Abstract
Genetically modified mice have been a major source of information about the molecular control of Schwann-cell myelin formation, and the role of β-neuregulin 1 (NRG1) in this process in vivo. In vitro, on the other hand, Schwann cells from rats have been used in most analyses of the signaling pathways involved in myelination. To correlate more effectively in vivo and in vitro data, we used purified cultures of mouse Schwann cells in addition to rat Schwann cells to examine two important myelin-related signals, cyclic adenosine monophosphate (cAMP), and NRG1 and to determine whether they interact to control myelin differentiation. We find that in mouse Schwann cells, neither cAMP nor NRG1, when used separately, induced markers of myelin differentiation. When combined, however, they induced strong protein expression of the myelin markers, Krox-20 and P0. Importantly, the level of cAMP signaling was crucial in switching NRG1 from a proliferative signal to a myelin differentiation signal. Also in cultured rat Schwann cells, NRG1 promoted cAMP-induced Krox-20 and P0 expression. Finally, we found that cAMP/NRG1-induced Schwann-cell differentiation required the activity of the cAMP response element binding family of transcription factors in both mouse and rat cells. These observations reconcile observations in vivo and on neuron-Schwann-cell cultures with studies on purified Schwann cells. They demonstrate unambiguously the promyelin effects of NRG1 in purified cells, and they show that the cAMP pathway determines whether NRG1 drives proliferation or induces myelin differentiation.
Keywords: neuregulin, protein kinase A, CREB, proliferation, adenovirus, Krox-20/Egr2
Introduction
The generation of myelinating Schwann cells from immature Schwann cells is controlled by axonal signals (Jessen and Mirsky, 2005) and axonally derived NRG1 type III plays an important role in this process (Michailov et al., 2004; Nave and Salzer, 2006; Taveggia et al., 2005). Nevertheless, in purified Schwann cells that cultured without neurons, exogenously applied NRG1, either soluble or membrane-bound, does not induce myelin proteins. Instead, the cells proliferate, suggesting that other signals are likely to play a role in myelin differentiation, potentially modulating or combining with NRG1 signaling (Taveggia et al., 2005).
Elevation of intracellular cyclic adenosine monophosphate (cAMP) levels has long been implicated in promoting Schwann-cell differentiation (Jessen and Mirsky, 2005). This idea is based on numerous experiments showing that in cultured rat Schwann cells, activation of cAMP pathways induces expression of myelin-related molecules, including galactocerebroside (Gal-C), 04 antigen, E-cadherin, periaxin, Krox-20, Oct-6, nuclear factor-κB (NF-κB), protein zero (P0), and myelin basic protein (MBP) (Crawford et al., 2008; Lemke and Chao, 1988; Mirsky et al., 1990; Monje et al., 2009; Monuki et al., 1989; Morgan et al., 1991; Parkinson et al., 2003; Sobue and Pleasure, 1984; Yoon et al., 2008). Furthermore, cAMP elevation downregulates expression of proteins that are normally suppressed during myelination but which are expressed by immature Schwann cells, including the low-affinity neurotrophin receptor (p75NTR), neural-cell adhesion molecule, N-cadherin, glial fibrillary acidic protein, growth-associated protein-43, c-Jun, and Sox-2 (Crawford et al., 2008; Jessen et al., 1987; Mokuno et al., 1988; Morgan et al., 1991; Parkinson et al., 2004, 2008; Scherer et al., 1994).
In vivo evidence for a role for cAMP in Schwann-cell myelination comes from experiments showing that zebrafish lacking the transmembrane G-protein-coupled receptor Gpr 126 do not produce peripheral myelin, while this phenotype can be rescued by treating mutants with the cAMP-elevating agent, forskolin (Monk et al., 2009).
Further evidence for a role for cAMP in Schwann-cell myelination comes from studying one of its downstream effectors, protein kinase A (PKA). These experiments show that PKA activity is necessary for myelination in neuron-Schwann-cell co-cultures and that the peak of PKA activity in the sciatic nerve occurs around the onset of myelination (Howe and McCarthy, 2000; Yoon et al., 2008). Downstream, PKA phosphorylates the p65 subunit of the transcription factor NF-κB, which is necessary for myelination in Schwann-cell/neuron co-cultures, while recent results suggest that NF-κB is also activated by axonal-type III NRG1 during myelination (Limpert and Carter, 2010; Nickols et al., 2003; Yoon et al., 2008).
Here, we show that in cultured mouse Schwann cells strong expression of the myelin proteins Krox-20 and P0 is only induced by combined cAMP/NRG1 and not by either alone. Additionally, we find that the concentration of cyclic AMP is crucial to determining the outcome of NRG1 signaling. At low levels of cyclic AMP activation NRG1 promotes Schwann-cell proliferation without myelin differentiation, whereas a high concentration of cyclic AMP promotes differentiation. We show that cAMP response element binding (CREB) protein transcription factors are required for cyclic AMP/NRG1 effects on myelin differentiation in both rat and mouse Schwann cells.
Materials and Methods
Materials
Poly-D-lysine (PDL), poly-L-lysine (PLL), bromodeoxyuridine (BrdU), and 2′-O-dibutyryladenosine 3′:5′-cycli-cAMP (dbcAMP), triton X-100, Hoechst dye H33258, mouse monoclonal antibodies to β-galactosidase, and β-tubulin were from Sigma (Poole, UK). Other antibody sources were as follows: monoclonal anti-BrdU, alkaline phosphatase-conjugated antidigoxygenin: Roche Diagnostics (Burgess Hill, UK); monoclonal c-Jun antibody: BD Transduction Laboratories (Oxford, UK); monoclonal anti-GAPDH: Abcam (Cambridge, UK); rabbit anti-Krox-20: Covance (Harrogate, UK); rabbit anti-periaxin: Peter Brophy and Diane Sherman (Edinburgh University, UK) (Gillespie et al., 1994); monoclonal anti-P0: Astexx (Graz, Austria): rabbit anti-Sox-2: Chemicon (Chandlers Ford, UK); biotinylated anti-mouse Ig, streptavidin-Cy3, and streptavidin-FITC: Amersham Pharmacia Biotech (Amersham, UK); rhodamine-conjugated goat anti-mouse Ig: MP Biomedicals (Solon, OH); Cy3-conjugated donkey anti-rabbit Ig and Cy3-conjugated donkey antimouse Ig: Jackson Immunoresearch (Newmarket, UK); goat anti-rabbit and mouse Ig horseradish peroxidase-conjugated antibodies: Promega (Southampton, UK). 8-pCPT-2-O-Me-cAMP and 6-Bnz-cAMP were from BIOLOG (Bremen, Germany). Fetal calf serum (FCS) and horse serum (HS) were from Invitrogen (Paisley, UK) and Perbio (Cramlington, UK). TGFβ-1 and NRGβ-1 were from R&D Systems Europe (Abingdon, UK). Sources of other reagents have been documented elsewhere (Jessen et al., 1994; Meier et al., 1999; Parkinson et al., 2001, 2003, 2004, 2008).
Animals
Animal experiments follow UK Home Office Guidelines. Postnatal day (P) 3–5 ICR mice or Sprague– Dawley rats were used to generate purified mouse and rat Schwann-cell cultures (see below). Krox-20+/− mice in which a null allele is created in the Krox-20 gene by an in-frame insertion of the Escherichia coli lacZ gene were used to prepare purified cultures of Schwann cells containing one copy of the Krox-20 allele (Schneider-Manoury et al., 1993). Dr P. Charnay (École Normale Supèrieure, Paris) provided heterozygous animals.
Cell Culture and Adenoviral Infection Assays
Schwann cells from sciatic nerve and brachial plexus of P3 rats and P2 mice were purified as previously described (Brockes et al., 1979; Morgan et al., 1991; Stevens et al., 1998). The mouse and rat Schwann-cell purifications were similar except that 5% HS was used instead of 10% FCS, and cells were plated on PLL/laminin instead of PDL/laminin. For immunocytochemistry experiments, Schwann cells (5,000 cells/15 μL) drop were plated on PDL/laminin coated coverslips (rat) and PLL/laminin (mouse), and cultured in supplemented defined medium (Jessen et al., 1994) containing 10−6 M insulin (DM). Adenovirus expressing A-CREB (a dominant negative inhibitor of CREB formed by fusing an extension onto the CREB leucine zipper domain) and control adenovirus, both expressing green fluorescent protein (GFP), were from Dr J. Uney (Warburton et al., 2005). Adenoviral supernatants were prepared, purified using Vivapure AdenoPACK 500 (Sartorius Mechatronics) according to the manufacturer’s protocol, and tittered as described previously (Parkinson et al., 2001). Schwann cells were infected by adenoviral preparations in DM, 0.5% FCS, or alternatively HS and 2 μM forskolin. After 24 h, the medium was changed to DM and 0.5% FCS or HS. For Western blot analysis, Schwann-cell cultures were serum-purified and immunopanned (Dong et al., 1999) before being replated onto PDL/laminin or PLL/laminin-coated dishes in medium-containing DM, NRG-1 (10 ng/mL), forskolin (2 μM), and 0.5% FCS (rat) or 0.5% HS (mouse). Cells were allowed to proliferate until confluent. They were then placed in DM without mitogenic factors for 12 h before experimental reagents were added.
Western Blot Analysis
Cells were lysed either in 25 mM HEPES pH 7.5, 0.1 M NaCl, 1% Triton X-100, and proteinase and phosphatase inhibitors, or 25 mM Tris–HCl pH 7.4, 95 mM NaCl, 10 mM EDTA, 2% SDS, and proteinase and phosphatase inhibitors, and homogenized. Fifty micrograms of protein extracts were separated on 10% SDS–polyacrylamide gels, transferred onto nitrocellulose membranes (Hybond ECL; Amersham Pharmacia), blocked with 5% fat-free milk in Tris-buffered saline, and incubated with primary antibodies in this solution. Antibody to β-tubulin was used at 1:1,000; antibodies to Krox-20, c-Jun, and Sox-2 were used at 1:2,500; antibody to l-periaxin was used at 1:15,000.
In Situ Hybridization
A digoxigenin-labeled cDNA probe was used to detect P0 mRNA. Cells were fixed in 4% paraformaldehyde (PF) in PBS, using a previously described protocol (Lee et al., 1997).
Semiquantitative RT-PCR
RNA was isolated from P5 mouse sciatic nerves and from serum-purified mouse Schwann cells using Trizol reagent (Invitrogen) followed by DNase digestion and clean-up with RNeasy Microkit (Qiagen). Total RNA (400 ng) was reverse transcribed using Superscript II (Invitrogen) and random primers according to the Superscript II protocol. PCR was performed using the following primers: CREB1: forward, GTCTGTGGAT AGTGTAACTG; reverse, CATCAGTGGTCTGTGCATAC; activating transcription factor 1 (ATF1): forward, GGCTCTCGAGTTACGCTCTC; reverse, GTTGAACCA GGCTGAGATGC; cAMP response element modulator (CREM): forward, CTAGCAGAAGAAGCAACTCG: reverse, CATTATGAGGATGCTGCATCG.
Immunocytochemistry and Microscopy
Immunolabeling with BrdU and P0 polyclonal antibodies has been described previously (Morgan et al., 1991; Parkinson et al., 2003). For O4 and Gal-C (1:5), primary antibodies were added to unfixed cells for 1 h and then incubated with relevant secondary antibodies. They were postfixed with 4% PF in PBS for 10 min. For monoclonal P0 (1:500) immunocytochemistry, cells were fixed in 4% PF in PBS for 10 min, permeabilized in methanol at −20°C for 10 min, blocked in antibody diluting solution (ADS; PBS containing 10% donor calf serum, 0.1 M lysine, and 0.2% sodium azide) for 30 min, and then primary antibody diluted in ADS was applied overnight at 4°C. For all other antibodies, cells were fixed in 4% PF in PBS, pH 7.5, for 10 min. After fixation, cells were permeabilized and blocked in ADS supplemented with 0.2% Triton X-100 for 30 min. Primary antibody to c-Jun was used at 1:500, Krox-20 at 1: 250, β-galactosidase at 1:500, and L-periaxin at 1: 8,000. Secondary antibodies in ADS were applied for 30 min at room temperature. Coverslips were mounted in Citifluor (London, UK). To control for nonspecific labeling, primary antibodies were excluded from a single sample in each experiment. Immunofluorescent cells were visualized using a Nikon Optiphot-2 or a Nikon Eclipse E800 fluorescent microscope. Images were captured using a digital camera (DXM1200, Nikon) and ACT-1 acquisition software (Nikon). Images were imported into Adobe Photoshop version 8.0 or Corel Draw software.
Statistical Analysis
Data are presented as arithmetic mean ± standard deviation of at least three independent experiments. The Student’s t test and one-way analysis of variance (ANOVA) were used to measure statistical significance (GraphPad).
Results
DbcAMP and NRG1 Synergistically Induce High Levels of Krox-20 and P0 Protein in Mouse Schwann Cells But Neither Is Effective When Used Alone
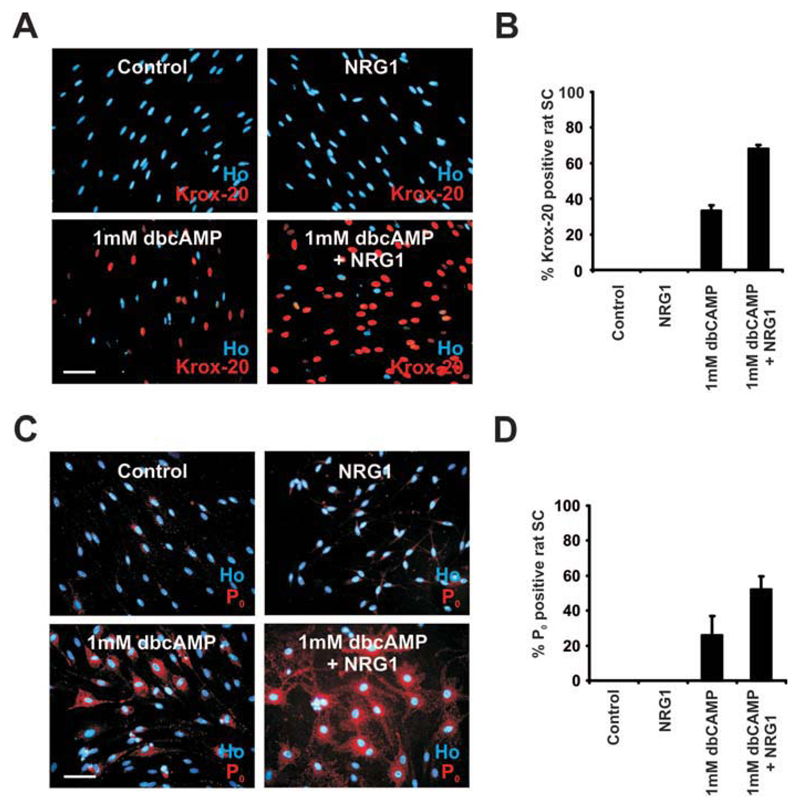
Elevation of cAMP levels alone, in the absence of serum or growth factors, is sufficient to induce myelin gene expression in cultured rat Schwann cells (Lemke and Chao, 1988; Morgan et al., 1991; Parkinson et al., 2003). Because little is known about the response of purified cultured mouse Schwann cells to cAMP, we examined whether dbcAMP also induced the myelin phenotype in these cells. We used the promyelin transcription factor Krox-20 to indicate myelin differentiation and dbcAMP at a concentration (1 mM) that induces myelin proteins in rat Schwann cells. Serum-purified mouse Schwann cells were cultured in DM with or without dbcAMP (1 mM) for 72 h. After this, protein was extracted from some of the cells and analyzed by Western blotting, whereas others were fixed for immunocytochemistry, using an antibody to Krox-20.
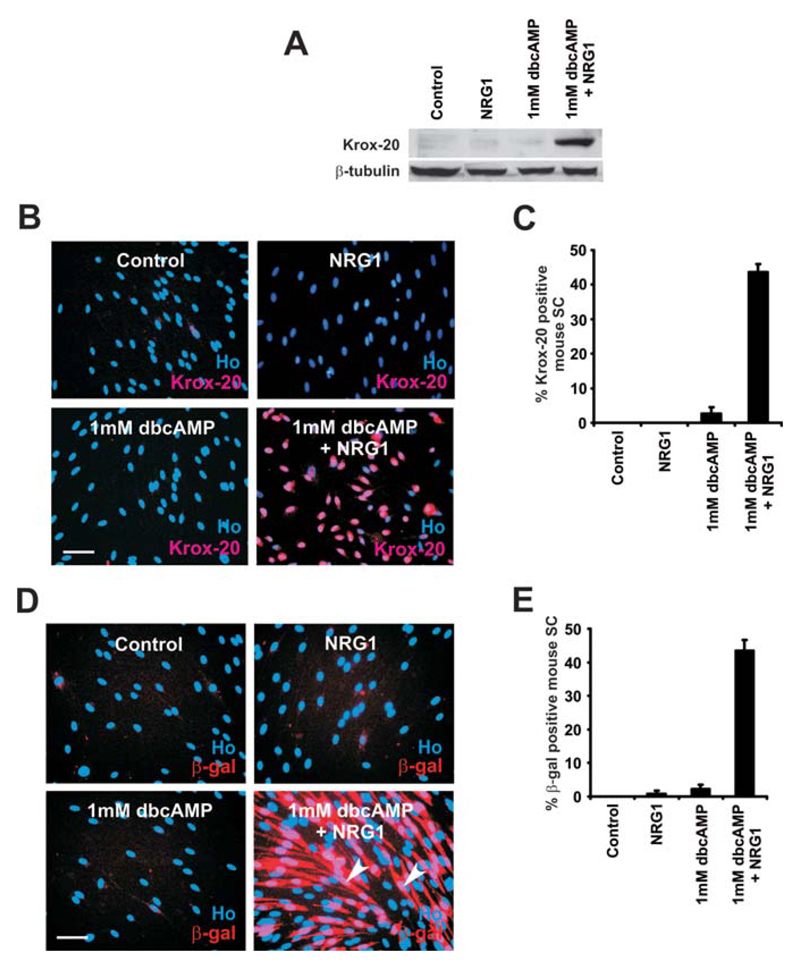
Surprisingly, Western blotting showed that treatment with dbcAMP for 72 h was insufficient to induce Krox-20 protein expression in mouse Schwann cells (Fig. 1A). Similarly, immunocytochemistry revealed that only 2.6 ± 1.9% of Schwann cells expressed weak nuclear Krox-20 after treatment with dbcAMP (n = 3; Fig. 1B,C). Extended treatment with dbcAMP for 120 h using either 1 or 2 mM dbcAMP failed to further induce nuclear Krox-20 (data not shown). This shows that in mouse Schwann cells, activation of cAMP pathways alone, even using very high-dbcAMP concentrations and extensive incubation periods, is insufficient to induce significant Krox-20 expression.
Fig. 1.
Induction of Krox-20 protein in mouse Schwann cells requires dbcAMP and NRG1 in combination. A: Western blot showing Krox-20 expression in mouse Schwann cells (expanded and passaged once) after 72h in NRG1 (20 ng/mL) alone, dbcAMP (1 mM) alone, dbcAMP (1 mM), and NRG1 (20 ng/ml) or DM with no additions (Control). β-Tubulin was used as a loading control. Note that high levels of Krox-20 are induced in cultures treated with combined dbcAMP/NRG1 and that neither dbcAMP alone nor NRG1 alone is sufficient to induce Krox-20 protein. B: Krox-20 expression visualized immunocytochemically in mouse Schwann cells cultured for 72 h with NRG1 alone, dbcAMP alone, combined dbcAMP/NRG1, or in DM alone (Control). Note that high levels of Krox-20 are only induced in cultures treated with combined dbcAMP/NRG1. Cells are co-labeled with Hoechst dye (Ho). Scale bar, 50 μm. C: Quantification of experiments shown in (B). Error bars: standard deviation of the mean. D: β-Gal expression visualized immunocytochemically in Schwann cells from Krox-20/lacZ ± nerves, cultured for 72 h with NRG1 alone, dbcAMP alone, dbcAMP/NRG1, or in DM (Control). High levels of β-Gal are only induced in cultures treated with combined dbcAMP/NRG1. Cells are co-labeled with Hoechst dye (Ho). Scale bar, 50 μm. E: Quantification of experiments shown in (D). Error bars: standard deviation of the mean. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In mice in vivo, axonally expressed NRG1 type III promotes myelin wrapping and Schwann-cell differentiation (Michailov et al., 2004; Nave and Salzer, 2006; Taveggia et al., 2005). Using the experimental design above, we therefore tested whether exogenous NRG1 (20 ng/mL) could induce differentiation in purified mouse Schwann-cell cultures on its own or in combination with dbcAMP (1 mM).
Treatment with NRG1 alone did not elevate Krox-20 levels. However, combined dbcAMP/NRG1 resulted in robust induction of Krox-20 protein using Western blot analysis (Fig. 1A). Additionally, we found that immunocytochemically 43.6 ± 2.3% of cells expressed strong nuclear Krox-20, a significant increase compared with control cells or cultures treated with dbcAMP alone or NRG1 alone (n = 3; P < 0.005; Fig. 1B,C). To substantiate this finding, we used Schwann cells from mice that were heterozygous for a Krox-20/lacZ chimaeric gene (referred to as Krox-20+/−; Schneider-Manoury et al., 1993). In these mice, lacZ gene expression reflects the normal Krox-20 gene expression (Murphy et al., 1996; Topilko et al., 1994). Krox-20+/− Schwann cells were purified, plated in DM, and treated as above for 72 h. Cells were then fixed and labeled with an antibody to β-gal, the lac-Z gene product. Schwann cells maintained in DM or NRG1 expressed virtually no β-gal, 2.2% ± 1.3% of Schwann cells expressed β-gal after dbcAMP treatment, while 43.5% ± 3.2% of Schwann cells treated with combined dbcAMP/NRG1 displayed strong β-gal expression (n = 4; P < 0.001; Fig. 1D,E). Thus, neither dbcAMP (1 or 2 mM) nor NRG1 (20 ng/mL) treatment alone induced significant levels of Krox-20. A combination of the two was required to induce strong Krox-20 expression.
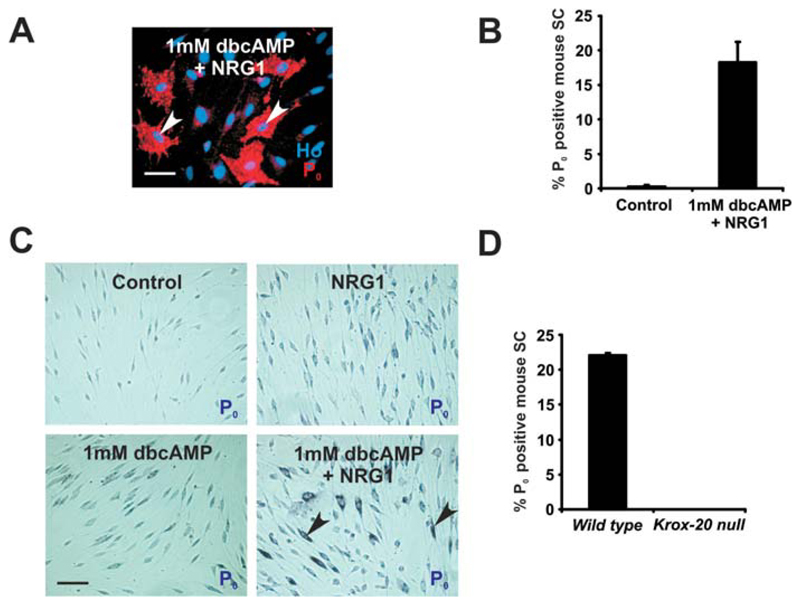
Because combined dbcAMP/NRG1 induces expression of Krox-20, we tested whether this treatment also induced strong expression of the major peripheral myelin protein P0. Purified mouse Schwann cells were treated with NRG1 alone, dbcAMP alone, combined dbcAMP/NRG1 or left in DM for 120 h. They were then fixed and labeled immunocytochemically for P0 protein expression. About 18.2% ± 3% of Schwann cells expressed high levels of P0, characteristic of a myelin-differentiated cell (Jessen and Mirsky, 2005) after 120 h of dbcAMP/NRG1 treatment, whereas these cells were very rarely seen in unstimulated cultures (n = 4; P < 0.01; Fig. 2A,B) or in cultures treated with dbcAMP alone (not shown). Other cultures were assessed for P0 mRNA expression after 72 h using identical conditions. Although some Schwann cells treated with either dbcAMP or NRG1 demonstrated slightly elevated levels of P0 mRNA, only cells that received the combination of the two factors expressed high levels of P0 mRNA (Fig. 2C).
Fig. 2.
Combined dbcAMP and NRG1 induces strong expression of P0 mRNA and protein in mouse Schwann cells. A: P0 expression visualized immunocytochemically in mouse Schwann cells treated for 120 h with dbcAMP/NRG1. Note the significant flattening of the P0 positive cells (two flattened cells are indicated by arrowheads). Cells are co-labeled with Hoechst dye (Ho). Scale bar, 50 μm. B: Quantification of the experiment in (A), comparing P0 induction in Schwann cells treated with a combined dbcAMP/NRG1 with Schwann cells cultured in DM alone (Control). Error bars: standard deviation of the mean. The P0 monoclonal antibody used here (Archelos et al., 1993) detects only high levels of P0 in cultured cells. Therefore, the percentage of P0-positive cells is lower than that of the Krox-20-positive cells (see Fig. 1), and the percentage of P0-positive cells induced by dbcAMP in rat Schwann cells in previous papers when a polyclonal P0 antibody was used (e.g., Lee et al., 1997; Morgan et al., 1991). C: P0 mRNA expression in mouse Schwann cells after culture in NRG1, dbcAMP, combined dbcAMP/NRG1 or DM (Control) for 72 h. Only combined dbcAMP/NRG1 treatment induces high levels of P0 mRNA, although low levels of induction can be seen in cells treated either with dbcAMP alone or with NRG1 alone. Arrowheads show Schwann cells expressing high levels of P0 mRNA. Scale bar, 50 μm. D: A substantial proportion of Schwann cells from wild-type mice cultured for 96 h in a combed dbcAMP/NRG1 express P0 protein, whereas Schwann cells from Krox-20−/− mice fail to do so. Error bars: standard deviation of the mean. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
P0 is a direct transcriptional target of Krox-20 and in the absence of Krox-20, and P0 gene expression is not upregulated by Schwann cells at the beginning of myelination (LeBlanc et al., 2006; Topilko et al., 1994). We therefore tested whether P0 induction by dbcAMP/NRG1 treatment relied on the expression of Krox-20. We stimulated Schwann cells from control or Krox-20−/− mice (Schneider-Manoury et al., 1993), with combined dbcAMP/NRG1 for 96 h and then fixed and labeled the cells with antibodies to P0. No Krox-20−/− Schwann cells expressed significant levels of P0 protein in response to dbcAMP/NRG1 (Fig. 2D), demonstrating that dbcAMP/NRG1 P0 upregulation requires prior induction of Krox-20.
In summary, these experiments show that treatment of mouse Schwann cells with combined dbcAMP (1 or 2 mM)/NRG1 (20 ng/mL) results in a strong induction of both Krox-20 and P0, while, surprisingly, treatment with 1 mM dbcAMP alone is insufficient to induce significant expression of myelin genes.
NRG1 Promotes DbcAMP-Induced Gal-C, O4, and Periaxin in Mouse Schwann Cells
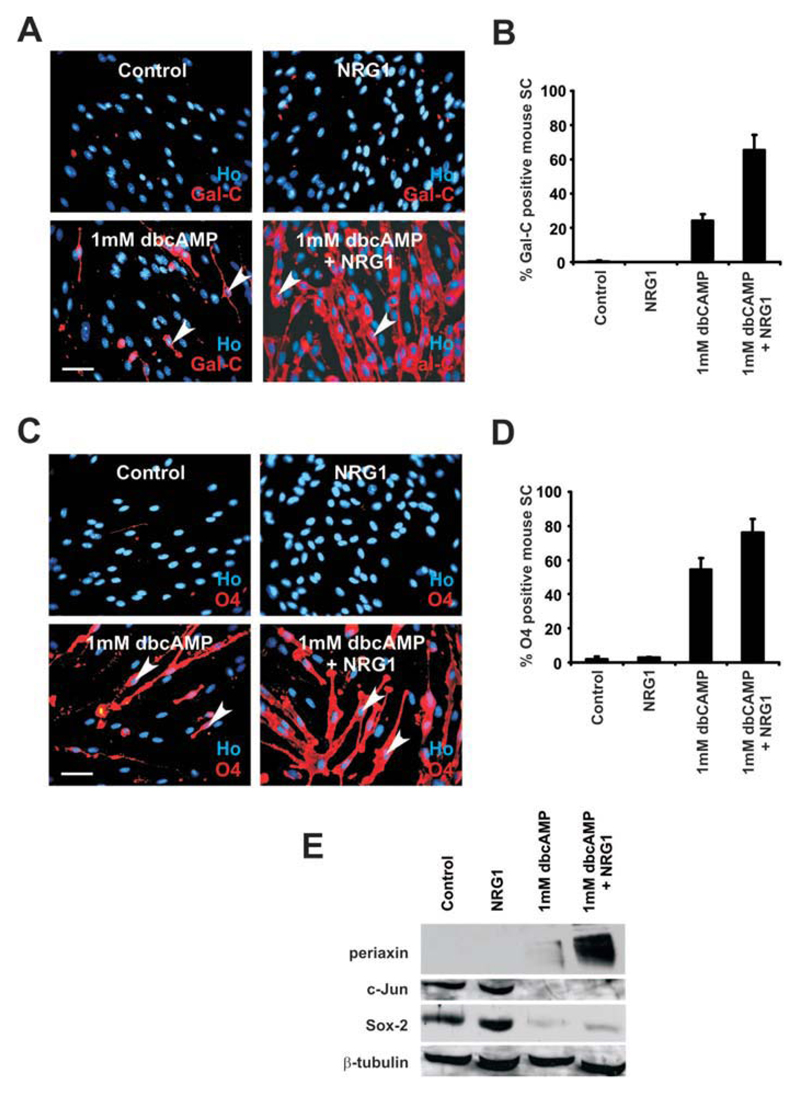
In rat Schwann cells, dbcAMP induces expression of early differentiation markers such as galactocerebroside (Gal-C), O4, and periaxin, which appear in vivo at the onset or just before myelination (Jessen and Mirsky, 2005; Jessen et al., 1985; Mirsky et al. 1990; Sobue et al., 1984, 1986). To further elucidate the role of dbcAMP and NRG1 in mouse Schwann-cell differentiation, we tested whether 72 h of treatment using the conditions used above would induce these markers. In dbcAMP alone, Gal-C is induced in 24.2% ± 3.7% of cells (n = 5; P < 0.001; Fig. 3A,B) and O4 is induced in 54.5% ± 6.6% of cells (n = 5; P < 0.0001; Fig. 3C,D). When cultured in combined dbcAMP/NRG1, the percentage of Schwann cells that express Gal-C and O4 increased to 65.5% ± 8.7% (n = 5; P < 0.001; Fig. 3A,B) and 76.2% ± 7.9% (n = 5; P < 0.01; Fig. 5C,D), respectively. Furthermore, western blotting demonstrated that high levels of periaxin protein were only induced by combined dbcAMP/NRG1 (Fig. 3E).
Fig. 3.
NRG1 increases dbcAMP induced galactocerebroside (Gal-C), O4, and periaxin expression in mouse Schwann cells. A: Gal-C expression visualized immunocytochemically in mouse Schwann cells cultured for 72 h with NRG1 alone, dbcAMP alone, combined dbcAMP/NRG1 or DM (Control). In general, Schwann cells treated with dbcAMP/NRG1 express more Gal-C than Schwann cells treated with dbcAMP alone, whereas cells cultured either in NRG1 alone or in DM (Control) do not express Gal-C. Arrowheads point to Gal-C positive Schwann cells in both dbcAMP alone and in dbcAMP/NRG1. Cells are co-labeled with Hoechst dye (Ho). Scale bar, 50 μm. B: Quantification of experiments illustrated in (A). Error bars: standard deviation of the mean. C: 04 expression shown immunocytochemically in mouse Schwann cells cultured for 72 h with NRG1 alone, dbcAMP alone, combined dbcAMP/NRG1 or DM (Control). In general, Schwann cells treated with dbcAMP/NRG1 combined express more 04 than Schwann cells treated with dbcAMP alone. Arrowheads point to 04-positive Schwann cells cultured in dbcAMP alone and in combined dbcAMP/NRG1. Cells are co-labeled with Hoechst dye (Ho). Scale bar, 50 μm. D: Quantification of the experiments illustrated in (C). Cells cultured in DM alone (Control) and cells treated with NRG1 alone do not express 04, while treatment with dbcAMP alone induces 04 in an intermediate proportion of cells compared with cells treated with dbcAMP/NRG1 combined. Error bars: standard deviation of the mean. E: Western blot of mouse Schwann cells cultured in NRG1 alone, dbcAMP alone, dbcAMP/NRG1 combined or in DM alone (Control) for 72 h. Blots were probed with antibodies to periaxin, c-Jun, and Sox-2. β-Tubulin was used as a loading control. Note that high levels of periaxin are induced in cells treated with combined dbcAMP/NRG1. Both c-Jun and Sox-2 levels are strongly reduced in cells treated with dbcAMP alone and in cells treated with combined dbcAMP/NRG1. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
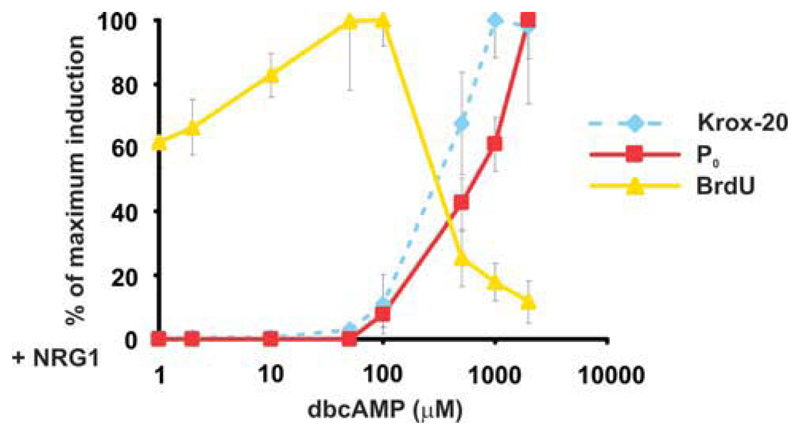
Fig. 5.
NRG1-induced appearance of nondividing, myelin cells is tightly correlated with reduction in proliferating, immature cells as the dbcAMP concentration increases. Purified mouse Schwann cells were exposed to NRG1 (20 ng/mL) at the dbcAMP concentrations indicated. For proliferation and Krox-20 immunolabeling, cells were exposed for 72 h. For the proliferation assay, BrdU was included for the last 7 h. For P0, cells were exposed for 120 h before immunolabeling. BrdU incorporation and expression of Krox-20 and P0 are expressed as a percentage of their own maximum, which was 52% ± 4.2% for BrdU, 49.3% ± 5.8% for Krox-20, and 22% ± 5.8% for Po. DbcAMP concentration (x-axis) is plotted on a log scale. Error bars: standard deviation of the mean. Statistical analysis was initially performed using one-way ANOVA with Tukey’s multiple comparison test. Note that optimal concentrations of dbcAMP for enhancement of NRG1-induced proliferation were an order of magnitude lower than optimal concentrations for Krox-20 induction. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
We also tested whether dbcAMP was sufficient to downregulate two inhibitors of Schwann-cell myelin differentiation, c-Jun and Sox-2 (Le et al., 2005; Parkinson et al., 2008). Western blotting demonstrated that in mouse Schwann cells treated with dbcAMP(1 mM) for 72 h, expression of both c-Jun and Sox-2 proteins is strongly inhibited (Fig. 3E).
In summary, although dbcAMP alone induced some expression of Gal-C, O4, and periaxin in mouse Schwann cells, addition of NRG1 greatly potentiated this effect. These observations provide further evidence for the role of NRG1 as a positive driver of differentiation in mouse Schwann cells. Notably, however, dbcAMP alone was sufficient to completely downregulate the inhibitors of myelin differentiation, c-Jun and Sox-2 (Le et al., 2005; Parkinson et al., 2008). Thus, dbcAMP can induce partial differentiation of mouse Schwann cells but requires NRG1 for further differentiation.
NRG1 Amplifies DbcAMP-induced Myelin Differentiation in Rat Schwann Cells
Although NRG1 promotes myelin differentiation in mouse Schwann cells in vivo (Nave and Salzer, 2006), to date, experiments with cultured Schwann cells from rats show that NRG1 has either neutral or inhibitory effects on cAMP-induced differentiation (Cheng and Mudge, 1996; Monje et al., 2009; Ogata et al., 2004). In light of our findings with mouse Schwann cells, we revisited this issue using cultured rat cells. To test whether the addition of NRG1 signaling would influence myelin gene expression in response to dbcAMP in rat Schwann cells, serum-purified cells were cultured in NRG1 (20 ng/mL) alone, dbcAMP (1 mM) alone, combined dbcAMP (1 mM)/NRG1 (20 ng/mL), or DM for 72 h. Cells were then fixed and immunolabeled for expression of Krox-20 and P0. We found that while 25.7% ± 11.1% of rat Schwann cells expressed Krox-20 and 33.1% ± 3.1% expressed P0 after treatment with 1 mM dbcAMP, this increased to 52% ± 7.7% and 68% ± 2.3%, respectively, in response to treatment with combined dbcAMP/NRG1 (n = 4; P < 0.001; Fig. 4A–D).
Fig. 4.
In rat Schwann cells also, NRG1 increases dbcAMP-induced Krox-20 and P0 expression. A: Krox-20 expression shown immunocytochemically in rat Schwann cells cultured for 72 h with NRG1 alone, dbcAMP alone, in combined dbcAMP/NRG1 or in DM (Control). In contrast to mouse cells, Krox-20 is induced both in cultures treated with dbcAMP alone and in cultures treated with combined dbcAMP/NRG1. A larger number of cells is induced by combined dbcAMP/NRG1. Note that the cells cultured in DM (Control) and cells treated with NRG1 alone do not express Krox-20. Cells are co-labeled with Hoechst dye (Ho). Scale bar, 50 μm. B: Quantification of the experiments shown in (A). A proportion of rat Schwann cells express Krox-20 after treatment with dbcAMP alone, whereas combined dbcAMP/NRG1 treatment increases the proportion of induced cells. Error bars: standard deviation of the mean. C: P0 protein expression shown immunocytochemically in rat Schwann cells cultured for 72 h with NRG1 alone, dbcAMP alone, combined dbcAMP/NRG1, or in DM (Control). In contrast to mouse cells, P0 is induced both in cultures treated with dbcAMP alone and in cultures treated with combined dbcAMP/NRG1. Cells are co-labeled with Hoechst dye (Ho). Scale bar, 50 μm. D: Quantification of the experiments shown in (C). In contrast to mouse cells, P0 is induced both in cultures treated with dbcAMP alone and in cultures treated with combined dbcAMP/NRG1. A greater proportion of P0 positive cells are, however, induced in cells treated with dbcAMP/NRG1. Cells are co-labeled with Hoechst dye (Ho). Scale bar, 50 μm. Error bars: standard deviation of the mean. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
These experiments, first, confirm that the activation of cAMP pathways is sufficient to induce myelin differentiation in rat Schwann cells, in agreement with previous work, although this is not the case in mouse cells. Second, they show that even in rat cells, it is clearly possible to reveal NRG1 as a signal that promotes myelin differentiation, although this has not been detected in previous studies using other experimental conditions.
The DbcAMP Concentration Determines Whether NRG1 Drives Proliferation or Differentiation
NRG1 is a Schwann-cell mitogen. But it also promotes myelination in vivo and can act as an essential myelin-differentiation signal in purified Schwann cells, as we show above. This suggests that the biological outcome of NRG1 signaling can be switched between promoting these two incompatible fates. We have found that this can be achieved by varying the strength of cyclic AMP activation.
To show this, we applied a constant dose of NRG1 (20 ng/mL) to cells exposed to different levels of cAMP activation (1μM-2 mM dbcAMP; see Fig. 5). We found that at zero (not shown) or low-dbcAMP concentrations, NRG1 maintained Schwann cells in a state of rapid proliferation that was maximal at 100 μM dbcAMP. Application of NRG1 at higher dbcAMP concentrations induced the appearance of an alternative cell population in increasing numbers, namely mitotically quiescent cells that expressed the myelin-related proteins P0 or Krox-20. The two cell populations, those synthesizing DNA on the one hand, and those expressing P0 or Krox-20 protein on the other, were strictly nonoverlapping (not shown), in line with previous work (Monje et al., 2009; Morgan et al., 1991). Figure 5 demonstrates that the increase in NRG1-induced myelin differentiation in the cultures was tightly correlated with a decrease in DNA synthesis as the dbcAMP concentration rose. This eventually brought the rate of proliferation well below that seen when NRG1 was applied alone (or in 1 μM dbcAMP).
These results show that cAMP pathways control the outcome of NRG1 signaling. When cAMP activation is weak, NRG1 drives Schwann-cell proliferation at the same time as the cells maintain the immature phenotype. When the cAMP activation is strong, NRG1 triggers the appearance of an increasing number of nondividing, myelin cells at the expense of the proliferating, immature population, which is correspondingly reduced.
Activation of PKA Is Sufficient to Replicate the Effects of DbcAMP on Mouse Schwann Cells
Protein kinase A (PKA) isoforms and guanine exchange proteins directly activated by cAMP (epacs) are downstream targets of cAMP (Bos, 2003). PKA activation is required for both rat Schwann-cell proliferation and differentiation in vitro (Howe and McCarthy, 2000; Monje et al., 2008; Yoon et al., 2008). On the other hand, epac activation does not influence rat Schwann-cell proliferation, although its role in differentiation is unknown (Monje et al., 2008).
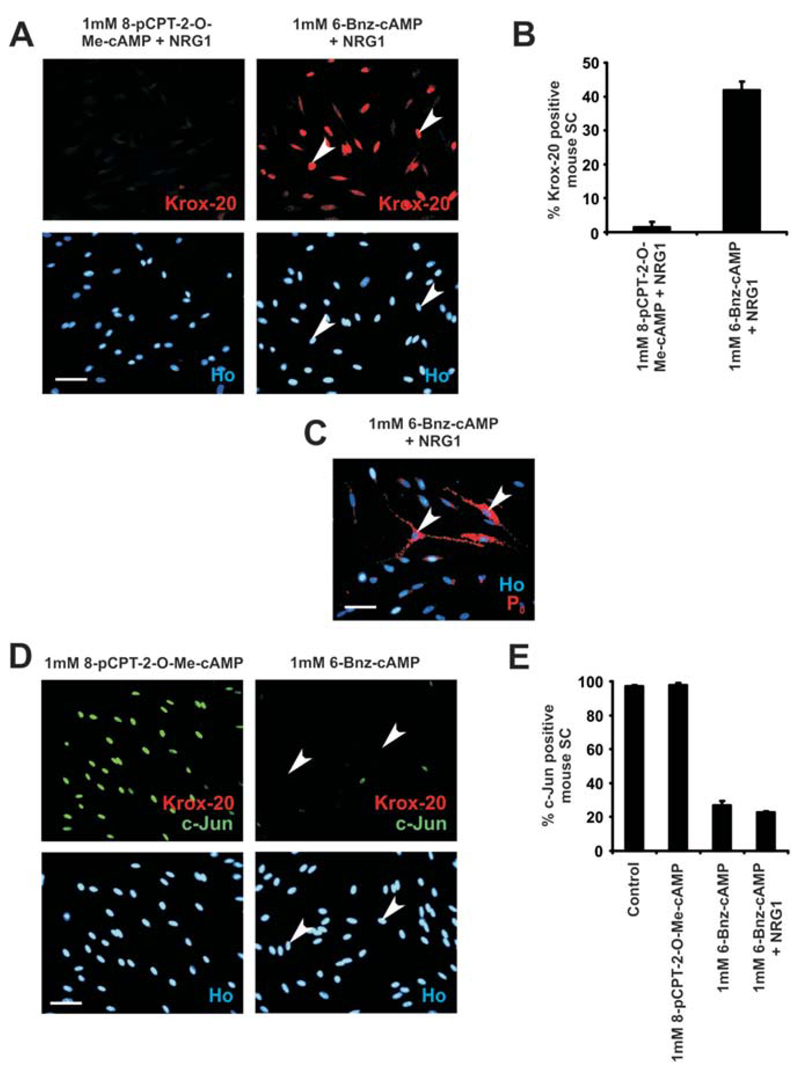
To determine whether either of these targets play a role in mouse Schwann cells, we used the cAMP analogue 6-Bnz-cAMP, which activates only PKA or the analogue 8-pCPT-2-O-Me-cAMP, which selectively activates the exchange factors, epac1 and 2 (Bos, 2003). Schwann cells were treated with each analogue separately, either on its own or in the presence of NRG1. After 72 h, cells were fixed and immunolabeled for Krox-20, P0, and c-Jun. In the presence of 6-Bnz-cAMP (1 mM) and NRG1 (20 ng/mL), 41.6% ± 2.8% of Schwann cells expressed Krox-20 (n = 4; P < 0.0001; Fig. 6A,B) and 8.2% ± 0.3% of Schwann cells expressed high levels of P0 (n = 4; P < 0.0001; Fig. 6C). In contrast, the combination of 8-pCPT-2-O-Me-cAMP (1 mM) and NRG1 failed to induce significant levels of either Krox-20 or P0 protein (Fig. 6A,B and data not shown). As with dbcAMP, neither 8-pCPT-2-O-Me-cAMP nor 6-Bnz-cAMP alone induced Krox-20 expression (Fig. 6D).
Fig. 6.
The PKA activator, 6-Bnz-cAMP, mimics the effects of dbcAMP on cultured mouse Schwann cells, while the epac activator 8-pCPT-2-O-Me-cAMP is ineffective. A: Krox-20 expression shown immunocytochemically in mouse Schwann cells cultured with 8-pCPT-2-O-Me-cAMP (1 mM) (to activate epac) combined with NRG1 (20 ng/mL) or with 6-Bnz-cAMP (1 mM) (to activate PKA) combined with NRG1 (20 ng/mL) for 72 h. High levels of Krox-20 are only induced in cultures treated with 6-Bnz-cAMP and NRG1. Arrowheads demonstrate Krox-20-positive Schwann cells. Cells are co-labeled with Hoechst dye (Ho). Scale bar, 50 μm. B: Quantification of the experiments shown in (A). High levels of Krox-20 are only induced in cells cultured in combined 6-Bnz-cAMP/NRG1. Error bars: standard deviation of the mean. C: P0 protein expression shown immunocytochemically in mouse Schwann cells cultured for 72 h with 6-Bnz-cAMP/NRG1. Arrowheads point to P0-positive Schwann cells. Cells are co-labeled with Hoechst dye (Ho). Scale bar, 50 μm. D: Mouse Schwann cells double-immunolabeled with antibodies to c-Jun and Krox-20 after treatment with 8-pCPT-2-O-Me-cAMP or 6-Bnz-cAMP for 72 h. Activation of PKA with 6-Bnz-cAMP alone without NRG1 is sufficient to suppress c-Jun protein levels, whereas cells continue to express c-Jun when they are cultured with 8-pCPT-2-O-Me-cAMP. Krox-20 is not induced by either compound. Arrowheads show c-Jun-negative Schwann cells after treatment with 6-Bnz-cAMP. Cells are co-labeled with Hoechst dye (Ho). Scale bar, 50 μm. E: Quantification of the proportion of mouse Schwann cells expressing c-Jun when cultured with 1 mM 8-pCPT-2-O-Me-cAMP or 6-Bnz-cAMP alone, with combined 6-Bnz-cAMP/NRG1 or in DM (Control) for 72 h. Cells cultured either with 6-Bnz-cAMP alone or with combined 6-Bnz-cAMP/NRG1 show greatly reduced levels of c-Jun. Error bars: standard deviation of the mean. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In line with this, after treatment of Schwann cells for 72 h with 6-Bnz-cAMP only 26.7% ± 2.7% of Schwann cells expressed c-Jun, compared with 97% ± 0.7% of control Schwann cells (n = 3; P < 0.05), while 8-pCPT-2-O-Me-cAMP failed to reduce c-Jun expression. The percentage of c-Jun positive Schwann cells also remained significantly reduced when 6-Bnz-cAMP and NRG1 were used in combination (n = 3; P < 0.0001; Fig. 6D,E).
Together, these results demonstrate that activation of PKA is sufficient to downregulate c-Jun expression in mouse Schwann cells and in combination with NRG1 signaling, PKA activation induces both Krox-20 and P0. These results also suggest that the alternative cyclic AMP pathway using epac 1 and 2 is unlikely to play a role in these events.
The CREB Family of Transcription Factors Are Required for cAMP/NRG1-induced Schwann Cell Differentiation
We have shown that activation of PKA replicates the effects of dbcAMP on mouse Schwann-cell myelin differentiation (see Fig. 6). We therefore investigated the effects of some downstream targets of PKA on differentiation. The CREB family of transcription factors is the major target of PKA, which phosphorylates and activates them (Tasken and Aandahl, 2004). Main members of this family include CREB1, CREM, and ATF1, which bind as dimers to cAMP-responsive elements (CREs) in target genes (Mayr and Montminy, 2001). CREB1 has previously been identified in rat Schwann cells and their precursors in vivo and in vitro and is phosphorylated at Ser-133 in response to stimuli such as NRG1, axonal membranes, and ATP treatment (Lee et al., 1999; Stevens and Fields, 2000; Stewart, 1995; Tabernero et al., 1998).
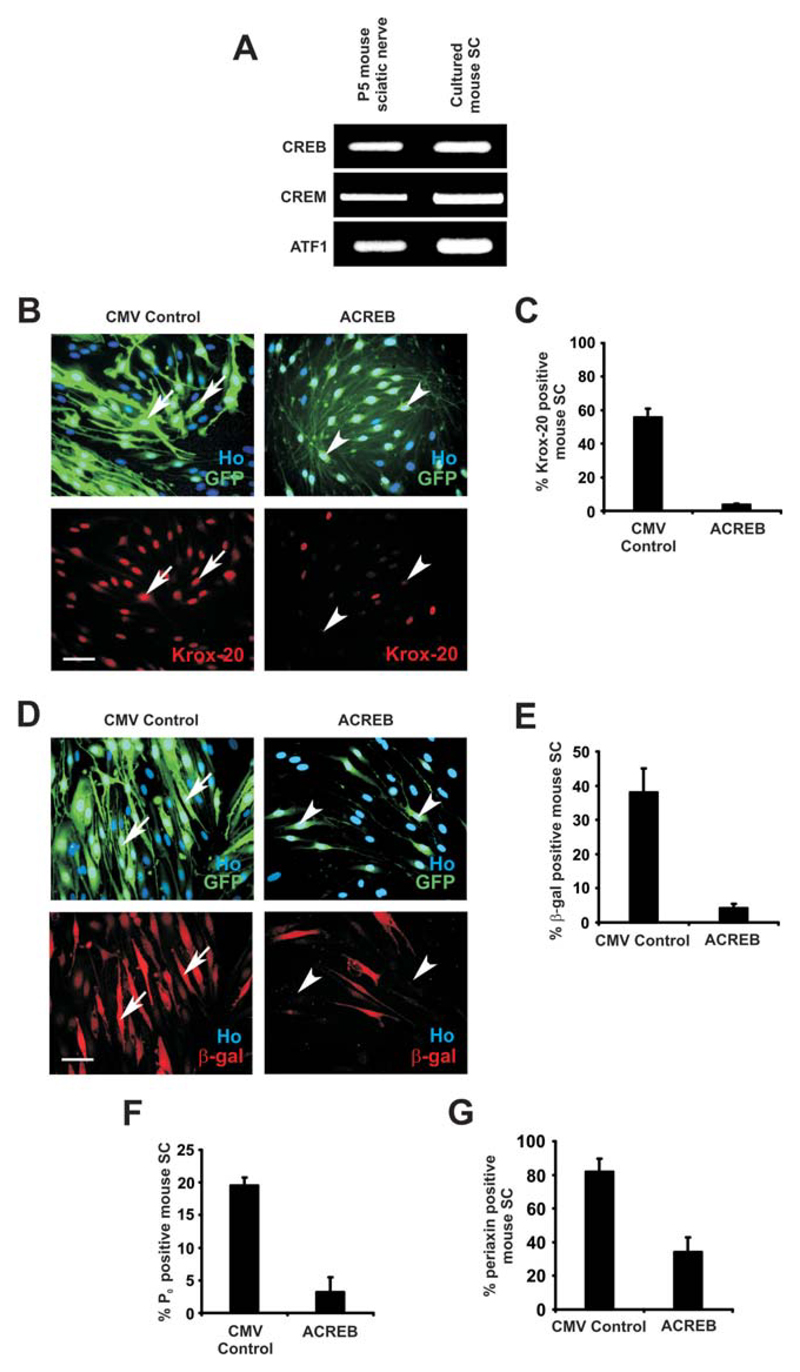
We used RT-PCR to examine whether other CREB family members were expressed by Schwann cells and found that that CREB1, CREM, and ATF1 are all present in extracts from P5 mouse sciatic nerves or cultured mouse Schwann cells (Fig. 7A).
Fig. 7.
The CREB family of transcription factors is required for dbcAMP/NRG1 induced mouse Schwann cell myelin differentiation. A: RT-PCR for mRNA expression of CREB1, CREM, and ATF-1 in P5 mouse sciatic nerve and cultured primary mouse Schwann cells. mRNA for all three genes is expressed both in the nerve and in cultured cells. B–G: Cells were treated with either A-CREB or CMV adenoviruses in DM containing forskolin (2 μM) for 48 h and then dbcAMP(1 mM)/NRG1(20 ng/mL) for a further 48 h before fixation and immunolabeling. B: Induction of Krox-20 protein by dbcAMP/NRG1 is severely decreased after adenoviral infection with A-CREB virus compared with the CMV-control. Krox-20 immunolabeling of mouse Schwann cells after infection with CMV-control and A-CREB adenoviruses. Arrows show CMV-control infected Schwann cells that are positive for GFP and Krox-20. Arrowheads demonstrate A-CREB-infected Schwann cells that are GFP positive but Krox-20 negative. Cells are co-labeled with Hoechst (Ho). Scale bar, 50 μm. C: Quantification of the experiments above. Error bars: standard deviation of the mean. D: β-Gal expression (indicative of Krox-20 gene expression) induced by dbcAMP/NRG1 is severely reduced following infection with A-CREB virus compared with cells treated with CMV control virus. Primary mouse Krox-20/lacZ+/− Schwann cells immunolabeled for β-Gal were treated with 2 μM forskolin for 48 h and subsequently exposed to combined dbcAMP/NRG1 for 48 h. Arrows point to CMV-control infected Schwann cells that are positive for GFP and β-Gal. Arrowheads demonstrate A-CREB-infected Schwann cells that are GFP-positive but β-Gal-negative. Cells are co-labeled with Hoechst (Ho). Scale bar, 50 μm. E: Quantification of the experiments above. Error bars: standard deviation of the mean. F: Induction of P0 by dbcAMP/NRG1 is severely decreased after A-CREB adenoviral infection compared with CMV-control. Error bars: standard deviation of the mean. G: Induction of periaxin by dbcAMP/NRG1 is decreased after A CREB adenoviral infection compared with CMV-control. Error bars: standard deviation of the mean. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To test whether the CREB family is required for cAMP/NRG1-induced Schwann-cell differentiation, we used an adenovirus co-expressing a dominant negative form of CREB with GFP (A-CREB) and a control virus expressing only GFP (CMV control; Warburton et al., 2005). Purified mouse Schwann cells were plated and infected with either A-CREB or control viruses in DM, 0.5% HS, and 2 mM forskolin for 48 h, including replacement with the same medium at 24 h. Schwann cells were then treated with combined dbcAMP (1 mM) and NRG1 (20 ng/mL) in DM for a further 48 h before cells were fixed and labeled by immunocytochemistry. Krox-20 expression was strongly inhibited in the presence of A-CREB. Only 3.6% ± 0.6% cells of A-CREB-infected cells were Krox-20 positive compared with 55.7% ± 5.3% Krox-20 positive cells in CMV control infected cells (n = 4; P < 0.001; Fig. 7B,C). Similarly, the A-CREB adenovirus strongly inhibited β-gal expression in Krox-20+/− Schwann cells induced by dbcAMP/NRG1. Only 4.1% ± 1.4% of A-CREB-infected cells were β-gal-positive compared with 38% ± 7.1% of control cells (n = 4; P < 0.01; Fig. 7D,E), a 9.3-fold drop in expression. Expression of A-CREB also significantly inhibited dbAMP/NRG1-induced P0 expression, because only 3.2% ± 2.3% of A-CREB-infected cells were P0 positive, while 19.5% ± 1.3% of control-infected cells were P0 positive (n = 3; P < 0.05; Fig. 7F). In addition, A-CREB strongly reduced periaxin induction by dbAMP/NRG1. Only 34.1% ± 8.8% of A-CREB-infected cells were periaxin-positive compared with 81.9% ± 7.6% positive cells infected with control virus (n = 4; P < 0.001; Fig. 7G).
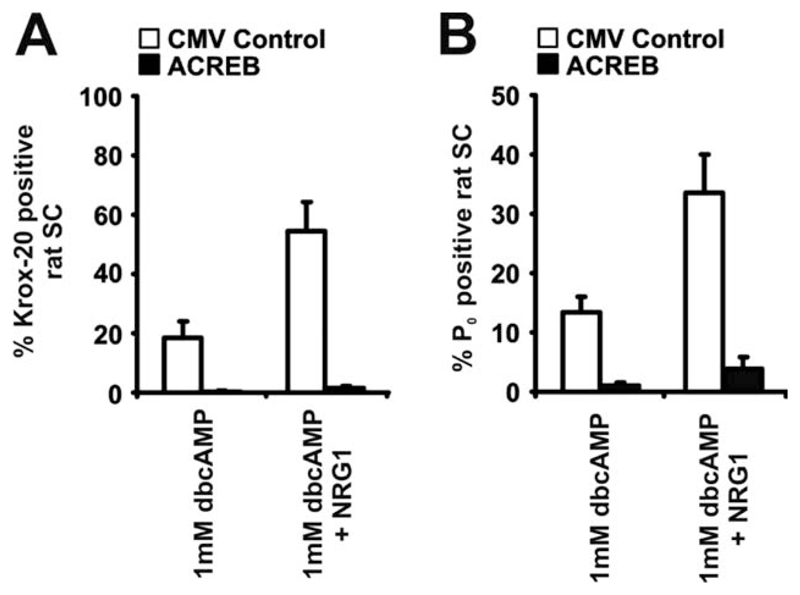
To confirm the role of CREB in Schwann-cell differentiation, we tested whether expression of A-CREB could inhibit myelin differentiation in cultured rat Schwann cells. Purified P3 Schwann cells were infected with A-CREB or control viruses for 24 h, followed by incubation for a further 48 h in DM plus dbcAMP (1 mM) or in dbcAMP (1 mM) and NRG1 (20 ng/mL). Cells were then fixed and immunolabeled with antibodies to Krox-20. About 18.6% ± 5.5% and 54.6% ± 9.6% of control infected cells expressed Krox-20 when treated with dbcAMP or dbcAMP/NRG1 respectively, in the presence of A-CREB, whereas Krox-20 was barely induced (n = 3; P < 0.001; Fig. 8A). In parallel experiments, cells infected with either A-CREB or control viruses were treated under the same experimental conditions before fixation and immunolabeling for P0. This revealed that 13.4% ± 2.6% and 33.5% ± 6.4% of control cells treated with either dbcAMP alone or dbcAMP/NRG1, respectively, expressed P0, in comparison with cells infected with A-CREB that failed to upregulate significant P0 protein (n = 3; P < 0.001; Fig. 8B).
Fig. 8.
In rat Schwann cells, the CREB family of transcription factors is required for dbcAMP and dbcAMP/NRG1-induced myelin differentiation. A, B: The proportion of (A) Krox-20-positive and (B) P0-positive cells is severely decreased in rat Schwann cells after A-CREB adenoviral infection compared with the CMV-control. Cells were induced for 72 h in dbcAMP (1 mM) or in combined dbcAMP(1 mM)/NRG1(20 ng/mL). Error bars: standard deviation of the mean. Error bars: standard deviation of the mean.
In conclusion, these experiments demonstrate that the CREB family of transcription factors is required for dbcAMP/NRG1-induced myelin differentiation in both cultured mouse and rat Schwann cells.
Discussion
Although NRG1 clearly promotes myelination by Schwann cells in contact with axons in vivo or in myelinating co-cultures, it has proved difficult to demonstrate that NRG1 promotes myelin differentiation when presented to purified cells in vitro, when the typical outcome is proliferation. This suggests the existence of a mechanism that modulates NRG1-activated cascades and has prompted a search for pathways that can determine the outcome of NRG1 signaling in Schwann cells (Nave and Salzer, 2006). Here, we show that in purified cultures of Schwann cells from mouse nerves, the promyelin effects of NRG1 can be revealed unambiguously and identify the cAMP pathway as a critical modulator of NRG1 signaling. Additionally, the promyelin effects of NRG1 can be seen in rat Schwann-cell cultures under appropriate experimental conditions. We also demonstrate a requirement for the CREB family of transcription factors in myelin differentiation in cultured rodent Schwann cells.
In vivo and in neuron-Schwann-cell co-cultures, NRG1 type III promotes Schwann-cell ensheathment of axons and myelin thickness. It is likely that the first of these functions, the control of appropriate axon-Schwann-cell relationships including radial sorting, requires juxtacrine signaling by NRG1 type III present on the axonal surface. On the other hand, the myelination process itself and myelin thickness can also be promoted by soluble forms of NRG1, whether type III or II (Michailov et al., 2004; Syed et al., 2010; Taveggia et al., 2005). Despite this, neither membrane attached nor soluble NRG1 type III or II induce expression of myelin proteins in purified Schwann-cell cultures from rats or mice. Instead, NRG1 induces proliferation (Chen et al., 2006; Taveggia et al., 2005).
We find that in mouse Schwann cells, the choice between these contradictory effects of NRG1, proliferation, and myelin differentiation is controlled by the strength of cAMP signaling. The combination of low dbcAMP and NRG1 drives proliferation in cells with the immature (premyelin) phenotype, while in high dbcAMP, the mitogenic effects of NRG1 are suppressed and NRG1 instead activates myelin genes.
Using lower concentrations of NRG1 did not allow use of lower cAMP levels to induce myelin genes. Rather, myelin protein expression was always promoted when NRG1 was increased. This was true at both low and high-cAMP activation levels (4 and 10 μM forskolin; Supp. Info. Fig. 1).
cAMP pathways have often been implicated in Schwann-cell myelination on the basis of their ability to induce myelin protein expression in rat Schwann-cell cultures. This is not the case in mouse cells where the presence of NRG1 in addition to high levels of dbcAMP is obligatory for expression of the key myelin-related proteins P0 and Krox-20. These findings demonstrate an unexpected difference between Schwann cells from rats and mice and help resolve conflicting literature, which, in different experimental settings, variously reveals NRG1 as a key Schwann-cell mitogen, an essential myelination signal or a signal that is neutral with respect to myelin differentiation (Cheng and Mudge, 1996; Monje et al., 2009; Morgan et al., 1994; Morrissey et al., 1995; Nave and Salzer, 2006).
Although Notch signaling and TGFβ signaling through the TGFβ type II receptor have been directly shown to drive Schwann-cell proliferation in developing nerves in vivo (D’Antonio et al., 2006; Woodhoo et al., 2009), there is good evidence that NRG1 is also involved in this process as an axon-associated Schwann-cell mitogen (Nave and Salzer, 2006). This probable in vivo effect of NRG1 is readily modeled in purified Schwann cells and is amplified by low-level activation of cAMP pathways, as we have shown here. The notion that NRG1 is a mitogen in vivo means that the effect of NRG1 on Schwann cells must change around birth, because at that time, Schwann cells fall out of division and start myelination, which is also promoted by NRG1. The present finding that this promyelin effect of NRG1 can be modeled in vitro, by strong co-activation of cAMP pathways, suggests a way in which cAMP pathways might act in vivo. This involves activation of cAMP pathways in Schwann cells of embryonic nerves, presumably by axon-associated signals that gradually increase in intensity into the myelination period. In the period before myelination, low-cAMP levels should amplify the mitogenic effects of NRG1 (and perhaps other mitogens), but later, increasing cAMP signaling would inactivate the mitogenic effect of NRG1 and switch NRG1 to promoting myelination.
In agreement with others, we find that the effects of cAMP are mediated by cAMP activation of PKA (Howe and McCarthy, 2000; Kim et al., 1997; Yoon et al., 2008). It remains to be explained how low-varying levels of cAMP generate different biological outcomes through this pathway. It will also be important to determine whether different cAMP levels, through these or other mechanisms, alter NFκB signaling or the relative strength of PI3 kinase or ERK activation, because this balance is likely to help determine the myelination status of Schwann cells (Limpert and Carter, 2010; Ogata et al., 2004; Syed et al., 2010).
Our finding that NRG1 can boost cAMP-induced differentiation in cultured rat Schwann cells was surprising in view of previously published observations, which reported either negative or neutral effects of NRG1 on myelin-related differentiation induced by cAMP elevation (Cheng and Mudge, 1996; Monje et al., 2009; Ogata et al., 2004). However, there are key differences between their experiments and ours. Ogata et al. (2004) stimulated rat Schwann cells for 24 h with NRG1 and 3 μM forskolin and assessed myelin gene RNA. They concluded that NRG1 inhibited mRNA induction of myelin-associated glycoprotein (MAG), P0, and MBP. However, 3 μM forskolin causes relatively weak activation of cAMP pathways (judged by the lower levels of myelin proteins induced by 3 μM forskolin than by 20 μM forskolin or 1 mM cAMP analogues in rat Schwann cells [Morgan et al., 1991, unpublished observations)]. The observed reduction in myelin gene mRNA in these experiments is therefore likely to be due to the fact that Schwann cells are proliferating at this low level of cAMP activation (see present results) and will not express significant levels of myelin genes under these conditions (Morgan et al., 1991). Monje et al. (2009) treated rat Schwann cells with a high concentration of dbcAMP (1 mM) in combination with NRG1 (20 ng/mL) for 72 h. They concluded that NRG1 neither reduced nor increased myelin protein induction by dbcAMP demonstrated by counts of MAG positive Schwann cells. It is likely to be significant in this context that MAG appears very early in myelination. When we examined another early marker, periaxin, we failed to find a significant effect of NRG1 on the amount of periaxin protein induced by dbcAMP (1 mM) in rat Schwann cells, in line with the observations of Monje et al. (2009) on MAG expression (Supp. Info. Fig. 2). This suggests that NRG1 boosts in particular expression of proteins that are involved in myelin sheath formation, rather than controlling early indicators of myelin differentiation.
Our findings suggest that the CREB family of transcription factors has a role in Schwann-cell differentiation, because the dominant-negative A-CREB construct blocked the induction of Krox-20 and P0 in both rat and mouse Schwann cells. CREB is phosphorylated at Ser-133 in cultured rat Schwann cells after prolonged contact with neurite membranes (Lee et al., 1999), suggesting that CREB phosphorylation is regulated by axonal contact in vivo. CREB is also phosphorylated at Ser-133 in response to NRG1 treatment in rat Schwann cells, while addition of 2 mM forskolin and NRG1 induces a more sustained phosphorylation of CREB (Rahmatullah et al., 1998; Tabernero et al., 1998). It has also been postulated that CREB activation has a role in Schwann-cell proliferation (Rahmatullah et al., 1998), although we found that A-CREB expression does not block NRG-1 or low cAMP/NRG1-induced rat Schwann-cell proliferation (Wanek and Arthur-Farraj, unpublished). A CREB null mutation causes lethality in mice late in embryogenesis (Rudolph et al., 1998), thus conditional inactivation of CREB and potentially other family members, such as CREM and ATF-1, would be a useful future approach to determine the role of the CREB family in Schwann cells.
Supplementary Material
Additional Supporting Information may be found in the online version of this article.
Acknowledgments
We thank Peter Brophy, Diane Sherman, and James Uney for gifts of reagents and Patrick Charnay for transgenic mice.
Grant sponsors: Wellcome Trust Programme Grant, MRC PhD Scholarship.
References
- Archelos JJ, Roggenbuck K, Schneider-Schaulies J, Linington C, Toyka KV, Hartung HP. Production and characterization of monoclonal antibodies to the extracellular domain of P0. J Neurosci Res. 1993;35:46–53. doi: 10.1002/jnr.490350107. [DOI] [PubMed] [Google Scholar]
- Bos JL. Epac: A new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Chen S, Velardez MO, Warot X, Yu ZX, Miller SJ, Cros D, Corfas G. Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function. J Neurosci. 2006;26:3079–3086. doi: 10.1523/JNEUROSCI.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Mudge AW. Cultured Schwann cells constitutively express the myelin protein P0. Neuron. 1996;16:309–319. doi: 10.1016/s0896-6273(00)80049-5. [DOI] [PubMed] [Google Scholar]
- Crawford AT, Desai D, Gokina P, Basak S, Kim HA. E-cadherin expression in postnatal Schwann cells is regulated by the cAMP-dependent protein kinase A pathway. Glia. 2008;56:1637–1647. doi: 10.1002/glia.20716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antonio M, Droggiti A, Feltri ML, Roes J, Wrabetz L, Mirsky R, Jessen KR. TGFβ type II receptor signaling controls Schwann cell death and proliferation in developing nerves. J Neurosci. 2006;26:8417–8427. doi: 10.1523/JNEUROSCI.1578-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Sinanan A, Parkinson D, Parmantier E, Mirsky R, Jessen KR. Schwann cell development in embryonic mouse nerves. J Neurosci Res. 1999;56:334–348. doi: 10.1002/(SICI)1097-4547(19990515)56:4<334::AID-JNR2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Gillespie CS, Sherman DL, Blair GE, Brophy PJ. Periaxin, a novel protein of myelinating Schwann cells with a possible role in axonal ensheathment. Neuron. 1994;12:497. doi: 10.1016/0896-6273(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Howe DG, McCarthy KD. Retroviral inhibition of cAMP-dependent protein kinase inhibits myelination but not Schwann cell mitosis stimulated by interaction with neurons. J Neurosci. 2000;20:3513–3521. doi: 10.1523/JNEUROSCI.20-10-03513.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Brennan A, Morgan L, Mirsky R, Kent A, Hashimoto Y, Gavrilovic J. The Schwann cell precursor and its fate: A study of cell death and differentiation during gliogenesis in rat embryonic nerves. Neuron. 1994;12:509–527. doi: 10.1016/0896-6273(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R, Morgan L. Myelinated, but not unmyelinated axons, reversibly down-regulate N-CAM in Schwann cells. J Neurocytol. 1987;16:681–688. doi: 10.1007/BF01637659. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Morgan L, Brammer M, Mirsky R. Galactocerebroside is expressed by non-myelin-forming Schwann cells in situ. J Cell Biol. 1985;101:1135–1143. doi: 10.1083/jcb.101.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HA, DeClue JE, Ratner N. cAMP-dependent protein kinase A is required for Schwann cell growth: Interactions between the cAMP and neuregulin/tyrosine kinase pathways. J Neurosci Res. 1997;49:236–247. [PubMed] [Google Scholar]
- Le N, Nagarajan R, Wang JY, Araki T, Schmidt RE, Milbrandt J. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc Natl Acad Sci USA. 2005;102:2596–2601. doi: 10.1073/pnas.0407836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc SE, Jang SW, Ward RM, Wrabetz L, Svaren J. Direct regulation of myelin protein zero expression by the Egr2 transactivator. J Biol Chem. 2006;281:5453–5460. doi: 10.1074/jbc.M512159200. [DOI] [PubMed] [Google Scholar]
- Lee M, Brennan A, Blanchard A, Zoidl G, Dong Z, Tabernero A, Zoidl C, Dent MA, Jessen KR, Mirsky R. P0 is constitutively expressed in the rat neural crest and embryonic nerves and is negatively and positively regulated by axons to generate non-myelinforming and myelin-forming Schwann cells, respectively. Mol Cell Neurosci. 1997;8:336–350. doi: 10.1006/mcne.1996.0589. [DOI] [PubMed] [Google Scholar]
- Lee MM, Badache A, DeVries GH. Phosphorylation of CREB in axon-induced Schwann cell proliferation. J Neurosci Res. 1999;55:702–712. doi: 10.1002/(SICI)1097-4547(19990315)55:6<702::AID-JNR5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Lemke G, Chao M. Axons regulate Schwann cell expression of the major myelin and NGF receptor genes. Development. 1988;102:499–504. doi: 10.1242/dev.102.3.499. [DOI] [PubMed] [Google Scholar]
- Limpert AS, Carter BD. Axonal neuregulin 1 type III activates NF-κB in Schwann cells during myelin formation. J Biol Chem. 2010;285:16614–16622. doi: 10.1074/jbc.M109.098780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- Meier C, Parmantier E, Brennan A, Mirsky R, Jessen KR. Developing Schwann cells acquire the ability to survive without axons by establishing an autocrine circuit involving insulin-like growth factor, neurotrophin-3, and platelet-derived growth factor-BB. J Neurosci. 1999;19:3847–3859. doi: 10.1523/JNEUROSCI.19-10-03847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Mirsky R, Dubois C, Morgan L, Jessen KR. 04 and A007-sulfatide antibodies bind to embryonic Schwann cells prior to the appearance of galactocerebroside; regulation of the antigen by axon-Schwann cell signals and cAMP. Development. 1990;109:105–116. doi: 10.1242/dev.109.1.105. [DOI] [PubMed] [Google Scholar]
- Mokuno K, Sobue G, Reddy UR, Wurzer J, Kreider B, Hotta H, Baron P, Ross AH, Pleasure D. Regulation of Schwann cell nerve growth factor receptor by cyclic adenosine 3′,5′-monophosphate. J Neurosci Res. 1988;21:465–472. doi: 10.1002/jnr.490210237. [DOI] [PubMed] [Google Scholar]
- Monje PV, Athauda G, Wood PM. Protein kinase A-mediated gating of neuregulin-dependent ErbB2–ErbB3 activation underlies the synergistic action of cAMP on Schwann cell proliferation. J Biol Chem. 2008;283:34087–34100. doi: 10.1074/jbc.M802318200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje PV, Rendon S, Athauda G, Bates M, Wood PM, Bartlett-Bunge M. Non-antagonistic relationship between mitogenic factors and cAMP in adult Schwann cell re-differentiation. Glia. 2009;57:947–961. doi: 10.1002/glia.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk K, Naylor SG, Glenn TD, Mercurio S, Perlin JR, Dominguez C, Moens CB, Talbot WS. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science. 2009;325:1402–1405. doi: 10.1126/science.1173474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monuki ES, Weinmaster G, Kuhn R, Lemke G. SCIP: A glial POU domain gene regulated by cAMP. Neuron. 1989;3:783–793. doi: 10.1016/0896-6273(89)90247-x. [DOI] [PubMed] [Google Scholar]
- Morgan L, Jessen KR, Mirsky R. The effects of cAMP on differentiation of cultured Schwann cells: Progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP-, N-CAM-, NGF-receptor-) depends on growth inhibition. J Cell Biol. 1991;112:457–467. doi: 10.1083/jcb.112.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P, Topilko P, Schneider-Manoury S, Seitanidou T, Baron-Van Evercooren A, Charnay P. The regulation of Krox-20 expression reveals important steps in the control of peripheral glial cell development. Development. 1996;122:2847–2857. doi: 10.1242/dev.122.9.2847. [DOI] [PubMed] [Google Scholar]
- Nave KA, Salzer JL. Axonal regulation of myelination by neuregulin 1. Curr Opin Neurobiol. 2006;16:492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Nickols JC, Valentine W, Kanwal S, Carter BD. Activation of the transcription factor NF-κB in Schwann cells is required for peripheral myelin formation. Nat Neurosci. 2003;6:161–167. doi: 10.1038/nn995. [DOI] [PubMed] [Google Scholar]
- Ogata T, Iijima S, Hoshikawa S, Miura T, Yamamoto S, Oda H, Nakamura K, Tanaka S. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J Neurosci. 2004;24:6724–6732. doi: 10.1523/JNEUROSCI.5520-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, Feltri ML, Wrabetz L, Behrens A, Mirsky R, Jessen KR. c-Jun is a negative regulator of myelination. J Cell Biol. 2008;181:625–637. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson DB, Bhaskaran A, Droggiti A, Dickinson S, D’Antonio M, Mirsky R, Jessen KR. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J Cell Biol. 2004;164:385–394. doi: 10.1083/jcb.200307132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson DB, Dickinson S, Bhaskaran A, Kinsella MT, Brophy PJ, Sherman DL, Sharghi-Namini S, Duran Alonso MB, Mirsky R, Jessen KR. Regulation of the myelin gene periaxin provides evidence for Krox-20-independent myelin-related signalling in Schwann cells. Mol Cell Neurosci. 2003;23:13–27. doi: 10.1016/s1044-7431(03)00024-1. [DOI] [PubMed] [Google Scholar]
- Parkinson DB, Dong Z, Bunting H, Whitfield J, Meier C, Marie H, Mirsky R, Jessen KR. Transforming growth factor beta (TGFβ) mediates Schwann cell death in vitro and in vivo: Examination of c-Jun activation, interactions with survival signals, and the relationship of TGFβ-mediated death to Schwann cell differentiation. J Neurosci. 2001;21:8572–8585. doi: 10.1523/JNEUROSCI.21-21-08572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmatullah M, Schroering A, Rothblum K, Stahl RC, Urban B, Carey DJ. Synergistic regulation of Schwann cell proliferation by heregulin and forskolin. Mol Cell Biol. 1998;18:6245–6252. doi: 10.1128/mcb.18.11.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph D, Tafuri A, Gass P, Hammerling GJ, Arnold B, Schutz G. Impaired fetal T cell development and perinatal lethality in mice lacking the cAMP response element binding protein. Proc Natl Acad Sci USA. 1998;95:4481–4486. doi: 10.1073/pnas.95.8.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SS, Xu YT, Roling D, Wrabetz L, Feltri ML, Kamholz J. Expression of growth-associated protein-43 kD in Schwann cells is regulated by axon-Schwann cell interactions and cAMP. J Neurosci Res. 1994;38:575–589. doi: 10.1002/jnr.490380510. [DOI] [PubMed] [Google Scholar]
- Schneider-Manoury S, Topilko P, Seitandou T, Levi G, Cohen-Tannoudji M, Pournin S, Babinet C, Charnay P. Disruption of Krox-20 results in alteration of rhombomeres 3 and 5 in the developing hindbrain. Cell. 1993;75:1199–1214. doi: 10.1016/0092-8674(93)90329-o. [DOI] [PubMed] [Google Scholar]
- Sobue G, Pleasure D. Schwann cell galactocerebroside induced by derivatives of adenosine 3′,5′-monophosphate. Science. 1984;224:72–74. doi: 10.1126/science.6322307. [DOI] [PubMed] [Google Scholar]
- Sobue G, Shuman S, Pleasure D. Schwann cell responses to cyclic AMP: Proliferation, change in shape, and appearance of surface galactocerebroside. Brain Res. 1986;362:23–32. doi: 10.1016/0006-8993(86)91394-6. [DOI] [PubMed] [Google Scholar]
- Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science. 2000;287:2267–2271. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- Stevens B, Tanner S, Fields RD. Control of myelination by specific patterns of neural impulses. J Neurosci. 1998;18:9303–9311. doi: 10.1523/JNEUROSCI.18-22-09303.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart HJ. Expression of c-Jun, Jun B, Jun D, cAMP response element binding protein by Schwann cells and their precursors in vivo and in vitro. Eur J Neurosci. 1995;7:1366–1375. doi: 10.1111/j.1460-9568.1995.tb01128.x. [DOI] [PubMed] [Google Scholar]
- Tabernero A, Stewart HJ, Jessen KR, Mirsky R. The neuron-glia signal beta neuregulin induces sustained CREB phosphorylation on Ser-133 in cultured rat Schwann cells. Mol Cell Neurosci. 1998;10:309–322. doi: 10.1006/mcne.1998.0662. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topilko P, Schneider-Manoury S, Levi G, Baron-Van Evercooren A, Chennoufi ABY, Seitanidou T, Babinet C, Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371:796. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Glover CP, Massey PV, Wan H, Johnson B, Bienemann A, Deuschle U, Kew JN, Aggleton JP, Bashir ZI, Uney J, et al. cAMP responsive element-binding protein phosphorylation is necessary for perirhinal long-term potentiation and recognition memory. J Neurosci. 2005;25:6296–6303. doi: 10.1523/JNEUROSCI.0506-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C, Korade Z, Carter BD. Protein kinase A-induced phosphorylation of the p65 subunit of nuclear factor-κB promotes Schwann cell differentiation into a myelinating phenotype. J Neurosci. 2008;28:3738–3746. doi: 10.1523/JNEUROSCI.4439-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.