Abstract
Objectives
To evaluate the 12-month costs and quality-adjusted life years (QALYs) gained to the Italian National Health Service of facilitated access to a website for hazardous drinkers compared with a standard face-to-face brief intervention (BI).
Design
Randomised 1:1 non-inferiority trial.
Setting
Practices of 58 general practitioners (GPs) in Italy.
Participants
Of 9080 patients (>18 years old) approached to take part in the trial, 4529 (49·9%) logged on to the website and 3841 (84.8%) undertook online screening for hazardous drinking. 822 (21.4%) screened positive and 763 (19.9%) were recruited to the trial.
Interventions
Patients were randomised to receive either a face-to-face BI or access via a brochure from their GP to an alcohol reduction website (facilitated access).
Primary and secondary outcome measures
The primary outcome is the cost per QALY gained of facilitated access compared with face-to-face. A secondary analysis includes total costs and benefits per 100 patients, including number of hazardous drinkers prevented at 12 months.
Results
The average time required for the face-to-face BI was 8 min (95% CI 7.5 min to 8.6 min). Given the maximum time taken for facilitated access of 5 min, face-to-face is an additional 3 min: equivalent to having time for another GP appointment for every three patients referred to the website. Complete case analysis adjusting for baseline the difference in QALYs for facilitated access is 0.002 QALYs per patient (95% CI −0.007 to 0.011).
Conclusions
Facilitated access to a website to reduce hazardous drinking costs less than a face-to-face BI given by a GP with no worse outcomes. The lower cost of facilitated access, particularly in regards to investment of time, may facilitate the increase in provision of BIs for hazardous drinking.
Trial registration number
NCT01638338;Post-results.
Keywords: health economics, substance misuse, information technology, world wide web technology
Strengths and limitations of this study.
The cost-effectiveness analysis uses individual patient data to evaluate the short-term costs and benefits of a way to increase the implementation of brief interventions (BI) for hazardous and harmful drinkers in primary care.
Follow-up rates exceeded 90% at 3 months and 80% at 12 months.
Limited data were collected as part of the trial on time taken in standard Italian GP appointments and cost of a GP in Italy, so assumptions based on data from the literature were required.
The results were extrapolated to the English National Health Services, hence caution should be exercised interpreting these findings given differences between the Italian and English NHS.
The results of the analysis are dependent on assumptions made regarding the number of patients who receive a face-to-face BI or the number of patients who access the website.
Introduction
Consumption of alcohol is a risk factor for premature mortality,1 with growing evidence of the significant negative health impact of alcohol consumption, including increased risk of cancer.2 The WHO has identified the European region as having the highest rates of alcohol-related ill health across the globe.2 Brief interventions (BIs) have been found to be effective in reducing alcohol consumption in primary care populations3 leading to recommendations for their implementation in primary care, including in the Italian National Guidelines.4 Delivering a face-to-face standard BI alongside screening the Italian population for hazardous drinking is potentially cost-effective to the Italian National Health Service (INHS), with the potential to prevent 7200 alcohol-related deaths over 30 years and 91 700 alcohol-related hospitalisations.5 Despite strong evidence of their potential benefit, the implementation of BIs in primary care across Europe has been limited.6 This may be due to the significant upfront investment required to deliver face-to-face BIs in the form of GP or other primary care staff time, of which there is finite availability.
As a result, an alternative approach may be required to deliver BIs in primary care, one that is less of a burden on clinician time and is easier to implement. Facilitated access, where a clinician directs patients to a website for alcohol reduction, has the potential to provide similar benefits to a face-to-face BI but potentially with a lower upfront investment in time and hence cost. Although there is evidence regarding the potential impact of BIs on reducing alcohol consumption and hence anticipated long-term health benefits, there is less evidence for their impact on short-term costs and health-related quality of, particularly in, an Italian primary healthcare setting.5 This information is required to identify strategies to improve the implementation of BIs in the INHS.
The aim of this health economic evaluation is to evaluate the short-term cost savings to the INHS of facilitated access to a website for hazardous and harmful drinkers compared with a standard face-to-face BI over 12 months. Hazardous drinkers are defined as people with an alcohol consumption level that is potentially detrimental to their health and is measured using the Alcohol Use Disorders Identification Test (AUDIT).7 These will be reported alongside potential benefits. Face-to-face BI for hazardous drinking has been recommended for widespread implementation in the English National Health System (NHS), but that evidence suggests that this has not happened.6 We have therefore included a secondary analysis of the potential cost savings to the English NHS of facilitated access to a website to provide additional information to NHS policy-makers.
Methods
EFAR trial
Effectiveness of primary care based Facilitated Access to alcohol Reduction website - a non-inferiority randomized controlled trial-Friuli Venezia Giulia (EFAR-FVG) is a randomised 1:1 trial, with the primary aim of testing for non-inferiority of a face-to-face BI for hazardous and harmful drinkers delivered by a GP (face-to-face BI) compared with facilitated access to an interactive website for reducing hazardous and harmful drinking (facilitated access). GPs from the region of Northern Italy, Friuli-Venezia, were recruited via the official register for the region. Patients aged ≥18 years and who did not meet any of the exclusion criteria for the trial were recruited to the trial by being given a trial brochure and encouraged by their GP to access a healthy lifestyle website. Patients who accessed the website were asked to complete the short Alcohol Use Disorders Identification Test (AUDIT-C).8 9 The AUDIT-C is comprises three questions to identify probable hazardous or harmful drinking, with a lower threshold score of 5 for men and 4 for women. Patients scoring at the threshold and above on the AUDIT-C were advised of their risk via a personalised message from their GP and advised to enter the study. Following consent to the study, patients completed baseline questionnaires and were randomised to face-to-face BI or facilitated access (the GP gives the patient a leaflet that directs them to the website) to a version of the Down Your Drink Website (www.downyourdrink.org.uk) adapted for an Italian audience. Further details of the EFAR-FVG trial10 11 and Down Your Drink website12 can be found elsewhere.
Costs
The aim of this analysis is to assess the short-term resource impact of facilitated access to a website. There is unlikely to be a significant immediate health benefit to patients as a result of reductions in alcohol consumption given the long-term impact and health risk of hazardous and harmful drinking. As a result the only resource use collected as part of the trial was time spent by GPs delivering the standard face-to-face BI as this is likely to be the main source of cost savings. GPs indicated if the face-to-face BI took <5 min, 5–10 min or >10 min. The cost per minute of a GP appointment was then multiplied by 5, 10 or 15 min for each patient to obtain the cost per patient of the face-to-face BI. The time and cost of screening was not included given that it was assumed to be the same in both groups. GPs were also asked to report how long it took them to refer patients to the website.
The cost of a GP appointment was taken from the Italian study published by Gerzeli et al 13 and was estimated at €11 an appointment for 2010 costs. No healthcare cost inflation index for Italy could be located, so instead the English healthcare cost inflation index was applied to bring the cost to 2015/2016 values14 at €12 an appointment. Assuming an average appointment length of 9 min,15 this equates to a cost per minute of €1.27. The primary analysis for costs is from the Italian healthcare perspective. A secondary analysis evaluating the potential cost savings for the English NHS costs has also been conducted to provide hypothetical information on the probability of the intervention is cost-effective in England. As reported in Hobbs et al 15 study of 101.8 million GP consultations carried out in English GPs, the average duration of a GP appointment in England is 9.2 min at a cost of £31.14 The significantly higher cost of GP time in the English NHS compared with INHS is likely to be a result of higher salaries and overhead costs in the English NHS.
All GPs attended a 1-day training session for the delivery of a face-to-face BI for hazardous and harmful drinking using motivational interviewing, with an average cost per GP participant of €51 for the cost of trainers, resources and room hire. The cost of an honorarium and travel costs for experts leading the training (€10 971), and cost of the GP’s time attending the training (at €533 per GP per day) was also included in the cost of training.
The cost of adapting the website was collected as part of the trial at a total cost of €35 000. GPs were asked to familiarise themselves with use of the website prior to start of the trial at a cost per GP of €76.
Quality-adjusted life years
Quality-adjusted life years (QALYs) represent a measure of mortality and morbidity over time, anchored at 1 for perfect health and 0 for death, with 1 year spent in perfect health equal to 1 QALY. They are used to assist healthcare policy-makers with decisions about the implementation of new interventions in healthcare in an equitable and standardised way. The cost of the new intervention minus current practice is divided by the additional QALYs generated by the new intervention to calculate the cost per QALY gained, with a lower mean cost per QALY being preferable. The new intervention might also dominate current practice by resulting in more QALYs for a lower average cost per patient. The EuroQol EQ-5D16 and its associated preference-based tariff17 is the most common way to calculate QALYs in most developed countries.
Euroqol EQ-5D 5 level (EQ-5D-5L)18 was administered to all patients in the trial to complete at baseline, 3 months and 12 months. Patients were asked to complete questionnaires online in the first instance, but for some patients questionnaires were completed over the phone following multiple attempts to contact the patient to complete the questionnaire online. The 5-level version of the EQ-5D was chosen given recent evidence of a reduced ceiling effect compared with the 3 level.19 Time trade-off values for the EQ-5D-5L were used to calculate patient-level utility tariffs. As no Italian weights are currently available in the cross-walk or time trade-off value sets for the EQ-5D-5L, the time trade-off algorithm for the UK was applied.20
Patient-level QALYs were calculated from baseline, 3-month and 12-month patient-level utility scores, adjusting for timing of follow-ups to calculate the area under the curve. Adjustments were not patient specific and were counted specifically as 3 months and 12 months regardless of when the patient actually completed the questionnaire so as not to introduce bias from delayed responses. As responses at all three time points are required to calculate QALYs, values reported are for complete case analysis (patients who have complete EQ-5D-5L responses for all three time points). The mean QALYs per patient reported have been adjusted for baseline EQ-5D-5L utility values using linear regression analysis and including a coefficient for randomisation.21 CIs are from 1000 bootstrap replications.
Cases of hazardous or harmful drinking prevented
As hazardous drinkers also include a generally healthy population, with potential QALY losses occurring in the far future as a result of future chronic alcohol-related health problems, the EQ-5D has been found to be insensitive to changes in hazardous drinking at the point of behaviour change for risk reduction.22 As a result, additional analyses of costs versus cases of hazardous or harmful drinking prevented have been included. Patients completed the 10 question version of the AUDIT (AUDIT-10) at baseline, 3 months and 12 months, with hazardous or harmful drinking defined as a score ≥8. Cases of hazardous or harmful drinking prevented at 12 months have been calculated using the data from the main paper for the trial using AUDIT-10 data at 12 months.11 This was converted to cases prevented per 1000 patients by calculating the percentage of patients that are hazardous or harmful drinkers at 12 months in the face-to-face BI, changing this to a rate per 1000 patient years and applying the OR reported in the main clinical paper11 for 12 months.
Sensitivity analysis: missing data
It was assumed that data at follow-up time points were missing at random. Additional analyses have been conducted using alternative ways to account for missing data in QALYs. This includes an incomplete case analysis using all data collected, not just complete cases, to calculate QALYs and calculating QALYs imputing missing data using chained equations as recommended in Hunter et al.23
Cost-effectiveness plane and cost-effectiveness acceptability curve
Results from the bootstrapped, complete case, QALYs were used to generate the cost-effectiveness plane (CEP) and cost-effectiveness acceptability curve (CEAC).
To represent the uncertainty in costs the average duration of the appointment was calculated using the proportion of patients who had appointments of different lengths and the Dirichlet process.24 The cost was varied using a random number generated in Excel and the gamma distribution assuming that the SE is equal to the mean cost of an appointment (€12 for IHNS and £31 in the UK). An average cost per appointment was then generated for each of the 1000 simulated iterations and for Italian and UK costs.
The CEAC is calculated using the formula (β1)WTP−(C1−C2), where β1 is the beta coefficient for the treatment effect from one of the iterations of the bootstrapped linear regressions adjusting for baseline EQ-5D-5L utility scores, WTP is the willingness to pay for a QALY gained, C1 is the average cost per patient of facilitated access and C2 is cost of the average cost per patient of the face-to-face BI. The probability that facilitated access is cost-effective compared with the face-to-face BI for a given WTP for a QALY is based on the proportion of times the formula is positive from the 1000 bootstrap iterations combined with the 1000 simulated iterations.
No discount rate was applied to the analysis given the 12-month time horizon.
Hypothesis testing
Given the hypothesis of non-inferiority between the two groups as the primary analysis for the trial, it was assumed that there would be no difference in QALYs or cases of hazardous or harmful drinking between the two groups. Instead the analysis focuses on the potential benefit per 1000 patients with facilitated access to the alcohol reduction website compared with a brief face-to-face BI. As no information of the average GP appointment duration in Italy is available, it has been assumed that the average appointment duration in Italy is similar to that of the English NHS of 9 min.
Results
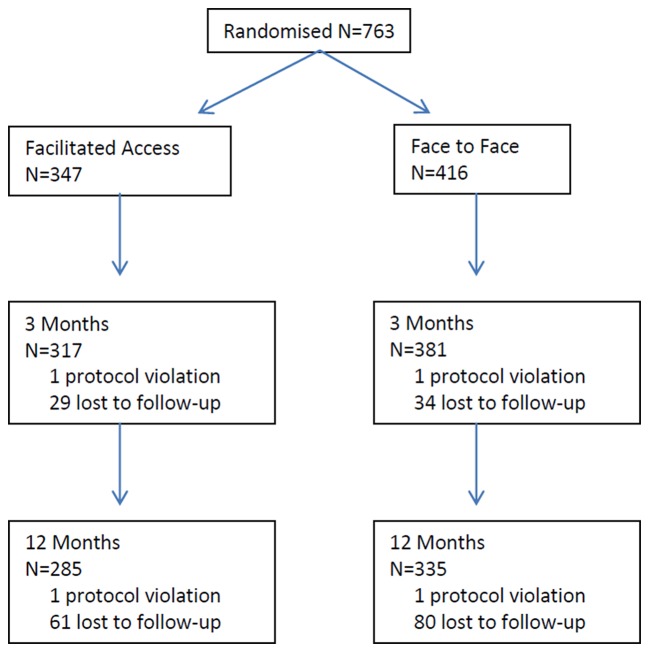
Patient numbers and loss to follow-up for the trial are reported in figure 1. Further patient demographics can be found in the main trial findings paper11.
Figure 1.
Consort diagram. Patient progress through trial for the primary outcome.
Costs
Of the 416 patients allocated to the face to face BI group, 304 (73%) received the BI from their GP. Information on the duration of each BI was recorded by the GPs using a questionnaire. For 171 patients (56.3%), the BI took <5 min, 5–10 min for 87 patients (28.6%) and >10 min for 46 patients (15.1%). The average time required for the face-to-face BI is 8 min (95% CI 7.5 min to 8.6 min). The amount of time spent facilitating access to the website was <5 min. Based on this, we made the conservative estimate that facilitated access required 5 min of a GP’s time. The difference of 3 min between the two groups is equivalent to having time for another appointment for every three patients referred to the website.
The average cost per patient of a face-to-face BI, including patients randomised to face-to-face BI but who did not receive the face-to-face appointment was €10,16 per patient (95% CI €9,53 to €10,92). If patients who did not receive the face-to-face BI are excluded from the analysis, the average cost is €11,10 per patient (95% CI €10,52 to €11,69).
The average cost per GP of training to deliver the face-to-face BI was €774. The cost per patient of training for face-to-face BI is dependent on how many patients the GP delivers a face-to-face BI to. Assuming that GPs could have provided a face-to-face BI to patients in either group, the total number of interventions they could have provided was 763, or 13 patients per GP, resulting in an average cost per patient of €60 for training for face-to-face BI.
The total cost of website development and piloting was €47 408, including the cost of GPs familiarising themselves with the website. Assuming 763 patients received facilitated access, the cost per patient of the website is €62,13. Each patient was also given a leaflet from the GP directing them to the website at a total cost per patient of €0,51. In the most conservative scenario (lowest possible cost difference between the two groups) of €70 per patient for face-to-face BI (€60 for training and €10,16 for the GP time to deliver the BI) and facilitated access costs €68 per patient (5 min for facilitated access, the cost of the leaflet and an additional cost per patient of updating the website of €62), facilitated access costs €2 less per patient compared with face-to-face BI.
In the least conservative estimate (highest possible cost difference between the two groups), we assume that the cost of the website approaches zero given that there is no upper limit to the number of patients who could feasibly access the website. Instead the only costs for facilitated access are the cost of the leaflet (€0,51 per patient), GP time referring patients to the website (5 min at a cost of €6,35 per patient) and time spent familiarising themselves with the website (€5,86). In the least conservative estimate we assume that the cost per patient for face-to-face BI is €71 (€60 for training and €11,10 for the GP time to deliver the BI) and facilitated access costs €13 per patient (5 min for facilitated access and an additional cost of the leaflet and GPs time familiarise themselves with the website of €6,37) facilitated access results in a cost saving of €58 per patient compared with face-to-face BI.
Utility scores and QALYs
The results for mean complete case analysis for utility scores and QALYs are reported in table 1.
Table 1.
Mean utility scores and QALYs for face-to-face and facilitated access
| Face-to-face | Facilitated access | |||||||
| Baseline | 3 months | 12 months | QALYs | Baseline | 3 months | 12 months | QALYs | |
| N | 415 | 381 | 335 | 331 | 346 | 317 | 285 | 275 |
| Mean | 0.914 | 0.942 | 0.938 | 0.937 | 0.913 | 0.942 | 0.938 | 0.937 |
| SE | 0.004 | 0.004 | 0.004 | 0.004 | 0.005 | 0.004 | 0.005 | 0.004 |
| 95% CI—lower | 0.905 | 0.934 | 0.93 | 0.929 | 0.903 | 0.934 | 0.929 | 0.929 |
| 95% CI—upper | 0.923 | 0.949 | 0.948 | 0.944 | 0.923 | 0.95 | 0.948 | 0.945 |
QALYs, quality-adjusted life years.
There was no significant difference in QALYs between facilitated access to the website and the face-to-face BI. Complete case analysis and adjusting for baseline the difference in QALYs for facilitated access minus face-to-face BI was 0.002 per patient (95% CI −0.007 to 0.011). At a willingness to pay (WTP) of €25 000 per QALY gained, as recommended by the INHS,25 facilitated access could cost an additional €50 per patient on average compared with face-to-face BI and be considered cost-effective. At the lower end of the CI where facilitated access results in a QALY decrement of −0.007 QALYs over 1 year, facilitated access would need to save €175 per patient to be cost-effective. The difference in utility scores and QALYs is described in table 2. Based on the results of the multiple imputation analysis, facilitated access can cost an additional €15 compared with face-to-face BI and be considered cost-effective (95% CI −€225 to €250).
Table 2.
Difference in health utility of facilitated access compared with face-to-face BI at 3 months and 12 months and adjusted difference in QALYs
| Analysis | Estimate | Lower 95% CI | Upper 95% CI | p Value |
| Complete case | ||||
| 3-month EQ-5D-5L | 0.003 | −0.010 | 0.013 | 0.658 |
| 12-month EQ5D 5 L | −0.0003 | −0.013 | 0.012 | 0.960 |
| QALYS (adjusted) | 0.002 | −0.007 | 0.01 | 0.622 |
| Incomplete case | ||||
| 3-month EQ-5D-5L | 0.0006 | −0.011 | 0.012 | 0.914 |
| 12-month EQ-5D-5L | 0.0004 | −0.013 | 0.013 | 0.955 |
| QALYS (from means) | 0.0003 | |||
| Multiple imputation | ||||
| 3-month EQ-5D-5L | 0.0006 | −0.010 | 0.012 | 0.913 |
| 12-month EQ-5D-5L | 0.0005 | −0.012 | 0.013 | 0.935 |
| QALYS (adjusted) | 0.0006 | −0.009 | 0.01 | 0.901 |
EQ-5D-5L, Euroqol EQ-5D 5 level; QALYs, quality-adjusted life years.
Cost-effectiveness plane and cost-effectiveness acceptability curve
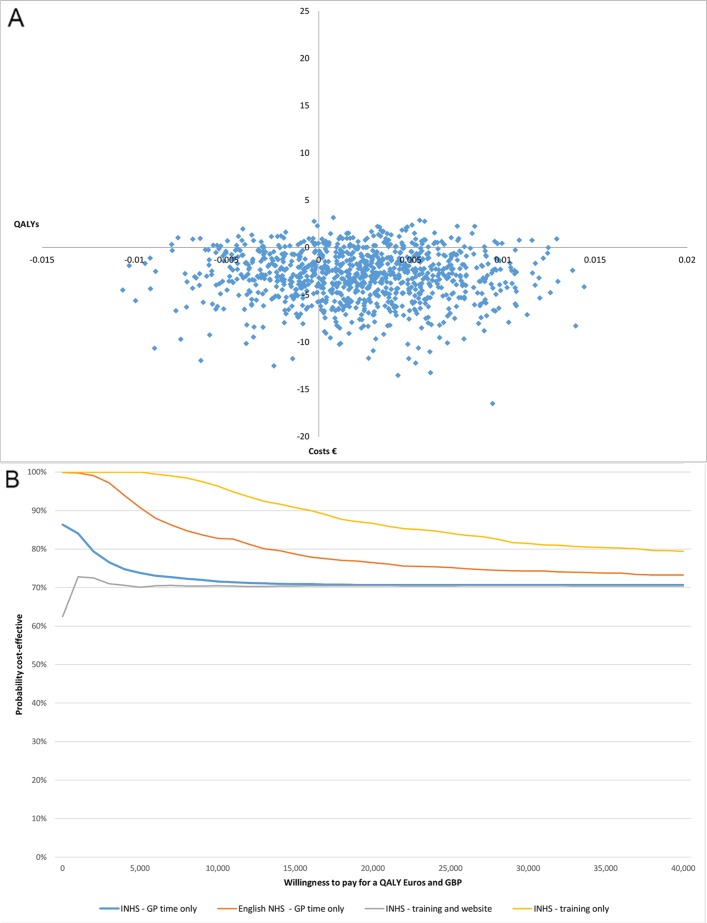
Results of the CEP and CEAC are reported in figure 2. There is a 70% probability that the intervention is cost-effective from an INHS cost perspective when all relevant costs are included (intervention delivery, training and website development) at a WTP for a QALY of €25 000, and an 84% probability if only the cost of training (excluding website development costs) are included. There is a 75% probability that the website is cost-effective compared with face-to-face BI if English NHS costs are used and intervention costs only are included, at a WTP for a QALY of £25 000.
Figure 2.
(A) Cost effectiveness acceptability plane (Italian costs only). (B) Cost-effectiveness acceptability plane (Italian and UK costs). Blue, INHS GP time only; orange, English NHS; grey, INHS training and website; yellow, INHS training only. GP, general practitioners; INHS, Italian National Health Service; NHS, National Health Service.
Benefits per 1000 patients referred
The results from the AUDIT analysis are reported in the clinical paper.11 At 12 months, there was no significant difference between two groups in the number of hazardous or harmful drinkers with an OR of 0.94 (95% CI 1.432 to 0.621). At 12 months, of the patients randomised to face-to-face BI, 26.3% were hazardous or harmful drinkers (AUDIT-10 ≥8) or 263 patients per 1000. Change this to a rate of 263 patients per 1000 patient years and applying an OR of 0.94, 18 patients per 1000 patient years are prevented from hazardous or harmful drinking if they were given facilitated access instead of a face-to-face BI. Facilitated access compared with the face-to-face BI also results in time for an additional 333 appointments.
Question 10 in the AUDIT-10 asks patients if a healthcare professional has recommended that they reduce their drinking. Potentially as a result of the nature of the intervention (a GP discussing their drinking with them) this was the question most frequently with a score >0 compared with other questions on the AUDIT. In the face-to-face group, 40% of patients answered >0 to question 10% and 31% in the intervention group at 12 months. If this is taken into account and a lower threshold of 7 for hazardous drinking applied, 16% of patients fall above the threshold for risky drinking in the face-to-face group and 18% in the facilitated access group with an OR of 1.9 (95% CI 1 to 3.7). This equates to 158 additional hazardous or harmful drinkers per 1000 patient years for facilitated access compared with face-to-face BI.
Discussion
Our findings indicate that in the INHS system, the chance that facilitated access to a website to reduce hazardous drinking is cost-effective compared with a face-to-face BI delivered by a GP is between 70% and 84%. However, these numbers are dependent on assumptions made about the number of patients given facilitated access versus those given a face-to-face BI given the high up-front costs of website modification or training, respectively. The costs per patient decrease as more patients access each treatment.
Although no data on the long-term benefits were included as part of this trial, other modelling studies in Italy have looked at the potential long-term benefits of BIs. Angus et al 5 modelled screening of the adult Italian population and providing a standard BI for those identified as hazardous drinkers over 10 years. They estimated that 32% of population receive the intervention at a cost of €411 million, with a potential cost saving of €370 million and a QALY gain of 75 200. This translates to an incremental cost-effectiveness ratio (ICER) of 550 per QALY gained. Given that facilitated access to a website costs significantly less than the standard BI across a whole population, it is likely that population-level screening for hazardous drinking and a facilitated access to a website is potentially cost saving. The lower cost in terms of time required of facilitated access compared with face-to-face may also increase the probability that BIs are implemented in the INHS.
Given the low level of implementation in the English NHS and the higher cost per hour of English GPs, if the findings from the Italian study were equivalent in England, there would be an even greater probability that facilitated access is cost-effective compared with a face-to-face BI. This result though should be interpreted with caution and points to the need for additional research in this area in England.
Strengths and weaknesses
Limited resource use data were collected as part of the trial. In particular there were no data on what impact access to the website had on follow-up GP appointments. If patients had concerns about the information they accessed on the website, it is the possible that they went to see their GP for additional advice, representing an additional cost that was not included in this analysis. Conversely, any cost savings as a result of prevention of alcohol related admissions were not captured as part of this study. If the wider costs to society beyond healthcare are considered, the cost to the economy of productivity losses as a result of alcohol-related days off work and loss of productivity were also not included. A trial of an online BI implemented in the work place found that the intervention group were less likely to have sick leave and for less days in total, although not significantly so.26 This though represents an important consideration for inclusion for trials in this area and population group.
Obtaining high-quality information on the cost of GP time in Italy was challenging, with availability only of limited information on GP time and costs and no published national costs.5 As a result, there is limited information to use for costing. However, the two sources used to cost GP time resulted in a similar value per minute of GP time suggesting consistency in the way GP time is costed in Italy. The lack of availability of an Italian tariff for the EQ-5D-5L is also a limitation of this study. An Italian tariff developed for the 3-level version of the EQ-5D found that Italian valuations were higher, particularly for more severe health states.27 Further research would be required to evaluate the implications for studies similar to these.
We have used data from the English NHS to estimate the potential cost-effectiveness of the intervention in an English primary care population. Previous trials of online interventions for reducing hazardous drinking in the UK have found it challenging to achieve high enough rates of follow-up to enable reliable measurement of effectiveness and cost-effectiveness.22 It is not possible to be sure that there would be a similar level of effectiveness of facilitated access compared with face-to-face BI in England, but the cost savings are likely to be similar to those projected here if GPs take a similar amount of time to conduct a BI.
The use of the AUDIT-10 as an outcome measure to measure the effectiveness of treatments for hazardous or harmful drinking is questionable. This is due to the problem with question 10 in the AUDIT which asks whether a healthcare professional has suggested reducing drinking. This is more likely to receive a positive response for patients screened for hazardous drinking and provided with any form of intervention—face-to-face BI or facilitated access. Advice is potentially more memorable at face-to-face BI and hence the reversal of results when this question was removed. This is discussed further in the main clinical paper11.
Conclusions
There is a high probability that facilitated access to a website to reduce alcohol consumption could deliver more benefits for fewer resources given that it costs less than the standard face-to-face BI. Additional benefits may also include an increase in the rates of delivery of BI via facilitated access given the lower time requirement for GPs compared with face-to-face BI.
Supplementary Material
Acknowledgments
The study was jointly supported by the Italian Ministry of Health and by the Region Friuli-Venezia Giulia, Italy (grant number: D25E12002900003). The funders had no role in the conception, preparation, review, approval, or submission of this manuscript. We thank Dr Alaman Allamani for chairing of the Trial Steering Committee.
Footnotes
Contributors: PW, PS and RDV: conceived the study and together with NF developed the design. PS, PW, RDV, CT, CL and RMcG: were responsible for the development of the website. PS, FS, RDV and CT: were responsible for follow-up of patients. RH: was responsible for the analysis and wrote the first draft. All authors: contributed to its revision and final approval.
Funding: The study was jointly supported by the Italian Ministry of Health and by the Region Friuli-Venezia Giulia, Italy (grant number: D25E12002900003). The funders had no role in the conception, preparation, review, approval, or submission of this manuscript. We thank Dr Alaman Allamani for chairing of the Trial Steering Committee.
Competing interests: PW has intellectual property rights for www.downyourdrink.org.uk, is Chief Medical Advisor to the UK charity Drinkaware and has provided private consultancy on the topic of screening and brief interventions to several agencies. CL is the cofounder and Chief Executive Officer at Lumos Medica Srl, which provides software solutions for clinical trials. The other authors declare no competing interests.
Ethics approval: Ethical Committee of the Azienda Sanitaria 4 Medio Friuli, Udine, IT. The protocol was approved on 14 June 2012 by the Independent Local Ethics Committee for Clinical Research of the Health Services Agency No 2 Isontina, Italy.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Anonymised trial data are held on secure servers at University College London. For access to the data please contact the corresponding author. Access will be granted subject to approval by the steering committee.
Collaborators: The EFAR Study Team include Giosué Acampora, Maria Angela Bravo, Chiara Bregant, Gianna Bunello, Leonardo Butà, Lucia Casatta, Castellarin, Egidio Chamouni, Moshé Benyamin, Maria Pia Cisilin, Clemente Condello, Nelly Drigani, Tiziano Ermacora, Alessandro Falcidia, Adriana Fasiolo, Angelo Florio, Mauro Gubiani, Gianni Iacuzzo, Edi Ietri, Giuseppe Latella, Guglielmo Lucca, Ciro Mamolo, Carmelo Matera, Lucia Mei, Francesca Melon, Emilio Mezzasalma, Isabella Milan, Carlo Miotti, Gian Franco Panizzo, Francesca Pighin, Raffaela Principato, Pierina Revignas, Antonella Rolff, Ivana Rupalti, Rosanna Sellibara, Michela Sereni, Gianfranco Silverii, Giuseppe Taglialatela, Chiara Toffoletti, Massimo Toffolo, Laura Ivana Tonelli, Simone Trevisani, Roberto Troisi, Gianni Tubaro, Roberto Vallini, Elisabetta Zappalà and Antonio Zappi.
References
- 1. World Health Organisation. Status Report on Alcohol and Health in 35 European Countries. 2013. http://www.euro.who.int/__data/assets/pdf_file/0017/190430/Status-Report-on-Alcohol-and-Health-in-35-European-Countries.pdf (accessed 2 Aug 2016).
- 2. Connor J. Alcohol consumption as a cause of cancer. Addiction 2017;112 10.1111/add.13477 [DOI] [PubMed] [Google Scholar]
- 3. Kaner EF, Beyer F, Dickinson HO, et al. . Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev 2007;2:CD004148 10.1002/14651858.CD004148.pub3 [DOI] [PubMed] [Google Scholar]
- 4. d SM. Piano Nazionale Alcol E Salute. Roma 2007. [Google Scholar]
- 5. Angus C, Scafato E, Ghirini S, et al. . Cost-effectiveness of a programme of screening and brief interventions for alcohol in primary care in Italy. BMC Fam Pract 2014;15:26 10.1186/1471-2296-15-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colom J, Scafato E, Segura L, et al. . Brief interventions implementation on alcohol from the European health systems perspective. Front Psychiatry 2014;5:161 10.3389/fpsyt.2014.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Babor T, Higgins-Biddle J, Sounders J, et al. . The alcohol use disorders identification test. Guidelines for use in primary care 2001. http://apps.who.int/iris/bitstream/10665/67205/1/WHO_MSD_MSB_01.6a.pdf (accessed Aug 2016).
- 8. Frank D, DeBenedetti AF, Volk RJ, et al. . Effectiveness of the AUDIT-C as a screening test for alcohol misuse in three race/ethnic groups. J Gen Intern Med 2008;23:781–7. 10.1007/s11606-008-0594-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Struzzo P, De Faccio S, Moscatelli E. Identificazione precoce dei bevitori a rischio in Assistenza Sanitaria Primaria in Italia: adattamento del Questionario AUDIT e verifica dell’efficacia dell’uso dello short-AUDIT nel contest nazionale. Bollettino delle Farmacodipendenze e Alcolismo 2006. http://www.unicri.it/wwk/publications/dacp/index.php [Google Scholar]
- 10. Struzzo P, Scafato E, McGregor R, et al. . A randomised controlled non-inferiority trial of primary care-based facilitated access to an alcohol reduction website (EFAR-FVG): the study protocol. BMJ Open 2013;3:e002304 10.1136/bmjopen-2012-002304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wallace P, Struzzo P, Della Vedova R, et al. . Randomised controlled non-inferiority trial of primary care-based facilitated access to an alcohol reduction website. BMJ open 2017:2016–14576.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Linke S, McCambridge J, Khadjesari Z, et al. . Development of a psychologically enhanced interactive online intervention for hazardous drinking. Alcohol Alcohol 2008;43:669–74. 10.1093/alcalc/agn066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gerzeli S, Rognoni C, Quaglini S, et al. . Cost-effectiveness and cost-utility of beclomethasone/formoterol versus fluticasone propionate/salmeterol in patients with moderate to severe asthma. Clin Drug Investig 2012;32:253–65. 10.2165/11598940-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 14. Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury, UK: University of Kent, 2016;227. [Google Scholar]
- 15. Hobbs FDR, Bankhead C, Mukhtar T, et al. . Clinical workload in UK primary care: a retrospective analysis of 100 million consultations in England, 2007-14. Lancet 2016;387:2323–30. 10.1016/S0140-6736(16)00620-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. EuroQol. What is EQ-5D. 2016. http://www.euroqol.org/home.html (accessed Aug 2016).
- 17. EuroQol. Valuation of EQ-5D. 2016. http://www.euroqol.org/about-eq-5d/valuation-of-eq-5d.html (accessed Aug 2016).
- 18. EuroQol. EQ-5D-5L 2016. http://www.euroqol.org/eq-5d-products/eq-5d-5l.html (accessed Aug 2016)
- 19. Janssen MF, Pickard AS, Golicki D, et al. . Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717–27. 10.1007/s11136-012-0322-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Devlin N, Shah K, Feng Y, et al. . Valuing Health-Related Quality of Life: An EQ-5D-5L Value Set for England. 2016. https://www.ohe.org/publications/valuing-health-related-quality-life-eq-5d-5l-value-set-england (accessed Aug 2016). [DOI] [PMC free article] [PubMed]
- 21. Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487–96. 10.1002/hec.944 [DOI] [PubMed] [Google Scholar]
- 22. Essex HN, White IR, Khadjesari Z, et al. . Quality of life among hazardous and harmful drinkers: EQ-5D over a 1-year follow-up period. Qual Life Res 2014;23:733–43. 10.1007/s11136-013-0521-7 [DOI] [PubMed] [Google Scholar]
- 23. Hunter RM, Baio G, Butt T, et al. . An educational review of the statistical issues in analysing utility data for cost-utility analysis. Pharmacoeconomics 2015;33:355–66. 10.1007/s40273-014-0247-6 [DOI] [PubMed] [Google Scholar]
- 24. Briggs A, Sculpher M, Claxton K. Decision Modelling fo Health Economic Evaluation. Oxford Univesrity Press: Oxford, 2006. [Google Scholar]
- 25. Fattore G. A proposal for guidelines for the economic evaluation of health interventions in Italy. PharmacoEconomics Italian Research Articles 2009;11:83–93. [Google Scholar]
- 26. Khadjesari Z, Freemantle N, Linke S, et al. . Health on the web: randomised controlled trial of online screening and brief alcohol intervention delivered in a workplace setting. PLoS One 2014;9:e112553 10.1371/journal.pone.0112553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scalone L, Cortesi PA, Ciampichini R, et al. . Italian population-based values of EQ-5D health states. Value Health 2013;16:814–22. 10.1016/j.jval.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 28. Wallace P, Struzzo P, Della Vedova R, et al. . Randomised controlled non-inferiority trial of primary care-based facilitated access to an alcohol reduction website. BMJ open 2017:014576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.