Abstract
The transcription factor Krox-20 (Egr2) is a master regulator of Schwann cell myelination. In mice from which calcineurin B had been excised in cells of the neural crest lineage, calcineurin–nuclear factor of activated T cells (NFAT) signaling was required for neuregulin-related Schwann cell myelination (Kao et al. [2009] Immunity 12:359–372). Whether NFAT signaling required simultaneous elevation of intracellular cAMP levels was not explored. In vivo, Krox-20 expression requires continuous axon–Schwann cell signaling that in Schwann cell cultures can be mimicked by elevation of intracellular cAMP. We have investigated the role of the calcineurin–NFAT pathway in Krox-20 induction in purified rat Schwann cell cultures. Activation of this pathway requires elevation of intracellular Ca2+ levels. The calcium ionophore A23187 or ionomycin was used to increase intracellular Ca2+ levels in Schwann cell cultures that had been treated with dibutyryl cAMP to induce Krox-20. Increase in Ca2+ levels significantly potentiated Krox-20 induction, determined by Krox-20 immunolabeling of individual cells and Western blotting. Levels of the myelin proteins periaxin and P0 were also elevated. The potentiating effect was blocked by cyclosporin A, a specific blocker of the calcineurin–NFAT pathway. We found that, in the absence of cAMP elevation, treatment with A23187 alone failed to induce Krox-20 expression, indicating that NFAT upregulation of Krox-20 requires elevation of cAMP levels in Schwann cells. P-VIVIT, another specific inhibitor of calcineurin–NFAT interaction, blocked Krox-20 induction in response to dibutyryl cAMP and ionophore. HA-NFAT1 (1–460)-GFP translocated to the nucleus on treatment with dibutyryl cAMP with or without added ionophore. NFAT isoforms 1–4 were detected in purified Schwann cells by quantitative RT-PCR.
Keywords: PNS, P-VIVIT, myelin, Egr2, myelin protein, zero, periaxin
Calcineurin–nuclear factor of activated T cells (NFAT) signaling promotes Schwann cell myelination via the transcription factor Krox-20 (Kao et al., 2009), but how this novel signaling system interacts with other pathways that control expression of myelin genes and proteins is unknown. We have investigated whether the cAMP pathway, a long-established inducer of myelin differentiation, interacts with calcineurin–NFAT signaling to control myelination.
Krox-20 is a master regulator of Schwann cell myelination (Topilko et al., 1994; Zorick et al., 1999; Decker et al., 2006). In cultured Schwann cells, it is induced in response to cAMP and in turn upregulates myelin-associated genes, causes withdrawal from the cell cycle, and protects cells from death (Nagarajan et al., 2001; Jessen and Mirsky, 2002; Parkinson et al., 2003, 2004). cAMP has been implicated in the action of G-protein-coupled receptor 26 in inducing myelination in zebrafish, and this receptor is essential for timely myelination in mammals (Monk et al., 2009, 2011). In contrast to cultured rat Schwann cells, in which neuregulin-1 (NRG1) potentiates myelin gene induction by cAMP, in mouse Schwann cells Krox-20 protein induction requires combined cAMP and NRG1 (Murphy et al., 1996; Parkinson et al., 2003, 2004; Arthur-Farraj et al., 2011). In mouse neuron–Schwann cell cocultures, NRG1 is required for Schwann cell ensheathment and myelination, whereas in vivo it regulates myelin sheath thickness and the number of axons/Schwann cell unit in unmyelinated fibers (Michailov et al., 2004; Taveggia et al., 2005). Nevertheless, when presented in membrane-bound form to cultured Schwann cells, NRG1 fails to induce significant levels of myelin genes or proteins (Taveggia et al., 2005), suggesting that myelination requires an additional signal.
The calcineurin–NFAT pathway induces Krox-20 in T cells and Schwann cells (Rengarajan et al., 2000; Kao et al., 2009; Lazarevic et al., 2009). A sustained increase in intracellular Ca2+ levels activates cytoplasmic calcineurin. This promotes dephosphorylation and nuclear translocation of NFAT, where it complexes with other proteins, including Krox-20, to become transcriptionally active (Cameron et al., 1995; Hogan et al., 2003). The Krox-20 promoter and upstream enhancer elements contain NFAT binding sites, and reporter assays indicate that Krox-20 is an NFAT target gene and that calcineurin is upregulated in Schwann cells expressing Krox-20 (Rengarajan et al., 2000; Nagarajan et al., 2001; Kao et al., 2009). In mice lacking the calcineurin regulatory subunit calcineurin B1 in neural crest derivatives, NFAT dephosphorylation and activation fail, Schwann cell Krox-20 levels are lower, and radial sorting and myelination are delayed. NFAT activation occurs at least in part through NRG1 signaling. NRG1 addition to neuron–Schwann cell cocultures promotes activation of NFAT isoforms, and the Krox-20 myelin-specific enhancer (MSE) and promoter are stimulated by co-operative activity of NFATc4 and SOX10, a transcription factor required for Krox-20 upregulation and myelination (Pierano et al., 2000; Finzsch et al., 2010). NFAT also synergizes with SOX10 to activate the promoter of the major myelin protein P0. Thus, in response to NRG1, NFATs act by upregulating Krox-20 and also act directly on P0, which is itself regulated by a combination of Krox-20 and SOX10 (Jang and Svaren, 2009; Kao et al., 2009; Finzsch et al., 2010).
Calcineurin–NFAT activity is blocked by cyclosporin A, which indirectly represses NFAT by inhibiting calcineurin activity (Liu et al., 1991; Okamura et al., 2000). We used purified rat Schwann cell cultures and an in vitro model of myelination to investigate the relative roles of the calcineurin–NFAT pathway and cAMP elevation in induction of Krox-20 and its target genes, periaxin and P0. We found that, in the absence of cAMP elevation, increasing Ca2+ levels failed to induce Krox-20 expression, indicating that in Schwann cells NFAT upregulation of Krox-20 requires elevation of cAMP levels.
Materials and Methods
Materials
Polyclonal rabbit anti-Krox-20 antibody was from Covance (Crawley, United Kingdom). Mouse monoclonal antibody to HA was from Roche (Burgess Hill, United Kingdom), goat anti-rabbit Ig fluorescein isothiocyanate (FITC) antibody was from MP Biomedicals (Illkirch, France), and donkey anti-mouse Ig cy3 was from Jackson Immunoresearch (Newmarket, United Kingdom). Periaxin antibody was a gift from Peter Brophy and Diane Sherman (Neuroscience Centre, Edinburgh University, Edinburgh, United Kingdom), and P0 polyclonal antibody was made in the laboratory as previously described (Morgan et al., 1994). Calcium ionophore (A23187), cyclosporin A, ionomycin, transferrin, selenium, putrescine, triiodothyronine (T3), thyroxine (T4), progesterone, dexamethasone, insulin, cytosine arabinoside, hyaluronidase, laminin, poly-D-lysine (PDL), lysine, sodium azide, Triton X-100, bovine serum albumin (BSA), Hoechst nuclear stain, dimethyl sulfoxide, dibutyryl cAMP (dbcAMP), paraformaldehyde (PF), and glycine were obtained from Sigma-Aldrich (Poole, United Kingdom). Forskolin was from Calbiochem (Nottingham, United Kingdom). Dulbecco’s modified Eagle’s medium (DMEM), Ham’s F12 medium, trypsin, L-glutamine, penicillin and streptomycin, and Leibovitz L-15 medium were obtained from Invitrogen (Paisley, United Kingdom). Human recombinant β-neuregulin1 (NRG1) was from R&D Systems (Minneapolis, MN; catalog No. 396-HB-050; EGF domain with BSA carrier protein; reconstituted in PBS containing 1% BSA and further diluted 1:500 in DMEM or DM for experimental use). Citifluor was from Citifluor Ltd. (London, United Kingdom). Collagenase was from Worthington Biochemical Corporation (Lorne Laboratories, Reading, United Kingdom). Fetal calf serum (FCS) was from TCS Cellworks (Poole, United Kingdom). The serum-free supplemented defined medium used in this study was a modification of the medium of Bottenstein and Sato (1979). A 1:1 mixture of DMEM mg/liter glucose) and Ham’s F12 was supplemented with transferrin (100 lg/ml), BSA (0.035%), putrescine (16 μg/ml), T3 (10.1 ng/ml), T4 (400 ng/ml), progesterone (60 ng/ml), dexamethasone (38 ng/ml), glutamine (2 mM), penicillin (100 U/ml) and streptomycin (100 U/ml), T3 (10.1 ng/ml), and selenium (160 ng/ml; Dong et al., 1999).
Schwann Cell Culture
Schwann cells were prepared from sciatic nerves and brachial plexus from 3-day-old (P3) Sprague-Dawley rats (maintained at University College London Biological Services Unit in accordance with Home Office regulations) as described previously (Brockes et al., 1979; Morgan et al., 1991). After desheathing and dissociation in a trypsin/collagenase mixture, Schwann cells were cultured in 13 mM PDL/laminin-coated tissue culture dishes in DMEM containing 10% FCS plus cytosine arabinoside (10−5 M) for 3 days to remove contaminating fibroblasts (Arthur-Farraj et al., 2011). Cells obtained from these dishes are subsequently described as serum-purified Schwann cells. These cells were cultured, unless otherwise stated, in serum-free supplemented defined medium (Jessen et al., 1994; Dong et al., 1999) containing 10−6 M insulin, referred to as defined medium (DM). The serum-purified cultures are >99% pure when monitored immunocytochemically using S-100 labeling to identify Schwann cells and Hoechst dye to identify total nuclei in the culture. In some experiments, Schwann cells were purified by immunopanning prior to culture in cytosine arabinoside (Dong et al., 1999). The different experimental treatments were carried out with serum-purified Schwann cells plated on 13-mm PDL/laminin-coated glass coverslips (Morgan et al., 1991). Cells were initially plated at a density of 5,000 cells/15 µl drop and subsequently cultured in a final volume of 400 μl. The cells were either cultured in DM alone or DM plus dbcAMP (10−3 M), with or without A23187 (100 nM), dbcAMP plus cyclosporin (100 ng/ml), and dbcAMP plus A23187 (100 nM) and cyclosporin A (100 ng/ml) for 24–36 hr. In some experiments, ionomycin (0.65 µm) was used instead of A23187.
Immunocytochemistry
For Krox-20, periaxin, or hemagglutinin (HA) immunolabeling, cells were fixed in 4% PF for 10 min, permeabilized in 0.5% Triton in phosphate-buffered saline (PBS) for 1 hr, and blocked in 0.2% Triton in antibody diluting solution (10% calf serum, 0.1 M lysine in PBS) for 2 hr (Krox-20) or 1 hr (periaxin, HA) at room temperature. For P0 labeling, cells were fixed in 1 M HCl for 10 min, then treated with 0.1 M sodium borate for 10 min, followed by antibody diluting solution. The cells were subsequently incubated with Krox-20 antibody (1:250) or alternatively with periaxin antibody (1:8,000), polyclonal P0 (1:500), or HA antibodies (1:200) in antibody dilution solution overnight at 4°C and finally with FITC or goat anti-rabbit Ig Cy3 or goat anti-mouse Ig Cy3 antibodies (1:600–1,000) in antibody diluting solution containing Hoechst dye (1:1,000) for 30 min at room temperature before mounting in Citfluor antifade mounting medium.
Microscopy and Quantification
Results were based on a minimum of three separate experiments using triplicate coverslips unless otherwise stated. Experimental results were quantified by using a fluorescence light microscope (Nikon Eclipse E800). For immunocytochemical experiments, on every coverslip, at least 500 Hoechst-labeled Schwann cells were counted. The percentage of Krox-20-positive cells (based on nuclear staining) or of periaxin- or P0-positive cells was computed as a proportion of the total Hoechst-stained Schwann cell nuclei for each coverslip. Statistical analysis for all experiments was performed in Microsoft Excel, and a two-tailed Student’s t-test was used to determine statistical significance. P < 0.05 was considered as statistically significant.
Western Blotting
Purified, immunopanned Schwann cells were plated onto 90-mm PDL/laminin-coated tissue culture plastic dishes as described previously (Lee et al., 1997) and cultured in 3% FCS in DMEM with NRG1 (10 ng/ml) and forskolin (2 µm) until confluent, then trypsinized and plated onto 6 × 60 mm dishes and cultured in the same medium until confluent. They were subsequently placed in DM containing 0.5% FCS for 24 hr prior to treatment with DM containing dbcAMP (10−3 M), with or without A23187 (100 nM), and dbcAMP plus A23187 (100 nM) and cyclosporin (100 ng/ml) for 24–36 hr prior to harvesting. Extracts from cells were prepared and blotted as described previously (Parkinson et al., 2004). Primary antibody dilutions were rabbit anti-Krox-20 (1:2,000), rabbit anti-periaxin (1:15,000), and rabbit anti-tubulin (1:1,000), and blots were incubated overnight at 4°C. Membranes were washed, and secondary antibody (goat anti-rabbit HRP 1:2,000; Promega, Southampton, United Kingdom) in this solution was added for 2 hr. Specific protein complexes were revealed by using ECL Plus chemiluminescent reagent (Amersham Biosciences, Little Chalfont, United Kingdom; Morgan et al., 1991; Parkinson et al., 2004). Blots were quantified in Image J.
Transfection Experiments
Schwann cells were transfected using the Mirus, TransIT-LT1 transfection system according to the manufacturer’s instructions (Mirus Bio Corp., Madison, WI). The following plasmids were used: plasmids 11106, GFP-VIVIT; plasmid 11107, HA-NFAT1(1–460)-GFP (Addgene, Cambridge, MA) and pEGFP-N1 (Clontech, Palo Alto, CA), which was used as a control for the GFP-VIVIT and HA-NFAT1(460)-GFP plasmids. In brief, purified cells (4,500/15 μl drop) were plated on coverslips in 3% FCS in DMEM with NRG1 (10 ng/ml) and forskolin (2 µm) overnight. For transfection, they were changed into DM containing 0.5% FCS. After 24 hr, either 10−3 M dbcAMP or 4 µm forskolin was added to some wells to induce a myelinating phenotype (Morgan et al., 1991), and some wells also contained either A23187 or alternatively ionomycin (0.65 µm), plus or minus cyclosporin, as indicated. To examine movement of HA-NFAT1(1–460)-GFP into the nucleus, cells were fixed 16 hr later with 4% PF in PBS for 10 min. In some experiments, cells were immunolabeled with antibodies to HA to confirm that cells visualized using the GFP tag were also labeled with the HA tag. For experiments with the inhibitory plasmid P-VIVIT and the control plasmid, dbcAMP and ionophore were added 24 hr after transfection and fixed 24 hr later in 4% PF in PBS, followed by immunolabeling with Krox-20 antibodies. The percentage of Krox-20/GFP-positive cells was compared with the percentage of Krox-20/GFP-negative cells on the same coverslip to estimate the relative inhibition caused by the P-VIVIT plasmid.
Quantitative RT-PCR
Total RNA was extracted from two separate preparations of purified Schwann cells prepared as described above from P3 rats using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. RNA was DNAse treated, purified by passing through an RNAeasy minicolumn according to the manufacturer’s instructions (Qiagen, Crawley, United Kingdom), and quantified using a NanoDrop spectro-photometer. The 260/230 readings were >2. The integrity of the RNA was confirmed, with an RNA quality indicator (RQI) of 10 using an Experion RNA StdSens chip (Bio-Rad, Hemel Hempstead, United Kingdom). One microgram of RNA in 20 μl total volume was reverse transcribed into cDNA using both oligo-dT and random primers with Superscript II reverse transcriptase. An RT minus was also performed. Primers for NFATc1, −c2, −c3, and −c4 were used exactly as described by Kim and Usachev (2009) for qRT-PCR. Glyceraldehyde 3-phosphate dehydgrogenase (gapdh), hypoxanthine guanine phosphoribosyl transferase (hprt), and tyrosine 3-monooxygenase/tryptophan 5-monooxygenase (ywhaz) were used as stable reference genes for the amplification with sequences described by Langnaese et al. (2008). Real-time PCR was performed with SYBR Green Jumpstart Taq readymix (Sigma) in a Chromo4 System (Bio-Rad). The program for qRT-PCR was 94°C for 2 min, 94°C for 15 sec, 60°C for 30 sec, followed by 72°C for 30 sec, 39 times. The melting curve of PCR amplicons generated one peak. The primers were optimized for efficiency prior to use. All standard curves were constructed by using fivefold dilutions of cDNA. All primer efficiencies were within 95–105%. Data were analyzed in Opticon monitor 3 software and fold changes determined by using the Livak and Schmittgen (2001) method.
Results
The Ca2+ Ionophore A23187 Potentiates Krox-20 Expression in Schwann Cells Cultured in the Presence of Agents That Mimic Elevation of Intracellular cAMP
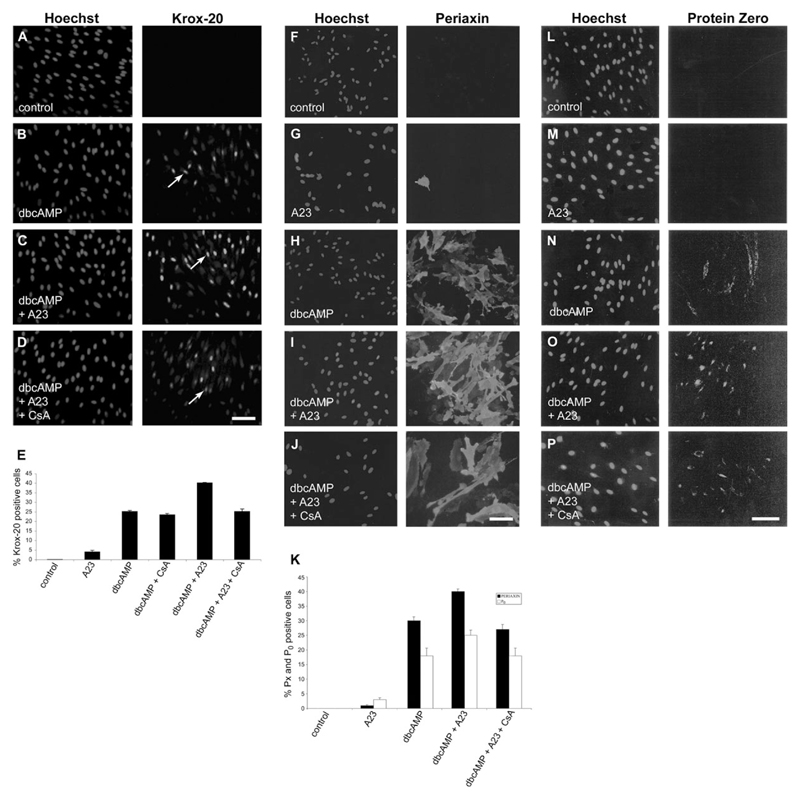
Elevation of intracellular cAMP in quiescent Schwann cells cultured under serum-free conditions in DM induces Krox-20 expression and myelin-related differentiation (Parkinson et al., 2004). This model was used to investigate the role of signaling pathways induced by Ca2+ in myelin-related differentiation in Schwann cells. Serum-purified Schwann cells were cultured in DM alone or in DM plus Ca2+ ionophore A23187 or plus the cAMP analog dbcAMP or plus dbcAMP and ionophore for 24 hr. The cultures were immunolabeled with Krox-20 antibodies and Hoechst dye (Fig. 1A–D). The results are quantified in Figure 1E. No Krox-20 immunolabeling was seen in Schwann cells cultured in DM alone (n = 4; Fig. 1A,E). Minimal induction (3.7 ± 0.33) was seen in cultures treated with ionophore alone (n = 3; Fig. 1E). In the cell cultures exposed to dbcAMP alone, a mean of 24% ± 4.93% (n = 4) Krox-20-immunolabeled cells was observed (Fig. 1B,E), in line with previous work (Parkinson et al., 2004). In cultures in which the Ca2+ ionophore A23187 (100 nM) was added together with dbcAMP (1 mM), Krox-20 induction was significantly enhanced (P < 0.006) to a mean of 39% ± 1.67% (n = 4) Krox-20-immunolabeled cells, and labeling in individual cells appeared stronger (Fig. 1C,E). A similar elevation of Krox-20 induction was seen when ionomycin, another Ca2+ ionophore, was added instead of A23187 (not shown). In agreement with the results of Kao et al. (2009) and Arthur-Farraj et al. (2011), we also found that addition of NRG1 to cultures treated with dbcAMP induced an increase in the number of cells that expressed Krox-20, to levels similar to those induced when ionophore was used in combination with dbcAMP, whereas NRG1 used in the absence of dbcAMP failed to induce significant expression of Krox-20. Furthermore, addition of ionophore to cultures treated with NRG1 and dbcAMP in combination failed to increase the number of cells expressing Krox-20 (not shown). The findings show that Ca2+ potentiates dbcAMP-induced Krox-20 induction in purified Schwann cell cultures. They are also consistent with the idea that NRG1 promotes cAMP-driven Schwann cell differentiation by elevating intracellular Ca2+ levels.
Fig. 1.
Krox-20 expression induced in response to dbcAMP is potentiated by the Ca2+ ionophore A23187. A–D: Left panels: purified Schwann cells labeled with Hoechst nuclear stain; right panels: cells immunolabeled with Krox-20 antibody (examples arrowed). A: Culture treated with DM and high insulin. B: Culture treated with DM and high insulin plus dbcAMP (1 mM). C: Culture treated with DM and high insulin plus dbcAMP and Ca2+ ionophore A23187 (100 nM). D: Culture treated with DM and high insulin plus dbcAMP, Ca2+ ionophore A23187, and cyclosporin A (CsA; 100 ng/ml). E: Graph showing the percentage of Krox-20-positive cells induced in each culture condition indicated below the x-axis. The percentage of Krox-20-positive cells induced was significantly different between cells treated with dbcAMP alone and cells treated with dbcAMP plus ionophore (P < 0.001). It was also significantly different when cells treated with dbcAMP plus ionophore were compared with cells treated with dbcAMP, ionophore, and CsA to inhibit calcineurin activation (P < 0.001). There was no significant difference when cells treated with dbcAMP alone were compared with cells treated with dbcAMP plus CsA or with cells treated with dbcAMP, ionophore, and CsA. Note that cyclosporin A inhibits the potentiation of Krox-20 expression induced by ionophore and that Krox-20 is minimally induced in the presence of ionophore alone. F–J: Left panels: purified Schwann cells labeled with Hoechst nuclear stain; right panels: cells immunolabeled with periaxin antibody. F: Culture treated with DM and high insulin. G: Culture treated with DM and high insulin plus ionophore A23187. H: Culture treated with DM and high insulin plus dbcAMP (1 mM). I: Culture treated with DM and high insulin plus dbcAMP and Ca2+ ionophore A23187 (100 nM). J: Culture treated with DM and high insulin plus dbcAMP, Ca2+ ionophore A23187, and CsA (100 ng/ml). K: Graph showing the percentage of immunlolabeled periaxin- and P0-positive cells induced under similar conditions. The percentage of positive cells induced was significantly different between cells treated with dbcAMP alone and cells treated with dbcAMP plus ionophore (periaxin: P < 0.04; P0: P < 0.03); it was also significantly different when cells treated with dbcAMP plus ionophore were compared with cells treated with dbcAMP, ionophore, and CsA (periaxin: P < 0.02; P0: P < 0.03). There was no significant difference between cells treated with dbcAMP alone and cells treated with dbcAMP, ionophore, and CsA. Error bars show standard error of the mean. L–P: Left panels: purified Schwann cells labeled with Hoechst nuclear stain; right panels: cells immunolabeled with P0 antibody. L: Culture treated with DM and high insulin. M: Culture treated with DM and high insulin plus ionophore A23187. N: Culture treated with DM and high insulin plus dbcAMP (1 mM). O: Culture treated with DM and high insulin plus dbcAMP and Ca2+ ionophore A23187 (100 nM). P: Culture treated with DM and high insulin plus dbcAMP, Ca2+ ionophore A23187, and CsA (100 ng/ml). Scale bars = 50 µm.
Cyclosporin A Inhibits the Effect of the Ca2+ Ionophore A23187 on Krox-20 Levels in dbcAMP-Induced Schwann Cells
To investigate whether Ca2+ promotes Krox-20 expression through the calcineurin–NFAT pathway in our system, we tested whether cyclosporin A, a specific inhibitor of calcineurin, could inhibit Ca2+-induced Krox-20 induction. Serum-purified Schwann cells were plated in DM, DM containing dbcAMP, or dbcAMP and Ca2+ ionophore A23187 in the presence or absence of cyclosporin A. Cyclosporin A abolished (P < 0.006) the potentiating effect of the ionophore but had no effect on the number of Schwann cells expressing Krox-20 (23% ± 2.04%; n = 6) in cultures plated in the presence of dbcAMP alone, although overall labeling in individual cells appeared somewhat weaker (Fig. 1D,E).
These experiments show that the Ca2+-activated calcineurin–NFAT pathway promotes Krox-20 expression in Schwann cells provided that cAMP levels in Schwann cells are elevated. This indicates that the promyelin effects of calcineurin–NFAT signaling depend on simultaneous activation of cAMP pathways in Schwann cells.
The Ca2+ Ionophore A23187 Potentiates Periaxin and P0 Expression in Schwann Cells Cultured in the Presence of dbcAMP
In addition to elevating Krox-20 levels, exposure of purified Schwann cells to dbcAMP elevates levels of the myelin-associated proteins periaxin and P0, most probably because periaxin and P0 are Krox-20 target genes (Nagarajan et al., 2002; Parkinson et al., 2003). To determine whether treatment with the ionophore A23187 would also increase the number of cells expressing high levels of periaxin or P0 and whether this also depended on the NFAT pathway, we cultured Schwann cells in DM alone or plus ionophore; or plus dbcAMP; or plus dbcAMP and ionophore; or plus dbcAMP, ionophore, and cyclosporin, followed by immunolabeling with periaxin or P0 antibodies (Fig. 1F–J [periaxin], L–P [P0]). Very few Schwann cells expressed periaxin or P0 when cells were cultured in DM alone or in DM containing ionophore (Fig. 1F,G [periaxin], L,M [P0]). The results are quantified in Figure 1K. Many cells were periaxin or P0 positive when cells were treated with dbcAMP (Fig. 1H,N), and the number of positive cells was boosted by addition of ionophore (Fig. 1I,N). Addition of cyclosporin to the culture medium prevented this effect, resulting in 27.1% ± 2.5% periaxin-positive cells and 17.7% ± 2.5% P0-positive cells, a return to just below the figures obtained with dbcAMP alone (Fig. 1J,P).
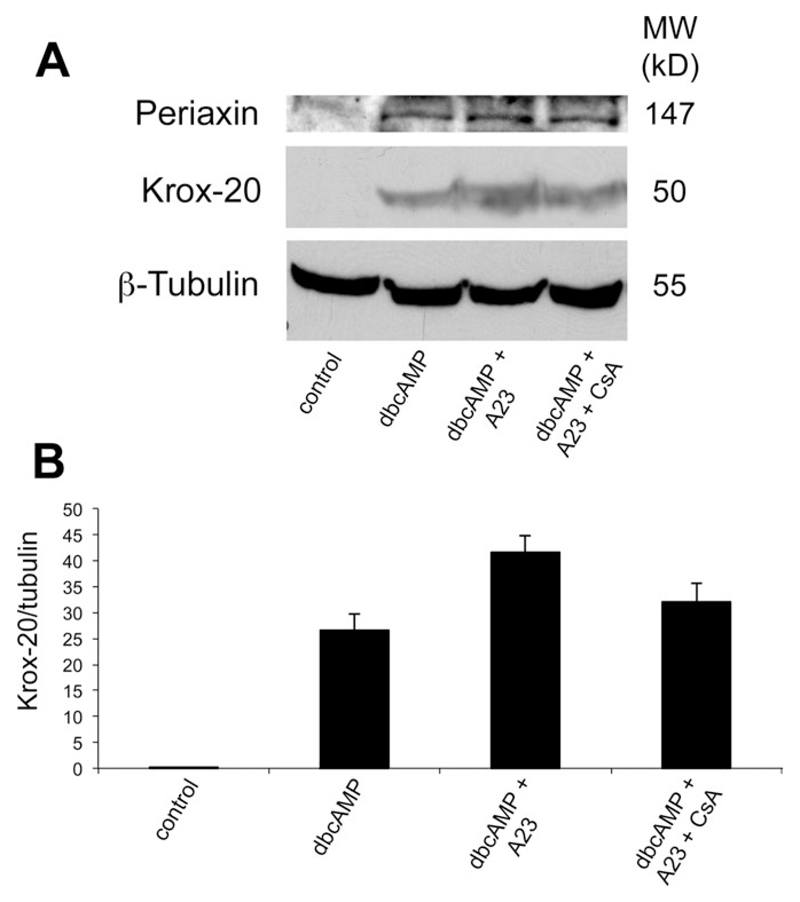
Effects of the Ca2+ Ionophore A23187 and Cyclosporin A on Krox-20 Induction Can Be Detected by Western Blot Analysis
Protein extracts from purified Schwann cells cultured in DM alone, with added dbcAMP, with added dbcAMP plus the Ca2+ ionophore A23187, or with added dbcAMP plus the ionophore and cyclosporin were analyzed by Western blotting and probed sequentially with antibodies to Krox-20 and β-tubulin, to control for protein loading levels on the blot (Fig. 2A,B). No Krox-20 was detectable in the untreated cells, whereas significant induction was seen under all other conditions, with highest levels detectable in the cells treated with dbcAMP and ionophore. Addition of cyclosporin to cells treated with dbcAMP and ionophore resulted in a return toward Krox-20 levels seen in dbcAMP alone, supporting the results obtained immunocytochemically. Periaxin levels followed a similar although less marked trend (Fig. 2A).
Fig. 2.
Western immunoblot showing that Krox-20 protein is induced by dbcAMP and is potentiated by Ca2+ ionophore. A: Protein extracts of purified Schwann cells treated with DM alone, with added dbcAMP, with added dbcAMP plus the Ca2+ ionophore A23187, and with added dbcAMP plus A23187 and cyclosporin (lane 4) for 36 hr were subjected to SDS-gel electrophoresis and probed sequentially with antibodies to Krox-20, periaxin, and β-tubulin to control for protein loading levels on the blot. Note that Krox-20 is undetectable in the untreated cells, whereas a significant induction is seen under all other conditions. B: Densitometry of the Krox-20 levels for the average of three Western blots. Highest levels are detectable in the cells treated with dbcAMP and ionophore. Note that, in the presence of cyclosporin, the increase in Krox-20 levels seen in cells treated with dbcAMP and Ca2+ ionophore is reduced as expected if cyclosporin is blocking the calcineurin–NFAT pathway.
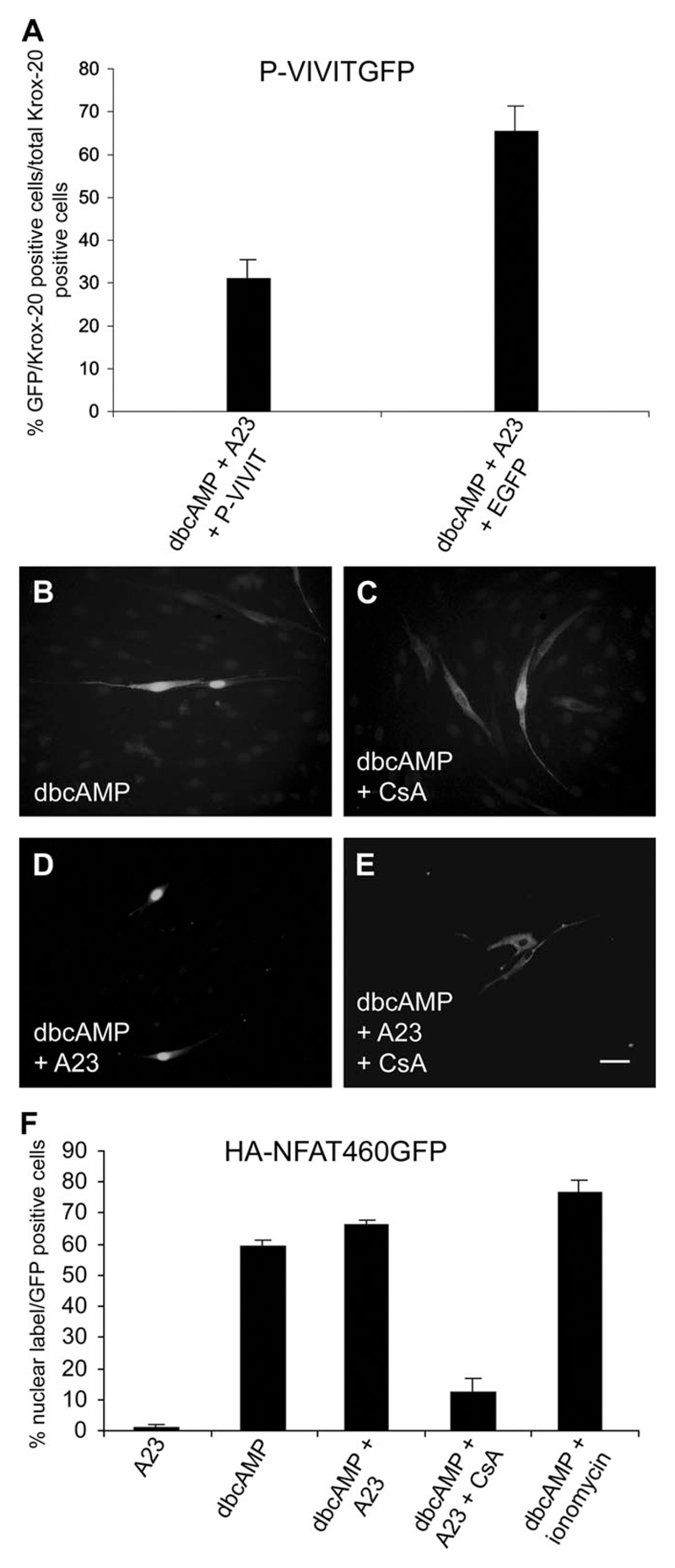
Krox-20 Induction Can Be Inhibited by P-VIVIT
Although cyclosporin is widely used as an inhibitor of the calcineurin–NFAT signaling pathway, more specific inhibitors exist (Aramburu et al., 1999). To confirm results with cyclosporin, we used a GFP plasmid containing the P-VIVIT sequence. This sequence, which interferes specifically with the interaction between calcineurin and NFAT, is a highly selective inhibitor of NFAT-dependent expression of target genes in T cells, a cell type in which NFAT also induces Krox-20 (Aramburu et al., 1999). We therefore tested whether expression of P-VIVIT interfered with the induction of Krox-20 protein in response to dbcAMP and calcium ionophore, as predicted if the NFAT pathway is involved in potentiating Krox-20 expression. We found that fewer transfected cells expressing P-VIVITGFP expressed Krox-20 in response to dbcAMP and calcium ionophore than untransfected cells within the same culture that did not contain P-VIVITGFP. The number of cells induced to express Krox-20 in the presence of P-VIVITGFP was reduced to 31.3% ± 4.41% of the number of untransfected cells in the same culture that expressed Krox-20 in the presence of dbcAMP and A23187. The pEGFP-N1 plasmid used to control for inhibition of Krox-20 induction by the plasmid backbone alone reduced induction to 65.5% ± 5.8% of that seen in uninfected cells (n = 2; Fig. 3A). Therefore, P-VIVIT induces suppression of the Krox-20 response to dbcAMP and ionophore. These experiments confirm the synergistic interactions between cAMP and the calcineurin–NFAT signaling in myelin differentiation.
Fig. 3.
Cultures treated with dbcAMP and ionophore were transfected with GFP-VIVIT or control plasmid EGFP-N1 and immunolabeled with Krox-20 antibodies. Alternatively, cultures were treated with dbcAMP with or without ionophore and CsA and transfected with HA-NFAT1(1–460)-GFP. A: The percentage of cells that coexpressed GFP-VIVIT and Krox-20 or that coexpressed the control EGFP-N1 plasmid and Krox-20 was expressed as a percentage of the total number of Krox-20-positive cells within the same culture. VIVIT-GFP induces significant suppression of Krox-20 compared with untransfected cells (P < 0.03), whereas EGFP-N1 induces milder suppression. B: Cells were treated with dbcAMP for 16 hr after transfection with HA-NFAT1(1–460)-GFP, which is clearly visible in the nucleus, indicating that this treatment alone induces translocation of NFAT. C: Treatment with dbcAMP and CsA prevented translocation to the nucleus and HA-NFAT1(1–460)-GFP remained in the cytoplasm. D: Treatment with dbcAMP and Ca2+ ionophore again induced HA-NFAT1(1–460)-GFP nuclear translocation. E: Treatment with dbcAMP, Ca2+ ionophore, and CsA blocked translocation to the nucleus. Here the HA-NFAT1(1–460)-GFP was visualized by using antibodies to the N-terminal HA tag rather than the C-terminal GFP tag. HA-labeled cells showed complete overlap with GFP-labeled cells, indicating that either tag can be used for visualization. Transfection rates ranged from 1% to 10% of total cells on the coverslip, so that only one or two fluorescent cells appear within each field. F: Graph showing the percentage of GFP-positive transfected cells with a GFP-labeled nucleus under the conditions described above. Scale bars = 50 µm.
NFAT Translocates to the Schwann Cell Nucleus
To act as a transcription factor, NFAT must be translocated to the nucleus. To explore translocation of NFATs to the nucleus, we transfected Schwann cells with a plasmid HA-NFAT1 (NFATc2)(1–460)-GFP, which in other cell types translocates to the nucleus when intracellular calcium levels are elevated (Aramburu et al., 1998). This plasmid can be visualized within cells directly by GFP fluorescence or indirectly by immunolabeling for the HA tag that is located at the C terminus of the NFAT sequence (Fig. 3B–E). We found no cells with nuclear labeling in cells cultured in DM alone (not shown). Very few cells with nuclear labeling were seen in the presence of A23187 alone (1.1% ± 1.02%), showing that in Schwann cells, unlike in some other cell types, nuclear translocation of NFAT requires cAMP elevation. For cells that had been treated with 1 mM dbcAMP, we found significant translocation to the nucleus (59.3% ± 1.90%; Fig. 3B,F). In the combined presence of dbcAMP and either the Ca2+ ionophore A23187 or ionomycin, however, the percentage of cells with nuclear labeling was increased to 66.3% ± 1.53% or 76.6% ± 4.25% respectively, although the difference between the percentage of cells with nuclear label after treatment with dbcAMP alone and with dbcAMP plus ionophore was not significantly different (Fig. 3D, F). In the presence of cyclosporin, translocation to the nucleus was blocked in response to dbcAMP elevation alone and in response to combined dbcAMP and A23187 (12.3% ± 4.51% and 11.9% ± 1.77% respectively; Fig. 3C,E,F).
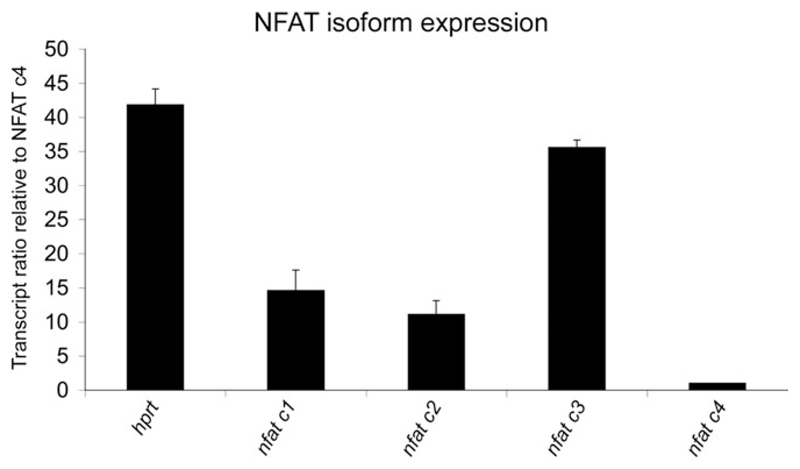
NFAT Isoforms Are Detectable in Schwann Cells by Quantitative RT-PCR
Our interpretation of the results described above, namely, that Ca2+ promotes Krox-20 expression in Schwann cells via a process that depends on NFAT, pre-supposes that Schwann cells express NFAT isoforms. To confirm the presence of NFAT isoforms in rat Schwann cells, we used qRT-PCR. All four isoforms were detected. Judging from the cycle threshold (Ct) values, which are inversely correlated with the logarithm of the initial copy number of the transcript being amplified, NFATc3 was the most abundant isoform, followed by NFATc1 and NFATc2, which were expressed in more or less equal amounts, with NFAT c4 in much lower abundance (Ct values: c1 = 22.8 ± 0.66; c2 = 23.3 ± 0.57; c3 = 20.6 ± 0.17; c4 = 26.6 ± 0.26; hprt = 20.1 ± 0.27; Fig. 4). These observations strengthen the conclusion that in Schwann cells the combined elevation of cAMP and Ca2+ activates the NFAT pathway.
Fig. 4.
Quantitative real-time PCR shows that purified cultured Schwann cells express NFAT isoforms nfatc1, –2, –3, and –4. Hprt was used as an endogenous control. Results were normalized to the level of nfatc4, which was the least abundant isoform. Nfatc3 was most abundant, with intermediate levels of nfatc1 and –2.
Discussion
In addition to crucial roles in the differentiation of Schwann cells in the PNS and in the immune system, the calcineurin–NFAT signaling pathway plays an important modulatory role in the differentiation program of a number of cell types both in vivo and in vitro, including osteoclasts and pancreatic β-cells (Hogan et al., 2003; Heit et al., 2006). Because of the importance of Krox-20, together with its interacting NAB proteins, as a key regulator of Schwann cell myelination (Topilko et al., 1994; Nagarajan et al., 2002; Le et al., 2005; Decker et al., 2006), we investigated the relationship between Krox-20 induction and activation of the calcineurin–NFAT pathway in purified cultured Schwann cells.
Our results show that only Schwann cells in which intracellular levels of dbcAMP have been elevated respond to increased levels of intracellular calcium by increasing the proportion of cells that express Krox-20, periaxin, and P0. Furthermore, elevation of intracellular cAMP is required for NFAT translocation to the nucleus. At present we do not understand the pathways involved in this response, although the response might involve direct activation of calcium signaling by dbcAMP, a possibility that remains to be tested. Elevation of intracellular Ca2+ is not sufficient on its own to promote Krox-20 expression via the calcineurin–NFAT pathway. Our findings mirror the response seen when rat Schwann cells are cultured in a combination of dbcAMP and NRG1. NRG1 alone does not increase levels of Krox-20 protein significantly, whereas addition of NRG1 to cultures that have been treated with dbcAMP increases the induction of Krox-20 over that seen with dbcAMP alone (Arthur-Farraj et al., 2011). When Schwann cells are cultured in dbcAMP and NRG1, there is no increase in Krox-20 protein expression in response to increased Ca2+ levels (not shown). Taken together, these results indicate cooperativity between cAMP- and Ca2+-induced calcineurin–NFAT pathways, for which precedents exist in other cell types (see, e.g., Soleimanpour et al., 2010). A simplified diagram of some of the pathways involved is shown in Figure 5.
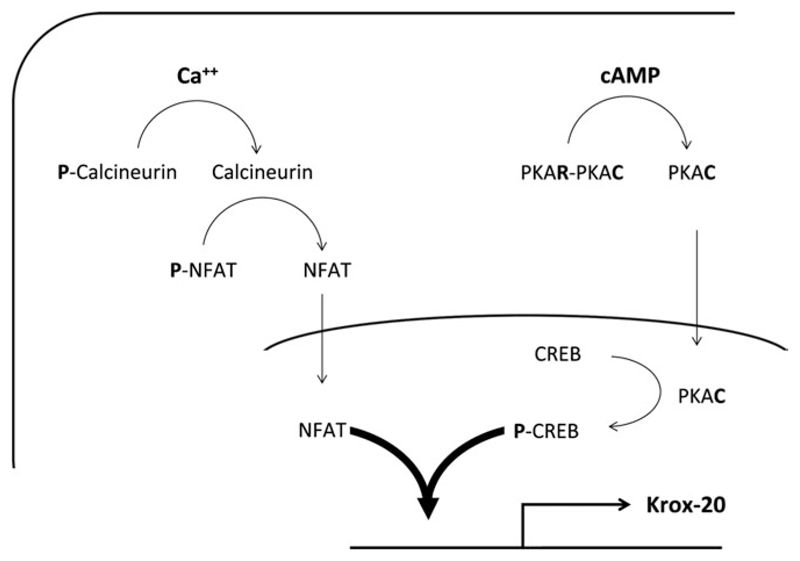
Fig. 5.
Diagram showing some of the pathways involved in the interactions among NFAT, cAMP, and Krox-20 elevation in Schwann cells. On calcium activation, the calcium phosphatase calcineurin is dephosphorylated and interacts with NFAT isoforms to dephosphorylate them. This causes translocation to the nucleus, where interaction with the Krox-20 promoter can occur to promote transcription. Phosphorylation of CREB is required for Krox-20 activation, and it is suggested that synergistic interaction of P-CREB and NFAT further promotes Krox-20 activation. The role of cAMP elevation in activating calcineurin and NFAT activation and translocation to the nucleus in Schwann cells is not understood and is therefore not depicted, although it is possible that elevation of intracellular cAMP acts directly to elevate calcium levels and thereby activate calcineurin- or other calcium-dependent signaling pathways.
As described earlier, experiments using calcineurin B1-deficient mice have revealed an important role for NRG1 signaling in the calcineurin–NFAT response. Addition of NRG1 to dorsal root ganglion (DRG)–Schwann cell cocultures rapidly increases Ca2+ levels in Schwann cells and activates NFATc3 and –c4 isoforms detected immunocytochemically by dephosphorylating them (Kao et al., 2009). We found that nfatc1–c4 isoforms are present in rat Schwann cells, nfatc3 being the predominant isoform and nfatc4 being expressed at the lowest level.
Cyclosporin, a well-characterized inhibitor of the calcineurin–NFAT pathway, abolishes the potentiation of the Krox-20 response by ionophore, although it does not affect the number of cells expressing Krox-20 in response to dbcAMP. In our immunocytochemical and Western blot experiments, we also found that neither cyclosporin nor FK506 (not shown) could block induction of Krox-20 induced by dbcAMP alone. Furthermore, P-VIVIT, a selective inhibitor of the NFAT-calcineurin interaction (Aramburu et al., 1999), substantially reduced Krox-20 induction in response to dbcAMP plus ionophores. It should be noted that transfection of the control pEGFP-N1 plasmid alone caused some suppression of the Krox-20 response.
Transfection of HA-NFAT1(1–460)-GFP into Schwann cell cultures showed that treatment with dbcAMP alone was, however, sufficient to induce nuclear translocation in a substantial proportion of transfected cells, although addition of either A23187 or ionomycin potentiated the response. In both cases, the response was blocked by cyclosporin, suggesting that initial NFAT translocation to the nucleus depends on cAMP activation of calcineurin. This is further potentiated by Ca2+ activation of calcineurin. The finding that Krox-20 induction is not blocked by cyclosporin although NFAT translocation to the nucleus is blocked suggests that it is unlikely that induction of Krox-20 by cAMP is channeled exclusively through the calcineurin–NFAT pathway. This is supported by other evidence, first, because the hypomyelinating phenotype induced by lack of the calcineurin B subunit is much less severe than that seen in Krox-20 null mice, in which a complete arrest of myelination is seen, and, second, because activation of the cAMP response element-binding (CREB) family of proteins is required for Krox-20 induction in rat Schwann cells, and these proteins are likely to have direct effects on the Krox-20 promoter (Topilko et al., 1994; Arthur-Farraj et al., 2011). Three members of this family, CREB, CREM, and ATF1, are expressed by Schwann cells (Stewart, 1995; Arthur-Farraj et al., 2011). Furthermore, CRE elements have been identified in the Oct-6 enhancer MSE, and Oct-6 and Sox10 synergize to activate the Schwann cell enhancer (mSCE) of the Krox-20 gene (Ghislain and Charnay, 2006; Svaren and Meijer, 2008; Jagalur et al., 2011).
Calcineurin regulates several transcription factors other than NFAT, including the CREB coactivator TORC2, and some effects of cyclosporin involve neither calcineurin nor NFAT (Kiani et al., 2000; Screaton et al., 2004). Nevertheless, compelling evidence indicates that the calcineurin–NFAT pathway is the principal molecular target for these inhibitors, and the results obtained using P-VIVIT strongly suggest that cyclosporin exerts its effects via inhibition of the NFAT–calcineurin interaction in our experiments. This conclusion is supported by the fact that the Krox-20 promoter contains NFAT binding sites, and the observed nuclear translocation of NFAT in Schwann cells (Liu et al., 1991; Abbott et al., 1998; Graef et al., 2001; Kao et al., 2009).
It is possible that c-Jun may also be involved in the regulation of NFAT in Schwann cells because JNK/c-Jun has been reported to phosphorylate NFATc1 and NFATc3 and to promote export of NFATc3 from the nucleus (Chow et al., 1997). We have recently shown that c-Jun is a crucial regulator of the phenotype of the repair Schwann cell that is generated after nerve transection to promote nerve regeneration and that it suppresses myelin differentiation in cultured cells (Parkinson et al., 2004, 2008; Jessen and Mirsky, 2008; Arthur-Farraj et al., 2012). Schwann cell c-Jun is suppressed by Krox-20 in vivo and by elevation of cAMP in vitro. Krox-20 suppression of c-Jun could therefore also contribute to increased levels of nuclear NFATc3 (Parkinson et al., 2004, 2008).
The present work complements and extends further the in vitro findings that elevation of intracellular cAMP in quiescent purified rat Schwann cell cultures induces Krox-20 expression and myelin related differentiation (Morgan et al., 1991; Parkinson et al., 2003, 2004, 2008; Monje et al., 2009, 2010; Arthur-Farraj et al., 2011). Our findings suggest that the Ca2+-activated calcineurin–NFAT pathway may participate in the neuron–Schwann cell signaling cascade in partnership with the cAMP pathway to induce the expression of Krox-20. This suggestion is consistent with previous studies showing that, upon electrical stimulation, DRG neurons cocultured with Schwann cells release both ATP and adenosine, which in turn bind to P2Y purinergic receptors that elevate intracellular Ca2+- or cAMP-linked A2 adenosine receptors to affect proliferation and differentiation of Schwann cells, albeit at the premyelinating stage (Colomar and Amedee, 2001; Stevens and Fields, 2000; Stevens et al., 2004).
In conclusion, our findings indicate that Krox-20 induction in cultured Schwann cells requires cAMP elevation. Schwann cells express NFAT isoforms 1–4, and our study shows that the calcineurin–NFAT pathway alone is insufficient to stimulate Krox-20 expression in Schwann cells. This pathway does, however, potentiate Krox-20 expression induced by dbcAMP in Schwann cell cultures in vitro, an effect that is blocked by the calcineurin inhibitors cyclosporin A and P-VIVIT.
Acknowledgments
We thank Peter Brophy and Diane Sherman for antibodies and Jane Pendjiky for help with the illustrations.
Contract grant sponsor: Commonwealth Scholarship Commission (to M.J.K.); Contract grant sponsor: Wellcome Trust (to K.R.J., R.M.); Contract grant sponsor: MRC (to K.R.J., R.M.).
References
- Abbott KL, Friday BB, Thaloor D, Murphy TJ, Pavlath GK. Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells. Mol Biol Cell. 1998;9:2905–2916. doi: 10.1091/mbc.9.10.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramburu J, Garcia-Cózar F, Raghavan A, Okamura H, Rao A, Hogan PG. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol Cell. 1998;1:627–637. doi: 10.1016/s1097-2765(00)80063-5. [DOI] [PubMed] [Google Scholar]
- Aramburu J, Yaffe MB, López-Rodríguez C, Cantley LC, Hogan PG, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- Arthur-Farraj P, Wanek K, Hantke J, Davis CM, Jayakar A, Parkinson DB, Mirsky R, Jessen KR. Mouse Schwann cells need both NRG1 and cyclic AMP to myelinate. Glia. 2011;59:720–733. doi: 10.1002/glia.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman R, Turmaine M, Wicher GK, et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Cameron AM, Steiner JP, Roskams AJ, Ali SM, Ronnett GV, Snyder SH. Calcineurin associated with the inositol 1,4,5-trisphosphate receptor–FKBP12 complex modulates Ca2+ flux. Cell. 1995;83:463–472. doi: 10.1016/0092-8674(95)90124-8. [DOI] [PubMed] [Google Scholar]
- Chow CW, Rincón M, Cavanagh J, Dickens M, Davis RJ. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science. 1997;278:1638–1641. doi: 10.1126/science.278.5343.1638. [DOI] [PubMed] [Google Scholar]
- Colomar A, Amedee T. ATP stimulation of P2X7 receptors activates three different ionic conductances on cultured mouse Schwann cells. Eur J Neurosci. 2001;14:927–936. doi: 10.1046/j.0953-816x.2001.01714.x. [DOI] [PubMed] [Google Scholar]
- Decker L, Desmarquet-Trin-Dinh C, Taillebourg E, Ghislain J, Vallat JM, Charnay P. Peripheral myelin maintenance is a dynamic process requiring constant Krox20 expression. J Neurosci. 2006;26:9771–9779. doi: 10.1523/JNEUROSCI.0716-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Sinanan A, Parkinson D, Parmantier E, Mirsky R, Jessen KR. Schwann cell development in embryonic mouse nerves. J Neurosci Res. 1999;56:334–348. doi: 10.1002/(SICI)1097-4547(19990515)56:4<334::AID-JNR2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Finzsch M, Schreiner S, Kichko T, Reeh P, Tamm ER, Bösl MR, Meijer D, Wegner M. Sox10 is required for Schwann cell identity and progression beyond the immature Schwann cell stage. J Cell Biol. 2010;189:701–712. doi: 10.1083/jcb.200912142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghislain J, Charnay P. Control of myelination in Schwann cells: a Krox20 cis-regulatory element integrates Oct6, Brn2 and Sox10 activities. EMBO Rep. 2006;7:52–58. doi: 10.1038/sj.embor.7400573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef IA, Chen F, Chen L, Kuo A, Crabtree GR. Signals transduced by Ca2+/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell. 2001;105:863–875. doi: 10.1016/s0092-8674(01)00396-8. [DOI] [PubMed] [Google Scholar]
- Heit JJ, Apelqvist AA, Gu X, Winslow MM, Neilson JR, Crabtree GR, Kim SK. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006;443:345–349. doi: 10.1038/nature05097. [DOI] [PubMed] [Google Scholar]
- Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- Jagalur NB, Ghazvini M, Mandemakers W, Driegen S, Maas A, Jones EA, Jaegle M, Grosveld F, Svaren J, Meijer D. Functional dissection of the Oct6 Schwann cell enhancer reveals an essential role for dimeric Sox10 binding. J Neurosci. 2011;31:8585–8594. doi: 10.1523/JNEUROSCI.0659-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SW, Svaren J. Induction of myelin protein zero by early growth response 2 through upstream and intragenic elements. J Biol Chem. 2009;284:20111–20120. doi: 10.1074/jbc.M109.022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Signals that determine Schwann cell identity. J Anat. 2002;200:367–376. doi: 10.1046/j.1469-7580.2002.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56:1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Brennan A, Morgan L, Mirsky R, Kent A, Hashimoto Y, Gavrilovic J. The Schwann cell precursor and its fate: a study of cell death and differentiation during gliogenesis in rat embryonic nerves. Neuron. 1994;12:509–527. doi: 10.1016/0896-6273(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Kao SC, Wu H, Xie J, Chang CP, Ranish JA, Graef IA, Crabtree GR. Calcineurin/NFAT signaling is required for neuregulin-regulated Schwann cell differentiation. Science. 2009;323:651–654. doi: 10.1126/science.1166562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani A, Rao A, Aramburu J. Manipulating immune responses with immunosuppressive agents that target NFAT. Immunity. 2000;12:359–372. doi: 10.1016/s1074-7613(00)80188-0. [DOI] [PubMed] [Google Scholar]
- Kim M-S, Ushachev YM. Mitochondrial Ca2+ cycling facilitates activation of the transcription factor NFAT in sensory neurons. J Neurosci. 2009;29:12101–12114. doi: 10.1523/JNEUROSCI.3384-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langnaese K, John R, Schweizer H, Ebmeyer U, Keilhoff G. Selection of reference genes for quantitative real-time PCR in a rat asphyxial cardiac arrest model. BMC Mol Biol. 2008;9:53. doi: 10.1186/1471-2199-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic V, Zullo AJ, Schweitzer MN, Staton TL, Gallo EM, Crabtree GR, Glimcher LH. The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells. Nat Immunol. 2009;10:306–313. doi: 10.1038/ni.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le N, Nagarajan R, Wang JY, Svaren J, LaPash C, Araki T, Schmidt RE, Milbrandt J. Nab proteins are essential for peripheral nervous system myelination. Nat Neurosci. 2005;8:932–940. doi: 10.1038/nn1490. [DOI] [PubMed] [Google Scholar]
- Lee M, Brennan A, Blanchard A, Zoidl G, Dong Z, Tabernero A, Zoidl C, Dent MA, Jessen KR, Mirsky R. P0 is constitutively expressed in the rat neural crest and embryonic nerves and is negatively and positively regulated by axons to generate non-myelin-forming and myelin-forming Schwann cells, respectively. Mol Cell Neurosci. 1997;8:336–350. doi: 10.1006/mcne.1996.0589. [DOI] [PubMed] [Google Scholar]
- Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–delta delta Ct) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Monje PV, Rendon S, Athauda G, Bates M, Wood PM, Bunge MB. Non-antagonistic relationship between mitogenic factors and cAMP in adult Schwann cell re-differentiation. Glia. 2009;57:947–961. doi: 10.1002/glia.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje PV, Soto J, Bacallao K, Wood PM. Schwann cell dedifferentiation is independent of mitogenic signaling and uncoupled to proliferation: role of cAMP and JNK in the maintenance of the differentiated state. J Biol Chem. 2010;285:31024–1036. doi: 10.1074/jbc.M110.116970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk KR, Naylor SG, Glenn TD, Mercurio S, Perlin JR, Dominguez C, Moens CB, Talbot WS. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science. 2009;325:1402–1405. doi: 10.1126/science.1173474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk KR, Oshima K, Jörs S, Heller S, Talbot WS. Gpr126 is essential for peripheral nerve development and myelination in mammals. Development. 2011;138:2673–2680. doi: 10.1242/dev.062224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan L, Jessen KR, Mirsky R. The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (O4+) to a myelin phenotype (P0+, GFAP−, N-CAM−, NGF- receptor−) depends on growth inhibition. J Cell Biol. 1991;112:457–467. doi: 10.1083/jcb.112.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan L, Jessen KR, Mirsky R. Negative regulation of the P0 gene in Schwann cells: suppression of P0 mRNA and protein induction in cultured Schwann cells by FGF2 and TGF beta 1, TGF beta 2 and TGF beta 3. Development. 1994;120:1399–1409. doi: 10.1242/dev.120.6.1399. [DOI] [PubMed] [Google Scholar]
- Murphy P, Topilko P, Schneider-Manoury S, Seitanidou T, Baron Van Evercooren A, Charnay P. The regulation of Krox-20 expression reveals important steps in the control of peripheral glial cell development. Development. 1996;122:2847–2857. doi: 10.1242/dev.122.9.2847. [DOI] [PubMed] [Google Scholar]
- Nagarajan R, Svaren J, Le N, Araki T, Watson M, Milbrandt J. EGR2 mutations in inherited neuropathies dominant-negatively inhibit myelin gene expression. Neuron. 2001;30:355–368. doi: 10.1016/s0896-6273(01)00282-3. [DOI] [PubMed] [Google Scholar]
- Nagarajan R, Le N, Mahoney H, Araki T, Milbrandt J. Deciphering peripheral nerve myelination by using Schwann cell expression profiling. Proc Natl Acad Sci U S A. 2002;99:8998–9003. doi: 10.1073/pnas.132080999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura H, Aramburu J, García-Rodríguez C, Viola JP, Raghavan A, Tahiliani M, Zhang X, Qin J, Hogan PG, Rao A. Concerted de-phosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol Cell. 2000;6:539–550. doi: 10.1016/s1097-2765(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Parkinson DB, Dickinson S, Bhaskaran A, Kinsella MT, Brophy PJ, Sherman DL, Sharghi-Namini S, Duran Alonso MB, Mirsky R, Jessen KR. Regulation of the myelin gene periaxin provides evidence for Krox-20-independent myelin-related signalling in Schwann cells. Mol Cell Neurosci. 2003;23:13–27. doi: 10.1016/s1044-7431(03)00024-1. [DOI] [PubMed] [Google Scholar]
- Parkinson DB, Bhaskaran A, Droggiti A, Dickinson S, D’Antonio M, Mirsky R, Jessen KR. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J Cell Biol. 2004;164:385–394. doi: 10.1083/jcb.200307132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, Feltri ML, Wrabetz L, Behrens A, Mirsky R, Jessen KR. c-Jun is a negative regulator of myelination. J Cell Biol. 2008;181:625–637. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierano RI, Goerich DE, Riethmacher D, Wegner M. Protein zero gene expression is regulated by the glial transcription factor Sox10. Mol Cell Biol. 2000;20:3198–3209. doi: 10.1128/mcb.20.9.3198-3209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengarajan J, Mittelstadt PR, Mages HW, Gerth AJ, Kroczek RA, Ashwell JD, Glimcher LH. Sequential involvement of NFAT and Egr transcription factors in FasL regulation. Immunity. 2000;12:293–300. doi: 10.1016/s1074-7613(00)80182-x. [DOI] [PubMed] [Google Scholar]
- Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S, Guzman E, Niessen S, Yates JR, III, Takemori H, Okamoto M, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Soleimanpour SA, Crutchlow MF, Ferrari AM, Raum JC, Groff DN, Rankin MM, Liu C, De León DD, Naji A, Kushner JA, Stoffers DA. Calcineurin signaling regulates human islet β-cell survival. J Biol Chem. 2010;285:40050–40059. doi: 10.1074/jbc.M110.154955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science. 2000;287:2267–2271. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- Stevens B, Ishibashi T, Chen JF, Fields RD. Adenosine: an activity-dependent axonal signal regulating MAP kinase and proliferation in developing Schwann cells. Neuron Glia Biol. 2004;1:23–34. doi: 10.1017/s1740925x04000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart HJ. Expression of c-Jun, Jun B, Jun D and cAMP response element binding protein by Schwann cells and their precursors in vivo and in vitro. Eur J Neurosci. 1995;7:1366–1375. doi: 10.1111/j.1460-9568.1995.tb01128.x. [DOI] [PubMed] [Google Scholar]
- Svaren J, Meijer D. The molecular machinery of myelin gene transcription in Schwann cells. Glia. 2008;56:1541–1551. doi: 10.1002/glia.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, Babinet C, Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371:796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- Zorick TS, Syroid DE, Brown A, Gridley T, Lemke G. Krox-20 controls SCIP expression, cell cycle exit and susceptibility to apoptosis in developing myelinating Schwann cells. Development. 1999;126:1397–1406. doi: 10.1242/dev.126.7.1397. [DOI] [PubMed] [Google Scholar]