Abstract
Purpose
To assess the evolution of retinal findings in patients with membranoproliferative glomerulonephritis (MPGN) by funduscopy, intravenous fluorescein angiography and optical coherence tomography.
Observations
Three women and one man were followed for a period of 1.5–37 years. Four patients (8 eyes) had drusen detected at first fundus exam at age 24, 29, 50 and 55. Three patients (6 eyes) had diffuse thickening of Bruch's membrane, and two patients (3 eyes) had detachment of the retinal pigment epithelium with serous retinal detachment. Drusen tended to widen over a period of 10-year follow-up in one case.
Conclusions and importance
Drusen remain the ocular stigmata for MPGN occuring at an early age. The retinal disease is progressive with gradual thickening of Bruch's membrane and occurrence of retinal pigment epithelium detachment.
Keywords: Drusen, Macular degeneration, Membranoproliferative glomerulonephritis, Retinal pigment epithelial detachment
1. Introduction
Membranoproliferative glomerulonephritis (MPGN) is a rare progressive glomerular disease with poor prognosis. It was previously classified ultrastructurally into 3 patterns based on location of electron dense deposits: subendothelial deposits for Type I, diffuse dense deposits in the glomerular basement membrane for Type II, and both subepithelial and subendothelial deposits for Type III. More recently and based on the role of complement in its pathogenesis,1 many researchers proposed reclassifying MPGN into immunoglobulin-mediated disease (activation of classical complement pathway) and non-immunoglobulin-mediated disease (activation of the alternative complement pathway).2, 3, 4 The vast majority of MPGN cases represent immune-complex disease while a minority (around 5%) represent complement-mediated C3 glomerulonephritis.2
We present the findings of a case series of MPGN collected through soliciting cases to members of the Pan American Retina & Vitreous Society (Table 1).
Table 1.
Clinical summary of cases with membranoproliferative glomerulonephritis with positive eye exam.
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Age at first presentation | 52 | 14 | 24 | 52 |
| Gender | Female | Female | Male | Female |
| Race | Caucasian | Caucasian | Caucasian | Caucasian |
| Systemic disease | Diabetes mellitus; HTN | Diabetes mellitus; HTN | HTN | Monoclonal gammopathy |
| Type of MPGN | II* | Probably Primary** | II* | Probably Secondary* |
| Duplication of basal lamina of glomerulus | Yes | Yes | Yes | Yes |
| Antibodies present (IgG, IgM) | No | Yes | Yes | Yes |
| Complement present (C3) | Yes | Yes | Yes | Yes |
| Smoker | No | No | No | No |
| Age at dx of MPGN | 50 | 28 | 24 | 55 |
| HTN duration (years) | 2 | 26 | 3 | No HTN |
| Hemoglobin level serum | 11.0 | 13.0 | 11.1 | NA |
| Serum creatinine mg/dl | 2.2 | 1.6 | 1.5 | 5.0 |
| Oral corticosteroid intake | Yes | Yes | No | Yes |
| Mycophenolate mofetil intake | Yes | Yes | No | Yes |
| Age at first diagnosis of drusen | 50 | 29 | 24 | 55 |
| Initial vision | 20/25 OD 20/20 OS | 20/20 OD 20/30 OS | 20/20 OD 20/20 OS | 20/60 OD 20/40 OS |
| Final vision | 20/25 OD 20/20 OS | 20/30 OD 20/30 OS | 20/20 OD 20/20 OS | 20/200 OD 20/40 OS |
| Length of follow-up (years) | 1.5 | 37 | 2.5 | 2.0 |
| Drusen type (laminar) | Yes | Yes | Yes | Yes |
| Drusen location | Diffuse till equator | Diffuse till equator | Macula | Posterior pole |
| Drusen size (microns) | 50 | 200 | 25 | 50 |
| Fundus Autofluorescence | Yes | Yes | No | Yes |
| Diffuse thickening of Bruch's membrane | Yes | Yes | No | Yes |
| Serous retinal detachment | No | Yes | No | Yes |
| RPE detachment | No | Yes | No | Yes |
| Choroidal new vessel | No | No | No | No |
Abbreviations: HTN, systemic hypertension; MPGN, membranoproliferative glomerulonephritis; NA, not assessed; OD, right eye; OS, left eye, RPE, retinal pigment epithelium (one asterisk refers to documentation by transmission electron microscopy, and 2 asterisks refers to negative workup for secondary causes of MPGN).
2. Findings
Case 1
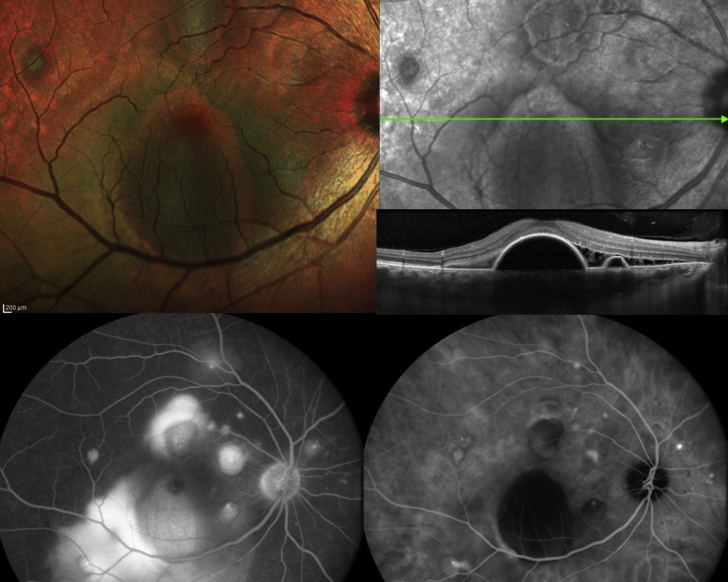
This 53-year-old Caucasian woman with MPGN II was asymptomatic with best corrected visual acuity of 20/25 (right eye) and 20/20 (left eye). Funduscopy revealed bilateral diffuse drusen (Fig. 1) involving uniquely the posterior pole (Fig. 2) with retinal pigment epithelium (RPE) alterations in the right eye on fluorescein angiography (Fig. 2) and diffusely thickened Bruch’s membrane (Fig. 3).
Case 2
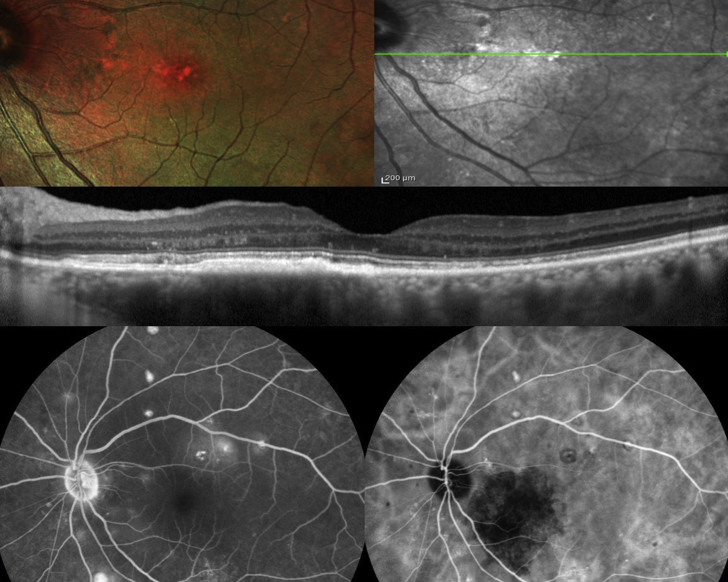
This 50-year-old Caucasian woman, product of first cousin parents, was followed since age 13 in the Motility clinic for blurred vision of 20/40 attributed to esophoria and hyperopia. At age 24, she was started on oral corticosteroid and various immunosuppressive regimens to treat the glomerulonephritis. At age 27, renal biopsy revealed 30% loss of glomeruli with linear deposit at the glomerular basement membrane but negative immunofluorescence for C3 and immunoglobulins. Drusen were first detected at age 29 concomitant with a second kidney biopsy compatible with MPGN (positive immunofluorescence for complement C3 and IgM). At age 30, visual loss in the left eye was attributed to central serous chorioretinopathy. There was subfoveal RPE detachment that resolved after 6 months. Ten years later, there was progression of drusen along with diffuse marked thickening of Bruch’s membrane (Fig. 4, Fig. 5, Fig. 6, Fig. 7) with preservation of vision at 20/30 level in both eyes.
Case 3
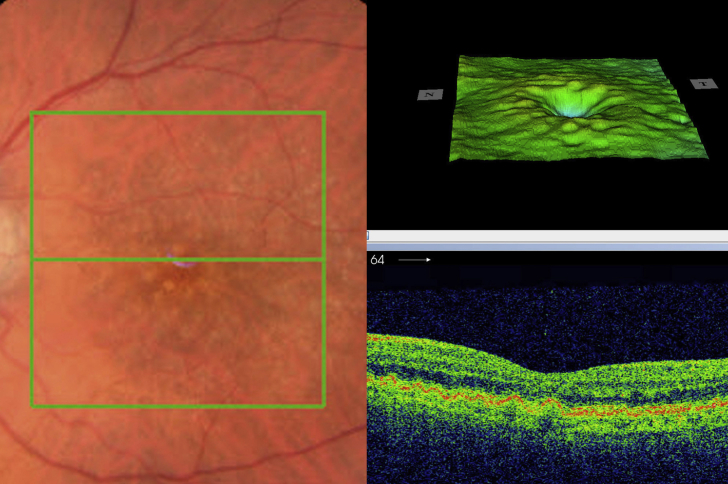
This asymptomatic 24-year-old Caucasian man with MPGN II had drusen in the macular region (Fig. 8) that did not change over a follow-up of 3 years. Spectral domain optical coherence tomography (SD-OCT) revealed normal looking Bruch’s membrane (Fig. 8).
Case 4
This 52-year-old Caucasian woman had proteinuria (2.6g/24 hour) with decreased kidney function. A kidney biopsy revealing dense deposits in the glomerular basement membrane with positive immunofluorescence for IgG and C3. Since then she was maintained on Prednisone 30mg daily. One year later, 3 monthly intravitreal bevacizumab injections failed to flatten multiple RPE detachments in the left eye. Systemic corticosteroid therapy was replaced by mycophenolate mofetil. Tacrolimus was also added to stop progressive proteinuria. Three years later, the RPE detachment have worsened in both eyes and responded partially with 6 monthly ranibizumab injections to the right eye. Cyclophosphamide and rituximab could not stop progressive renal failure and a second renal biopsy detected MPGN with monoclonal kappa deposits and bone marrow establishing the diagnosis of monoclonal gammopathy. Two years later (8 years after initial presentation) (Fig. 9), visual acuity was 20/200 in the right eye from multiple RPE detachments. Three monthly intravitreal aflibercept injections aggravated the detachment. Thereafter the left eye had similar pathology (RPE detachment) (Fig. 10) and visual acuity improved from 20/63 to 20/40 after 3 bolus of intravenous infliximab. Peritoneal dialysis was started with a proteinuria of 14g/dl and creatinine of 3.5mg/dl.
Fig. 1.
Case 1-Color and autofluorescence of Case 1 showing posterior pole drusen in both eyes. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Case 1- Fluorescein angiography of the right eye and left eye shows hyperfluorescence of the drusen with drusen localized to the posterior pole (bottom left) and diseased retinal pigment epithelium (bottom right).
Fig. 3.
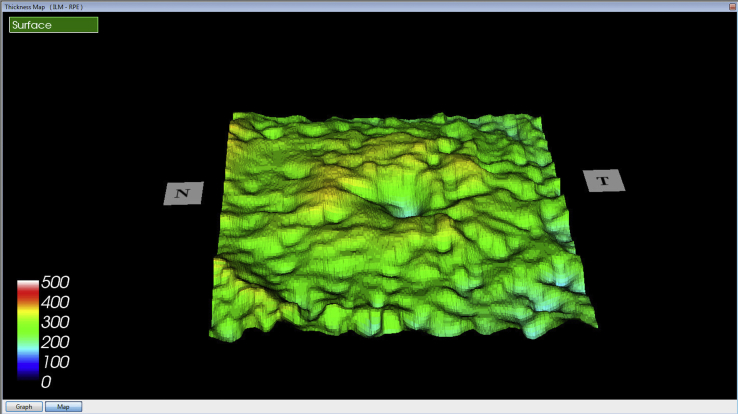
Case 1- Markedly thickened Bruch's membrane with elevation of retinal pigment epithelium at sites of drusen on Optical coherence tomography of both eyes.
Fig. 4.
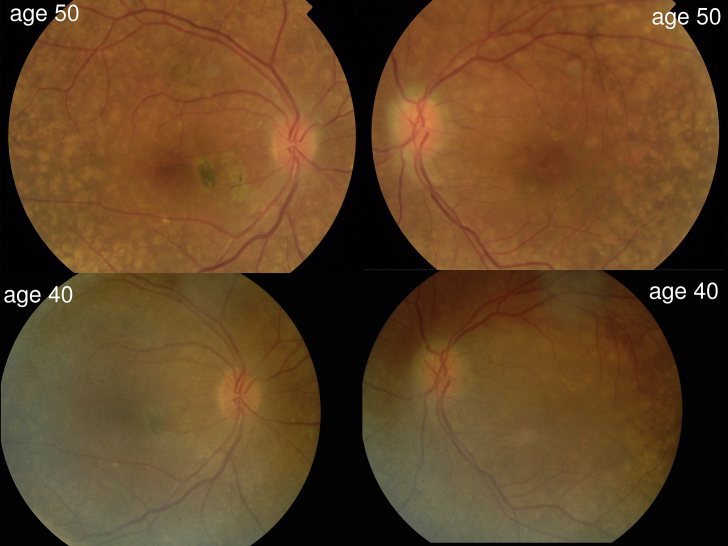
Case 2- Color photographs of the posterior pole in both eyes showing enlargement of drusen over 10-year-follow-up. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
Case 2- Autofluorescence showing bilateral diffuse stippled mottling of the retinal pigment epithelium, along with patches of sick retinal pigment epithelium in the right macula at the site of previous serous detachment of the retina.
Fig. 6.
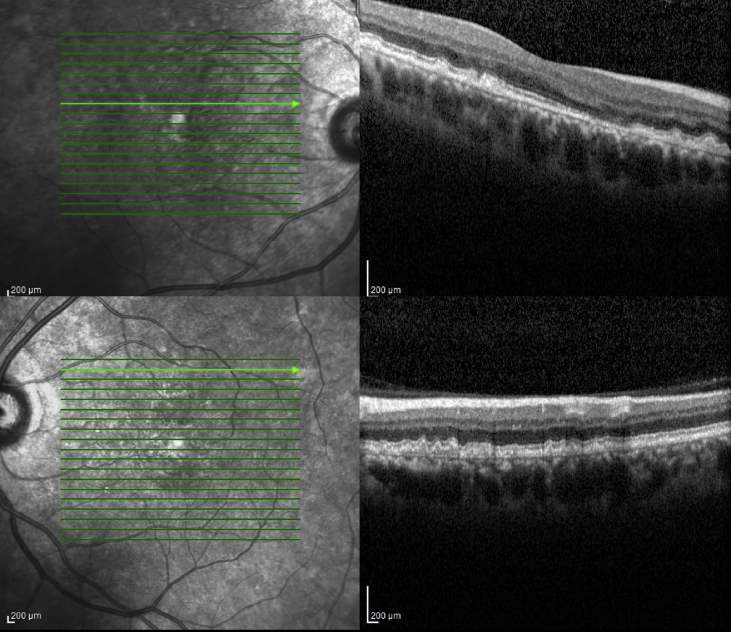
Case 2- Diffuse thickening of Bruch's membrane with nodular elevations at sites of drusen on Optical coherence tomography of the right macula.
Fig. 7.
Case 2- Retinal pigment epithelium-internal limiting membrane Optical coherence tomography view of the left eye shows nodular elevations of retinal pigment epithelium corresponding to evenly spaced drusen.
Fig. 8.
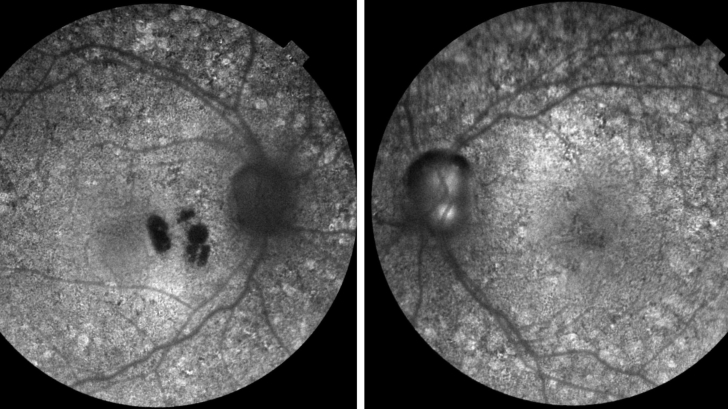
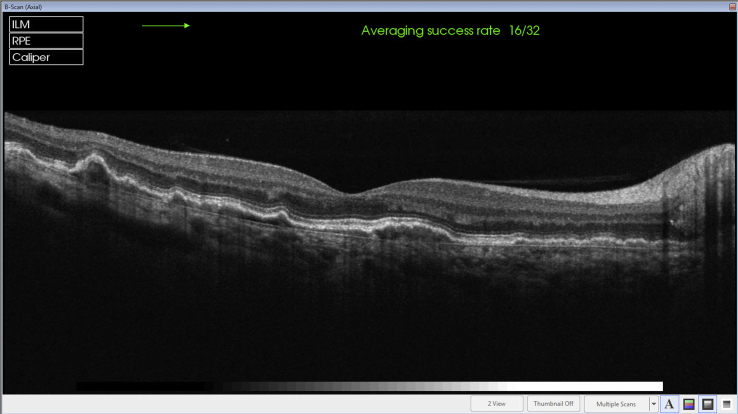
Case 3- Fine drusen in the macular region in both eyes (Top) with no thickening of Bruch's membrane of the right macula (Bottom).
Fig. 9.
Case 4- Right posterior pole showing multiple detachment of retinal pigment epithelium (Top left), well outlined on Optical coherence tomography (Top right), with many pinpoint leakage noted on fluorescein angiography (Bottom left) and indocyanine green (Bottom right). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 10.
Case 4- Left posterior pole showing multiple alterations of retinal pigment epithelium with drusen (Top left) well outlined on Optical coherence tomography with diffuse thickening of Bruch's membrane (Top right); many pinpoint leakage sites are noted outside the arcade on fluorescein angiography (Bottom left) and indocyanine green (Bottom right). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3. Discussion
We presented 4 cases of MPGN that illustrate a variety of presentations from symptomatic (Case 2, Case 4) to asymptomatic (Case 1, Case 3). Pigment epithelial detachment in Case 4 may be related to the use of systemic corticosteroids. The common denominator in this case series was the presence of drusen at an early age (Table 1). Drusen tended to coalesce forming a diffusely thickened basement membrane (Case 1) (Fig. 3, Fig. 6) or focally with detachment of the RPE (Case 1) or recurrent central serous chorioretinopathy (Case 2, Case 4).5, 6, 7 Drusen have been described in MPGN as diffuse8, 9 and often evenly spaced (Fig. 7) resembling drusen in age related macular degeneration (AMD) (Fig. 11).10, 11 Drusen were reported most often with type II and occasionally with other types.12 Drusen are rarely observed in subjects below age 50 years.13 The two major types of early onset drusen include basal laminar drusen and dominant drusen. Basal laminar drusen represent small scattered round yellowish macular lesions visualized by fluorescein angiography as ‘stars in the sky’ appearance and are associated with MPGN. On the other hand, dominant drusen appear as small radial drusen with autosomal dominant inheritance that is caused by a mutation in the EGF containing fibrillin-like extracellular matrix protein 1 (EFEMP1) gene.13 Thickened Bruch's membrane in the young is seen in MPGN and in other entities like pseudoxanthoma elasticum with angioid streaks (thickened calcified membrane).14 The drusen in MPGN are similar to glomerular deposits in their subepithelial location, and their composition from complement components and immunoglobulins, and also similar to drusen in age-related macular degeneration in location (Fig. 11), composition and associated complement deposits.15, 16 The variability of the drusen size noted in the current series may probably reflect the stage of the disease with early onset drusen being small, few and macular in location, while in advanced cases, there is coalescence of drusen, involvement of more peripheral areas, and more autofluorescence.
Fig. 11.
(unpublished case)- This 79-year-old woman presented with visual loss from a right eye choroidal new vessel formation. The left macula had diffuse drusen (Left). Retinal pigment epithelium-internal limiting membrane view shows an evenly pitted pattern similar to case 2 (Right top). Optical coherence tomography (horizontal section) showed the density of the drusen by the dome-shaped even spaced elevations of the retinal pigment epithelium (Right bottom).
MPGN II is a very rare disease (2–3 cases per million) affecting both genders equally and diagnosed commonly in children between 5 and 15 years of age.1, 2 The most common findings are hematuria and proteinuria with half of the patients ending in renal failure 10 years after diagnosis.1, 2 Diagnosis requires renal biopsy with C3 but not IgG demonstrable by immunofluorescence. Systemic stigmata include partial lipodystrophy or drusen of the retinal pigment epithelium.17
The retinal findings in MPGN may represent a model for AMD because both AMD and MPGN share a common pathophysiology: uncontrolled systemic activation of the alternative pathway of the complement cascade.15, 16, 17, 18 There are potential triggers resulting in complement system dysfunction such as complement factor H mutations,19 antibodies against complement factor H, and autoantibodies against C3bBb (C5 convertase) that are frequently detected in MPGN.20
Several groups contributed to our further understanding of drusen in MPGN. Leys et al.21, 22, 23 defined the clinical and angiographic findings in MPGN emphasizing the long term complications of the disease that happen at an early age, such as choroidal neovascularization. Likewise, Savige et al.20 presented the long term visual effects (from 2 to 40 years) associated with C3 glomerulopathies. In late disease, peripheral vision was restricted, central vision was distorted, and there were scotomas from either sub-retinal choroidal neovascular membranes, RPE detachment or atypical serous retinopathy. According to Dalvin et al.,3, 4 drusen are more common in complement-mediated MPGN (39% of patients) than in immune complex-mediated MPGN (9% of patients). This same group emphasized the new classification as relevant to nephrology, and more so to ophthalmology and that complement mediated MPGN need routine screening by ophthalmologists.3, 4 In addition, patients with basal laminar drusen are at risk to develop MPGN, and it is recommended that patients with extensive basal laminar drusen undergo screening for renal dysfunction.19 MPGN is an uncommon cause of chronic nephritis involving primarily children or young adults and characterized histologically by: (1) proliferation of mesangial and endothelial cells and expansion of the mesangial matrix, (2) thickening of the peripheral capillary walls by subendothelial immune deposits and/or intramembranous dense deposits, and (3) mesangial interposition into the capillary wall with the double-contour or tram-track appearance.
Past systemic therapy for MPGN have met with little success such as plasmapheresis (suppress C3NeF activity), IV immunoglobulin, B cell suppression.24, 25, 26, 27 Two drugs appear promising: eculizumab and compstatin. Eculizumab, a monoclonal antibody, binds C5 and halt formation of membrane attack complex and appear beneficial in delaying nephropathy progression.24, 25 Compstatin, a peptide that binds C3 protein, has been developed as a potential treatment that targets the complement system.26 Administered intra-vitreally, the drug has completed phase I trials. A small study in a primate model of AMD found improvement of drusen in four subjects.27 Treatment of choroidal neovascularization in MPGN requires vascular endothelial growth factor antagonists.28, 29, 30
Limitations of our study include its retrospective nature, and small number of cases. However, this study confirms that drusen remain the ocular stigmata for MPGN occurring at an early age. The retinal disease progress from drusen, gradual thickening of Bruch's membrane and occurrence of retinal pigment epithelium detachment or choroidal new vessels.
Patient consent
Consent to publish the case reports was not obtained. This report does not contain any personal information that could lead to the identification of the patients. The study received IRB approval at the Rafic Hariri University Hospital.
Funding
None.
Conflict of interest
The authors have no financial disclosures.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
Contributor Information
Ahmad M. Mansour, Email: ammansourmd@gmail.com.
Luiz H. Lima, Email: LUIZLIMA9@gmail.com.
J. Fernando Arevalo, Email: Arevalojf2020@gmail.com.
Miguel Hage Amaro, Email: miguelhamaro@yahoo.com.br.
Virginia Lozano, Email: vlozano77@hotmail.com.
Alaa Bou Ghannam, Email: alaa.bghannam@gmail.com.
Errol W. Chan, Email: ewechan@gmail.com.
References
- 1.Fakhouri F., Fremeaux-Bacchi V., Noel L.H. C3 glomerulopathy: a new classification. Nat Rev Nephrol. 2010;6:494–499. doi: 10.1038/nrneph.2010.85. [DOI] [PubMed] [Google Scholar]
- 2.Woo S.A., Ju H.Y., Kwon S.H. Reanalysis of membranoproliferative glomerulonephritis patients according to the new classification: a multicenter study. Kidney Res Clin Pract. 2014;33:187–191. doi: 10.1016/j.krcp.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalvin L.A., Fervenza F.C., Sethi S., Pulido J.S. Manifestations of complement-mediated and immune complex-mediated membranoproliferative glomerulonephritis: a comparative consecutive series. Ophthalmology. 2016;123:1588–1594. doi: 10.1016/j.ophtha.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Dalvin L.A., Fervenza F.C., Sethi S., Pulido J.S. Shedding light on fundus drusen associated with membranoproliferative glomerulonephritis: breaking stereotypes of types I, II, and III. Retin Cases Brief Rep. 2016;10:72–78. doi: 10.1097/ICB.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 5.Polk T.D., Kimura A.E., Park D., Gass J.D. Subretinal fluid associated with membranoproliferative glomerulonephritis. Arch Ophthalmol. 1997;115:927–928. doi: 10.1001/archopht.1997.01100160097022. [DOI] [PubMed] [Google Scholar]
- 6.Ulbig M.R., Riordan-Eva P., Holz F.G., Rees H.C., Hamilton P.A. Membranoproliferative glomerulonephritis type II associated with central serous retinopathy. Am J Ophthalmol. 1993;116:410–413. doi: 10.1016/s0002-9394(14)71397-2. [DOI] [PubMed] [Google Scholar]
- 7.Ritter M., Bolz M., Haidinger M. Functional and morphological macular abnormalities in membranoproliferative glomerulonephritis type II. Br J Ophthalmol. 2010;94:1112–1114. doi: 10.1136/bjo.2009.159475. [DOI] [PubMed] [Google Scholar]
- 8.Venkatesh P., Sony P., Garg S.P. Optical coherence tomography of fundus findings in type II mesangiocapillary glomerulonephritis. Eye (Lond) 2006;20:497–499. doi: 10.1038/sj.eye.6701897. [DOI] [PubMed] [Google Scholar]
- 9.Batioğlu F., Müftüoğlu O., Atmaca L. Optical coherence tomography of fundus abnormalities associated with type II membranoproliferative glomerulonephritis. Retina. 2003;23:261–262. doi: 10.1097/00006982-200304000-00026. [DOI] [PubMed] [Google Scholar]
- 10.Duvall-Young J., Short C.D., Raines M.F., Gokal R., Lawler W. Fundus changes in mesangiocapillary glomerulonephritis type II: clinical and fluorescein angiographic features. Br J Ophthalmol. 1989;73:900–906. doi: 10.1136/bjo.73.11.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duvall-Young J., MacDonald M.K., McKechnie N.M. Fundus changes in (type II) mesangiocapillary glomerulonephritis simulating drusen: a histopathological report. Br J Ophthalmol. 1989;73:297–302. doi: 10.1136/bjo.73.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han D.P., Sievers S. Extensive drusen in type I membranoproliferative glomerulonephritis. Arch Ophthalmol. 2009;127:577–579. doi: 10.1001/archophthalmol.2009.38. [DOI] [PubMed] [Google Scholar]
- 13.Guigui B., Leveziel N., Martinet V. Angiography features of early onset drusen. Br J Ophthalmol. 2011;95:238–244. doi: 10.1136/bjo.2009.178400. [DOI] [PubMed] [Google Scholar]
- 14.Mansour A.M., Henkind P. Marked thickening of Bruch's membrane in a 17-year-old man with angioid streaks. Eye (Lond) 1998;12:898–900. doi: 10.1038/eye.1998.230. [DOI] [PubMed] [Google Scholar]
- 15.Mullins R.F., Russell S.R., Anderson D.H., Hageman G.S. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. [PubMed] [Google Scholar]
- 16.D'Souza Y.B., Jones C.J., Short C.D. Oligosaccharide composition is similar in drusen and dense deposits in membranoproliferative glomerulonephritis type II. Kidney Int. 2009;75:824–827. doi: 10.1038/ki.2008.658. [DOI] [PubMed] [Google Scholar]
- 17.D'Souza Y., Short C.D., McLeod D. Long-term follow-up of drusen-like lesions in patients with type II mesangiocapillary glomerulonephritis. Br J Ophthalmol. 2008;92:950–953. doi: 10.1136/bjo.2007.130138. [DOI] [PubMed] [Google Scholar]
- 18.Colville D., Guymer R., Sinclair R.A. Visual impairment caused by retinal abnormalities in mesangiocapillary (membranoproliferative) glomerulonephritis type II (“dense deposit disease”) Am J Kidney Dis. 2003;42:E2–E5. doi: 10.1016/s0272-6386(03)00665-6. [DOI] [PubMed] [Google Scholar]
- 19.van de Ven J.P., Boon C.J., Fauser S. Clinical evaluation of 3 families with basal laminar drusen caused by novel mutations in the complement factor H gene. Arch Ophthalmol. 2012;130:1038–1047. doi: 10.1001/archophthalmol.2012.265. [DOI] [PubMed] [Google Scholar]
- 20.Savige J., Amos L., Ierino F. Retinal disease in the C3 glomerulopathies and the risk of impaired vision. Ophthalmic Genet. 2016;37:369–376. doi: 10.3109/13816810.2015.1101777. [DOI] [PubMed] [Google Scholar]
- 21.Leys A., Vanrenterghem Y., Van Damme B., Snyers B., Pirson Y., Leys M. Fundus changes in membranoproliferative glomerulonephritis type II. A fluorescein angiographic study of 23 patients. Graefes Arch Clin Exp Ophthalmol. 1991;229:406–410. doi: 10.1007/BF00166300. [DOI] [PubMed] [Google Scholar]
- 22.Leys A., Vanrenterghem Y., Van Damme B., Snyers B., Pirson Y., Leys M. Sequential observation of fundus changes in patients with long standing membranoproliferative glomerulonephritis type II (MPGN type II) Eur J Ophthalmol. 1991;1:17–22. doi: 10.1177/112067219100100104. [DOI] [PubMed] [Google Scholar]
- 23.Leys A., Vanrenterghem Y., Van Damme B., Snyers B., Pirson Y., Leys M. Subretinal neovascular membranes associated with chronic membranoproliferative glomerulonephritis type II. Graefes Arch Clin Exp Ophthalmol. 1990;228:499–504. doi: 10.1007/BF00918479. [DOI] [PubMed] [Google Scholar]
- 24.Daina E., Noris M., Remuzzi G. Eculizumab in a patient with dense-deposit disease. N Engl J Med. 2012;366:1161–1163. doi: 10.1056/NEJMc1112273. [DOI] [PubMed] [Google Scholar]
- 25.Vivarelli M., Pasini A., Emma F. Eculizumab for the treatment of dense-deposit disease. N Engl J Med. 2012;366:1163–1165. doi: 10.1056/NEJMc1111953. [DOI] [PubMed] [Google Scholar]
- 26.Ricklin D., Lambris J.D. Compstatin: a complement inhibitor on its way to clinical application. Adv Exp Med Biol. 2008;632:273–292. doi: 10.1007/978-0-387-78952-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chi Z.L., Yoshida T., Lambris J.D., Iwata T. Suppression of drusen formation by compstatin, a peptide inhibitor of complement C3 activation, on cynomolgus monkey with early-onset macular degeneration. Adv Exp Med Biol. 2010;703:127–135. doi: 10.1007/978-1-4419-5635-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karampelas M., Kopsachilis N., Chang J.H., Pal B. Patient with type II membranoproliferative glomerulonephritis: intravitreal bevacizumab therapy for choroidal neovascularization. Ophthalmologe. 2012;109:791–793. doi: 10.1007/s00347-012-2550-y. [DOI] [PubMed] [Google Scholar]
- 29.McCullagh D., Silvestri G., Maxwell A.P. Treatment of choroidal neovascularisation secondary to membranoproliferative glomerulonephritis type II with intravitreal ranibizumab. BMJ Case Rep. 2014 doi: 10.1136/bcr-2013-010247. 2014 Jun 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farah S.E., Fazelat A., Frei G. Treatment of subretinal neovascular membrane in a patient with membranoproliferative glomerulonephritis type II. Ophthalmic Surg Lasers Imaging. 2009;40:416–418. doi: 10.3928/15428877-20096030-13. [DOI] [PubMed] [Google Scholar]