ABSTRACT
Cell wall-modifying enzymes have been previously investigated in charophyte green algae (CGA) in cultures of uniform age, giving limited insight into their roles. Therefore, we investigated the in situ localisation and specificity of enzymes acting on hemicelluloses in CGA genera of different morphologies and developmental stages. In vivo transglycosylation between xyloglucan and an endogenous donor in filamentous Klebsormidium and Zygnema was observed in longitudinal cell walls of young (1 month) but not old cells (1 year), suggesting that it has a role in cell growth. By contrast, in parenchymatous Chara, transglycanase action occurred in all cell planes. In Klebsormidium and Zygnema, the location of enzyme action mainly occurred in regions where xyloglucans and mannans, and to a lesser extent mixed-linkage β-glucan (MLG), were present, indicating predominantly xyloglucan:xyloglucan endotransglucosylase (XET) activity. Novel transglycosylation activities between xyloglucan and xylan, and xyloglucan and galactomannan were identified in vitro in both genera. Our results show that several cell wall-modifying enzymes are present in CGA, and that differences in morphology and cell age are related to enzyme localisation and specificity. This indicates an evolutionary significance of cell wall modifications, as similar changes are known in their immediate descendants, the land plants.
This article has an associated First Person interview with the first author of the paper.
KEY WORDS: Charophyte green algae, Mixed-linkage β-glucan, Transglycosylation, Xyloglucan transglucosylase hydrolase, Xylan, Xyloglucan
Summary: The distribution of major hemicelluloses and cell wall-modifying enzymes in three charophyte green algae (CGA), representing the ancestors of land plants, relate to algal morphology and developmental stage.
INTRODUCTION
A shared feature of plants and most green algae is that their cells are surrounded by cell walls, which are a diverse composite of complex polysaccharides and crucial for plant function and survival (Popper et al., 2011). In particular, walls of late diverged charophyte green algae (CGA, e.g. Zygnematophyceae, Charophyceae) and land plants exhibit chemical similarities, while more ancient CGA (e.g. Klebsormidiophyceae) lack some of the components found in their descendants (Table S1). This supports the hypothesis that the entire land plant lineage evolved from a single group within the CGA, namely the Zygnematophyceae (Wickett et al., 2014), which were able to colonize terrestrial habitats about 460 million years ago (Becker and Marin, 2009). Consequently, it has been proposed that land plants inherited the major cell wall components from their algal ancestors (Domozych et al., 2012) with a cell wall considered a prerequisite for terrestrial survival (Harholt et al., 2016). It has been shown recently that flexible cell walls mediated by desiccation-induced callose deposition in Klebsormidium (Herburger and Holzinger, 2015) or the specific occurrence of pectic substances in the macroalgae Ulva compressa (Holzinger et al., 2015) coincide with elevated desiccation tolerance in aero-terrestrial or intertidal habitats, respectively. This suggests that modulating the cell wall architecture and composition in response to abiotic stress was crucial for the survival of algal colonizers of terrestrial habitats. Although the cell walls of various CGA have been explored over the past decades, there are many remaining questions regarding the localisation and metabolism of specific wall components.
Polysaccharides of plant cell walls are synthesized by glycosyltransferases (GTs) within Golgi bodies (hemicelluloses and pectins) or at the plasma membrane (cellulose and callose) and are secreted into the cell wall (Scheller and Ulvskov, 2010; Harholt et al., 2010). In plant cell walls, specific enzymes modify the hemicelluloses, for example by hydrolysis or transglycosylation (Franková and Fry, 2013). Hemicelluloses are a group of polysaccharides that interact, typically through hydrogen bonds, with cellulose microfibrils (Carpita and Gibeaut, 1993; Park and Cosgrove, 2012). While hydrolases cleave glycosidic bonds in the backbone of cell wall polysaccharides (e.g. the β-1→4-bond between d-glucopyranose residues in xyloglucan), transglycosylases cut a polysaccharide chain (donor) and reattach it to an acceptor substrate (Rose et al., 2002). The latter can be either an endogenous cell wall polysaccharide or an exogenous oligosaccharide (Fry, 1997). Xyloglucan is one of the most abundant hemicelluloses in the primary cell walls of non-commelinid flowering plants (Fry, 2011). Processing by xyloglucan endotransglucosylase hydrolase (XTH; EC 2.4.1.207) aids the incorporation of newly synthesized xyloglucan into the cell wall (Thompson et al., 1997), loosening of cell walls during expansive cell growth (Fry et al., 1992; Van Sandt et al., 2007), shrinkage of tension wood fibres in trees in response to gravitropism (Nishikubo et al., 2007), and fruit growth and ripening (Han et al., 2015). Other donor substrates for transglycosylases are mannans, mixed-linkage (1→3,1→4)-β-d-glucan (MLG), cellulose and, to a lesser extent, xylans (Schröder et al., 2004; Fry et al., 2008a; Simmons et al., 2015; Shinohara, et al., 2017). Transglycosylation activity between xyloglucan and either xyloglucan (xyloglucan:xyloglucan endotransglucosylase activity; XET) or MLG (MLG:xyloglucan endotransglucosylase activity; MXE) has also been demonstrated in extracts of some charophytes in vitro (Fry et al., 2008a). Furthermore, blotting algal thalli onto paper coated with sulphorhodamine-labelled xyloglucan oligosaccharides (XyGO-SRs) (tissue prints) suggested that there was transglycosylase activity in vitro in growth zones of the macroalgae Chara (Charophyta) and Ulva (Chlorophyta) (Van Sandt et al., 2007a). While the tissue-printing technique provides a good spatial estimation of transglycosylase activities at the tissue level (e.g. Olsen et al., 2016), it is less precise than in vivo techniques that are able to resolve enzyme action at the cellular level (Vissenberg et al., 2000). For green algae, the resolution of transglycosylase action at the cellular level is still missing. This has resulted in a considerable knowledge gap, particularly for filamentous and unicellular green algae that are too small for the tissue-printing technique to be applied. Knowledge of the precise spatiotemporal localisation of wall-modifying enzymes would provide valuable new insights into the mechanisms of cell growth in simple multicellular plants.
The present study focuses on three members of the CGA, Klebsormidium, Zygnema and Chara. The latter forms morphologically complex thalli and grows in the water body of lakes and ponds, while filamentous Klebsormidium and Zygnema occur worldwide in limnic and aero-terrestrial habitats and fulfil numerous important ecological functions as components of biological soil crusts (Elbert et al., 2012). With increasing age, cell walls of Zygnema and Klebsormidium undergo dramatic changes, such as an increase in diameter and the formation of additional layers (Mikhailyuk et al., 2014; Herburger et al., 2015; Pichrtová et al., 2016a). However, information is scarce regarding whether these morphological changes also involve changes in the chemical composition of the cell wall or the activity and specificity of cell wall-modifying enzymes. To date, algal cell or filament age as a factor influencing the architecture and composition of the cell wall, has received little attention. This is surprising since cell wall composition and the hemicelluloses (e.g. xyloglucan, mannans) incorporated into the wall are known to be altered in response to cell age (Métraux, 1982; Morrison et al., 1993). We investigated the donor substrate specificity and localisation of transglycanases in vitro and in vivo. This is the first study showing both the location of the transglycosylase action in vivo and at the cellular level in charophyte algae. Long-term cultivation experiments (up to 1 year) allowed us to compare enzyme activity/action in algae of different culture age and cells of different developmental stages. Based on observations of algal populations in various hydro- and aero-terrestrial habitats (e.g. Karsten et al., 2010; Pichrtová et al., 2014) and cultured algae (e.g. Herburger et al., 2015), we hypothesized that cell age changes: (1) the architecture of the cell wall (i.e. distribution of hemicelluloses), and (2) the activity and specificity of enzymes acting on cell wall polysaccharides. Possible biological functions of different hemicelluloses as well as implications for the high ecophysiological and evolutionary success of these algae are discussed.
RESULTS
Age-dependent cell wall thickening correlates with higher proportion of pectins or hemicelluloses
To test whether increasing cell age (1 month compared to 1 year) changes the cell wall composition of Klebsormidium and Zygnema S, the alcohol-insoluble residue (AIR) of algal filaments was fractionated (Fig. 1) and analysed. Zygnema S filaments possessed a larger pectin fraction when compared with Klebsormidium, with highest amounts being found in old filaments. In contrast, increasing cell age increased the total hemicellulose content of Klebsormidium. This suggests that age-dependent cell wall thickening in Zygnema is characterized by an increase in the pectin content, while in Klebsormidium cell walls are thickened by deposition of hemicelluloses.
Fig. 1.
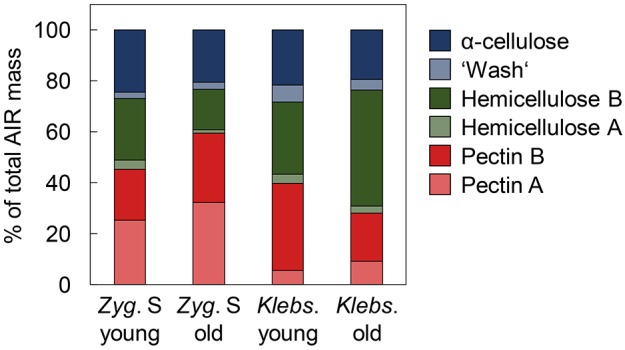
Fractionation of cell wall components from young and old Zygnema S and Klebsormidium crenulatum filaments into six classes. Classes are shown as percentages of total alcohol-insoluble residue (AIR). n=3 (s.d. <5%). Zyg. S, Zygnema S; Klebs., Klebsormidium. Young filaments are 1 month old; old filaments are 12 months old.
Zygnema S transglycanases accept a wider range of donor substrates than Chara and Klebsormidium in vitro
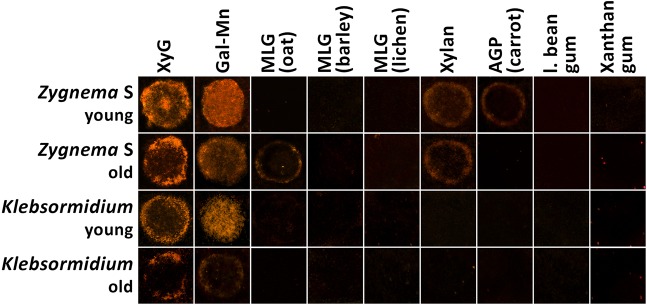
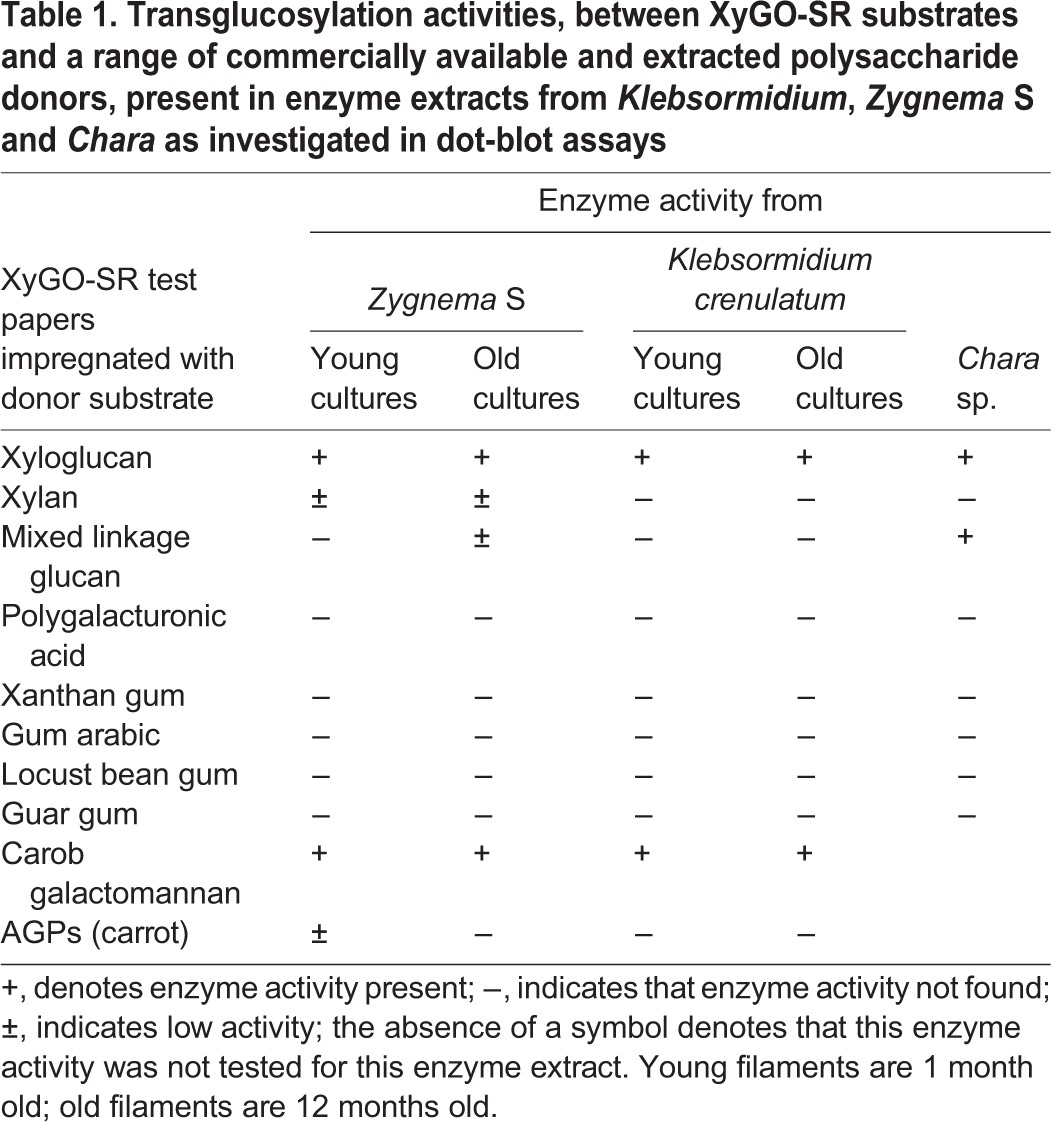
To estimate whether different hemicellulose contents in Klebsormidium and Zygnema S and in young and old filaments coincide with different substrate specificities of hemicellulose-modifying enzymes, a dot-blot assay testing the transglycosylase activities of extracted algal proteins was carried out. Extracts from freshly isolated Chara thalli were also analysed, but only confirmed previous results showing transgylcosylase activity between xyloglucan:xyloglucan and xyloglucan:MLG (e.g. Fry et al., 2008a). Zygnema S extracts exhibited transglycosylase activity towards all major hemicelluloses tested [xyloglucan, galactomannan, MLG (old extracts), xylan] and arabinogalactan proteins (AGPs; young extracts) (Fig. 2, Table 1). In contrast, the activity of extracts from Chara and Klebsormidium was restricted to xyloglucans and galactomannan only. Klebsormidium extracts showed the lowest detection signals (Table 1).
Fig. 2.
Dot-blot assay for transglycosylase activity of enzyme extracts of young and old Zygnema S and Klebsormidium crenulatum filaments. Test papers were coated with 1% (w/v) solutions of different cell wall polysaccharides and ∼5 mM XyGO-SR. Enzyme extracts (in 5 µl aliquots), were loaded on the test papers and incubated for 2 h before washing with an ethanol:formic acid:water (1:1:1, v/v/v) mixture for 2 h, rinsing twice with distilled water and drying overnight. XyGO-SR was visualized at 365 nm. Young filaments are 1 month old; old filaments are 12 months old.
Table 1.
Transglucosylation activities, between XyGO-SR substrates and a range of commercially available and extracted polysaccharide donors, present in enzyme extracts from Klebsormidium, Zygnema S and Chara as investigated in dot-blot assays
Transglycosylation between xyloglucan and endogenous donor substrates occurs in charophyte cell walls in vivo
Since transglycosylase activity (in vitro) was found in all charophytes analysed, algae were exposed to fluorescent XyGO-SRs to test for transglycosylase action (in vivo). Both, young Zygnema S and Klebsormidium filaments incorporated XyGO-SR fluorescence into their outer cell walls, including terminal cross cell walls, but not in the cell corners between individual cells or in the inner cross cell walls (Fig. 3A,E,F). This highly specific occurrence of enzyme action was also found in younger, growing cells and occasionally occurred in old Zygnema S filaments, but was not seen in the majority of cells (i.e. thick-walled pre-akinetes) within the same filaments (Fig. 3B). Old Klebsormidium filaments with thick cell walls were predominantly devoid of fluorescence (Fig. 3E) with the exception that some filaments contained dead cells that exhibited strong auto-fluorescence derived from cytoplasmic residue. The autofluorescence associated with dead cells is distinct from XyGO-SR fluorescence and was also observed in dead cells of control filaments (data not shown). In contrast to Zygnema S and Klebsormidium, parenchymatous Chara sp. incorporated XyGO-SRs into all cell planes with a maximum in younger cells towards the apex of the main axis and the branchlets, and in the walls of the stipulodes (Fig. 4).
Fig. 3.
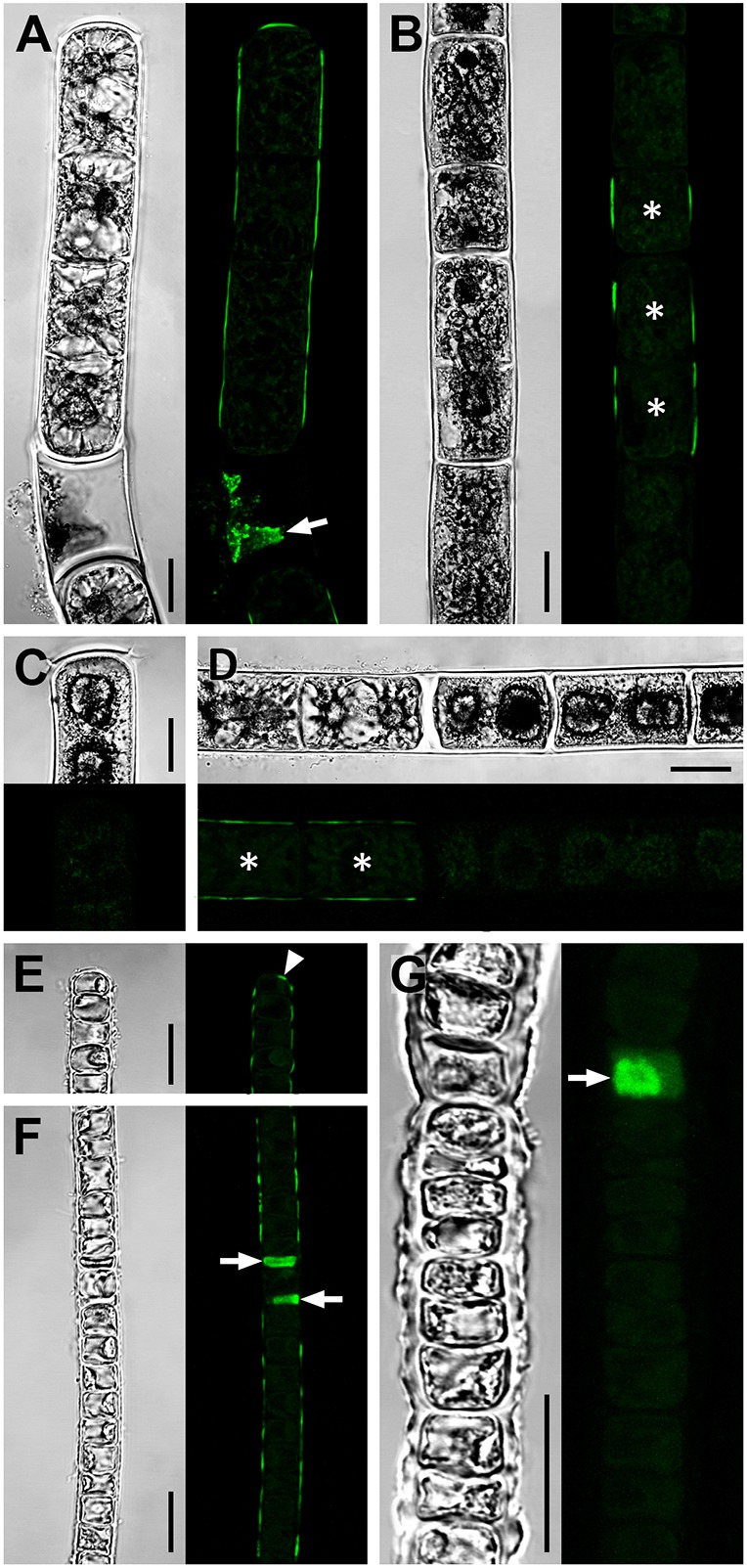
Transglycosylase action in young and old Zygnema S and Klebsormidium crenulatum filaments. Confocal micrographs showing integration of the fluorescent acceptor substrate XyGO-SR in young (A,E,F) and old (B–D,G) filaments of Zygnema S (A–D) and Klebsormidium crenulatum (E–G) indicative of transglycosylase action. After XyGO-SR incorporation, filaments were incubated in DMF to remove chlorophyll autofluorescence. Cells that are dead prior to XyGO-SR incubation were seen to contain fluorescent cytoplasmic residue (A,F,G; arrows). Corresponding bright-field images are also shown. (A) Filament with fluorescence in outer cell walls and a terminal cross cell wall. (B) Filament showing fluorescence in longitudinal cell walls of short cells (asterisks), but not in longer cells. (C) Terminal cell lacking fluorescence. (D) Filament with fluorescence in outer walls of two vegetative cells (asterisks) but not in adjacent pre-akinetes. (E,F) Filaments with fluorescence in outer cell walls including a terminal cross cell wall (arrowhead). (G) Filament lacking fluorescence in cell walls. Young filaments are 1 month old; old filaments are 12 months old. Scale bars: 10 µm.
Fig. 4.
Fluorescence microscopy images showing integration of the fluorescent acceptor substrate XyGO-SR into Chara sp. cell walls. After incubation in XyGO-SRs, Chara was washed in culture medium and viewed using the DAPI channel of an epifluorescence microscope at ×40 magnification. (A) Bright-field image. (B,C) Incorporation of XyGO-SRs in all cell walls. The walls of the stipulodes, and the cells towards the tip of the main axis and branchlets appeared to have incorporated the most XyGO-SRs and fluoresced the most strongly. (D) Control in which Chara sp. was incubated with non-fluorescent XyGOs.
Highly complex cell wall composition
Colocalisation of XyGO-SRs and transglycosylase action does not provide information regarding the presence of potential endogenous donor substrates. Therefore, a set of cell wall polymer-specific monoclonal antibodies (mAbs) was used to generate a spatial map for hemicellulose distribution in the cell walls of Zygnema S (Fig. 5) and Klebsormidium (Fig. 6). We labelled whole cells (Figs 5 and 6; Fig. S2A,B) and sections of high-pressure frozen filaments, the latter exposing cross cell walls directly to the mAbs (Fig. S2C–F). Results are summarized in Fig. S3. The outer and cross cell walls of young and old Zygnema S filaments (Fig. 5A–D, in Fig. 5A one optical section is shown, whereas in Fig. 5B–D z-projections of ∼50 optical sections are shown; Fig. S2C,D) labelled with LM15 (which recognises epitopes present in xyloglucan) and mAb 400-4 [which recognises epitopes present in (1→4)-β-mannans] colocalised with areas of XyGO-SR incorporation. Old filaments lacked mannan epitopes in cross walls (Fig. 5D). In contrast, MLG epitopes (mAb 400-3), showed a punctate labelling pattern in outer cell walls of young filaments (Fig. 5E, one optical section shown). Occasionally, MLG (mAb 400-3) was labelled as a band close to the expanded terminal cross cell walls in young Zygnema S filaments (Fig. 5F, z-stack) whereas old Zygnema S filaments showed stronger MLG labelling in some cells (Fig. 5G, z-stack). Xylan epitopes (mAb LM10) were restricted to H-shaped cell wall structures in both young and old Zygnema S filaments (Fig. 5H,I, z-stacks).
Fig. 5.
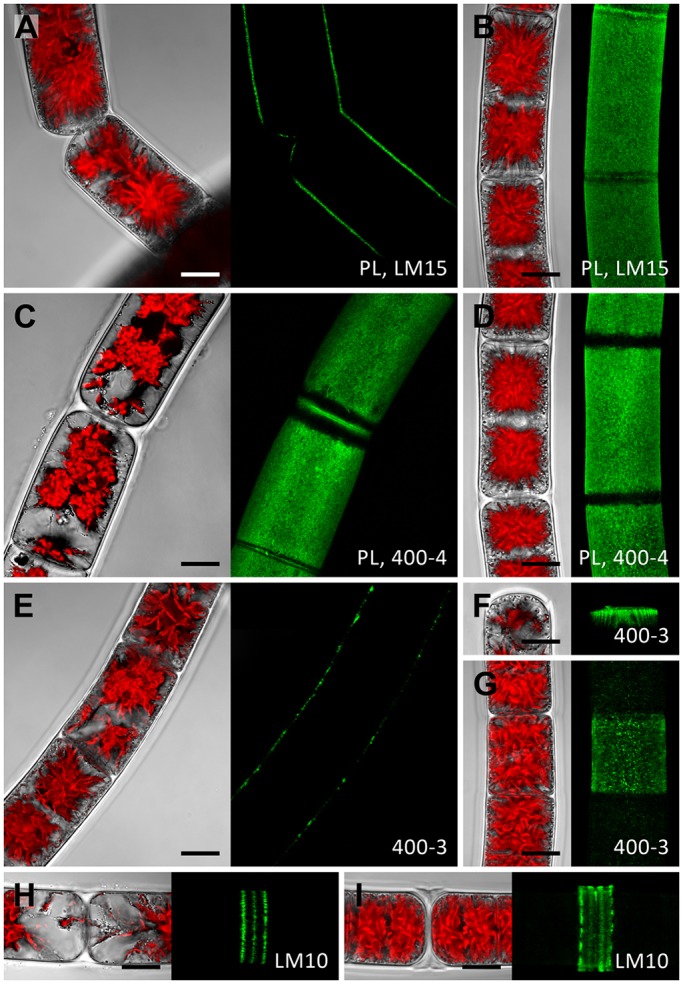
Whole-cell labelling of Zygnema S. Young (A,C,E,F,H) and old (B,D,G,I) filaments labelled with the monoclonal antibodies LM15, 400-4, 400-3 or LM10 (green). In the confocal micrographs in A and E, one optical section is shown, confocal micrographs in B–D and F–I show z-projections of ∼50 optical sections. The corresponding bright-field images include red chloroplast autofluorescence. (A) Detaching cells with staining in exposed cell walls. (B) Staining in outer and cross cell walls but not in ribbon-like zones close to cross cell walls. (C) Similar pattern to that shown in B. (D) Staining in outer cell walls. (E) Filament with patchy labelling in outer cell walls. (F) Circular staining underneath expanded terminal cross wall. (G) Central cell showing patchy straining, which is weak in adjacent cells. (H) H-shaped cell wall structure with staining in three distinct rings. (I) Prominent H-shaped cell wall structure with strong staining. Young filaments are 1 month old; old filaments are 12 months old. Scale bars: 10 µm.
Fig. 6.
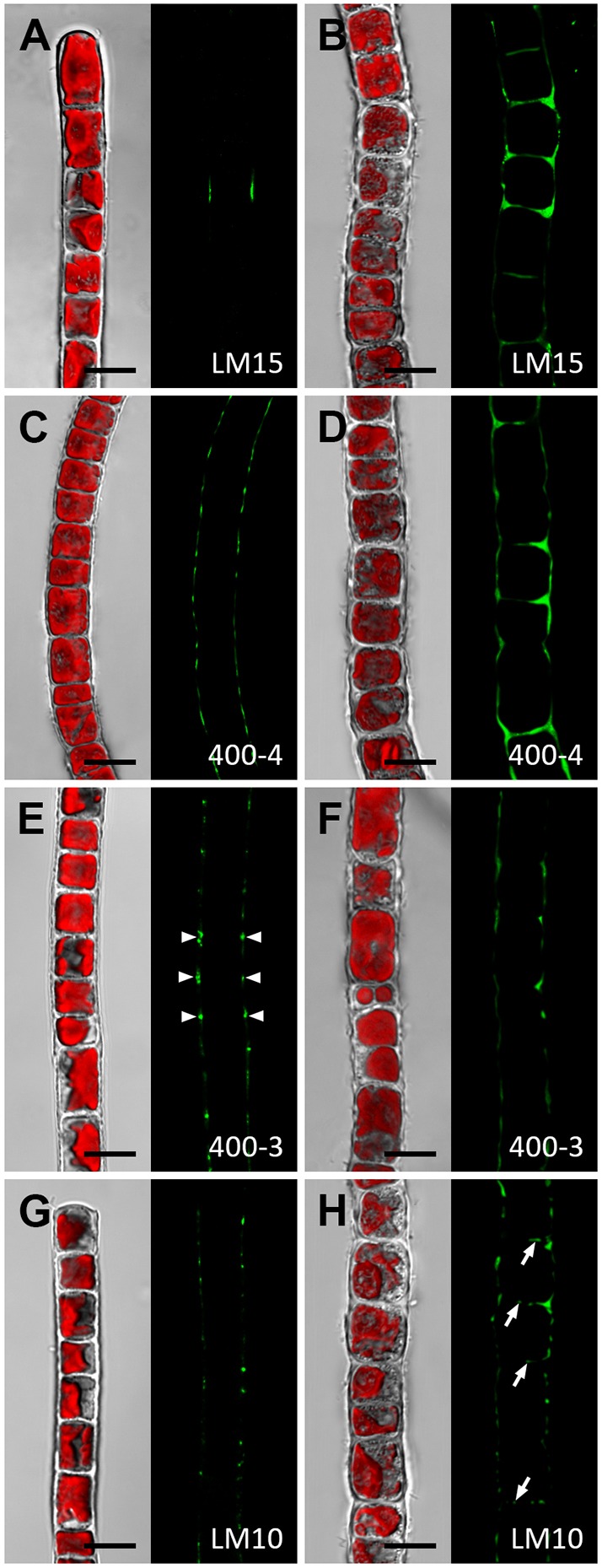
Whole-cell labelling of Klebsormidium crenulatum. Young (A,C,E,G) and old (B,D,G,H) filaments labelled with the monoclonal antibodies LM15, 400-4, 400-3 or LM10 (green) and visualised with a confocal microscope. The corresponding bright-field images include red chloroplast autofluorescence. (A) Filament with weak staining in restricted areas. (B) Intense labelling in thickened cell corners between individual cells and some staining in cross cell walls. (C) Staining in outer cell walls. (D) Intense staining in cell corners and thickened cross cell walls. (E,F) Staining in cell corners (arrowheads) and occasionally in longitudinal cell walls of longer cells. (G) Punctuate staining pattern in outer cell walls. (H) Similar appearance to cells shown in G; additionally, cross cell walls show staining (arrows). Young filaments are 1 month old; old filaments are 12 months old. Scale bars: 10 µm.
In contrast to Zygnema S, xyloglucan (mAb LM15) and mannan epitopes (mAb 400-4) were scarce in young Klebsormidium filaments (Fig. 6A,C; in Fig. 6 single optical sections are shown) with more intense labelling observed in the thickened cell walls of old filaments (Fig. 6B,D). MLG epitopes (mAb 400-3) were restricted to the cell corners between individual Klebsormidium cells (Fig. 6E,F) and xylan epitopes (mAb LM10) showed a punctuate distribution in outer cell walls with occasional labelling of the cross cell walls of old Klebsormidium filaments (Fig. 6H).
Pectate lyase (PL) treatment increased the strength of the antibody signal (Fig. S2A,B), but neither altered labelling patterns nor facilitated detachment of cells [i.e. single cells or small filaments (2–5 cells) were not enriched] in either Zygnema S or Klebsormidium (data not shown).
Transglycosylase activity changes with culture age
To test whether transglycosylase activities of extracts prepared from young and old algal filaments was accompanied by hydrolytic activity, the loss of viscosity of four different polysaccharide solutions was investigated (Fig. 7). Hydrolysis of xyloglucan was strongest after adding extracts prepared from young or old Zygnema S filaments, where the efflux time decreased to ∼13–16% after 1 day (Fig. 7A). In contrast, hydrolysis of galactomannan was greatest following treatment with extracts prepared from old Zygnema S and Klebsormidium filaments, decreasing efflux time to ∼5% after 5 h (Fig. 7B). Extracts from young Zygnema S filaments showed the highest hydrolytic activity towards MLG, reducing efflux time to <10% within 1 h (Fig. 7C). Extracts from young Klebsormidium filaments showed lower hydrolytic activity, which was absent in old Klebsormidium (Fig. 7C). Hydrolysis of xylan was only observed after adding young Zygnema S extracts and the efflux time decreased to ∼25% after 1 day (Fig. 7D).
Fig. 7.
Viscometric assay estimating the hydrolysis of four cell wall polysaccharides by enzyme extracts of young and old Zygnema S and Klebsormidium crenulatum filaments. Reaction mixtures contained 1% (w/v) polysaccharide, 300 mM Na succinate (pH 5.5) and 10% (v/v) dialysed algal enzyme extract. (A) Xyloglucan, (B) galactomannan, (C) mixed-linkage (1→3,1→4)-β-glucan (MLG) from oat, (D) xylan. n=3 (s.d. <5%). Young filaments are 1 month old; old filaments are 12 months old.
DISCUSSION
The present study provides new insights into algal cell wall metabolism by showing that transglycosylase actions are abundant in young filaments of both Klebsormidium and Zygnema S and restricted to longitudinal cell walls, where cell expansion occurs. In contrast, enzymatic actions decrease with increasing filament age. In vitro studies and immunolocalisation of the major hemicelluloses (xyloglucan, mannans, MLG and xylan) suggested that xyloglucan and mannans (Zygnema S) or xyloglucan (Klebsormidium) are the most likely donor substrates. These results show for the first time, that the hemicellulose network of early (Klebsormidium) and late (Zygnema) diverged CGA undergoes strong spatiotemporal changes and might be involved in survival strategies such as pre-akinete formation, regularly observed in Zygnema.
Cell wall composition changes with cell age
Land plant cell walls are remodelled in response to ageing and stresses (e.g. mechanical or chemical stresses). In contrast, the effect of cell age on the cell wall composition of CGA members had not been investigated, even though, for example, increasing cell age and environmental stress triggers the formation of resistant pre-akinetes in Zygnema, which are crucial for survival and involve changes of the cell wall morphology such as massive thickening (e.g. McLean and Pessoney, 1971; Herburger et al., 2015). As found in the present study, age-dependent cell wall thickening in Zygnema S predominantly consists of an increase in the pectin content, while in Klebsormidium cell walls are thickened by deposition of hemicelluloses.
However, in both genera, immunostaining of whole cells and sections of high-pressure frozen filaments with a set of cell wall polymer-specific monoclonal antibodies (mAbs) revealed an increased abundance of hemicellulose epitopes in thickened cell wall parts. In the case of Zygnema S, this was particularly true for MLG and xylan, while xyloglucan and mannan were detectable in both old and young filaments and had levels that were independent of cell size. This suggests that the latter two hemicelluloses are important cell wall components in the species investigated. Both xyloglucan and mannans are associated with homogalacturonan, since the labelling signal strongly increased upon unmasking with PL. This underpins the close relationship between Zygnematophyceae and land plants, where close physical proximity between xyloglucan and pectins, indicating stable interactions, were confirmed by 2D and 3D solid-state nuclear magnetic resonance (ssNMR) (Dick-Pérez et al., 2011). A considerable amount of xyloglucan might be covalently linked to pectins as shown for a range of angiosperm cell suspension cultures (Thompson and Fry, 2000; Popper and Fry, 2008). The restriction of xylan epitopes to thickened cell wall areas in both Zygnema S and Klebsormidium (H-shaped structures between individual cells) resembles findings in land plants (e.g. Nicotiana tabacum), where xylans can be abundant in thickened primary cell walls of collenchyma and epidermis cells (Hervé et al., 2009). As shown recently, land plant xylan interacts tightly with cellulose (Simmons et al., 2016) and its absence impairs cell wall strength and the vessel development. A role of xylan in strengthening algal cell walls is suggested by an increased binding of LM10 to old and thickened Klebsormidium cell walls, while young filaments that are characterised by highly flexible cell walls (Herburger and Holzinger, 2015) showed less binding. Detection of xylans in the early diverged CGA Klebsormidium reflects the occurrences of xylan metabolism-related genes in the recently published Klebsormidium flaccidum genome (Hori et al., 2014). In contrast to xylans, MLG is not a common cell wall component of flowering plants and is restricted to Poales, horsetails, some liverworts, red and brown algae and the CGA (Eder et al., 2008; Fry et al., 2008b; Sørensen et al., 2008; Salmeán et al., 2017; Popper and Tuohy, 2010). In young Zygnema S, a low concentration of MLG was present in outer cell walls, while the signal increased in old filaments and was particularly strong in walls of individual cells. A similar trend was observed in Klebsormidium (Fig. S2), where the epitopes were restricted to only a few cells. Occasionally, binding of mAb 400-3 was detected close to cross cell walls. Correspondingly, binding of mAb 400-3 in Klebsormidium was mostly restricted to the cell corners between individual cells. As shown for Equisetum, the MLG content correlates positively with developmental stage, since it predominates in secondary cell walls (Leroux et al., 2011; Sørensen et al., 2008). The restriction of MLG to secondary cell walls was also found in the green alga Micrasterias (Zygnematophyceae; Eder et al., 2008). Thus, incorporation of MLG in old filaments of Klebsormidium and Zygnema S might be an age-dependent process.
Different transglycosylase activities are associated with culture age
Extracts prepared from young (1 month) and old (1 year) Klebsormidium and Zygnema S cultures and field-collected Chara sp. were capable of incorporating XyGO-SR, therefore they exhibited xyloglucan endotransglycosylase activity. When compared with Klebsormidium and Chara, Zygnema S extracts showed the most lax donor substrate specificity (Fig. 2, Table 1). The restriction of transglycosylase activity toward MLG (i.e. MLG:xyloglucan endotransglucosylase activity; MXE) in old Zygnema S extracts is interesting because it corresponds to findings in Equisetum sp., where older tissues exhibit higher MXE:XET rates than younger tissues (e.g. young compared to old stems; Fry et al., 2008b; Mohler et al., 2013). This might be related to the higher MLG content in secondary cell walls of old Equisetum tissues (see previous paragraph). This suggests that MLG, and its processing by MXE action, may perform similar functions in Equisetum and Zygnematophyceae but not Klebsormidiophyceae (i.e. cell wall strengthening of older cells/tissues) (Fry et al., 2008b).
Intriguingly our cultures exhibited two further novel transglycosylase activities: (1) between xyloglucan and mannans (Klebsormidium, Zygnema S), and (2) between xyloglucan and xylan (Zygnema S). The capability to act on galactomannan is particularly interesting because, although mannan transglycosylase activity, which crafts mannan-based plant polysaccharides including galactomannan onto galactoglucomannan oligosaccharides, has been found in various land plants (Schröder et al., 2004), to our knowledge, no previous studies have reported transglycosylase reactions between galactomannan (donor) and xyloglucan oligosaccharides (acceptor) (McGregor et al., 2017). Although this novel activity needs further investigation, the ability of algae to process mannans by transglycosylation is plausible. By using immunolabelling techniques, we found mannans to occur abundantly in both Klebsormidium and Zygnema S cell walls. This confirms previous studies where mannans were detected in the cell walls of CGA in glycan microarray experiments (Sørensen et al., 2011). Additionally, although members of the CGA were not investigated, a recent study highlights the ancient evolution of the Endo Glucanase 16 (EG16) clade [within Glycoside Hydrolase family 16 (GH16)] as a class of enzymes that are capable of carrying out heterotransglycosylation reactions and have a broad substrate specificity (McGregor et al., 2017).
XET localisation is related to morphology
Localisation studies on Klebsormidium and Zygnema S filaments taken from young and old cultures suggest that transglycanase action is involved into the construction and growth of longitudinal cell walls. The main donor substrates might be xyloglucan and/or mannans, because epitopes of these hemicelluloses colocalised predominately with the sites of enzyme action. The idea that transglycanase activity is involved in cell wall growth of the species investigated is supported by the following observations: (1) that enzyme action is not detectable in cross cell walls, (2) that it is abundant in longitudinal cell walls of young filaments exhibiting expanding cells, but (3) that it is absent in thick-walled cells (pre-akinetes) of old Zygnema S filaments that had ceased growth. However, (4) transglycanase action was present in smaller (i.e. expanding) cells within the same filaments. In contrast to Klebsormidium and Zygnema S, transglycanase action in Chara was also found between individual cells (i.e. all cell planes). A tempting speculation is that these differences are linked to different body plans (parenchymatous versus filamentous) and mechanisms of cell division (phragmoplast versus cleavage/reduced phragmoplast; Staehelin and Hepler, 1996). Chara and land plants use a ‘true phragmoplast’, while cell division in Zygnema S and Klebsormidium occurs perpendicular to the length axis of the uniserate filaments by forming a centripetally encroaching septum between two daughter cells (Graham et al., 2000). Whereas in Klebsormidiophyceae centrosomes organize mitotic spindles, in Zygnematales, centripetal furrowing can be accompanied by the formation of a small cell plate in the cell centre, which is connected to the appearance of a rudimentary phragmoplast (Sawitzky and Grolig, 1995; Scherp et al., 2001; Yoon et al., 2010). Since Zygnematophyceae are considered the sister group to land plants, it has been proposed that the different mechanisms of cell division between Charophyceae/land plants and Zygnematophyceae result partially from reductions (e.g. less complex phragmoplast) in the latter (Buschmann and Zachgo, 2016). One such modification might be that XET is not involved in the construction of the cell plate as found in land plants, where xyloglucan occurs in the equatorial plane during late anaphase and shows a strong spatial correlation with XET action during cell plate formation (Yokoyama and Nishitani, 2001). Interestingly, similar to what is seen for cell plates, Chara cell walls are rich in non-methylesterified homogalacturonan (HG) and contain only low amounts of xyloglucan and cellulose (Sørensen et al., 2011). As proposed by Proseus and Boyer (2008), the ‘pectate cycle’ mediates non-enzymatic anisotropic growth of Chara cells, involving incorporation of HG and the formation of new Ca2+–HG links. Young Chara cells predominantly incorporate xyloglucan oligosaccharides into their cell walls, suggesting that transglycanase action is nevertheless involved in cell growth as well and accompanies the ‘pectate cycle’.
Hydrolytic activities also change with cell age
As well as transglycosylases, plants contain numerous enzymes that use water as an acceptor substrate resulting in the hydrolysis of a polysaccharide. Franková and Fry (2011) screened enzyme extracts from more than 50 land plants and revealed a variety of hydrolytic activities, including β-d-xylosidase, endo-(1→4)-β-d-xylanase, β-d-mannosidase and endo-(1→4)-β-d-mannanase, α-d-xylosidase activities. As shown by a viscometric assay, extracts prepared from young cultures caused more rapid scission of xyloglucan, MLG and xylan compared with extracts from older cultures. However, when galactomannan was added as a substrate, old Klebsormidium and Zygnema S extracts had higher hydrolytic activities. Mannose-containing polysaccharides are considered among the main hemicelluloses in CGA (Popper, 2008). Land plant mannans serve numerous biological functions (Liepmann et al., 2007 and references therein), including as structural elements and energy reserves (Moreira and Filho, 2008). Thus, it is possible that the high capability of both Klebsormidium and Zygnema S to degrade mannans allows mobilization of energy reserves. Old filaments of both Klebsormidium (K.H., unpublished data) and Zygnema S (Herburger et al., 2015), have considerably lower photosynthetic performance compared with young filaments, as shown by microscopic Imaging-PAM (Fig. S4). Thus, mannans in old filaments might serve as an additional easily accessible energy reservoir, and cover along with lipids (Pichrtová et al., 2016b) the high metabolic costs when pre-akinetes start germinating, accompanied by a high cell division rate (Pichrtová et al., 2016a). Furthermore, mannans (and/or other cell wall polysaccharides) might be partially removed from the cell wall by hydrolysis to gain the building blocks for newly formed cell wall areas.
Functional role of hemicelluloses in filamentous CGA
The specific occurrence of some hemicelluloses in the contact zone of individual cells (MLG, xylans) and cross cell walls (xyloglucan, mannans) of Zygnema S and Klebsormidium supports the hypothesis that these polysaccharides play an important role in cell–cell attachment (Ikegaya et al., 2008). Treating Spirogyra sp. (Zygnematophyceae) filaments with cellulase or removing pectin from the cell wall does not cause cell detachment; however, adding exogenous xyloglucan promotes attachment of the cell wall to experimentally induced rhizoids, suggesting that xyloglucan might be involved in cell–cell attachment (Ikegaya et al., 2008). Furthermore, cell detachment did not increase following PL treatment of either Klebsormidium or Zygnema S. Thus, pectins (homogalacturonan) might be important for the attachment of algae to surfaces (Domozych et al., 2014), and certainly for the mucilage production in Zygnematophyceae (e.g. Eder et al., 2008; Eder and Lütz-Meindl, 2010), but not for cell–cell attachment. PL treatment did not influence binding of the mAbs LM15 and 400-4 to the cell walls of Klebsormidium. This is perhaps not surprising since, although genes involved in the homogalacturonan biosynthesis occur in Klebsormidium flaccidum (Hori et al., 2014), they may not be (highly) transcribed and the pectin fraction of Klebsormidium lacks high amounts of galacturonic acid (Domozych et al., 1980; O'Rourke et al., 2015) and homogalacturonan epitopes (Sørensen et al., 2011).
Conclusion
The present study reports the first in vivo determination of the sites of transglycanase action for xyloglucan as acceptor substrate in charophyte green algae (CGA). Additional (hetero)-transglycanase activities were found to exist in CGA members between xyloglucan and (1) MLGs, (2) xylans or (3) mannans. Although CGA have similar cell wall compositions to those of land plants, they exhibit conspicuous structural and chemical changes, including in transglycanase specificities, in response to ageing and stress. Long-term cultivation experiments allowed us to gain new insights into algal cell wall metabolism showing that the hemicellulose content and distribution change, and that transglycanase action is more abundant in young filaments of Klebsormidium and Zygnema S. Furthermore, transglycanase action appeared to be associated with morphology as it was restricted to longitudinal cell walls, where cell expansion occurs in filamentous CGA (Klebsormidium and Zygnema S), but was found in anticlinal and periclinal cell walls in parenchymatous Chara.
MATERIALS AND METHODS
Algal material and long-term cultivation
Young (1 month) and old (1 year) cultures of Zygnema sp. ‘Saalach’ (‘Zygnema S’; SAG 2419; Herburger et al., 2015) and Klebsormidium crenulatum (‘Klebsormidium’; SAG 2415; Karsten et al., 2010) were maintained on 1.5% agar plates or in 250 ml Erlenmeyer flasks (subsamples of old Klebsormidium). Zygnema S was cultivated in Bold's Basal Medium (BBM, Bischoff and Bold, 1963) and Klebsormidium in modified BBM (3 NMBBM; Starr and Zeikus, 1993). Culture conditions were described in detail elsewhere (Herburger et al., 2015). Chara sp. were collected from Eglington canal, Galway (53°16′35.1″N 9°03′32.1″W), in September 2015 and January 2016 and washed in BBM to remove any co-occurring algae and bacteria. The Chara specimens were then viewed under a light microscope, and undamaged and uncontaminated specimens selected for analysis.
Preparation of alcohol-insoluble residue and cell wall fractioning
The alcohol-insoluble residue (AIR) was prepared according to O'Rourke et al. (2015). Filaments of young and old Zygnema S and Klebsormidium (0.8–1 g fresh mass) were washed thoroughly with distilled water (dH2O), frozen in liquid nitrogen, ground with a mortar and pestle, stirred in five volumes of 70% ethanol containing 1% (v/w) formic acid for 16 h and centrifuged at 5000 g for 10 min. The pellet was washed five times in 70% ethanol, once in acetone and was then air dried. The AIR was stirred in phenol:acetic acid:water (2:1:1, w/v/v) at 70°C for 1 h, washed in ethanol to remove proteins and separated into six fractions according to O'Rourke et al. (2015) [i.e. two pectin fractions (extracted in ammonium oxalate at 100°C for 2 h or 16 h), hemicellulose A (insoluble in 6 M NaOH after 72 h) and B (soluble in NaOH), pooled washings (‘wash’; soluble in buffer, pH 4) and the inextractable residue (‘α-cellulose’)].
Enzyme extraction
Total buffer-extractable protein from young and old Zygnema S and Klebsormidium was prepared according to Fry et al. (2008a). Briefly, 0.9–1.5 g of algal fresh mass was ground in 4.8–8 ml ice-cold extraction buffer [10 mM CaCl2, 300 mM Na succinate (pH 5.5), 2 mM ascorbate, 15% (v/v) glycerol, 3% (w/v) polyvinylpolypyrrolidone], kept on ice for 2 h, filtered through Miracloth (Merck Millipore, Tullagreen, Carrigtwohill, Ireland) and centrifuged at 12,000 g for 10 min (4°C). Extracts were dialysed against dH2O and either used immediately for in vitro assays or stored at −20°C.
Source of polysaccharides for dot-blot and viscometric assays
Tamarind xyloglucan, carob galactomannan (high viscosity), beechwood xylan, mixed-linkage (1→3,1→4)-β-glucan (MLG) from oat (high viscosity), barley (high viscosity) and Icelandic moss (‘lichenan’) were purchased from Megazyme (Wicklow, Ireland), and xanthan gum and locust bean gum from Ceratonia siliqua seeds from Sigma-Aldrich (Steinheim, Germany). Arabinogalactan proteins (AGPs) were extracted from young carrots as described by Popper (2011).
Transglycosylase activity – dot-blot assay
A fluorescent dot-blot assay was used to estimate transglycosylase activity in vitro (Fry, 1997; Chormova et al., 2015). Whatman No. 1 filters (Whatman, Dassel, Germany) were coated with nine different biologically relevant cell wall polysaccharides [1% (w/v) in dH2O; see previous paragraph], left to dry and coated with sulphorhodamine-labelled xyloglucan oligosaccharides (XyGO-SRs; ∼5 µM) prepared according to Kosík and Farkaš (2008) from enzymatically digested tamarind xyloglucan (Sulová et al., 1995). Test papers were loaded with 5 µl of algal enzyme extract and incubated in darkness at ∼20°C between acetate sheets to maintain humidity for 2 h. Papers were washed in ethanol:formic acid:water (1:1:1, v/v/v) for 1.5 h, rinsed twice with dH2O and dried overnight. Orange fluorescence emitted by bound XyGO-SR was visualized by using a CX-20 work station [excitation 365 nm; Spectronics Corp., Westbury (NY), USA] connected to a Nikon Coolpix 8400 camera (Nikon Corp., Tokyo, Japan). Test papers lacking XyGO-SR or polysaccharide coating, or loaded with heat-inactivated enzyme extracts served as controls.
In vivo localization of transglycosylase activity
In vivo incorporation of XyGO-SR into algal cell walls was visualized according to Vissenberg et al. (2000) with modifications. Young and old filaments of Zygnema S or Klebsormidium or freshly collected Chara sp. were incubated in 1 ml culture medium (Zygnema S and Klebsormidium, BBM or 3 NMBBM, pH 5.5; Chara media described by Zhu and Boyer, 1992) containing 5 µM XyGO-SR for 2 h. Filaments were washed with ethanol:formic acid:water (6:0.4:4, v/v/v) for 10 min and with 5% (v/v) formic acid overnight. Zygnema S and Klebsormidium filaments were rinsed twice with culture medium and incubated in 1 ml dimethylformamide (DMF) either for 2 min (Klebsormidium) or 4 min (Zygnema S) to reduce autofluorescence by extracting photosynthetic pigments (Fig. S1). Chara were washed twice with culture medium. Incorporated XyGO-SR in Zygnema S and Klebsormidium was visualized with a Zeiss Pascal 5 confocal laser-scanning microscope (CLSM) equipped with an argon laser [excitation 488 nm, emission 560 nm long pass (LP), false colour green] on a Zeiss Axiovert 200 M microscope. A corresponding bright-field image was collected in a second channel. Incorporated XyGO-SR in Chara sp. was visualised by using the DAPI channel (excitation, 320–390 nm; emission, 430–490 nm) of an Olympus 1X51, X-Cite series 120 and imaged using an Olympus DP71 camera. Control groups contained xyloglucan oligosaccharides lacking the sulphorhodamine group.
Immunolabelling
Young and old filaments of Zygnema S and Klebsormidium were chemically fixed [3% (v/v) paraformaldehyde, 1 h], blocked [with 1% (w/v) bovine serum albumin (BSA) for 1 h; Sigma-Aldrich], washed and incubated under continuous shaking for 2 h in monoclonal antibodies [mAbs; 1:6 in phosphate buffered saline (PBS)] purchased from Biosupplies (400 series) or Plant Probes (LM series). The mABs bind to xyloglucan (LM15; Marcus et al., 2008), (1→4)-β-mannan (400-4; Pettolino et al., 2001), mixed-linkage (1→3, 1→4)-β-d-glucan (400-3; Meikle et al., 1994) or to unsubstituted/low-substituted (1→4)-β-xylan (LM10; McCartney et al., 2005). Filaments were blocked again [0.5% (w/v) BSA, 30 min] and incubated in the secondary antibody (1:100 in PBS for 2 h): Alexa Fluor 488-conjugated goat anti-mouse IgG (g1) (Thermo Fisher Sci., Waltham, MA) for 400-3 and 400-4; FITC goat anti-rat-IgG (whole molecule) (Sigma-Aldrich) for LM10 and LM15. To test whether epitopes are masked by pectins preventing binding of mAbs (Marcus et al., 2008) enzymatic unmasking by incubating algal filaments in 4 units ml−1 pectate lyase for 18 h (PL; E-PLYCJ, Megazyme) prior to chemical fixation was performed (Domozych et al., 2014). Filaments were examined with a confocal laser-scanning microscope (excitation, 488 nm; emission, 505–550 nm band pass, false coloured green and 560 nm long pass, false coloured red). Up to 50 optical sections through a filament allowed generation of z-stacks. A corresponding bright-field image was collected in a third channel and merged with the false colour red image. As a control, the primary antibody was omitted or heat-inactivated prior use.
Cryofixation and labelling of semi-thin sections
Cryofixation using a Leica EMPACT high-pressure freezer (Leica Microsysteme), freeze substitution in a Leica EM AFS and embedding in LR-White (London Resin Company Ltd.) was carried out according to Lütz-Meindl and Aichinger (2004). From fixed material (1-month-old filaments of Zygnema S or Klebsormidium), semi-thin sections were prepared by using a Leica Ultramicrotome (Leica Microsystems GmbH), transferred to ten-well polylysine-coated slides (Thermo Fisher Scientific), and labelled with the mAbs LM15 and 400-4 as described in Herburger and Holzinger (2015). Some sections were enzymatically unmasked by PL (E-PLYCJ) incubation. Fluorescence of secondary antibodies (see previous paragraph) was visualized with a confocal laser-scanning microscope.
Viscometric assay of hydrolytic activity of enzyme extract
Hydrolytic activity of algal enzyme extracts was tested with a viscometric assay as described by Fry (1998). Reaction mixtures contained 10% (v/v) enzyme extract and 1% (w/v) polysaccharide (xyloglucan, MLG, galactomannan or xylan) in buffer (10 mM CaCl2, 300 mM Na succinate, pH 5.5). Mixtures were sucked into a 1 ml vertical glass pipette and an efflux time of 0.8 ml liquid as a function of incubation time up to 1 day was monitored. Efflux time was expressed as percentage of control assays, which contained heat-inactivated enzyme extracts.
Microscopic Imaging-PAM
The effective quantum yield of PSII [Y(II), 620 nm] and near-infrared remission (NIR, 780 nm) of young (1 month culture) and old (1 year culture) Zygnema S and Klebsormidium filaments were visualised with an Imaging-PAM (M-series, Heinz Walz GmbH) connected to a modified Axio Scope A.1 epifluorescence microscope equipped with a Zeiss Fluar 40×1.3 NA objective and CCD Camera IMAG-K6 (Herburger and Holzinger, 2015).
Supplementary Material
Acknowledgements
The authors acknowledge expert technical assistance in algal cultivation by Beatrix Jungwirth, University of Innsbruck. Prof. Dr Michael Schagerl (University of Vienna, Austria) is acknowledged for allowing access to his microscopic Imaging-PAM. Prof. Dr Ursula Lütz-Meindl (University of Salzburg, Austria) is thanked for access to her high-pressure freezing and freeze substitution device, and Ancuela Andosch for technical assistance. Dr Stian Olsen [UiT (The Arctic University of Norway, Norway)] is kindly thanked for preparation of the XyGO-SRs used in the experiments carried out at NUI Galway.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: Z.A.P., A.H.; Methodology: K.H., Z.A.P.; Investigation: K.H., L.M.R.; Writing - original draft: K.H., Z.A.P., A.H.; Writing - review & editing: K.H., Z.A.P., A.H.; Supervision: Z.A.P., A.H.; Project administration: A.H., Z.A.P.; Funding acquisition: A.H.
Funding
This study was supported by Austrian Science Fund (grant P 24242-B16 and I 1951-B16 to A.H.). K.H. received a travelling grant from the University of Innsbruck. Z.A.P. gratefully acknowledges funding from Science Foundation Ireland (Research Frontiers Programme, grant 11/RFP/EOB/3345). Deposited in PMC for immediate release.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.203208.supplemental
References
- Becker B. and Marin B. (2009). Streptophyte algae and the origin of embryophytes. Ann. Bot. 103, 999-1004. 10.1093/aob/mcp044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff H. W. and Bold H. C. (1963). Phycological studies IV. Some soil algae from Enchanted Rock and related algal species, pp. 1-95. Austin: University of Texas. [Google Scholar]
- Buschmann H. and Zachgo S. (2016). The evolution of cell division: from streptophyte algae to land plants. Trends Plant Sci. 21, 872-883. 10.1016/j.tplants.2016.07.004 [DOI] [PubMed] [Google Scholar]
- Carpita N. C. and Gibeaut D. M. (1993). Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 3, 1-30. 10.1111/j.1365-313X.1993.tb00007.x [DOI] [PubMed] [Google Scholar]
- Chormova D., Franková L., Defries A., Cutler S. R. and Fry S. C. (2015). Discovery of small molecule inhibitors of xyloglucan endotransglucosylase (XET) activity by high-throughput screening. Phytochemistry 117, 220-236. 10.1016/j.phytochem.2015.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick-Pérez M., Zhang Y., Hayes J., Salazar A., Zabotina O. A. and Hong M. (2011). Structure and interactions of plant cell-wall polysaccharides by two-and three-dimensional magic-angle-spinning solid-state NMR. Biochemistry 50, 989-1000. 10.1021/bi101795q [DOI] [PubMed] [Google Scholar]
- Domozych D. S., Stewart K. D. and Mattox K. R. (1980). The comparative aspects of cell wall chemistry in the green algae (Chlorophyta). J. Mol. Evol. 15, 1-12. 10.1007/BF01732578 [DOI] [PubMed] [Google Scholar]
- Domozych D. S., Ciancia M., Fangel J. U., Mikkelsen M. D., Ulvskov P. and Willats W. G. T. (2012). The cell walls of green algae: a journey through evolution and diversity. Front. Plant Sci. 3, 82 10.3389/fpls.2012.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domozych D. S., Sørensen I., Popper Z. A., Ochs J., Andreas A., Fangel J. U., Pielach A., Sacks C., Brechka H., Ruisi-Besares P. et al. (2014). Pectin metabolism and assembly in the cell wall of the charophyte green alga Penium margaritaceum. Plant Physiol. 165, 105-118. 10.1104/pp.114.236257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder M. and Lütz-Meindl U. (2010). Analyses and localization of pectin-like carbohydrates in cell wall and mucilage of the green alga Netrium digitus. Protoplasma 243, 25-28. 10.1007/s00709-009-0040-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder M., Tenhaken R., Driouich A. and Lütz-Meindl U. (2008). Occurrence and characterization of arabinogalactan-like proteins and hemicelluloses in Micrasterias (Streptophyta). J. Phycol. 44, 1221-1234. 10.1111/j.1529-8817.2008.00576.x [DOI] [PubMed] [Google Scholar]
- Elbert W., Weber B., Burrows S., Steinkamp J., Büdel B., Andreae M. O. and Pöschl U. (2012). Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat. Geosci. 5, 459-462. 10.1038/ngeo1486 [DOI] [Google Scholar]
- Franková L. and Fry S. C. (2011). Phylogenetic variation in glycosidases and glycanases acting on plant cell wall polysaccharides, and the detection of transglycosidase and trans-β-xylanase activities. Plant J. 67, 662-681. 10.1111/j.1365-313X.2011.04625.x [DOI] [PubMed] [Google Scholar]
- Franková L. and Fry S. C. (2013). Biochemistry and physiological roles of enzymes that ‘cut and paste’ plant cell-wall polysaccharides. J. Exp. Bot. 64, 3519-3550. 10.1093/jxb/ert201 [DOI] [PubMed] [Google Scholar]
- Fry S. C. (1997). Novel ‘dot-blot'assays for glycosyltransferases and glycosylhydrolases: optimization for xyloglucan endotransglycosylase (XET) activity. Plant J. 11, 1141-1150. 10.1046/j.1365-313X.1997.11051141.x [DOI] [Google Scholar]
- Fry S. C. (1998). Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem. J. 332, 507-515. 10.1042/bj3320507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry S. C. (2011). Cell wall polysaccharide composition and covalent cross-linking. Annual Plant Reviews: Plant Polysaccharides, Biosynthesis and Bioengineering, Vol. 41 (ed. Ulvskov P.), pp. 1-42. Oxford: Blackwell Publishing. [Google Scholar]
- Fry S. C., Smith R. C., Renwick K. F., Martin D. J., Hodge S. K. and Matthews K. J. (1992). Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochem. J. 282, 821-828. 10.1042/bj2820821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry S. C., Mohler K. E., Nesselrode B. H. W. A. and Franková L. (2008a). Mixed-linkage β-glucan: xyloglucan endotransglucosylase, a novel wall-remodelling enzyme from Equisetum (horsetails) and charophytic algae. Plant J. 55, 240-252. 10.1111/j.1365-313X.2008.03504.x [DOI] [PubMed] [Google Scholar]
- Fry S. C., Nesselrode B. H. W. A., Miller J. G. and Mewburn B. R. (2008b). Mixed-linkage (1→3, 1→4)-β-d-glucan is a major hemicellulose of Equisetum (horsetail) cell walls. New Phytol. 179, 104-115. 10.1111/j.1469-8137.2008.02435.x [DOI] [PubMed] [Google Scholar]
- Graham L. E., Cook M. E. and Busse J. S. (2000). The origin of plants: body plan changes contributing to a major evolutionary radiation. Proc. Natl. Acad. Sci. USA 97, 4535-4540. 10.1073/pnas.97.9.4535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Zhu Q., Zhang Z., Meng K., Hou Y., Ban Q., Suo J. and Rao J. (2015). Analysis of xyloglucan endotransglycosylase/hydrolase (XTH) genes and diverse roles of isoenzymes during persimmon fruit development and postharvest softening. PLoS ONE 10, e0123668 10.1371/journal.pone.0123668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harholt J., Suttangkakul A. and Scheller H. V. (2010). Biosynthesis of pectin. Plant Physiol. 153, 384-395. 10.1104/pp.110.156588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harholt J., Moestrup Ø. and Ulvskov P. (2016). Why plants were terrestrial from the beginning. Trends Plant Sci. 21, 96-101. 10.1016/j.tplants.2015.11.010 [DOI] [PubMed] [Google Scholar]
- Herburger K. and Holzinger A. (2015). Localization and quantification of callose in the streptophyte green algae Zygnema and Klebsormidium: correlation with desiccation tolerance. Plant Cell Physiol. 56, 2259-2270. 10.1093/pcp/pcv139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herburger K., Lewis L. A. and Holzinger A. (2015). Photosynthetic efficiency, desiccation tolerance and ultrastructure in two phylogenetically distinct strains of alpine Zygnema sp. (Zygnematophyceae, Streptophyta): role of pre-akinete formation. Protoplasma 252, 571-589. 10.1007/s00709-014-0703-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé C., Rogowski A., Gilbert H. J. and Paul Knox J. (2009). Enzymatic treatments reveal differential capacities for xylan recognition and degradation in primary and secondary plant cell walls. Plant J. 58, 413-422. 10.1111/j.1365-313X.2009.03785.x [DOI] [PubMed] [Google Scholar]
- Holzinger A., Herburger K., Kaplan F. and Lewis L. A. (2015). Desiccation tolerance in the chlorophyte green alga Ulva compressa: does cell wall architecture contribute to ecological success? Planta 242, 477-492. 10.1007/s00425-015-2292-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K., Maruyama F., Fujisawa T., Togashi T., Yamamoto N., Seo M., Sato S., Yamada T., Mori H., Tajima N. et al. (2014). Klebsormidium flaccidum genome reveals primary factors for plant terrestrial adaptation. Nat. Commun. 5, 3978 10.1038/ncomms4978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya H., Hayashi T., Kaku T., Iwata K., Sonobe S. and Shimmen T. (2008). Presence of xyloglucan-like polysaccharide in Spirogyra and possible involvement in cell–cell attachment. Phycol. Res. 56, 216-222. 10.1111/j.1440-1835.2008.00503.x [DOI] [Google Scholar]
- Karsten U., Lütz C. and Holzinger A. (2010). Ecophysiological performance of the aeroterrestrial green alga Klebsormidium crenulatum (Klebsormidiophyceae, Streptophyta) isolated from an alpine soil crust with an emphasis on desiccation stress. J. Phycol. 46, 1187-1197. 10.1111/j.1529-8817.2010.00921.x [DOI] [PubMed] [Google Scholar]
- Kosík O. and Farkaš V. (2008). One-pot fluorescent labeling of xyloglucan oligosaccharides with sulforhodamine. Anal. Biochem. 375, 232-236. 10.1016/j.ab.2007.11.025 [DOI] [PubMed] [Google Scholar]
- Leroux O., Knox J. P., Masschaele B., Bagniewska-Zadworna A., Marcus S. E., Claeys M., van Hoorebeke L. and Viane R. L. L. (2011). An extensin-rich matrix lines the carinal canals in Equisetum ramosissimum, which may function as water-conducting channels. Ann. Bot. 108, 307-319. 10.1093/aob/mcr161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepman A. H., Nairn C. J., Willats W. G., Sørensen I., Roberts A. W. and Keegstra K. (2007). Functional genomic analysis supports conservation of function among cellulose synthase-like a gene family members and suggests diverse roles of mannans in plants. Plant Physiol. 143, 1881-1893. 10.1104/pp.106.093989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütz-Meindl U. and Aichinger N. (2004). Use of energy-filtering transmission electron microscopy for routine ultrastructural analysis of high-pressure-frozen or chemically fixed plant cells. Protoplasma 223, 155-162. 10.1007/s00709-003-0033-3 [DOI] [PubMed] [Google Scholar]
- Marcus S. E., Verhertbruggen Y., Hervé C., Ordaz-Ortiz J. J., Farkas V., Pedersen H. L., Willats W. T. G. and Knox P. J. (2008). Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 8, 60 10.1186/1471-2229-8-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney L., Marcus S. E. and Knox J. P. (2005). Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J. Histochem. Cytochem. 53, 543-546. 10.1369/jhc.4B6578.2005 [DOI] [PubMed] [Google Scholar]
- McGregor N., Yin V., Tung C.-C., Van Petegem F. and Brumer H. (2017). Crystallographic insight into the evolutionary origins of xyloglucan endo-transglycosylases and endo-hydrolases. Plant J. 89, 651-670. 10.1111/tpj.13421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean R. J. and Pessoney G. F. (1971). Formation and resistance of akinetes of Zygnema. In Contributions in Phycology (ed. Parker B. C. and Brown R. M. Jr), pp. 145-152. Lawrence, KS: Allen. [Google Scholar]
- Meikle P. J., Hoogenraad N. J., Bonig I., Clarke A. E. and Stone B. A. (1994). A (1→3, 1→4)-β-glucan-specific monoclonal antibody and its use in the quantification and immunocytochemical location of (1→3, 1→4)-β-glucans. Plant J. 5, 1-9. 10.1046/j.1365-313X.1994.5010001.x [DOI] [PubMed] [Google Scholar]
- Métraux J.-P. (1982). Changes in cell-wall polysaccharide composition of developing Nitella internodes. Planta 155, 459-466. 10.1007/BF01607568 [DOI] [PubMed] [Google Scholar]
- Mikhailyuk T., Holzinger A., Massalski A. and Karsten U. (2014). Morphology and ultrastructure of Interfilum and Klebsormidium (Klebsormidiales, Streptophyta) with special reference to cell division and thallus formation. Eur. J. Phycol. 49, 395-412. 10.1080/09670262.2014.949308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler K. E., Simmons T. J. and Fry S. C. (2013). Mixed-linkage glucan: xyloglucan endotransglucosylase (MXE) re-models hemicelluloses in Equisetum shoots but not in barley shoots or Equisetum callus. New Phytol. 197, 111-122. 10.1111/j.1469-8137.2012.04371.x [DOI] [PubMed] [Google Scholar]
- Moreira L. R. S. and Filho E. X. F. (2008). An overview of mannan structure and mannan-degrading enzyme systems. Appl. Microbiol. Biotechnol. 79, 165-178. 10.1007/s00253-008-1423-4 [DOI] [PubMed] [Google Scholar]
- Morrison J. C., Greve L. C. and Richmond P. A. (1993). Cell wall synthesis during growth and maturation of Nitella internodal cells. Planta 189, 321-328. 10.1007/BF00194428 [DOI] [PubMed] [Google Scholar]
- Nishikubo N., Awano T., Banasiak A., Bourquin V., Ibatullin F., Funada R., Brumer H., Teeri T. T., Hayashi T., Sundberg B. et al. (2007). Xyloglucan endo-transglycosylase (XET) functions in gelatinous layers of tension wood fibers in poplar—a glimpse into the mechanism of the balancing act of trees. Plant Cell Physiol. 48, 843-855. 10.1093/pcp/pcm055 [DOI] [PubMed] [Google Scholar]
- Olsen S., Striberny B., Hollmann J., Schwacke R., Popper Z. and Krause K. (2016). Getting ready for host invasion: elevated expression and action of xyloglucan endotransglucosylases/hydrolases in developing haustoria of the holoparasitic angiosperm Cuscuta. J. Exp. Bot. 67, 695-708. 10.1093/jxb/erv482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke C., Gregson T., Murray L., Sadler I. H. and Fry S. C. (2015). Sugar composition of the pectic polysaccharides of charophytes, the closest algal relatives of land-plants: presence of 3-O-methyl-d -galactose residues. Ann. Bot. 116, 225-236. 10.1093/aob/mcv089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. B. and Cosgrove D. J. (2012). A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases. Plant Physiol. 158, 1933-1943. 10.1104/pp.111.192880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettolino F. A., Hoogenraad N. J., Ferguson C., Bacic A., Johnson E. and Stone B. A. (2001). A (1→4)-β-mannan-specific monoclonal antibody and its use in the immunocytochemical location of galactomannans. Planta 214, 235-242. 10.1007/s004250100606 [DOI] [PubMed] [Google Scholar]
- Pichrtová M., Hájek T. and Elster J. (2014). Osmotic stress and recovery in field populations of Zygnema sp. (Zygnematophyceae, Streptophyta) on Svalbard (High Arctic) subjected to natural desiccation. FEMS Microbiol. Ecol. 89, 270-280. 10.1111/1574-6941.12288 [DOI] [PubMed] [Google Scholar]
- Pichrtová M., Hájek T. and Elster J. (2016a). Annual development of mat-forming conjugating green algae Zygnema spp. in hydro-terrestrial habitats in the Arctic. Polar Biol. 39, 1653-1662. [Google Scholar]
- Pichrtová M., Arc E., Stöggl W., Kranner I., Hájek T., Hackl H. and Holzinger A. (2016b). Formation of lipid bodies and changes in fatty acid composition upon pre-akinete formation in Arctic and Antarctic Zygnema (Zygnematophyceae, Streptophyta) strains. FEMS Microbiol. Ecol. 92, fiw096 10.1093/femsec/fiw096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper Z. A. (2008). Evolution and diversity of green plant cell walls. Curr. Opin. Plant Biol. 11, 286-292. 10.1016/j.pbi.2008.02.012 [DOI] [PubMed] [Google Scholar]
- Popper Z. A. (2011). Extraction and detection of arabinogalactan proteins. In The Plant Cell Wall: Methods and Protocols (ed. Popper Z. A.), pp. 245-254. New York City: Humana Press. [DOI] [PubMed] [Google Scholar]
- Popper Z. A. and Fry S. C. (2008). Xyloglucan−pectin linkages are formed intra-protoplasmically, contribute to wall-assembly, and remain stable in the cell wall. Planta 227, 781-794. 10.1007/s00425-007-0656-2 [DOI] [PubMed] [Google Scholar]
- Popper Z. A. and Tuohy M. G. (2010). Beyond the green: understanding the evolutionary puzzle of plant and algal cell walls. Plant Physiol. 153, 373-383. 10.1104/pp.110.158055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper Z. A., Michel G., Hervé C., Domozych D. S., Willats W. G. T., Tuohy M. G., Kloareg B. and Stengel D. B. (2011). Evolution and diversity of plant cell walls: from algae to flowering plants. Annu. Rev. Plant. Biol. 62, 567-590. 10.1146/annurev-arplant-042110-103809 [DOI] [PubMed] [Google Scholar]
- Proseus T. E. and Boyer J. S. (2008). Calcium pectate chemistry causes growth to be stored in Chara corallina: a test of the pectate cycle. Plant Cell Environ. 31, 1147-1155. 10.1111/j.1365-3040.2008.01829.x [DOI] [PubMed] [Google Scholar]
- Rose J. K. C., Braam J., Fry S. C. and Nishitani K. (2002). The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant Cell Physiol. 43, 1421-1435. 10.1093/pcp/pcf171 [DOI] [PubMed] [Google Scholar]
- Salmeán A. A., Duffieux D., Harholt J., Qin F., Michel G., Czjzek M., Willats W. G. T. and Hervé C. (2017). Insoluble (1→3),(1→4)-β-d-glucan is a component of cell walls in brown algae (Phaeophyceae) and is masked by alginates in tissues. Sci. Rep. 7, 2880 10.1038/s41598-017-03081-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawitzky H. and Grolig F. (1995). Phragmoplast of the green alga Spirogyra is functionally distinct from the higher plant phragmoplast. J. Cell Biol. 130, 1359-1371. 10.1083/jcb.130.6.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller H. V. and Ulvskov P. (2010). Hemicelluloses. Annu. Rev. Plant Biol. 61, 263-289. 10.1146/annurev-arplant-042809-112315 [DOI] [PubMed] [Google Scholar]
- Scherp P., Grotha R. and Kutschera U. (2001). Occurrence and phylogenetic significance of cytokinesis-related callose in green algae, bryophytes, ferns and seed plants. Plant Cell Rep. 20, 143-149. 10.1007/s002990000301 [DOI] [PubMed] [Google Scholar]
- Schröder R., Wegrzyn T. F., Bolitho K. M. and Redgwell R. J. (2004). Mannan transglycosylase: a novel enzyme activity in cell walls of higher plants. Planta 219, 590-600. 10.1007/s00425-004-1274-x [DOI] [PubMed] [Google Scholar]
- Shinohara N., Sunagawa N., Tamura S., Yokoyama R., Ueda M., Igarashi K. and Nishitani K. (2017). The plant cell-wall enzyme AtXTH3 catalyses covalent cross-linking between cellulose and cello-oligosaccharide. Sci. Rep. 7, 46099 10.1038/srep46099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons T. J., Mohler K. E., Holland C., Goubet F., Franková L., Houston D. R., Hudson A. D., Meulewaeter F. and Fry S. C. (2015). Hetero-trans-β-glucanase, an enzyme unique to Equisetum plants, functionalizes cellulose. Plant J. 83, 753-769. 10.1111/tpj.12935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons T. J., Mortimer J. C., Bernardinelli O. D., Pöppler A. C., Brown S. P., Dupree R. and Dupree P. (2016). Folding of xylan onto cellulose fibrils in plant cell walls revealed by solid-state NMR. Nat. Commun. 7, 13902 10.1038/ncomms13902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen I., Pettolino F. A., Wilson S. M., Doblin M. S., Johansen B., Bacic A. and Willats W. T. G. (2008). Mixed-linkage (1→3), (1→4)-β-d-glucan is not unique to the Poales and is an abundant component of Equisetum arvense cell walls. Plant J. 54, 510-521. 10.1111/j.1365-313X.2008.03453.x [DOI] [PubMed] [Google Scholar]
- Sørensen I., Pettolino F. A., Bacic A., Ralph J., Lu F., O'Neill M. A., Fei Z., Rose J. K. C., Domozych D. S. and Willats W. G. T. (2011). The charophycean green algae provide insights into the early origins of plant cell walls. Plant J. 68, 201-211. 10.1111/j.1365-313X.2011.04686.x [DOI] [PubMed] [Google Scholar]
- Staehelin L. A. and Hepler P. K. (1996). Cytokinesis in higher plants. Cell 84, 821-824. 10.1016/S0092-8674(00)81060-0 [DOI] [PubMed] [Google Scholar]
- Starr R. C. and Zeikus J. A. (1993). UTEX — the culture collection of algae at the University of Texas at Austin 1993 list of cultures. J. Phycol. 29, 1-106. 10.1111/j.0022-3646.1993.00001.x [DOI] [Google Scholar]
- Sulová Z., Lednicka M. and Farkas V. (1995). A colorimetric assay for xyloglucan-endotransglycosylase from germinating seeds. Anal. Biochem. 229, 80-85. 10.1006/abio.1995.1381 [DOI] [PubMed] [Google Scholar]
- Thompson J. E. and Fry S. C. (2000). Evidence for covalent linkage between xyloglucan and acidic pectins in suspension-cultured rose cells. Planta 211, 275-286. 10.1007/s004250000287 [DOI] [PubMed] [Google Scholar]
- Thompson J. E., Smith R. C. and Fry S. C. (1997). Xyloglucan undergoes inter-polymeric transglycosylation during binding to the plant cell wall in vivo: evidence from 13C:3H dual labelling and isopycnic centrifugation in caesium trifluoroacetate. Biochem. J. 327, 699-708. 10.1042/bj3270699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sandt V. S., Stieperaere H., Guisez Y., Verbelen J. P. and Vissenberg K. (2007). XET activity is found near sites of growth and cell elongation in bryophytes and some green algae: new insights into the evolution of primary cell wall elongation. Ann. Bot. 99, 39-51. 10.1093/aob/mcl232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissenberg K., Martinez-Vilchez I. M., Verbelen J. P., Miller J. G. and Fry S. C. (2000). In vivo colocalization of xyloglucan endotransglycosylase activity and its donor substrate in the elongation zone of Arabidopsis roots. Plant Cell 12, 1229-1237. 10.1105/tpc.12.7.1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickett N. J., Mirarab S., Nguyen N., Warnow T., Carpenter E., Matasci N., Ayyampalayam S., Barker M. S., Burleigh J. G., Gitzendanner M. A. et al. (2014). Phylotranscriptomic analysis of the origin and early diversification of land plants. Proc. Natl. Acad. Sci. USA 111, E4859-E4868. 10.1073/pnas.1323926111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama R. and Nishitani K. (2001). Endoxyloglucan transferase is localized both in the cell plate and in the secretory pathway destined for the apoplast in tobacco cells. Plant Cell Physiol. 42, 292-300. 10.1093/pcp/pce034 [DOI] [PubMed] [Google Scholar]
- Yoon M.-C., Han J.-W., Hwang M.-S. and Kim G.-H. (2010). Cytoskeletal changes during nuclear and cell division in the freshwater alga Zygnema cruciatum (Chlorophyta, Zygnematales). Algae 25, 197-204. 10.4490/algae.2010.25.4.197 [DOI] [Google Scholar]
- Zhu G. L. and Boyer J. S. (1992). Enlargement in Chara studied with a turgor clamp. Plant Physiol. 100, 2071-2080. 10.1104/pp.100.4.2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.