Abstract
Early epidemiologic studies of estrogen metabolism measured only 2-hydroxyestrone and 16α-hydroxyestrone and relied on direct enzyme immunoassays without purification steps. Eight breast cancer studies have used these assays with prospectively collected blood or urine samples. Results were inconsistent, and generally not statistically significant; but the assays had limited specificity, especially at the low concentrations characteristic of postmenopausal women. To facilitate continued testing in population-based studies of the multiple laboratory-based hypotheses about the roles of estrogen metabolites, a novel liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay was developed to measure concurrently all 15 estrogens and estrogen metabolites in human serum and urine, as unconjugated and total (glucuronidated+sulfated+unconjugated) concentrations. The assay has high sensitivity (lower limit of quantitation ~1–2 pmol/L), reproducibility (coefficients of variation generally ≤5%), and accuracy. Three prospective studies utilizing this comprehensive assay have demonstrated that enhanced 2-hydroxylation of parent estrogens (estrone+estradiol) is associated with reduced risk of postmenopausal breast cancer. In the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) cohort, the serum ratio of 2-hydroxylation pathway metabolites to parent estrogens was associated with a 28% reduction in breast cancer risk across extreme deciles (p-trend=.05), after adjusting for unconjugated estradiol and breast cancer risk factors. Incorporating this ratio into a risk prediction model already including unconjugated estradiol improved absolute risk estimates substantially (by ≥14%) in 36% of the women, an encouraging result that needs replication. Additional epidemiologic studies of the role of estrogen metabolism in the etiology of hormone-related diseases and continued improvement of estrogen metabolism assays are justified.
Keywords: breast cancer, endogenous steroid hormones, estradiol, estrogen metabolites, hormonal carcinogenesis, 2-hydroxyestrone, 16α-hydroxyestrone
Endogenous estrogen and breast cancer risk
It is widely recognized that endogenous estrogen is associated with increased risk of postmenopausal breast cancer. Persuasive evidence comes from a pooled analysis of individual participant data from nine prospective studies [1]. Included were 663 breast cancer cases and 1765 matched controls, all postmenopausal and not taking exogenous hormones at cohort entry. Breast cancer risk was statistically significantly increased, by 30–50%, with a doubling of circulating estradiol, bioavailable estradiol [estradiol not bound to sex hormone-binding globulin (SHBG)], free estradiol (estradiol not bound to SHBG or albumin), estrone, or estrone sulfate. Across extreme quintiles of circulating estradiol, relative risk (RR) doubled [RR=2.00; 95% confidence interval (CI)=1.47 to 2.71; p-trend<.001]. This strong positive association had been suspected, but not demonstrated in epidemiologic studies until the 1990s, because of the limited sensitivity and accuracy of estradiol assays at the low concentrations characteristic of postmenopausal women.
More recently, in a pooled analysis including seven prospective studies, 767 premenopausal breast cancer cases and 1699 matched controls, none of whom were taking exogenous hormones at cohort entry, endogenous estrogen was associated with increased risk of premenopausal disease, though not as strongly as with postmenopausal disease [2]. Breast cancer risk was statistically significantly increased, by 20–30%, with a doubling of circulating estradiol, free estradiol, or estrone, Across extreme quintiles of circulating estradiol, relative risk increased by 40% (RR=1.41; 95% CI=1.02 to 1.95; p-trend=.004. The ongoing pooled analysis of postmenopausal breast cancer now includes more than 5000 cases, practically all the data available worldwide. Nonetheless, while statistical power has become less of a problem, achieving adequate accuracy and specificity for the estradiol assays remains a challenge [3].
Early research on estrogen metabolism
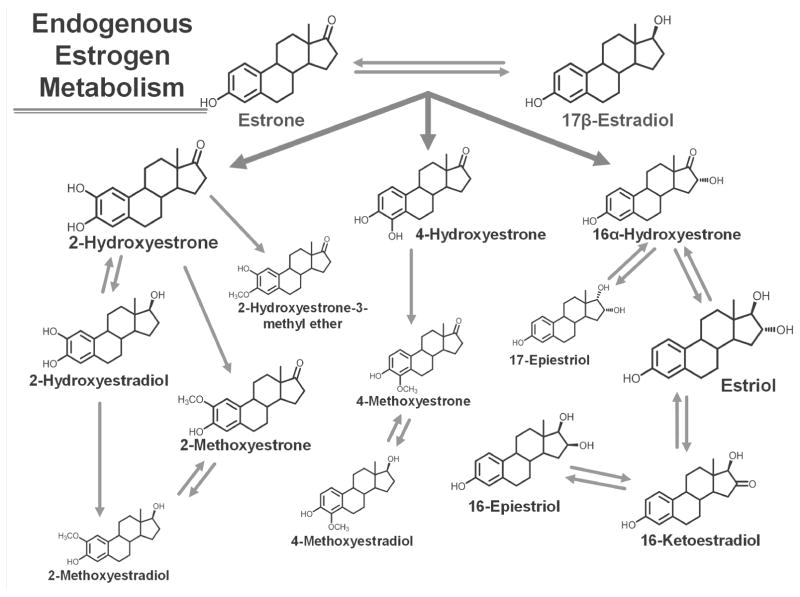
The contribution of estrogen metabolism to the development of breast cancer is much more ambiguous than that of estradiol and estrone, the parent estrogens. The parent estrogens can be irreversibly hydroxylated at the 2-, 4-, or 16-position of the steroid ring (Figure 1). Reactive catechol estrogen metabolites, metabolites with adjacent hydroxyl groups on the steroid ring, are formed through 2-hydroxylation and 4-hydroxylation, but can be converted to less reactive compounds by methylation. Estradiol, estrone, and estrogen metabolites can exist in conjugated forms, which are covalently linked to glucuronide, sulfate, or glutathione residues, or unconjugated forms. The conjugated forms are believed to be important in bioavailability, specifically estrogen storage, cellular transport, and excretion. Almost always, when circulating estradiol is assayed for an epidemiologic study, only the unconjugated form is measured.
Figure 1.
Estrogen metabolism pathways. The parent estrogens, estrone and estradiol, can be irreversibly hydroxylated at the C-2, C-4, or C-16 positions of the steroid ring. The relative abundance of the estrogen or estrogen metabolite in serum from postmenopausal women is indicated by the relative size of the chemical structure. The structures are for the unconjugated forms of the estrogens and estrogen metabolites.
Multiple hypotheses, based on laboratory experiments, exist about the role of specific estrogen metabolites and estrogen metabolism profiles in the etiology of breast cancer [4–6]. Both estrogen receptor-mediated mechanisms involving increased mitosis and proliferation and estrogen receptor-independent mechanisms involving direct DNA damage have been proposed. However, estrogen metabolism remained largely unexplored in epidemiologic studies until recently because no robust analytic methods were available to accurately and reproducibly characterize estrogen metabolism in large population-based studies.
More than 30 years ago, Jack Fishman and Leon Bradlow published one of the first epidemiologic studies of estrogen metabolism and breast cancer [7]. Included were 33 breast cancer cases and 10 controls; all the women were postmenopausal or perimenopausal. Estrogen metabolism was measured retrospectively, after breast cancer diagnosis. A novel in vivo radiometric method had been developed in order to measure the total oxidative metabolism of estrogen, independent of further biotransformations and conjugation pathways. Radioactive forms of estradiol, labeled with tritium in the 17α, 2-, or 16α-positions of the steroid ring, were injected into the women; and the rate and extent of oxidation --- at the 17-position, which converts estradiol to estrone, and at the 2- and 16-positions, which, respectively, estimate the 2-hydroxylation and 16α-hydroxylation pathways --- were measured in serial bloods. Oxidation at the 16-position was statistically significantly increased, by 60%, among the cases, but essentially the same at the 17- and 2-postions. These results indicated that increased formation of 16α-hydroxyestrone and its downstream metabolites was positively associated with breast cancer. This positive association was provocative since clinical, laboratory, and epidemiologic studies had previously suggested that enhanced formation of estriol, the most abundant 16-pathway metabolite, from estrone and estradiol, might be inversely associated with breast cancer risk [8,9].
Extensive laboratory research over the next decade led to the hypothesis, promoted by Leon Bradlow and collaborators, that the ratio of 2-hydroxyestrone to 16α-hydroxyestrone measures the balance between these two competing estradiol oxidation pathways and is a biomarker of reduced breast cancer risk [10]. This hypothesis could be tested in epidemiologic studies once inexpensive, high throughput enzyme immunoassays (EIA) that could measure 2-hydroxyestrone and 16α-hydroxyestrone in stored urine and blood samples became available [11]. The urinary and serum/plasma EIA that were developed were direct assays and did not involve extraction or other purification steps [11–15]. The correlation between urinary and plasma measures of 2-hydroxyestrone, 16α-hydroxyestrone, and the 2:16 ratio, in concurrently collected samples from 511 premenopausal women, was fair to moderate (Spearman r = 0.60, 0.22, and 0.52, respectively; all p<.0001) [15]. Since practically all the estrogens and estrogen metabolites in urine are conjugated, the urinary EIA for both metabolites enzymatically hydrolyzed glucuronide and sulfate residues with Helix pomatia extract; the serum/plasma EIA for 2-hydroxyestrone, but not 16α-hydroxyestrone, also included an enzymatic hydrolysis [12,15].
Prospective studies of 2-hydroxyestrone, 16α-hydroxyestrone, and breast cancer
Eight cohort studies, using serum, plasma, or urine collected and stored at study baseline, prior to cancer diagnosis, have investigated whether breast cancer risk is associated with the 2-hydroxyestrone:16α-hydroxyestrone ratio (Table 1) [16–23]. All studies used the EIA described above for the two metabolites. None of the women were taking exogenous hormones, either oral contraceptives or menopausal hormone therapy, at study baseline. Results were not consistent, in either premenopausal or postmenopausal women. In general, neither the relative risks across extreme quantiles nor the tests for trend reached statistical significance. Though the more recent studies were relatively large, including over 300 cases [20–23], only the largest study, the analysis in the Women’s Health Initiative – Hormone Trials, which included 793 breast cancer cases and 1685 controls, showed a statistically significant association, a 28% increase in risk comparing the highest to lowest quintile of the 2:16 ratio (95% CI=1.00 to 1.63; p-trend=.03) [22].
Table 1.
Prospective studies of 2-hydroxyestrone and 16α-hydroxyestrone and breast cancer risk
| Cohort1 - country | Publication year | Cases / controls | Menopausal status at time of specimen collection | Assay | RR (95% CI)2 for 2:16 ratio: high vs. low quantile3 | p-trend3 |
|---|---|---|---|---|---|---|
| Guernsey - UK [16] | 1998 | 60 / 184 | premenopausal | urine | 0.75 (0.35 – 1. 62) | --- |
| 42 / 139 | postmenopausal | EIA | 0.71 (0.29 – 1.75) | --- | ||
| ORDET - Italy [17] | 2000 | 67 / 264 | premenopausal | urine | 0.55 (0.23 – 1.32) | --- |
| 71 / 274 | postmenopausal | EIA | 1.31 (0.53 – 3.18) | --- | ||
| SOF - USA [18] | 2003 | 272 / 291 | postmenopausal | serum | 1.17 (0.73 – 1.87) | --- |
| EIA | ||||||
| Diet, Cancer, and Health - Denmark [19] | 2005 | 197 / 1974 | postmenopausal | urine | 0.94 (0.69 – 1.26) | NS |
| EIA | for doubling | |||||
| NHS - USA [20] | 2008 | 340 / 675 | postmenopausal | plasma | 1.30 (0.87 – 1.95) | .35 |
| EIA | ||||||
| NYUWHS - USA [21] | 2009 | 377 / 377 | premenopausal | serum | 1.13 (0.68 – 1.87) | .51 |
| EIA | ||||||
| WHI-HT - USA [22] | 2012 | 793 / 1685 | postmenopausal | serum | 1.28 (1.00 – 1.63) | .03 |
| EIA | ||||||
| NYUWHS - USA | 2014 | 499 / 499 | postmenopausal | serum/plasma | 1.13 (0.74 – 1.73) | .88 |
| NSMSC - Sweden [23] | EIA |
Guernsey: Guernsey III cohort; ORDET: Hormones and Diet in the Etiology of Breast Tumors; SOF: Study of Osteoporotic Fractures; NHS: Nurses’ Health Study; NYUWHS: New York University Women’s Health Study; WHI-HT: Women’s Health Initiative-Hormone Trials; NSMSC: Northern Sweden Mammary Screening Cohort.
RR: relative risk. Relative risks are adjusted for study design matching factors and breast cancer risk factors as in the original manuscript. 95% CI: 95% confidence interval.
Statistically significant RR and trends are in bold. NS: not statistically significant.
Includes only those women not on menopausal hormone therapy at study entry.
Since breast cancer is now viewed as a heterogeneous disease, the most recent studies of the 2:16 ratio explored associations by steroid hormone receptor status [19–23]. Results continued to be inconsistent. In the New York University Women’s Health Study (NYUWHS) cohort, the positive association with the ratio was noticeably stronger for estrogen receptor-positive (ER+) tumors in premenopausal women but became inverse for ER+ tumors in postmenopausal women (in premenopausal women, adjusted RR across extreme quartiles of ratio=2.15; 95% CI=0.9 to 5.3 for ER+ and 1.18; 95% CI=0.2 to 6.5 for estrogen receptor-negative (ER−) tumors; in postmenopausal women, adjusted RR for doubling of ratio=0.81; 95% CI=0.6 to 1.1 for ER+ and 1.17, 95% CI=0.5 to 2.6 for ER− tumors) [21,23]. In the Nurses’ Health Study (NHS) cohort also, adjusted relative risk across extreme quartiles of the ratio became inverse for postmenopausal ER+ progesterone receptor-positive (PR+) breast cancer (RR=0.88; 95% CI=0.5 to 1.5) [20]. In the Women’s Health Initiative – Hormone Trials (WHI-HT) study, adjusted relative risks across extreme quartiles remained positive for postmenopausal ER+PR+ and ER-PR− breast cancer, but lost statistical significance (RR=1.24; 95% CI=0.9 to 1.7 and RR=1.10; 95% CI=0.6 to 2.0, respectively) [22].
All of these analyses relied on direct EIA to measure 2-hydroxyestrone and 16α-hydroxyestrone, which may be problematic. Urine samples from 530 women were used to compare state-of-the-art EIA kits [13,14], run at an experienced laboratory, with a liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay for 15 estrogens/estrogen metabolites [24]. For absolute concentrations of each metabolite, the ranking of the women agreed quite well in premenopausal women, with Spearman correlation coefficients of 0.8 to 0.9, but only moderately well in postmenopausal women, with correlation coefficients of 0.4 to 0.6 (Table 2) [25]. For the 2-hydroxyestrone:16α-hydroxyestrone ratio, the correlation was 0.6 to 0.7 in premenopausal women, but only 0.2 in postmenopausal women. Absolute concentrations for both metabolites were consistently higher with the EIA than the LC-MS/MS assay, with mean concentrations 2 to 4 times as high in premenopausal women and 7 to 12 times as high in postmenopausal women (Table 2) [25]. These results suggested that the EIA for 2-hydroxyestrone and 16α-hydroxyestrone were not as specific as hoped, and might detect additional steroids or other interfering compounds, especially at the low estrogen levels characteristic of postmenopausal women. Although this comparison of EIA with LC-MS/MS was performed with urine samples, it is likely that in serum and plasma, which are more complex matrices, EIA would perform even less well, relative to LC-MS/MS.
Table 2.
Comparison of enzyme Immunoassay (EIA) and liquid chromatography-tandem mass spectrometry (LC-MS/MS) measures of urinary 2-hydroxyestrone and 16α-hydroxyestrone1
| Estrogen metabolite | Premenopausal luteal women N = 264 |
Premenopausal non-luteal women N = 98 |
Postmenopausal women N = 168 |
|---|---|---|---|
| Spearman correlation coefficient | |||
|
| |||
| 2-Hydroxyestrone | 0.81 | 0.89 | 0.37 |
| 16α-Hydroxyestrone | 0.86 | 0.89 | 0.62 |
| 2:16 ratio2 | 0.68 | 0.60 | 0.17 |
|
| |||
| Geometric mean concentration, in pmol / mg creatinine EIA / LC-MS/MS | |||
|
| |||
| 2-Hydroxyestrone | 48 / 25 | 31 / 14 | 19 / 2.9 |
| 16α-Hydroxyestrone | 32 / 11 | 24 / 6.5 | 14 / 1.2 |
12-hour overnight urines from controls participating in a population-based case-control study of breast cancer among Asian-American women, aged 20 – 55 years [25].
Ratio of 2-hydroxyestrone to 16α-hydroxyestrone.
Comprehensive assessment of estrogen metabolism by LC-MS/MS
To facilitate further exploration of the contribution of individual differences in estrogen metabolism to cancer etiology, Regina Ziegler, Larry Keefer, Xia Xu, and Timothy Veenstra developed a novel LC-MS/MS method for measuring concurrently the approximately 15 endogenous estrogens and estrogen metabolites (Fig. 1; all 15 referred to as EM) in human urine and serum [24,26]. The method is accurate, reproducible, sensitive enough to quantify the low levels of EM in many postmenopausal women, and simple and robust enough to be used in large epidemiologic studies. The procedure includes an optional enzymatic hydrolysis, extraction, a single chemical derivatization, and LC-MS/MS. If the sample is enzymatically hydrolyzed with Helix pomatia extract, glucuronide and sulfate residues are cleaved from the EM and the assay measures total (glucuronidated + sulfated + unconjugated) concentrations of each EM. If the sample is not hydrolyzed, the assay measures only unconjugated concentrations of each EM. Since practically all EM in urine are conjugated, only total EM is measured in urine samples; in serum samples, both total and unconjugated EM are measured, and conjugated EM is calculated by subtraction. The derivatization step adds a bulky, positively charged dansyl [5-(dimethylamino)naphthalene-1-sulfonyl] moiety to the reactive phenolic hydroxyl characteristic of all estrogens and estrogen metabolites. Dansylation, an especially clever part of the technique, improves sensitivity substantially since mass spectrometry cannot effectively separate uncharged, lipophilic compounds, such as unconjugated steroids. These assays rely on stable isotope dilution to correct for loss or degradation. Six to nine isotopically labeled unconjugated EM are added at the start of the assay procedure, prior to enzymatic hydrolysis or extraction. To ensure stability while optimizing assay conditions, 13C isotopes have been substituted for the 2H isotopes originally used. Over time, volume requirements have been reduced. A total of 0.3 mL of serum or urine is now required for assaying either total EM or unconjugated EM.
With improved technique and equipment, the reproducibility and sensitivity of the EM assays have become better. In serum samples from postmenopausal women, total laboratory coefficients of variation, based on blinded quality control samples, including all steps of the procedure, and combining within- and between-batch variation, were <5% for each EM, in total or unconjugated form, and <3% for estrone and estradiol [27]. Laboratory variability for the serum and urine assays has been substantially less than variation within a population. Intraclass correlation coefficients, a measure of the percent of total variability due to interindividual differences, were ≥95% (except for urinary 17-epiestriol) in premenopausal women, in postmenopausal women, and in men [28,29]. In serum the lower limit of quantitation, defined as the lowest concentration at which reproducible, reliable readings can be obtained, was 1 to 2 pmol/L (e.g. for estradiol, 0.27 to 0.54 pg/mL) [27].
Assay accuracy is difficult to assess objectively. Results from additivity experiments in which known amounts of each of the 15 unconjugated EM were added to charcoal-stripped urine and serum samples have been published [24,26]. At 8 pg of each EM per mL of serum, “accuracy”, defined as the percent of the quantity added that was actually measured, was reasonable and ranged from 91 to 113% [26]. However, charcoal would have removed steroids and other interfering compounds from the serum and improved “accuracy”. In addition, “accuracy” may deteriorate at lower concentrations. Participation in the Centers for Disease Control and Prevention Hormone Standardization Program (HoSt) for estradiol [30,31] is providing the bias of the serum assay over time in measuring absolute concentrations of unconjugated estradiol. However, the accuracy of the assay in measuring total estradiol or any of the other EM is not provided. Ultimately, confidence in the accuracy of the assay is based on peak resolution, signal-to-noise ratio, and absolute recovery, at physiologically meaningful concentrations. The EM assay is being optimized for heparin-plasma samples, and will eventually be extended to breast tissue. In both situations, additional purification step(s) beyond the dichloromethane extraction currently practiced will be necessary to ensure accurate, reproducible, sensitive measurement. At present, EM assays using improved versions of the published techniques [24,26] are being conducted at the Frederick National Laboratory for Cancer Research (Frederick, MD) and Craft Technologies, Inc. (Wilson, NC).
Although this LC-MS/MS assay was developed to assess estrogen metabolism, its sensitivity enables circulating unconjugated estradiol to be measured at the low concentrations characteristic of postmenopausal women. An assay that reliably distinguishes serum estradiol concentrations in the low postmenopausal range (<110 pmol/L; <30 pg/mL), and at the even lower concentrations found in women being treated for breast cancer with aromatase inhibitors (~4 pmol/L; ~1 pg/mL), can be an important prognostic tool in the management of breast cancer, osteoporosis and bone fracture, cardiovascular disease, and possibly cognitive dysfunction (32,33). In addition, sensitive, accurate measurement of circulating estradiol is critical for epidemiologic studies of endogenous estrogen and disease risk and survival, as well as studies of the lifestyle, environmental, and genetic determinants of endogenous estrogen exposure (32). Historically, epidemiologic and clinical studies of estradiol have relied on radioimmunoassays and EIA, which could include extraction and/or chromatography (indirect methods) or no purification (direct methods). These methods, particularly the direct assays, are generally not accurate or sensitive enough to measure circulating estradiol at low postmenopausal levels (32,33).
Prospective studies of estrogen metabolism and postmenopausal breast cancer
Four cohort studies of estrogen metabolism and breast cancer have been published; another has been submitted; and a sixth is in progress (Table 3) [27, 34–36]. Each has utilized serum, plasma, or urine collected at study baseline, prior to cancer diagnosis, and assayed the 15 EM found in blood and urine with the LC-MS/MS technique described above. None of the women were using oral contraceptives or menopausal hormone therapy at study baseline. In each study, molar concentrations of individual EM were summed to form metabolic pathway groups, based on biochemistry, metabolism, and prior hypotheses. All 15 EM were summed as a measure of overall estrogen exposure. To compensate for the moderate-to-high correlation among individual EM and metabolic pathway groups, ratios of metabolic groups were emphasized in analyses. The initial US studies [27,34], as well as that in Shanghai women, who have breast cancer incidence rates 35% those in the United States [37], were agnostic and comprehensive in evaluating hypotheses. None of the four published studies has analyzed results by steroid hormone receptor status.
Table 3.
Prospective studies of estrogen metabolism and breast cancer risk
| Cohort1 - country | Publication year | Cases / controls | Menopausal status at time of specimen collection | Assay |
|---|---|---|---|---|
| NHS II - USA [34] | 2012 | 247 / 485 | premenopausal | urine LC-MS/MS |
| PLCO - USA [27] | 2012 | 277 / 423 | postmenopausal | serum LC-MS/MS |
| Columbia MO - USA [35] | 2014 | 215 / 215 | mostly postmenopausal | serum LC-MS/MS |
| B-FIT - USA [36] | 2014 | 407 / 496 | postmenopausal | serum LC-MS/MS |
| SWHS - China | submitted | 402 / 402 | postmenopausal | urine LC-MS/MS |
| NHS - USA | in progress | 346 / 692 | postmenopausal | plasma LC-MS/MS |
| NHS II - USA2 | in progress | 188 / 385 | premenopausal | urine LC-MS/MS |
NHS II: Nurses’ Health Study II; PLCO: Prostate, Lung, Colorectal, and Ovarian Cancer Screening Study; Columbia MO: Columbia, Missouri Serum Bank; B-FIT: Breast and Bone Follow-up to the Fracture Intervention Trial; SWHS: Shanghai Women’s Health Study; NHS: Nurses’ Health Study.
Expansion of 2012 NHS II study.
In the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) cohort, relative risks of postmenopausal invasive breast cancer were calculated for individual EM, EM grouped by metabolic pathway, and pathway ratios, comparing the highest to lowest deciles, with multivariate Cox proportional hazards models [27]. Nearly all individual EM and metabolic groups were associated with increased risk of breast cancer (Table 4). Unconjugated estradiol was strongly associated with risk [RR=2.07; 95% CI=1.19 to 3.62; p-trend=.01), a finding consistent with the pooled analysis of prospective data available worldwide (1). No parent estrogen, estrogen metabolite, or metabolic group remained statistically significantly associated with breast cancer risk after adjusting for unconjugated estradiol (Table 4). However, three ratios remained statistically significantly associated with risk. The 2-pathway:parent estrogens ratio (RR=0.66; 95% CI=0.51 to 0.87; p-trend=.003) and 2-pathway:16-pathway ratio (RR=0.62; 95% CI=0.45 to 0.86; p-trend=.005) were each associated with decreased risk, and the 4-pathway catechols:4-pathway methylated catechols ratio (RR=1.34; 95% CI=1.04 to 1.72; p-trend=.02) was associated with increased risk (Table 4). The first and third of these ratios remained statistically significantly associated with risk even after adjustment for unconjugated estradiol. In fact, when these two ratios and unconjugated estradiol were all included in a single model, the two ratios remained statistically significantly associated with risk, and the estradiol association lost statistical significance [27].
Table 4.
Relative risk (RR) of postmenopausal invasive breast cancer by serum estrogens/estrogen metabolites (EM) in the PLCO cohort
| EM measure | Adjusted for breast cancer risk factors1 | Also adjusted for unconjugated estradiol2 | ||
|---|---|---|---|---|
|
| ||||
| RR across deciles3 | p-trend4 | RR across deciles3 | p-trend4 | |
| All EM | 1.76 | 0.02 | 1.35 | 0.38 |
| Parent estrogens | 1.73 | 0.02 | 1.38 | 0.30 |
| Conjugated estrone | 1.62 | 0.02 | 1.32 | 0.29 |
| Unconjugated estrone | 1.77 | 0.03 | 1.02 | 0.97 |
| Conjugated estradiol | 1.28 | 0.20 | 1.12 | 0.59 |
| Unconjugated estradiol | 2.07 | 0.01 | --- | --- |
|
| ||||
| 2-hydroxylation pathway | 1.73 | 0.08 | 1.08 | 0.85 |
| 4-hydroxylation pathway | 1.81 | 0.04 | 1.31 | 0.46 |
| 16-hydroxylation pathway | 1.74 | 0.02 | 1.33 | 0.39 |
|
| ||||
| 2-pathway:4-pathway | 0.86 | 0.34 | 0.85 | 0.27 |
| 2-pathway:16-pathway | 0.62 | 0.005 | 0.69 | 0.07 |
| 4-pathway:16-pathway | 0.90 | 0.26 | 0.97 | 0.76 |
|
| ||||
| 2-pathway:parent estrogens | 0.66 | 0.003 | 0.72 | 0.05 |
| 4-pathway:parent estrogens | 0.87 | 0.12 | 0.94 | 0.52 |
| 16-pathway:parent estrogens | 0.52 | 0.07 | 0.64 | 0.23 |
|
| ||||
| 2-catechols:methylated 2-catechols | 1.15 | 0.32 | 1.06 | 0.69 |
| 4-catechols:methylated 4-catechols | 1.34 | 0.02 | 1.31 | 0.03 |
Adjusted for study design matching factors (age at study entry, date of blood collection) and breast cancer risk factors (age at menarche, parity/age at birth of first child, age at natural menopause, type of menopause, family history of breast cancer, personal history of benign breast disease, previous use of menopausal hormone therapy) [27].
Adjusted for unconjugated estradiol, as well as the study design matching factors and breast cancer risk factors listed above.
RR correspond to a unit increase in the log-transformed EM measure with the logarithmic bases chosen so that a unit increase in the logarithm corresponds approximately to an increase in the EM measure from the 10th to the 90th percentile in study controls. Statistically significant RR are in bold.
P for trend is based on a two-sided Wald test of the coefficient associated with a unit increase in the log-transformed EM measure. Statistically significant trends are in bold.
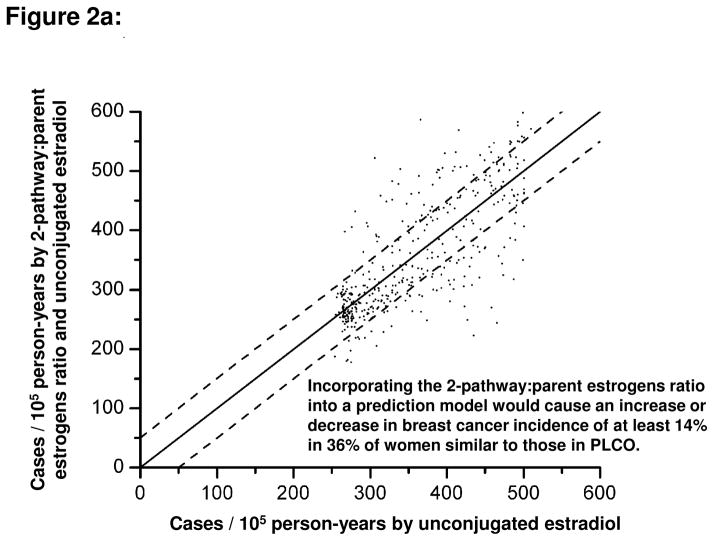
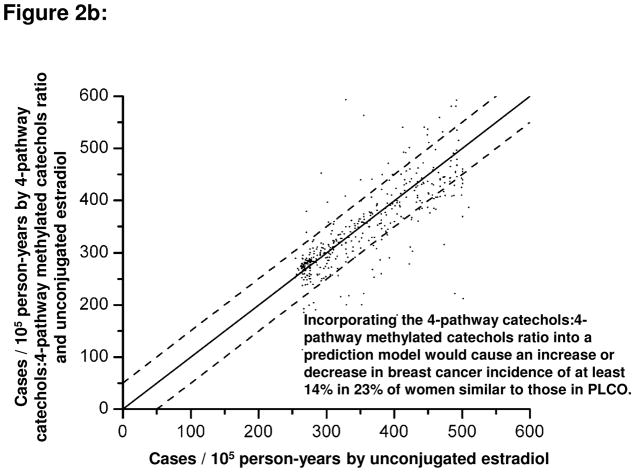
An intriguing question was whether information about estrogen metabolism profiles, if added to information about circulating concentrations of unconjugated estradiol, would alter estimates of absolute risk of breast cancer for individual women. A hypothetical population of postmenopausal women with estrogen metabolism profiles similar to those observed in the PLCO cohort was considered [27]. Addition of the 2-pathway:parent estrogens ratio to a model containing unconjugated estradiol increased or decreased, by at least 14%, the absolute breast cancer risk estimate for 36% of the women (Figure 2a). Addition of the 4-pathway catechols:4-pathway methylated catechols ratio to a model containing unconjugated estradiol changed, by at least 14%, the absolute risk estimate for 30% of the women (Figure 2b). Addition of all EM, the sum of all 15 parent estrogens and estrogen metabolites, to a model containing unconjugated estradiol changed the absolute risk estimate by at least 14% for only 14% of the women [27]. If these ratios of estrogen metabolism pathways are confirmed as predictors of breast cancer risk, they might provide clues to mechanisms of breast carcinogenesis and suggest targets for preventive interventions.
Figure 2.
Comparison of estimated absolute risks of breast cancer (expressed as incidence rates, in cases per 105 person-years) for each control subject in the PLCO cohort. Estimated risks were based on Cox proportional hazards models for the estrogen metabolism profile of each control. One model included unconjugated estradiol only (x-axis), and the other model included both unconjugated estradiol and an additional estrogen metabolism measure (y-axis): (A) the ratio of the 2-pathway to parent estrogens; (B) the ratio of 4-pathway catechols to 4-pathway methylated catechols. Absolute risk estimates were calibrated using breast cancer incidence rates for white women, aged 60–64 years, in the 2004–2006 Surveillance, Epidemiology, and End Results population (357 cases per 105 person-years) [27]. Each dot represents the two estimated absolute risks for one control. If the two risks are equal, they will fall on a diagonal line. Upper and lower dashed lines demarcate risk predictions that differ by at least 50 cases per 105 person-years (14%). For the Cox models, unconjugated estradiol and metabolic pathway ratios were log-transformed and the following covariates were included: age at study entry, period of blood collection, age at menarche, combined parity and age at birth of first child, age at natural menopause, type of menopause, first-degree family history of breast cancer, personal history of benign breast disease, and previous use of menopausal hormone therapy [27].
In the Columbia, Missouri Serum Bank cohort, risk of postmenopausal breast cancer was also calculated for individual EM, metabolic pathway groups, and pathway ratios and compared the highest to lowest quartiles [35]. All EM combined and parent estrogens were associated with increased risk, but each of the three hydroxylation pathways was associated with reduced risk (Table 5). None of these relative risks or tests for trend was statistically significant. Of the pathway ratios, moderately strong, but statistically nonsignificant, inverse associations (RR=0.6–0.7 across extreme quartiles) were noted for 2-pathway:parent estrogens, 2-pathway:16-pathway, and 2-pathway:4-pathway, largely because of the 29% (95% CI=0.40 to 1.27) reduction in risk across extreme quartiles of the 2-pathway (p-trend=0.10) (Table 5). Whether these relationships were independent of unconjugated estradiol was not explored because unconjugated estradiol was only weakly associated with risk (RR=1.06). However, including unconjugated estrone in the models did not attenuate risk by >10% [35]. No clear patterns in risk were reported for the catechols:methylated catechols ratio. Nonetheless, risk was increased at high catechol concentrations and reduced at high methylated catechol concentrations. For both 2-pathway catechols and the single 4-pathway catechol, breast cancer risk was elevated in the fourth quartile, compared to the first, and reduced for two of the three 2-pathway methylated catechols and both 4-pathway methylated catechols [35].
Table 5.
Relative risk (RR) of postmenopausal breast cancer by serum estrogens/estrogen metabolites (EM) in the Columbia, Missouri cohort
| EM measure | Adjusted for breast cancer risk factors1 | |
|---|---|---|
|
| ||
| RR across quartiles2 | p-trend3 | |
| All EM | 1.34 | NS |
| Parent estrogens | 1.46 | NS |
| Conjugated estrone | 1.35 | NS |
| Unconjugated estrone | 1.57 | NS |
| Conjugated estradiol | 1.48 | NS |
| Unconjugated estradiol | 1.06 | NS |
|
| ||
| 2-hydroxylation pathway | 0.71 | 0.10 |
| 4-hydroxylation pathway | 0.89 | NS |
| 16-hydroxylation pathway | 0.94 | NS |
|
| ||
| 2-pathway:4-pathway | 0.60 | 0.10 |
| 2-pathway:16-pathway | 0.63 | 0.10 |
| 4-pathway:16-pathway | 1.00 | NS |
|
| ||
| 2-pathway:parent estrogens | 0.72 | 0.11 |
| 4-pathway:parent estrogens | 0.84 | NS |
| 16-pathway:parent estrogens | 0.94 | NS |
Adjusted for study design matching factors (age at blood collection, years from blood collection to menopause, time of day at blood collection) and breast cancer risk factors identified as confounders (age at menarche, parity/age at birth of first child, type of menopause, family history of breast cancer, body mass index) [35].
RR compare women in the highest quartile of the EM measure to women in the lowest quartile, with quartiles based on the distribution among the study controls. None of the RR is statistically significant.
None of the p-values for trend is statistically significant. Low p-values are presented, even though statistically nonsignificant. Method for calculating p-trend not presented in [35]
In the Breast and Bone Follow-up to the Fracture Intervention Trial (B-FIT) cohort also, risk of postmenopausal breast cancer was calculated for individual EM, metabolic pathway groups, and pathway ratios and compared the highest to lowest quintiles [36]. Invasive and in situ disease were included. As in the PLCO cohort, parent estrogens, 2-pathway, 4-pathway, and 16-pathway were all positively associated with risk, with statistically significant trends for parent estrogens (p-trend=.01) and the 16-pathway (p-trend=.02) (Table 6). As in the PLCO analysis, the 2-pathway:parent estrogens ratio and the 2-pathway:16-pathway ratio were statistically significantly associated with reduced risk (RR=0.69, 95% CI=0.46 to 1.05; p-trend=.01 and RR=0.60; 95% CI=0.40 to 0.90; p-trend=.002, respectively). Similarly strong, inverse, statistically significant trends were also observed in B-FIT for the 4-pathway:parent estrogens ratio and the 4-pathway:16-pathway ratio, which suggested that enhanced hydroxylation at the 2- or 4-position might lower risk. Contrary to the PLCO findings, the ratios of catechols to methylated catechols in the 2-pathway and 4-pathway were each associated with reduced risk (RR=0.68, 95% CI=0.45 to 1.02; p-trend=.05 and RR=0.89; 95% CI=0.57 to1.38; p-trend=.28, respectively). Whether these associations of estrogen metabolism profiles with breast cancer risk were independent of the recognized strong relationship of unconjugated estradiol with risk could not be evaluated. Only total concentrations of each EM were assayed, and thus unconjugated estradiol was not measured. However, adjustment of the models for total estradiol did not change estimates by >10% [36].
Table 6.
Relative risk (RR) of postmenopausal breast cancer by serum estrogens/estrogen metabolites (EM) in the B-FIT cohort
| EM measure | Not adjusted for breast cancer risk factors1 | |
|---|---|---|
|
| ||
| RR across quintiles2 | p-trend3 | |
| All EM4 | --- | --- |
| Parent estrogens | 1.80 | 0.01 |
| Total estrone4 | 1.48 | 0.04 |
| Unconjugated estrone4 | --- | --- |
| Total estradiol4 | 1.86 | 0.04 |
| Unconjugated estradiol4 | --- | --- |
|
| ||
| 2-hydroxylation pathway | 1.54 | 0.14 |
| 4-hydroxylation pathway | 1.40 | 0.12 |
| 16-hydroxylation pathway | 1.88 | 0.02 |
|
| ||
| 4-pathway:2-pathway5 | 0.83 | 0.44 |
| 2-pathway:16-pathway | 0.60 | 0.002 |
| 4-pathway:16-pathway | 0.57 | 0.002 |
|
| ||
| 2-pathway:parent estrogens | 0.69 | 0.01 |
| 4-pathway:parent estrogens | 0.61 | 0.004 |
| 16-pathway:parent estrogens | 0.73 | 0.18 |
|
| ||
| 2-catechols:methylated 2-catechols | 0.68 | 0.05 |
| 4-catechols:methylated 4-catechols | 0.89 | 0.28 |
Adjusted for study design matching factors (clinical center, trial participation status). Not adjusted for breast cancer risk factors since none were identified as confounders. Race, education, age at menarche, parity/age at birth of first child, breast feeding, years since menopause, family history of breast cancer, prior use of menopausal hormone therapy, body mass index, and alcohol consumption, as well as year of blood draw and time since blood draw, did not change RR estimates by >10% [36].
RR compare women in the highest quintile of the EM measure to women in the lowest quintile, with quintiles based on the distribution in the study cohort. Statistically significant RR are in bold.
P for trend is based on models including the EM measure quintiles as an ordinal variable. Statistically significant trends are in bold.
Only total concentrations of individual EM were assayed so unconjugated estrone and unconjugated estradiol were not measured. Similarly, conjugated estrone and conjugated estradiol could not be estimated. All EM was not calculated.
In summary, each of these three prospective studies of postmenopausal breast cancer suggests that enhanced 2-hydroxylation is associated with reduced risk. This consistent finding does not support the laboratory-based hypothesis that 2-pathway catechols produce semiquinones and quinones which through redox cycling generate reactive oxygen species that damage DNA [38,39]. However, this finding does agree with the hypothesis that 2-pathway catechols, in contrast to estradiol and 16-pathway estrogen metabolites, do not increase estrogen receptor-mediated signaling and cell proliferation in the breast since they bind weakly to the receptor [39–41]. The reduced risk associated with enhanced 2-hydroxylation is also consistent with several experiments suggesting that 2-pathway catechols are preferentially excreted, relative to parent estrogens [42,43]. Thus, 2-hydroxylation could reduce breast cancer risk simply by decreasing the concentration of bioactive unconjugated estradiol in circulation and in the breast.
The increased breast cancer risk associated with the ratio of 4-pathway catechols to 4-pathway methylated catechols in the PLCO cohort supports the laboratory-based hypothesis that DNA adducts derived from 4-pathway catechols are unstable, depurinating, and highly mutagenic but this reactivity of 4-pathway catechols is blocked by methylation [39,44]. While the Columbia, MO analysis also provides modest evidence for this hypothesis, the B-FIT analysis does not.
All three epidemiologic studies, especially the PLCO analysis, suggest that the associations with estrogen metabolism profiles, as measured by pathway ratios, are independent of the strong positive associations of unconjugated estradiol and estrone with postmenopausal breast cancer risk [1]. In other words, these biomarkers of estrogen metabolism may provide new information about mechanisms of breast carcinogenesis.
Prospective studies of estrogen metabolism and premenopausal breast cancer
One prospective study of estrogen metabolism and breast cancer has focused on premenopausal, not postmenopausal, breast cancer (Table 3). Conducted in NHS II, this nested case-control study measured total (glucuronidated + sulfated + unconjugated) concentrations of the 15 EM, adjusted for creatinine, in carefully timed mid-luteal phase urines from premenopausal women [34]. Most of the cases (89%) were still premenopausal at diagnosis; both invasive and in situ disease were included. None of the participants had used exogenous hormones or been pregnant or lactating within the six months prior to urine collection. Urinary estrone and estradiol levels were each strongly, statistically significantly, and inversely associated with risk in multivariate models [top vs. bottom quartile RR: estrone=0.52; 95% CI=0.30 to 0.88; p-curvature for non-linear trend=.01; estradiol=0.51; 95% CI=0.30 to 0.86; p-trend=.005]. Inverse, although statistically nonsignificant, trends were also observed with the 2- and 4-pathways, but not the 16-pathway, which was not associated with risk at all. Both the 2-pathway:parent estrogens ratio and the 2-pathway:16-pathway ratio were inversely, but statistically nonsignificantly, associated with risk; while the 16-pathway:parent estrogens ratio was marginally statistically significantly positively associated (p-trend=.06). Thus this cohort study of estrogen metabolism and breast cancer in premenopausal women concurs with the three cohort studies in postmenopausal women and also suggests that enhanced 2-hydroxylation may be protective.
Modulating estrogen metabolism
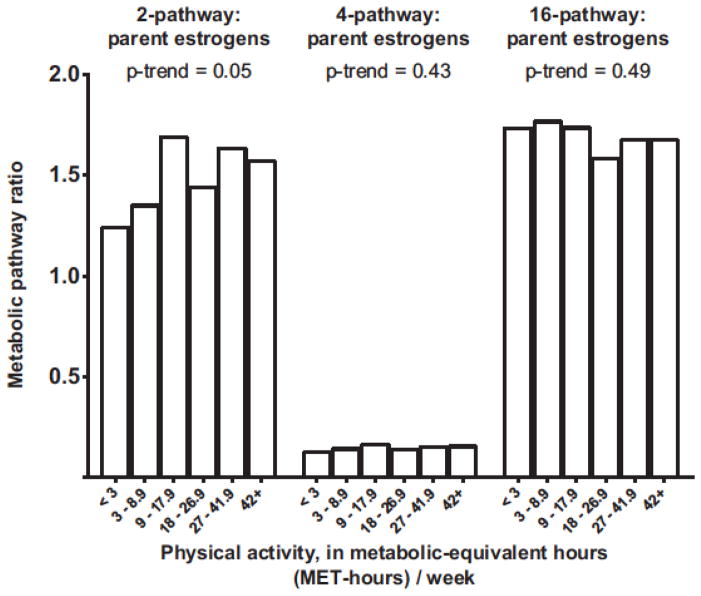
If enhanced 2-hydroxylation of parent estrogens really does reduce the risk of breast cancer, we will want to know how genetics and lifestyle determine individual estrogen metabolism profiles, and whether we can modify the profiles. To begin to address this question, the luteal phase urinary EM data for the controls in the NHS II study [34] were combined with comparable data from a biomarker reproducibility study in NHS II [45] in order to explore correlates and possible determinants of estrogen production and metabolism in 603 premenopausal women. One of many potential determinants evaluated was physical activity. Total recreational physical activity was assessed periodically by interview in NHS II; the activity estimates preceding and following the urine collection, which were separated by four years, were combined to estimate adult physical activity. High physical activity [42+ metabolic equivalent-hours (MET-hours)/week vs. <3 MET-hours/week] was associated with an 11% lower level of urinary parent estrogens (p-trend=.16) and a 15% lower level of urinary estradiol (p-trend=.03) [46], which is consistent with the inverse relationships reported by cohort studies of luteal phase circulating parent estrogens and physical activity [47]. High physical activity was also associated with increases in the 2-pathway:parent estrogens ratio (27% higher for 42+ MET-hours/week vs. <3 MET-hours/week; p-trend=.05) and 2-pathway:16-pathway ratio (31% higher; p-trend=.09). No associations with the 4-pathway:parent estrogens (p-trend=.43) or 16-pathway:parent estrogens ratios (p-trend=.49) were apparent (Figure 3) (46). While these results, derived from the most comprehensive cross-sectional examination of estrogen metabolism and physical activity to date, suggest that vigorous physical activity might increase 2-hydroxylation, they need to be replicated and should be cautiously interpreted.
Figure 3.
Geometric means for the 2-pathway:parent estrogens ratio, 4-pathway:parent estrogens ratio, and 16-pathway:parent estrogens ratio by level of adult recreational physical activity. Total concentrations of the 15 estrogens/estrogen metabolites (EM) were measured in luteal phase urines from 603 premenopausal control participants in NHS II [46]. Total recreational physical activity was assessed periodically by interview in NHS II; and estimates at two points of time, preceding and following the urine collection and separated by four years, were combined. Generalized linear models were used to calculate geometric means and included as covariates age at urine collection, actual luteal day at collection, first morning urine, body mass index, alcohol consumption, and usual menstrual cycle length. P for trend was calculated by modeling the medians of the physical activity categories as a continuous variable.
Summary
Experimental, epidemiologic, and clinical research demonstrate convincingly that endogenous estrogens are involved in the etiology of breast cancer. However, until recently the contribution of estrogen metabolism remained largely unexplored in epidemiologic studies despite multiple hypotheses, based on laboratory experiments, about the roles of specific estrogen metabolites and metabolic pathways. No robust analytic methods were available to accurately characterize estrogen metabolism profiles in large population-based studies. The first cohort studies of estrogen metabolism and breast cancer measured only 2-hydroxyestrone and 16α-hydroxyestrone and relied on direct enzyme immunoassays without purification steps. Results were inconsistent, and generally not statistically significant; but the assays had limited specificity, especially at the low concentrations characteristic of postmenopausal women. Recently a novel LC-MS/MS assay was developed to measure concurrently all 15 estrogens and estrogen metabolites in human serum and urine, in conjugated and unconjugated forms, with high sensitivity, reproducibility, and accuracy. Three prospective studies utilizing this comprehensive assay have now demonstrated that enhanced 2-hydroxylation of parent estrogens is associated with reduced risk of postmenopausal breast cancer. A similar pattern was also noted in a prospective study of premenopausal breast cancer. In the postmenopausal breast cancer studies, the associations with ratios of estrogen metabolism pathways appeared independent of the recognized association of unconjugated estradiol with increased risk. If these biomarkers of estrogen metabolism are confirmed as reliable predictors of breast cancer risk, they might not only provide clues to mechanisms of breast carcinogenesis but also become useful clinically in prevention and treatment. Additional epidemiologic studies of the role of estrogen metabolism in the etiology of breast cancer and other hormone-related diseases and continued improvement of assays that accurately and comprehensively assess estrogen metabolism are justified.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Key T, Appleby P, Barnes I, Reeves G Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–16. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 2.Endogenous Hormones and Breast Cancer Collaborative Group. Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ, Barricarte A, Berrino F, Krogh V, Sieri S, Brinton LA, Dorgan JF, Dossus L, Dowsett M, Eliassen AH, Fortner RT, Hankinson SE, Helzlsouer KJ, Hoff man-Bolton J, Comstock GW, Kaaks R, Kahle LL, Muti P, Overvad K, Peeters PH, Riboli E, Rinaldi S, Rollison DE, Stanczyk FZ, Trichopoulos D, Tworoger SS, Vineis P. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013;14:1009–19. doi: 10.1016/S1470-2045(13)70301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endogenous Hormones and Breast Cancer Collaborative Group. Steroid hormone measurements from different types of assays in relation to body mass index and breast cancer risk in postmenopausal women: reanalysis of eighteen prospective studies. Steroids. 2015 doi: 10.1016/j.steroids.2014.09.001. ??:????–?? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yue W, Yager JD, Wang JP, Jupe ER, Santen RJ. Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids. 2013;78:161–70. doi: 10.1016/j.steroids.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Yager JD. Mechanisms of estrogen carcinogenesis. Steroids. 2015 ??:????-?? [Google Scholar]
- 6.Santen RJ. Estrogen metabolites and breast cancer. Steroids. 2015 doi: 10.1016/j.steroids.2014.08.003. ??:????-?? [DOI] [PubMed] [Google Scholar]
- 7.Schneider J, Kinne D, Fracchia A, Pierce V, Anderson KE, Bradlow HL, Fishman J. Abnormal oxidative metabolism of estradiol in women with breast cancer. Proc Natl Acad Sci USA. 1982;79:3047–51. doi: 10.1073/pnas.79.9.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemon HM. Endocrine influences on human mammary cancer formation. A critique. Cancer. 1969;23:781–90. doi: 10.1002/1097-0142(196904)23:4<781::aid-cncr2820230407>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 9.MacMahon B, Cole P, Brown J. Etiology of human breast cancer: a review. J Natl –Cancer Inst. 1973;50:21–42. doi: 10.1093/jnci/50.1.21. [DOI] [PubMed] [Google Scholar]
- 10.Bradlow HL, Davis DL, Lin G, Sepkovic D, Tiwari R. Effects of pesticides on the ratio of 16 alpha/2-hydroxyestrone: a biologic marker of breast cancer risk. Environ Health Perspect. 1995;103(Suppl 7):147–50. doi: 10.1289/ehp.95103s7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziegler RG, Rossi SC, Fears TR, Bradlow HL, Adlercreutz H, Sepkovic D, Kiuru P, Wahala K, Vaught JB, Donaldson JL, Falk RT, Fillmore CM, Siiteri PK, Hoover RN, Gail MH. Quantifying estrogen metabolism: an evaluation of the reproducibility and validity of enzyme immunoassays for 2-hydroxyestrone and 16alpha-hydroxyestrone in urine. Environ Health Perspect. 1997;105(Suppl 3):607–14. doi: 10.1289/ehp.97105s3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klug TL, Bradlow HL, Sepkovic DW. Monoclonal antibody-based enzyme immunoassay for simultaneous quantitation of 2- and 16a-hydroxyestrone in urine. Steroids. 1994;59:648–55. doi: 10.1016/0039-128x(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 13.Bradlow HL, Sepkovic DW, Klug T, Osborne MP. Application of an improved ELISA assay to the analysis of urinary estrogen metabolites. Steroids. 1998;63:406–13. doi: 10.1016/s0039-128x(98)00041-5. [DOI] [PubMed] [Google Scholar]
- 14.Falk RT, Rossi SC, Fears TR, Sepkovic DW, Migella A, Adlercreutz H, Donaldson J, Bradlow HL, Ziegler RG. A new ELISA kit for measuring urinary 2-hydroxyestrone, 16alpha-hydroxyestrone, and their ratio: reproducibility, validity, and assay performance after freeze-thaw cycling and preservation by boric acid. Cancer Epidemiol Biomarkers Prev. 2000;9:81–7. [PubMed] [Google Scholar]
- 15.Bradlow HL, Jernström H, Sepkovic DW, Klug TL, Narod SA. Comparison of plasma and urinary levels of 2-hydroxyestrogen and 16 alpha-hydroxyestrogen metabolites. Mol Genet Metab. 2006;87:135–46. doi: 10.1016/j.ymgme.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Meilahn EN, De Stavola B, Allen DS, Fentiman I, Bradlow HL, Sepkovic DW, Kuller LH. Do urinary oestrogen metabolites predict breast cancer? Guernsey III cohort follow-up. Br J Cancer. 1998;78:1250–5. doi: 10.1038/bjc.1998.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muti P, Bradlow HL, Micheli A, Krogh V, Freudenheim JL, Schünemann HJ, Stanulla M, Yang J, Sepkovic DW, Trevisan M, Berrino F. Estrogen metabolism and risk of breast cancer: a prospective study of the 2:16alpha-hydroxyestrone ratio in premenopausal and postmenopausal women. Epidemiology. 2000;11:635–40. doi: 10.1097/00001648-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Cauley JA, Zmuda JM, Danielson ME, Ljung BM, Bauer DC, Cummings SR, Kuller LH. Estrogen metabolites and the risk of breast cancer in older women. Epidemiology. 2003;14:740–4. doi: 10.1097/01.ede.0000091607.77374.74. [DOI] [PubMed] [Google Scholar]
- 19.Wellejus A, Olsen A, Tjonneland A, Thomsen BL, Overvad K, Loft S. Urinary hydroxyestrogens and breast cancer risk among postmenopausal women: a prospective study. Cancer Epidemiol Biomarkers Prev. 2005;14:2137–42. doi: 10.1158/1055-9965.EPI-04-0934. [DOI] [PubMed] [Google Scholar]
- 20.Eliassen AH, Missmer SA, Tworoger SS, Hankinson SE. Circulating 2-hydroxy- and 16alpha-hydroxy estrone levels and risk of breast cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:2029–35. doi: 10.1158/1055-9965.EPI-08-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arslan AA, Shore RE, Afanasyeva Y, Koenig KL, Toniolo P, Zeleniuch-Jacquotte A. Circulating estrogen metabolites and risk for breast cancer in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2009;18:2273–9. doi: 10.1158/1055-9965.EPI-09-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackey RH, Fanelli TJ, Modugno F, Cauley JA, McTigue KM, Brooks MM, Chlebowski RT, Manson JE, Klug TL, Kip KE, Curb JD, Kuller LH. Hormone therapy, estrogen metabolism, and risk of breast cancer in the Women’s Health Initiative Hormone Therapy Trial. Cancer Epidemiol Biomarkers Prev. 2012;21:2022–32. doi: 10.1158/1055-9965.EPI-12-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arslan AA, Koenig KL, Lenner P, Afanasyeva Y, Shore RE, Chen Y, Lundin E, Toniolo P, Hallmans G, Zeleniuch-Jacquotte A. Circulating estrogen metabolites and risk of breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2014;23:1290–7. doi: 10.1158/1055-9965.EPI-14-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X, Veenstra TD, Fox SD, Roman JM, Issaq HJ, Falk R, Saavedra JE, Keefer LK, Ziegler RG. Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem. 2005;77:6646–54. doi: 10.1021/ac050697c. [DOI] [PubMed] [Google Scholar]
- 25.Faupel-Badger JM, Fuhrman BJ, Xu X, Falk RT, Keefer LK, Veenstra TD, Hoover RN, Ziegler RG. Comparison of liquid chromatography-tandem mass spectrometry, RIA, and ELISA methods for measurement of urinary estrogens. Cancer Epidemiol Biomarkers Prev. 2010;19:292–300. doi: 10.1158/1055-9965.EPI-09-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:7813–21. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- 27.Fuhrman BJ, Schairer C, Gail MH, Boyd-Morin J, Xu X, Sue LY, Buys SS, Isaacs C, Keefer LK, Veenstra TD, Berg CD, Hoover RN, Ziegler RG. Estrogen metabolism and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2012;104:326–39. doi: 10.1093/jnci/djr531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falk RT, Xu X, Keefer L, Veenstra TD, Ziegler RG. A liquid chromatography-mass spectrometry method for the simultaneous measurement of 15 urinary estrogens and estrogen metabolites: assay reproducibility and interindividual variability. Cancer Epidemiol Biomarkers Prev. 2008;17:3411–8. doi: 10.1158/1055-9965.EPI-08-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuhrman BJ, Xu X, Falk RT, Dallal CM, Veenstra TD, Keefer LK, Graubard BI, Brinton LA, Ziegler RG, Gierach GL. Assay reproducibility and interindividual variation for 15 serum estrogens and estrogen metabolites measured by liquid chromatography-tandem mass spectrometry. Cancer Epidemiol Biomarkers Prev. 2014;23:2649–57. doi: 10.1158/1055-9965.EPI-14-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vesper HW, Botelho JC, Vidal ML, Rahmani Y, Thienpont LM, Caudill SP. High variability in serum estradiol measurements in men and women. Steroids. 2014;82:7–13. doi: 10.1016/j.steroids.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vesper HW, Botelho JC, Wang Y. Challenges and improvements in testosterone and estradiol testing. Asian J Androl. 2014;16:178–84. doi: 10.4103/1008-682X.122338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosner W, Hankinson SE, Sluss PM, Vesper HW, Wierman ME. Challenges to the measurement of estradiol: an endocrine society position statement. J Clin Endocrinol Metab. 2013;98:1376–87. doi: 10.1210/jc.2012-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JS, Ettinger B, Stanczyk FZ, Vittinghoff E, Hanes V, Cauley JA, Chandler W, Settlage J, Beattie MS, Folkerd E, Dowsett M, Grady D, Cummings SR. Comparison of methods to measure low serum estradiol levels in postmenopausal women. J Clin Endocrinol Metab. 2006;91:3791–7. doi: 10.1210/jc.2005-2378. [DOI] [PubMed] [Google Scholar]
- 34.Eliassen AH, Spiegelman D, Xu X, Keefer LK, Veenstra TD, Barbieri RL, Willett WC, Hankinson SE, Ziegler RG. Urinary estrogens and estrogen metabolites and subsequent risk of breast cancer among premenopausal women. Cancer Res. 2012;72:696–706. doi: 10.1158/0008-5472.CAN-11-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falk RT, Brinton LA, Dorgan JF, Fuhrman BJ, Veenstra TD, Xu X, Gierach GL. Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: a nested case-control study. Breast Cancer Res. 2013;15:R34. doi: 10.1186/bcr3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dallal CM, Tice JA, Buist DS, Bauer DC, Lacey JV, Jr, Cauley JA, Hue TF, Lacroix A, Falk RT, Pfeiffer RM, Fuhrman BJ, Veenstra TD, Xu X, Brinton LA B~FIT Research Group. Estrogen metabolism and breast cancer risk among postmenopausal women: a case-cohort study within B~FIT. Carcinogenesis. 2014;35:346–55. doi: 10.1093/carcin/bgt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziegler RG, Anderson WF, Gail MH. Increasing breast cancer incidence in China: the numbers add up. J Natl Cancer Inst. 2008;100:1339–41. doi: 10.1093/jnci/djn330. [DOI] [PubMed] [Google Scholar]
- 38.Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Estrogens as endogenous genotoxic agents--DNA adducts and mutations. J Natl Cancer Inst Monogr. 2000;27:75–93. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- 39.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–82. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 40.Bradlow HL, Hershcopf R, Martucci C, Fishman J. 16 alpha-hydroxylation of estradiol: a possible risk marker for breast cancer. Ann N Y Acad Sci. 1986;464:138–51. doi: 10.1111/j.1749-6632.1986.tb16001.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhu BT, Han GZ, Shim JY, Wen Y, Jiang XR. Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: Insights into the structural determinants favoring a differential subtype binding. Endocrinology. 2006;147:4132–50. doi: 10.1210/en.2006-0113. [DOI] [PubMed] [Google Scholar]
- 42.Kono S, Merriam GR, Brandon DD, Loriaux DL, Lipsett MB, Fujino T. Radioimmunoassay and metabolic clearance rate of catecholestrogens, 2-hydroxyestrone and 2–hydroxyestradiol in man. J Steroid Biochem. 1983;19:627–33. doi: 10.1016/0022-4731(83)90228-5. [DOI] [PubMed] [Google Scholar]
- 43.Pfeiffer E1, Graf E, Gerstner S, Metzler M. Stimulation of estradiol glucuronidation: a protective mechanism against estradiol-mediated carcinogenesis? Mol Nutr Food Res. 2006;50:385–9. doi: 10.1002/mnfr.200500198. [DOI] [PubMed] [Google Scholar]
- 44.Bransfield LA, Rennie A, Visvanathan K, Odwin SA, Kensler TW, Yager JD, Friesen MD, Groopman JD. Formation of two novel estrogen guanine adducts and HPLC/MS detection of 4-hydroxyestradiol-N7-guanine in human urine. Chem Res Toxicol. 2008;21:1622–30. doi: 10.1021/tx800145w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eliassen AH, Ziegler RG, Rosner B, Veenstra TD, Roman JM, Xu X, Hankinson SE. Reproducibility of fifteen urinary estrogens and estrogen metabolites over a 2- to 3-year period in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2009;18:2860–8. doi: 10.1158/1055-9965.EPI-09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matthews CE, Fortner RT, Xu X, Hankinson SE, Eliassen AH, Ziegler RG. Association between physical activity and urinary estrogens and estrogen metabolites in premenopausal women. J Clin Endocrinol Metab. 2012;97:3724–33. doi: 10.1210/jc.2012-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tworoger SS, Missmer SA, Eliassen AH, Barbieri RL, Dowsett M, Hankinson SE. Physical activity and inactivity in relation to sex hormone, prolactin, and insulin-like growth factor concentrations in premenopausal women. Cancer Causes Control. 2007;18:743–52. doi: 10.1007/s10552-007-9017-5. [DOI] [PubMed] [Google Scholar]