Abstract
Dyslexia can have different manifestations: this has motivated different theories on its nature, on its underlying brain bases and enduring controversies on how to best treat it. The relative weight of the different manifestations has never been evaluated using both behavioural and fMRI measures, a challenge taken here to assess the major systems called into play in dyslexia by different theories.
We found that adult well-compensated dyslexics were systematically impaired only in reading and in visuo-phonological tasks, while deficits for other systems (e.g. motor/cerebellar, visual magnocellular/motion perception) were only very occasional. In line with these findings, fMRI showed a reliable hypoactivation only for the task of reading, in the left occipito-temporal cortex (l-OTC).
The l-OTC, normally a crossroad between the reading system and other systems did not show the same level of intersection in dyslexics; yet, it was not totally silent because it responded, in segregated parts, during auditory phonological and visual motion perception tasks.
This minimal behavioural and functional anatomical comorbidity demonstrates that a specific deficit of reading is the best description for developmental dyslexia, at least for adult well compensated cases, with clear implications for rehabilitation strategies. The reduced intersection of multiple systems in the l-OTC suggests that dyslexics suffer from a coarser connectivity, leading to disconnection between the multiple domains that normally interact during reading.
Keywords: comorbidity, developmental dyslexia, disconnection hypothesis, fMRI, occipito-temporal cortex
1. Introduction
Developmental dyslexia is currently defined as a specific learning disorder characterized by persistent – at least 6 month - difficulties in reading accuracy, fluency and/or comprehension, despite normal learning opportunities (World Health Organization, 2013).
The nature of the cognitive and neurophysiological mechanisms underlying dyslexia has been the focus of lively debate (Nicolson et al., 2001; Snowling, 2001; Stein, 2001). Four major classical alternative hypotheses describe dyslexia as (i) a phonological disorder that also affects the decoding of orthography to phonology (Frith, 1999; Snowling, 2001; Ramus et al., 2003), (ii) a disorder of the magnocellular pathways (Galaburda, 1993; Eden et al., 1996b; Hari and Renvall, 2001; Stein, 2001), (iii) dysfunction of the visuospatial attention system1 (Facoetti et al., 2000; Facoetti et al., 2006; Peyrin et al., 2011; Gabrieli and Norton, 2012) or (iv) a cerebellar dysfunction (Fawcett et al., 2001; Nicolson et al., 2001; Bishop, 2002; Rae et al., 2002). Surprisingly, a modular hypothesis that posits a specific deficit in the translation of orthography to phonology, that is independent of meta-phonological operations (Cossu et al., 1993a), has never gained great popularity nor has it inspired imaging studies. One other theory, the disconnection theory of dyslexia (Paulesu et al., 1996; Rosen et al., 2000; Silani et al., 2005), has also not yet been fully developed.
The availability of supporting evidence for each of these hypotheses may cast doubts on the meaningfulness of the dyslexic syndrome as a specific developmental disorder that affects the reading acquisition with a unitary mechanism. Indeed, all these hypotheses2 were originally somewhat supported by behavioural, anatomical and anatomofunctional data. For example, some studies were taken in support of what is historically known as the visual/magnocellular hypothesis, which was originally benchmarked by a reduced activation of area V5/MT in dyslexic subjects (Eden et al., 1996a; Demb et al., 1998)2; other studies, however, did not confirm the hypoactivation of area MT/V5 (Vanni et al., 1997). Recently Eden and colleagues (Olulade et al., 2013) reconsidered the role of V5/MT and concluded that it was less likely to be dysfunctional if the reference groups are subjects matched for reading age. Olulade and colleagues (2013; see also Goswami, 2015 for a review) propose that some of the deficits seen for elementary perceptual tasks might be the consequence of a reduced reading experience rather than the underlying cause.
Similar considerations apply to the cerebellar hypothesis (Nicolson et al., 2001): together with reports of “grey matter”3 volumetric reductions (Brown et al., 2001; Brambati et al., 2004; Eckert et al., 2005; Kronbichler et al., 2008) there have been VBM studies with no such findings (Hoeft et al., 2007; Pernet et al., 2009a&b4; Raschle et al., 2011; Steinbrinck et al., 2008; Vinckenbosch et al., 2005). Similarly, functional imaging studies have shown contrasting results on this matter: after the initial description of a reduced cerebellar activation for motor tasks (Nicolson et al., 1999), there have been a few replications (Menghini et al., 2006; Baillieux et al., 2009; Yang et al., 2013): yet, several meta-analyses on dyslexia failed to confirm that finding (see Richlan et al., 2009, Paulesu et al., 2014 and Martin et al., 2016), while Linkersdörfer et al. (2012) in their meta-analysis of VBM data, and PET/fMRI reading data, report the challenging finding of a cerebellar common area where activations were stronger while grey matter density was lower for dyslexics.
There are several possible explanations for the contrasting “evidence” on the brain foundations behind dyslexia and its very neurocognitive nature. For example, one possible cause of the divergent results may be a variability in the recruitment/diagnostic criteria: in some studies only highly compensated dyslexics, frequently taken from university populations, were recruited to limit the possibility of spurious comorbidities, including the one of specific language impairment (e. g. Brunswick et al., 1999; McCrory et al., 2000; Ruff et al., 2002; McCrory et al., 2005; Pekkola et al., 2006; Dufor et al., 2007; MacSweeney et al., 2009; Olulade et al., 2012); in other studies, no specification was given on the level of compensation of the subjects who were recruited from dyslexia clinics (Menghini et al., 2006; Conway et al., 2008; Kast et al., 2011). Historically, some of the studies, including the first study by one of us (Paulesu et al., 1996) were biased towards a given theory and only a limited set of theory-relevant behaviours were tested5.
The age of the participants may be another important source of noise as younger subjects may appear to have a greater comorbidity simply because some of the systems under investigation are still under development. Small sample sizes with limited power may have also played a role in generating observations that proved difficult to replicate (Poldrack et al., 2017).
What would be an effective strategy to address these issues successfully?
First and foremost, it is important to test the different hypotheses in the same sample of subjects. At the behavioural level this has been done in at least six studies (Ramus et al., 2003; White et al., 2006; Reid et al., 2007; Heim et al., 2008; Menghini et al., 2010). Even though a comparative study on comorbidity across different age ranges is lacking, the aforementioned studies can be tentatively summarized as follows: (1) observation of a broad comorbidity may be more frequent in children (Heim et al., 2008; Heim et al., 2010; Menghini et al., 2010) than in adults; and (2) the strategy of recruiting highly compensated dyslexics (usually university students) may have helped to identify dyslexics with limited comorbidity because the studies of adults (Ramus et al., 2003; Reid et al., 2007) concur in showing a core reading and phonological deficit with only occasional co-morbidities.
A second requirement would be to assess the same issues using functional anatomical techniques as well. Indeed, some of the theories of dyslexia are anatomical in nature (e.g. the cerebellar or the visual-perception/dorsal stream hypotheses) and behavioural tests alone simply cannot challenge the implied neural systems explicitly. Today we can take advantage of almost 30 years of functional imaging studies of normal reading (reviews and meta-analyses in Cattinelli et al., 2013; Taylor et al., 2013) and on dyslexia (reviews and meta-analyses, for example, in Maisog et al., 2008; Richlan et al., 2009; Paulesu et al., 2014) whereby we have a broad knowledge of the functional properties of the brain regions involved in normal reading (see also Cohen et al., 2004; Danelli et al., 2013) and the brain area where hypoactivation is most likely to be observed in dyslexia (e.g. the left occipito-temporal cortex; see Richlan et al., 2009; Paulesu et al., 2014; Martin et al., 2016). Furthermore the combination of fMRI and behavioural testing allows us to assess compensatory phenomena, successful compensation or compensatory attempts (Berlingeri et al., 2010; Berlingeri et al., 2013): these should manifest in the form of hyperactivations in the pathological group with normal performance, for the former case, and pathological performance in the latter (see also, Reuter-Lorenz and Cappell, 2008).
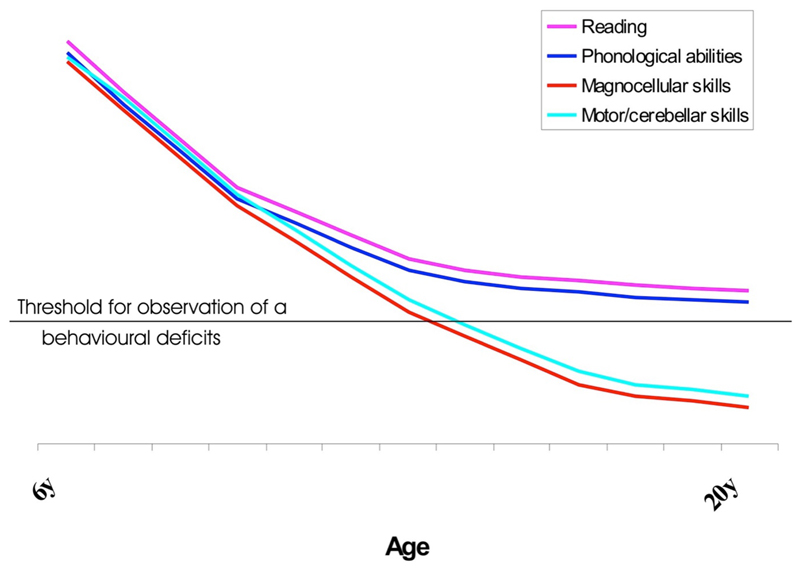
Until an explicit longitudinal study of comorbidity in dyslexia is not available6, the same combined fMRI/behavioural approach may provide insights on a possible developmental trajectory of cognitive maturation in dyslexics. For example, Figure 1 shows a possible theoretical account of the behavioural dynamic changes in dyslexics as they would emerge from the aforementioned behavioural literature. During childhood, the dyslexic child may sit behind schedule in the maturation of several systems (for convenience only four systems are mentioned); but the only systems to be persistently pathological would be those identified by studies of comorbidity in adults (e.g. Ramus et al., 2003; Reid et al., 2007)7.
Figure 1.
Hypothetic developmental changes in co-morbidities in dyslexia as a focal or as a more diffused disorder in relationship with hypothetical different time courses of maturation of various systems called into play in reading. The figure provides a hypothetical fit of the different outcomes of behavioural comorbidity studies in children (see, for example, Heim et al., 2008) and comorbidity studies in adults (see, for example, Ramus et al., 2003).
Why this might be remains a matter of speculation: perhaps the motor and visuo-magnocellular and dorsal-visual systems are not truly pathological during childhood or, maybe, compensation of these systems is more likely because of their anatomically more distributed nature, compared to the encapsulated left hemispheric reading system (Shaywitz et al., 2003; Bishop, 2013). Alternatively, the development of some systems (e.g. the oculomotor and attentional systems) is only delayed because reduced reading experience prevents the maturation of other systems that are normally boosted when reading is fluent (review in Goswami, 2015).
What counts here is that a combined functional anatomical and behavioural assessment of well-compensated adult dyslexics may help to decide, in a more explicit manner, whether multiple deficits (overt or hidden by compensation) persist into adulthood in dyslexia and whether they are necessary for the reading disorder to persist. To test this empirically, we assessed whether the well-known dysfunctional anatomical pattern observed in dyslexia for single word or pseudo-word reading (the left occipito-temporal hypoactivation; see Richlan et al., 2009; Linkersdörfer et al. (2012) or Paulesu et al., 2014 for a recent meta-analyses) (1) replicates in a new sample and (2) whether such replication is accompanied by significant comorbidity in behavioural and functional imaging measures.
1.2. Aims of the study
The aim of this study was to investigate comorbidity8 in adult dyslexics. Our approach is more complete than those used previously because we integrated behavioural and fMRI data and tested whether the well documented malfunctioning of the left ventral occipito-temporal cortex during reading, was accompanied by other neurofunctional deficits in tasks and brain regions that are not associated with reading9. In order not to bias our investigation towards a particular type of dyslexia, subjects were recruited on the grounds of having a clinical diagnosis of dyslexia based on their reading skills since their childhood. To minimize the risk of spurious comorbidities, we tested adult well-compensated dyslexics, the same strategy adopted, for example, in the behavioural comorbidity study of Ramus et al. (2003).
In addition to evaluating our subjects with a vast array of tasks, we assessed their brain functionality during four different tasks: (1) reading, (2) auditory rhyming, (3) visuo-motion perception (a test that challenges the visual dorsal stream from its roots and should depend, even though not exclusively, on the visual magnocellular system), and (4) a motor sequence learning task, which is normally accompanied by cerebellar activations (Danelli et al., 2013).
With this repertoire of behavioural and fMRI tasks we were in the position to challenge some major theories of dyslexia and their predictions regarding the presence or absence of specific comorbidities.
Predictions
On the basis of our own and others’ previous meta-analytical work, we anticipated our data would replicate the hypoactivation of the left occipito-temporal cortex during reading, a finding that was found in an overwhelming number of imaging experiments across different cultures (Paulesu et al., 2001; Hu et al., 2010) and in groups of dyslexics of all ages (see for a meta-analyses; Maisog et al., 2008; Richlan et al., 2009; Martin et al., 2016). If endophenotypes of dyslexia exist for the task of reading, this is definitively a strong candidate.
The different theories of dyslexia make different predictions on what should be observed in a sample of dyslexics who, because of their compensation, should display a specific dyslexic deficit: for example, a strictly modular reading deficit hypothesis would predict deficits for reading tasks in reading specific brain regions only, (e.g. the left occipitotemporal cortex). On the other hand, a general phonological theory would predict the co-existence of important auditory phonological deficits together with fMRI hypoactivations during the auditory phonological task. The cerebellar and the visual motion perception hypotheses postulate the additional presence of motor specific deficits and /or visual motion perception deficits together with specific brain malfunctions.
Although we did not commit ourselves to any of these hypotheses from the outset, we made all possible efforts to put ourselves in the position to find reliable results using sensitive measures to decide among the aforementioned scenarios.
In challenging these hypotheses, we capitalized on our previous work in normal readers in which we mapped the reading, phonological, visuo-motion perception and motor learning systems and we also assessed the degree of topographical convergence of those systems in the brain with particular reference to the left occipito-temporal cortex (Danelli et al., 2013): as in that previous paper, we took the presence/absence of overlaps of multiple systems in this region as an indication of functional/anatomical connectivity though convergence there (see Zeki and Shipp, 1988). We hypothesized that dyslexics may suffer from a reduction in such connectivity.
This mapping allowed us to produce an in depth characterization of the normal functional nature of the brain regions with reduced activation during reading in the dyslexic subjects, and to generate a more specific hypothesis about the nature of the neurocognitive deficit behind dyslexia. In addition, by having a relatively vast array of tasks we were able to test whether any brain region that was malfunctioning for reading in dyslexia was generally silent or responsive to other stimulations.
As the reader shall see, there is one clear winner among the aforementioned hypotheses with the additional suggestion of a reduced connectivity within the left ventral occipitotemporal cortex as an important trait of the disorder.
2. Methods
2.1. Participants
To minimize the risk of recruiting subjects with multiple uncorrelated deficits, all subjects were in the highest range of education. They comprised 23 healthy right-handed Italian university students [F=12, M=11; agemean(s.d.)=20.6 (2.29)] with at least 13 years of schooling, and 20 subjects fulfilling a diagnosis of dyslexia [F=5, M=15; agemean(s.d.)=21.2 (5.2)], matched with the normal controls for educational level (Mann-Whitney U-test, Z-value=-1.67, p=.09, |r|=.25).
The subjects with dyslexia were recruited on the grounds of their past medical/school history and the presence of a previous diagnosis of developmental dyslexia based on their reading skills. Accordingly, the recruitment of the dyslexics was not biased towards the presence of any additional sign (e.g. phonological, motor, etc.).
2.2. Neuropsychological tests
All subjects, controls and dyslexics, were tested with following tests.
General intelligence: Wechsler Adult Intelligence Scale Revised (WAIS-R; Wechsler, 1981).
Simple “visuo-verbal simple vocal reaction time” (VOT task): Simple vocal-reaction times were measured by asking subjects to say ‘pronti’ (“ready”) as quickly as possible every time a small dot appeared on a computer screen at random intervals. This measure served as a reference baseline for what is implied form detection of a simple visual stimulus to the generation of the utterance of a bi-syllabic word.
Reading: single word- and pseudo-word reading for disyllabic stimuli (reaction times and accuracy) as in Paulesu et al. (2001).
Elementary auditory perception: discrimination of pairs of pure tones (from the fMRI scans).
Phonology: digit span (from the WAIS-R); auditory discrimination of letter names (from the fMRI scans); spoonerisms test, digit naming and picture naming tasks (see also Paulesu et al., 2001).
“Magnocellular” and dorsal stream visual tests: contrast discrimination; speed discrimination with both low-frequency and high-frequency Gabor patches; coherent motion perception tasks.
“Cerebellar tests”: sequence motor learning task (from the fMRI scans).
A detailed description of these tasks is reported in the supplementary materials (section sm-1) or in the description of the fMRI tasks.
For all reaction time tasks the individual median of the RTs were used for further analysis.
2.3. fMRI tasks
During 4 separate fMRI scans, participants performed (1) a pseudo-word reading task, in which subjects were instructed to mentally read each bi-syllabic pseudo-word (2) an auditory letter-name rhyming task, in which subjects were instructed to respond when two letter names rhymed and, in the baseline, when two pure tones were matched for pitch (3) a visual motion stimulation task, in which subjects stared at a fixation point while a low-frequency Gabor patch was either stationary (baseline) or moving randomly across the screen (experimental condition), and finally, (4) a motor sequence learning task, in which subjects were instructed to learn a sequence of 8 key presses, receiving specific auditory feedback for correct or wrong taps. A detailed description of the tasks is reported in the supplementary materials (section sm-2, see also Danelli et al., 2013).
2.4. fMRI data acquisition
MRI scans were performed on a 1.5 T Marconi-Philips Infinion Scanner or with a General Eletric Signa HD-XT scanner, using an Echo Planar Imaging (EPI) gradient echo sequence (Flip angle = 90°; TE = 60msec; TR = 3000 msec; FOV = 240x240; matrix = 64 x 64) and a 8channel phased array coil. A substantially identical number of controls and dyslexic was scanned with each scanner (Controls: Infinion #12; Signa #11; Dyslexics Infinion #7; Signa:#13; X2 (2, N = 43) = 1.28, p=.26).
The selected volume consisted of 35 contiguous, interleaved, axial and coplanar with ACPC line, images (thickness = 5 mm; interslice gap = 0 mm), acquired every 3 seconds. The four-fMRI experiments described below involved 60 fMRI scans collected in alternating 30-second blocks of 10 scans of baseline and experimental task. Ten initial “dummy” scans were acquired and then discarded. For all participants, the sample anatomical space included the entire cerebral hemispheres and the cerebellum down to -40 below the bicommissural plane.
2.5. Behavioural data analysis
Individual behavioural performance on I.Q., phonological skills, reading skills, magnocellular and motor skills (see tables 1) were assessed using the Crawford and Howell's method (Crawford et al., 1998). Because there were many variables under statistical testing, a Family Wise Error (FWER) correction was adopted10. We studied the pattern of between-variable correlations in the control group by means of Spearmann’s rho tests. The Spearmann’s rho correlation matrices are reported in table sm-1 and sm-2, respectively. The global level of significance of the two patterns of correlation was tested by means of Bartlett’s sphericity test (Bartlett, 1937).
Table 1.
Demographic, behavioural group data for I.Q., reading and phonological, “dorsal visual stream” tasks, and fMRI group data for motor/cerebellar and phonological tasks are reported (red bold: p<.001 uncorrected p-value; blue bold: .001<p<0.01 uncorrected p-value, green bold: .01<p<.05 uncorrected p-value).
Moreover, raw data of each dyslexic are summarized. Black Bold: indicates raw data that showed a significant differences from the control group using the Crawford and Howell's method (p<.05 uncorrected threshold); Orange bold: indicates raw data that showed a significant differences from the control group using the Crawford and Howell's method (FWE corrected value; see correlation analyses in section 2.5 for details).
| Controls | Dyslexics | OF (f) | EC (f) | DF (f) | IB (f) | AB (f) | NB (m) | MG (m) | MK (m) | MM (m) | DC (m) | DG (m) | ODA (m) | MB (m) | LB (m) | CV (m) | FS (m) | FD (m) | LDP (m) | TDP (m) | PN (m) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 21 (2.3) | 21 (5.2) | 25 | 17 | 20 | 39 | 19 | 22 | 27 | 27 | 18 | 18 | 18 | 20 | 24 | 19 | 18 | 19 | 18 | 18 | 18 | 20 |
| Education | 14 (2) | 13 (2) | 13 | 12 | 13 | 18 | 13 | 13 | 18 | 13 | 12 | 12 | 12 | 14 | 17 | 13 | 13 | 12 | 12 | 12 | 12 | 14 |
|
| ||||||||||||||||||||||
| Verbal I.Q. | 120 (7.2) | 112 (8) | 113 | WISC | 115 | 122 | 113 | 125 | 122 | 112 | 115 | 105 | 116 | 122 | 124 | 108 | 101 | 99 | 102 | 108 | 107 | 107 |
| Performance I.Q. | 122 (8.6) | 121 (9.7) | 115 | WISC | 128 | 144 | 126 | 131 | 119 | 127 | 126 | 113 | 111 | 120 | 132 | 115 | 114 | 103 | 130 | 123 | 110 | 120 |
| Voice-Onset Time (msec) | 375 (59) | 376 (83.5) | 427 | 301 | 485 | 360 | 328 | 273 | 351 | 296 | 320.5 | 235.5 | 407 | 390 | 500 | 309 | 359 | 578 | 453 | 359 | 407 | 375 |
| Pseudo-word reading (msec) | 536 (67) | 876 (135.7) | 772 | 942 | 1141 | 898.5 | 563 | 839 | 795 | 923.5 | 821 | 781.5 | 812 | 867 | 1141 | 945.5 | 875 | 992 | 921 | 742.5 | 719 | 960.5 |
| Pseudo-word reading (errors) | 0.5 (0.8) | 1.3 (1.4) | 0 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 4 | 2 | 0 | 0 | 0 | 2 | 2 | 1 | 4 | 2 | 4 |
| Word reading (msec) | 472 (51.2) | 656 (115.6) | 716 | 620 | 906 | 742.5 | 531 | 504 | 623 | 654.5 | 656 | 591 | 539 | 578 | 836 | 617 | 578 | 672 | 641 | 547 | 562 | 890 |
| Word reading (errors) | 0.1 (0.3) | 0.4 (0.6) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 1 |
| Spoonerism (seconds) | 54 (25.8) | 141 (81.5) | 86 | 88 | 92 | 86 | 85 | 166 | 118 | 272 | 206 | 106 | 62 | 87 | 86 | 260 | 171 | 68 | 371 | 99 | 152 | 168 |
| Spoonerism (errors) | 2 (1.7) | 6 (4) | 4 | 2 | 1 | 2 | 13 | 9 | 10 | 0 | 3 | 8 | 5 | 6 | 5 | 6 | 9 | 4 | 4 | 1 | 15 | 7 |
| Digit naming (seconds) | 14 (3.7) | 20 (4.8) | 21 | 22 | 14.5 | 20 | 15.5 | 14.5 | 16.5 | 20.5 | 21 | 18 | 15 | 13 | 19 | 24.5 | 17.5 | 22 | 31.5 | 17.5 | 24.5 | 28.5 |
| Picture naming (msec) | 636 (63.4) | 740 (88) | 826 | 691 | 781 | 812 | 688 | 553 | 680 | 640 | 691 | 686 | n.e. | 781 | 820 | 812 | 703 | 921 | 766 | 656 | 719 | 843 |
| Contrast sensitivity with low-frequency Gabor(% of increased contrast) | 3 (1.7) | 5 (5.7) | n.e. | n.e. | 1 | 7 | 1 | n.e. | 5 | 2 | 7 | 1 | 1 | 2 | n.e. | 22 | 6 | n.e. | 1 | 10 | n.e. | 8 |
| Contrast sensitivity with high-frequency Gabor (% of increased contrast) | 19 (46.6) | 47 (65) | n.e. | n.e. | 125 | 0 | 143 | n.e. | 0 | 0 | 0 | 144 | 109 | 0 | n.e. | 0 | 4 | n.e. | 130 | 0 | n.e. | 0 |
| Speed discrimination with low-frequency Gabor (% of increased speed) | 133 (7.6) | 135 (5.2) | n.e. | n.e. | 141 | 130 | 141 | n.e. | 140 | 142 | 138 | 137 | 129 | 130 | n.e. | 129 | 140 | n.e. | 132 | 135 | n.e. | 130 |
| Speed discrimination with high-frequency Gabor (% of increased speed) | 230.6 (27.7) | 225 (23.8) | n.e. | n.e. | 242 | 242 | 200 | n.e. | 242 | 229 | 242 | 203 | 242 | 242 | n.e. | 242 | 165 | n.e. | 202 | 217 | n.e. | 231 |
| Coherent motion perception (% of increased proportion of moving dots) | 35 (5.9) | 35 (4.6) | n.e. | n.e. | 40 | 32 | 40 | n.e. | 33 | 31 | 31 | 39 | 40 | 40 | n.e. | 40 | n.r. | n.e. | 31 | 29 | n.e. | 32 |
|
| ||||||||||||||||||||||
| Motor learning(correct taps/40 trials) | 26 (4.8) | 25 (7) | 29 | 18 | n.r. | 26 | n.r. | 31 | 30 | 29 | 27 | n..r | 24 | 30 | 23 | 8 | 27 | 10 | 15 | 20 | 25 | 28 |
| Motor learning(lack of corrections /40 trials) | 5 (5.9) | 6 (6.9) | 3 | 10 | n..r | 1 | n.r. | 4 | 3 | 0 | 2 | n.r. | 5 | 1 | 7 | 26 | 2 | 17 | 13 | 7 | 4 | 1 |
| Tone discrimination (d-prime) | 4 (0.7) | 3 (1.2) | 3.8 | n.r. | 1.7 | 4.6 | 4.6 | 4 | 2.8 | n.r. | n.r | 1.6 | 4.6 | 4 | 2.4 | 3.2 | 4.6 | 1.4 | 1.7 | 4 | 2.6 | 1.7 |
| Syllable rhyming(d-prime) | 4 (1.4) | 3 (1.3) | 4.6 | n.r. | 4.6 | 4.6 | 0.6 | 2.8 | 4.6 | n.r. | n.r | 1.7 | 3.2 | 2.5 | 4.6 | 1.9 | 2.8 | 4.6 | 1.7 | 4 | 3.6 | 1.9 |
| Tone discrimination (reaction times) | 1.3 (.12) | 1.4 (.04) | 1.3 | n.r. | 1.6 | 1.1 | 1.2 | 1.2 | 1.3 | n.r. | n.r | 1.2 | 1.4 | 1.3 | 1.6 | 1.6 | 1.4 | 1.4 | 1.6 | 1.4 | 1.3 | 1.5 |
| Syllable rhyming(reaction times) | 1.4 (.16) | 1.5 (.12) | 1.5 | n.r. | 1.5 | 1.4 | 1.4 | 1.3 | 1.3 | n.r. | n.r | 1.4 | 1.7 | 1.4 | 1.8 | 1.4 | 1.5 | 1.5 | 1.6 | 1.6 | 1.5 | 1.5 |
CS: contrast sensitivity. SD: Speed discrimination. n.e.: not executed (the subject did not performed the task). n.r.: not recorded (the subject performed the fMRI task, but the behavioral response was not recorded because of a response box malfunction).
On the basis of the correlation matrices, we identified the following families of variables to generate family specific thresholds:
Family 1: word-reading, pseudo-word reading, VOT task, picture naming, rhyme (d-prime); for this family the corrected alpha level was set to .01;
Family 2: digit naming and spoonerisms; for this family the corrected alpha level was set to 0.025;
Family 3: verbal and performance I.Q.; for this family the corrected alpha level was set to 0.025;
Family 4: correct taps and lack of corrections; for this family the corrected alpha level was set to 0.025;
Family 5: contrast discrimination for magnocellular stimuli, contrast discrimination for parvocellular stimuli, speed discrimination for magnocellular stimuli and coherent motion perception; for this family the corrected alpha level was set to .0125;
Family 6: speed discrimination with parvocellular stimuli (alpha level .05 – i.e. independent measure).
Group comparisons (dyslexics versus controls) were performed using multiple Mann-Whitney U tests with the software SPSS (http://www-01.ibm.com/software/it/analytics/spss). For the non-parametric between-groups comparisons, the magnitude of the effect sizes was calculated according to the equation:
Comparison of different naming skills.
A two-way 2x3 ANOVA with one between-group factor (controls, dyslexics) and one within-group factor (word-reading, pseudo-word reading and picture naming) was calculated to compare different kinds of naming skills (reading words, pseudo-words, picture naming). The partial eta squared was also computed to measure the magnitude of the effect sizes.
2.6. fMRI data analysis11
After standard pre-processing of the fMRI data (Friston et al., 1995) the experimental conditions were modelled in a block-design and the condition-specific effects were estimated using the General Linear Model as implemented in SPM8 (Wellcome Department of Imaging Neuroscience, London, UK). For each subject and task, images were converted from DICOM to NIFTI, realigned (default options in SPM8 saved in “estimate and reslice” batch) to remove movement artefacts and normalized into an MRI stereotactic space (using standard SPM8 procedures and the EPI template). Images were then convolved in space with a three-dimensional isotropic Gaussian kernel (10 mm FWHM) to improve the signal-to-noise ratio. The data were also high-pass filtered with a cut-off period of 128 sec.
Finally, individual effects, i.e. the contrast-images estimated at the first level, were entered in a random-effect analysis for group-level inference (Friston, 2005). These analyses conform to a second-level ANOVA with a between-group factor (two-levels, controls and dyslexics, with unequal variance) and a within-group factor (four-levels with equal variance) that modelled the four task effects: (1) pseudo-word reading versus baseline (2) auditory rhyme detection versus baseline (3) motion perception versus baseline (4) motor sequence learning versus baseline. To this end we used the full factorial routine in SPM8 with correction for global signal omitted by default.
Whole brain analyses
Simple effects, conjunction effects and between-group comparisons were assessed for each task and pairs of tasks. For the between group analyses, we also calculated higher order group-by-task interactions which allowed us to test the specificity of any given difference in a given task (e.g. for pseudo-word reading) with reference to other tasks (e.g. auditory phonological processing). The form of such interactions was therefore, for example, [(reading – rhyming)controls > (reading-rhyming)dyslexics]. All these analyses were performed first on the entire brain volume.
Small volume corrected analyses
The main between-group comparisons were also tested on the 9 clusters identified in the recent meta-analysis by Paulesu et al. (2014), which was based on 53 previous imaging studies on dyslexia. The aim of the small-volume-corrected analyses was to test to what extent new results would be consistent with a substantial body of previous independent empirical work summarized by the clusters identified in that meta-analysis12. The 9 clusters for small volume correction included, the left ventral occipito-temporal cortices, lateral middle and superior temporal cortices, left dorsal-parietal and premotor cortices (see table 2a in Paulesu et al. 2014, and the supplementary figure SF-1 of this article).
Table 2. Whole-brain analyses.
(1) brain areas hypo-activated in dyslexics during pseudo-word reading (between-group comparisons at p<.05 FWE-corrected at voxel-level), and (2) brain areas that showed a group-by-task interaction effects (p<.05 FWE-corrected at cluster-level after a voxel-wise threshold of p<0.001 uncorrected). The two most significant local-maxima in SPM are reported for each anatomical area. The number of voxels in the cluster is also reported.
|
MNI Coordinates
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Z score | Cluster-size | x | y | z | Z score | |
|
| |||||||||
| Brain regions | Left hemisphere | Right hemisphere | |||||||
| 1. Between-group comparisons | |||||||||
|
| |||||||||
| a. Pseudo-word reading | |||||||||
| Fusiform gyrus | -42 | -50 | -16 | 5.4 | 279 | ||||
| -40 | -54 | -10 | 5.2 | 279 | |||||
|
| |||||||||
| 2. Group-by-task interactions | |||||||||
|
| |||||||||
| a. (reading – rhyming)controls > (reading-rhyming)dyslexics | |||||||||
| Inf. temporal gyrus+ | -42 | -46 | -14 | 4.0 | 383 | ||||
| Fusiform gyrus+ | -42 | -52 | -10 | 4.1 | 383 | ||||
| -38 | 50 | 14 | 5.2 | 383 | |||||
|
| |||||||||
| b. (reading – visual motion perception)controls > (reading - visual motion perception)dyslexics | |||||||||
| Mid. temporal gyrus+ | -46 | -64 | -2 | 3.7 | 733 | ||||
| -42 | -60 | -2 | 3.5 | 733 | |||||
| Inf. temporal gyrus | -54 | -60 | -12 | 3.6 | 733 | ||||
| -58 | -60 | -10 | 3.5 | 733 | |||||
| Fusiform gyrus | -44 | -46 | -22 | 4.4 | 733 | ||||
“In depth” analyses: normal and pathological convergence of multiple systems in reading areas
Finally, we characterized the properties of the brain regions that were significantly less active in the dyslexics for the task of reading.
First, we assessed such properties in the normal controls: we replicated the approach published in Danelli et al. (2013) where we quantified the level of intersection of the four systems challenged by our tasks in the left ventral occipito-temporal cortex. This established the normal level of convergence of multiple systems in the region that proved hypoactive in this sample of dyslexics. First we isolated the region of hypoactivation in the dyslexics for the reading task (p<0.001 FWER corrected for cluster extent). Second, within this region, we sought conjunctions between the reading task and each other task. Finally we isolated again what we called reading per-se regions (Danelli et al., 2013), namely voxels that within the mask were active while not showing even trends for activation in any other task (no voxels above the p<0.05 uncorrected threshold)13 or were significantly more active for reading than in all other tasks combined14.
Second, for the same region, we evaluated whether the dyslexic subjects activate this region in other tasks. An image containing the pseudo-word reading hypo-activations of the dyslexics was used as a mask for each simple effect to further evaluate whether the dysfunction of this brain region was either specifically associated with a reading task or it was task-independent.
Statistical thresholds
All analyses were thresholded at p<0.05 after voxel wise family wise error (FWE) correction either across the whole brain or in our regions of interest. If this correction was not met, a p<0.05 FWE cluster-wise correction was adopted after an uncorrected voxel-wise threshold of p<0.001. Accordingly, all results described were all corrected for multiple comparisons, using state of the art approaches.
3. Results
3.1. Behavioural data
All behavioural data are summarized in Table 1.
3.1.1. Group analyses
Between-group differences that survived a correction for multiple comparisons were the reading times for words and pseudo-words, several phonological tasks (spoonerisms task; digit naming; picture naming), and the Arithmetic subtest of the WAIS. Other nominally significant results (e.g. the Digit-Symbol Coding or Similarities subtest of the WAIS) did not survive the correction for multiple comparisons.
In the majority of the other tests, and crucially the visual magnocellular/dorsal stream tests, or the motor learning test, there was no significant difference when a corrected threshold was applied. A detailed description of all the group-comparisons is reported in supplementary materials (section sr-3.1).
Comparisons of the effect sizes across different tasks
The tasks that provided the strongest discrimination of dyslexics and controls (using non-parametric |r| values > 0.5; Field, 2005) were those involving visuo-ortho-phonological integration either explicitly (e.g. naming tasks) or implicitly (e.g. spoonerism tasks).
A comparison of different kinds of naming skills (word-reading, pseudo-word reading and picture naming), measured with the same RT latency procedure, also revealed an interesting pattern that discriminated dyslexics from controls. Although response times were fastest for reading words in both groups, controls showed slower responses for picture naming than pseudoword reading whereas dyslexics were slower for pseudowords than picture naming (see table 1 and supplementary figure SF-2). This group difference was formally demonstrated by a two-way 2x3 ANOVA with one between-group factor (controls, dyslexics) and one within-group factor (tasks) showed significant group (F(1-40)=77, p<.001, n2p=.659) and task (F(2-80)=76, p<.001, n2p=.654) effects and group-by-task interaction effects (F(2-80)=45, p=.001, n2p=.532).
3.1.2. Single-subject behavioural analyses
All subjects with dyslexia, with one exception, showed a lengthening of vocal reaction times for pseudo-word reading. Half of dyslexics showed a pathological performance in, at least, one phonological task – in particular, the majority showed a lengthening of their processing times for the spoonerisms task. Only occasional deviations emerged for “visual magnocellular”/dorsal stream tests and motor/cerebellar tasks (see table 1 and section sr-3.2 in the supplementary materials for details).
For the group of normal controls, deviant performances were observed only very occasionally.
3.2. fMRI results
3.2.1. Behavioural patterns during fMRI
For pseudo-word reading subjects reported to have read all the stimuli, consistently throughout the task. This was ensured, a priori, by selecting a stimulus presentation rate that was longer than the VOT of the slowest dyslexic.
In the auditory rhyming task, neither accuracy nor reaction times differed significantly between controls and dyslexics in the auditory rhyming task (Mann-Whitney U-testaccuracy, Z-value=-1.3, p=.2, |r|=.21; Mann-Whitney U-testspeed, Z-value=-1.9, p=.06, |r|=.31) and in the tone-discrimination task (Mann-Whitney U-testaccuracy, Z-value=-1.9, p=.05, |r|=.31; Mann-Whitney U-testspeed, Z-value=-1.6, p=.1, |r|=.26).
For the visual motion stimulation task, subjects confirmed to have clearly perceived the moving Gabor patches while fixating the centre of the virtual display.
For the motor learning task, there was no significant difference between controls and dyslexics either in the number of correct taps (Mann-Whitney U-test, Z-value=-.67, p=.5, |r|=.11), nor in the lack of corrections (Mann-Whitney U-test, Z-value=-.81, p=.42, |r|=.13).
3.2.2. fMRI results
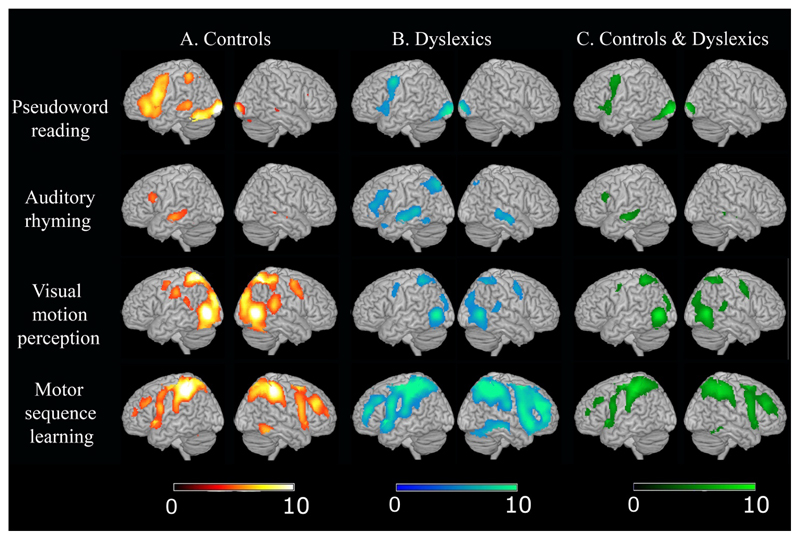
Results of the within-group fMRI effects for each task are described in details in supplementary materials (section sr-4.1, see also figure 2: columns A and B). Here it is worth noting that our fMRI data from controls replicated previous observations on similar tasks. This includes activation in (i) the occipito-temporal cortex during pseudoword reading, (ii) the dorsal visual stream from area MT/V5, up to parietal cortex and the connected dorsal oculomotor cortices for the visual motion task; and (iii) the anticipated cerebellar activations during the sequence motor learning task.
Figure 2.
Activation patterns for normal readers and dyslexic readers. On the right-hand side commonalities between normal readers and developmental dyslexics are reported. Effects were thresholded at p<.05 corrected for multiple comparisons (FWER-corrected). On the bottom, the colour scales indicate the significance of the SPMs[Z] maps. Only voxels surviving a FWER p<0.05 threshold are visualized.
3.2.2.1. Common task effect for controls and dyslexics
A detailed description of these results is in the supplementary materials (section sr-4.2; see also figure 2: column C). Here it is worth mentioning that, among other effects, there was common activation in (i) the left inferior temporal cortex during the auditory phonological task (the same region was hypoactivated in dyslexics for reading – see below), (ii) area V5/MT for the moving Gabor patches stimulation and (iii) the cerebellum for the motor learning task.
3.2.2.2. Between-group comparisons
Whole brain volume analyses
Pseudoword reading was the only task where activation was significantly different in the controls and dyslexics. Dyslexics showed reduced activation (i.e. hypoactivation) in the left occipito-temporal cortex (table 2.1) (p<0.001 FWER corrected at voxel-level). No other between-group differences (hypoactivations or as hyperactivations) emerged between controls and dyslexics in the other tasks (auditory rhyming, visual-magnocellular and motor-cerebellar tasks). This task specific effect was confirmed by a group-by-task interaction in the left ventral occipito-temporal cortex for the [reading > rhyming] and for the [reading > visual motion perception] comparisons (table 2.2). These effects were significant, respectively, at p=.02 and p=.004 FWER corrected for cluster size after voxel level thresholding at p<0.001. No other interaction effects were found. These results indicate that the best neuro-anatomical marker for dyslexia was observed in the left occipitotemporal cortex during reading.
Small-volume corrected analyses based on the meta-analysis of Paulesu et al. (2014)
Apart from the obvious replication of a left ventral occipito-temporal cortex hypoactivation (all three clusters revealed in Paulesu et al. 2014, clusters L5, L6 and L2315, were significantly less active in dyslexics; see table 3.1) there was a further small-volume corrected finding in the dorsal left parietal cortex in cluster L30: this emerged for the [reading > rhyming] (stereotactic coordinates: X= -40; y=-44; Z=42, ; SVC p = 0.03; see table 3.2-a) and for the [reading > visual motion perception] (stereotactic coordinates: X= -38; y=-42; Z=42; SVC p = 0.05; table 3.2-b) higher-order interactions.
Table 3. Small-volume corrected analyses.
(1) between-group and (2) higher-order group-by-task comparisons were tested on the 9 clusters identified in a recent meta-analysis by Paulesu et al. (2014). Here, we report the results that survive at p<.05 FWE corrected in the Small-Volume-Correction analyses. The p-values are corrected. All local-maxima in SPM are reported.
| Brain regions | MNI Coordinates | # Cluster (see Paulesu et al., 2014) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Z score | p-value | |||||
| 1. Between-group comparisons | |||||||||
|
| |||||||||
| controls > dyslexics for reading | |||||||||
| Inf. temporal gyrus | -46 | -60 | -8 | 4.4 | .001 | L6 (x=-50; y=-61; z=-9) | |||
| Fusiform gyrus | -42 | -50 | -16 | 5.4 | <.001 | L23 (x=-45; y=-49; z=-15) | |||
| -42 | -56 | -18 | 5.0 | <.001 | L5 (x=-41; y=-60; z=-18) | ||||
| Inf. occipital gyrus | -48 | -58 | -14 | 4.3 | .001 | L6 (x=-50; y=-61; z=-9) | |||
|
| |||||||||
| 2. Group-by-task interactions | |||||||||
|
| |||||||||
| a. (reading – rhyming)controls > (reading-rhyming)dyslexics | |||||||||
| Inf. parietal lobule | -40 | -44 | 42 | 3.4 | .039 | L30 (x=-43; y=-40; z=46) | |||
| Inf. temporal gyrus | -42 | -46 | -14 | 4.0 | .005 | L23 (x=-45; y=-49; z=-15) | |||
| Fusiform gyrus | -42 | -50 | -12 | 4.0 | .005 | L23 (x=-45; y=-49; z=-15) | |||
|
| |||||||||
| b. (reading – motion perception)controls > (reading-motion perception)dyslexics | |||||||||
| Inf. parietal lobule | -38 | -42 | 42 | 3.3 | .050 | L30 (x=-43; y=-40; z=46) | |||
| Inf. temporal gyrus | -54 | -60 | -12 | 3.6 | .025 | L6 (x=-50; y=-61; z=-9) | |||
| -50 | -64 | -4 | 3.5 | .029 | L6 (x=-50; y=-61; z=-9) | ||||
| Fusiform gyrus | -42 | -48 | -18 | 4.3 | .002 | L23 (x=-45; y=-49; z=-15) | |||
| -42 | -56 | -20 | 3.6 | .025 | L5 (x=-41; y=-60; z=-18) | ||||
| Inf. occipital gyrus | -48 | -58 | -14 | 3.4 | .045 | L6 (x=-50; y=-61; z=-9) | |||
3.2.2.3. In depth functional analyses
These analyses addressed two questions.
Question (1) what are the normal functional properties of the occipito-temporal cortices that were more active in normal readers for reading? What is the level of intersection of multiple systems in such regions?
Question (2) Does the cortical region hypoactivated in dyslexics for reading respond in the other tasks?
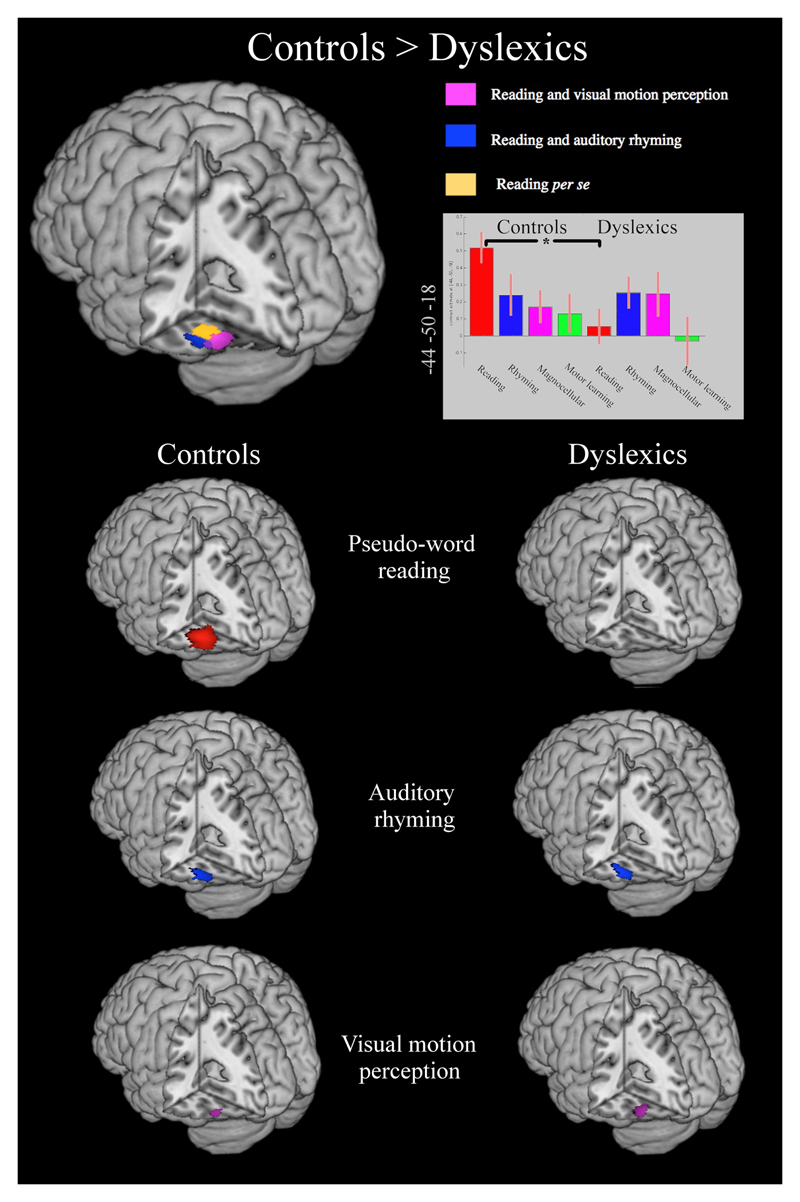
The analyses addressing question 1, using the approach of Danelli et al. (2013), showed that the brain region that is hypoactive in this sample of dyslexics, normally contains three functionally distinct sub-regions:
-
(i)
a more posterior portion localized in the lateral part of the fusiform gyrus where activations for reading and visuo-motion perception overlap (table 4–b and purple areas in the upper part of figure 3).
-
(ii)
a more anterior and lateral portion localized in the posterior part of the inferior temporal gyrus that responds to both reading and auditory phonological processing (table 4-a and blue areas in the upper part of figure 3), a region called LIMA (lateral inferior-temporal multimodal area) by Cohen et al. (2004).
-
(iii)
an anterior ventro-medial portion of the fusiform gyrus specifically activated for pseudo-word reading with no overlapping response with that seen for the auditory phonological task nor for the visual motion perception task (table 4-c and yellow areas in the upper part of figure 3). This region may be equivalent to the one described by Cohen et al. (2002) as the visual word-form area (VWFA).
Table 4.
In depth functional analyses (1): normal organization of the left occipito-temporal region according to the methodology of Danelli et al. (2013). This region is hypoactive for reading in the present sample of dyslexics.
| Brain regions | MNI Coordinates | |||
|---|---|---|---|---|
|
| ||||
| x | y | z | Z score | |
|
| ||||
| Controls > Dyslexics in reading | ||||
| a. Reading & Rhyming | ||||
| Inf. temporal gyrus | -48 | -50 | -22 | 4.8 |
|
| ||||
| b. Reading & Visual Motion Perception | ||||
| Fusiform gyrus | -42 | -54 | -18 | 5.2 |
| -42 | -54 | -10 | 5.2 | |
|
| ||||
| c. Reading per se | ||||
| Inf. temporal gyrus | -40 | -46 | -16 | 7.2* |
| Fusiform gyrus | -34 | -46 | -22 | 6.1* |
| -40 | -50 | -20 | 5.8^ | |
This effect was calculated on the reading data exclusively masked on the other three tasks data thresholded at 0.05 uncorrected.
This region was identified in the controls by comparing the reading task with all the other tasks with the linear contrast “3 -1 -1 -1” for reading, rhyming, visuo-motion perception and motor learning respectively.
Figure 3.
In the upper part of the figure, the reduced activations for pseudo-word reading in dyslexics (p<.05 FWE-corrected). All regions mapped were significantly less active in the dyslexic readers for reading. The colour code represents the level of intersection seen in the normal controls between reading and auditory rhyming (blue areas) or reading and visual motion detection task (purple areas) in controls. In yellow, the area specifically involved in pseudo-word reading task for controls.
In the lower part of the figure, activations of each task for each group are reported for the occipito-temporal areas. Dyslexics showed activations for the auditory phonological task (blue areas), for the visual motion perception task (purple areas), but not for reading (red areas in the controls).
The analyses addressing question 2, showed that in dyslexics, within the region that is hypoactivated for reading (areas in red in the lower part of figure 3 and table 5-a), there were activations for the auditory phonological processing more laterally (areas in blue in the lower part of figure 3 and table 5-b) and posteriorly for the visual motion perception task (purple areas in the lower part of figure 3; see also table 5-c), but, of course, no intersection with the reading task as the region is not significantly activated for reading in dyselxics.16
Table 5.
In depth functional analyses (2): functional characterization of the regions hypo-activated by dyslexics during reading.
|
| ||||||||
| Brain regions | MNI Coordinates |
|||||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | Z score | x | y | z | Z score | |
|
|
||||||||
| Left occipito-temporal activations | ||||||||
|
| ||||||||
| Controls | Dyslexics | |||||||
|
| ||||||||
| a. Pseudo-word reading | ||||||||
| Fusiform gyrus | -42 | -56 | -20 | >8 | ------------------ | |||
| -38 | -48 | -24 | >8 | ------------------ | ||||
|
| ||||||||
| b. Auditory rhyming | ||||||||
| Inf. temporal gyrus | -52 | -48 | -20 | 4.7 | -50 | -50 | -12 | 5.83 |
| -48 | -44 | -14 | 5.54 | |||||
|
| ||||||||
| c. Visual motion perception | ||||||||
| Fusiform gyrus | -42 | -64 | -18 | 6.36 | ------------------ | |||
| Inf. occipital gyrus | -50 | -74 | -4 | >8 | -48 | -72 | -4 | >8 |
| ------------------ | -48 | -68 | -2 | >8 | ||||
|
| ||||||||
CS: contrast sensitivity. SD: Speed discrimination. n.e.: not executed (the subject did not performed the task). n.r.: not recorded (the subject performed the fMRI task, but the behavioral response was not recorded because of a response box malfunction).
To summarize, part of the region of hypoactivation for reading in dyslexia responded normally during simple auditory phonological processing and for the moving Gabor patch. On the other hand, the medial portion, that may correspond to the VWFA in normal controls, was not significantly activated by our dyslexics in any of our tasks.
4. Discussion
How many behavioural deficits in dyslexia?
The first aim of this study was to evaluate co-morbidity in adult well-compensated developmental dyslexics. To our knowledge, this is the first such an attempt in adult readers using both behavioural and functional imaging techniques.
Our results clearly show that, in adult subjects with a history of dyslexia, the neuropsychological pattern is dominated by a reading decoding difficulty that manifests even at a single bi-syllabic word or pseudo-word level, and is accompanied by phonological deficits (Snowling, 1981; Pennington et al., 1990; Swan and Goswami, 1997a, b; Ramus et al., 2003).
However, the phonological deficit was not present in a uniform manner across all phonological tasks. It was most evident in phonological tasks that require access to phonology from visual inputs (picture and digit naming tasks) or tasks like the spoonerisms that are facilitated by (i) orthographic knowledge and (ii) phonological segmentations skills that develop when orthographic-to-phonological decoding strategies are mastered (see, for example, Bradley and Bryant, 1983).
In other phonological tasks, e.g. the digit span task of the WAIS, the difference between the groups was not as significant or not significant at all as in the case of the simple auditory phonological task used during fMRI (see below). This finding is perfectly compatible with recent longitudinal observations by Caravolas et al. (2012) in developing children from different cultures and consistent with previous cross-cultural work in adults (Paulesu et al., 2001).
Furthermore, when a formal comparison was made between two tasks that share the general process of recoding from visual input into phonology, that is the pseudo-word reading and the picture naming tasks, dyslexics showed a reversed pattern of reaction times when compared with normal controls: normal controls were considerably slower in naming pictures (by 90 msec on average), dyslexics were much slower in naming pseudo-words (110 msec slower than for picture naming).
On the contrary, no group difference emerged for the auditory phonological task that involved discrimination of letter-names during fMRI, even though processing load in this task was similar to the pseudo-word reading task because it required the manipulation of at least two syllables (the letter name – e.g.: [pi], [di], [èf-fe], [ʒè-ta] - and the reference letter name [bi]). Accordingly, auditory phonological tasks, that require minimal reliance on orthographic codes, were performed flawlessly by adult well-compensated dyslexics.
Taken together, these findings confirm the phonological deficit normally seen in dyslexia (Ramus et al., 2003; Marinelli et al., 2011). Of the many phonological behaviours, phonological retrieval from visual input is particularly problematic for dyslexics and particularly so when orthographic strings are involved.
In contrast, behavioural deficits in tasks designed to challenge the other systems under investigation (visual magnocellular/visual dorsal stream; motor/cerebellar) were only observed occasionally for individual subjects.
This pattern is highly reminiscent of the one described by Ramus et al. (2003) or with the observations of Reid et al. (2007) if one considers that having relatively small samples of patients exposes to the risk of missing the observation of specific cases which, by all means, occur with a very low frequency.
To summarize the behavioural results, our co-morbidity study shows that, as much as this might seem obvious, reading deficits are of utmost prominence in dyslexia. This core deficit is accompanied by phonological difficulties particularly in tasks that require orthographic knowledge or phonological retrieval from visual input such as letter strings. As normally observed in cultures with transparent orthographies, the reading disorder was in the form of a speed-dyslexia, as subjects made only occasional errors (Landerl et al., 1997; Paulesu et al., 2001; Hutzler and Wimmer, 2004).
These findings suggest that one should observe a functional anatomical deficit in regions not only involved in reading, conceived as a visual task, but also in the integration of visual and phonological codes (Cohen et al., 2004; Danelli et al., 2013). In contrast, the same behavioural pattern predicts little if any reduction of brain activation in simple auditory phonological tasks or indeed all other non-reading tasks. Functional imaging is needed to test these predictions and search for evidence of compensatory activity during non-reading tasks that might be indicative of abnormal processing that was not evident in behavioural measures. Compensatory activity is expected to be observed as hyperactivations (see for example the literature on graceful aging; Cabeza et al., 2002; Berlingeri et al., 2010; Berlingeri et al., 2013; Zapparoli et al., 2013; Zapparoli et al., 2016). If we had observed hyperactivation during non-reading tasks, we would have concluded that a broad comorbidity persists into adulthood, but for some domains this is only detectable at the endophenotypical functional anatomical level in the form of a compensatory process. We did not, however, find evidence to support this hypothesis.
How many functional anatomical deficits in dyslexia?
The functional imaging data complement the behavioural ones in a revealing way. These new fMRI results replicate a well-established finding in showing a reduced activation of the left inferior temporal/fusiform region and neighbouring extra-striate cortices for reading. A similar pattern has been seen in several previous studies of reading in dyslexia across multiple age-ranges. For example, the meta-analysis of left-occipito-temporal cortex data summarized in Paulesu et al. (2014) was derived from 273 controls and 251 dyslexics from 17 studies. The sample of dyslexics investigated in the current study are therefore similar to the populations described so far in the imaging literature for this particular endophenotype.
By providing an array of behavioural and functional neuroimaging investigations, our results permit a deeper and more discriminatory interpretations of previous data.
The key observation we are focusing on is` the lack of significant functional anatomical differences (hypoactivation or hyperactivations) in the non-reading tasks (i.e. the visual magnocellular, simple auditory phonological and cerebellar tasks).17 If we had observed hyperactivations, with equivalent behaviour, we would have suggested some form of successful compensation in a still deficient system. However, this was not the case which implies that systems thought to play a causal role in dyslexia, like the visual-magnocellular account or the cerebellar account, may finally reach maturation despite enduringly malfunction in the decoding system for reading18. We therefore have no evidence that our dyslexics differed in domains outside those of reading, phonological retrieval from visual stimuli or orthographic processing, in the context of strong evidence that behaviour and brain activation were abnormal during the reading task.
To summarize, our interpretation of the present data with well compensated dyslexics excludes a causal link between the enduring reading disorder and an enduring broad deficit in visual magnocellular or dorsal stream. This is consistent with the proposal that deficits in elementary perceptual tasks might be the consequence rather than cause of reduced reading experience (Olulade et al., 2013). However, our data are also compatible with the likely possibility that perceptual deficits will slow reading acquisition in childhood (see figure 1) and prevent effective compensation in adult dyslexics (see also Franceschini et al., 2012; Gori et al., 2016).
A generic phonological theory is also weakened by our findings that well-compensated adult dyslexics were perfectly capable of performing a simple auditory phonological discrimination task, providing that this did not involve phonological retrieval from visual input, as in the picture-naming task (McCrory et al., 2005). They also had a normal digit span. This indicates that, although subtle phonological deficits can be found in some pre-school children at risk for dyslexia and these predict the disorder (for recent meta-analysis, see Snowling and Melby-Lervåg, 2016), not all kinds of phonological abilities correlate with the ease of reading acquisition (Caravolas et al., 2012) and some phonological tasks are better compensated than others.
We have also argued above that difficulties on the spoonerism task may be a consequence of dyslexics having weaker orthographic knowledge and phonological segmentation abilities because of inefficient orthographic to phonological decoding strategies. This suggests that the integration between phonology and other representations is a crucial part of an enduring disorder in its pure form together with the maturation of the underlying neuronal substrates19.
Our proposal for a relatively pure reading disability in well-compensated dyslexics is also supported by the dissection of the functional properties of the L-OTC. In normal controls, this area appears to be a crossroad of different functional systems (Tarkiainen et al., 1999; Xue and Poldrack, 2007; Danelli et al., 2013). In particular, our normal readers activated the more anterior and lateral component in the inferior-temporal region (LIMA, Cohen et al., 2004) during reading and auditory phonological tasks, and the more posterior lateral fusiform during reading and visual motion perception. The intersection of activation across tasks in these two regions contrasts to the response in the more medial region described as a visual word-form area (VWFA) that is not responsive during visual motion or auditory language tasks and therefore appears to be more selective for reading (Cohen et al., 2002; but see Price and Devlin, 2003; Twomey et al., 2011). Irrespective of what specific function VWFA plays, its co-activation with LIMA and the posterior fusiform during normal reading suggests that activation in all three of these regions might need to be functionally integrated for fast and efficient reading (see Zeki and Shipp, 1988 for a discussion of integration by convergence in this region). Evidence that this integration process has broken down in dyslexics is provided by our observation that dyslexics were able to activate LIMA during auditory phonological tasks, and the posterior fusiform during visual motion perception, but showed hypoactivation in all three regions during reading.
Finally, the “cerebellar” hypothesis is also not supported by our data.
“Reading is Reading is Reading”? A revival of the disconnection hypothesis of dyslexia and the modular nature of the decoding deficit of dyslexia
Although our evidence does not support any of the major theories of dyslexia in their extreme formulation, is there any other hypothesis that might be reinforced by the present findings? In a more than twenty years-old paper, Cossu and Marshall (Cossu et al., 1993b) contended that dyslexia is the result of a deficit limited to the reading system. Their evidence was based on the observation of hyperlexic Down syndrome subjects who were unable to perform phonological awareness tasks while being extremely good at reading. Their evidence was criticized (see, for example, the reactions by Morton and Frith, 1993), yet Cossu et al. (1993a) provided a vehement response in a commentary called Reading is Reading is Reading.
Their theory is still viable if we put it in a broader context. Our data on adult dyslexics, with a fairly pure form of dyslexia, definitively points to the lack of maturation in a specialized print-to-phonology interface. Moreover, we have shown how the deficit can persist when other putative deficits are not observed or have been resolved. The reason behind the lack of maturation of the left occipito-temporal cortical decoding module remains mysterious, and possibly it has genetic origins (see Peterson and Pennington, 2015 for a review; see also Skeide et al., 2015): we propose that a perturbed connectivity between visual cortical analysers and auditory phonological representations may be a crucial factor. In the present data, the L-OTC of normal subjects is a crossroad of several systems while in dyslexic subjects it is not. Integration through a form of functional anatomical convergence, is one of the processes used by the brain to integrate multidimensional stimuli (Zeki and Shipp, 1988) but it is clearly not operating affectively in dyslexics, at least in this brain region.
Perturbed functional connectivity in dyslexia has already been postulated on the basis of PET activation data from visual phonological tasks (Paulesu et al., 1996), DTI MRI data (Klingberg et al., 2000; Deutsch et al., 2005; Zhao et al., 2016), VBM data (Silani et al., 2005) and pathological animal models (Galaburda, 1993). Recent seed based functional connectivity analyses of fMRI data collected when reading or at rest further support this concept (Schurz et al., 2015). The pathological data from Galaburda’s group suggest that the disconnectional impact of dyslaminations and ectopias in dyslexia, provide a microanatomical explanation of the bases for disconnection. Reduced connectivity would prove particularly critical for the task of reading, causing dyslexia, because reading requires the de-novo integration of multiple other systems (visual, semantic phonological) that may each independently be involved in many other tasks.
Functional disconnection in the reading system can also explain why dyslexics fail on auditory phonological tasks that are facilitated by orthographic awareness, like in complex rhyming tasks (Seidenberg and Tanenhaus, 1979). It is well known that complex auditory phonological awareness tasks are associated with left occipito-temporal cortical activations (Démonet et al., 1994). The same disconnection hypothesis may also explain why dyslexics also struggle with tasks that require phonological retrieval from non-orthographic stimuli as reported by McCrory et al., (2005).
While one could argue that less compensated dyslexics may show a broader degree of comorbidity, the present study shows that dyslexia, in a relatively pure form, exists and it is accompanied by specific behavioural and dysfunctional anatomical patterns.
The present data and interpretations have a very obvious practical implication: reading is the best test for dyslexia because it is systematically accompanied by pathological behaviour and tangible and reproducible functional anatomical signs. It is also very likely that practice and training in orthographic-to-phonological awareness is the best rehabilitation program for dyslexia (McArthur et al., 2015) because it may activate connections between the visual and auditory systems.
Supplementary Material
Highlights.
We found a very specific deficit for reading in adult well compensated dyslexics
Other deficits were primarily in tasks requiring access to phonology from vision
This pattern went with reduced activation of the left occipitotemporal cortex (l-OTC)
Multiple systems converge in the l-OTC for normal readers but less so for dyslexics
We argue that dyslexics lack a l-OTC multiple domain integration needed for reading
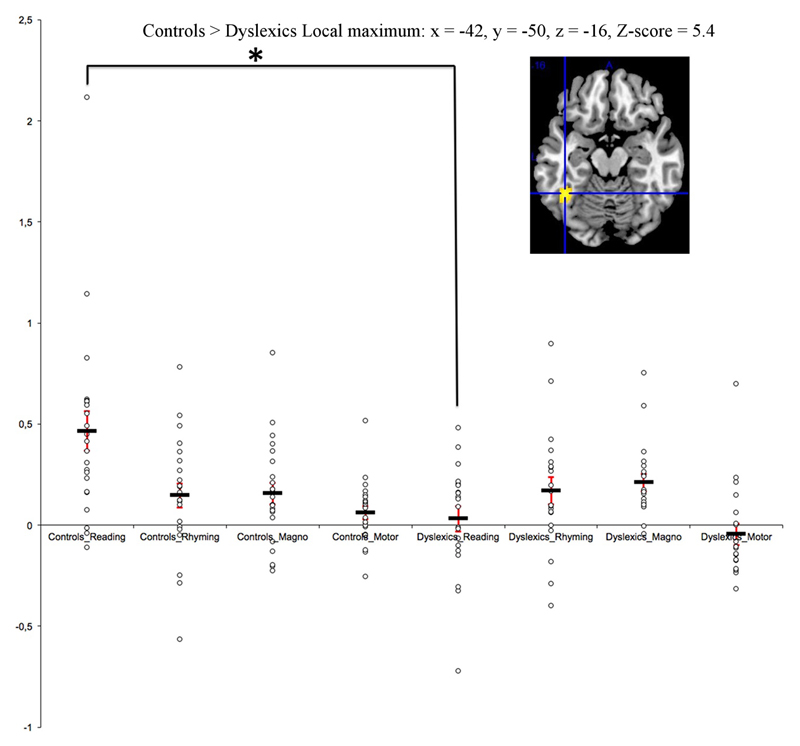
Figure 4.
The scatter plots (with the SEMs) for the key region where we found a FWER corrected reduced activation in dyslexics specifically for the reading task. The same regions showed the high-level interaction (Reading > Rhyming)Controls > (Reading > Rhyming)Dyslexics described in table 2.
Acknowledgements
We are grateful to Dr. Laura Paganelli and Dr. Gilda Pesenti for referring some of their patients to us, and a special thanks to all the volunteers for their participation. Professor C.J. Price is supported by a Wellcome PRF grant 205103/Z/16/Z.
Footnotes
We do not test explicitly this hypothesis in this article. We rather tested the visual motion perception system, the stem of the dorsal visual pathway that feeds into the visual attentional system. As the reader shall see, the patterns of activation associated with a visual-motion perception task activate most of the dorsal visual stream involved in visuo-spatial attention.
There is some controversy on whether testing the functioning of magnocellular system, of the visual-motion perception system and the dorsal visual-attentional system are equivalent or comparable matters. For example, there is now evidence to suggest that visual motion perception and the functioning of the dorsal stream are not solely depending on magnocellular input (for discussions, see Skottun, 2015).
VBM is unable to differentiate normal grey matter from dyslaminated grey matter, ectopias or scars, all microscopical pathological hallmarks of dyslexia (Galaburda et al., 1985): accordingly, any VBM finding in dyslexia should be treated with some caution.
Instead of a focal grey matter reduction, Pernet et al. (2009a) found reversed correlations between behavioural variables and grey matter cerebellar density in their dyslexics. In a further description of their data, Pernet et al. (2009b) found that dyslexics could have lower or higher “grey matter” density than controls, rather than a systematic cerebellar grey matter reduction.
Of course, any experiment, in which only one possible explanation of developmental dyslexia is tested, makes it impossible to draw conclusions on whether phonological, visual-motion perception or cerebellar deficits co-occur in dyslexia.
Admittedly, the ultimate tests for causality are longitudinal or training studies, some of which have found positive links between “magnocellular” / visuospatial abilities even prior to reading (Franceschini et al., 2012; Gori et al., 2015 & 2016).
The temporal pattern illustrated in Figure 1 could be fully tested only in a longitudinal study.
Throughout the paper, the wording comorbidity is used to mean the co-occurrence of deficits in distinct functional systems (e.g. phonological and visual motion perception systems) rather than the co-occurrence of two syndromes (e.g. dyslexia and attention deficit hyperactivity disorder).
Activation of area MT/V5 is never seen when reading single words or pseudo-words in central presentation, two tasks in which dyslexic are pathologically slow. A dysfunction of area MT/V5 in a visual motion perception task would be a deficit in a task/domain associated with dyslexia for a region not immediately associable with the task of reading.
The adoption of a Bonferroni correction would have been unnecessarily too harsh and it would be based on the assumption that each task-specific comparison between a dyslexic and the group of control would be independent, something that from a methodological and psychometrical point of view is difficult to maintain.
As this paper is primarily concerned with inferences at a population level, we deliberately do not describe individual fMRI data. Such description will be the subject of a companion paper on the diagnostic value of different fMRI tests in dyslexia at the individual level.
The small volume corrected –ROI- analyses allowed us to test specific a priori anatomical hypotheses, and were justified because the ROIs were derived from independent data.
This effect was calculated on the reading data exclusively masked on the other three tasks data thresholded at 0.05 uncorrected.
This region was identified in the controls by comparing the reading task with all the other tasks with the linear contrast “3 -1 -1 -1” for reading, rhyming, visuo-motion perception and motor learning respectively.
The cluster labels used for the small volume correction are those presented by Paulesu et al. (2014) in table 2.
We have preliminary data showing that, for picture naming the vast majority of this area is normally active in dyslexia with a small but significant region of hypoactivation corresponding to the mesial fusiform region labelled as VWFA by Cohen et al. (2002). This has previously been noted by McCrory et al. (2005).
It is important to stress that our data go beyond a naïf claim as if we had demonstrated the null hypothesis for the non-reading tasks, something that would be problematic to state firmly: rather, as there were higher level group by task interactions, we also provide explicit evidence of a greater fMRI activation difference for the reading task, compared with any other tasks tested, particularly for the left occipito-temporal cortex.
This pattern of results is also compatible with Olulade et al. (2013) or Goswami’s (2015) interpretation of the causal link between a limited reading experience and a delayed maturation of the visual magnocellular system: our subjects were well-compensated dyslexics with considerable reading training throughout their life.
The same argument could be taken if one considers the other end of the spectrum between elementary visual processing, the integration between phonology and vision and auditory phonology itself. Much as described by Ziegler et al. (2010) in children, our dyslexics did not show systematic elementary visual deficits, nor they were delayed in generating a stereotyped utterance in response to a visual stimulus; yet, they were impaired when the visual tasks involved rapid naming, i.e., the integration of visual inputs with stimulus specific phonological codes, as in reading or picture naming.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Baillieux H, Vandervliet EJ, Manto M, Parizel PM, De Deyn PP, Mariën P. Developmental dyslexia and widespread activation across the cerebellar hemispheres. Brain and Language. 2009;108:122–132. doi: 10.1016/j.bandl.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Bartlett MS. Properties of sufficiency and statistical tests. Proceedings of the Royal Statistical Society Series A. 1937;160:268–282. [Google Scholar]
- Berlingeri M, Bottini G, Danelli L, Ferri F, Traficante D, Sacheli L, Colombo N, Sberna M, Sterzi R, Scialfa G, Paulesu E. With time on our side? Task-dependent compensatory processes in graceful aging. Experimental Brain Research. 2010;205:307–324. doi: 10.1007/s00221-010-2363-7. [DOI] [PubMed] [Google Scholar]
- Berlingeri M, Danelli L, Bottini G, Sberna M, Paulesu E. Reassessing the HAROLD model: is the hemispheric asymmetry reduction in older adults a special case of compensatory-related utilisation of neural circuits? Experimental Brain Research. 2013;224:393–410. doi: 10.1007/s00221-012-3319-x. [DOI] [PubMed] [Google Scholar]
- Bishop DV. Cerebellar abnormalities in developmental dyslexia: cause, correlate or consequence? Cortex. 2002;38:491–498. doi: 10.1016/s0010-9452(08)70018-2. [DOI] [PubMed] [Google Scholar]
- Bishop DV. Cerebral asymmetry and language development: cause, correlate, or consequence? Science. 2013;340:1230531. doi: 10.1126/science.1230531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley L, Bryant PE. Categorizing sounds and learning to read: A causal connection. Nature. 1983;301:419–421. [Google Scholar]
- Brambati SM, Termine C, Ruffino M, Stella G, Fazio F, Cappa SF, Perani D. Regional reductions of gray matter volume in familial dyslexia. Neurology. 2004;63:742–745. doi: 10.1212/01.wnl.0000134673.95020.ee. [DOI] [PubMed] [Google Scholar]
- Brown WE, Eliez S, Menon V, Rumsey JM, White CD, Reiss AL. Preliminary evidence of widespread morphological variations of the brain in dyslexia. Neurology. 2001;56:781–783. doi: 10.1212/wnl.56.6.781. [DOI] [PubMed] [Google Scholar]
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: A search for Wernicke's Wortschatz? Brain. 1999;122(Pt 10):1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Caravolas M, Lervåg A, Mousikou P, Efrim C, Litavsky M, Onochie-Quintanilla E, Salas N, Schöffelová M, Defior S, Mikulajová M, Seidlová-Málková G, et al. Common patterns of prediction of literacy development in different alphabetic orthographies. Psychological Science. 2012;23:678–686. doi: 10.1177/0956797611434536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattinelli I, Borghese A, Gallucci M, Paulesu E. Reading the reading brain: A new meta-analysis of functional imaging data on reading. Journal of Neurolinguistics. 2013;26:214–238. [Google Scholar]
- Cohen L, Jobert A, Le Bihan D, Dehaene S. Distinct unimodal and multimodal regions for word processing in the left temporal cortex. Neuroimage. 2004;23:1256–1270. doi: 10.1016/j.neuroimage.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehéricy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Conway T, Heilman KM, Gopinath K, Peck K, Bauer R, Briggs RW, Torgesen JK, Crosson B. Neural substrates related to auditory working memory comparisons in dyslexia: an fMRI study. Journal of the International Neuropsychological Society. 2008;14:629–639. doi: 10.1017/S1355617708080867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossu G, Rossini F, Marshall JC. Reading is reading is reading. Cognition. 1993a;48:297–303. doi: 10.1016/0010-0277(93)90016-o. [DOI] [PubMed] [Google Scholar]
- Cossu G, Rossini F, Marshall JC. When reading is acquired but phonemic awareness is not: a study of literacy in Down's syndrome. Cognition. 1993b;46:129–138. doi: 10.1016/0010-0277(93)90016-o. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Howell DC, Garthwaite PH. Payne and Jones revisited: estimating the abnormality of test score differences using a modified paired samples t test. Journal of Clinical and Experimental Neuropsychology. 1998;20:898–905. doi: 10.1076/jcen.20.6.898.1112. [DOI] [PubMed] [Google Scholar]
- Danelli L, Berlingeri M, Bottini G, Ferri F, Vacchi L, Sberna M, Paulesu E. Neural intersections of the phonological, visual magnocellular and motor/cerebellar systems in normal readers: implications for imaging studies on dyslexia. Human Brain Mapping. 2013;34:2669–2687. doi: 10.1002/hbm.22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Boynton GM, Heeger DJ. Functional magnetic resonance imaging of early visual pathways in dyslexia. Journal of Neuroscience. 1998;18:6939–6951. doi: 10.1523/JNEUROSCI.18-17-06939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JD, Wandell B. Children's reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex. 2005;41:354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- Dufor O, Serniclaes W, Sprenger-Charolles L, Démonet JF. Top-down processes during auditory phoneme categorization in dyslexia: a PET study. Neuroimage. 2007;34:1692–1707. doi: 10.1016/j.neuroimage.2006.10.034. [DOI] [PubMed] [Google Scholar]
- Démonet JF, Price C, Wise R, Frackowiak RS. A PET study of cognitive strategies in normal subjects during language tasks. Influence of phonetic ambiguity and sequence processing on phoneme monitoring. Brain. 1994;117(Pt 4):671–682. doi: 10.1093/brain/117.4.671. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Wilke M, Eckert M, Richards T, Berninger V. Anatomical signatures of dyslexia in children: unique information from manual and voxel based morphometry brain measures. Cortex. 2005;41:304–315. doi: 10.1016/s0010-9452(08)70268-5. [DOI] [PubMed] [Google Scholar]
- Eden GF, VanMeter JW, Rumsey JM, Maisog JM, Woods RP, Zeffiro TA. Abnormal processing of visual motion in dyslexia revealed by functional brain imaging. Nature. 1996a;382:66–69. doi: 10.1038/382066a0. [DOI] [PubMed] [Google Scholar]
- Eden GF, VanMeter JW, Rumsey JM, Zeffiro TA. The visual deficit theory of developmental dyslexia. Neuroimage. 1996b;4:S108–117. doi: 10.1006/nimg.1996.0061. [DOI] [PubMed] [Google Scholar]
- Facoetti A, Paganoni P, Turatto M, Marzola V, Mascetti GG. Visual-spatial attention in developmental dyslexia. Cortex. 2000;36:109–123. doi: 10.1016/s0010-9452(08)70840-2. [DOI] [PubMed] [Google Scholar]
- Facoetti A, Zorzi M, Cestnick L, Lorusso ML, Molteni M, Paganoni P, Umilta C, Mascetti GG. The relationship between visuo-spatial attention and nonword reading in developmental dyslexia. Cognitive Neuropsychology. 2006;23:841–855. doi: 10.1080/02643290500483090. [DOI] [PubMed] [Google Scholar]
- Fawcett AJ, Nicolson RI, Maclagan F. Cerebellar tests differentiate between groups of poor readers with and without IQ discrepancy. Journal of Learning Disabilities. 2001;34:119–135. doi: 10.1177/002221940103400203. [DOI] [PubMed] [Google Scholar]
- Field A. Discovering statistics using SPSS. 2nd ed. Thousand Oaks, CA, US: Sage Publications; 2005. [Google Scholar]
- Friston KJ. Models of brain function in neuroimaging. Annual Review of Psychology. 2005;56:57–87. doi: 10.1146/annurev.psych.56.091103.070311. [DOI] [PubMed] [Google Scholar]
- Franceschini S, Gori S, Ruffino M, Pedrolli K, Facoetti A. A causal link between visual spatial attention and reading acquisition. Current Biology. 2012;22:814–819. doi: 10.1016/j.cub.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Frith U. Paradoxes in the definition of dyslexia. Dyslexia. 1999;5:192–214. [Google Scholar]
- Gabrieli JD, Norton ES. Reading abilities: importance of visual-spatial attention. Current Biology. 2012;22:R298–299. doi: 10.1016/j.cub.2012.03.041. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Sherman GF, Rosen GD, Aboitiz F, Geschwind N. Developmental dyslexia: four consecutive patients with cortical anomalies. Annual Neurology. 1985;18(2):222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- Galaburda AM. Neuroanatomic basis of developmental dyslexia. Neurologic Clinics. 1993;11:161–173. [PubMed] [Google Scholar]
- Gori S, Seitz AR, Ronconi L, Franceschini S, Facoetti A. Multiple causal links between magnocellular-dorsal pathway deficit and developmental dyslexia. Cerebral Cortex. 2016;26:4356–4369. doi: 10.1093/cercor/bhv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami U. Sensory theories of developmental dyslexia: three challenges for research. Nature Review Neuroscience. 2015;16:43–54. doi: 10.1038/nrn3836. [DOI] [PubMed] [Google Scholar]
- Hari R, Renvall H. Impaired processing of rapid stimulus sequences in dyslexia. Trends in Cognitive Sciences. 2001;5:525–532. doi: 10.1016/s1364-6613(00)01801-5. [DOI] [PubMed] [Google Scholar]
- Heim S, Grande M, Pape-Neumann J, van Ermingen M, Meffert E, Grabowska A, Huber W, Amunts K. Interaction of phonological awareness and 'magnocellular' processing during normal and dyslexic reading: behavioural and fMRI investigations. Dyslexia. 2010;16:258–282. doi: 10.1002/dys.409. [DOI] [PubMed] [Google Scholar]
- Heim S, Tschierse J, Amunts K, Wilms M, Vossel S, Willmes K, Grabowska A, Huber W. Cognitive subtypes of dyslexia. Acta Neurobiologiae Experimentalis (Wars) 2008;68:73–82. doi: 10.55782/ane-2008-1674. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, McMillon G, Kolchugina G, Black JM, Faizi A, Deutsch GK, et al. Functional and morphometric brain dissociation between dyslexia and reading ability. Proceedings of the National Academy of Sciences of the USA. 2007;104:4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Lee HL, Zhang Q, Liu T, Geng LB, Seghier ML, Shakeshaft C, Twomey T, Green DW, Yang YM, Price CJ. Developmental dyslexia in Chinese and English populations: dissociating the effect of dyslexia from language differences. Brain. 2010;133(Pt 6):1694–706. doi: 10.1093/brain/awq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzler F, Wimmer H. Eye movements of dyslexic children when reading in a regular orthography. Brain and Language. 2004;89:235–242. doi: 10.1016/S0093-934X(03)00401-2. [DOI] [PubMed] [Google Scholar]
- Kast M, Bezzola L, Jäncke L, Meyer M. Multi- and unisensory decoding of words and nonwords result in differential brain responses in dyslexic and nondyslexic adults. Brain and Language. 2011;119:136–148. doi: 10.1016/j.bandl.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Wimmer H, Staffen W, Hutzler F, Mair A, Ladurner G. Developmental dyslexia: Gray matter abnormalities in the occipitotemporal cortex. Human Brain Mapping. 2008;29:613–625. doi: 10.1002/hbm.20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landerl K, Wimmer H, Frith U. The impact of orthographic consistency on dyslexia: a German-English comparison. Cognition. 1997;63:315–334. doi: 10.1016/s0010-0277(97)00005-x. [DOI] [PubMed] [Google Scholar]
- Linkersdörfer J, Lonnemann J, Lindberg S, Hasselhorn M, Fiebach CJ. Grey matter alterations co-localize with functional abnormalities in developmental dyslexia: an ALE meta-analysis. PLoS One. 2012;7(8):e43122. doi: 10.1371/journal.pone.0043122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacSweeney M, Brammer MJ, Waters D, Goswami U. Enhanced activation of the left inferior frontal gyrus in deaf and dyslexic adults during rhyming. Brain. 2009;132:1928–1940. doi: 10.1093/brain/awp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF. A meta-analysis of functional neuroimaging studies of dyslexia. Annals of the New York Academy of Sciences. 2008;1145:237–259. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- Marinelli CV, Angelelli P, Di Filippo G, Zoccolotti P. Is developmental dyslexia modality specific? A visual-auditory comparison of Italian dyslexics. Neuropsychologia. 2011;49:1718–1729. doi: 10.1016/j.neuropsychologia.2011.02.050. [DOI] [PubMed] [Google Scholar]
- Martin A, Kronbichler M, Richlan F. Dyslexic Brain Activation Abnormalities in Deep and Shallow Orthographies: A Meta-Analysis of 28 Functional Neuroimaging Studies. Human Brain Mapping. 2016;37:2676–2699. doi: 10.1002/hbm.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur G, Castles A, Kohnen S, Larsen L, Jones K, Anandakumar T, Banales E. Sight Word and Phonics Training in Children With Dyslexia. Journal of Learning Disabilities. 2015;48:391–407. doi: 10.1177/0022219413504996. [DOI] [PubMed] [Google Scholar]
- McCrory E, Frith U, Brunswick N, Price C. Abnormal functional activation during a simple word repetition task: A PET study of adult dyslexics. Journal of Cognitive Neuroscience. 2000;12:753–762. doi: 10.1162/089892900562570. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, Mechelli A, Frith U, Price CJ. More than words: a common neural basis for reading and naming deficits in developmental dyslexia? Brain. 2005;128:261–267. doi: 10.1093/brain/awh340. [DOI] [PubMed] [Google Scholar]
- Menghini D, Finzi A, Benassi M, Bolzani R, Facoetti A, Giovagnoli S, Ruffino M, Vicari S. Different underlying neurocognitive deficits in developmental dyslexia: a comparative study. Neuropsychologia. 2010;48:863–872. doi: 10.1016/j.neuropsychologia.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Menghini D, Hagberg GE, Caltagirone C, Petrosini L, Vicari S. Implicit learning deficits in dyslexic adults: an fMRI study. Neuroimage. 2006;33:1218–1226. doi: 10.1016/j.neuroimage.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Morton J, Frith U. What lesson for dyslexia from Down's syndrome? Comments on Cossu, Rossini, and Marshall (1993) Cognition. 1993;48:289–296. doi: 10.1016/0010-0277(93)90045-w. discussion 297-303. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Berry EL, Jenkins IH, Dean P, Brooks DJ. Association of abnormal cerebellar activation with motor learning difficulties in dyslexic adults. Lancet. 1999;353:1662–1667. doi: 10.1016/S0140-6736(98)09165-X. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Dean P. Developmental dyslexia: the cerebellar deficit hypothesis. Trends in Neurosciences. 2001;24:508–511. doi: 10.1016/s0166-2236(00)01896-8. [DOI] [PubMed] [Google Scholar]
- Olulade OA, Gilger JW, Talavage TM, Hynd GW, McAteer CI. Beyond phonological processing deficits in adult dyslexics: atypical FMRI activation patterns for spatial problem solving. Developmental Neuropsychology. 2012;37:617–635. doi: 10.1080/87565641.2012.702826. [DOI] [PubMed] [Google Scholar]
- Olulade OA, Napoliello EM, Eden GF. Abnormal visual motion processing is not a cause of dyslexia. Neuron. 2013;79:180–190. doi: 10.1016/j.neuron.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Danelli L, Berlingeri M. Reading the dyslexic brain: multiple dysfunctional routes revealed by a new meta-analysis of PET and fMRI activation studies. Frontiers in Human Neuroscience. 2014;8:830. doi: 10.3389/fnhum.2014.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Démonet JF, Fazio F, McCrory E, Chanoine V, Brunswick N, Cappa SF, Cossu G, Habib M, Frith CD, Frith U. Dyslexia: cultural diversity and biological unity. Science. 2001;291:2165–2167. doi: 10.1126/science.1057179. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RS, Frith CD. Is developmental dyslexia a disconnection syndrome? Evidence from PET scanning. Brain. 1996;119(Pt 1):143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Pekkola J, Laasonen M, Ojanen V, Autti T, Jääskeläinen IP, Kujala T, Sams M. Perception of matching and conflicting audiovisual speech in dyslexic and fluent readers: an fMRI study at 3 T. Neuroimage. 2006;29:797–807. doi: 10.1016/j.neuroimage.2005.09.069. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Van Orden GC, Smith SD, Green PA, Haith MM. Phonological processing skills and deficits in adult dyslexics. Child Development. 1990;61:1753–1778. [PubMed] [Google Scholar]
- Pernet CR, Andersson J, Paulesu E, Demonet JF. When all hypotheses are right: a multifocal account of dyslexia. Human Brain Mapping. 2009a;30:2278–2292. doi: 10.1002/hbm.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet CR, Poline JB, Demonet JF, Rousselet GA. Brain classification reveals the right cerebellum as the best biomarker of dyslexia. BMC Neuroscience. 2009b;10:67. doi: 10.1186/1471-2202-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RL, Pennington BF. Developmental dyslexia. Annual Review of Clinical Psychology. 2015;11:283–307. doi: 10.1146/annurev-clinpsy-032814-112842. [DOI] [PubMed] [Google Scholar]
- Peyrin C, Démonet JF, N'Guyen-Morel MA, Le Bas JF, Valdois S. Superior parietal lobule dysfunction in a homogeneous group of dyslexic children with a visual attention span disorder. Brain and Language. 2011;118:128–138. doi: 10.1016/j.bandl.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, Nichols TE, Poline JB, Vul E, Yarkoni T. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nature Reviews. Neuroscience. 2017;18(2):115–126. doi: 10.1038/nrn.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word form area. Neuroimage. 2003;19:473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Rae C, Harasty JA, Dzendrowskyj TE, Talcott JB, Simpson JM, Blamire AM, Dixon RM, Lee MA, Thompson CH, Styles P, Richardson AJ, et al. Cerebellar morphology in developmental dyslexia. Neuropsychologia. 2002;40:1285–1292. doi: 10.1016/s0028-3932(01)00216-0. [DOI] [PubMed] [Google Scholar]
- Ramus F, Rosen S, Dakin SC, Day BL, Castellote JM, White S, Frith U. Theories of developmental dyslexia: insights from a multiple case study of dyslexic adults. Brain. 2003;126:841–865. doi: 10.1093/brain/awg076. [DOI] [PubMed] [Google Scholar]
- Raschle NM, Chang M, Gaab N. Structural brain alterations associated with dyslexia predate reading onset. Neuroimage. 2011;57:742–749. doi: 10.1016/j.neuroimage.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AA, Szczerbinski M, Iskierka-Kasperek E, Hansen P. Cognitive profiles of adult developmental dyslexics: theoretical implications. Dyslexia. 2007;13:1–24. doi: 10.1002/dys.321. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science. 2008;17(3):177–182. [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Human Brain Mapping. 2009;30(10):3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen GD, Burstein D, Galaburda AM. Changes in efferent and afferent connectivity in rats with induced cerebrocortical microgyria. Journal of Comparative Neurology. 2000;418:423–440. [PubMed] [Google Scholar]
- Ruff S, Cardebat D, Marie N, Démonet JF. Enhanced response of the left frontal cortex to slowed down speech in dyslexia: an fMRI study. Neuroreport. 2002;13:1285–1289. doi: 10.1097/00001756-200207190-00014. [DOI] [PubMed] [Google Scholar]
- Schurz M, Wimmer H, Richlan F, Ludersdorfer P, Klackl J, Kronbichler M. Resting-State and Task-Based Functional Brain Connectivity in Developmental Dyslexia. Cerebral Cortex. 2015;25:3502–3514. doi: 10.1093/cercor/bhu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg MS, Tanenhaus MK. Orthographic effects on rhyme monitoring. Journal of Experimental Psychology: Human Learning and Memory. 1979;5:546–554. [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Pugh KR, Holahan JM, Marchione KE, Fletcher JM, Lyon GR, et al. Neural systems for compensation and persistence: young adult outcome of childhood reading disability. Biological Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Silani G, Frith U, Demonet JF, Fazio F, Perani D, Price C, Frith CD, Paulesu E. Brain abnormalities underlying altered activation in dyslexia: a voxel based morphometry study. Brain. 2005;128:2453–2461. doi: 10.1093/brain/awh579. [DOI] [PubMed] [Google Scholar]
- Skeide MA, Kirsten H, Kraft I, Schaadt G, Müller B, Neef N, Brauer J, Wilcke A, Emmrich F, Boltze J, Friederici AD. Genetic dyslexia risk variant is related to neural connectivity patterns underlying phonological awareness in children. Neuroimage. 2015;118:414–421. doi: 10.1016/j.neuroimage.2015.06.024. [DOI] [PubMed] [Google Scholar]
- Skottun BC. The need to differentiate the magnocellular system from the dorsal stream in connection with dyslexia. Brain and Cognition. 2015;95:62–66. doi: 10.1016/j.bandc.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Snowling MJ. Phonemic deficits in developmental dyslexia. Psychological Research. 1981;43:219–234. doi: 10.1007/BF00309831. [DOI] [PubMed] [Google Scholar]
- Snowling MJ. From language to reading and dyslexia. Dyslexia. 2001;7:37–46. doi: 10.1002/dys.185. [DOI] [PubMed] [Google Scholar]
- Snowling MJ, Melby-Lervåg M. Oral language deficits in familial dyslexia: A meta-analysis and review. Psychological Bulletin. 2016;142:498–545. doi: 10.1037/bul0000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrink C, Vogt K, Kastrup A, Muller HP, Juengling FD, Kassubek J, Riecker A. The contribution of white and gray matter differences to developmental dyslexia: insights from DTI and VBM at 3.0 T. Neuropsychologia. 2008;46:3170–3178. doi: 10.1016/j.neuropsychologia.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Stein J. The magnocellular theory of developmental dyslexia. Dyslexia. 2001;7:12–36. doi: 10.1002/dys.186. [DOI] [PubMed] [Google Scholar]
- Swan D, Goswami U. Phonological awareness deficits in developmental dyslexia and the phonological representations hypothesis. Journal of Experimental Child Psychology. 1997a;66:18–41. doi: 10.1006/jecp.1997.2375. [DOI] [PubMed] [Google Scholar]
- Swan D, Goswami U. Picture naming deficits in developmental dyslexia: the phonological representations hypothesis. Brain and Language. 1997b;56:334–353. doi: 10.1006/brln.1997.1855. [DOI] [PubMed] [Google Scholar]
- Tarkiainen A, Helenius P, Hansen PC, Cornelissen PL, Salmelin R. Dynamics of letter string perception in the human occipitotemporal cortex. Brain. 1999;122(Pt 11):2119–2132. doi: 10.1093/brain/122.11.2119. [DOI] [PubMed] [Google Scholar]
- Taylor JS, Rastle K, Davis MH. Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychological Bulletin. 2013;139:766–791. doi: 10.1037/a0030266. [DOI] [PubMed] [Google Scholar]
- Twomey T, Kawabata Duncan KJ, Price CJ, Devlin JT. Top-down modulation of ventral occipito-temporal responses during visual word recognition. Neuroimage. 2011;55:1242–1251. doi: 10.1016/j.neuroimage.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanni S, Uusitalo MA, Kiesilä P, Hari R. Visual motion activates V5 in dyslexics. Neuroreport. 1997;8:1939–1942. doi: 10.1097/00001756-199705260-00029. [DOI] [PubMed] [Google Scholar]
- Vinckenbosch E, Robichon F, Eliez S. Gray matter alteration in dyslexia: converging evidence from volumetric and voxel-by-voxel MRI analyses. Neuropsychologia. 2005;43:324–331. doi: 10.1016/j.neuropsychologia.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R. Wechler Adult Intelligence Scale - Revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- White S, Milne E, Rosen S, Hansen P, Swettenham J, Frith U, Ramus F. The role of sensorimotor impairments in dyslexia: a multiple case study of dyslexic children. Developmental Science. 2006;9(3):237–255. doi: 10.1111/j.1467-7687.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The international classification of diseases. 10th ed. Geneve: World Healt Organization; 1993. [Google Scholar]
- Xue G, Poldrack RA. The neural substrates of visual perceptual learning of words: implications for the visual word form area hypothesis. Journal of Cognitive Neuroscience. 2007;19:1643–1655. doi: 10.1162/jocn.2007.19.10.1643. [DOI] [PubMed] [Google Scholar]
- Yang Y, Bi HY, Long ZY, Tao S. Evidence for cerebellar dysfunction in Chinese children with developmental dyslexia: an fMRI study. International Journal of Neuroscience. 2013;123:300–310. doi: 10.3109/00207454.2012.756484. [DOI] [PubMed] [Google Scholar]
- Zapparoli L, Invernizzi P, Gandola M, Verardi M, Berlingeri M, Sberna M, De Santis A, Zerbi A, Banfi G, Bottini G, Paulesu E. Mental images across the adult lifespan: a behavioural and fMRI investigation of motor execution and motor imagery. Experimental Brain Research. 2013;224:519–540. doi: 10.1007/s00221-012-3331-1. [DOI] [PubMed] [Google Scholar]
- Zapparoli L, Saetta G, De Santis C, Gandola M, Zerbi A, Banfi G, Paulesu E. When I am (almost) 64: The effect of normal ageing on implicit motor imagery in young elderlies. Behavioural Brain Research. 2016;303:137–151. doi: 10.1016/j.bbr.2016.01.058. [DOI] [PubMed] [Google Scholar]
- Zeki S, Shipp S. The functional logic of cortical connections. Nature. 1988;335:311–317. doi: 10.1038/335311a0. [DOI] [PubMed] [Google Scholar]
- Zhao J, Thiebaut de Schotten M, Altarelli I, Dubois J, Ramus F. Altered hemispheric lateralization of white matter pathways in developmental dyslexia: Evidence from spherical deconvolution tractography. Cortex. 2016;76:51–62. doi: 10.1016/j.cortex.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Ziegler JC, Pech-Georgel C, Dufau S, Grainger J. Rapid processing of letters, digits and symbols: what purely visual-attentional deficit in developmental dyslexia? Developmental Science. 2010;13:F8–F14. doi: 10.1111/j.1467-7687.2010.00983.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.