Abstract
Purpose
Moraxella atlantae is a rare pathogen. Keratitis from this organism has never been specifically reported or described. In this report we provide the first clinical description and photograph of Moraxella atlantae infectious keratitis.
Observations
A 41 year-old man presented with a three day history of left eye pain. He was found to have a corneal ring ulcer and infiltrate from which Moraxella atlantae was cultured. The patient was successfully treated with intensive topical gatifloxacin (0.5%) and fortified tobramycin (1.5%); oral doxycycline was added to reduce corneal thinning. The patient's infection resolved with a residual scar and final best corrected visual acuity of 20/200 OS.
Conclusions and importance
Moraxella atlantae can present as a ring-shaped infectious corneal infiltrate and ulcer. Ring infiltrates have been observed with other microorganisms, including several other gram negative bacteria and classically, acanthamoeba. Frequently presumed to be purely immunologic, corneal ring infiltrates can have a number of other etiologies, including infectious and toxic. There are different types of immunologic rings as well, making differentiation of corneal rings sometimes difficult for the ophthalmologic generalist and subspecialist alike. In this paper we discuss characteristics of various corneal ring infiltrates, along with their immune pathophysiology. Infectious rings are distinguished from immunologic Wessely rings.
Keywords: Moraxella atlantae, Ring ulcer, Wessely ring, Ring infiltrate, Annular infiltrate, Keratitis
1. Introduction
Moraxella is a gram negative, oxidase positive diplobacillus that grows in pairs and chains. While it can be cultured elsewhere, Moraxella is predominantly an ear, nose, and throat, or ocular pathogen.1 Ocular infections can range from angular blepharoconjunctivitis to severe keratitis.2 Once associated with malnourished alcoholics and debilitated elderly individuals, Moraxella infections have since been reported in healthy patients with abnormalities of the eyelids, conjunctiva, and corneal epithelium.3 Risk factors for Moraxella keratitis include systemic disease such as diabetes and leprosy, and ocular factors such as blepharitis, dry eye, contact lens wear, herpes infection, glaucoma, thyroid eye disease, lagophthalmos, scleritis, trauma, penetrating keratoplasty, bullous keratopathy, and blind eye.2, 4
Several Moraxella species are known to infect the human eye including M. nonliquefaciens, M. lacunata, M. osloensis, and M. atlantae.1 M. liquifaciens, a common cause of corneal ulcers in the past, has now been grouped with M. lacunata.5 M. catarrhalis, previously categorized under genuses Neisseria and later Branhamella, has occasionally been implicated in conjunctivitis, ulcerative keratitis, pseudomembrane, and endophthalmitis.6, 7 Moraxella atlantae is an uncommon opportunistic pathogen8 -- of all Moraxella eye infections cultured and sent to the CDC from 1953 to 1980, only one percent were due to M. atlantae.1 To date, this species has been described in only two other patients, both bacteremic: one with lupus9 and one with metastatic rectal adenocarcinoma.8 Neither had ocular involvement. Since Moraxella isolates are often considered non-pathogenic or contaminants,1 there is the possibility that microbiology labs may not always speciate Moraxella infections. Additionally, Moraxella can be difficult to grow, due to wide sensitivity to antibiotics and fastidious growth requirements.3, 8 These factors may lead to a potential under-reporting of certain Moraxella species.8 Regardless, M. atlantae ocular infections are rare, and their typical presentation is unknown. In this paper we present the first clinical description of Moraxella atlantae keratitis, manifesting as an ulcerative ring infiltrate.
2. Case report
A 41 year-old man presented with 3 days of worsening pain and blurred vision in the left eye. He denied recent trauma or contact lens use, but had a remote history of flash burn from welding OU and metallic corneal foreign body OS. He smoked a pack per day and consumed alcohol to an unspecified degree.
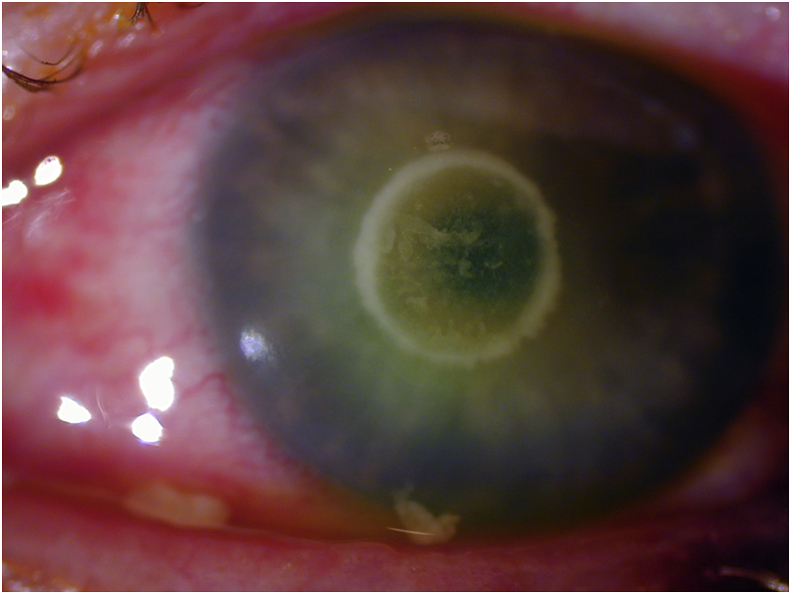
On examination vision was 20/30 OD and hand motion OS. Slit lamp exam of the left eye showed a corneal ulcer with a 4mm epithelial defect, an underlying circular infiltrate of the same size, 85–90% stromal thinning, and no hypopyon (Fig. 1). Based on clinical appearance, a gram-negative infection was initially suspected; the patient was prescribed intensive topical gatifloxacin (0.5%) and fortified tobramycin (1.5%). Oral doxycycline was added to reduce collagenolysis and corneal thinning. Corneal scrapings were taken, and cultures eventually grew Moraxella atlantae. In the course of his care, the patient displayed indications of alcohol abuse including unreliability following instructions and the smell of alcohol on his breath. He was followed closely for the first 2 weeks, and showed a positive clinical response to antibiotic therapy. However, he was then lost to follow-up for the next three months. When he returned, the ulcer had resolved with a residual scar and mild corneal thinning. Best corrected visual acuity had improved to 20/200.
Fig. 1.
Moraxella atlantae Ring Ulcer: Slit lamp photograph showing central corneal ring abscess.
3. Discussion
Moraxella keratitis typically presents as a painless and indolent localized oval-shaped ulcer with an undermined necrotic edge and gray infiltrate.2, 10 Infections are usually located in the inferocentral cornea, or may be marginal in association with angular blepharitis.2, 10 Ulcers, however, can also develop severe inflammation, frequently associated with hypopyon; occasionally hyperacute inflammation causes a hyphema to form within the hypopyon.2, 7 Untreated infections slowly progress to deeper corneal layers, accompanied by tissue destruction and possible perforation.3, 10 Ring infiltrate is an unusual finding in Moraxella corneal infections. In the largest review of 95 cases of Moraxella keratitis, there was no mention of ring infiltrate.2 There is one passing mention of ring infiltrate with Moraxella in the literature,11 without an accompanying citation, case description, or photograph. Our case report contains the first detailed description and clinical photograph of an infectious corneal ring caused by Moraxella atlantae.
We recently saw an additional case of ring-shaped keratitis involving Moraxella in our clinic. An elderly patient with rheumatoid arthritis recently presented after 3 days of eye irritation with an infectious ring ulcer and infiltrate due to Moraxella catarrhalis (clinical observation, unpublished). On day 7 as the infiltrate was fading with topical fortified antibiotic treatment, she exhibited a new ring, presumed to be immunologic, peripheral to the original infection. Her musculoskeletal ailments unfortunately precluded satisfactory positioning for slit lamp photos. This additional case indicates that different Moraxella species can cause ring infiltrates.
Once considered pathognomonic of Acanthamoeba infection,10, 12, 13, 14, 15 ring-shaped corneal infections have now been observed with a number other microorganisms. An annular herpetic ulcer appeared five days after implantation of intracorneal ring segments for keratoconus,16 and ring infiltrate has been reported as well.17 Both keratomycoses11, 12, 14, 16, 17, 18, 19 and atypical mycobacterial keratitis20, 21 can have various clinical appearances, including a circular shape. Nevertheless, acanthamoebae remain nine to eleven times more likely to form ring infiltrates than bacteria or fungi.12 Rings may be incomplete or even double, and their size, density, and shape may vary.10, 13, 22, 23, 24, 25 A ring infiltrate is often a later finding in Acanthamoeba keratitis,11, 28 especially when untreated, and has been considered immunologic.12, 15, 22, 24 Pathologic examination of the ring, however, reveals that the stroma contains not only polymorphonuclear leukocytes (PMN) but also amebic cysts.13
Bacterial ring infiltrates, as in our patient, have been observed most commonly with gram negative organisms such as Neisseria, Serratia, Escherichia, Klebsiella, Proteus, Capnocytophaga, and Pseudomonas.10, 17, 21, 23, 26 Infectious rings from gram negative organisms such as P. aeruginosa and E. coli may appear 24–48 hours after experimental corneal inoculation,27 and clinically within 2–3 days.28 Bacterial toxins, damaged host cells, complement, and immune complexes chemotactically attract inflammatory cells.11, 27, 28, 29, 30 On histopathologic analysis, gram negative rings show heavy aggregation and concentration of PMNs, especially at the ulcer periphery.21, 27, 28, 29, 30, 31 Bacterial proteases such as elastase, however, act to inhibit further centripetal PMN migration.28, 29 They concurrently promote the breakdown of collagen and proteoglycan with the help of bacterial exo- and endotoxins.32 These processes result in liquefactive necrosis and central tissue loss that accentuate the ring-like appearance.29, 31, 32, 33
Gram positive bacteria such as Staphylococcus,11 Streptococcus,15, 34 Bacillus cereus,15, 21 and Listeria monocytogenes30 have also been implicated in forming corneal rings. Though staphylococcal rings are often immunologic, an actively infectious ring infiltrate from resistant Staphylococcus species has been reported with positive cultures and rapid progression.11 A ring opacity was additionally observed in association with Streptococcus mitus infectious crystalline keratopathy, after 3 months of incomplete recognition and treatment.14 Corneal infection with Nocardia classically assumes a beaded wreath appearance,10, 17 although a confluent ring infiltrate is also possible.
It is important to differentiate infectious ring infiltrates from non-infectious immune rings, as both can form in the course of microbial keratitis.17, 19, 35 Signs such as pain, purulence, anterior chamber reaction, response to antibiotics over merely steroids alone, epithelial defect greater than 2mm, and necrosis are suggestive of infection, compared to the milder findings of an infiltrate outside the ulcer borders and response to topical steroids as seen with purely immunologic rings.10, 11, 12, 19, 30, 35, 36 Infectious ring infiltrates contain viable microorganisms invading host tissue,29, 30 and directly admixed within the host immunologic response. We suggest that such infiltrates be referred to as “infectious” rings, in contrast to sterile ring infiltrates, which are usually referred to as “immune” rings. This suggestion is to simplify terminology, and not to imply that infectious rings do not include an active immunologic component. Some have also used the term “ring abscess” to describe an infectious ring, particularly suppurative ones.13, 21, 23, 28, 34 An untreated infectious corneal ring may intensify and expand centrifugally,10, 34 whereas immunologic rings slowly migrate centripetally and ultimately fade.24, 28 Even immune rings can, however, also cause tissue damage and corneal scarring through delayed hypersensitivity.28
Non-infectious immunologic rings ensue from complement activation by either of two mechanisms: A type 3 hypersensitivity response marked by antibodies interacting with antigens diffusing outward from an inflammatory source (a true “Wessely Ring”37), or an antibody-independent mechanism.15, 23, 27, 35, 38, 39, 40 Immunologic components required for complement activation are present in the cornea itself.40 In the classical pathway, as described by Wessely, antibodies diffuse toward antigenic stimuli from the infection. Antibody-antigen complexes form and activate complement in a circular zone.36, 38, 39, 41 PMN are chemotactically attracted from the periphery and concentrate where complexes have precipitated.10, 24, 35, 36, 39, 41 The resultant inflammatory reaction creates a visible ring, typically separated from the central nidus of infection by clear space.2, 5, 21, 24, 35, 39 Wessely rings commonly appear at 10–14 days.23, 24, 28, 36, 39 They have been reported following many corneal infections including S. aureus,21 Herpes simplex,38, 41 Varicella Zoster,11, 42 Microsporidium,43 and nontuberculous Mycobacteria.10 Ring responses may appear early (1–5 days) if there has been previous exposure to the same bacterial antigen.27, 36, 39 In the setting of chronic non-bacterial infectious keratitis, rings have been observed after a longer delay—16 or more days in some Acanthamoeba44 and 2 months in a case of Microsporidium keratitis.43 These infiltrates also contain infectious microorganisms, and occur after protracted periods of no or incomplete therapy.13, 43 Although also referred to as “Wessely rings” by some authors,24 these annular infiltrates differ in ways from the classic Wessely ring, and their exact immunologic pathophysiology has not yet been fully elucidated.15
An antibody-independent, alternative complement activation pathway underlies other corneal immune reactions.15, 23, 27, 35, 39, 40 Ring formation was observed 43 hours after irritation in a contact lens user with culture-proven P. aeruginosa.36, 39 The authors espoused a bacterial endotoxin-initiated, properdin-mediated complement pathway, independent of antibody and immunoglobulin, consistent with immune ring appearance before 8 days.36, 39, 40 Gram negative cell wall endotoxins activate properidin, leading to antibody-independent complement activation and chemotactic attraction of neutrophils.27, 39 Properdin-mediated rings, like Wessely rings, are distinct from the actual infectious infiltrate and ulcer, and are located between the infiltrate and limbus with an intervening clear zone.36 Properdin appears to also play a role in early ring formation when Pseudomonas endotoxin is present even absent bacterial tissue invasion.39, 40
Sterile corneal rings have been observed with the antineoplastic drug Perifosine,45 topical anesthetic abuse,10, 24, 46 Behcet's Disease,47 contact lens overwear,48 recurrent corneal erosions,35, 49 post-refractive surgery50 and corneal crosslinking,51 lipid keratopathy,52, 53 posterior polymorphous dystrophy,54 corneal foreign bodies,14, 24 corneal burns,14, 24 and exposure to aquarium coral toxin.55 The pathophysiology behind these rings likely differs despite a superficially similar shape. However, disruption of the corneal epithelium and corneal exposure to microbial antigens presumably factors in some instances.35
4. Conclusions
Ring infiltrates in the setting of infectious keratitis have been classically associated with acanthamoeba, but now observed with a number of other microorganisms. In this report we provide the first clinical description and photograph of M. atlantae ulcerative keratitis, presenting as a ring-shaped infiltrate. Moraxella atlantae is an extremely rare eye pathogen. Potential predisposing risk factors may include a history of ocular trauma and suspected alcohol abuse. The early ring appearance at presentation prior to antibiotic therapy, with full symptoms and findings of infectious keratitis, and positive cultures all point to an infectious corneal ring, rather than a purely immunologic process. Differentiation of corneal rings can sometimes be difficult,10, 15, 27 for both the ophthalmologic generalist and subspecialist alike. Frequently presumed to be immunologic Wessely-like rings, corneal ring infiltrates can have other etiologies, including infectious and toxic. In this paper we expand discussion beyond our case report to review characteristics of various corneal ring infiltrates, along with their immune pathophysiology.
Patient consent
The patient and next of kin could not be located or contacted to sign consent to publish this report. The report does not contain any personal information that could lead to identification of the patient.
Funding
No funding or grant support.
Conflict of interest
The following authors have no financial disclosures: AB, TC.
Authors
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
Pranav Patel, MD assisted in literature search.
References
- 1.Graham D.R., Band J.D., Thornsberry C., Hollis D.G., Weaver R.E. Infections caused by Moraxella, Moraxella urethralis, Moraxella-like groups M-5 and M-6, and Kingella kingae in the United States, 1953-1980. Rev Infect Dis. 1990;12:423–431. doi: 10.1093/clinids/12.3.423. [DOI] [PubMed] [Google Scholar]
- 2.Das S., Constantinou M., Daniell M., Taylor H.R. Moraxella keratitis: predisposing factors and clinical review of 95 cases. Br J Ophthalmol. 2006;90:1236–1238. doi: 10.1136/bjo.2006.095182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cobo L.M., Coster D.J., Peacock J. Moraxella keratitis in a nonalcoholic population. Br J Ophthalmol. 1981;65:683–686. doi: 10.1136/bjo.65.10.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mian S.I., Malta J.B. Moraxella keratitis: risk factors, presentation, and management. Acta Ophthalmol. 2011;89:e208–209. doi: 10.1111/j.1755-3768.2009.01780.x. [DOI] [PubMed] [Google Scholar]
- 5.Tonjum T., Caugant D.A., Bovre K. Differentiation of Moraxella nonliquefaciens, M. lacunata, and M. bovis by using multilocus enzyme electrophoresis and hybridization with pilin-specific DNA probes. J Clin Microbiol. 1992;30:3099–3107. doi: 10.1128/jcm.30.12.3099-3107.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergren R.L., Tasman W.S., Wallace R.T., Katz L.J. Branhamella (Moraxella) catarrhalis endophthalmitis. Arch Ophthalmol. 1993;111:1169–1170. doi: 10.1001/archopht.1993.01090090021009. [DOI] [PubMed] [Google Scholar]
- 7.Whitcher J.P., Cevallos V. Moraxella, down but not out–the eye bug that won't go away. Br J Ophthalmol. 2006;90:1215–1216. doi: 10.1136/bjo.2006.100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Baere T., Muylaert A., Everaert E. Bacteremia due to Moraxella atlantae in a cancer patient. J Clin Microbiol. 2002;40:2693–2695. doi: 10.1128/JCM.40.7.2693-2695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchman A.L., Pickett M.J. Moraxella atlantae bacteraemia in a patient with systemic lupus erythematosis. J Infect. 1991;23:197–199. doi: 10.1016/0163-4453(91)92335-3. [DOI] [PubMed] [Google Scholar]
- 10.Keenan J.D., Mcleod S.D. Bacterial keratitis. In: Yanoff M., Duker J.S., editors. Ophthalmology. fourth ed. Saunders; Philadelphia: 2013. pp. 217–224. [Google Scholar]
- 11.Wallang B.S., Das S., Sharma S., Sahu S.K., Mittal R. Ring infiltrate in staphylococcal keratitis. J Clin Microbiol. 2013;51:354–355. doi: 10.1128/JCM.02191-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mascarenhas J., Lalitha P., Prajna N.V. Acanthamoeba, fungal, and bacterial keratitis: a comparison of risk factors and clinical features. Am J Ophthalmol. 2014;157:56–62. doi: 10.1016/j.ajo.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theodore F.H., Jakobiec F.A., Juechter K.B. The diagnostic value of a ring infiltrate in acanthamoebic keratitis. Ophthalmology. 1985;92:1471–1479. doi: 10.1016/s0161-6420(85)33830-7. [DOI] [PubMed] [Google Scholar]
- 14.McDonnell J.M., Gritz D.C., Hwang D., McDonnell P.J. Infectious crystalline keratopathy with ring opacity. Cornea. 1992;11:479–483. doi: 10.1097/00003226-199209000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Kent C. Common wisdom: science or myth? Rev Ophthalmol. 2012;19:46–57. [Google Scholar]
- 16.Rayward O., Arriola-Villalobos P., Cuiña-Sardiña R., Diaz-Valle D., Benítez-Del-Castillo J.M., García-Feijoo J. Annular herpetic keratitis after intracorneal ring segment implantation. Cornea. 2011;30:1286. doi: 10.1097/ICO.0b013e31821c9bb8. [DOI] [PubMed] [Google Scholar]
- 17.Garg P., Rao G.N. Corneal ulcer: diagnosis and management. Community Eye Health. 1999;12:21–23. [PMC free article] [PubMed] [Google Scholar]
- 18.Biser S.A.1, Perry H.D., Donnenfeld E.D., Doshi S.J., Chaturvedi V. Arthrographies keratitis mimicking acanthamoeba keratitis. Cornea. 2004;23:314–317. doi: 10.1097/00003226-200404000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Stein R.M., Clinch T.E., Cohen E.J., Genvert G.I., Arentsen J.J., Laibson P.R. Infected vs sterile corneal infiltrates in contact lens wearers. Am J Ophthalmol. 1988;105:632–636. doi: 10.1016/0002-9394(88)90056-6. [DOI] [PubMed] [Google Scholar]
- 20.Moore M.D., Newton C., Kaufman H.E. Chronic keratitis caused by Mycobacterium gordonae. Am J Ophthalmol. 1986;102:516–521. doi: 10.1016/0002-9394(86)90083-8. [DOI] [PubMed] [Google Scholar]
- 21.Hong A.R., Shute T.S., Huang A. BacterialKeratitis. In: Mannis M.J., Holland E.J., editors. Cornea: Fundamentals, Diagnosis, and Management. fourth ed. Elsevier; Edinburgh: 2017. pp. 875–901. [Google Scholar]
- 22.Illingworth C.D.1, Cook S.D. Acanthamoeba keratitis. Surv Ophthalmol. 1998;42:493–508. doi: 10.1016/s0039-6257(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 23.Shovlin J. Ring infiltrates and the contact lens patient: are they pathognomonic? Int Contact Lens Clin. 1989;16:25–27. [Google Scholar]
- 24.Arffa R.C., Grayson M. fourth ed. Mosby; St. Louis, Mo: 1997. Grayson's Diseases of the Cornea; pp. 675–676. 50-51,271-272. [Google Scholar]
- 25.Frangie J.M., Moore M.B. Parasitic keratitis, including acanthamoeba. In: Leibowitz H.M., Waring G.O., editors. Corneal Disorders: Clinical Diagnosis and Management. second ed. Saunders; Philadelphia: 1998. pp. 719–733. [Google Scholar]
- 26.Heidemann D.G., Pflugfelder S.C., Kronish J., Alfonso E.C., Dunn S.P., Ullman S. Necrotizing keratitis caused by Capnocytophaga ochracea. Am J Ophthalmol. 1988;105:655–660. doi: 10.1016/0002-9394(88)90060-8. [DOI] [PubMed] [Google Scholar]
- 27.Mondino B.J., Rabin B.S., Kessler E., Gallo J., Brown S.I. Corneal rings with gram-negative bacteria. Arch Ophthalmol. 1977;95:2222–2225. doi: 10.1001/archopht.1977.04450120128019. [DOI] [PubMed] [Google Scholar]
- 28.Ijiri Y., Yamamoto T., Kamata R. The role of Pseudomonas aeruginosa elastase in corneal ring abscess formation in pseudomonal keratitis. Graefes Arch Clin Exp Ophthalmol. 1993;231:521–528. doi: 10.1007/BF00921117. [DOI] [PubMed] [Google Scholar]
- 29.Van Horn D.L., Davis S.D., Hyndiuk R.A., Alpren T.V. Pathogenesis of experimental Pseudomonas keratitis in the Guinea pig: bacteriologic, clinical, and microscopic observations. Invest Ophthalmol Vis Sci. 1978;17:1076–1086. [PubMed] [Google Scholar]
- 30.Holbach L.M., Bialasiewicz A.A., Boltze H.J. Necrotizing ring ulcer of the cornea caused by exogenous Listeria monocytogenes serotype IV b infection. Am J Ophthalmol. 1988;106:105–106. doi: 10.1016/s0002-9394(14)76404-9. [DOI] [PubMed] [Google Scholar]
- 31.Rapuano C., Luchs J., Kim T. Corneal infections, inflammations, and infectious disorders. In: Krachmer J., editor. Anterior Segment: The Requisites in Ophthalmology. 1 ed. Mosby; St. Louis: 2000. p. 108. [Google Scholar]
- 32.Chusid M.J., Davis S.D. Pathogenesis of corneal and conjunctival infections. In: Tabbara K.F., Hyndiuk R.A., editors. Infections of the Eye: Basic Principles and Clinical Management. Little Brown & Co Inc; Boston: 1986. pp. 45–62. [Google Scholar]
- 33.Kreger A.S., Gray L.D. Purification of Pseudomonas aeruginosa proteases and microscopic characterization of pseudomonal protease-induced rabbit corneal damage. Infect Immun. 1978;19:630–648. doi: 10.1128/iai.19.2.630-648.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liesegang T.J., Samples J.R., Waller R.R. Suppurative interstitial ring keratitis due to streptococcus. Ann Ophthalmol. 1984;16:392–396. [PubMed] [Google Scholar]
- 35.Ionides A.C., Tuft S.J., Ferguson V.M., Matheson M.M., Hykin P.G. Corneal infiltration after recurrent corneal epithelial erosion. Br J Ophthalmol. 1997;81:537–540. doi: 10.1136/bjo.81.7.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rabinowitz S.M., Starr M.B., Gorman B.D., Kezirian G.M. Properdin-mediated immune ring formation associated with Pseudomonas keratitis. Arch Ophthalmol. 1987;105:173–174. doi: 10.1001/archopht.1987.01060020027013. [DOI] [PubMed] [Google Scholar]
- 37.Sery T.W., Pinkes A.H., Nagy R.M. Immune corenal rings. Invest Ophthalmol. 1962;1:672–685. [PubMed] [Google Scholar]
- 38.Meyers-Elliott R.H., Pettit T.H., Maxwell W.A. Viral antigens in the immune ring of Herpes simplex stromal keratitis. Arch Ophthalmol. 1980;98:897–904. doi: 10.1001/archopht.1980.01020030891018. [DOI] [PubMed] [Google Scholar]
- 39.Belmont J.B., Ostler H.B., Dawson C.R., Schwab I., Dulay D. Noninfectious ring-shaped keratitis associated with Pseudomonas aeruginosa. Am J Ophthalmol. 1982;93:338–341. doi: 10.1016/0002-9394(82)90536-0. [DOI] [PubMed] [Google Scholar]
- 40.Mondino B.J., Ratajczak H.V., Goldberg D.B., Schanzlin D.J., Brown S.I. Alternate and classical pathway components of complement in the normal cornea. Arch Ophthalmol. 1980;98:346–349. doi: 10.1001/archopht.1980.01020030342023. [DOI] [PubMed] [Google Scholar]
- 41.O'Day D.M., Jones B.R. Herpes simplex keratitis. In: Tasman W., Jaeger E.A., editors. vol. 4. J.B. Lippincott Co; Philadelphia: 1991. pp. 1–27. (Duane's Clinical Ophthalmology). (Chapter 19) [Google Scholar]
- 42.Khan A.O.1, Al-Assiri A., Wagoner M.D. Ring corneal infiltrate and progressive ring thinning following primary varicella infection. J Pediatr Ophthalmol Strabismus. 2008;45:116–117. doi: 10.3928/01913913-20080301-13. [DOI] [PubMed] [Google Scholar]
- 43.Thomas K.E., Purcell T.L., Tanzer D.J., Schanzlin D.J. Delayed diagnosis of microsporidial stromal keratitis: unusual Wessely ring presentation and partial treatment with medications against Acanthamoeba. BMJ Case Rep. 2011:2011. doi: 10.1136/bcr.08.2010.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao G., Sun S., Zhao J., Xie L. Genotyping of Acanthamoeba isolates and clinical characteristics of patients with Acanthamoeba keratitis in China. J Med Microbiol. 2010;59:462–466. doi: 10.1099/jmm.0.016667-0. [DOI] [PubMed] [Google Scholar]
- 45.Keenan J.D., Fram N.R., McLeod S.D., Strauss E.C., Margolis T.P. Perifosine-related rapidly progressive corneal ring infiltrate. Cornea. 2010;29:583–585. doi: 10.1097/ICO.0b013e3181b55cd8. [DOI] [PubMed] [Google Scholar]
- 46.Penna E.P., Tabbara K.F. Oxybuprocaine keratopathy: a preventable disease. Br J Ophthalmol. 1986;70:202–204. doi: 10.1136/bjo.70.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen S., Kremer I. Bilateral corneal immune ring opacity in Behcet's syndrome. Arch Ophthalmol. 1991;109:324–325. doi: 10.1001/archopht.1991.01080030026023. [DOI] [PubMed] [Google Scholar]
- 48.Klein P. Corneal immune ring as a complication of soft extended wear contact lens use. Optom Vis Sci. 1991;68:853–857. doi: 10.1097/00006324-199111000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Papathanassiou M., Gartry D. Sterile corneal ulcer with ring infiltrate and hypopyon after recurrent erosions. Eye (Lond) 2007;21:124–126. doi: 10.1038/sj.eye.6702438. [DOI] [PubMed] [Google Scholar]
- 50.Teichmann K.D., Cameron J., Huaman A., Rahi A.H., Badr I. Wessely-type immune ring following phototherapeutic keratectomy. J Cataract Refract Surg. 1996;22:142–146. doi: 10.1016/s0886-3350(96)80284-7. [DOI] [PubMed] [Google Scholar]
- 51.Ghanem R.C., Netto M.V., Ghanem V.C., Santhiago M.R., Wilson S.E. Peripheral sterile corneal ring infiltrate after riboflavin-UVA collagen cross-linking in keratoconus. Cornea. 2012;31:702–705. doi: 10.1097/ICO.0b013e318226da53. [DOI] [PubMed] [Google Scholar]
- 52.Waring G.O., III, Mbekeani J.N. Corneal degenerations. In: Leibowitz H.M., Waring G.O. III, editors. Corneal Disorders: Clinical Diagnosis and Manangement. second ed. WB Sauders Co.; Philadelphia: 1998. pp. 287–338. [Google Scholar]
- 53.Kenyon K.R., Navon S.E., Haritoglou C. Corneal manifestations of metabolic diseases. In: Krachmer J.H., Mannis M.J., Holland E.J., editors. second ed. vol. 1. Elsevier Mosby; Philadelphia: 2005. pp. 749–776. (Cornea). Fundamentals, Diagnosis and Management. [Google Scholar]
- 54.Witschel H., Sundmacher R., Theopold H., Jaeger W. Posterior polymorphous dystrophy of the cornea (Schlichting). An unusual clinical variant. Albr Von Graefes Arch Klin Exp Ophthalmol. 1980;214:15–25. doi: 10.1007/BF00414532. [DOI] [PubMed] [Google Scholar]
- 55.Moshirfar M., Khalifa Y.M., Espandar L., Mifflin M.D. Aquarium coral keratoconjunctivitis. Arch Ophthalmol. 2010;128:1360–1362. doi: 10.1001/archophthalmol.2010.206. [DOI] [PubMed] [Google Scholar]