Abstract
The AMP-activated protein kinase (AMPK) is a sensor of cellular energy status. It is activated, by a mechanism requiring the tumor suppressor LKB1, by metabolic stresses that increase cellular ADP:ATP and/or AMP:ATP ratios. Once activated, it switches on catabolic pathways that generate ATP, while switching off biosynthetic pathways and cell cycle progress. These effects suggest that AMPK activators might be useful for treatment and/or prevention of type 2 diabetes and cancer. Indeed, AMPK is activated by the drugs metformin and salicylate, the latter being the major breakdown product of aspirin. Metformin is widely used to treat diabetes, while there is epidemiological evidence that both metformin and aspirin provide protection against cancer. We review the mechanisms of AMPK activation by these and other drugs, and by natural products derived from traditional herbal medicines.
The AMP-activated protein kinase (AMPK) is a highly conserved sensor of cellular energy status, and genes encoding the three subunits of the kinase are found in essentially all eukaryotic genomes (Hardie, 2011). AMPK is switched on by metabolic stresses and xenobiotic compounds that cause a cellular energy imbalance, which is detected as increases in the ratios of ADP:ATP and AMP:ATP. Since the energy status of the cell is a crucial factor in all aspects of cell function, it is not surprising that AMPK has many downstream targets whose phosphorylation mediates dramatic changes in cell metabolism, cell growth and other functions. In general, AMPK switches on catabolic processes that provide alternative pathways to generate ATP, while switching off anabolic pathways and other processes consuming ATP, thus acting to restore cellular energy homeostasis. The kinase evolved in single-celled eukaryotes and is still involved in multicellular organisms in regulating energy balance in a cell-autonomous manner. However, it is now clear that new functions were acquired during the development of metazoans, so that AMPK is also regulated by hormones and adipokines that regulate energy balance at the whole body level, a topic reviewed in more detail elsewhere (Hardie, et al., 2012). Metabolic disorders such as obesity and type 2 diabetes, which are becoming increasingly prevalent in modern society, are essentially problems caused by a positive energy balance, and it was predicted - in a review written in the 1990s (Winder and Hardie, 1999) - that activators of AMPK might be useful for treating these disorders. This suggestion has been taken up by the pharmaceutical industry, which has been developing novel activators, but what was not predicted was that several existing drugs, as well as many natural plant products with reputed health benefits, would also turn out to be AMPK activators. Findings that AMPK caused inhibition of progress through the cell cycle (Imamura, et al., 2001), and that the mechanism of AMPK activation required the presence of the tumor suppressor LKB1 (Hawley, et al., 2003; Woods, et al., 2003; Shaw, et al., 2004), also introduced the idea (discussed further below) that AMPK activators might be useful in the prevention and/or treatment of cancer.
The major aim of this review is to discuss the various drugs and xenobiotics that regulate AMPK, to clarify the various mechanisms by which they achieve this, and to discuss the evidence that these agents might be useful in the treatment of obesity, type 2 diabetes and cancer.
AMPK: subunit structure and regulation
AMPK appears to exist in all eukaryotes as heterotrimeric complexes composed of a catalytic α subunit and regulatory β and γ subunits (Fig. 1). In mammals all three subunits have multiple isoforms (α1, α2; β1, β2; γ1, γ2, γ3) encoded by distinct genes, and all twelve possible heterotrimeric combinations are able to form when these are co-expressed in cells. The function of the different subunit isoforms remains unclear, although there is tissue-specific expression of some isoforms, and (as discussed below) there is evidence that different isoforms may target complexes to specific subcellular locations. Moreover, some AMPK-activating drugs show selectivity for certain isoform combinations.
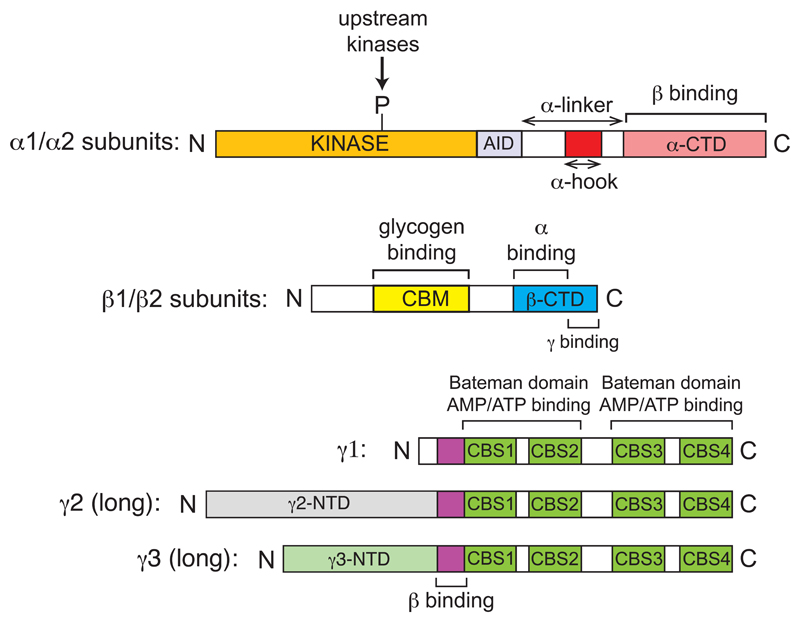
Figure 1.
Domain structure of the α, β and γ subunits of AMPK and their isoforms. KEY: α-subunits: AID, auto-inhibitory domain, α-CTD, C-terminal domain; β-subunits: CBM, carbohydrate-binding module; β-CTD, C-terminal domain; γ subunits: CBS1, CBS2, CBS3, CBS4, cystathione β-synthase repeats; γ2-NTD, N-terminal extension of “long” version of γ2 subunit; γ3-NTD, N-terminal extension of “long” version of γ3 subunit.
The catalytic α subunits
The catalytic subunits are encoded by two alternate genes in mammals (PRKAA1 and PRKAA2 in humans). The α1 isoform appears to be universally expressed, while α2 is more abundant in tissues such as skeletal and cardiac muscle, and appears to be absent in cells of the blood and endothelial cell lineages. Both isoforms contain conventional serine/threonine kinase domains at the N-terminus, and their kinase activity is increased >100-fold by phosphorylation of a conserved threonine residue within the “activation loop”, a sequence segment also critically involved in regulation of many other kinases (Johnson, et al., 1996). This threonine residue is usually referred to as Thr-172 due to its position in the original rat sequence (Hawley, et al., 1996), although the exact residue numbering may differ in other species. The upstream kinases that phosphorylate this site were first identified by whole kinome screens in yeast (Saccharomyces cerevisiae), where three protein kinases with partially redundant functions were identified (Hong, et al., 2003; Nath, et al., 2003; Sutherland, et al., 2003). Although there were no clear orthologs of these in the human kinome, searching using their kinase domain sequences revealed that the closest matches were to LKB1 and the two isoforms of the calmodulin-dependent kinase kinases, CaMKKα and CaMKKβ. Indeed, all three of these can act as upstream kinases in mammalian cells, although CaMKKα is less active that the other two (Hawley, et al., 2003; Woods, et al., 2003; Shaw, et al., 2004; Hawley, et al., 2005; Hurley, et al., 2005; Woods, et al., 2005). Thr-172 can also be phosphorylated by transforming growth factor-β activated protein kinase-1 (TAK1) (Momcilovic, et al., 2006; Herrero-Martin, et al., 2009), although the exact physiological significance of that mechanism remains uncertain.
The discovery of LKB1 as an upstream kinase was particularly interesting because LKB1 had previously been identified as the product of a tumor suppressor gene (Alessi, et al., 2006), and AMPK was its first downstream target to be identified. It was subsequently discovered that, in addition to the α1 and α2 subunits of AMPK, LKB1 also phosphorylates and activates twelve other enzymes with kinase domains closely related to those of AMPK, now known as the AMPK-related kinase or ARK family (Lizcano, et al., 2004). LKB1 is only active in the form of a complex with two other subunits, STRAD and MO25 (Hawley, et al., 2003). This complex appears to be constitutively active (Sakamoto, et al., 2004), with Thr-172 phosphorylation normally being regulated instead by binding of ligands to the substrate, AMPK (see below). By contrast, CaMKKβ appears to have a very low basal activity against AMPK in intact cells, and only phosphorylates Thr-172 in response to treatments that increase intracellular Ca2+ (Hawley, et al., 2005; Hurley, et al., 2005; Woods, et al., 2005).
The identities of the protein phosphatase(s) dephosphorylating Thr-172 are not fully resolved, although there is genetic evidence in S. cerevisiae that there are two phosphatases dephosphorylating this site. One is a complex between Glc7 (the single PP1 catalytic subunit) and its targeting subunit Reg1 (Sanz, et al., 2000), while the other is Sit4, the ortholog of the mammalian phosphatase PP6 (Ruiz, et al., 2011; Ruiz, et al., 2012). In MIN6 cells (a mouse pancreatic β cell line) evidence from siRNA knockdowns suggests that a complex between PP1 and the targeting subunit PPP1R6 dephosphorylates Thr-172 and, despite no clear sequence relationship, DNA encoding PPP1R6 complements a reg1 mutant yeast strain (Garcia-Haro, et al., 2010). It has also been reported that RNAi-based depletion of PPM1E (a member of the PPM family of protein phosphatases, which are not related to the PPP family containing PP1 and PP6) increases Thr-172 phosphorylation in HEK-293 cells (Voss, et al., 2011).
The kinase domain (KD) of AMPK is immediately followed by a small region termed the auto-inhibitory domain (AID) (Fig. 1). It was given this name because truncated α subunits containing the KD plus the AID have very low activity even when phosphorylated on Thr-172, while constructs containing the KD alone exhibit full activity (Goransson, et al., 2007; Pang, et al., 2007; Chen, et al., 2009). In a crystal structure of a fission yeast KD-AID construct, the AID forms a small domain with three α-helices, which interacts with the small and large lobes of the KD on the opposite face to the active site, appearing to constrain the kinase domain in a less active, “open” conformation. Mutations in the predicted KD:AID interface in a heterotrimeric complex from mammals also increased the basal activity in the absence of AMP, suggesting that the AID is involved in the mechanism of allosteric activation by AMP (Chen, et al., 2009). However, in the structure of an active mammalian heterotrimeric complex, the AID appeared to be unstructured (Xiao, et al., 2011), so its role in the overall regulation of the AMPK heterotrimer remains unclear.
The C-terminal region of the α subunit (α-CTD) forms a globular domain that interacts with the C-terminal domain of the β subunit, and is thus essential for formation of the αβγ complex (Crute, et al., 1998; Hudson, et al., 2003; Xiao, et al., 2007; Xiao, et al., 2011). The KD/AID and the α-CTD are joined by an linker peptide of extended conformation; in a recent structure (Xiao, et al., 2011) this linker wraps around the γ subunit, rather like two arms stretching out from the KD/AID and the α-CTD that hold the γ subunit in a tight embrace. The “clasped hands” of these arms are formed by a structure called the “α hook”, which contacts one of the regulatory nucleotides bound to the γ subunit (see below).
Several groups have reported that complexes containing the α2 isoform are enriched in the nucleus in different cell types (Salt, et al., 1998; Turnley, et al., 1999; Ai, et al., 2002), although α1 complexes do not appear to be completely excluded from the nucleus. A short putative nuclear localization sequence (KKIR, residues 224-227) has been highlighted towards the C-terminus of the α2 kinase domain (Suzuki, et al., 2007); in α1 the equivalent sequence is KKIC. Interestingly, there are well-defined and functional nuclear export sequences at the C-termini of both α1 and α2 (Kazgan, et al., 2010). It seems likely that both the α1 and α2 complexes shuttle in and out of the nucleus, although this has not been studied in detail.
The β subunits
The β1 and β2 subunits are encoded by two alternate genes in mammals (PRKAB1 and PRKAB2 in humans). Both are widely expressed, although β1 predominates in some tissues such as rodent liver (Thornton, et al., 1998). As already indicated, their C-terminal domains (β-CTD) interact with the α-CTD, and the β subunits then terminate with a short sequence that associates with the γ subunit via an inter-subunit β-sheet (Amodeo, et al., 2007; Townley and Shapiro, 2007; Xiao, et al., 2007; Xiao, et al., 2011). The β-CTD thus forms the “core” of the heterotrimeric complex, holding the α and γ subunits together. Both β subunits are subject to myristoylation at their N-termini (Oakhill, et al., 2010); this is required for the ability of AMP and ADP binding to enhance phosphorylation of Thr-172 (Oakhill, et al., 2010; Oakhill, et al., 2011). It has also been proposed that myristoylation affects the translocation of AMPK complexes containing α2 between the cytoplasm and the nucleus (Suzuki, et al., 2007).
Along with the β-CTD, the other β subunit region that is well conserved between species is the central carbohydrate-binding module (CBM, Fig. 1), originally termed the glycogen-binding domain (Hudson, et al., 2003; Polekhina, et al., 2003). This module is related to CBMs found in other proteins, where they are often fused to catalytic domains involved in the metabolism of glycogen and starch, and thus co-localize the catalytic domains with their polysaccharide substrates (Machovic and Janecek, 2006). Although the CBM was missing from two partial structures of the mammalian heterotrimer (Xiao, et al., 2007; Xiao, et al., 2011), it was present in a structure from budding yeast (Amodeo, et al., 2007). The CBM from rat β1 has also been crystallized on its own, and conserved residues involved in carbohydrate binding have been identified (Polekhina, et al., 2005). The CBM of β2 has a higher affinity for glycogen than that of β1 (Koay, et al., 2010).
Although it is clear that the β subunit CBMs cause AMPK complexes to bind to the surface of glycogen particles (Hudson, et al., 2003; Polekhina, et al., 2003; Bendayan, et al., 2009), the physiological role of this is not completely resolved. However, glycogen synthase (GS), the key enzyme of glycogen synthesis that is also bound to the glycogen particle, is a substrate for AMPK. Both the muscle and liver isoforms of glycogen synthase are phosphorylated by AMPK at N-terminal sites that cause their inactivation (Carling and Hardie, 1989; Jorgensen, et al., 2004; Bultot, et al., 2012), suggesting that one function of the CBM is to co-localize AMPK with this downstream target. Glycogen and synthetic oligosaccharides based on the structure of glycogen also inhibit AMPK, although inhibition by glycogen varies with different preparations of the polysaccharide (McBride, et al., 2009). One hypothesis is that AMPK acts as a glycogen sensor that regulates glycogen synthesis according to certain aspects of glycogen structure, although the latter are not yet well defined.
The γ subunits
The γ1, γ2 and γ3 subunit isoforms are encoded by three alternate genes in mammals (PRKAG1-3 in humans). The γ1 isoform appears to be expressed ubiquitously, whereas γ2 and γ3 are most abundant in muscle. The γ2 and γ3 isoforms contain N-terminal extensions that are unrelated to each other and are not present in γ1 (Fig. 1); in both cases these extensions can also occur as “long” and “short” forms due to the use of alternate transcriptional start sites (Lang, et al., 2000; Yu, et al., 2004). Recently, a third γ2 variant (γ2-3B) has been identified that lacks the amino acids encoded by exons 1-3 in the “long” form but has 32 unique residues encoded by an alternate exon, 3B (Pinter, et al., 2012). Both at the mRNA and the protein level, γ2 is expressed in adults in both cardiac and skeletal muscle, while γ3 appears to be restricted to skeletal muscle (Cheung, et al., 2000; Milan, et al., 2000; Yu, et al., 2004). However, AMPK activity has been immunoprecipitated from other tissues, such as brain, using an anti-γ3 antibody (Cheung, et al., 2000). Both γ3 and γ2-3B are also expressed in fetal mouse heart, and γ2-3B appears to account for almost half of total γ2 protein in adult mouse heart (Pinter, et al., 2012).
All three γ subunit isoforms contain at their C-termini four tandem repeats of a sequence known as a cystathione β-synthase (CBS) repeat; these are numbered CBS1-4 from the N- to the C-terminus (Fig. 1). CBS1 is immediately preceded by a short sequence involved in the interaction with the β subunit (Viana, et al., 2007; Xiao, et al., 2007). CBS repeats occur in around 20 other proteins in the human genome, invariably as tandem pairs. Single tandem pairs form structures known as Bateman domains, which are regulatory domains that bind adenosine-containing ligands, either AMP, ADP, ATP or, in the case of cystathione β-synthase itself, S-adenosyl methionine (Scott, et al., 2004). The γ subunits of AMPK are unusual in having four rather than two repeats, and these form two Bateman domains that assemble in a head-to-head manner (Amodeo, et al., 2007; Townley and Shapiro, 2007; Xiao, et al., 2007). Because of the four-fold symmetry, there are four clefts that could potentially bind adenine nucleotides; these are numbered according to the number of the CBS repeat bearing an aspartate residue that interacts with the nucleotide ribose ring. Site 2 is unoccupied (CBS2 lacks an aspartate), while site 4 contains AMP that is very tightly bound and non-exchangeable, and which may play a structural role. Sites 1 and 3 are the sites where AMP, ADP and ATP bind reversibly in competition with each other. They bind AMP, ADP and ATP with quite similar affinity, although site 1 appears to have a higher affinity for all three nucleotides (Scott, et al., 2004; Xiao, et al., 2011).
Canonical regulation of AMPK by adenine nucleotides and calcium ions
When cells are under metabolic stress (e.g. due to shortage of glucose or oxygen), or are treated with a pharmacological agent that inhibits ATP synthesis (e.g. almost any mitochondrial inhibitor), or are under stress due to accelerated ATP consumption (e.g. due to activation of motor proteins during muscle contraction), there will be an increase in the cellular ADP:ATP ratio. In unstressed cells the concentration of AMP is usually very low because the high ATP:ADP ratio drives the adenylate kinase reaction (ATP + AMP ↔ 2ADP) towards ADP synthesis. However, whenever the ADP:ATP ratio rises during energetic stress, the highly reversible adenylate kinase reaction will be partially displaced towards synthesis of AMP, causing a large rise in AMP. Increases in ADP and AMP (relative to ATP) are thus signals that cells are under energetic stress, and are the key signals that switch on AMPK in its canonical, energy-sensing role. In unstressed cells, the two exchangeable sites on the γ subunit (sites 1 and 3) are probably occupied by ATP. During a mild stress both ADP:ATP and AMP:ATP ratios will rise, but because AMP concentrations will still be low compared with ATP and ADP, it is most likely ADP that initially displaces ATP. The majority of cellular ATP is present as a complex in which the β and γ phosphates bind a Mg2+ ion (Mg.ATP2-), which has a lower affinity for the γ subunit than free ATP4- (Xiao, et al., 2011). Thus, when binding to the AMPK-γ subunits ADP does not have to compete with total ATP, but only with the less abundant ATP4-. Given its low concentration during a mild stress, AMP may only displace ADP or ATP as stress becomes more severe and its concentration rises further.
Binding of ADP or AMP causes a conformational change in the AMPK complex that both promotes phosphorylation of Thr-172 (Hawley, et al., 1995; Oakhill, et al., 2011), and inhibits dephosphorylation (Davies, et al., 1995; Suter, et al., 2006; Xiao, et al., 2011). Complete phosphorylation can cause >100-fold activation, although the degree of phosphorylation may never come close to this in vivo. It is important to note that ADP and AMP appear to affect phosphorylation and dephosphorylation entirely by binding to AMPK, rather than by regulating the upstream kinase or phosphatase. The effect of phosphorylation is further amplified by allosteric activation, which is triggered only by binding of AMP, and not ADP. It has been proposed (Xiao, et al., 2011) that the allosteric effect is due to binding of AMP to the high affinity site (site 1), whereas the effect on phosphorylation state is mediated by binding of ADP or AMP to the lower affinity site (site 3). However, mutations of the aspartate residues that bind the nucleotide ribose rings in sites 1 and 3, and even the non-exchangeable site (site 4), have been found to reduce both responses (Oakhill, et al., 2011). Thus, there appear to be complex, co-operative interactions between the nucleotides bound at these three sites, which are not yet fully understood. It has also been proposed that protection against Thr-172 dephosphorylation is mediated by the “α hook” region of the α subunit; a structure of a complex with AMP bound at site 3 suggests that the α hook would be able to interact with AMP or ADP at this site, but that the extra phosphate on ATP might prevent this interaction (Xiao, et al., 2011).
The other “canonical” activation mechanism involves the phosphorylation of Thr-172 by CaMKKβ in response to a rise in intracellular Ca2+. This occurs, for example, in response to depolarization of neurons (Hawley, et al., 2005), activation of receptors coupled to phosphatidylinositol-specific phospholipases, and hence Ca2+ release, in various cell types (Stahmann, et al., 2006; Thornton, et al., 2008; Yang, et al., 2011), and activation of the antigen receptor in T lymphocytes (Tamas, et al., 2006). This mechanism can occur in the absence of any change in cellular nucleotides, although the two canonical mechanisms (AMP/ADP and Ca2+) can also synergize with each other (Fogarty, et al., 2010).
Downstream targets and use of AMPK activators in diabetes and cancer
AMPK phosphorylates serine residues surrounded by a well-defined recognition motif (Scott, et al., 2002; Gwinn, et al., 2008). Space constraints do not permit a comprehensive discussion of the entire literature on downstream targets, and the reader is referred to other recent reviews (Hardie, 2007; Steinberg and Kemp, 2009; Hardie, 2011; Mihaylova and Shaw, 2011; Hardie, et al., 2012). However, Figs 2-4 summarize some well characterized targets that are also briefly discussed below, with one or two representative references provided in each case.
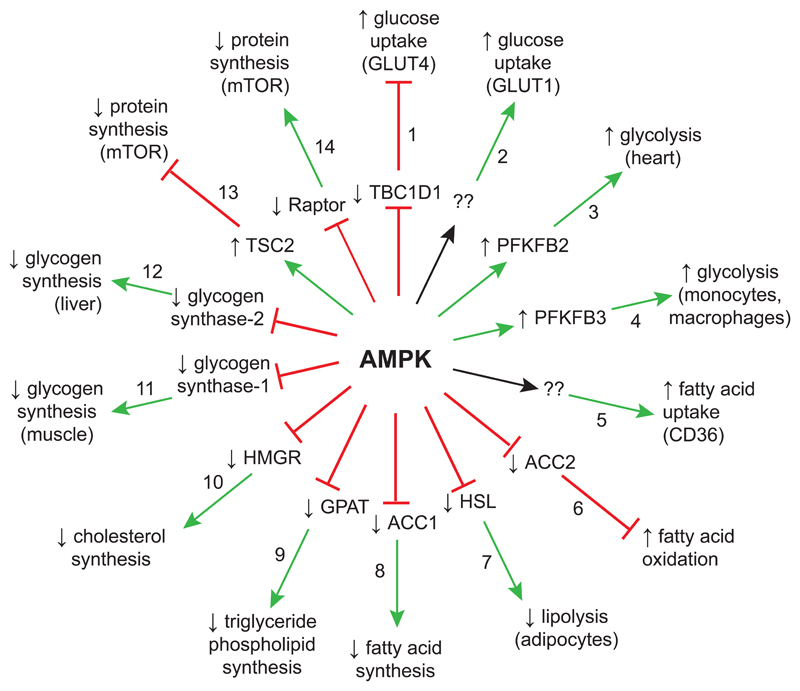
Figure 2.
Acute metabolic effects of AMPK activation. Upward/downward arrows indicate that the target protein (inner wheel) or pathway (outer wheel) are activated/inactivated. See text for numbering and key to acronyms. Green arrows on the “spokes” of the wheel indicate activation, red lines with a bar at the end indicate inhibition, while black arrows and “??” indicate that the direct target protein that explains the effect is not known.
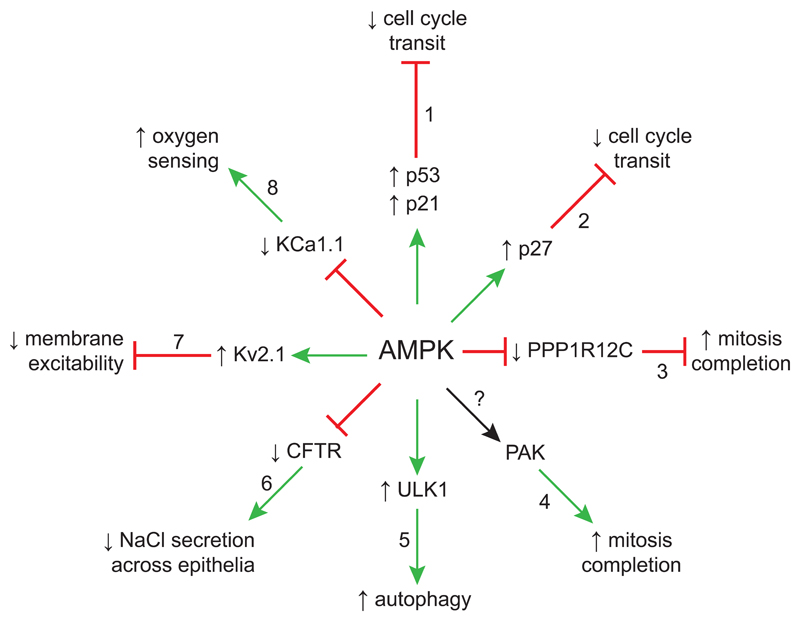
Figure 4.
Effects of AMPK activation other than on metabolism and/or transcription. Upward/downward arrows indicate that the functions of the target protein (inner wheel) or target process (outer wheel) are activated/inactivated.
Fig. 2 involves targets involved in the acute regulation of metabolism. Progressing clockwise around the spokes of the “wheel”, starting at 12 o’clock:
-
1)
AMPK activates GLUT4-mediated glucose uptake in muscle via phosphorylation of TBC1D1 (Frosig, et al., 2010), a Rab-GAP protein that under basal conditions inhibits fusion of intracellular GLUT4-containing vesicles with the plasma membrane. Phosphorylation by AMPK triggers 14-3-3 binding, causing TBC1D1 to dissociate from the intracellular vesicles.
-
2)
AMPK also activates glucose uptake in other cells by activation of GLUT1 already present on the membrane (Barnes, et al., 2002). However, GLUT1 does not appear to be a direct target for AMPK, and the molecular mechanism remains unknown.
-
3)
AMPK activates glycolysis in cardiac myocytes by phosphorylation and activation of the PFKFB2 isoform of 6-phosphofructo-2-kinase, which catalyzes the synthesis of fructose-2,6-bisphosphate, a key activator of the glycolytic enzyme 6-phosphofructo-1-kinase (Marsin, et al., 2000).
-
4)
AMPK also activates glycolysis in monocytes and macrophages by phosphorylation and activation of the PFKFB3 isoform of 6-phosphofructo-2-kinase. This may allow macrophages to generate ATP efficiently in regions of infection or injury that are hypoxic (Marsin, et al., 2002).
-
5)
AMPK activates fatty acid uptake via translocation of the transporter CD36 to the plasma membrane, although the molecular mechanism remains unknown (Habets, et al., 2009).
-
6)
AMPK activates fatty acid oxidation by phosphorylating and inactivating the mitochondria-associated isoform of acetyl-CoA carboxylase (ACC2), thus lowering malonyl-CoA, an inhibitor of fatty acid uptake into mitochondria via the carnitine:palmityl-CoA transferase system (Merrill, et al., 1997).
-
7)
Lipolysis (hydrolysis of triglyceride stores) in adipose tissue is catalyzed by desnutrin/ATGL, which removes the first fatty acid, and hormone-sensitive lipase (HSL), which removes the second (Zimmermann, et al., 2004). AMPK inhibits lipolysis in rodent and human adipocytes by phosphorylation of HSL, antagonizing its activation by cyclic AMP-dependent protein kinase and its translocation to the surface of the lipid droplet (Garton, et al., 1989; Daval, et al., 2005; Bourron, et al., 2010). Since lipolysis is a catabolic pathway, it might seem illogical that AMPK should inhibit it. However, this may be a mechanism to limit the production during lipolysis of free fatty acids; if these are allowed to accumulate they recycle into triglycerides, creating a futile cycle that consumes ATP. Consistent with this, AMPK is activated in 3T3-L1 adipocytes by cyclic AMP-elevating agents and this is associated with increases in cellular AMP:ATP ratios, but both effects are blunted by inhibition of lipolysis or of the acyl-CoA synthetase required for recycling of fatty acids back to triglycerides (Gauthier, et al., 2008). Somewhat paradoxically, AMPK has been reported to phosphorylate and activate desnutrin/ATGL in mammalian cells (Ahmadian, et al., 2011), although in nematode worms the enzyme appears to be inhibited by AMPK phosphorylation (Narbonne and Roy, 2009), which is more consistent with the known effects of AMPK on HSL function in mammals.
-
8)
AMPK inhibits fatty acid synthesis by directly phosphorylating and inactivating the cytosolic isoform of acetyl-CoA carboxylase (ACC1) (Davies, et al., 1992); phosphospecific antibodies that recognize the key phosphorylation sites on both ACC1 and ACC2 are widely used as markers for AMPK activation.
-
9)
AMPK inhibits triglyceride and phospholipid synthesis by causing inactivation of the first enzyme involved in their synthesis, glycerol phosphate acyl transferase (GPAT); it remains unclear whether GPAT is a direct substrate for AMPK (Muoio, et al., 1999).
-
10)
AMPK inhibits isoprenoid/cholesterol synthesis by direct phosphorylation and inactivation of 3-hydroxy-3-methylglutaryl CoA reductase (HMGR) (Clarke and Hardie, 1990).
-
11)
AMPK inhibits muscle glycogen synthesis by phosphorylation and inactivation of glycogen synthase-1 (Jorgensen, et al., 2004).
-
12)
AMPK inhibits liver glycogen synthesis by phosphorylation and inactivation of glycogen synthase-2 (Bultot, et al., 2012).
-
13)
AMPK inhibits protein synthesis by phosphorylating tuberous sclerosis complex protein-2 (TSC2), causing Rheb (a small G protein that activates mammalian target-of-rapamycin complex-1, mTORC1) to be converted to its inactive GDP-bound form (Inoki, et al., 2003).
-
14)
AMPK also inhibits protein synthesis by directly phosphorylating Raptor, a subunit of the mTORC1 complex (Gwinn, et al., 2008).
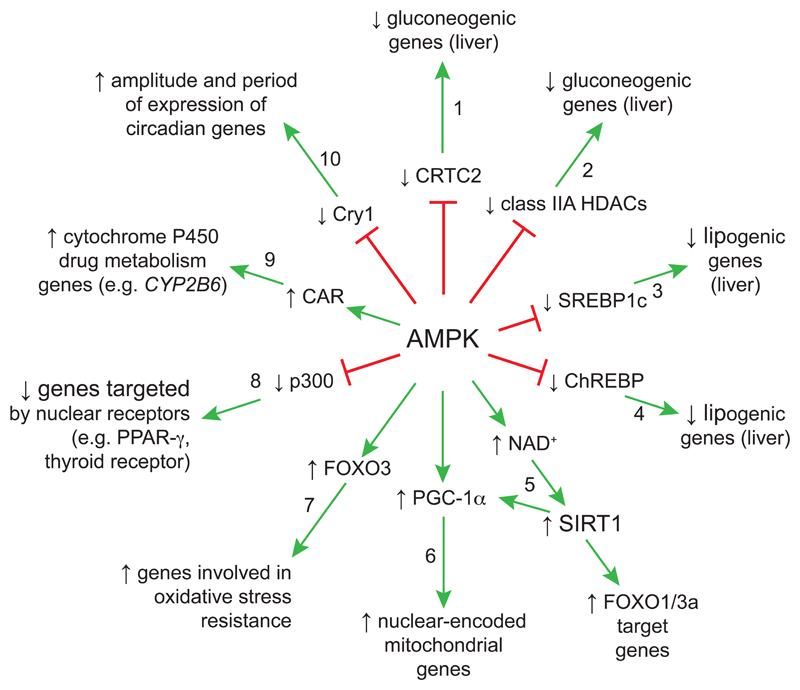
In addition to these acute effects on metabolism, AMPK also has numerous effects on transcription, which are summarized in Fig. 3. Progressing clockwise around the spokes of the wheel, starting at 12 o’clock:
-
1)
AMPK phosphorylates cyclic AMP response element binding protein- (CREB-) regulated transcription co-activator-2 (CRTC2, formerly called TORC2), causing it to bind 14-3-3 proteins, thus retaining it in the cytoplasm and inhibiting activation of genes encoding gluconeogenic enzymes in the liver (Koo, et al., 2005).
-
2)
AMPK phosphorylates class IIa lysine deacetylases (HDAC-4, -5 and -7), causing them to bind 14-3-3 proteins that retain them in the cytoplasm, and preventing formation of heterodimers with class I lysine deacetylases (e.g. HDAC3). The latter activate transcription of specific genes, including gluconeogenic genes, and this therefore represents a second mechanism [see (1) above] by which AMPK down-regulates gluconeogenesis (Mihaylova, et al., 2011).
-
3)
AMPK phosphorylates sterol response element binding protein-1c (SREBP-1c), preventing its proteolytic processing and nuclear translocation, and thus inhibiting transcription of lipogenic genes, including those encoding ACC-1 and fatty acid synthase (Li, et al., 2011).
-
4)
AMPK phosphorylates carbohydrate response element binding protein (ChREBP), inhibiting its DNA binding and consequent activation of transcription of lipogenic genes, including the liver isoform of pyruvate kinase (Kawaguchi, et al., 2002).
-
5)
AMPK activates the class III deacetylase SIRT1, which deacetylates FOXO-1 and -3, activating genes modulated by those transcription factors [see (7) below]. SIRT1 also deacetylates and activates PGC-1α [see (6) below]. The mechanism by which AMPK activates SIRT1 remains uncertain, although it has been proposed that effects of AMPK on metabolism increase the cellular concentration of NAD+, a co-substrate of SIRT1 (Canto, et al., 2009).
-
6)
AMPK activation causes phosphorylation of peroxisome proliferator-activated receptor-γ (PPARγ) coactivator-1α (PGC-1α), a “master regulator” of expression of mitochondrial genes that triggers mitochondrial biogenesis. The phosphorylation sites identified do not closely conform to the AMPK recognition motif so this may not be a direct effect, but this phosphorylation is believed to activate transcription of PGC-1α by a positive feedback loop acting on its own promoter (Jager, et al., 2007). In addition, PGC-1α is deacetylated and activated by SIRT1 in response to AMPK activation [see (5) above].
-
7)
AMPK directly phosphorylates several sites on the transcription factor FOXO3 [see also (5) above], activating transcription of many genes, including genes involved in resistance to oxidative stress (Greer, et al., 2007).
-
8)
AMPK phosphorylates the transcriptional co-activator p300, reducing its interaction with, and thus inhibiting transcription induced by, nuclear hormone receptors such as PPAR-γ and the thyroid hormone receptor (Yang, et al., 2001).
-
9)
AMPK activation induces transcription from genes modulated by the constitutive androstane receptor (CAR), particularly those (e.g. CYP2B6) encoding cytochrome P450 enzymes that metabolize xenobiotic compounds, such as phenobarbital (Rencurel, et al., 2005).
-
10)
AMPK phosphorylates the cryptochrome protein Cry1, triggering its degradation and thus relieving its inhibitory effects on the BMAL1:CLOCK heterodimer, a transcription factor involved in the establishment of circadian rhythms of gene expression (Lamia, et al., 2009).
Figure 3.
Effects of AMPK activation on transcription. Upward/downward arrows indicate that the target transcription factor or co-activator (inner wheel) or target genes (outer wheel) are activated/inactivated. Note that SIRT1 (on spoke 5) deacetylates both FOXO family transcription factors and the transcriptional co-activator PGC-1α.
In addition to the effects on expression of messenger RNAs described above, AMPK phosphorylates the RNA polymerase I transcription factor TIF-1A, thus inhibiting the production of ribosomal RNA (Hoppe, et al., 2009). In proliferating cells, rRNA accounts for around 80-90% of all RNA synthesis, so this mechanism would conserve ATP, while also inhibiting cell growth under conditions of energy limitation.
Fig. 4 summarizes regulation by AMPK of some targets involved in cellular processes other than metabolism or transcription. Progressing clockwise around the spokes of the wheel, starting at 12 o’clock:
-
1)
AMPK activation triggers phosphorylation of p53 at Ser-15 (human sequence numbering). This site does not fit the AMPK recognition motif so this may not be a direct phosphorylation, but it causes p53 stabilization and thus activates transcription of p21WAF1, a cyclin-dependent kinase inhibitor that triggers G1:S phase cell cycle arrest (Imamura, et al., 2001; Jones, et al., 2005).
-
2)
AMPK activation causes phosphorylation of another cyclin-dependent kinase inhibitor, p27KIP1, at a C-terminal threonine residue; this site does not fit the AMPK consensus motif so this may not be a direct phosphorylation. However, it causes p27KIP1 stabilization, inducing G1:S phase cell cycle arrest and autophagy (Liang, et al., 2007).
-
3)
AMPK phosphorylates PPP1R12C, a regulatory subunit of protein phosphatase-1 (PP1) that targets PP1 to dephosphorylate myosin regulatory light chain (MRLC). Phosphorylation triggers binding of 14-3-3 proteins to PPP1R12C and this may prevent the phosphatase complex from dephosphorylating MRLC. Phosphorylation of PPP1R12C appears to be necessary for completion of mitosis, as discussed further under (4) below (Banko, et al., 2012).
-
4)
AMPK also phosphorylates PAK2, a protein kinase that phosphorylates MRLC at the N-terminal site that is dephosphorylated by the PPP1R12C:PP1 complex; phosphorylation of MRLC activates the ATPase activity of non-muscle myosin, triggering the movement of this motor protein along actin fibres. Although it is not yet clear whether phosphorylation by AMPK regulates PAK2 activity, MRLC phosphorylation is required for completion of mitosis (Banko, et al., 2012). This helps to explain defects in chromosomal segregation observed in Drosophila larvae that lack AMPK (Lee, et al., 2007), and is consistent with findings that AMPK is found to be phosphorylated at the mitotic apparatus in M phase cells (Vazquez-Martin, et al., 2011). Although mitosis is an energy-requiring event and it might appear paradoxical that AMPK should activate it, it may be crucial for cells undergoing mitosis to complete the process if energy stress occurs while it is happening, so that they can arrest in a more orderly manner in the following G1 phase instead.
-
5)
AMPK phosphorylates the protein kinase ULK1, triggering autophagy. Autophagy is critical to recycle amino acids and other components in cells starved of nutrients, while autophagy of mitochondria (mitophagy) recycles components from damaged mitochondria that are no longer efficiently producing ATP (Egan, et al., 2011).
-
6)
AMPK phosphorylates and inactivates the cystic fibrosis transmembrane regulator (CFTR), a Cl- channel gated by ATP that is involved in trans-epithelial salt transport (Hallows, et al., 2003). Salt transport is an energy-requiring process, so this would represent an energy-conserving mechanism.
-
7)
AMPK phosphorylates the voltage-gated, delayed rectifier K+ channel, Kv2.1, in its C-terminal tail; phosphorylation sensitizes the channel so that it opens at lower extents of membrane depolarization. This reduces membrane excitability and the consequent firing of action potentials in central neurons. Since firing of action potentials in the brain is a major consumer of energy, this mechanism may protect neurons during metabolic stress (Ikematsu, et al., 2011).
-
8)
AMPK phosphorylates another voltage-gated K+ channel, KCa1.1 (also known as BKCa); in this case the phosphorylation inactivates the channel. This is believed to be part of the oxygen-sensing mechanism in type I cells of the carotid body: hypoxia activates AMPK, causing inactivation of KCa1.1 that then facilitates opening of voltage-gated Ca2+ channels. Increases in cell Ca2+ cause neurotransmitter release from the type I cells, triggering firing of the carotid sinus nerve that signals to the brain, where corrective changes in breathing patterns are initiated (Ross, et al., 2011).
In summary, AMPK activation has numerous and complex effects on cell function, although these can mostly be viewed as mechanisms to restore cellular energy homeostasis when it becomes disrupted. By direct phosphorylation of metabolic enzymes and by effects on transcription, AMPK switches on catabolic pathways such as the uptake of glucose and fatty acids, and their metabolism by mitochondrial oxidation and (in the case of glucose) by glycolysis. In addition, AMPK switches off biosynthetic pathways such as the synthesis of glucose, glycogen and lipids in the liver. By promoting muscle glucose uptake and metabolism and by inhibiting hepatic glucose production, AMPK activation can explain the anti-hyperglycemic actions of the drug metformin, which is used to treat type 2 diabetes and is discussed further below. In addition, type 2 diabetes is thought to be primarily caused by insulin resistance, which is strongly associated with excess triglyceride storage in liver and muscle. By switching off the synthesis of fatty acids and triglycerides and enhancing fat oxidation instead, AMPK activation might also explain the insulin-sensitizing actions of metformin.
The synthesis of fatty acids, triglycerides, cholesterol, RNA (especially rRNA) and proteins are all up-regulated in rapidly proliferating cells such as tumor cells. AMPK activation switches off all of these pathways, and would therefore be expected to exert a cytostatic, anti-tumor effect, reinforced by its ability to cause cell cycle arrest. These effects of AMPK might explain, at least in part, the tumor suppressor effects of the upstream kinase LKB1 (Alessi, et al., 2006), as well as findings that metformin usage reduces the risk of cancer in diabetics (Evans, et al., 2005), and that metformin and other AMPK activators (phenformin, A-769662, see below) delay the onset of tumorigenesis in mouse models (Huang, et al., 2008). However, AMPK may represent a “double-edged sword” in cancer in that, once a tumor has arisen, it might exert a cell-autonomous effect to protect tumor cells against the actions of cytotoxic agents. AMPK activators might therefore be deleterious in the treatment (as opposed to the prevention) of cancer. Consistent with this idea, in an siRNA screen of protein kinases whose loss was synthetically lethal with c-Myc activation in U2OS cells, two major hits obtained were AMPK-α1 and the AMPK-related kinase ARK5 (NUAK1). These results suggest that these kinases help to protect cells against the metabolic stresses caused by c-Myc activation (Liu, et al., 2012). Similarly, it has been suggested that AMPK protects cells against the metabolic stress caused by glucose deprivation, and promotes anchorage-independent growth and solid tumor formation in vivo, by reducing NADPH consumption by lipid synthesis and thus preserving the nucleotide for protection against oxidative stress (Jeon, et al., 2012).
Activation of AMPK by drugs and xenobiotics
In this section we review the mechanisms by which AMPK is activated by pharmacologically active agents. Some of these are drugs in clinical use in conventional medicine, while others are natural products present in traditional medicines, or derived from food or beverages claimed to have “nutraceutical” properties. The majority of these were in use for these purposes before it was realized that they were activators of AMPK. An important tool in establishing their mechanisms of action was a pair of isogenic cell lines derived from HEK-293 cells, which express AMPK complexes containing γ2 with or without a single substitution in site 3 (R531G) that renders the complex insensitive to the effects of ADP and AMP on phosphorylation (Hawley, et al., 2010). The mutant cells will be referred to below as RG cells. Agents that act by increasing cellular ADP or AMP fail to activate AMPK in the RG cells, whereas agents that act by other mechanisms (e.g. the Ca2+ ionophore A23187, which increases intracellular Ca2+ and activates CaMKKβ) activate AMPK in the RG cells to the same extent as in the wild type cells. Other ancillary approaches used in this study were to measure changes in ADP:ATP ratio by capillary electrophoresis of acid extracts, and the use of an extracellular flux analyzer to measure oxygen uptake (Hawley, et al., 2010).
5-Aminoimidazole-4-carboxamide riboside
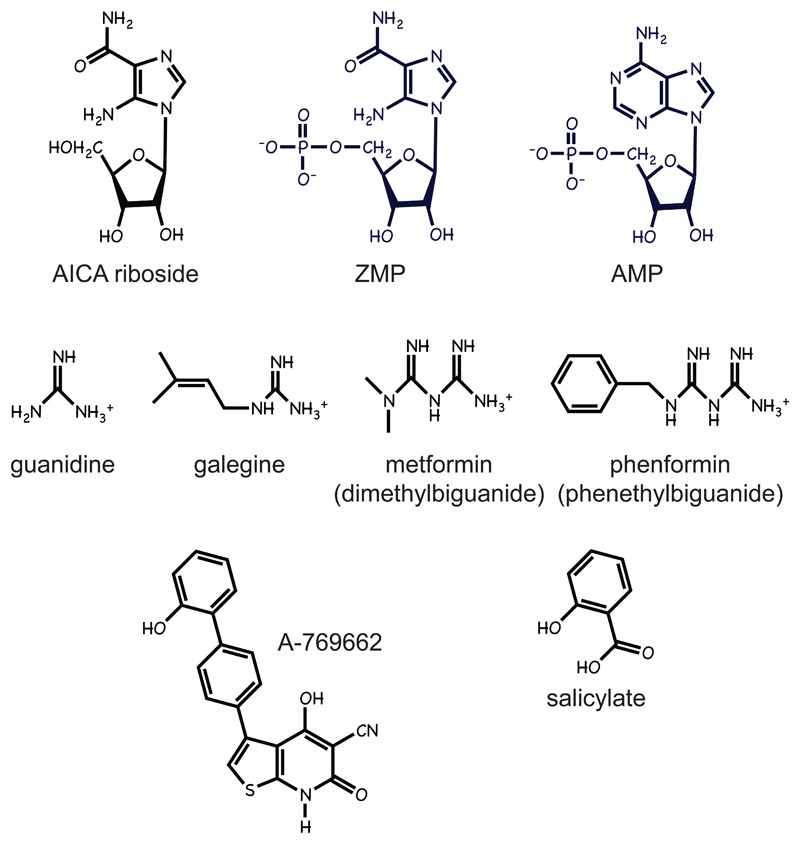
5-Aminoimidazole-4-carboxamide riboside (AICA riboside) is an adenosine analogue taken up into cells by adenosine transporters and phosphorylated by adenosine kinase to the equivalent ribotide, ZMP (Fig. 5), which mimics all three effects of AMP on the AMPK complex (Corton, et al., 1995). Because ZMP binds to site 3 on the γ subunit in a similar manner to AMP (Day, et al., 2007), AICA riboside does not activate AMPK in RG cells although, unlike AMPK activators inhibiting mitochondrial function, it does not cause changes in ADP:ATP ratio or oxygen uptake (Hawley, et al., 2010). Although ZMP is ≈50-fold less potent as an activator than AMP, AICA riboside activates AMPK in most cells because ZMP can accumulate to millimolar concentrations within the cell (Corton, et al., 1995). Because it is effective in vivo (although it is not orally available and has to be injected), AICA riboside has been much used to identify physiological processes regulated by AMPK in animal models.
Figure 5.
Structures of some AMPK-activating compounds and related compounds.
Another interesting feature of AICA riboside is that, under the name “acadesine”, it was used in small-scale clinical trials in humans undergoing coronary artery bypass graft surgery, where it appeared to provide some benefit (Mangano, 1997). These trials were undertaken before it was realized that AICA riboside activated AMPK, and at the time it was viewed as a means to rapidly replenish the total cellular adenine nucleotide content. Another interesting point is that ZMP is a naturally occurring intermediate in cellular purine nucleotide synthesis and is metabolized by the enzyme AICAR transformylase, which catalyzes the penultimate step in the synthesis of the purine nucleotide IMP. AICA riboside tends to work better as an AMPK-activating agent in quiescent, primary cells than in rapidly proliferating cells, possibly because the latter have a higher rate of purine synthesis so they more rapidly metabolize ZMP. Consistent with this, the anti-cancer agent methotrexate (an anti-folate drug that inhibits AICAR transformylase) sensitizes tumour cells to the AMPK-activating and growth-inhibitory effects of AICA riboside (Beckers, et al., 2006). Pemetrexed, another anti-folate drug used in the treatment of lung cancer, is a more selective inhibitor of AICAR transformylase, and it can cause accumulation of ZMP and activation of AMPK even in the absence of exogenous AICA riboside (Racanelli, et al., 2009). These findings raise the possibility that some effects of anti-folate drugs used to treat cancer might be mediated by AMPK.
Metformin, phenformin and galegine
Metformin is currently the first choice drug for treatment of type 2 diabetes. Because of the very high prevalence of this disorder, it is currently prescribed to more than 100 million people, or more than 1% of the world population. Metformin is derived from guanidine which, along with galegine (Fig. 5), is a natural product found in the plant Galega officinalis (also known as Goat’s Rue). Goat’s Rue was described in the 18th century as a herbal medicine to treat “symptoms such as thirst and frequent urination” (Hill, 1772). Interestingly, it is classed as a noxious weed in the USA because it is poisonous to herbivores, which presumably includes goats! Guanidine is quite toxic, but galegine (an isoprenyl derivative) is a potent AMPK activator (Mooney, et al., 2008) and was tested for treatment of diabetes in humans, with some success, in the 1920s (Muller and Reinwein, 1927). Metformin (dimethylbiguanide) is a biguanide derivative that was synthesized in the 1920s and found to be effective in lowering blood glucose in animals (Hesse and Taubmann, 1929). However, the success of insulin as a therapy for diabetes, developed in the same decade, put further studies of the biguanide drugs on hold. Metformin and its sister drug, phenformin (phenethylbiguanide, Fig. 5), were finally introduced into clinical practice in the 1960s, by which time the distinction between type 1 and type 2 diabetes had been made. Phenformin was withdrawn from the US market in 1978 due to a rare but life-threatening side effect of lactic acidosis, but use of metformin has greatly increased, particularly when impressive results were reported in the UK Prospective Diabetes Study (UKPDS, 1998).
Although the major effect of metformin was known to be a reduction of the high hepatic glucose production in type 2 diabetics (Hundal, et al., 2000), its molecular mechanism remained obscure until it was reported that it activated AMPK in isolated hepatocytes (Zhou, et al., 2001). The drug did not, however, activate AMPK or affect its phosphorylation by upstream kinases and phosphatases in cell-free systems (Hawley, et al., 2002), suggesting that it acted indirectly. Metformin inhibits Complex I of the respiratory chain (El-Mir, et al., 2000; Owen, et al., 2000), suggesting that it might activate AMPK by inhibiting ATP synthesis and thus increasing cellular ADP and AMP. This was confirmed when it was found that RG cells were completely resistant to the effects of metformin to activate AMPK; the same was observed for phenformin and galegine. As expected from this mechanism, all three agents also increased the cellular ADP:ATP ratio and inhibited oxygen uptake (Hawley, et al., 2010). There remains some controversy as to the extent to which the therapeutic actions of metformin are mediated by AMPK. In favor of this idea, mice with a conditional liver knockout of LKB1, where liver AMPK is no longer activated by metformin, failed to display the anti-hyperglycemic effects of metformin (Shaw, et al., 2005). However, mice with a conditional liver knockout of both AMPK catalytic subunits (α1 and α2) still displayed some responses to metformin in vivo, and acute inhibition of glucose production by metformin in hepatocytes isolated from the mice was still observed (Foretz, et al., 2010). A potential explanation of the latter effect is that fructose-1,6-bisphosphatase, one of the key enzymes of gluconeogenesis, is inhibited by AMP. By inhibiting mitochondrial respiration and increasing AMP and ADP, metformin could clearly also modulate AMP- or ADP-sensitive enzymes other than AMPK, such as fructose-1,6-bisphosphatase.
Thiazolidinediones
Representatives of another major class of anti-diabetic drug, the thiazolidinediones, including rosiglitazone (Fryer, et al., 2002), pioglitazone (Saha, et al., 2004) and troglitazone (Lebrasseur, et al., 2006), also activate AMPK in intact cells. Although thiazolidinediones have a known direct target, i.e. the transcription factor PPAR-γ, activation of AMPK in mammalian tissues appears to be too rapid to be explained by effects on transcription (Lebrasseur, et al., 2006). One major effect of thiazolidinediones, in this case acting via PPAR-γ, is thought to be the induction and release of the insulin-sensitizing hormone, adiponectin, from adipocytes. Consistent with this, markers of insulin resistance were improved by treatment with pioglitazone (10 mg/kg) in genetically obese, leptin-deficient (ob/ob) mice, but this effect was lost if the adiponectin gene was also knocked out (Kubota, et al., 2006). Interestingly, however, some amelioration of disease markers was still seen in the adiponectin-null mice at slightly higher doses of pioglitazone (30 mg/kg) (Kubota, et al., 2006); it is tempting to speculate that this adiponectin-independent effect was mediated by cell-autonomous activation of AMPK. In any case, the insulin-sensitizing effects of adiponectin are most likely mediated by the ability of the hormone to activate AMPK and stimulate fat oxidation in liver and muscle (Yamauchi, et al., 2002). Thus, thiazolidinediones activate AMPK both by a humoral, adiponectin-dependent mechanism and by a cell-autonomous, adiponectin-independent mechanism. It is also clear that the latter is caused (like the effect of metformin) by inhibition of Complex I of the respiratory chain (Brunmair, et al., 2004). Thus, thiazolidinediones fail to activate AMPK in RG cells, increase the cellular ADP:ATP ratio, and inhibit oxygen uptake (Fryer, et al., 2002; Hawley, et al., 2010).
2-Deoxyglucose
2-Deoxyglucose (2DG) is taken up into cells by glucose transporters and converted by hexokinase to 2-deoxyglucose-6-phosphate, which is not metabolized by glycolysis beyond that point. Although usually described as an inhibitor of glycolysis, it may activate AMPK in part by depleting ATP due to its rapid and uncontrolled phosphorylation by hexokinase. As expected from this mechanism, 2DG caused increases in ADP:ATP and failed to activate AMPK in RG cells, although unlike AMPK activators acting on mitochondrial function it did not inhibit oxygen uptake (Hawley, et al., 2010). Interestingly, 2DG can be metabolized to some extent by the pentose phosphate pathway, and it has recently been suggested that the generation of NADPH by this route could represent an AMPK-independent mechanism by which 2DG protects cells against the effects of glucose deprivation (Jeon, et al., 2012).
A-769662 and salicylate
Given that AMPK is an attractive target for drugs aimed at type 2 diabetes, it is not surprising that pharmaceutical companies have conducted high-throughput screens using their compound libraries. Abbott Laboratories developed a thienopyridone compound, A-769662 (Fig. 5), which caused allosteric activation of purified AMPK in cell-free assays. Although the oral availability of this compound is poor, when injected intraperitoneally it had metabolic effects expected for an AMPK activator, i.e. increased fat oxidation in normal rats, and reduced weight gain, decreased plasma glucose and triglycerides, and decreased liver triglycerides in obese (ob/ob) mice (Cool, et al., 2006). Interestingly, A-769662 mimics at least two of the effects of AMP on the AMPK system, i.e. allosteric activation and protection against Thr-172 dephosphorylation, yet it is clear that it binds at different site(s). Thus, it did not displace [3H]AMP from the AMPK-γ2 subunit in a scintillation proximity assay (Goransson, et al., 2007), and did not activate AMPK complexes containing the β2 subunit (Scott, et al., 2008), or complexes where the β1 subunit carried an S108A mutation (Sanders, et al., 2007), both of which remain fully sensitive to activation by AMP. Moreover, A-769662 still activates AMPK in RG cells, unlike agents that increase AMP and ADP (Hawley, et al., 2010; Hawley, et al., 2012).
Why should the AMPK complex have a distinct binding site for A-769662, a purely synthetic molecule? It seemed likely there is a natural ligand that binds at that site, and one such natural ligand is salicylate (Fig. 5). Salicylate was traditionally extracted from willow bark (Stone, 1763) although it is in fact produced by many plants as a hormone used in a defense response triggered by infection by fungi and other pathogens (Reymond and Farmer, 1998). Acetyl salicylate (ASA, trade name aspirin) is a derivative that is easier to take orally than salicylate, although it is rapidly broken down to salicylate once it enters the circulation (Higgs, et al., 1987). While cyclo-oxygenases (COX1 and COX2) are the established targets for aspirin, it has been reported recently that salicylate (although not aspirin) is a direct activator of AMPK at concentrations that are reached in human plasma after oral treatment with high-dose aspirin (Hawley, et al., 2012). Although a much smaller molecule and a less potent activator of AMPK than A-769662, salicylate shows some structural resemblance to it (Fig. 5). It is clear that salicylate binds at a site that overlaps with that used by A-769662, because: (i) both compounds cause allosteric activation, with salicylate antagonizing the larger effect of A-769662; (ii) effects of both compounds are greatly reduced in AMPK complexes containing the β2 rather than the β1 isoform, and are blocked by an S108A mutation in β1 (Hawley, et al., 2012). Moreover, both agents clearly have effects in vivo that are mediated by AMPK, because administration of either compound to mice led to an enhanced switch to fat oxidation on withdrawal of food, effects that were abolished in β1 knockout mice (Hawley, et al., 2012). Although salicylate is not directly synthesized by humans, it has probably been a component of the diet since humans evolved, especially since at one time humans might have often eaten moldy vegetable matter. It is tempting to speculate that some of the apparent protection against cancer provided by regular aspirin use (Rothwell, et al., 2012; Rothwell, et al., 2012) might be mediated by AMPK.
Phenobarbital
Phenobarbital is another drug that was in clinical use long before it was known to activate AMPK. It is a particularly effective inducer of cytochrome P450 enzymes that catalyze oxidation of hydrophobic drugs, a key initial step in the process that renders them more water soluble for excretion. The gene CYP2B6, encoding one cytochrome P450 in humans, is induced by the nuclear hormone receptor CAR (constitutive androstane receptor). It has been reported that phenobarbital activates AMPK and that other AMPK activators (AICA riboside, metformin) also induce CYP2B6 in a human hepatoma cell line, while in hepatocytes from AMPK-null mice phenobarbital failed to induce Cyp2b10, the mouse ortholog (Rencurel, et al., 2005; Rencurel, et al., 2006). Thus, induction of this P450 enzyme by phenobarbital is mediated by AMPK activation. Experiments with wild type and RG mutant HEK-293 cells confirmed that phenobarbital activates AMPK, like biguanides and thiazolidinediones, because it is an inhibitor of the respiratory chain (Hawley, et al., 2010). Mitochondria contain several very large multiprotein complexes, including the four respiratory chain complexes as well as the ATP synthase. It seems likely that there might be many binding sites within these complexes where hydrophobic, xenobiotic compounds could bind and cause some inhibition of ATP production. An interesting speculation is that AMPK is being used in this case as a general sensor of mitochondrial poisons that might need to be eliminated by cytochrome P450 metabolism.
Nutraceuticals and traditional medicines
Over recent years, a bewildering variety of natural plant products, some of which are present in foods and beverages used by humans, have been reported to activate AMPK in intact cells. Many of these are reputed to have health benefits and are being touted as “nutraceuticals”. Equally, there have been several reports of AMPK activation by plant products that are components of traditional medicines. As well as galegine and salicylate (discussed above) these plant products include resveratrol from grapes and red wine (Baur, et al., 2006), epigallocatechin-3-gallate from green tea and capsaicin from Chili peppers (Hwang, et al., 2005), curcumin from turmeric (Lim, et al., 2009) and garlic oil from Allium sativum (Ki, et al., 2007), as well as two compounds derived from traditional Chinese medicine, berberine from Chinese Goldthread (Turner, et al., 2008) and hispidulin from Snow Lotus (Lin, et al., 2010). Although some of these can be classed as polyphenols, they have very varied structures and it was at first difficult to see how they could all be AMPK activators. However, it has now been shown that several inhibit mitochondrial function, either inhibiting the respiratory chain [berberine (Turner, et al., 2008)] or the F1 ATP synthase [resveratrol and EGCG (Zheng and Ramirez, 2000)], or acting as uncouplers [curcumin (Lim, et al., 2009)]. Consistent with the idea that this is how they activate AMPK, berberine and resveratrol decreased the ATP:ADP ratio in cultured cells and failed to activate AMPK in cells expressing the AMP/ADP-insensitive RG mutant (Hawley, et al., 2010).
Why should so many plants produce compounds that are mitochondrial inhibitors and hence AMPK activators? These compounds are generally secondary metabolites, and some appear to have roles in defense against pathogens, or in deterring grazing by insect or animal herbivores. For example, resveratrol is produced in grapes in response to fungal infection (Romero-Perez, et al., 2001), while (as discussed above) Goat’s Rue (a source of galegine) is classed as a noxious weed in the USA because it is poisonous to herbivores. As discussed in the previous section, the respiratory chain and ATP synthase might have many potential binding sites for hydrophobic, xenobiotic compounds, and the production of mitochondrial poisons might be a good general mechanism for plants to deter infection by pathogens or grazing by herbivores. However, a side effect of this is that many of these compounds would also be AMPK activators and hence may have medicinal uses.
PT1
Another small molecule activator of AMPK, termed PT1, was isolated via a screen of compounds that activated an AMPK-α1 construct containing just the KD and the AID (Pang, et al., 2008). The compound activated the KD-AID construct and a complete α1β1γ1 heterotrimer but failed to activate a construct containing the KD alone, suggesting that its binding site was in the cleft between the KD and the AID. Consistent with this, PT1 caused phosphorylation of ACC in L6 myotubes without altering the AMP:ATP ratio, and also promoted ACC phosphorylation and lowered lipid content in HepG2 cells (Pang, et al., 2008).
Inhibition of AMP-metabolizing enzymes
An alternative approach to AMPK activation would be to inhibit the downstream metabolism of ADP and AMP, analogous to using inhibitors of phosphodiesterases such as sildenafil to increase cyclic nucleotides. Indeed, down-regulation of expression of 5'-nucleotidases that convert AMP to adenosine, i.e. NT5C2 in cultured human myotubes or NT5C1A in mouse muscle, activated AMPK and triggered ACC phosphorylation (Kulkarni, et al., 2011). The validity of this approach has also been examined by over-expressing the 5'-nucleotidase cN-IA, and the AMP deaminases AMPD1 and AMPD2 (which convert AMP to IMP) in HEK-293 cells. Over-expression of all three enzymes decreased the activation of AMPK by oligomycin in the cells, although over-expression of cN-IA had the largest effect (Plaideau, et al., 2012). Taken together, these results suggest that 5'-nucleotidase inhibitors might be useful as AMPK activators.
Conclusions
The evidence presented in this review suggests that AMPK-activating drugs are not only effective in treating type 2 diabetes, but might also be effective in providing protection against cancer. If this appears “too good to be true”, it does not stop there because it has recently also been suggested that AMPK activators reduce the accumulation of the Aβ peptides in cell culture models of Alzheimer’s disease, and thus might be useful for treatment of that disease (Salminen, et al., 2011). One caveat is that, while there is growing evidence that AMPK provides protection against the development of cancer, it is perhaps an unjustified leap of faith to say that AMPK activators would also be useful in the treatment of cancer once it has arisen. Indeed, there is now evidence that AMPK protect cells against the metabolic stress caused by over-expression of c-Myc (Liu, et al., 2012), while the LKB1-AMPK pathway also protects cultured cells against metabolic stress caused by glucose starvation (Jeon, et al., 2012). Thus, AMPK activators might be both a “friend and foe” in cancer, providing protection against its development, while at the same time making tumor cells harder to kill using cytotoxic agents once tumors have arisen.
Although it is generally easier to develop agents that are enzyme inhibitors rather than enzyme activators (as most indications for AMPK demand), the wide variety of agents that do activate AMPK when applied to intact cells show that development of AMPK activators is an achievable objective. Most of the existing agents activate AMPK indirectly by inhibiting ATP production, whether by inhibiting oxidative phosphorylation (metformin, phenformin, galegine, thiazolidinediones, resveratrol, berberine) or glycolysis (2-deoxyglucose), thus increasing cellular ADP:ATP and AMP:ATP ratios. Exceptions to this are: (i) AICA riboside (acadesine), a pro-drug that is converted to the active derivative, ZMP, which binds at the adenine nucleotide-binding sites; (ii) A-769662 and salicylate, which bind to a common site that, while not fully defined, is distinct from the adenine nucleotide-binding sites; and (iii) PT1, which appears to bind between the KD and the AID on the α subunit (Pang, et al., 2008). Some of these, including acadesine and A-769662, have poor oral availability, but salicylate is orally available, and the best bet to develop novel AMPK activators may be to target the salicylate-binding site. Finally, one should not discount the utility of AMPK inhibitors, although the only chemical inhibitor targeting the kinase domain reported to date [compound C (Zhou, et al., 2001)] has very poor selectivity for AMPK (Bain, et al., 2007). If AMPK does indeed protect tumor cells against cell death induced by cytotoxic agents, then combinations of AMPK inhibitors with cytotoxic drugs might be worth considering.
Highlights.
AMP-activated protein kinase (AMPK) is a sensor of cellular energy status
drugs that activate AMPK have potential in type 2 diabetes and in cancer
many existing drugs and xenobiotics (e.g. metformin, salicylate) activate AMPK
most, but not all, AMPK-activating xenobiotics inhibit mitochondrial function
Acknowledgements
Studies in the authors’ laboratory have been supported by the Wellcome Trust, by Cancer Research UK, and by the companies (AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Johnson & Johnson, Merck Serono and Pfizer) that support the Division of Signal Transduction Therapy at the University of Dundee.
References
- Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 2011;13:739–748. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai H, Ihlemann J, Hellsten Y, Lauritzen HP, Hardie DG, Galbo H, Ploug T. Effect of fiber type and nutritional state on AICAR- and contraction-stimulated glucose transport in rat muscle. Am J Physiol. 2002;282:E1291–E1300. doi: 10.1152/ajpendo.00167.2001. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Sakamoto K, Bayascas JR. Lkb1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- Amodeo GA, Rudolph MJ, Tong L. Crystal structure of the heterotrimer core of Saccharomyces cerevisiae AMPK homologue SNF1. Nature. 2007;449:492–495. doi: 10.1038/nature06127. [DOI] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banko MR, Allen JJ, Schaffer BE, Wilker EW, Tsou P, White JL, Villen J, Wang B, Kim SR, Sakamoto K, et al. Chemical genetic screen for AMPKalpha2 substrates uncovers a network of proteins Involved in mitosis. Mol Cell. 2012;45:1–15. doi: 10.1016/j.molcel.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG, Foufelle F, Carling D, Hardie DG, Baldwin SA. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK) J Cell Sci. 2002;115:2433–2442. doi: 10.1242/jcs.115.11.2433. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers A, Organe S, Timmermans L, Vanderhoydonc F, Deboel L, Derua R, Waelkens E, Brusselmans K, Verhoeven G, Swinnen JV. Methotrexate enhances the antianabolic and antiproliferative effects of 5-aminoimidazole-4-carboxamide riboside. Mol Cancer Ther. 2006;5:2211–2217. doi: 10.1158/1535-7163.MCT-06-0001. [DOI] [PubMed] [Google Scholar]
- Bendayan M, Londono I, Kemp BE, Hardie GD, Ruderman N, Prentki M. Association of AMP-activated protein kinase subunits with glycogen particles as revealed in situ by immunoelectron microscopy. J Histochem Cytochem. 2009;57:963–971. doi: 10.1369/jhc.2009.954016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourron O, Daval M, Hainault I, Hajduch E, Servant JM, Gautier JF, Ferre P, Foufelle F. Biguanides and thiazolidinediones inhibit stimulated lipolysis in human adipocytes through activation of AMP-activated protein kinase. Diabetologia. 2010;53:768–778. doi: 10.1007/s00125-009-1639-6. [DOI] [PubMed] [Google Scholar]
- Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, Roden M, Gnaiger E, Nohl H, Waldhausl W, et al. Thiazolidinediones, like metformin, Inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes. 2004;53:1052–1059. doi: 10.2337/diabetes.53.4.1052. [DOI] [PubMed] [Google Scholar]
- Bultot L, Guigas B, Von Wilamowitz-Moellendorff A, Maisin L, Vertommen D, Hussain N, Beullens M, Guinovart JJ, Foretz M, Viollet B, et al. AMP-activated protein kinase phosphorylates and inactivates liver glycogen synthase. Biochem J. 2012 doi: 10.1042/BJ20112026. [DOI] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD(+) metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling D, Hardie DG. The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim Biophys Acta. 1989;1012:81–86. doi: 10.1016/0167-4889(89)90014-1. [DOI] [PubMed] [Google Scholar]
- Chen L, Jiao ZH, Zheng LS, Zhang YY, Xie ST, Wang ZX, Wu JW. Structural insight into the autoinhibition mechanism of AMP-activated protein kinase. Nature. 2009;459:1146–1149. doi: 10.1038/nature08075. [DOI] [PubMed] [Google Scholar]
- Cheung PCF, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase γ subunit isoforms and their role in AMP binding. Biochem J. 2000;346:659–669. [PMC free article] [PubMed] [Google Scholar]
- Clarke PR, Hardie DG. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 1990;9:2439–2446. doi: 10.1002/j.1460-2075.1990.tb07420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside: a specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem. 1998;273:35347–35354. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- Daval M, Diot-Dupuy F, Bazin R, Hainault I, Viollet B, Vaulont S, Hajduch E, Ferre P, Foufelle F. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem. 2005;280:25250–25257. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- Davies SP, Carling D, Munday MR, Hardie DG. Diurnal rhythm of phosphorylation of rat liver acetyl-CoA carboxylase by the AMP-activated protein kinase, demonstrated using freeze-clamping. Effects of high fat diets. Eur J Biochem. 1992;203:615–623. doi: 10.1111/j.1432-1033.1992.tb16591.x. [DOI] [PubMed] [Google Scholar]
- Davies SP, Helps NR, Cohen PTW, Hardie DG. 5'-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2Cα and native bovine protein phosphatase-2Ac. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- Day P, Sharff A, Parra L, Cleasby A, Williams M, Horer S, Nar H, Redemann N, Tickle I, Yon J. Structure of a CBS-domain pair from the regulatory gamma1 subunit of human AMPK in complex with AMP and ZMP. Acta Crystallogr D Biol Crystallogr. 2007;63:587–596. doi: 10.1107/S0907444907009110. [DOI] [PubMed] [Google Scholar]
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty S, Hawley SA, Green KA, Saner N, Mustard KJ, Hardie DG. Calmodulin-dependent protein kinase kinase-beta activates AMPK without forming a stable complex - synergistic effects of Ca2+ and AMP. Biochem J. 2010;426:109–118. doi: 10.1042/BJ20091372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosig C, Pehmoller C, Birk JB, Richter EA, Wojtaszewski JF. Exercise-induced TBC1D1 Ser237 phosphorylation and 14-3-3 protein binding capacity in human skeletal muscle. J Physiol. 2010;588:4539–4548. doi: 10.1113/jphysiol.2010.194811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer LG, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct pathways. J Biol Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- Garcia-Haro L, Garcia-Gimeno MA, Neumann D, Beullens M, Bollen M, Sanz P. The PP1-R6 protein phosphatase holoenzyme is involved in the glucose-induced dephosphorylation and inactivation of AMP-activated protein kinase, a key regulator of insulin secretion, in MIN6 beta cells. FASEB J. 2010;24:5080–5091. doi: 10.1096/fj.10-166306. [DOI] [PubMed] [Google Scholar]
- Garton AJ, Campbell DG, Carling D, Hardie DG, Colbran RJ, Yeaman SJ. Phosphorylation of bovine hormone-sensitive lipase by the AMP-activated protein kinase. A possible antilipolytic mechanism. Eur J Biochem. 1989;179:249–254. doi: 10.1111/j.1432-1033.1989.tb14548.x. [DOI] [PubMed] [Google Scholar]
- Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS, Ruderman NB. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J Biol Chem. 2008;283:16514–16524. doi: 10.1074/jbc.M708177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goransson O, McBride A, Hawley SA, Ross FA, Shpiro N, Foretz M, Viollet B, Hardie DG, Sakamoto K. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem. 2007;282:32549–32560. doi: 10.1074/jbc.M706536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282:30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets DD, Coumans WA, El Hasnaoui M, Zarrinpashneh E, Bertrand L, Viollet B, Kiens B, Jensen TE, Richter EA, Bonen A, et al. Crucial role for LKB1 to AMPKalpha2 axis in the regulation of CD36-mediated long-chain fatty acid uptake into cardiomyocytes. Biochim Biophys Acta. 2009;1791:212–219. doi: 10.1016/j.bbalip.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Hallows KR, Kobinger GP, Wilson JM, Witters LA, Foskett JK. Physiological modulation of CFTR activity by AMP-activated protein kinase in polarized T84 cells. Am J Physiol Cell Physiol. 2003;284:C1297–C1308. doi: 10.1152/ajpcell.00227.2002. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated protein kinase--an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver, and identification of threonine-172 as the major site at which it phosphorylates and activates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green KA, Mustard KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The anti-diabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51:2420–2425. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Selbert MA, Goldstein EG, Edelman AM, Carling D, Hardie DG. 5'-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J Biol Chem. 1995;270:27186–27191. doi: 10.1074/jbc.270.45.27186. [DOI] [PubMed] [Google Scholar]
- Herrero-Martin G, Hoyer-Hansen M, Garcia-Garcia C, Fumarola C, Farkas T, Lopez-Rivas A, Jaattela M. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 2009;28:677–685. doi: 10.1038/emboj.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse G, Taubmann G. Die wirkung des biguanids und seiner derivate aud den zuckerstoffwechsel. Arch Exp Path Pharm. 1929;142:290–308. [Google Scholar]
- Higgs GA, Salmon JA, Henderson B, Vane JR. Pharmacokinetics of aspirin and salicylate in relation to inhibition of arachidonate cyclooxygenase and antiinflammatory activity. Proc Natl Acad Sci USA. 1987;84:1417–1420. doi: 10.1073/pnas.84.5.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. The Vegetable System, or the internal structure and life of plants. London: 1772. [Google Scholar]
- Hong SP, Leiper FC, Woods A, Carling D, Carlson M. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc Natl Acad Sci U S A. 2003;100:8839–8843. doi: 10.1073/pnas.1533136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe S, Bierhoff H, Cado I, Weber A, Tiebe M, Grummt I, Voit R. AMP-activated protein kinase adapts rRNA synthesis to cellular energy supply. Proc Natl Acad Sci USA. 2009;106:17781–17786. doi: 10.1073/pnas.0909873106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Wullschleger S, Shpiro N, McGuire VA, Sakamoto K, Woods YL, McBurnie W, Fleming S, Alessi DR. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412:211–221. doi: 10.1042/BJ20080557. [DOI] [PubMed] [Google Scholar]
- Hudson ER, Pan DA, James J, Lucocq JM, Hawley SA, Green KA, Baba O, Terashima T, Hardie DG. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Current Biol. 2003;13:861–866. doi: 10.1016/s0960-9822(03)00249-5. [DOI] [PubMed] [Google Scholar]
- Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmoldulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- Hwang JT, Park IJ, Shin JI, Lee YK, Lee SK, Baik HW, Ha J, Park OJ. Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochem Biophys Res Commun. 2005;338:694–699. doi: 10.1016/j.bbrc.2005.09.195. [DOI] [PubMed] [Google Scholar]
- Ikematsu N, Dallas ML, Ross FA, Lewis RW, Rafferty JN, David JA, Suman R, Peers C, Hardie DG, Evans AM. Phosphorylation of the voltage-gated potassium channel Kv2.1 by AMP-activated protein kinase regulates membrane excitability. Proc Natl Acad Sci USA. 2011;108:18132–18137. doi: 10.1073/pnas.1106201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H. Cell cycle regulation via p53 phosphorylation by a 5'-AMP activated protein kinase activator, 5-aminoimidazole- 4-carboxamide-1-beta-d- ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun. 2001;287:562–567. doi: 10.1006/bbrc.2001.5627. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1{alpha} Proc Natl Acad Sci USA. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LN, Noble MEM, Owen DJ. Active and inactive protein kinases: structural basis for regulation. Cell. 1996;85:149–158. doi: 10.1016/s0092-8674(00)81092-2. [DOI] [PubMed] [Google Scholar]
- Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F, Schjerling P, Vaulont S, Hardie DG, Hansen BF, et al. The α2-5'AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes. 2004;53:3074–3081. doi: 10.2337/diabetes.53.12.3074. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K. Mechanism for fatty acid "sparing" effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem. 2002;277:3829–3835. doi: 10.1074/jbc.M107895200. [DOI] [PubMed] [Google Scholar]
- Kazgan N, Williams T, Forsberg LJ, Brenman JE. Identification of a nuclear export signal in the catalytic subunit of AMP-activated protein kinase. Mol Biol Cell. 2010;21:3433–3442. doi: 10.1091/mbc.E10-04-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki SH, Choi JH, Kim CW, Kim SG. Combined metadoxine and garlic oil treatment efficaciously abrogates alcoholic steatosis and CYP2E1 induction in rat liver with restoration of AMPK activity. Chem Biol Interact. 2007 doi: 10.1016/j.cbi.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Koay A, Woodcroft B, Petrie EJ, Yue H, Emanuelle S, Bieri M, Bailey MF, Hargreaves M, Park JT, Park KH, et al. AMPK beta subunits display isoform specific affinities for carbohydrates. FEBS Lett. 2010;584:3499–3503. doi: 10.1016/j.febslet.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1114. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Kubota T, Kumagai H, Itoh S, Satoh H, Yano W, Ogata H, Tokuyama K, Takamoto I, et al. Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and -independent pathways. J Biol Chem. 2006;281:8748–8755. doi: 10.1074/jbc.M505649200. [DOI] [PubMed] [Google Scholar]
- Kulkarni SS, Karlsson HK, Szekeres F, Chibalin AV, Krook A, Zierath JR. Suppression of 5'-nucleotidase enzymes promotes AMP-activated protein kinase (AMPK) phosphorylation and metabolism in human and mouse skeletal muscle. J Biol Chem. 2011;286:34567–34574. doi: 10.1074/jbc.M111.268292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T, Yu L, Tu Q, Jiang J, Chen Z, Xin Y, Liu G, Zhao S. Molecular cloning, genomic organization, and mapping of PRKAG2, a heart abundant gamma(2) subunit of 5'-AMP-activated protein kinase, to human chromosome 7q36 [In Process Citation] Genomics. 2000;70:258–263. doi: 10.1006/geno.2000.6376. [DOI] [PubMed] [Google Scholar]
- Lebrasseur NK, Kelly M, Tsao TS, Farmer SR, Saha AK, Ruderman NB, Tomas E. Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am J Physiol Endocrinol Metab. 2006;291:E175–E181. doi: 10.1152/ajpendo.00453.2005. [DOI] [PubMed] [Google Scholar]
- Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, Lee SH, Shong M, Kim JM, Kim J, et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447:1017–1020. doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, et al. AMPK phosphorylates and Inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- Lim HW, Lim HY, Wong KP. Uncoupling of oxidative phosphorylation by curcumin: implication of its cellular mechanism of action. Biochem Biophys Res Commun. 2009;389:187–192. doi: 10.1016/j.bbrc.2009.08.121. [DOI] [PubMed] [Google Scholar]
- Lin YC, Hung CM, Tsai JC, Lee JC, Chen YL, Wei CW, Kao JY, Way TD. Hispidulin potently inhibits human glioblastoma multiforme cells through activation of AMP-activated protein kinase (AMPK) J Agric Food Chem. 2010;58:9511–9517. doi: 10.1021/jf1019533. [DOI] [PubMed] [Google Scholar]
- Liu L, Ulbrich J, Muller J, Wustefeld T, Aeberhard L, Kress TR, Muthalagu N, Rycak L, Rudalska R, Moll R, et al. Deregulated MYC expression induces dependence upon AMPK-related kinase 5. Nature. 2012;483:608–612. doi: 10.1038/nature10927. [DOI] [PubMed] [Google Scholar]
- Lizcano JM, Göransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Mäkelä TP, Hardie DG, et al. LKB1 is a master kinase that activates 13 protein kinases of the AMPK subfamily, including the MARK/PAR-1 kinases. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machovic M, Janecek Sh. The evolution of putative starch-binding domains. FEBS Lett. 2006;580:6349–6356. doi: 10.1016/j.febslet.2006.10.041. [DOI] [PubMed] [Google Scholar]
- Mangano DT. Effects of acadesine on myocardial infarction, stroke, and death following surgery. A meta-analysis of the 5 international randomized trials. The Multicenter Study of Perioperative Ischemia (McSPI) Research Group. J Am Med Ass. 1997;277:325–332. doi: 10.1001/jama.277.4.325. [DOI] [PubMed] [Google Scholar]
- Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Current Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- Marsin AS, Bouzin C, Bertrand L, Hue L. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J Biol Chem. 2002;277:30778–30783. doi: 10.1074/jbc.M205213200. [DOI] [PubMed] [Google Scholar]
- McBride A, Ghilagaber S, Nikolaev A, Hardie DG. The glycogen-binding domain on AMP-activated protein kinase is a regulatory domain that allows the kinase to act as a sensor of glycogen structure. Cell Metab. 2009;9:23–34. doi: 10.1016/j.cmet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill GM, Kurth E, Hardie DG, Winder WW. AICAR decreases malonyl-CoA and increases fatty acid oxidation in skeletal muscle of the rat. Am J Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud PD, Yu RT, Alvarez JG, Downes M, Evans RM, Montminy M, Shaw RJ. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 2011;145:607–621. doi: 10.1016/j.cell.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milan D, Jeon JT, Looft C, Amarger V, Robic A, Thelander M, Rogel-Gaillard C, Paul S, Iannuccelli N, Rask L, et al. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science. 2000;288:1248–1251. doi: 10.1126/science.288.5469.1248. [DOI] [PubMed] [Google Scholar]
- Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281:25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- Mooney MH, Fogarty S, Stevenson C, Gallagher AM, Palit P, Hawley SA, Hardie DG, Coxon GD, Waigh RD, Tate RJ, et al. Mechanisms underlying the metabolic actions of galegine that contribute to weight loss in mice. Br J Pharmacol. 2008;153:1669–1677. doi: 10.1038/bjp.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H, Reinwein H. Zur pharmakologie des Galegins. Arch Exp Path Pharm. 1927;125:212–228. [Google Scholar]
- Muoio DM, Seefeld K, Witters LA, Coleman RA. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem J. 1999;338:783–791. [PMC free article] [PubMed] [Google Scholar]
- Narbonne P, Roy R. Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature. 2009;457:210–214. doi: 10.1038/nature07536. [DOI] [PubMed] [Google Scholar]
- Nath N, McCartney RR, Schmidt MC. Yeast Pak1 kinase associates with and activates Snf1. Mol Cell Biol. 2003;23:3909–3917. doi: 10.1128/MCB.23.11.3909-3917.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakhill JS, Chen ZP, Scott JW, Steel R, Castelli LA, Ling N, Macaulay SL, Kemp BE. beta-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK) Proc Natl Acad Sci USA. 2010;107:19237–19241. doi: 10.1073/pnas.1009705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakhill JS, Steel R, Chen ZP, Scott JW, Ling N, Tam S, Kemp BE. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332:1433–1435. doi: 10.1126/science.1200094. [DOI] [PubMed] [Google Scholar]
- Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348:607–614. [PMC free article] [PubMed] [Google Scholar]
- Pang T, Xiong B, Li JY, Qiu BY, Jin GZ, Shen JK, Li J. Conserved alpha-helix acts as autoinhibitory sequence in AMP-activated protein kinase alpha subunits. J Biol Chem. 2007;282:495–506. doi: 10.1074/jbc.M605790200. [DOI] [PubMed] [Google Scholar]
- Pang T, Zhang ZS, Gu M, Qiu BY, Yu LF, Cao PR, Shao W, Su MB, Li JY, Nan FJ, et al. Small molecule antagonizes autoinhibition and activates AMP-activated protein kinase in cells. J Biol Chem. 2008;283:16051–16060. doi: 10.1074/jbc.M710114200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter K, Grignani RT, Czibik G, Farza H, Watkins H, Redwood C. Embryonic expression of AMPK gamma subunits and the identification of a novel gamma2 transcript variant in adult heart. J Mol Cell Cardiol. 2012;53:342–349. doi: 10.1016/j.yjmcc.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaideau C, Liu J, Hartleib-Geschwindner J, Bastin-Coyette L, Bontemps F, Oscarsson J, Hue L, Rider MH. Overexpression of AMP-metabolizing enzymes controls adenine nucleotide levels and AMPK activation in HEK293T cells. FASEB J. 2012;26:2685–2694. doi: 10.1096/fj.11-198168. [DOI] [PubMed] [Google Scholar]
- Polekhina G, Gupta A, Michell BJ, van Denderen B, Murthy S, Feil SC, Jennings IG, Campbell DJ, Witters LA, Parker MW, et al. AMPK β-Subunit targets metabolic stress-sensing to glycogen. Current Biol. 2003;13:867–871. doi: 10.1016/s0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- Polekhina G, Gupta A, van Denderen BJ, Feil SC, Kemp BE, Stapleton D, Parker MW. Structural basis for glycogen recognition by AMP-activated protein kinase. Structure (Camb) 2005;13:1453–1462. doi: 10.1016/j.str.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Racanelli AC, Rothbart SB, Heyer CL, Moran RG. Therapeutics by cytotoxic metabolite accumulation: pemetrexed causes ZMP accumulation, AMPK activation, and mammalian target of rapamycin inhibition. Cancer Res. 2009;69:5467–5474. doi: 10.1158/0008-5472.CAN-08-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rencurel F, Foretz M, Kaufmann MR, Stroka D, Looser R, Leclerc I, da Silva Xavier G, Rutter GA, Viollet B, Meyer UA. Stimulation of AMP-activated protein kinase is essential for the induction of drug metabolizing enzymes by phenobarbital in human and mouse liver. Mol Pharm. 2006;70:1925–1934. doi: 10.1124/mol.106.029421. [DOI] [PubMed] [Google Scholar]
- Rencurel F, Stenhouse A, Hawley SA, Friedberg T, Hardie DG, Sutherland C, Wolf CR. AMP-activated protein kinase mediates phenobarbital induction of CYP2B gene expression in hepatocytes and a newly derived human hepatoma cell line. J Biol Chem. 2005;280:4367–4373. doi: 10.1074/jbc.M412711200. [DOI] [PubMed] [Google Scholar]
- Reymond P, Farmer EE. Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol. 1998;1:404–411. doi: 10.1016/s1369-5266(98)80264-1. [DOI] [PubMed] [Google Scholar]
- Romero-Perez AI, Lamuela-Raventos RM, Andres-Lacueva C, de La Torre-Boronat MC. Method for the quantitative extraction of resveratrol and piceid isomers in grape berry skins. Effect of powdery mildew on the stilbene content. J Agric Food Chem. 2001;49:210–215. doi: 10.1021/jf000745o. [DOI] [PubMed] [Google Scholar]
- Ross FA, Rafferty JN, Dallas ML, Ogunbayo O, Ikematsu N, McClafferty H, Tian L, Widmer H, Rowe IC, Wyatt CN, et al. Selective expression in carotid body Type I cells of a single splice variant of the large conductance calcium- and voltage-activated potassium channel confers regulation by AMP-activated protein kinase. J Biol Chem. 2011;286:11929–11936. doi: 10.1074/jbc.M110.189779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, Lee R, Belch JF, Wilson M, Mehta Z, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012a;379:1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012b;379:1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Liu Y, Xu X, Carlson M. Heterotrimer-independent regulation of activation-loop phosphorylation of Snf1 protein kinase involves two protein phosphatases. Proc Natl Acad Sci USA. 2012;109:8652–8657. doi: 10.1073/pnas.1206280109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Xu X, Carlson M. Roles of two protein phosphatases, Reg1-Glc7 and Sit4, and glycogen synthesis in regulation of SNF1 protein kinase. Proc Natl Acad Sci USA. 2011;108:6349–6354. doi: 10.1073/pnas.1102758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman NB. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun. 2004;314:580–585. doi: 10.1016/j.bbrc.2003.12.120. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab. 2004;287:E310–E317. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K, Haapasalo A, Soininen H, Hiltunen M. AMP-activated protein kinase: a potential player in Alzheimer's disease. J Neurochem. 2011;118:460–474. doi: 10.1111/j.1471-4159.2011.07331.x. [DOI] [PubMed] [Google Scholar]
- Salt IP, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, Hardie DG. AMP-activated protein kinase - greater AMP dependence, and preferential nuclear localization, of complexes containing the α2 isoform. Biochem J. 1998;334:177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MJ, Ali ZS, Hegarty BD, Heath R, Snowden MA, Carling D. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J Biol Chem. 2007;282:32539–32548. doi: 10.1074/jbc.M706543200. [DOI] [PubMed] [Google Scholar]
- Sanz P, Alms GR, Haystead TA, Carlson M. Regulatory interactions between the Reg1-Glc7 protein phosphatase and the Snf1 protein kinase. Mol Cell Biol. 2000;20:1321–1328. doi: 10.1128/mcb.20.4.1321-1328.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW, Norman DG, Hawley SA, Kontogiannis L, Hardie DG. Protein kinase substrate recognition studied using the recombinant catalytic domain of AMP-activated protein kinase and a model substrate. J Mol Biol. 2002;317:309–323. doi: 10.1006/jmbi.2001.5316. [DOI] [PubMed] [Google Scholar]
- Scott JW, van Denderen BJ, Jorgensen SB, Honeyman JE, Steinberg GR, Oakhill JS, Iseli TJ, Koay A, Gooley PR, Stapleton D, et al. Thienopyridone drugs are selective activators of AMP-activated protein kinase beta1-containing complexes. Chem Biol. 2008;15:1220–1230. doi: 10.1016/j.chembiol.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahmann N, Woods A, Carling D, Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta. Mol Cell Biol. 2006;26:5933–5945. doi: 10.1128/MCB.00383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- Stone E. An account of the success of the bark of the willow in the cure of agues. Phil Trans. 1763;53:195–200. [Google Scholar]
- Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. Dissecting the role of 5'-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- Sutherland CM, Hawley SA, McCartney RR, Leech A, Stark MJ, Schmidt MC, Hardie DG. Elm1p is one of three upstream kinases for the Saccharomyces cerevisiae SNF1 complex. Curr Biol. 2003;13:1299–1305. doi: 10.1016/s0960-9822(03)00459-7. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Okamoto S, Lee S, Saito K, Shiuchi T, Minokoshi Y. Leptin stimulates fatty acid oxidation and peroxisome proliferator-activated receptor alpha gene expression in mouse C2C12 myoblasts by changing the subcellular localization of the alpha2 form of AMP-activated protein kinase. Mol Cell Biol. 2007;27:4317–4327. doi: 10.1128/MCB.02222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas P, Hawley SA, Clarke RG, Mustard KJ, Green K, Hardie DG, Cantrell DA. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006;203:1665–1670. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton C, Sardini A, Carling D. Muscarinic receptor activation of AMP-activated protein kinase inhibits orexigenic neuropeptide mRNA expression. J Biol Chem. 2008 doi: 10.1074/jbc.M708987200. [DOI] [PubMed] [Google Scholar]
- Thornton C, Snowden MA, Carling D. Identification of a novel AMP-activated protein kinase β subunit isoform which is highly expressed in skeletal muscle. J Biol Chem. 1998;273:12443–12450. doi: 10.1074/jbc.273.20.12443. [DOI] [PubMed] [Google Scholar]
- Townley R, Shapiro L. Crystal structures of the adenylate sensor from fission yeast AMP-activated protein kinase. Science. 2007;315:1726–1729. doi: 10.1126/science.1137503. [DOI] [PubMed] [Google Scholar]
- Turner N, Li JY, Gosby A, To SW, Cheng Z, Miyoshi H, Taketo MM, Cooney GJ, Kraegen EW, James DE, et al. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57:1414–1418. doi: 10.2337/db07-1552. [DOI] [PubMed] [Google Scholar]
- Turnley AM, Stapleton D, Mann RJ, Witters LA, Kemp BE, Bartlett PF. Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem. 1999;72:1707–1716. doi: 10.1046/j.1471-4159.1999.721707.x. [DOI] [PubMed] [Google Scholar]
- UKPDS. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- Vazquez-Martin A, Oliveras-Ferraros C, Cufi S, Menendez JA. Polo-like kinase 1 regulates activation of AMP-activated protein kinase (AMPK) at the mitotic apparatus. Cell cycle. 2011;10:1295–1302. doi: 10.4161/cc.10.8.15342. [DOI] [PubMed] [Google Scholar]
- Viana R, Towler MC, Pan DA, Carling D, Viollet B, Hardie DG, Sanz P. A conserved sequence immediately N-terminal to the Bateman domains in AMP-activated protein kinase gamma subunits is required for the interaction with the beta subunits. J Biol Chem. 2007;282:16117–16125. doi: 10.1074/jbc.M611804200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M, Paterson J, Kelsall IR, Martin-Granados C, Hastie CJ, Peggie MW, Cohen PT. Ppm1E is an in cellulo AMP-activated protein kinase phosphatase. Cellular Signalling. 2011;23:114–124. doi: 10.1016/j.cellsig.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. The AMP-activated protein kinase, a metabolic master switch: possible roles in Type 2 diabetes. Am J Physiol. 1999;277:E1–E10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Xiao B, Heath R, Saiu P, Leiper FC, Leone P, Jing C, Walker PA, Haire L, Eccleston JF, Davis CT, et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449:496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;6:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yang W, Hong YH, Shen XQ, Frankowski C, Camp HS, Leff T. Regulation of transcription by AMP-activated protein kinase. Phosphorylation of p300 blocks its interaction with nuclear receptors. J Biol Chem. 2001;276:38341–38344. doi: 10.1074/jbc.C100316200. [DOI] [PubMed] [Google Scholar]
- Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Fujii N, Hirshman MF, Pomerleau JM, Goodyear LJ. Cloning and characterization of mouse 5'-AMP-activated protein kinase gamma3 subunit. Am J Physiol Cell Physiol. 2004;286:C283–C292. doi: 10.1152/ajpcell.00319.2003. [DOI] [PubMed] [Google Scholar]
- Zheng J, Ramirez VD. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br J Pharmacol. 2000;130:1115–1123. doi: 10.1038/sj.bjp.0703397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]