476 | 0.289 | 0.782 | 0.004 |
| Hazardous/harmful drinkers at baseline n=218 (OR) | 0.772 | 0.431 | 1.383 | 0.382 |
Test for interaction between the groups, p=0.192.
Table 7 shows the proportions of participants classified as hazardous/harmful drinkers at 3 months and 12 months using a cut point of >7 on the AUDIT questionnaire with question 10 removed.
Table 7.
Proportions of hazardous/harmful drinking as defined by >7 points on Alcohol Use Disorders Identification Test with question 10 removed
| Time period, n in follow-up | Face-to-face, n (%) | Facilitated, n (%) |
| Baseline, n=761 | 93 (22.4) | 79 (22.8) |
| 3 months, n=698 | 28 (7.4) | 32 (10.1) |
| 12 months, n=620 | 27 (8.1) | 35 (12.3) |
Table 8 shows the results of further continuous and categorical analyses based on the AUDIT-C questions. Neither analysis supported non-inferiority of facilitated access.
Table 8.
Continuous and categorical analyses based on the Alcohol Use Disorders Identification Test (AUDIT-C) questions
| Analysis | Estimate | Lower 95% CI | Upper 95% CI | p Value |
| Risky drinkers on AUDIT-C (OR) | 1.555 | 2.127 | 1.136 | 0.006 |
| Difference in mean AUDIT-C score | −0.185 | −0.396 | 0.027 | 0.087 |
AUDIT-C, Alcohol Use Disorders Identification Test-C.
EQ-5D
The results of the EQ-5D are reported in a separate paper (Hunter et al, Randomised non-inferiority trial of primary care-based facilitated access to an alcohol reduction website: cost effectiveness analysis bmjopen in pressmanuscript ID: bmjopen-2016–0 14 577).
Discussion
As far as we are aware, this is the first trial comparing effectiveness of facilitated access by GPs to an alcohol reduction website with delivery of face-to-face BI. It has demonstrated that this approach can be successfully implemented in general practice, with 58 participating GPs each providing facilitated access to an average of more than 150 patients, and nearly half of the patients subsequently following their GP’s advice to log on and undertake screening. Furthermore, the great majority of patients randomised to facilitated access to the website went on to engage actively, downloading several pages and making multiple entries. The ODHIN trial which tested the relative impact on GP screening and BI activity of providing access to an alcohol reduction website (eBI), financial incentives and education and training, found that eBI was not associated with increased rates of activity.22 However, the training and familiarisation with the website offered to the GPs was almost certainly less rigorous than in the EFAR-FVG trial.23 Furthermore, the organisation of general practice in the five countries where ODHIN trial was conducted may have been less favourable to GP facilitated access.
The trial has a number of limitations. Fewer participants were recruited than the figure defined by the power calculation, and more importantly the AUDIT-C screening tool performed poorly as a predictor of hazardous or harmful drinking as defined by a score of ≥8 points on the AUDIT. This meant that the trial population included only a minority (29.6%) of hazardous/harmful drinkers as defined by an AUDIT score of ≥8. The resultant threshold effect was almost certainly responsible at least in part for the only modest reductions seen in the proportions of hazardous/harmful drinkers in both groups. The use of AUDIT-C cut points of 5 for men and 4 for women would have been expected to lead to the inclusion of substantially higher proportions of hazardous and harmful drinkers as defined by a score of 8 or more on the AUDIT.24 25 The AUDIT-C has also been validated in Italian populations and found to perform similarly.10 However a recent paper has suggested that higher cut points should be used to reliably identify risky drinkers.26 In addition, the statistical analyses assumed a similar clustering effect for both treatment conditions at the practice level, through fitting practice level random effects. It could be argued that differences in the intervention could lead to difference in the clustering within practices; however, our assumption a priori, based on substantial relevant experience, was that shared practice characteristics were likely to be the dominant factors in our analysis. Given the overall nature of the results and their interpretation, we have not undertaken further supportive analyses on this question.
The trial did not observe the scale of reduction in the proportions of hazardous or harmful drinkers in the patients following BI by their GPs which had informed our sample size calculation. Instead, there was a paradoxical increase in the proportion of patients in the face-to-face BI group categorised as hazardous or harmful drinkers at 3 months though this was not maintained at 12 months. We postulated that this was largely due to bias introduced by the final AUDIT question which asks about advice to reduce drinking from a healthcare professional, and might therefore be expected to elicit a positive response in the short-term following face-to-face BI. This hypothesis was supported by failure to confirm non-inferiority when the final question was omitted in the post hoc analysis.
The main strengths of the study include the size of the study population, numbers of GPs involved, high levels of facilitated access activity and high follow-up rates at both 3 months (91.5%) and 12 months (81.2%). The use of the Internet to deliver all components of the trial with the exception of the face-to-face intervention for patients in the control group reduced the cost of the trial, ensured consistency of conduct of all phases, avoided errors of transcription and enabled real-time tracking of trial activity by the study team. Furthermore, there were no reported breaches of data security.
Analysis of all prespecified outcome measures demonstrated evidence of non-inferiority for facilitated access versus face-to-face BI. On the face of it, this implies that a simple message given by the GP to the patient during facilitated access combined with provision of the log-on code for the alcohol reduction website was no less effective in prompting behavioural change than a 5–10 min BI delivered face-to-face. This is consistent with the findings of a number of studies, most notably the SIPS trial which found the outcomes in patients screening positive hazardous or harmful drinking provided with a patient information leaflet were no worse than for those given 5 min of structured brief advice or 20 min of brief lifestyle counselling.27 These findings are also consistent with much of the growing literature on the effectiveness of digital interventions indicating that users benefit from online alcohol interventions and that this approach may be particularly useful for groups less likely to access traditional alcohol-related services, such as women, young people and at-risk users.28
However, the reliability of the conclusions from the primary analyses is seriously called into question by the results of the post hoc analyses performed in order to deal with the presumptive evidence of response bias in the face-to-face group. When these were performed using both a subset of the questions on the AUDIT omitting question 10 and the three questions of the AUDIT-C, the results no longer supported the conclusion of non-inferiority of facilitated access. This raises real questions about the reliability of the trial’s main findings, and further research will be needed to determine whether these can be replicated. Alternative cut points on the screening AUDIT-C could be used to ensure the inclusion of greater proportions of hazardous/harmful drinkers in future studies, and an alternative outcome measure such as the timeline follow-back questionnaire29 could be used in order to avoid bias introduced by the AUDIT. It would also be helpful to replicate the trial in general practice settings involving larger clinical teams and greater numbers of registered patients. At least one such trial is currently underway in Catalunya, Spain.30 Additional study is also needed to improve understanding of the mechanisms underlying the impact of facilitated access and the conditions required to optimise it, including the role played by online GP personalisation.
Supplementary Material
Acknowledgments
The study was jointly supported by the Italian Ministry of Health and by the Region Friuli-Venezia Giulia, Italy (grant number: D25E12002900003). The funders had no role in the conception, preparation, review, approval or submission of this manuscript. We thank Dr Donatella Ferrante and Dr Francesco Marcatto for their support with the analyses and Dr Alaman Allamani for chairing of the Trial Steering Committee.
Footnotes
Contributors: PW, PS and RDV conceived the study and together with NF developed the design. PS, PW, RDV, CT, CL and RMG were responsible for the development of the website, and PW, PS, FS, RDV, CT were responsible for the management of the study and follow-up of patients. NF was responsible for statistical analyses and RH for the health economic analyses. PW, PS and NF wrote the first draft and RH, PW, PS and NF contributed to its revision. ES participated in the management of the study and contributed to the review of the manuscript and its final approval, together with all the other authors.
Funding: This study was jointly supported by the Italian Ministry of Health and by the regional school for the training in Primary Care of the Region Friuli-Venezia Giulia, Italy (grant number: D25E12002900003). The funders had no direct influence over the design or conduct of the study.
Competing interests: PW has intellectual property rights for www.downyourdrink.org.uk, is chief medical advisor to the UK charity Drinkaware and has provided private consultancy on the topic of screening and brief interventions to several agencies. CL is the co-founder and chief executive officer at Lumos Medica Srl, which provides software solutions for clinical trials. The other authors declare no competing interests.
Patient consent: No personal medical information about an identifiable living individual is included in this paper.
Ethics approval: Isontina Independent Local Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Anonymised trial data is held on secure servers at University College London. To access the data, please contact the corresponding author, supplying study protocol and approval.
References
- 1. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009;373:2223–33. 10.1016/S0140-6736(09)60746-7 [DOI] [PubMed] [Google Scholar]
- 2. Roerecke M, Rehm J. Alcohol use disorders and mortality: a systematic review and meta-analysis. Addiction 2013;108:1562–78. 10.1111/add.12231 [DOI] [PubMed] [Google Scholar]
- 3. Kaner EF, Beyer F, Dickinson HO, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev 2007;2:CD004148 10.1002/14651858.CD004148.pub3 [DOI] [PubMed] [Google Scholar]
- 4. Colom J, Scafato E, Segura L, et al. Brief interventions implementation on alcohol from the European health systems perspective. Front Psychiatry 2014;5:161 10.3389/fpsyt.2014.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson P, Bendtsen P, Spak F. Implementation science: a scientific report describing the methods, results and conclusions of the ODHIN randomized controlled trial. Barcelona, Spain, 2015. [Google Scholar]
- 6. Anderson P, Chisholm D, Fuhr DC. Effectiveness and cost-effectiveness of policies and programmes to reduce the harm caused by alcohol. Lancet 2009;373:2234–46. 10.1016/S0140-6736(09)60744-3 [DOI] [PubMed] [Google Scholar]
- 7. Crane D, Garnett C, Brown J, et al. Behavior change techniques in popular alcohol reduction apps: content analysis. J Med Internet Res 2015;17:e118 10.2196/jmir.4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wallace P, Murray E, McCambridge J, et al. On-line randomized controlled trial of an internet based psychologically enhanced intervention for people with hazardous alcohol consumption. PLoS One 2011;6:e14740 10.1371/journal.pone.0014740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elliott JC, Carey KB, Bolles JR. Computer-based interventions for college drinking: a qualitative review. Addict Behav 2008;33:994–1005. 10.1016/j.addbeh.2008.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donoghue K, Patton R, Phillips T, et al. The effectiveness of electronic screening and brief intervention for reducing levels of alcohol consumption: a systematic review and meta-analysis. J Med Internet Res 2014;16:e142 10.2196/jmir.3193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nair NK, Newton NC, Shakeshaft A, et al. A systematic review of digital and computer-based alcohol intervention pograms in primary care. Curr Drug Abuse Rev 2015;8:111–8. 10.2174/1874473708666150916113538 [DOI] [PubMed] [Google Scholar]
- 12. Struzzo P, Scafato E, McGregor R, et al. A randomised controlled non-inferiority trial of primary care-based facilitated access to an alcohol reduction website (EFAR-FVG): the study protocol. BMJ Open 2013;3:12 10.1136/bmjopen-2012-002304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frank D, DeBenedetti AF, Volk RJ, et al. Effectiveness of the AUDIT-C as a screening test for alcohol misuse in three race/ethnic groups. J Gen Intern Med 2008;23:781–7. 10.1007/s11606-008-0594-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 1993;88:791–804. 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- 15. Struzzo P, De Faccio S, Moscatelli E. Identificazione precoce dei bevitori a rischio in assistenza sanitaria primaria in Italia: adattamento del Questionario AUDIT e verifica dell’efficacia dell’uso dello short-AUDIT nel contest nazionale. Bollettino delle Farmacodipendenze e Alcolismo, 2006. http://www.unicri.it/wwk/publications/dacp/index.php. [Google Scholar]
- 16. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337–43. 10.3109/07853890109002087 [DOI] [PubMed] [Google Scholar]
- 17. Linke S, McCambridge J, Khadjesari Z, et al. Development of a psychologically enhanced interactive online intervention for hazardous drinking. Alcohol Alcohol 2008;43:669–74. 10.1093/alcalc/agn066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lygidakis C, Wallace P, Tersar C, et al. Download your doctor: implementation of digitally-mediated personal physician presence to enhance patient engagement with a health-promoting internet application. JMIR Res Protoc 2016;5:e36 10.2196/resprot.5232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller WR, Rollnick S. Talking oneself into change: Motivational interviewing, stages of change, and therapeutic process. J Cogn Psychother 2004;18:299–308. 10.1891/jcop.18.4.299.64003 [DOI] [Google Scholar]
- 20. Babor TF, Higgins-Biddle JC, Saunders JB, et al. AUDIT: The Alcohol Use Disorders Identification Test. Guidelines for use in primary care. 2001. http://whqlibdoc.who.int/hq/2001/WHO_MSD_MSB_01.6a.pdf (Last accessed August 2015).
- 21. Hunter R, Freemantle N, Wallace P, et al. Randomised controlled non-inferiority trial of primary care-based facilitated access to an alcohol reduction website: cost effectiveness analysis. BMJ Open 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anderson P, Bendtsen P, Spak F, et al. Improving the delivery of brief interventions for heavy drinking in primary health care: outcome results of the Optimizing Delivery of Health Care Intervention (ODHIN) five-country cluster randomized factorial trial. Addiction 2016;111:1935–45. 10.1111/add.13476 [DOI] [PubMed] [Google Scholar]
- 23. Bendtsen P, Müssener U, Karlsson N, et al. Implementing referral to an electronic alcohol brief advice website in primary healthcare: results from the ODHIN implementation trial. BMJ Open 2016;6:e010271 10.1136/bmjopen-2015-010271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rumpf HJ, Hapke U, Meyer C, et al. Screening for alcohol use disorders and at-risk drinking in the general population: psychometric performance of three questionnaires. Alcohol Alcohol 2002;37:261–8. 10.1093/alcalc/37.3.261 [DOI] [PubMed] [Google Scholar]
- 25. Dawson DA, Grant BF, Stinson FS, et al. Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcohol Clin Exp Res 2005;29:844–54. 10.1097/01.ALC.0000164374.32229.A2 [DOI] [PubMed] [Google Scholar]
- 26. Khadjesari Z, White IR, McCambridge J, et al. Validation of the AUDIT-C in adults seeking help with their drinking online. Addict Sci Clin Pract 2017;12:1–11. 10.1186/s13722-016-0066-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaner E, Bland M, Cassidy P, et al. Effectiveness of screening and brief alcohol intervention in primary care (SIPS trial): pragmatic cluster randomised controlled trial. BMJ 2013;346:e8501 10.1136/bmj.e8501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elison S, Davies G, Ward J. Effectiveness of computer-assisted therapy for substance dependence using breaking free online: subgroup analyses of a heterogeneous sample of service users. JMIR Ment Health 2015;2:e13 10.2196/mental.4355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sobell LC, Agrawal S, Annis H, et al. Cross-cultural evaluation of two drinking assessment instruments: alcohol timeline followback and inventory of drinking situations. Subst Use Misuse 2001;36:313–31. 10.1081/JA-100102628 [DOI] [PubMed] [Google Scholar]
- 30. López-Pelayo H, Wallace P, Segura L, et al. A randomised controlled non-inferiority trial of primary care-based facilitated access to an alcohol reduction website (EFAR Spain): the study protocol. BMJ Open 2014;4:e007130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.