Abstract
Purpose
To describe a case of bilateral conjunctivitis and cicatricial ectropion associated with dupilumab therapy for atopic dermatitis.
Observations
Severe hyperemia of the conjunctiva and eyelid margins, as well as cicatricial ectropion, began two months after starting weekly dupilumab injections for atopic dermatitis. The findings worsened over the next several months and improved after discontinuing dupilumab.
Conclusions and importance
Dupilumab is a promising intervention in the management of atopic dermatitis and asthma, however, little is known about its potential ocular adverse effects. We report the first case of dupilumab-associated ocular inflammation leading to cicatricial ectropion.
Keywords: Dupilumab, Cicatricial ectropion, Conjunctivitis, Ocular surface inflammation
1. Introduction
Dupilumab has recently shown promise in the treatment of such suspected type-2 helper T-cell mediated conditions as moderate to severe atopic dermatitis,[1], [2], [3], [4], [5] nasal polyps,6 and uncontrolled persistent asthma.[7], [8], [9] Dupilumab is a human monoclonal antibody that blocks the interleukin-4 receptor α subunit thereby inhibiting interleukin-4 (IL-4) and interleukin-13 (IL-13) signaling. IL-4 and IL-13 are secreted by CD4+ Th2 lymphocytes and drive several inflammatory processes including immunoglobulin (Ig) class switching from IgM antibodies to IgE antibodies leading to mast cell activation.7 Though generally well-tolerated, higher rates of allergic conjunctivitis have been reported by patients on dupilumab.10 We describe a case of ocular surface inflammation and severe eyelid inflammation leading to bilateral cicatricial ectropion in a patient receiving dupilumab. To our knowledge, this is the first report of eyelid inflammation leading to cicatricial ectropion associated with dupilumab.
2. Case description
A 61-year-old Caucasian male was referred to the oculoplastics service of the Cole Eye Institute for bilateral epiphora, eye pain, and cicatricial ectropion. The patient's past medical history was notable for atopic dermatitis being treated with weekly injections of dupilumab during the preceding nine months as part of a phase III clinical trial. Over the past ten years, the patient was treated with a variety of systemic immunosuppressive therapies for the management of his atopic dermatitis, including mycophenolate mofetil, cyclosporine, and prednisone. For most of the 10-year period the patient received mycophenolate mofetil 3000mg/day with minimal benefit and therefore received multiple adjunctive trials of cyclosporine. The patient experienced greater improvement in atopic dermatitis symptoms when taking cyclosporine 100mg/day, but when tapered off for concern of toxicity, his symptoms returned. Regarding oral prednisone therapy, the patient received 2 trials of 60mg/day bursts with slow tapers over a 4- month period. His atopic dermatitis was not controlled when tapered to below 20mg/day of prednisone. All of these medications had been stopped for several months prior to his presentation. Approximately two months after beginning dupilumab, the patient reported marked improvement in his skin findings, but noted eyelid redness, crusting, tearing, and intermittent blurred vision. His history included a trial of topical fluorometholone 1% and neomycin-polymyxin b-dexamethasone ophthalmic ointments, which provided minimal relief. His other medications included amlodipine, pravastatin, and metoprolol. The patient had no prior known drug allergies or significant ocular history.
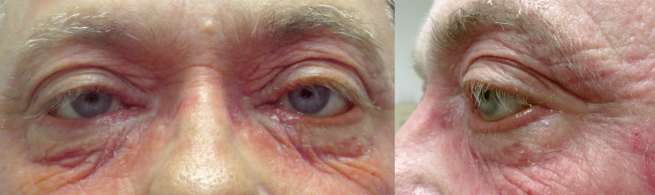
On presentation, best corrected visual acuity was 20/30 in each eye. Intraocular pressure was normal and there was no afferent pupillary defect. External examination revealed severe cicatricial ectropion with punctal stenosis of both lower eyelids, hyperemic eyelid margins, and significant conjunctival injection that extended beyond the interpalpebral zone of both eyes (Fig. 1). Dilation and irrigation of both lower punctae showed moderate punctal stenosis, but no evidence of canalicular or nasolacrimal duct obstruction. The upper punctae appeared normal, but were not dilated or irrigated. There was no evidence of intraocular inflammation.
Fig. 1.
External photos showing bilateral conjunctival hyperemia, upper eyelid edema, and cicatricial ectropion (left greater than right). Additionally, there is left-sided blepharoptosis.
The etiology of the patient's symptoms was thought to be an adverse reaction from dupilumab. The patient and his dermatologist opted to continue dupilumab treatment as it appeared to significantly improve his severe atopic dermatitis. Examination four months later demonstrated significant progression of the conjunctival injection and eyelid margin hyperemia, as well as the bilateral cicatricial ectropion. Dupilumab was discontinued and prednisone 60 mg orally per day was started. Two months after discontinuing dupilumab, on the same oral prednisone dose, the patient reported a 50% improvement in his symptoms and examination showed significant improvement in the degree of conjunctival injection, eyelid margin hyperemia, and ectropion. At follow-up three months later, on a dose of prednisone 10mg orally per day, the patient noted a 75% improvement in his ocular symptoms since stopping dupilumab. Examination showed resolution of the conjunctival injection and eyelid margin hyperemia, and improvement in the cicatricial ectropion to the pre-dupilumab position. Unfortunately, his atopic dermatitis symptoms returned with indurated skin of both lower eyelids and eczematous rash on all extremities and trunk (Fig. 2).
Fig. 2.
External photos showing bilateral resolution of conjunctivitis, eczematous reaction of lower eyelids and cheeks, and persistent cicatricial ectropion (left greater than right) following discontinuation of dupilumab and prednisone taper.
3. Discussion
Encouraging safety and efficacy data position dupilumab as a promising corticosteroid-sparing therapeutic for management of asthma8 and atopic dermatitis.[1], [10] Positive results in controlling symptoms of atopic dermatitis from phase III trials poise dupilumab to be the first FDA-approved biological systemic treatment for atopic dermatitis.[2], [10] Given that approximately 20% of children and 3% of adults worldwide suffer from atopic dermatitis, the therapeutic scope of this new treatment is wide-reaching.2
In published results from phase IIb trials, “conjunctival infections, irritations, and inflammation” were reported in 2–11% of patients receiving dupilumab depending on the dosing regimen compared to 3% in the placebo group.1 Data regarding ocular complications were further classified as allergic or infectious conjunctivitis in the phase III trials (SOLO1 and SOLO2). Rates of allergic conjunctivitis ranged from 3-5% in SOLO1 and 1% in SOLO2 compared to 1% in the placebo group of each study. Infectious conjunctivitis rates were similar at 3–5% in SOLO1 and 4% in SOLO2 compared to approximately 1% in each of the placebo groups. One patient discontinued treatment as a result of the conjunctivitis, but no further description was provided to characterize the severity of disease.10 It is important to note that not all patients were examined by an ophthalmologist and consequently these rates may not reflect the true incidence of each sub-type of conjunctivitis.11
It is unclear what factors may predispose patients to ocular complications from dupilumab, though it does seem that patients with severe atopic dermatitis or a history of allergic conjunctivitis were more likely to develop conjunctivitis.11 Unlike our patient, the severity of conjunctivitis in the majority (>90%) of patients was only mild or moderate.11 Clinically, the conjunctivitis and eyelid margin erythema in our patient was likely multifactorial. We believe that eyelid inflammation resulted in cicatricial ectropion and some degree of exposure conjunctivitis. However, the bright flush of the injection and its extent beyond the interpalpebral zone suggest a direct effect from the dupilumab itself. This is further evidenced by significant improvement in both the conjunctival injection and eyelid position after discontinuation of dupilumab. Our patient had no known history of allergic conjunctivitis or ocular atopy. Simpson et al. described two clinical presentations, which include blepharoconjunctivitis involving atopic dermatitis of the eyelids and periorbita and conjunctivitis with limbal injection.11 These may not represent distinct phenotypes as our patient appeared to have characteristics of both, though one could argue that the conjunctival changes observed in this patient were secondary to surface exposure from ectropion formation rather than an independent inflammatory process.
The underlying mechanism for the development of conjunctivitis in these patients remains unknown but may be specific to atopic dermatitis as this side effect interestingly was not noted in patients with asthma or nasal polyposis on dupilumab.11 Some have hypothesized that these cases may simply represent a manifestation of atopic keratoconjunctivitis (AKC) caused by the increased activity of AKC-specific ligands due to IL-4 and IL-13 blockade.12 Others suggest that local undertreatment may be contributory as there seems to be an inverse relationship between conjunctivitis and serum concentrations of dupilumab.11 In our patient, conjunctival injection was likely secondary to a combination of discrete ocular surface inflammation from dupilumab itself as well as secondary to exposure from bilateral cicatricial ectropion. As dupilumab becomes more widely available, the ophthalmic community may play an important role in recognizing, treating and characterizing the adverse ocular effects from this medication.
4. Conclusions
Dupilumab, a novel agent in the management of moderate-to-severe forms of asthma and atopic dermatitis, can cause significant conjunctival injection, eyelid margin hyperemia, and cicatricial ectropion. To our knowledge, this is the first report to describe the development of cicatricial ectropion in the setting of dupilumab treatment.
5. Patient consent
The patient provided both oral and written consent for use of his medical history and images in this publication.
Acknowledgements and disclosures
Funding
No funding or grant support.
Conflict of interest
The following authors have no financial disclosures: ACB, ADB, JDP.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
References
- 1.Thaçi D., Simpson E.L., Beck L.A. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387:40–52. doi: 10.1016/S0140-6736(15)00388-8. [DOI] [PubMed] [Google Scholar]
- 2.D'erme A.M. The beginning of biological treatment era in the atopic dermatitis management. Dermatol Ther. 2016;29:208–209. doi: 10.1111/dth.12277. [DOI] [PubMed] [Google Scholar]
- 3.Simpson E.L., Bieber T., Eckert L. Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74:491–498. doi: 10.1016/j.jaad.2015.10.043. [DOI] [PubMed] [Google Scholar]
- 4.Simpson E.L., Gadkari A., Worm M. Dupilumab therapy provides clinically meaningful improvement in patient-reported outcomes (PROs): a phase IIb, randomized, placebo-controlled, clinical trial in adult patients with moderate to severe atopic dermatitis (AD) J Am Acad Dermatol. 2016;75(3):506–515. doi: 10.1016/j.jaad.2016.04.054. [DOI] [PubMed] [Google Scholar]
- 5.Beck L.A., Thaçi D., Hamilton J.D. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 6.Bachert C., Mannent L., Naclerio R.M. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315:469–479. doi: 10.1001/jama.2015.19330. [DOI] [PubMed] [Google Scholar]
- 7.Vatrella A., Fabozzi I., Calabrese C., Maselli R., Pelaia G. Dupilumab: a novel treatment for asthma. J Asthma Allergy. 2014;7:123–130. doi: 10.2147/JAA.S52387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wenzel S., Castro M., Corren J. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388:31–44. doi: 10.1016/S0140-6736(16)30307-5. [DOI] [PubMed] [Google Scholar]
- 9.Wenzel S., Ford L., Pearlman D. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 10.Simpson E.L., Bieber T., Guttman-yassky E. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 11.Simpson E.L., Akinlade B., Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090–1091. doi: 10.1056/NEJMc1700366. [DOI] [PubMed] [Google Scholar]
- 12.Mennini M., Dahdah L., Fiocchi A. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090. doi: 10.1056/NEJMc1700366. [DOI] [PubMed] [Google Scholar]